Abstract
Actinobacillus pleuropneumoniae, a porcine respiratory tract pathogen, has been shown to express transferrin-binding proteins and urease during infection. Both activities have been associated with virulence; however, their functional role for infection has not yet been elucidated. We used two isogenic A. pleuropneumoniae single mutants (ΔexbB and ΔureC) and a newly constructed A. pleuropneumoniae double (ΔureC ΔexbB) mutant in aerosol infection experiments. Neither the A. pleuropneumoniae ΔexbB mutant nor the double ΔureC ΔexbB mutant was able to colonize sufficiently long to initiate a detectable humoral immune response. These results imply that the ability to utilize transferrin-bound iron is required for multiplication and persistence of A. pleuropneumoniae in the porcine respiratory tract. The A. pleuropneumoniae ΔureC mutant and the parent strain both caused infections that were indistinguishable from one another in the acute phase of disease; however, 3 weeks postinfection the A. pleuropneumoniae ΔureC mutant, in contrast to the parent strain, could not be isolated from healthy lung tissue. In addition, the local immune response—as assessed by fluorescence-activated cell sorter and enzyme-linked immunosorbent spot analyses—revealed a significantly higher number of A. pleuropneumoniae-specific B cells in the bronchoalveolar lavage fluid (BALF) of pigs infected with the A. pleuropneumoniae ΔureC mutant than in the BALF of those infected with the parent strain. These results imply that A. pleuropneumoniae urease activity may cause sufficient impairment of the local immune response to slightly improve the persistence of the urease-positive A. pleuropneumoniae parent strain.
Actinobacillus pleuropneumoniae is the etiologic agent of porcine pleuropneumonia, a highly infectious disease of fattening pigs occurring worldwide (12). A number of putative virulence factors, such as Apx toxins, capsule, lipopolysaccharide (LPS), the ability to utilize transferrin-bound iron, and urease, have been described elsewhere (18). To date, conclusive evidence obtained by challenge experiments has been presented to confirm the role of Apx toxins and capsular material. A spontaneous Apx toxin-negative A. pleuropneumoniae strain was shown to be avirulent (14), and this result was supported later by using transposon mutagenesis (36) as well as by an isogenic A. pleuropneumoniae apxC insertion mutant (29). Also, capsule-deficient A. pleuropneumoniae strains obtained by chemical mutagenesis were shown to be attenuated (22), and this result was confirmed by reconstituting virulence properties and capsule formation upon transformation with a recombinant plasmid (39). Also, it was shown recently that the [Cu,Zn]-superoxide dismutase is not required for virulence (35). For other putative virulence factors, such as LPS (1, 3, 4), and the utilization of transferrin-bound iron (15, 17, 40), no conclusive challenge experiments have been performed to date. With respect to urease, data are inconclusive; urease-negative A. pleuropneumoniae mutants have been found to produce acute infection (37), whereas in a low dose challenge trial reported recently, a urease-negative mutant was found to be unable to establish infection (7).
The construction of two isogenic A. pleuropneumoniae mutants, one that was unable to utilize transferrin-bound iron (ΔexbB) (38) and one that was urease negative (ΔureC) (28), was reported previously. For the present communication we have constructed an isogenic double (ΔureC ΔexbB) mutant, and we performed an aerosol challenge on A. pleuropneumoniae-free pigs using these three mutants and the parent strain. We show a possible role for urease in chronic A. pleuropneumoniae infection and demonstrate that utilization of transferrin-bound iron is important for A. pleuropneumoniae virulence.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains, plasmids, and primers used in this work are listed in Table 1. Escherichia coli strains were cultured in Luria-Bertani medium supplemented with the appropriate antibiotic (ampicillin, 100 μg/ml); for cultivation of E. coli β2155 (ΔdapA), diaminopimelic acid (1 mM) (Sigma Chemical Company, Deisenhofen, Germany) was added. A. pleuropneumoniae serotype 7 parent and mutant strains were grown in PPLO medium (Difco GmbH, Augsburg, Germany) supplemented with NAD (10 μg/ml) (Merck AG, Darmstadt, Germany), l-glutamine (100 μg/ml) (Serva, Heidelberg, Germany), l-cysteine hydrochloride (260 μg/ml) (Sigma), l-cystine dihydrochloride (10 μg/ml) (Sigma), dextrose (1 mg/ml), and Tween 80 (0.1%). Iron restriction was induced by the addition of 2,2′-dipyridyl (100 μM) (Sigma).
