Abstract
Objectives
The management of incidental findings of FDG‐avid tonsils on PET/CT (IFT) is unclear. We aimed to explore the prevalence of malignancy in IFT, identify risk factors for malignancy, and calculate optimal cutoffs of maximum standardized uptake values (SUVmax) to discriminate between benign and malignant lesions.
Methods
All patients who were tonsillectomized at our institution because of IFT from October 2011 to December 2020 were included. Patients undergoing PET/CT due to suspected tonsillar disease or cancer of unknown primary were excluded.
Results
In total, 77 patients were included, of which 11 (14%) of them had IFT malignancy. Dysphagia (p = 0.019) and alcohol abuse (p = 0.035) were associated with malignancy. Absolute SUVmax cutoff (≥9: sensitivity 100%; specificity 53%) was superior to SUVmax side‐to‐side ratio (≥1.5: sensitivity 64%; specificity 70%) to discriminate between benign and malignant lesions.
Conclusion
We recommend tonsillectomy for patients with IFT displaying SUVmax ≥ 9.0, ratio ≥ 1.5, or symptoms or findings suggesting malignancy.
Level of Evidence
3 Laryngoscope, 132:2370–2378, 2022
Keywords: incidental, FDG‐PET/CT, tonsil, malignancy, cancer
INTRODUCTION
Two‐deoxy‐2‐[18F]flouro‐D‐glucose (FDG) positron emission tomography/computed tomography (PET/CT) is a sensitive modality for diagnosing and staging of patients with different types of cancer. The number of applications for FDG‐PET/CT continues to increase for oncological and non‐oncological conditions, 1 and thus also the number of incidental focal FDG‐avid findings. Further knowledge on how to manage these findings is therefore important in the pursuit for identifying critical diseases and limiting unnecessary interventions.
Diffuse FDG uptake in Waldeyer's ring is considered a physiological finding and uni‐ or bilaterally increased FDG uptake in the palatine tonsil(s) is predominately related to various benign conditions including inflammation. 2 , 3 , 4 However, focally increased FDG uptake, and especially asymmetrically increased FDG uptake, in the palatine tonsils has been described as highly suspicious for squamous cell carcinoma (SCC). 5 Unfortunately, there is currently no consensus on how to define pathologically increased FDG uptake in the palatine tonsils, and evaluations are therefore still based on individual assessments of asymmetry and an overall evaluation of FDG uptake on PET/CT.
The maximum standardized uptake value (SUVmax) is a metric for quantifying FDG uptake in tissue on PET/CT. Previous studies suggest different cutoff values of absolute SUVmax (range 4.6–8.0) and SUVmax side‐to‐side ratios (range 1.24–1.6) between tonsils to discriminate between benign and malignant tonsillar lesions. 5 , 6 , 7 , 8 The weakness of these studies is that they also included patients with head and neck carcinoma of unknown primary (CUP) and suspected or histologically proven head and neck primary cancer cases as well as patients with “true” incidental focal FDG‐avid palatine tonsil(s) (IFT) in patients without suspected tonsillar disease and thus mixed different cohorts of patients undergoing FDG‐PET/CT with very different prevalence of tonsillar malignancy.
Based on the lack of knowledge on the prevalence of malignancy and evidence for how to manage IFT, our institution recommended patients with IFT to undergo tonsillectomy unless there are cogent contraindications. With the increasing number of patients with IFT, the question is whether the current management is the most optimal solution, or a more precise method for identifying patients with a high or low risk of malignancy based on demographic, clinical, and scan specific data is available.
The aims of the current study were to (1) explore the prevalence of malignancy in IFT, (2) identify potential risk factors for malignancy, and (3) calculate cutoff values for absolute and side‐to‐side ratios of SUVmax to discriminate benign and malignant lesions in IFT.
MATERIALS AND METHODS
Study Population
The medical records of all 754 patients aged ≥30 years, who were tonsillectomized (uni‐ or bilaterally) for any reason at the Department of Otorhinolaryngology, Head and Neck Surgery, Aarhus University Hospital between October 1, 2011, and December 31, 2020, were retrospectively investigated.
All patients with IFT were included in the study. IFT was defined as an unexpected increase of FDG uptake in one or both palatine tonsils in patients undergoing PET/CT for any reason but suspected tonsillar disease or as part of CUP, as these patients were considered a very different clinical entity with a different (higher) probability of tonsillar malignancy compared with “true” IFT.
For the current study, the following symptoms and findings were considered suggestive of tonsillar malignancy: throat pain or soreness, otalgia (ipsilateral to IFT), dysphagia, tonsillar asymmetry, visible tumor/ulceration, palpable tumor/induration, and tonsillar hyperemia.
The study was approved by the Danish Data Protection Agency (1‐16‐02‐604‐20) and the Danish Patient Safety Authority (1‐45‐70‐27‐21). Informed consent for controls was obtained under the umbrella of the HYPOTHESIS study and approved by the Central Denmark Region Committees on Health Research Ethics (1‐10‐72‐188‐19).
FDG‐PET/CT Imaging
Maximum standardized uptake values (SUVmax) were measured bilaterally in the tonsil region, and the ratios of the SUVmax between the tonsils were calculated in Pmod version 4.006 using the semi‐quantification tools present in PBAS. PET/CT scan specific data are provided in Supporting Information, Appendix 1, in the online version of this article. Currently, there is no clear definition of increased uptake in the palatine tonsil(s). The initial evaluation was therefore based on individual assessments of asymmetry and an overall evaluation of FDG uptake.
