Abstract
Background
Increased attention has been focused on the associations of periodontal disease with the onset and progression of cognitive impairment. Although the associations are likely to be multifactorial, few studies have explored the role of mitochondrial dysfunction in the periodontitis‐dementia link.
Methods
Cross‐sectional data of 1,883 participants aged ≥60 years in the National Health and Nutrition Examination Survey 2011‒2014 were analyzed. The following data were collected: 1) general information on sociodemographic, behavioral, and health‐related factors; 2) periodontal status (mean attachment loss [AL] and mean probing depth [PD]); 3) mitochondrion‐derived biomarker of mitochondrial dysfunction (blood sample concentration of methylmalonic acid [MMA]); 4) cognitive function (Consortium to Establish a Registry for Alzheimer's disease immediate recall [CERAD‐IR] and delay recall [CERAD‐DR], animal fluency test, and digit symbol substitution test [DSST]). Mediation analysis weighted for complex survey design was used to assess the effect of MMA on the association of periodontal status with cognitive function after adjusting for potential confounders.
Results
Participants with Stage III and IV periodontitis had lower scores on cognitive performance and higher MMA levels than those with Stages I/II periodontitis. Circulating MMA was significantly associated with CERAD‐DR (weighted β [SE] = −0.076 [0.011]) and DSST (weighted β [SE] = −0.039 [0.009]), which mediated 9.9% and 6.0% of the total association of mean PD with cognitive function. Moreover, MMA mediated 11.7% and 5.8% of the association of mean AL with CERAD‐DR and DSST, respectively.
Conclusion
The findings suggest that MMA, a biomarker of mitochondrial dysfunction, plays a mediating role in the link between periodontitis and cognitive impairment in older adults aged ≥60 years.
Keywords: cognitive function, elderly, methylmalonic acid (MMA), mitochondrial dysfunction, periodontitis
1. INTRODUCTION
Periodontitis affects the connective tissues around the teeth and leads to tooth loss, occlusal dysfunction, and general health problems. As the prevalence of periodontitis increases with age, moderate‐to‐severe periodontitis affects almost two‐thirds (64%) of the older population in the US. 1 Increasing evidence suggests that periodontal inflammation induces oxidative stress locally and attenuates antioxidant capacity peripherally. 2 , 3 The mechanism underlying this phenomenon is recruitment of white blood cells, predominantly polymorphonuclear neutrophils (PMN), 4 triggered by the subgingival biofilm. For defense against periodontal pathogens, the “hyperactivated” PMN phenotype is accompanied by an overproduction of reactive oxygen species (ROS). 4 Increased ROS production in the mitochondria further leads to mitochondria damage and oxidative stress. Many lines of evidence suggest the redox balance disruption caused by periodontitis can explain the development of systemic diseases (e.g., obesity, diabetes mellitus, and atherosclerosis). 5 , 6 Also, the shared pathology via oxidative stress and mitochondrial dysfunction may link periodontitis and neurodegenerative diseases in older adults. 7
Cognitive impairment is a common phenomenon in older people and can be seen as a prodrome of dementia, such as Alzheimer disease (AD). 8 The primary pathogenesis of AD is linked to the accumulation of amyloid‐beta (Aβ) into plaques in the brain. Intracellular Aβ accumulation may aggravate mitochondrial dysfunction and oxidative stress. 9 Specifically, intracellular accumulation of oligomeric Aβ affects mitochondrial function owing to enhanced ROS release and mitochondrial fragmentation. 10 , 11 Impaired mitochondrial production of adenosine triphosphate (ATP) – which provides energy – disturbs axonal transportation, resulting in synaptic dysfunction and ultimately triggering cell death. 12
Mitochondria‐derived methylmalonic acid (MMA) is considered a biomarker of mitochondrial dysfunction. 13 Defects in mitochondrial function, hinder ATP production, and energy disturbance may affect MMA metabolism and induce tissue accumulation of MMA. 14 A recent epidemiological study suggests that the circulating level of MMA is a strong predictor of all‐cause and cardiovascular mortality in the general population. 13 Increased MMA level is also associated with renal dysfunction, heart failure, and neurodegenerative disease. 14 Serum MMA and Vitamin B12 concentrations were predictive of the cognitive decline rate in older adults. 15 Given that MMA is a Vitamin B12‐dependent metabolite, 16 mitochondrial dysfunction and oxidative stress might be involved in physiological and pathological processes.
Several studies show that periodontitis is associated with accelerated cognitive impairment and an increased risk for dementia. 17 A large retrospective cohort study indicated that participants with untreated periodontal disease were at greater risk of developing dementia. 18 Studies from our group and others have highlighted the mediating role of low‐grade inflammation and bacterial invasion in the association between periodontal inflammation and cognitive impairment. 19 , 20 , 21 However, only a few studies have focused on the systemic effect of an increased oxidative burden on the periodontitis–dementia link. Therefore, we aimed to study whether circulating MMA mediates the link between periodontal status and cognitive performance among older adults.
2. MATERIALS AND METHODS
2.1. Study design and population
A cross‐sectional study using the data from the National Health and Nutrition Examination Survey (NHANES) 2011–2012 and 2013–2014 collecting periodontal and cognitive data was conducted. NHANES is a representative survey performed in 2‐year cycles among civilians in the US. The survey contains health and nutrition information from a stratified, multistage probability sample of non‐institutionalized civilians in the US. 22 All data collection procedures for the survey were approved by the National Center for Health Statistics research ethics review board. Written informed consent was obtained from all NHANES participants. 23 The present study was exempt from ethics review because it consists of publicly released NHANES data. We followed the reporting of observational studies in epidemiology (STROBE) criteria to report this study. From the total participants of NHANES 2011–2012 and 2013–2014, elderly individuals (aged ≥60 years) with at least one tooth, who had undergone cognitive function assessments, had received a complete periodontal examination, and had completed laboratory tests for oxidative biomarkers, were included in the present study population.
2.2. Periodontal status assessment
Trained and calibrated examiners conducted periodontal probing examinations in mobile examination centers. Participants who had at least one tooth in the dentition were eligible for a full‐mouth periodontal examination of all four quadrants. Attachment loss (AL) and probing depth (PD) were measured at six sites per tooth using a Hu‐Friedy PCP‐2 periodontal probe. 24 PD and AL have shown an acceptable level of reliability, with inter‐class correlation coefficients ranging from 0.80–0.90 and 0.79–0.86, respectively. 24 Mean PD and mean AL were computed for each participant. These continuous variables were scaled by z‐score transformation. The number of missing teeth was also recorded in the dentition examination.