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5αF′ | F′ endA1 hsdR17 (rK− mK−) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] | 30 |
| HB101 | F− Δ(gpt-proA)62 leuB6 supE44 ara-14 galK2 lacY1 Δ(mcrC-mrr) rpsL20 (Strr) xyl-5 mtl-1 recA13 | 33 |
| β2155 | thrB1004 pro thi strA hsdS lacZΔM15 (F′ lacZΔM15 laqIqtraD36 proA+ proB+) ΔdapA::erm (Ermr) recA::RP4-2-tet (Tcr)::Mu-km (Kmr) λ pir | 9 |
| A. pleuropneumoniae | ||
| AP76 (wt) | A. pleuropneumoniae serotype 7 strain kindly provided by the Western College of Veterinary Medicine, Saskatoon, Canada | 2 |
| A. pleuropneumoniae ΔexbB | Unmarked exbB-negative knockout mutant of A. pleuropneumoniae AP76 | 38 |
| A. pleuropneumoniae ΔureC | Unmarked ureC-negative knockout mutant of A. pleuropneumoniae AP76 | 28 |
| A. pleuropneumoniae ΔexbB ΔureC | Unmarked exbB- and ureC-negative knockout mutant of A. pleuropneumoniae AP76 | This work |
| Plasmid | ||
| pBMK1 | Transconjugation vector based on pBluescript SK with mobRP4, a polycloning site, Kmr, and transcriptional fusion of the omlA promoter with the sacB gene | 28 |
| Primers | ||
| BA7 | CAA TGG ATC CAT TTT ATC TTC TTC AGG C; primer (internal BamHI site) upstream of the exbB gene | 38 |
| RE1 | AAG TTT AAA ATG CAT ATT GC; primer overlapping the start codon of the tbpB gene | 38 |
| ureC2 | GTA AGG ATC CAT TAA CAA TCC CAC GCA GTC AGT AT; primer (internal BamHI site) comprising positions 997 to 1022 of the urease operon (reference 6) | 28 |
| ureX | TCA TGT CGA CTA GAA CAA GAA ATA ACG CTG TGC AA; primer with internal SalI site comprising positions 2686 to 2711 of the urease operon (reference 6) | 28 |
Manipulation of DNA and construction of an A. pleuropneumoniae double deletion mutant.
DNA-modifying enzymes were purchased from New England Biolabs (Bad Schwalbach, Germany) and used according to the manufacturer's instructions. Taq polymerase was purchased from GIBCO-BRL Life Technologies (Karlsruhe, Germany). DNA for PCR and Southern blotting, as well as plasmid DNA, was prepared by standard protocols (33). Transformations, gel electrophoresis, PCR, and Southern blotting were done by standard procedures (33), and pulsed-field gel electrophoresis (PFGE) of A. pleuropneumoniae was performed as described previously (27). The A. pleuropneumoniae double (ΔureC ΔexbB) mutant was constructed using the A. pleuropneumoniae urease-negative (ΔureC) mutant (28) as the recipient strain. The plasmids used, as well as conjugation, selection, and counterselection procedures, have been described previously (28, 38).
Virulence studies.
Thirty-two outbred pigs 8 to 9 weeks of age were purchased from an A. pleuropneumoniae-free herd (no clinical symptoms, no serological response in the ApxII–enzyme-linked immunosorbent assay [ELISA] [24]), randomly assigned to four groups, and cared for in accordance with the principles outlined in the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (European Treaty Series, no. 123: http://conventions.coe.int/treaty/EN/Menuprincipal.htm. Groups were housed in separate isolation units with controlled temperature and ventilation. Infections were carried out in an aerosol chamber built according to the descriptions of Jacobsen et al. (23), with four pigs at a time. For aerosol infection, the A. pleuropneumoniae parent strain and the isogenic mutants were grown with shaking for approximately 3 h at 37°C to an optical density (OD) at 660 nm of 0.4. The culture was placed on ice, diluted 1:300 in ice-cold NaCl (150 mM), and kept on ice until use (for a maximum of 2 h). Immediately prior to aerosolization, bacteria were further diluted 1:100 in ice-cold NaCl (150 mM), resulting in living cell counts of 9.6 × 104/ml (ΔureC ΔexbB mutant), 15 × 104/ml (ΔexbB mutant), 9.7 × 104/ml (ΔureC mutant), and 7.4 × 104/ml (wild type [wt]); upon aerosolization these concentrations correspond to approximately 102 A. pleuropneumoniae cells per liter of aerosol in the chamber, a dose which had been titrated for the A. pleuropneumoniae parent strain (wild type) to induce severe but not fatal disease in this challenge model, at a total exposure time of 45 min (2 min of aerosolization, 10 min of incubation, 30 min of removal of bacteria through filters at a rate corresponding to ten complete exchanges of the air volume). Blood samples were taken on day 7 before infection (immediately upon arrival in the facility), as well as on days 7, 14, and 21 postinfection.
At postmortem analysis, lung lesion scores were determined according to the method described by Hannan et al. (19). Briefly, the size and position of lesions were mapped on a diagram representing the seven lung lobes, with each lobe allotted a maximum possible score of 5. Then, by assessing the pneumonic area for each lobe as a fraction of 5 (resulting in a maximum score of 35 for the complete lung), the lung lesion score was calculated. The bacteriological examination included surface swabs of affected and unaffected lung tissue, tonsils, bronchial lymph nodes, and heart muscle on supplemented PPLO agar, as well as on Gassner and Columbia sheep blood (CSB) agar. The degree of total bacterial colonization (growth on CSB agar), as well as colonization by enterobacteria (growth on Gassner agar) and by A. pleuropneumoniae-like bacteria (minimal growth with distinct hemolysis on CSB, good growth on supplemented PPLO agar) was assessed as +++ (confluent), ++ (>100 colonies), and + (<100 colonies). Some individual A. pleuropneumoniae-like colonies were subcultured on supplemented PPLO agar and confirmed by a slide agglutination test and PCR analysis using exbB- and ureC-specific primers.