As a reference control population, we used an equal number of patients ≥30 years (n = 77, 49% males, mean age 60 years) who had an FDG‐PET/CT performed at the Department of Nuclear Medicine, Aarhus University Hospital, as part of their clinical evaluation for malignant or inflammatory disease (suspected lung cancer (n = 36), lymphoma (n = 11), occult cancer (n = 8), infection (n = 7), vasculitis (n = 6), sarcoidosis (n = 5), colon cancer (n = 4)). Controls undergoing FDG‐PET/CT because of suspected tonsillar disease or as part of CUP were excluded.
Statistical Analysis
The Student's t‐test was used for analyzing normally distributed, continuous variables and the Kruskal–Wallis test for non‐normally distributed, continuous variables. The Fisher's exact test was used for categorical variables in between‐groups comparisons. The normality of data was assessed using quantile–quantile (QQ) plots. Receiver operating characteristic analysis (ROC) was used to identify the optimal SUVmax cutoff values for differentiating benign and malignant IFT. Results were considered statistically significant when p < 0.05. All statistical analyses were performed using STATA version 15.1.
RESULTS
In total, 77 patients undergoing bilateral (n = 60) or unilateral (n = 17) tonsillectomy because of IFT were included. Tonsillar histology examinations revealed malignancy in 11 (14%) patients. The clinical characteristics of patients with and without IFT malignancy are shown in Table I.
TABLE I.
Clinical Characteristics of Patients with Incidental Focal FDG‐Avid Palatine Tonsils (IFT) on FDG‐PET/CT Stratified by Tonsillar Histological Findings with Calculated Odds Ratios (OR) for Potential Risk Factors for Malignancy.
| Histological Findings | ||||
|---|---|---|---|---|
| Variable | Malignant (n = 11) | Benign (n = 66) | OR (95% CI) | p‐value |
| Age (years), mean (SD) | 63.2 (7.4) | 64.0 (13.3) | — | 0.85 a |
| Gender, n (%) | ||||
| Male | 8 (73%) | 29 (44%) | 3.4 (0.7–21.3) | 0.11 b |
| Female | 3 (27%) | 37 (56%) | ||
| History of malignancy, n (%) | 5 (45%) | 26 (39%) | 1.3 (0.3–5.6) | 0.75 b |
| History of head and neck radiotherapy, n (%) | 0 | 2 (3%) | — | 1.00 b |
| Previous tonsillectomy, n (%) | 0 | 5 (8%) | — | 1.00 b |
| Smoking status, n (%) | ||||
| Current | 5 (45%) | 16 (24%) | 2.6 (0.5–11.7) | 0.16 b |
| Previously | 5 (45%) | 25 (38%) | 1.4 (0.3–6.0) | 0.74 b |
| Never | 1 (9%) | 25 (38%) | 0.16 (0.004–1.3) | 0.09 b |
| Smoking load c (pack years), mean (SD) | 37.9 (18.9) | 38.3 (22) | — | 0.96 a |
| Alcohol abuse d (current/previous), n (%) | 3 (27%) | 3 (5%) | 7.9 (0.9–66.6) | 0.035 b |
| Oropharyngeal symptoms, n (%) | ||||
| None | 7 (64%) | 56 (85%) | 0.3 (0.06–1.8) | 0.11 b |
| Throat pain/soreness | 2 (18%) | 10 (15%) | 1.2 (.1–7.4) | 0.68 b |
| Otalgia e | 1 (9%) | 0 | — | 0.14 b |
| Dysphagia | 2 (18%) | 0 | — | 0.019 b |
| Objective findings, n (%) | ||||
| No suspicion | 4 (36%) | 41 (62%) | 0.3 (0.1–1.6) | 0.18 b |
| Tonsillar asymmetry | 5 (45%) | 20 (30%) | 1.9 (0.4–8.5) | 0.32 b |
| Visible tumor/ulceration | 2 (18%) | 3 (5%) | 4.7 (0.3–45.6) | 0.15 b |
| Palpable tumor | 4 (36%) | 8 (12%) | 4.1 (0.7–20.8) | 0.063 b |
| Tonsillar hyperemia | 2 (18%) | 3 (5%) | 4.7 (0.3–45.6) | 0.15 b |
| Elevated biochemical parameters f , n (%) | ||||
| C‐reactive protein | 3 (27%) | 9 (14%) | 2.4 (0.3–12.5) | 0.36 b |
| Leucocyte count | 2 (18%) | 7 (11%) | 1.9 (0.2–12.1) | 0.61 b |
| Neutrophil count | 1 (9%) | 3 (5%) | 2.1 (0.04–29.0) | 0.47 b |
| Lymphocyte count | 1 (9%) | 2 (3%) | 3.2 (0.05–65.6) | 0.37 b |
| Side of IFT | 0.37 (0.05–4.5) | 0.26 b | ||
| Unilateral | 9 | 61 | ||
| Bilateral | 2 | 5 | ||
CI = confidence interval; SD = standard deviation; SUVmax = maximum standardized uptake value.
Students t‐test.
Fisher's exact test.
One pack year: Smoking 20 cigarettes per day for 1 year.
Current or former alcohol intake above recommendations set by the Danish Health Authority (women: 7 units per week; men: 14 units per week) and signs of alcohol addiction for more than 1 month or more than one time during a 12‐month period. One unit is 12 grams of alcohol.