For the purpose of this study, the 2018 World Workshop Classification System was used to classify periodontitis. 25 First, periodontitis was diagnosed when the interdental AL ≥1 mm in ≥2 non‐adjacent teeth or AL in the buccal/lingual sites was ≥3 mm with PD >3 mm in ≥2 teeth. 26 Second, in terms of periodontitis severity, the maximum AL of 1–2 mm was defined as Stage I periodontitis; 3–4 mm as Stage II; ≥5 mm as Stage III/IV. Third, the complexity of management was also evaluated. Patients with Stage II periodontitis were reclassified as Stage III if the maximum PD was ≥6 mm. Patients with Stage III periodontitis were reclassified as Stage IV if tooth number was <20. 27 Due to the small proportion of participants with Stage I periodontitis (4.6%) in the present study, Stage I and II were combined into one category. In brief, we classified the patients with periodontitis as Stage I/II, Stage III, and Stage IV.
2.3. Cognitive function assessment
Cognitive function assessment was conducted among participants aged ≥60 years. A series of cognitive tests were used in the NHANES 2011–2014, including the tests developed by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD), animal fluency test (AFT), and digit symbol substitution test (DSST). For all performed cognitive tests as described below, higher scores indicate better cognitive performance. All scores were standardized using z‐score transformation.
The CERAD word learning test assesses immediate and delayed recall of new verbal information. 28 , 29 The test consists of two parts: three learning trials, during which participants were asked to read aloud 10 words and subsequently recall as many as possible; and a delayed trial, which was conducted after the AFT and DSST. The CERAD scores are the number of words the participants can recall in three learning trials and one delayed trial, which were designed to assess immediate (ranging from 0 to 30) and delayed (ranging from 0 to 10) ability to learn verbal information, respectively.
The AFT assesses categorical verbal fluency. 30 Participants were instructed to name as many animals as possible within 1 minute and could obtain one point for each named animal. The AFT score was the total number of points obtained.
The DSST, a subtest of the Wechsler Adult Intelligence Scale, 31 was administered to assess executive function and processing speed. 32 Participants were asked to match as many symbols paired with numbers as possible within 2 minutes. Following the standard scoring method, a score was given for each correctly matched symbol (with the maximum score being 133).
2.4. Mediator and covariate assessment
Concentration of circulating MMA is considered a biomarker of mitochondrial dysfunction. 13 Blood samples of participants for measuring MMA concentration were collected via venipuncture in mobile examination centers. Serum MMA was measured by gas chromatography–mass spectrophotometry with cyclohexanol derivatization. In brief, the internal standard solution was added to 75 μL of serum, and MMA was extracted using liquid–liquid extraction. Subsequently, the extracted acid was derivatized with butanol to produce a dibutyl ester. Then, the derivatized sample was reconstituted in acetonitrile–water. MMA was chromatographically separated from other compounds and measured by liquid chromatography–mass spectrometry using multiple reaction monitoring. MMA z‐scores were calculated using the mean and standard deviation of the laboratory value.
A variety of covariates were selected because they were hypothesized to be related to periodontitis and cognitive impairment among older adults. 21 Trained interviewers collected sociodemographic information, health‐related behaviors, and comorbid conditions from in‐person household interviews. These covariates included: 1) sociodemographic variables (age [year], sex [male or female], race or ethnicity, educational attainment [≤high school, college, or > college], and annual household income [<20,000$, 20,000–75,000$, or >75,000$]). We categorized the race or ethnicity as non‐Hispanic White, non‐Hispanic Black, and others (including Mexican American, other Hispanic, and other races [e.g., multi‐racial]); 2) behavioral factors (smoking status [non‐smoker, former smoker, or current smoker], alcohol intake [≥12 drinks/year or <12 drinks/year], and dental visit [<1 year, 1–3 years, or >3 years]); and 3) health conditions (diabetes mellitus, hypertension, dyslipidemia, obesity, abdominal adiposity, cardiovascular disease, and cancer). We described the detailed definitions and categories of health conditions in Supplementary Table S1 in the online Journal of Periodontology, as the previous study. 33
2.5. Statistical analysis
All statistical analyses were adjusted for survey design and weighting variables to account for the complex sampling design. We constructed new sample weights (the original 2‐year sample weight multiplied by 0.5) to account for the use of combined NHANES cycles. The means, proportions, and standard deviations (SD) were calculated and reported to describe the participant characteristics. Comparisons among Stage I/II, III, and IV periodontitis were performed using one‐way ANOVA for continuous variables and the Chi‐square test for categorical variables. Linear regression models were developed to investigate the relationship of Stage I/II, III, and IV periodontitis with the four cognitive assessments (the z scores of CERAD‐immediate recall [CERAD‐IR], CERAD‐delayed recall [CERAD‐DR], AFT, and DSST). The crude model did not adjust for confounders. We developed age‐, sex‐, and race‐ or ethnicity‐adjusted models, and fully adjusted models. The final model was adjusted for sociodemographic variables (age, sex, race or ethnicity, educational attainment, and annual household income), behavioral factors (smoking status, alcohol intake, and dental visit), health‐related factors (diabetes mellitus, hypertension, dyslipidemia, obesity, abdominal adiposity, cardiovascular disease, and cancer), white blood cell count, and the number of lost teeth. Results are presented as estimated effect sizes (β) and standard error (SE).
Mediation analysis was used to investigate whether the associations between periodontal parameters (mean PD and mean AL) and cognitive test scores are mediated by mitochondrial dysfunction or oxidative stress (the z score of MMA, Vitamin B12, and folate). As shown in Figure 1, mediation framework was established on three pathways (a, b, and c), as previous reported. 21 Partial mediation analysis was adopted as the effect of exposure on outcome (path c in Figure 1) was not reduced to zero. The process modeling outlined by Hayes (PROCESS macro v3.5) 34 was performed to estimate the indirect effect of the exposure (X) on the outcome (Y) mediated via the mediator (M). The proportion mediated was calculated using the following formula: .
FIGURE 1.