BALF.
Pigs were anesthetized by intramuscular application of azaperone (2 mg/kg of body weight) followed by an intramuscular injection of ketamine (15 mg/kg) and immobilized as previously described (21). To obtain bronchoalveolar lavage fluid (BALF), a flexible bronchoscope (type XP20; Olympus, Hamburg, Germany) was introduced into the bronchus of the right posterior cranial lobe. The tip of the bronchoscope was pushed into a wedge position to seal the bronchus. Twenty milliliters of isotonic NaCl (prewarmed to 30°C) was injected and recovered by applying a suction force of 20 to 50 kPa using a specially designed vacuum pump (Endoaspirator; System Endoparts, Georg Paudrach, Hanover, Germany). This washing process was repeated five times, and an average of 90 ml of BALF was obtained. The BALF was kept on ice for up to 2 h until the bacteriological status was assessed. Briefly, 1 ml of BALF was centrifuged (6,000 × g, 10 min), and the pellet was resuspended in 60 μl of NaCl (150 mM). Twenty microliters was plated on supplemented PPLO agar, as well as on Gassner and CSB agar, and plates were interpreted as described above. In addition, the total bacterial number as well as the number of A. pleuropneumoniae cells was assessed by serial 10-fold dilutions of nonconcentrated BALF and plating on CSB and supplemented PPLO agar.
ELISAs.
The generalized humoral immune response of pigs was determined in two different ELISAs. In order to assess antibody levels directed against the ApxIIA toxin, a standardized ELISA based on the recombinant A. pleuropneumoniae ApxIIA protein as the solid-phase antigen was employed (24). In order to assess antibody levels directed against outer membrane components, an ELISA based on the detergent extract of an iron-restricted A. pleuropneumoniae wild-type (wt) culture (16) as the solid-phase antigen was used. The detergent extract was diluted 1:50 in carbonate buffer (50 mM [pH 9.6]); Polysorb 96-microwell plates (Nunc, Roskilde, Denmark) were coated with 100 μl of diluted extract per well at 4°C for 16 h without subsequent blocking. Plates were washed with PBST (150 mM phosphate-buffered saline [PBS] [pH 7.2] containing 0.05% Tween 20) before the addition of serum, conjugate, and chromogen. Sera were initially diluted 1:100 and then diluted twofold further in PBST in the plates. An internal positive control (a pool of sera taken at 3 weeks postinfection from pigs infected with A. pleuropneumoniae wt cells) and a negative control (a pool of sera taken from pigs prior to infection) were used on each plate. Serum dilutions and goat anti-pig peroxidase conjugate (Dianova, Hamburg, Germany) were each incubated for 1 h at room temperature. The ELISA was developed using 2,2-azino-di-[3-ethylbenzithiazoline sulfonate] (ABTS) (Boehringer, Mannheim, Germany) as a substrate. The test was considered valid when the OD of the negative serum at a 1:100 dilution was lower than the OD of the positive serum at a 1:12,800 dilution. The titer given is the serum dilution with an OD higher than twice the OD of the negative control serum at a 1:100 dilution.
Fluorescence-activated cell sorter and enzyme-linked immunosorbent (ELI) spot analyses.
Eighty milliliters of BALF was centrifuged (400 × g, 10 min), and the cells were washed once in PBS and then resuspended in 1.5 ml of PBS. Using phase-contrast microscopy (500-fold magnification) and a hemocytometer, the numbers of lymphocytes, red blood cells, and other nucleated cells (including macrophages and granulocytes) were determined. An indirect immunofluorescence staining method for lymphocyte subpopulations in the BALF cells was performed using monoclonal porcine-specific antibodies against CD3 (8E6; VMRD, Pullman, Wash.), γ/δ T cells (MAC320; R. M. Binns, Babraham, United Kingdom), immunoglobulin A (IgA) (MCA638), IgG1 (MCA635), and IgM (MCA637) (all Igs were from Serotec, Oxford, United Kingdom). Goat anti-mouse isotype-specific phycoerythrin conjugates were used as secondary antibodies (Southern Biotechnologies, Birmingham, Ala.). Using a flow cytometer (FACScan; Becton Dickinson, Heidelberg, Germany) within the lymphocyte gate, the percentage of positive cells for the different markers was determined based on 5,000 analyzed events.