Ipsilateral to IFT.
Biochemical parameters were not investigated in 61 (79%) patients in relation to the FDG‐PET/CT. Normal range: C‐reactive protein (<8 mg/L), leucocyte count (3.5–10 × 109 cells/L), neutrophil count (2–7 × 109 cells/L), lymphocyte count (1.3–3.5 × 109 cells/L).
Bold values signifies p < 0.05.
Symptoms and Findings
Patients with at least one symptom suggestive of malignancy had an increased prevalence of tonsillar malignancy (4/14, 29%, sensitivity 36%; specificity 85%) compared with patients with no symptoms (7/63, 11%) (p = 0.11, Fisher's exact test). Similarly, patients with at least one clinical finding suggestive of malignancy (7/32, 22%, sensitivity 64%; specificity 62%) had an increased prevalence of tonsillar malignancy compared with patients with no such findings (4/45, 9%) (p = 0.18). However, both findings did not reach statistical significance. The sensitivity and specificity for the combination of at least one symptom or objective finding suggesting tonsillar malignancy was 73% and 55%, respectively. Three (27%) patients with tonsillar malignancy had neither symptoms nor clinical signs suggesting tonsillar malignancy (two patients with T1N0M0 SCC and one patient with tonsillar lymphoma).
Histology of Tonsillar Malignancy in IFT
Eleven patients were diagnosed with palatine tonsillar malignancy. Eight (73%) patients had SCC (p16 overexpression was found in four cases) involving the one (n = 7) or both (n = 1) tonsils and three (27%) patients had malignant lymphoma involving the one (n = 1) or both (n = 2) palatine tonsils. The TN staging of patients with SCC is shown in Table II. No distant metastases were found in patients with tonsillar SCC.
TABLE II.
TN Staging a of Eight Patients with Incidental Focal FDG‐Avid Palatine Tonsil(s) on FDG‐PET/CT with Squamous Cell Carcinoma (SCC) in One (n = 7) or Both Tonsils (n = 1) (Nine Tumors in Total).
| N0 | N1 | N2b | |
|---|---|---|---|
| T1 | 4 | 2 | 1 |
| T2 | 1 | ||
| T3 | 1 |
One patient had synchronous bilateral SCC.
According to UICC cancer staging, 8th ed.
Side of IFT in Patients with Tonsillar Malignancy
Two patients with tonsillar malignancy had bilateral ITF: one patient with SCC and synchronous high‐grade dysplasia in the contralateral tonsil and one patient with bilateral lymphoma. The remaining nine patients with tonsillar malignancy had unilateral IFT. Two of nine patients with unilateral IFT had bilateral malignancy: one patient had synchronous bilateral SCC (T2N1 tumor on the left side, SUVmax 13.9 and T1N0 tumor on the right side, SUVmax 5.93, SUVmax ratio 2.34) and one patient had bilateral lymphoma. One patient with benign histology had unilateral mild dysplasia (with ipsilateral IFT).
SUVmax Values
The mean absolute SUVmax in IFT tonsils with malignancy was significantly higher than those in IFT without malignancy and measurements in the control group (both p < 0.0001, Kruskal–Wallis test) (Table III). Similarly, the mean SUVmax ratio in IFT tonsils with malignancy was significantly higher than that of IFT without malignancy (p < 0.0001) and that of the control group (p = 0.012, Kruskal–Wallis test). Distribution plots of SUVmax absolute values and ratios among patients and controls are presented in Figure 1.
TABLE III.
SUVmax Measurements of Incidental Focal FDG‐Avid Palatine Tonsils (IFT) on FDG‐PET/CT in 77 Patients Stratified by Tonsillar Histological Findings and a Control Group.
| Variable | Malignant (n = 11) | Benign (n = 66) | Control group (n = 77) |
|---|---|---|---|
| Absolute SUVmax | |||
| Mean (SD) | 15.33 (5.10) | 9.66 (2.99) | 6.37 (2.01) |
| Range | [9.87–26.22] | [5.3–25.21] | [2.55–11.67] |
| SUVmax ratio | |||
| Mean (SD) | 1.69 (0.55) | 1.39 (0.21) | 1.09 (0.06) |
| Range | [1.10–3.23] | [1.01–1.90] | [1.00–1.26] |
CI = confidence interval; OR = odds ratio.
Fig. 1.