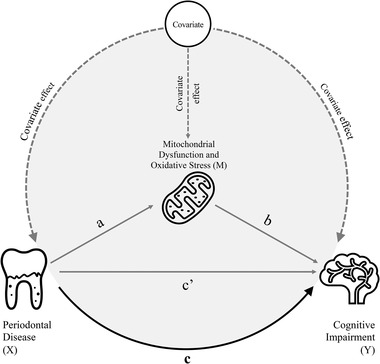
Theoretical diagram of the mediation model for the association between periodontal disease (exposure) and cognitive impairment (outcome) with the biomarker of mitochondrial dysfunction or oxidative stress as a mediator. Path a: regress “mediator (M)” on “exposure (X)” to examine if “exposure (X)” is a significant predictor of the “mediator (M).” If not, then it is unlikely to mediate anything. Path b: regress “outcome (Y)” on “mediator (M)” to test if the “mediator (M)” is significantly associated with “outcome (Y).” If not, then it is unlikely to mediate anything. Path c: regress “outcome (Y)” on “exposure (X)” to test if the “exposure (X)” is significantly predictor of “outcome (Y)” (total effect). Path c': regress “outcome (Y)” on both “exposure (X)” and “mediator (M)” to test if “mediator (M)” is a significant predictor of “outcome (Y)” and to observe whether the association between “exposure (X)” and “outcome (Y)” is attenuated when the “mediator (M)” is included (direct effect)
We performed a sensitivity analysis to demonstrate the robustness of the results. (1) Periodontitis was defined as described by the Centers for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP). 35 The details of the case definition are described in Supplementary Table S2 in the online Journal of Periodontology. Vitamin B12 and folate insufficiency had a relationship to cognitive decline. Thus, we defined serum Vitamin B12 and folate as biomarkers of oxidative stress in the mediation model. Considering the low percentage of missing data from the covariates analyzed (<5%), complete‐case multivariable regression was conducted. The cut‐off for statistical significance was set at P <0.05. Statistical analyses were performed using software.*
3. RESULTS
3.1. General characteristics
Out of 19,931 participants of the NHANES 2011–2014, 2,813 adults aged ≥60 years have a complete periodontal examination. Individuals with missing data on cognitive function and oxidative biomarker assessments (NHANES 2011‒2012: n = 512; 2013–2014: n = 418) were subsequently excluded. Finally, a total of 1,883 participants could be included in the study population (Figure 2). The participants’ mean (SD) age was 68.6 (6.6), 50.3% were men, and 47.2% were non‐Hispanic White individuals. Table 1 displays the characteristics of the study population using the 2018 World Workshop Classification system. There were 564 (29.9%) and 422 (22.4%) participants diagnosed with Stage III and Stage IV periodontitis, respectively. Compared with older adults aged ≥60 years with Stage I/II periodontitis, those with Stage III and IV periodontitis were men, current smokers, less educated, had a lower income and less dental care, and were more likely to have diabetes mellitus (all P < .001). The MMA increased with increasing staging of periodontitis (P < .001). In contrast, the cognitive function scores decreased with worsening periodontal health (P < .001). The population characteristics by CDC/AAP case definition are also described in Supplementary Table S3 in the online Journal of Periodontology.
FIGURE 2.
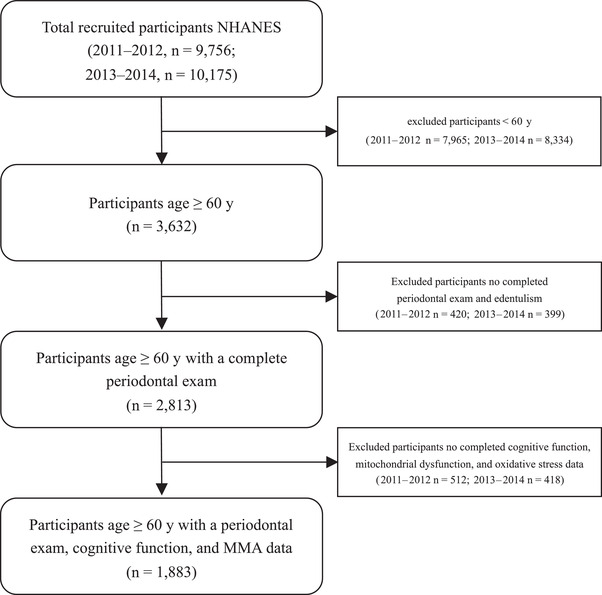
Flow diagram of participants in the National Health and Nutrition Examination Survey study (2011–2014)
TABLE 1.
Characteristics of the study population overall and stratified by periodontal status a
| Overall | Stage I/II | Stage III | Stage IV | P value d | |
|---|---|---|---|---|---|
| Number | 1,883 | 897 | 564 | 422 | |
| Weighted number | 35,954,260 | 20,797,036 | 9,509,648 | 5,647,577 | |
| Continuous variables, mean (SD) | |||||
| Age (year) | 68.60 (6.61) | 68.49 (6.55) | 68.60 (6.66) | 68.84 (6.68) | 0.669 |
| Body mass index (kg/m2) | 28.92 (6.24) | 28.91 (6.11) | 28.81 (5.94) | 29.07 (6.90) | 0.811 |
| Waist‐height ratio (cm/cm) | 0.61 (0.08) | 0.61 (0.09) | 0.61 (0.08) | 0.62 (0.09) | 0.364 |
| Systolic blood pressure (mmHg) | 132.32 (18.59) | 131.01 (17.48) | 132.53 (17.85) | 134.85 (21.42) | 0.002 |
| Diastolic blood pressure (mmHg) | 68.38 (13.75) | 68.60 (13.02) | 69.20 (12.90) | 66.83 (16.10) | 0.023 |
| Non‐HDL cholesterol (mg/dl) | 138.01 (40.48) | 138.97 (40.75) | 137.04 (39.92) | 137.29 (40.72) | 0.619 |
| Glycohemoglobin (%) | 6.03 (1.08) | 5.90 (0.84) | 6.043 (1.11) | 6.30 (1.40) | <0.001 |
| White blood cell count | 6.89 (2.41) | 6.67 (1.96) | 7.06 (2.92) | 7.13 (2.49) | 0.001 |
| Vitamin B12 (pg/mL) | 551 (829.32) | 567 (623.10) | 548 (1218.67) | 522 (506.9) | 0.007 |
| RBC folate (ng/mL) | 588.89 (267.79) | 621.34 (262.33) | 571.16 (275.08) | 544.51 (261.19) | <0.001 |
| CERAD immediate recall | 18.00 (4.75) | 19.41 (4.66) | 17.22 (4.36) | 16.04 (4.47) | <0.001 |
| CERAD delayed recall | 6.29 (2.39) | 7.01 (2.30) | 5.94 (2.25) | 5.25 (2.29) | <0.001 |