Cells from the BALF were assayed for antibody-secreting cells (ASC) of the different immunoglobulin isotypes (IgA, IgG1, and IgM) and for A. pleuropneumoniae-specific ASC of the different isotypes by ELI spot analysis (11). Briefly, nitrocellulose-bottomed 96-well plates (MAHB-N45; Millipore, Eschborn, Germany) were coated with an A. pleuropneumoniae antigen preparation (detergent extract of an iron-restricted A. pleuropneumoniae wt culture, diluted 1:10) in PBS for 2 h at 37°C. The plates were washed and blocked using RPMI 1640 containing 5% fetal calf serum. After removal of the block, BALF cells were added, and the plates were incubated overnight at 37°C in a moist atmosphere (5% CO2). The cells were removed by intense rinsing (PBS with 0.05% Tween 20), and monoclonal antibodies against porcine IgA, IgG1, and IgM (see above) were added for 2 h at 37°C. An anti-mouse IgG1 alkaline phosphatase-labeled conjugate (Southern Biotechnologies) was used as the secondary reagent. The color reaction was carried out using alkaline phosphatase buffer (0.1 M Tris, 0.15 M NaCl, 0.05 M MgCl2 [pH 9.5]) containing nitroblue tetrazolium (30 μg/ml) (Sigma) and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (16 μg/ml; Sigma). The frequency of ELI spots was counted using a stereomicroscope (30-fold magnification) and expressed as the number of spots per 106 lymphocytes. The means and standard deviations for the lymphocyte subsets, as well as for the ELI spots, were calculated; differences with P of <0.05 in the nonparametric Wilcoxon test were considered significant.
RESULTS
Construction of an isogenic A. pleuropneumoniae double (ΔureC ΔexbB) mutant.
An A. pleuropneumoniae double (ΔureC ΔexbB) mutant was constructed based on an A. pleuropneumoniae single (ΔureC) mutant and confirmed by PCR analyses, Southern blotting, PFGE, urease assay, and Western blotting (Fig. 1). Thereby it was shown that the Kanr sacB cassette can be used to successively introduce multiple site-specific mutations into one A. pleuropneumoniae parent strain.
FIG. 1.
Analysis of A. pleuropneumoniae AP76 wt (lanes 1), ΔureC (lanes 2), ΔexbB (lanes 3), and ΔureC ΔexbB (lanes 4) strains. Lanes M, size markers; lanes N, negative controls for PCR. (A) PCR using primers ureC2 and ureX (left) and RE1 and BA7 (right). (B) Southern blot analysis using the ureC gene (left) and the exbB gene (right) as probes. The ureC probe is cut by BstEII and SphI, with BstEII located outside and SphI located within the deletion site. The exbB probe is not cut by EcoRV and PacI, with EcoRV located outside and PacI located within the deletion site. (C) PFGE of ApaI-, AscI-, and NotI-digested DNA showing that no gross rearrangements have occurred. (D) Coomassie blue-stained gel (left) and Western blots developed with serum directed against the TbpB protein (middle) and the ExbB protein (right) of whole cell lysates obtained from cultures grown under iron-restricted conditions, showing that TbpB expression in the exbB mutants (ΔexbB and ΔureC ΔexbB) is unaffected. The open arrowhead indicates the position of the TbpB protein; the solid arrowhead indicates the position of the ExbB protein.
Bacterial reisolation kinetics and pathomorphological changes in challenged pigs.
BALF was sampled 1 week before as well as 1 and 3 weeks after challenge. Colonization by bacteria other than A. pleuropneumoniae, as assessed by the total count of CFU, was highly variable among individual pigs (<10/ml to 5 × 105/ml), but no significant differences were found among the four challenge groups or between the different sampling points. Upon challenge with the A. pleuropneumoniae ΔexbB and ΔureC ΔexbB mutants, no A. pleuropneumoniae cells could be reisolated from BALF 1 or 3 weeks after challenge. Upon challenge with the A. pleuropneumoniae ΔureC mutant and the wt strain, the challenge strain was reisolated from BALF on days 7 and 21 from the majority of pigs in these challenge groups (Table 2). No consistent difference with respect to the number of A. pleuropneumoniae colonies was observed between the two groups or between days 7 and 21. The correct pheno- and genotype of isolates were confirmed by urease testing and PCR analysis.
TABLE 2.
Virulence of A. pleuropneumoniae parent and isogenic mutant strains following aerosol challenge
| A. pleuropneumoniae challenge strain | No. of animals with lung lesions/total no. | Arithmetic mean ± standard deviation of lung lesion scorea | No. of animals with A. pleuropneumoniae reisolated after challenge from:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| BALF
|
Tonsilbc | Lymph nodeb | Heartb | Lungb
|
|||||
| Day 7 | Day 21 | Pneumonic | Intact | ||||||
| ΔureC ΔexbB mutant | 0/8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ΔexbB mutant | 0/8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ΔureC mutant | 7/8 | 5.66 ± 4.02 | 6 | 5 | 1 | 5 | 2 | 7 | 0 |
| wt | 8/8 | 6.64 ± 2.95 | 4 | 7 | 1 | 4 | 0 | 8 | 4 |
The lung lesion score was determined as described by Hannan et al. (19).
Tissues were examined at postmortem analysis.