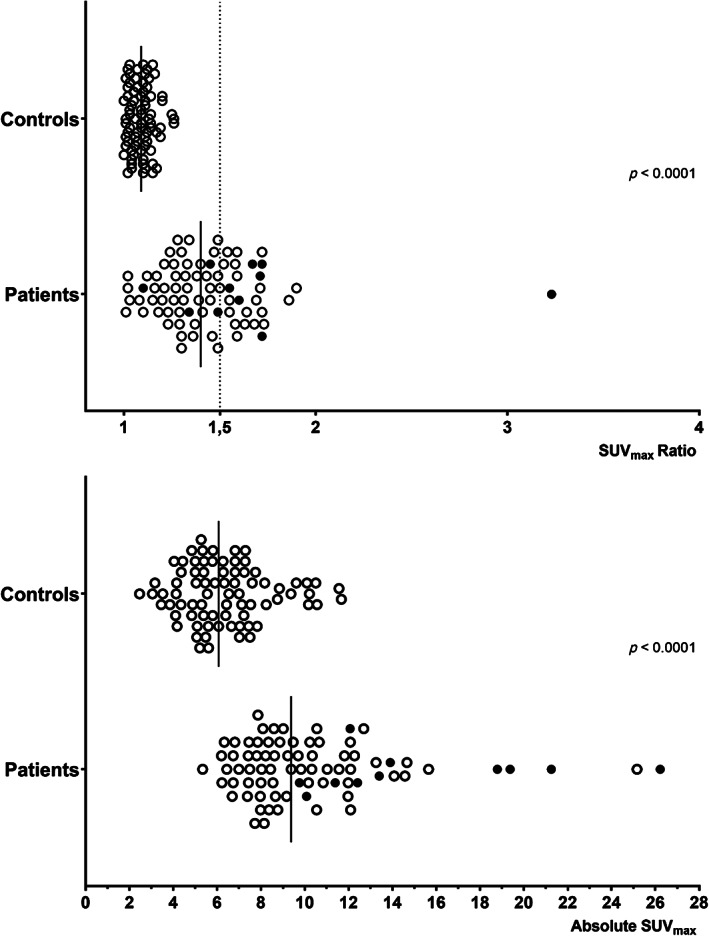
Distribution of SUVmax (maximum standardized uptake values) ratios and absolute values among patients and controls. Black circles represent histologically verified cases of tonsillar malignancy and white circles represent benign tonsils in the patient population.
ROC analyses showed that absolute SUVmax value was a better predictor of malignancy than SUVmax ratio between the tonsils (Figs. 2 and 3). The area under the curve (AUC) for absolute SUVmax was 0.87 (95% CI: 0.77–0.96) compared with 0.74 (95% CI: 0.57–0.90) for SUVmax ratio. Using absolute SUVmax ≥ 9 as cutoff, the sensitivity for tonsillar malignancy was 100% and the specificity was 53%. Positive predictive value (PPV) was 26% and negative predictive value (NPV) was 100%. Using an optimal SUVmax ratio ≥ 1.5 as cutoff, the sensitivity for tonsillar malignancy was 64% and the specificity was 70%. PPV was 26% and NPV 92%. The ROC analysis of the combination of both factors (SUVmax ≥ 9.0 and ratio ≥ 1.5) showed that if at least one of the factors was present, the sensitivity was 100% and specificity 38%, whereas both factors being present yielded a sensitivity of 64% and specificity 85%.
Fig. 2.
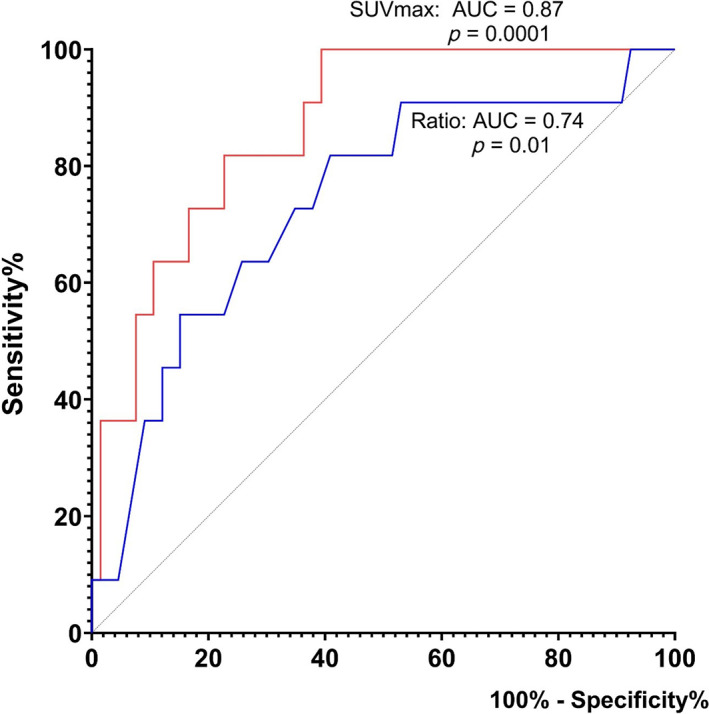
ROC curve analyses of absolute SUVmax values (red) and SUVmax ratio between tonsils (blue) as predictors of malignancy in the patient group. ROC, receiver operating characteristic curve; SUVmax, maximum standardized uptake values; AUC, area under the curve. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Fig. 3.