| Animal fluency test | 17.04 (5.49) | 17.98 (5.64) | 16.88 (5.37) | 15.26 (4.85) | <0.001 |
| Digit symbol substitution test | 47.90 (17.22) | 53.75 (16.45) | 45.87 (16.26) | 38.19 (14.96) | <0.001 |
| Methylmalonic acid (nmol/L) b | 179 (105) | 162 (95) | 191 (107) | 199 (215) | <0.001 |
| Number of lost teeth b | 6 (10) | 3 (7) | 4 (3) | 16 (5) | <0.001 |
| Mean PD (mm) | 1.47 (0.62) | 1.13 (0.34) | 1.70 (0.60) | 1.89 (0.76) | <0.001 |
| Mean AL (mm) | 2.22 (1.29) | 1.40 (0.42) | 2.49 (0.93) | 3.59 (1.59) | <0.001 |
| Categorical variables, n (%) | |||||
| Male | 947 (50.3) | 345 (38.5) | 358 (63.5) | 244 (57.8) | <0.001 |
| Race or ethnicity e | <0.001 | ||||
| Non‐Hispanic White | 889 (47.2) | 517 (57.6) | 236 (41.8) | 136 (32.2) | |
| Non‐Hispanic Black | 419 (22.3) | 142 (15.8) | 137 (24.3) | 140 (33.2) | |
| Others | 575 (30.5) | 238 (26.5) | 191 (33.9) | 146 (34.6) | |
| Education level c | <0.001 | ||||
| ≤High school | 835 (44.3) | 298 (33.2) | 254 (45.1) | 283 (67.2) | |
| College | 553 (29.4) | 299 (33.3) | 168 (29.8) | 86 (20.4) | |
| >College | 493 (26.2) | 300 (33.4) | 141 (25.0) | 52 (12.4) | |
| Annual household income c | <0.001 | ||||
| <20,000$ | 393 (21.7) | 134 (15.6) | 121 (22.2) | 138 (33.9) | |
| 20,000–75,000$ | 970 (53.5) | 456 (53.0) | 300 (55.1) | 214 (52.6) | |
| >75,000$ | 449 (24.8) | 271 (31.5) | 123 (22.6) | 55 (13.5) | |
| Smoking habit c | <0.001 | ||||
| Non‐smoker | 982 (52.2) | 542 (60.4) | 273 (48.5) | 167 (39.7) | |
| Former smoker | 682 (36.3) | 311 (34.7) | 201 (35.7) | 170 (40.4) | |
| Current smoker | 217 (11.5) | 44 (4.9) | 89 (15.8) | 84 (20.0) | |
| Alcohol intake ≥12 drinks/year c | 1,305 (69.3) | 614 (68.8) | 404 (72.0) | 287 (68.3) | 0.339 |
| Time since the last dental visit c | <0.001 | ||||
| Less than 1 year | 1,268 (67.4) | 704 (78.5) | 352 (62.4) | 212 (50.4) | |
| 1–3 years | 290 (15.4) | 116 (12.9) | 87 (15.4) | 87 (20.7) | |
| More than 3 years | 324 (17.2) | 77 (8.6) | 125 (22.2) | 122 (29.0) | |
| Obesity c | 679 (36.1) | 323 (36.0) | 205 (36.7) | 151 (36.2) | 0.971 |
| Abdominal adiposity c | 609 (32.3) | 282 (32.1) | 186 (33.9) | 141 (35.3) | 0.493 |
| Hypertension c | 597 (31.7) | 260 (29.1) | 189 (33.6) | 148 (35.2) | 0.046 |
| Dyslipidemia | 535 (28.4) | 261 (29.1) | 159 (28.2) | 115 (27.3) | 0.779 |
| Diabetes mellitus c | 370 (19.6) | 140 (15.6) | 114 (20.2) | 116 (27.6) | <0.001 |
| Cardiovascular disease c | 325 (17.3) | 147 (16.4) | 89 (15.8) | 89 (21.1) | 0.059 |
| Cancer c | 361 (19.2) | 193 (21.6) | 96 (17.1) | 72 (17.1) | 0.046 |
Periodontitis was defined by 2018 World Workshop Classification System.
Non‐normal distribution continuous variable, median (interquartile range).
Missing values for total study: education (n = 2; <1%), income (n = 71; 3.8%), smoking (n = 2; <1%), alcohol (n = 9; <1%), dental visit (n = 1; <1%), obesity (n = 11; <1%), abdominal adiposity (n = 56; 3.0%), hypertension (n = 6; <1%), diabetes (n = 5; <1%), cardiovascular disease (n = 1; <1%), and cancer (n = 3; <1%).
All P values were calculated with a two‐sided significance level of 0.05.
Race or ethnicity was categorized as non‐Hispanic White, non‐Hispanic Black, and others (including Mexican American, other Hispanic, and other races [e.g., multi‐racial]).
AL, attachment loss; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; HDL, high‐density lipoprotein; PD, probing depth; RBC, red blood cell; SD, standard deviation.
3.2. Periodontitis and cognitive function
Unadjusted and adjusted associations between periodontitis and cognitive function z‐scores are shown in Table 2. In the weighted univariable analyses, both Stage III and IV periodontitis showed inverse associations with four cognitive functioning tests, compared with Stage I/II periodontitis. Among these associations, the effect size in the AFT was relatively minor. A comparable pattern was observed in the multivariable models adjusted for age, sex, and race or ethnicity. In the weighted multivariable adjusted models, Stage III periodontitis was significantly associated with worse immediate verbal memory (CERAD‐IR: weighted β [SE] = −0.229 [0.023]), worse delayed verbal memory (CERAD‐DR: weighted β [SE] = −0.269 [0.023]), worse verbal fluency (as measured by AFT: weighted β [SE] = −0.080 [0.025]), and worse processing speed (DSST: weighted β [SE] = −0.139 [0.019]). Similarly, Stage IV periodontitis showed a statistically significant association – after adjustment of all covariates – with cognitive function test scores, except that of AFT (Table 2). In the sensitivity analysis, similar results were observed using the CDC/AAP case definition (see Supplementary Table S4 in online Journal of Periodontology).