In tonsil cultures, the presence of A. pleuropneumoniae was likely to be masked frequently by the heavy load of concomitant bacterial growth.
The bacteriological examination of tonsils, lung lymph nodes, hearts, pneumonic lesions (if present), and intact lungs at the end of the challenge experiment (3 weeks after challenge) revealed that all pigs challenged with the A. pleuropneumoniae ΔexbB or ΔureC ΔexbB mutant were culture negative (Table 2). From pigs challenged with the A. pleuropneumoniae wt and ΔureC strains, A. pleuropneumoniae was consistently reisolated from pneumonic lesions in pure culture, with surface smears showing dense (++) or confluent (+++) growth in 13 of 16 pigs. Reisolation from the hearts and tonsils succeeded sporadically, with no differences between the groups. The lymph nodes were culture positive for the wt strain and the ΔureC mutant for four and five pigs, respectively. The morphologically intact lung tissue was culture positive for four pigs challenged with the wt strain, whereas it was culture negative for all pigs challenged with the ΔureC mutant (Table 2).
Systemic and local immune response of challenged pigs.
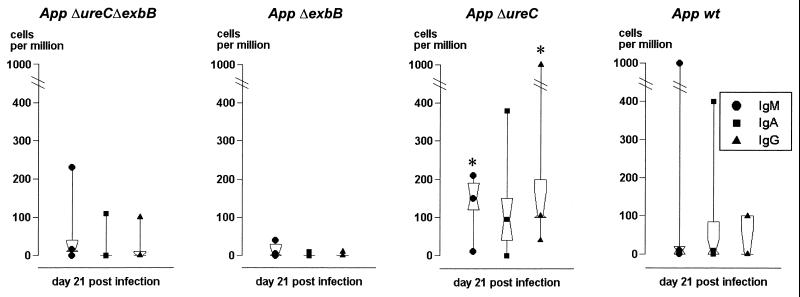
The systemic immune response was determined with two ELISA systems, using recombinant ApxIIA protein or detergent extract as the solid-phase antigen. Among the pigs challenged with the A. pleuropneumoniae ΔexbB or ΔureC ΔexbB mutant, none developed a detectable immune response (Fig. 2). In contrast, all pigs challenged with the A. pleuropneumoniae ΔureC mutant or the wt strain showed a strong humoral immune response in both systems, and no significant difference was observed between the groups (Fig. 2). The local immune response was studied based on the cells recovered from BALF. The total BALF volume (approximately 90 ml) contained ∼20 × 106 nonlymphoid cells (mainly macrophages and granulocytes) and ∼4 × 106 lymphocytes for all groups. These cell numbers remained at a comparable level throughout the entire challenge study. Using fluorescence-activated cell sorter analysis, lymphocytes were differentiated before challenge, as well as 1 and 3 weeks after challenge, into T cells (CD3+) and IgM-, IgA-, and IgG-expressing ASC, with no obvious differences among the groups. Using the ELI spot assay with an A. pleuropneumoniae detergent extract as the solid-phase antigen, A. pleuropneumoniae-specific ASC were detected 3 weeks after challenge. The number of specific ASC was clearly increased in pigs challenged with the A. pleuropneumoniae ΔureC mutant compared to all other groups; this increase was significant (P < 0.05) for IgM- and IgG-secreting cells (IgM, 10 to 210 ASC/106 cells; IgG, 40 to 1,000 ASC/106 cells) (Fig. 3).
FIG. 2.
Humoral immune response of pigs challenged with the A. pleuropneumoniae (App) parent strain and isogenic mutants 7 days before as well as 7 and 21 days after challenge. The antibody response was assessed with two ELISAs, using the recombinant ApxIIA protein (Apx-ELISA) or a detergent extract (Extract-ELISA) as the solid-phase antigen. The immune response was expressed in ELISA units (based on an external standard) for the standardized Apx-ELISA, with serum activities of more than 30 ELISA units considered positive; for the Extract-ELISA, the immune response was expressed as serum titer in comparison to an internal control. The central square within the hourglass shape represents the geometric mean, the hinges present the values in the middle of each half of data, and the top and bottom squares mark the maximum and minimum values, respectively.
FIG. 3.
Local immune response of pigs challenged with the A. pleuropneumoniae (App) parent strain and isogenic mutants 21 days after challenge. The immune response was assessed by ELI spot analysis differentiating between IgM (●), IgA (■), and IgG (▴) ASC. Significant differences are indicated by an asterisk. The dot, square, or triangle within the hourglass shape represents the geometric mean, the hinges present the values in the middle of each half of data, and the top and bottom symbols mark the maximum and minimum values, respectively.
DISCUSSION
The goal of this study was to determine the roles of putative A. pleuropneumoniae virulence factors in persistence and chronic infection. For our approach of using isogenic mutants, two target genes, exbB and ureC, were chosen. The exbB gene was selected because it has been shown previously that deletion of this gene completely inhibits utilization of transferrin-bound iron without preventing the expression of the TbpB protein (38), which is known to be a protective antigen (32). Since the expression of TbpB protein is particularly high in acute infection and decreases during the course of disease (20) and since it has been shown that A. pleuropneumoniae can obtain iron from siderophores of other bacteria (10) and, in addition, is able to bind hemin and hemoglobin (3), we hypothesized that the isogenic exbB mutant (ΔexbB) would be attenuated in the acute phase of disease but would still be able to persist.