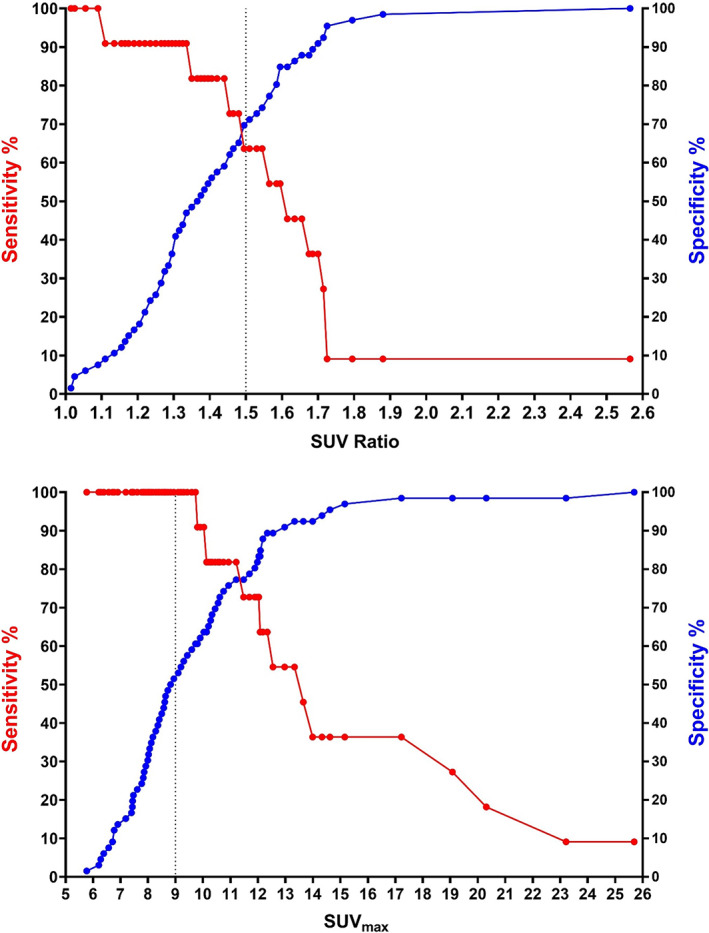
Graphical depiction of sensitivity and specificity in relation to absolute SUVmax values and SUVmax ratio values in the patient group. Using a cutoff value of SUVmax ≥9, the sensitivity for malignancy was 100% and the specificity was 53%. Using a cutoff value of SUVratio ≥1.5, the sensitivity for malignancy was 64% and the specificity was 70%. SUVmax, maximum standardized uptake value. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Indications for FDG‐PET/CT and Primary Cancer Findings
Patients with IFT were scanned for various oncological (84%) and non‐oncological (16%) conditions (Table IV). No significant associations between the prevalence of tonsillar malignancy and indications for FDG‐PET/CT were found. Histological findings among the 77 patients with IFT are shown in Table V.
TABLE IV.
Indications for FDG‐PET/CT and the Primary Sites of Suspected Disease Stratified by Histological Findings among 77 Patients with Incidental Focal FDG‐Avid Palatine Tonsils (IFT).
| Variable | Total (n = 77) | Malignant (n = 11) | Benign (n = 66) |
|---|---|---|---|
| Indication for FDG‐PET/CT, n (%) | |||
| Suspected malignant disease | 58 (75%) | 9 (82%) | 49 (74%) |
| Suspected benign disease | 11 (14%) | 1 (9%) | 10 (15%) |
| Follow‐up, malignant disease | 7 (9%) | — | 7 (11%) |
| Follow‐up, benign disease | 1 (1%) | 1 (9%) | — |
| Primary sites of suspicion, n (%) | |||
| Lung cancer | 24 (31%) | 4 (36%) | 20 (30%) |
| Head and neck cancer | 16 (20%) | 1 (9%) | 15 (23%) |
| Urogenital cancer | 11 (14%) | 2 (18%) | 9 (14%) |
| Soft tissue/bone cancer a | 4 (5%) | — | 4 (6%) |
| Hematologic cancer | 2 (3%) | — | 2 (3%) |
| Malignant melanoma | 2 (3%) | — | 2 (3%) |
| Gastrointestinal cancer | 2 (3%) | 1 (9%) | 1 (2%) |
| Pleural cancer | 2 (3%) | 1 (9%) | 1 (2%) |
| Prostate cancer | 1 (1%) | — | 1 (2%) |
| Other b | 13 (17%) | 2 (18%) | 11 (17%) |
No significant associations between the prevalence of tonsillar malignancy and indications for FDG‐PET/CT were found: Suspected malignant disease (16% malignancy) versus suspected benign disease (9%) (p = 1.00, Fisher's exact test), suspected or follow‐up of malignant disease (14%) versus suspected or follow‐up of benign disease (17%) (p = 0.68), lung cancer (17%) versus other indications (13%) (p = 0.73), head and neck cancer (6%) versus other indications (16%) (p = 0.44), and urogenital cancer (18%) versus other indications (14%) (p = 0.65).
CI = confidence interval; OR = odds ratio.
Chondroblastic osteosarcoma of maxilla, sarcoma (unspecified), thymoma (unspecified), and type B2 thymoma.
Infection (n = 7), rheumatologic disease (n = 4), and paraneoplastic syndrome (n = 2).
TABLE V.
Histological Findings in 77 Patients with Incidental Focal FDG‐Avid Palatine Tonsils (IFT) on FDG‐PET/CT.
| Variable | N (%) |
|---|---|
| Head and neck cancer | 19 (25%) |
| Oral cavity cancer | 10 (13%) |
| IFT | 8 (10%) |
| Base of tongue cancer | 1 (1%) |
| Lung cancer | 12 (16%) |
| Urogenital cancer | 10 (13%) |
| Lymphoma | 4 (5%) |
| IFT | 3 (4%) |
| Outside of tonsils | 1 (1%) |
| Gastrointestinal cancer | 3 (4%) |
| Soft tissue/bone cancer a | 3 (4%) |
| Malignant melanoma | 2 (3%) |
| Prostate cancer | 2 (3%) |
| Hepatic/biliary cancer | 1 (1%) |
| Pleural cancer | 1 (1%) |
| Breast cancer | 1 (1%) |
| No malignancy | 28 (36%) |
Fifty‐eight Primary Cancers were found in 49 (64%) patients. Nine patients had synchronous cancers: In five patients, SCC in IFT was found synchronous to cancer(s) with other locations: base of tongue (contralateral to SCC in IFT, n = 1), floor of mouth (ipsilateral to SCC in IFT, n = 1), lung (n = 1), urogenital (n = 1), and both urogenital and breast (n = 1). One patient had synchronous bilateral SCC in IFT (scan indication: suspected lung cancer) and five patients with malignancy in IFT had no other primary cancer (scan indications: suspected lung cancer [n = 2], gastrointestinal cancer [n = 1], pleural cancer [n = 1], and follow‐up of benign disease [n = 1]).