TABLE 2.
Weighted associations between periodontitis a and cognitive functions
| Weighted β coefficient (SE) | |||
|---|---|---|---|
| Unadjusted | Age‐, sex‐, and race‐ or ethnicity‐adjusted | Fully adjusted b | |
| CERAD immediate recall c | |||
| Stage I/II | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Stage III | −0.449 (0.024) | −0.294 (0.022) | −0.229 (0.023) |
| Stage IV | −0.766 (0.029) | −0.617 (0.028) | −0.331 (0.036) |
| CERAD delayed recall c | |||
| Stage I/II | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Stage III | −0.484 (0.024) | −0.334 (0.023) | −0.269 (0.023) |
| Stage IV | −0.737 (0.030) | −0.606 (0.028) | −0.355 (0.038) |
| Animal fluency test c | |||
| Stage I/II | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Stage III | −0.232 (0.026) | −0.118 (0.025) | −0.080 (0.025) |
| Stage IV | −0.601 (0.032) | −0.450 (0.031) | 0.041 (0.039) |
| Digit symbol substitution test c | |||
| Stage I/II | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Stage III | −0.420 (0.023) | −0.223 (0.020) | −0.139 (0.019) |
| Stage IV | −0.845 (0.028) | −0.615 (0.025) | −0.144 (0.031) |
Periodontitis was defined by the 2018 World Workshop Classification System.
Adjusted for age, sex, race or ethnicity, education, income, health behaviors (smoking habit, alcohol consumption, and dental visit), white blood cell count, number of lost teeth, and health conditions (abdominal adiposity, obesity, hypertension, dyslipidemia, dysglycemia, cardiovascular disease, and cancer).
CERAD immediate recall is a test of immediate verbal memory; CERAD delayed recall is a test of delayed verbal memory; animal fluency test is a test of verbal fluency; and digit symbol substitution test is a test of processing speed. Then, these continuous scores of cognitive tests were transformed to z‐score as outcome variables in the linear regression model.
Bold indicates P value <0.05.
CERAD, Consortium to Establish a Registry for Alzheimer's Disease; SE, standard error.
3.3. Mediation analysis
Mediation analysis was performed with full adjustment for potential confounders. Circulating MMA level was statistically significantly associated with mean PD (weighted β [SE] = 0.170 [0.013], P < .001) and mean AL (weighted β [SE] = 0.202 [0.015], P < .001, Table 3). After adjustment for covariates and mean PD, the MMA level was inversely associated with delayed verbal memory (as measured by CERAD‐DR; weighted β [SE] = −0.076 [0.011], P < .001) and processing speed (as measured by DSST; weighted β [SE] = −0.039 [0.009], P < .001, Table 3). Comparable results were obtained when mean AL was regarded as an exposure variable. The proportion of the association of mean PD and mean AL with verbal memory mediated by MMA ranged from 9.9% to 11.7% (Table 3). The indirect effect of both mean PD and mean AL on processing speed mediated by MMA was statistically significant, with the proportion mediated being 6.0% and 5.8%, respectively. Sensitivity analysis showed the mediating effect of Vitamin B12 is consistent with MMA (see Supplementary Table S5 in online Journal of Periodontology). In comparison, folate was not found to mediate the relationship between periodontal parameters and cognitive test scores (see Supplementary Table S6 in online Journal of Periodontology).
TABLE 3.
Mediating role of methylmalonic acid on the association between periodontal parameters and cognitive functions a
| Weighted β coefficient (SE) | CERAD‐IR | CERAD‐DR | AFT | DSST |
|---|---|---|---|---|
| Exposure: mean probing depth (PD) | ||||
| Path a (X→M) | 0.170 (0.013)*** | 0.170 (0.013)*** | 0.170 (0.013)*** | 0.170 (0.013)*** |
| Path b (M∣X→Y) | −0.012 (0.010) | −0.076 (0.011)*** | −0.027 (0.014) | −0.039 (0.009)*** |
| Path c (X→Y) | −0.146 (0.013)*** | −0.131 (0.013)*** | 0.044 (0.024) | −0.117 (0.011)*** |
| Path c' (X∣M→Y) | −0.144 (0.013)*** | −0.118 (0.013)*** | 0.040 (0.023) | −0.110 (0.011)*** |
| Indirect effect (X→M→Y) | −0.002 (0.002) | −0.013 (0.003)* | 0.004 (0.002) | −0.007 (0.003)* |
| Proportion mediated b | NA c | 9.9% | NA c | 6.0% |
| Exposure: mean attachment loss (AL) | ||||
| Path a (X→M) | 0.202 (0.015)*** | 0.202 (0.015)*** | 0.202 (0.015)*** | 0.202 (0.015)*** |
| Path b (M∣X→Y) | −0.016 (0.010)*** | −0.078 (0.011)*** | 0.019 (0.011) | −0.038 (0.009)*** |
| Path c (X→Y) | −0.121 (0.015)*** | −0.137 (0.015)*** | 0.034 (0.014)* | −0.138 (0.012)*** |
| Path c' (X∣M→Y) | −0.120 (0.015)*** | −0.121 (0.015)*** | 0.032 (0.014)* | −0.130 (0.012)*** |
| Indirect effect (X→M→Y) | −0.001 (0.002) | −0.016 (0.004)* | 0.002 (0.003) | −0.008 (0.002)* |
| Proportion mediated b | NA c | 11.7% | NA c | 5.8% |
Indicates P value < 0.05; ***indicates P value < 0.001.
“exposure” (X): mean PD or mean AL; “mediator (M)”: methylmalonic acid; “outcome” (Y): assessment scores of cognitive functions. Mean PD or mean AL was to z‐score as exposure variables. Methylmalonic acid was to z‐score as a mediator in the mediation analysis. CERAD‐IR is a test of immediate verbal memory; CERAD‐DR is a test of delayed verbal memory; AFT is a test of verbal fluency; and DSST is a test of processing speed. These assessment scores of cognitive tests were transformed to z‐score as outcome variables in the mediation analysis.
Analytical steps of mediation model include: path a represents the effect of “exposure” (X) on “mediator (M)”; path b represents the effect of “mediator (M)” on cognitive test scores “outcome” (Y); path c represents the total effect of “exposure” (X) on “outcome” (Y), without the adjustment for “mediator (M)”; and path c’ represents the direct effect of “exposure” (X) on “outcome” (Y), after adjustment for “mediator (M).”; indirect effect represents an effect of “exposure (X)” on “(outcome) Y” mediated by mediator (M). Proportion mediated = .