The ureC gene was selected because acute A. pleuropneumoniae infection can occur upon experimental infection using a high challenge dose of a urease-negative A. pleuropneumoniae mutant (7, 37), and a urease-negative field strain has been recovered in one case of natural infection (5). For other pathogens causing respiratory tract infections, either no effect (in the case of Bordetella bronchiseptica [25]) or a slight decrease in multiplication and persistence (in the case of Mycobacterium bovis [31]) has been observed. We hypothesized that urease might support the persistence and shedding of A. pleuropneumoniae by locally counteracting the reactive decrease of pH occurring upon infection. Since the RTX toxin of E. coli is known to be inhibited by subneutral pH values (34), a local urease-mediated return to physiological pH might maintain or restore the toxic efficacy of the A. pleuropneumoniae Apx toxin and thereby impair local defense mechanisms of the host, particularly in the late stage of infection. Based on these considerations, we also constructed the A. pleuropneumoniae double (ΔureC ΔexbB) mutant which, according to our hypotheses, would be attenuated over the entire course of disease.
The results we obtained with the A. pleuropneumoniae ΔexbB mutant contradicted our prediction. The complete absence of these mutants in BALF after only 1 week after infection and the lack of any specific humoral or local immune response implies that the ExbBD-mediated uptake of transferrin-bound iron is required for A. pleuropneumoniae virulence. The iron uptake via exogenous siderophores (10) is not sufficient to facilitate colonization of the respiratory tract by A. pleuropneumoniae or, alternatively, also depends on the ExbBD transporter function. This likely dependence on transferrin-bound iron is supported by results with Neisseria gonorrhoeae showing that transferrin receptor mutants were unable to cause infection in human volunteers (8).
The results obtained in the challenge experiment with the A. pleuropneumoniae ΔureC mutant confirmed previous results with respect to acute infection (7, 37) and supported the hypothetical role of urease in chronic infection. The A. pleuropneumoniae ΔureC mutant could not be isolated from unaltered lung tissue 3 weeks after challenge (Table 2), and this finding was further supported by the ELI spot assay showing a significantly higher number of A. pleuropneumoniae-specific B cells in the BALF from ΔureC-infected pigs than in the BALF from pigs infected with the parent strain (Fig. 3). This difference could be due to a more effective antigen uptake and presentation by dendritic cells in the airways (26), thereby leading to an increased number of ASC; this possibility is supported by the urease function hypothesized above. The similar numbers of total B and T cells do not contradict this potential explanation, as the lytic activity of Apx toxin is concentration dependent and would therefore be expected to primarily affect A. pleuropneumoniae-specific cells. To substantiate this hypothesis, however, additional challenge trials using different challenge doses should be performed.
A major obstacle in preventing A. pleuropneumoniae disease is the serovar-specific protection induced upon immunization with bacterins. One way to successfully overcome this problem is the use of attenuated live vaccines (13, 29). However, licensing of isogenic mutants containing an antibiotic resistance marker for use in livestock might be difficult to obtain. Therefore, the feasibility of successively introducing multiple mutations without antibiotic markers into the same parent strain might prove valuable for future A. pleuropneumoniae vaccine development. Based on our results, a urease-negative phenotype introduced as one mutation might be advantageous, as it facilitates a simple differentiation from common A. pleuropneumoniae isolates and, in addition, might reduce shedding of such a putative live vaccine.
ACKNOWLEDGMENTS
This work was supported by grant GE522/3-1 from the Deutsche Forschungsgemeinschaft, Bonn, Germany. W.T. is a fellow of the Mahanakorn University of Technology, Bangkok, Thailand.