Chondroblastic osteosarcoma of maxilla, malignant osteoclastoma, and type B2 thymoma.
DISCUSSION
The prevalence of malignancy in 77 patients with IFT was 14%. To our knowledge, this is the first study to investigate the prevalence of malignancy in IFT. Noteworthy, a significant proportion of patients with tonsillar malignancy did not have symptoms (64%) or clinical findings (36%) suggestive of tonsillar malignancy. Hence, tonsillar malignancy in IFT could not be ruled out by anamnesis and objective examination.
Risk Factors for Tonsillar Malignancy in IFT
We found that anamnestic dysphagia (p = 0.019) and alcohol abuse (current or past) (p = 0.035) were significantly associated with malignancy in IFT. However, most patients with malignancy in IFT had neither dysphagia (82%) nor a history of alcohol abuse (73%). Tonsillar asymmetry (noted in 30% of patients with benign histology) was not statistically associated with malignancy.
Incidental FDG‐Avid Findings
A number of studies recommend that incidental FDG‐avid findings on FDG‐PET/CT are evaluated for further examination despite the occurrence of false‐positive findings, as these foci frequently represent neoplasms unrelated to the primary indication for FDG‐PET/CT. 9 , 10 Britt et al. investigated 293 patients with head and neck cancer undergoing FDG‐PET/CT and found 134 incidental findings in 106 patients, with 35 (26%) foci being related to malignancy. 9 Hadad et al. investigated 670 consecutive cancer patients who had undergone FDG‐PET/CT for known or suspected malignant disease and found 35 incidental foci in 29 patients with abnormal increased FDG uptake. Twenty‐eight foci (80%) were deemed clinically significant, including four malignant, 18 premalignant, and six benign lesions. 10
Conditions and Factors Influencing Tonsillar FDG Uptake Findings
Several conditions may increase FDG uptake within the upper airways and the palatine tonsils including infection, inflammation, recent surgery/biopsy, and radiotherapy. In contrast, observed low FDG uptake may be related to low FDG‐avidity of some tumors (i.e., some salivary gland tumors and necrotic neoplasms), inadequate PET/CT scanner resolution, and obscuration (i.e., dental hardware). 3 , 4 , 11 Tonsillar FDG uptake is negatively influenced by smoking and advancing age, but the side‐to‐side correlation is unaltered by these factors. 12 In addition, human papilloma virus (HPV)‐positive oropharyngeal cancers were found to demonstrate lower FDG uptake compared with HPV‐negative tumors 13 and smaller tonsillar malignancies may present without increased FDG uptake. 14 Therefore, we advocate for tonsillectomies in all patients with symptoms or objective findings suggesting tonsillar malignancy despite normal FDG uptake on PET/CT.
Identifying Tonsillar Malignancy using SUVmax in FDG‐PET/CT
Investigating 299 adult patients without known or suspected disease in the head and neck region, Birkin et al. concluded that side‐to‐side tonsillar SUVmax ratios in the range 0.70–1.36 should be considered normal (1st–99th percentiles) in patients not suspected to have tonsillar malignancy. 12
No previous studies have been conducted exploring tonsillar findings in IFT and, therefore, no studies report on SUVmax values to discriminate benign from malignant lesions within IFT. Somewhat related, a number of studies compare tonsillar SUVmax values between patients with or without tonsillar malignancy in cohorts of patients with cervical CUP or head and neck cancer. 5 , 6 , 7 , 8 Pencharz et al. included patients with cervical CUP comparing those found to have tonsillar primary (n = 25) with patients with other primary site malignancies (n = 50). The authors concluded that tonsillar side‐to‐side SUVmax ratio ≥ 1.6 was highly suspicious (62% sensitivity; 100% specificity) for tonsillar cancer. 5
From a cohort of 157 patients with head and neck cancer and similar risk factors for SCC (such as smoking history), Davison et al. compared 26 tonsillar SCC patients with 26 patients with non‐tonsillar disease. Perfect (100%) sensitivity and specificity were found for identifying tonsillar malignancy using SUVmax ratio cutoff ≥1.48. The mean SUVmax was significantly higher in tonsils with SCC (9.4) than in non‐malignant tonsils (3.0) (p < 0.0001) with an optimal cutoff value for absolute SUVmax of 4.6 (sensitivity 88%; specificity 74%). 6
Including four groups of patients (A: clinical apparent tonsillar SCC; B: CUP with occult tonsillar SCC; C: CUP without tonsillar SCC; D: healthy controls), Lee et al. found significantly higher absolute and ratios of SUVmax values in malignant tonsils (A + B) compared with benign tonsils (C + D) (both p < 0.01). Optimal cutoff values to discriminate malignant from benign tonsils using absolute and ratios of SUVmax were 6.89 (sensitivity 85%; specificity 91%) and 1.323 (85% sensitivity and specificity) in group A versus D and 5.85 (sensitivity 71%; specificity 88%) and 1.244 (sensitivity 71%; specificity 75%) in group B versus C, respectively. The authors concluded that both absolute SUVmax values and tonsillar side‐to‐side SUVmax ratios may be helpful in the clinical setting. 7
Investigating 112 patients with head and neck cancer, Nakamura et al. reported an optimal cutoff value for absolute SUVmax ≥ 8.0 (sensitivity 68%; specificity 88%) and side‐to‐side tonsillar ratio ≥ 1.5 (no ROC analysis was made) to differentiate between tonsils with malignancy from benign findings. 8