In path b, “mediator (M)” is not significantly associated with “outcome (Y),” indicating it is unlikely to mediate anything. Mediation model is not appropriate.
All models were adjusted for sociodemographic variables, health behaviors, health conditions, white blood cell count, and the number of lost teeth.
AFT, animal fluency test; CERAD‐DR, Consortium to Establish a Registry for Alzheimer's Disease Delayed Recall; CERAD‐IR, Consortium to Establish a Registry for Alzheimer's Disease Immediate Recall; DSST, digit symbol substitution test; NA, not applicable; SE, standard error.
4. DISCUSSION
This study aimed to study whether circulating MMA mediates the link between periodontal status and cognitive performance among older adults in the NHANES study cohort. Community‐dwelling older adults in the United States with Stage III and IV periodontitis exhibited worse cognitive performance than Stage I/II periodontitis. Mitochondrial dysfunction may play a mediating role in the association of periodontitis with poor delayed verbal memory and processing speed. This suggests that the mediating effect may have less influence on memory encoding but may be more related to memory consolidation and executive function. Future work is needed to evaluate the speculation.
The observed relationships between periodontitis and low cognitive performance are in line with a recent study using the NHANES data. 36 Previous studies investigated the relationship between mitochondrial dysfunction, periodontitis, and systemic conditions, including renal function, 37 glycemic status, 38 , 39 endothelial function, 40 , 41 and cardiovascular disease. 42 A recent cross‐sectional study on 770 patients reported a bidirectional and causal relationship between periodontal inflammation and Stage 3–5 chronic kidney disease. 37 Structural equation modeling showed that the observed relationship was significantly mediated by systemic oxidative stress (as evaluated by protein carbonyls and isoprostanes). However, concerning periodontal and neurodegenerative diseases, there are a lack of large‐scale epidemiological studies involving redox imbalance. An animal experimental study showed that apolipoprotein knockout (ApoE –/–) mice with a Porphyromonas gingivalis infection had higher oxidative stress levels than sham‐infected ApoE–/– mice. 43 Meanwhile, another animal study recently reported that salvianolic acid B protects against Porphyromonas gingivalis‐induced cognitive impairment by inhibiting the ROS and increasing antioxidative enzyme levels. 44 The mechanism underlying the findings from the animal studies is consistent with the assumed mechanism in the present population‐based study.
A biologically plausible mechanism for the observed associations is that periodontitis and cognitive impairment may share a pathogenic background via mitochondrial dysfunction. Circulating MMA, a mitochondrial intermediate metabolite, was recently suggested as a promising biomarker of mitochondrial dysfunction. 13 Relatively low amounts of MMA are produced in the brain because of the low methylmalonyl‐CoA mutase activity. Nevertheless, the intracerebral accumulation of MMA could lead to neurodegeneration and mitochondrial energy metabolism impairment in methylmalonic acidemia. 45 In vivo study, chronic MMA treated rats presented water maze behavioral deficits related to oxidative brain damage. 46 Fibroblasts from patients with methylmalonic aciduria, where MMA accumulates in tissues, tended to show an impaired mitochondrial morphology and decreased mitochondrial respiration compared with control cells. 47 Therefore, the present study identified MMA as a novel but biologically plausible biomarker of mitochondrial dysfunction.
Sensitivity analysis showed similar results when regarding Vitamin B12 as biomarker of oxidative stress. Previous research supports that elevated serum MMA accompanied by vitamin B12 deficiency is associated with cognitive impairment. 48 Vitamin B12 itself is thought to possess antioxidant properties that mostly manifest as (1) modulation of cytokines and growth factors, (2) direct/indirect scavenging of ROS, and (3) reduction of oxidative stress caused by homocysteine and advanced glycation end products. 49 Moreover, a prospective cohort study reported an inverse relationship between serum vitamin B12 and periodontitis progression. 50 The role of mitochondrial oxidative stress in periodontal destruction has been reviewed before. 51 Periodontitis‐derived pathogens alter energy production processes in the mitochondria and disrupt the balance between ROS production and antioxidant defenses. 6 In brief, shared features of periodontitis and cognitive impairments are mitochondrial oxidative stress, that reinforces each other.
The findings of the present study have substantial etiological and clinical implications. The interplay with oxidative stress and inflammation may be involved in the physiological processes and pathological processes of periodontitis and warrants further in‐depth study. A large‐scale cohort study suggested that maintaining periodontal health could be a cost‐effective way of reducing or delaying the development of dementia. 18 Mitochondrial dysfunction may be a potential mediator of the periodontitis–dementia link. Our findings suggest that interventions with antioxidative supplements can be performed among patients with periodontitis to simultaneously improve periodontal health and prevent the onset of cognitive dysfunction; however, the findings need to be confirmed in future research.
The present study has several strengths. To the best of our knowledge, this is the first population‐based study to reveal that mitochondrial dysfunction plays a mediating role in the periodontitis–dementia relationship. Notably, the observed relationship persisted after adjustment for potential confounders, including sociodemographic characteristics, lifestyle factors, and several health conditions. Moreover, sensitivity analysis makes our results robust and provides strength to our analysis. Lastly, four cognitive function measures (including CERAD‐IR, CERAD‐DR, DSST, and animal fluency test) were used to adequately assess the domain‐specific cognitive performance.
This study also has several limitations. Firstly, data on measures of gingival bleeding were not available in the NHANES 2011–2014. Gingival bleeding is regarded as an important indicator of an individual's activity and burden of periodontal inflammation when studying cognitive impairment. Secondly, there was no data on systemic inflammatory markers and cytokines (e.g., tumor necrosis factor‐α, interleukin, and C‐reactive protein) in the NHANES data set. Oxidative stress and inflammation are interrelated and mutually amplify each other in the pathophysiological processes. 6 Although white blood cell count is regarded as a marker of systemic inflammation and regressed in the multivariable model, it is hard to exclude the potential impact of the inflammatory mechanism on the observed relationship. Thirdly, the cross‐sectional study design did not allow us to determine causal and temporal relationships. Treatment studies with a long‐term follow‐up are needed to provide a more comprehensive picture of periodontitis and cognitive impairment and confirm the role of mitochondrial dysfunction in an underlying biological mechanism.