REFERENCES
- 1.Abul-Milh M, Paradis S E, Dubreuil J D, Jacques M. Binding of Actinobacillus pleuropneumoniae lipopolysaccharides to glycosphingolipids evaluated by thin-layer chromatography. Infect Immun. 1999;67:4983–4987. doi: 10.1128/iai.67.10.4983-4987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson C, Potter A A, Gerlach G F. Isolation and molecular characterization of spontaneously occurring cytolysin-negative mutants of Actinobacillus pleuropneumoniae serotype 7. Infect Immun. 1991;59:4110–4116. doi: 10.1128/iai.59.11.4110-4116.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belanger M, Begin C, Jacques M. Lipopolysaccharides of Actinobacillus pleuropneumoniae bind pig hemoglobin. Infect Immun. 1995;63:656–662. doi: 10.1128/iai.63.2.656-662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belanger M, Dubreuil D, Jacques M. Proteins found within porcine respiratory tract secretions bind lipopolysaccharides of Actinobacillus pleuropneumoniae. Infect Immun. 1994;62:868–873. doi: 10.1128/iai.62.3.868-873.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard P C, Walker R L, Gardner I. Pleuropneumonia in swine associated with a urease-negative variant of Actinobacillus pleuropneumoniae serotype 1. J Vet Diagn Investig. 1993;5:279–282. doi: 10.1177/104063879300500226. [DOI] [PubMed] [Google Scholar]
- 6.Bosse J T, MacInnes J I. Genetic and biochemical analyses of Actinobacillus pleuropneumoniae urease. Infect Immun. 1997;65:4389–4394. doi: 10.1128/iai.65.11.4389-4394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosse J T, MacInnes J I. Urease activity may contribute to the ability of Actinobacillus pleuropneumoniae to establish infection. Can J Vet Res. 2000;64:145–150. [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 9.Dehio C, Meyer M. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J Bacteriol. 1997;179:538–540. doi: 10.1128/jb.179.2.538-540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diarra M S, Dolence J A, Dolence E K, Darwish I, Miller M J, Malouin F, Jacques M. Growth of Actinobacillus pleuropneumoniae is promoted by exogenous hydroxamate and catechol siderophores. Appl Environ Microbiol. 1996;62:853–859. doi: 10.1128/aem.62.3.853-859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson K, Quiding-Järbrink M, Osek J, Möller Å, Björk S, Holmgren J, Czerkinsky C. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect Immun. 1998;66:5889–5896. doi: 10.1128/iai.66.12.5889-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenwick B, Henry S. Porcine pleuropneumonia. J Am Vet Med Assoc. 1994;204:1334–1340. [PubMed] [Google Scholar]
- 13.Fuller T E, Thacker B J, Duran C O, Mulks M H. A genetically-defined riboflavin auxotroph of Actinobacillus pleuropneumoniae as a live attenuated vaccine. Vaccine. 2000;18:2867–2877. doi: 10.1016/s0264-410x(00)00076-1. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach G-F, Anderson C, Rossi-Campos A, Potter A A. The role of the 103 kD Actinobacillus pleuropneumoniae cytolysin in virulence and the association of the encoding gene with insertion sequence-like elements. 1992. p. 199. . Proc. Int. Pig Vet. Soc. Congr. 12:199. [Google Scholar]
- 15.Gerlach G-F, Anderson C, Potter A A, Klashinsky S, Willson P J. Cloning and expression of a transferrin-binding protein from Actinobacillus pleuropneumoniae. Infect Immun. 1992;60:892–898. doi: 10.1128/iai.60.3.892-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goethe R, Gonzáles O F, Lindener T, Gerlach G-F. A novel strategy for protective Actinobacillus pleuropneumoniae vaccines: detergent extraction of cultures induced by iron restriction. Vaccine. 2000;19:966–975. doi: 10.1016/s0264-410x(00)00212-7. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez G C, Caamano D L, Schryvers A B. Identification and characterization of a porcine-specific transferrin receptor in Actinobacillus pleuropneumoniae. Mol Microbiol. 1990;4:1173–1179. doi: 10.1111/j.1365-2958.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 18.Haesebrouck F, Chiers K, Van Overbeke I, Ducatelle R. Actinobacillus pleuropneumoniae infections in pigs: the role of virulence factors in pathogenesis and protection. Vet Microbiol. 1997;58:239–249. doi: 10.1016/s0378-1135(97)00162-4. [DOI] [PubMed] [Google Scholar]
- 19.Hannan P C, Bhogal B S, Fish J P. Tylosin tartrate and tiamutilin effects on experimental piglet pneumonia induced with pneumonic pig lung homogenate containing mycoplasmas, bacteria and viruses. Res Vet Sci. 1982;33:76–88. [PubMed] [Google Scholar]
- 20.Hennig I, Teutenberg-Riedel B, Gerlach G F. Downregulation of a protective Actinobacillus pleuropneumoniae antigen during the course of infection. Microb Pathog. 1999;26:53–63. doi: 10.1006/mpat.1998.0249. [DOI] [PubMed] [Google Scholar]