To sum up, optimal cutoffs for separating malignant and benign lesions were in the range of 4.6–8.0 for absolute SUVmax and in the range of 1.24–1.6 for side‐to‐side tonsillar SUVmax ratio. 5 , 6 , 7 , 8
Bilateral Tonsillar Malignancy
The relative frequent (approximately 4%) occurrence of bilateral tonsillar malignancy in clinically normal tonsils has been described in previous studies. 15 , 16 In the current study, we found one (9%) patient with synchronous bilateral tonsillar SCC (unilateral IFT, contralateral SUVmax 5.93) and one (9%) patient with SCC in the one tonsil had high‐grade dysplasia in the contralateral tonsil (bilateral IFT). These observations underscore the importance of not relying solely on side‐to‐side tonsillar SUVmax asymmetry (ratio and absolute difference) for selecting patients for tonsillectomy. Furthermore, the findings underscore the importance of bilateral tonsillectomy in cases with suspected or proven tonsillar malignancy.
Strengths and Limitations
Because of the study design, we may have missed some patients with IFT as some patients with IFT may not have been tonsillectomized. However, throughout the study period, it was common practice to offer and recommend tonsillectomy for ITF at our institution. Furthermore, we assume that potential patients with a missed tonsillar SCC diagnosis at the time of IFT would have undergone tonsillectomy later and we did not identify any such cases. The number of patients included was limited and the calculated optimal SUVmax cutoff values are associated with uncertainty, and the power to identify significant risk factors was accordingly low. The limited number of patients is reflected in our cautious recommendations. Because of the retrospective nature of the study, anamnestic and objective findings relied on the description in the medical charts, which may not have been exhaustive. Occasionally, it is difficult to discriminate the tonsils from the surrounding muscular tissue on the FDG‐PET/CT and, therefore, determine if the highest found SUVmax was located within tonsillar tissue. This occasional uncertainty was managed by using the highest found SUVmax, resulting in potentially overestimated SUVmax values in both patients and controls.
A major study strength is the fact that we included patients with “true” IFT, thus excluding patients undergoing FDG‐PET/CT because of suspected or likely tonsillar disease. Furthermore, histological data were available in all patients. Different scanners and reconstruction techniques may affect absolute SUVmax values, but the external validity of our findings is increased by the fact that the FDG‐PET/CT scans were performed at four different hospitals over a relatively long period. In addition, the absolute SUVmax cutoff value was set at a conservatively high level.
Clinical Applicability
When dealing with IFT, the primary focus is to correctly diagnose patients with malignant tonsillar lesions as negligence to identify malignancy may reduce the chances of cure. Hence, high sensitivity is crucial for methods used to select patients for further diagnosis (tonsillectomy). However, most patients with IFT underwent FDG‐PET/CT due to known or suspected malignancy (84%), and tonsillectomy may postpone urgent treatment. In addition, tonsillectomy is associated with significant pain 17 and risk of hemorrhage. 18 Therefore, methods to lower the number needed to treat (NNT) without compromising safety are valuable.
In the current study, applying SUVmax ≥ 9.0 as cutoff to our cohort of patients with IFT, all malignancies were identified, and NNT was reduced considerably (from 7.0 to 3.8), saving approximately half of the patients from undergoing tonsillectomy. Using 10 for absolute SUVmax cutoff, the sensitivity for identifying cases of malignancy was 91% (specificity 64%). We advocate the importance of identifying all cases of malignancy at the cost of lowering the specificity, and thus performing some extra, potentially unnecessary tonsillectomies. Clinical assessment only (at least one symptom or objective finding suggesting malignancy) was unreliable in the detection of malignancy (sensitivity 73%; specificity 55%, NNT 8). Relying on symptoms or findings alone, NNT was 6 and 8, respectively.
Because the differences in absolute SUVmax are higher than SUVmax side‐to‐side ratio between scanners and no previous studies have explored cutoff values for “true” IFT, we support the use of both absolute SUVmax and SUVmax ratio to discriminate benign and malignant lesions. 5 , 7 , 8
CONCLUSIONS
We found that the prevalence of malignancy in 77 patients with IFT was 14%. Optimal cutoff for absolute SUVmax (≥9.0: sensitivity 100%; specificity 53%) was superior to the use of SUVmax ratio for the detection of malignancy in IFT. Because of the limited number of patients and to reduce the risk of missing a malignant diagnosis, we recommend that patients with IFT undergo bilateral tonsillectomy if they fulfill one or more of the following criteria: (1) SUVmax of IFT ≥ 9.0; (2) SUVmax ratio ≥ 1.5; (3) symptoms or findings suggesting tonsillar malignancy. Applying these criteria to our cohort, NNT was 5.6 (thus sparing 23% of patients from unnecessary tonsillectomy). More restrictive criteria may be preferable when more studies are conducted.