5. CONCLUSIONS
The present study suggests that increased severity of periodontitis is negatively associated with multiple measures of cognitive performance in older adults in the US. MMA accounted for a statistically significant proportion of the association of periodontitis with delayed verbal memory and processing speed. The present findings suggest a potential role for mitochondrial dysfunction in mediating the negative effect of periodontitis on cognitive function. Although future prospective studies need to confirm these preliminary results, the findings may have substantial public health relevance. Maintaining the periodontal health of the elderly may reduce the pathogenic effects of systemic oxidative burden on the progression of cognitive impairment to dementia.
AUTHOR CONTRIBUTIONS
An Li was responsible for study conception and design, data analysis and interpretation, statistical analyses, and manuscript drafting; Mi Du contributed to the study conception and design, data interpretation, and manuscript drafting; Yuntao Chen – a statistical consultant – contributed to the study design and statistical analyses and critically reviewed the manuscript; Luc A.M. Marks contributed to the data interpretation and critically reviewed the manuscript; Anita Visser contributed to the data interpretation and critically reviewed the manuscript; Shulan Xu contributed to the data interpretation and critically reviewed the manuscript; and Geerten‐Has E. Tjakkes contributed to the study conception and design and data interpretation and critically reviewed the manuscript. All authors gave final approval and agreed to be held accountable for all aspects of this work to ensure integrity and accuracy.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The authors thank the National Health and Nutrition Examination Survey (NHANES) staff and investigators. A special thanks to all who participated in the NHANES survey for making this study possible through their participation. An Li, as a postdoctoral researcher, conducted the study at Southern Medical University and University Medical Center Groningen with funding from the Science Research Cultivation Program of Stomatological Hospital, Southern Medical University, China (No. PY2021007). The authors also acknowledge the support from the Major Innovation Projects in Shandong Province, China (No. 2021SFGC0502) and funding support from the University of Groningen, The Netherlands. Ethical approval for this study was not required. The authors report no conflicts of interest related to this study.
Li A, Du M, Chen Y, et al. Periodontitis and cognitive impairment in older adults: The mediating role of mitochondrial dysfunction. J Periodontol. 2022;93:1302–1313. 10.1002/JPER.21-0620
[Correction added on 11 June 2022, after first online publication: In Figure 2 on page 1306, the age of the excluded participants was corrected to < 60 y.]
Footnotes
SPSS 26.0, IBM, Chicago, IL.
REFERENCES
- 1. Eke PI, Dye BA, Wei L, Thornton‐Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914‐920. 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- 2. Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515‐521. 10.1111/j.1600-051X.2004.00509.x [DOI] [PubMed] [Google Scholar]
- 3. Chapple IL, Milward MR, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr. 2007;137:657‐664. 10.1093/jn/137.3.657 [DOI] [PubMed] [Google Scholar]
- 4. Sczepanik FSC, Grossi ML, Casati M, et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol 2000. 2020;84:45‐68. 10.1111/prd.12342 [DOI] [PubMed] [Google Scholar]
- 5. Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: understanding its relation with other systemic diseases. Front Physiol. 2017;8:693. 10.3389/fphys.2017.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullon P, Newman HN, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000. 2014;64:139‐153. 10.1111/j.1600-0757.2012.00455.x [DOI] [PubMed] [Google Scholar]
- 7. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787‐795. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 8. Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551‐2561. 10.1001/jama.2014.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson DSA, Oliver PL. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel). 2020;9:743. 10.3390/antiox9080743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horowitz MP, Greenamyre JT. Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S551‐S568. 10.3233/JAD-2010-100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951‐957. 10.1172/JCI64125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo J, Cho H, Seol Y, et al. Power failure of mitochondria and oxidative stress in neurodegeneration and its computational models. Antioxidants (Basel). 2021;10:229. 10.3390/antiox10020229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang S, Liu Y, Liu J, et al. Mitochondria‐derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all‐cause and cardiovascular mortality in the general population. Redox Biol. 2020;37:101741. 10.1016/j.redox.2020.101741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stepien KM, Heaton R, Rankin S, et al. Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non‐metabolic disorders. J Clin Med. 2017;6:71. 10.3390/jcm6070071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72:361‐367. 10.1212/01.wnl.0000341272.48617.b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green R, Allen LH, Bjørke‐Monsen AL, et al. Vitamin B(12) deficiency. Nat Rev Dis Primers. 2017;3:17040. 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- 17. Nadim R, Tang J, Dilmohamed A, et al. Influence of periodontal disease on risk of dementia: a systematic literature review and a meta‐analysis. Eur J Epidemiol. 2020;35:821‐833. 10.1007/s10654-020-00648-x [DOI] [PubMed] [Google Scholar]
- 18. Lee YL, Hu HY, Huang LY, Chou P, Chu D. Periodontal disease associated with higher risk of dementia: population‐based cohort study in Taiwan. J Am Geriatr Soc. 2017;65:1975‐1980. 10.1111/jgs.14944 [DOI] [PubMed] [Google Scholar]
- 19. Beydoun MA, Beydoun HA, Hossain S, El‐Hajj ZW, Weiss J, Zonderman AB. Clinical and bacterial markers of periodontitis and their association with incident all‐cause and Alzheimer's disease dementia in a large national survey. J Alzheimers Dis. 2020;75:157‐172. 10.3233/JAD-200064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sparks Stein P, Steffen MJ, Smith C, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer's disease. Alzheimers Dement. 2012;8:196‐203. 10.1016/j.jalz.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li A, Chen Y, van der Sluis LWM, Schuller AA, Tjakkes GH. White blood cell count mediates the association between periodontal inflammation and cognitive performance measured by digit symbol substitution test among older U.S. adults. J Gerontol A Biol Sci Med Sci. 2021;76:1309‐1315. 10.1093/gerona/glaa223 [DOI] [PubMed] [Google Scholar]
- 22. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design. Vital Health Stat 2. 2014:2011‐2014. [PubMed] [Google Scholar]
- 23. Curtin LR, Mohadjer LK, Dohrmann SM, et al. The National Health and Nutrition Examination Survey: sample design, 1999–2006. Vital Health Stat 2. 2012:1‐39. [PubMed] [Google Scholar]
- 24. Dye BA, Afful J, Thornton‐Evans G, Iafolla T. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2011–2014. BMC Oral Health. 2019;19:95. 10.1186/s12903-019-0777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri‐implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S162‐S170. 10.1111/jcpe.12946 [DOI] [PubMed] [Google Scholar]
- 26. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159‐S172. 10.1002/JPER.18-0006 [DOI] [PubMed] [Google Scholar]
- 27. Jiao J, Jing W, Si Y, et al. The prevalence and severity of periodontal disease in Mainland China: data from the Fourth National Oral Health Survey (2015‐2016). J Clin Periodontol. 2021;48:168‐179. 10.1111/jcpe.13396 [DOI] [PubMed] [Google Scholar]
- 28. Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141‐145. 10.1001/archneur.1989.00520380041011 [DOI] [PubMed] [Google Scholar]
- 29. Fillenbaum GG, van Belle G, Morris JC, et al. Consortium to establish a registry for Alzheimer's disease (CERAD): the first twenty years. Alzheimers Dement. 2008;4:96‐109. 10.1016/j.jalz.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whiteside DM, Kealey T, Semla M, et al. Verbal fluency: language or executive function measure? Appl Neuropsychol Adult. 2016;23:29‐34. 10.1080/23279095.2015.1004574 [DOI] [PubMed] [Google Scholar]
- 31. Wechsler D, Wechsler adult intelligence scale‐revised (WAIS‐R): Psychological Corporation; 1981.