- 21.Hensel A, Pabst R, Bunka S, Petzoldt K. Oral and aerosol immunization with viable or inactivated Actinobacillus pleuropneumoniae bacteria: antibody response to capsular polysaccharides in bronchoalveolar lavage fluids (BALF) and sera of pigs. Clin Exp Immunol. 1994;96:91–97. doi: 10.1111/j.1365-2249.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inzana T J, Todd J, Veit H P. Safety, stability, and efficacy of noncapsulated mutants of Actinobacillus pleuropneumoniae for use in live vaccines. Infect Immun. 1993;61:1682–1686. doi: 10.1128/iai.61.5.1682-1686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen M J, Nielsen J P, Nielsen R. Comparison of virulence of different Actinobacillus pleuropneumoniae serotypes and biotypes using an aerosol infection model. Vet Microbiol. 1996;49:159–168. doi: 10.1016/0378-1135(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 24.Leiner G, Franz B, Strutzberg K, Gerlach G F. A novel enzyme-linked immunosorbent assay using the recombinant Actinobacillus pleuropneumoniae ApxII antigen for diagnosis of pleuropneumonia in pig herds. Clin Diagn Lab Immunol. 1999;6:630–632. doi: 10.1128/cdli.6.4.630-632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMillan D J, Medina E, Guzman C A, Walker M J. Expression of urease does not affect the ability of Bordetella bronchiseptica to colonise and persist in the murine respiratory tract. FEMS Microbiol Lett. 1999;178:7–11. doi: 10.1111/j.1574-6968.1999.tb13752.x. [DOI] [PubMed] [Google Scholar]
- 26.McWilliam A S, Napoli S, Marsh A M, Pemper F L, Nelson D J, Pimm C L, Stumbles P A, Wells T N, Holt P G. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oswald W, Konine D V, Rohde J, Gerlach G F. First chromosomal restriction map of Actinobacillus pleuropneumoniae and localization of putative virulence-associated genes. J Bacteriol. 1999;181:4161–4169. doi: 10.1128/jb.181.14.4161-4169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oswald W, Tonpitak W, Ohrt G, Gerlach G. A single-step transconjugation system for the introduction of unmarked deletions into Actinobacillus pleuropneumoniae serotype 7 using a sucrose sensitivity marker. FEMS Microbiol Lett. 1999;179:153–160. doi: 10.1111/j.1574-6968.1999.tb08721.x. [DOI] [PubMed] [Google Scholar]
- 29.Prideaux C T, Lenghaus C, Krywult J, Hodgson A L. Vaccination and protection of pigs against pleuropneumonia with a vaccine strain of Actinobacillus pleuropneumoniae produced by site-specific mutagenesis of the ApxII operon. Infect Immun. 1999;67:1962–1966. doi: 10.1128/iai.67.4.1962-1966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raleigh F A, Lech K, Brent R. Selected topics from classical bacterial genetics. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: Publishing Associates and Wiley Interscience; 1989. pp. 1.4.1–1.4.14. [DOI] [PubMed] [Google Scholar]
- 31.Reyrat J-M, Lopez-Ramirez G, Ofredo C, Gicquel B, Winter N. Urease activity does not contribute dramatically to persistence of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1996;64:3934–3936. doi: 10.1128/iai.64.9.3934-3936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi-Campos A, Anderson C, Gerlach G F, Klashinsky S, Potter A A, Willson P J. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine. 1992;10:512–518. doi: 10.1016/0264-410x(92)90349-o. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schmidt H, Maier E, Karch H, Benz R. Pore-forming properties of the plasmid-encoded hemolysin of enterohemorrhagic Escherichia coli O157:H7. Eur J Biochem. 1996;241:594–601. doi: 10.1111/j.1432-1033.1996.00594.x. [DOI] [PubMed] [Google Scholar]
- 35.Sheehan B J, Langford P R, Rycroft A N, Kroll J S. [Cu,Zn]-superoxide dismutase mutants of the swine pathogen Actinobacillus pleuropneumoniae are unattenuated in infections of the natural host. Infect Immun. 2000;68:4778–4781. doi: 10.1128/iai.68.8.4778-4781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tascon R I, Vazquez-Boland J A, Gutierrez-Martin C B, Rodriguez-Barbosa I, Rodriguez-Ferri E F. The RTX haemolysins ApxI and ApxII are major virulence factors of the swine pathogen Actinobacillus pleuropneumoniae: evidence from mutational analysis. Mol Microbiol. 1994;14:207–216. doi: 10.1111/j.1365-2958.1994.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 37.Tascon Cabrero R I, Vazquez-Boland J A, Gutierrez C B, Rodriguez-Barbosa J I, Rodriguez-Ferri E F. Actinobacillus pleuropneumoniae does not require urease activity to produce acute swine pleuropneumonia. FEMS Microbiol Lett. 1997;148:53–57. doi: 10.1111/j.1574-6968.1997.tb10266.x. [DOI] [PubMed] [Google Scholar]
- 38.Tonpitak W, Thiede S, Oswald W, Baltes N, Gerlach G F. Actinobacillus pleuropneumoniae iron transport: a set of exbBD genes is transcriptionally linked to the tbpB gene and required for utilization of transferrin-bound iron. Infect Immun. 2000;68:1164–1170. doi: 10.1128/iai.68.3.1164-1170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward C K, Lawrence M L, Veit H P, Inzana T J. Cloning and mutagenesis of a serotype-specific DNA region involved in encapsulation and virulence of Actinobacillus pleuropneumoniae serotype 5a: concomitant expression of serotype 5a and 1 capsular polysaccharides in recombinant A. pleuropneumoniae serotype 1. Infect Immun. 1998;66:3326–3336. doi: 10.1128/iai.66.7.3326-3336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilke M, Franz B, Gerlach G F. Characterization of a large transferrin-binding protein from Actinobacillus pleuropneumoniae serotype 7. Zentbl Vetmed Reihe B. 1997;44:73–86. doi: 10.1111/j.1439-0450.1997.tb00953.x. [DOI] [PubMed] [Google Scholar]