Supporting information
Appendix 1. Supplementary Information.
Editor's Note: This Manuscript was accepted for publication on February 17, 2022.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1. Scarsbrook AF, Barrington SF. PET‐CT in the UK: current status and future directions. Clin Radiol 2016;71:673–690. 10.1016/j.crad.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 2. Zincirkeser S, Sahin E, Halac M, Sager S. Standardized uptake values of normal organs on 18F‐fluorodeoxyglucose positron emission tomography and computed tomography imaging. J Int Med Res 2007;35:231–236. 10.1177/147323000703500207. [DOI] [PubMed] [Google Scholar]
- 3. Fukui MB, Blodgett TM, Snyderman CH, et al. Combined PET‐CT in the head and neck: part 2. Diagnostic uses and pitfalls of oncologic imaging. Radiographics 2005;25:913–930. 10.1148/rg.254045136. [DOI] [PubMed] [Google Scholar]
- 4. Purohit BS, Ailianou A, Dulguerov N, Becker CD, Ratib O, Becker M. FDG‐PET/CT pitfalls in oncological head and neck imaging. Insights Imaging 2014;5:585–602. 10.1007/s13244-014-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pencharz D, Dunn J, Connor S, et al. Palatine tonsil SUVmax on FDG PET‐CT as a discriminator between benign and malignant tonsils in patients with and without head and neck squamous cell carcinoma of unknown primary. Clin Radiol 2019;74:165.e17‐23. 10.1016/j.crad.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 6. Davison JM, Ozonoff A, Imsande HM, Grillone GA, Subramaniam RM. Squamous cell carcinoma of the palatine tonsils: FDG standardized uptake value ratio as a biomarker to differentiate tonsillar carcinoma from physiologic uptake. Radiology 2010;255:578–585. 10.1148/radiol.10091479. [DOI] [PubMed] [Google Scholar]
- 7. Lee HJ, Kim JS, Roh JL, et al. Utility of quantitative 18 F‐fluorodeoxyglucose uptake measurement to identify occult tonsillar carcinoma in patients with cervical metastasis of unknown primary tumors: a retrospective case‐control study. Clin Otolaryngol 2013;38:30–38. 10.1111/coa.12055. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura S, Okochi K, Murata Y, Shibuya H, Kurabayashi T. [18F]Fluorodeoxyglucose‐PET/CT differentiation between physiological and pathological accumulations in head and neck. Nucl Med Commun 2009;30:498–503. 10.1097/MNM.0b013e3283299a52. [DOI] [PubMed] [Google Scholar]
- 9. Britt CJ, Maas AM, Kennedy TA, Hartig GK. Incidental findings on FDG PET/CT in head and neck cancer. Otolaryngol Head Neck Surg 2018;158:484–488. 10.1177/0194599817742579. [DOI] [PubMed] [Google Scholar]
- 10. Hadad ZS, Afzelius P, Sørensen SM, Jurik AG. Clinical relevance of 18F‐FDG‐PET/CT incidental findings. Dan Med J 2020;67:A10190553. [PubMed] [Google Scholar]
- 11. Blodgett TM, Fukui MB, Snyderman CH, et al. Combined PET‐CT in the head and neck: part 1. Physiologic, altered physiologic, and artifactual FDG uptake. Radiographics 2005;25:897–912. 10.1148/rg.254035156. [DOI] [PubMed] [Google Scholar]
- 12. Birkin E, Moore KS, Huang C, et al. Determinants of physiological uptake of 18F‐fluorodeoxyglucose in palatine tonsils. Medicine (Baltimore) 2018;97:e11040. 10.1097/MD.0000000000011040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tahari AK, Alluri KC, Quon H, Koch W, Wahl RL, Subramaniam RM. FDG PET/CT imaging of oropharyngeal squamous cell carcinoma: characteristics of HPV positive and ‐negative tumors. Clin Nucl Med 2014;39:225–231. 10.1097/RLU.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee HS, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Clinical values for abnormal 18F‐FDG uptake in the head and neck region of patients with head and neck squamous cell carcinoma. Eur J Radiol 2014;83:1455–1460. 10.1016/j.ejrad.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 15. Rokkjaer MS, Klug TE. Prevalence of synchronous bilateral tonsil squamous cell carcinoma: a retrospective study. Clin Otolaryngol 2018;43:1–6. 10.1111/coa.12981. [DOI] [PubMed] [Google Scholar]
- 16. Nami Saber C, Grønhøj C, Jensen DH, et al. Synchronous, bilateral tonsillar carcinomas: patient characteristics and human papillomavirus genotypes. Oral Oncol 2017;74:105–110. 10.1016/j.oraloncology.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 17. Pruegsanusak K, Wongsuwan K, Wongkittithawon J. A randomized controlled trial for perioperative morbidity in microdebrider versus cold instrument dissection tonsillectomy. J Med Assoc Thail 2010;93:558–565. [PubMed] [Google Scholar]
- 18. Hessén Söderman AC, Ericsson E, Hemlin C, et al. Reduced risk of primary postoperative hemorrhage after tonsil surgery in Sweden: results from the National Tonsil Surgery Register in Sweden covering more than 10 years and 54,696 operations. Laryngoscope 2011;121:2322–2326. 10.1002/lary.22179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Supplementary Information.