- 32. Parkin AJ, Java RI. Deterioration of frontal lobe function in normal aging: influences of fluid intelligence versus perceptual speed. Neuropsychology. 1999;13:539‐545. 10.1037//0894-4105.13.4.539 [DOI] [PubMed] [Google Scholar]
- 33. Li A, Chen Y, Visser A, Marks LAM, Tjakkes GE. Combined association of cognitive impairment and poor oral health on mortality risk in older adults: results from the NHANES with 15 years of follow‐up. J Periodontol. 2021. 10.1002/JPER.21-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayes AF, Rockwood NJ. Regression‐based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav Res Ther. 2017;98:39‐57. 10.1016/j.brat.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 35. Eke PI, Page RC, Wei L, Thornton‐Evans G, Genco RJ. Update of the case definitions for population‐based surveillance of periodontitis. J Periodontol. 2012;83:1449‐1454. 10.1902/jop.2012.110664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Botelho J, Leira Y, Viana J, et al. The role of inflammatory diet and vitamin d on the link between periodontitis and cognitive function: a mediation analysis in older adults. Nutrients. 2021;13:924. 10.3390/nu13030924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma P, Fenton A, Dias IHK, et al. Oxidative stress links periodontal inflammation and renal function. J Clin Periodontol. 2021;48:357‐367. 10.1111/jcpe.13414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun X, Mao Y, Dai P, et al. Mitochondrial dysfunction is involved in the aggravation of periodontitis by diabetes. J Clin Periodontol. 2017;44:463‐471. [DOI] [PubMed] [Google Scholar]
- 39. Allen EM, Matthews JB, OH DJ, Griffiths HR, Chapple IL. Oxidative and inflammatory status in Type 2 diabetes patients with periodontitis. J Clin Periodontol. 2011;38:894‐901. 10.1111/jcpe.12711 [DOI] [PubMed] [Google Scholar]
- 40. Martinez‐Herrera M, López‐Domènech S, Silvestre FJ, et al. Chronic periodontitis impairs polymorphonuclear leucocyte‐endothelium cell interactions and oxidative stress in humans. J Clin Periodontol. 2018;45:1429‐1439. 10.1111/jcpe.13027 [DOI] [PubMed] [Google Scholar]
- 41. Martínez‐Herrera M, Abad‐Jiménez Z, Silvestre FJ, et al. Effect of non‐surgical periodontal treatment on oxidative stress markers in leukocytes and their interaction with the endothelium in obese subjects with periodontitis: a pilot study. J Clin Med. 2020;9:2117. 10.3390/jcm9072117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bullon P, Cordero MD, Quiles JL, et al. Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic Biol Med. 2011;50:1336‐1343. 10.1016/j.freeradbiomed.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 43. Rokad F, Moseley R, Hardy RS, et al. Cerebral oxidative stress and microvasculature defects in TNF‐α expressing transgenic and Porphyromonas gingivalis‐infected ApoE‐/‐ mice. J Alzheimers Dis. 2017;60:359‐369. 10.3233/JAD-170304 [DOI] [PubMed] [Google Scholar]
- 44. Liu J, Wang Y, Guo J, Sun J, Sun Q. Salvianolic acid B improves cognitive impairment by inhibiting neuroinflammation and decreasing Aβ level in Porphyromonas gingivalis‐infected mice. Aging (Albany NY). 2020;12:10117‐10128. 10.18632/aging.103306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Melo DR, Kowaltowski AJ, Wajner M, Castilho RF. Mitochondrial energy metabolism in neurodegeneration associated with methylmalonic acidemia. J Bioenerg Biomembr. 2011;43:39‐46. [DOI] [PubMed] [Google Scholar]
- 46. Pettenuzzo LF, Schuck PF, Wyse AT, et al. Ascorbic acid prevents water maze behavioral deficits caused by early postnatal methylmalonic acid administration in the rat. Brain Res. 2003;976:234‐242. 10.1007/s10863-011-9330-2 [DOI] [PubMed] [Google Scholar]
- 47. Brasil S, Richard E, Jorge‐Finnigan A, et al. Methylmalonic aciduria cblB type: characterization of two novel mutations and mitochondrial dysfunction studies. Clin Genet. 2015;87:576‐581. 10.1111/cge.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey RL, Jun S, Murphy L, et al. High folic acid or folate combined with low vitamin B‐12 status: potential but inconsistent association with cognitive function in a nationally representative cross‐sectional sample of US older adults participating in the NHANES. Am J Clin Nutr. 2020;112:1547‐1557. 10.1093/ajcn/nqaa239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van de Lagemaat EE, de Groot L, van den Heuvel E. Vitamin B(12) in relation to oxidative stress: a systematic review. Nutrients. 2019;11:482. 10.3390/nu11020482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zong G, Holtfreter B, Scott AE, et al. Serum vitamin B12 is inversely associated with periodontal progression and risk of tooth loss: a prospective cohort study. J Clin Periodontol. 2016;43:2‐9. 10.1111/jcpe.12483 [DOI] [PubMed] [Google Scholar]
- 51. Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160‐232. 10.1111/j.1600-0757.2006.00178.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION


