Abstract
Objective
To evaluate: (i) safety, (ii) feasibility, and medium‐term (iii) oncological and (iv) functional outcomes of salvage radical prostatectomy (sRP) for recurrent localised prostate cancer (PCa) following initial focal therapy using irreversible electroporation (IRE).
Patients and Methods
An international, multicentre and retrospective analysis of prospectively collected data of patients that underwent sRP for recurrent localised PCa after initial primary IRE treatment. Data were reported on (i) surgical complications, (ii) feasibility of sRP reported by surgeons, (iii) time interval between IRE and sRP and pathology results, and (iv) urinary continence, erectile function, and quality of life.
Results
In four participating centres, a total of 39 patients with a median (interquartile range [IQR]) age 64 (60–67) years were identified. No serious adverse events occurred during or following sRP and surgery was deemed feasible without difficulties. The median (IQR) time to recurrence following IRE was 14.3 (9.1–38.8) months. Pathology results showed localised disease in 21 patients (53.8%) and locally‐advanced disease in 18 (46.2%). Positive surgical margins (PSMs) were observed in 10 patients (25.6%), of which six (15.4%) had significant PSMs. A persistent detectable prostate‐specific antigen level was found in one case after sRP, caused by metastatic disease. One patient had a biochemical recurrence 6 months after sRP. These two cases, together with a PSM case, required additional therapy after sRP. After a median (IQR) follow‐up of 17.7 (11.8–26.4) months, urinary continence and erectile function were preserved in 34 (94.4%) and 18 patients (52.9%), respectively, while quality of life remained stable.
Conclusions
Salvage RP is safe and feasible for patients with recurrent localised PCa following initial IRE treatment. The medium‐term oncological and functional outcomes are similar to primary RP. Strict patient selection for focal therapy and standardised follow‐up is needed as some patients developed high‐grade disease.
Keywords: prostate cancer, irreversible electroporation, focal therapy, recurrence, salvage treatment, radical prostatectomy, #PCSM, #ProstateCancer, #uroonc
Introduction
Focal therapy (FT) is an upcoming alternative treatment for carefully selected patients with localised prostate cancer (PCa) within a clinical trial setting [1, 2]. FT has potential to obtain oncological control while preserving urinary, sexual and bowel function. Several FT modalities have been studied, these include high‐intensity focussed ultrasound (HIFU), cryotherapy, photodynamic therapy, laser ablation, and irreversible electroporation (IRE). Clinical trials of these techniques have shown promising short‐ to mid‐term results [3, 4, 5, 6, 7, 8].
Irreversible electroporation induces irreversible permeabilisation of the cell membrane, by using high‐voltage electrical pulses employed by two or more electrodes placed in the prostate, resulting in loss of homeostasis and consequential cell death [9]. Blazevski et al. [10] performed primary IRE in 123 patients in a prospective setting and demonstrated very low morbidity (98.8% pad‐free continent, 93% preserved sexual function) at the 12‐month follow‐up. Short‐term oncological control was 77.5% following initial IRE treatment. However, when allowing one re‐treatment with IRE, only six of 123 patients (4.8%) required salvage whole‐gland treatment at a median follow‐up of 36 months. This is considered a low local recurrence rate, when compared to other FT modalities shown by Ahdoot et al. [11], which ranged from 4% to 50%. Although, IRE allows for repeat treatment, salvage whole‐gland treatment with radiotherapy or salvage radical prostatectomy (sRP) is still the optimal approach for a curative setting in some patients in case of treatment failure.
Irreversible electroporation ablation effectuates a sharp demarcation of the ablation zone within the electrode configuration. However, an ablate and resect study following focal IRE treatment without curative intent, showed that the ablation zone extended outside the needle configuration [12]. The large ablation zone may compromise the resectability, potentially having an impact on functional outcomes. Yet, there is a paucity of studies reporting outcomes of sRP following initial IRE treatment. The aim of this study was to evaluate safety, feasibility, and medium‐term oncological and functional outcomes of sRP for recurrent localised PCa following initial IRE treatment.
Patients and Methods
Population
This is a retrospective subset analysis on a prospective trial cohort data of an international multicentre registry. Following approval from the Institutional Review Boards, patients were identified in the registry that underwent sRP for recurrent localised PCa after at least one IRE treatment of the prostate. Recurrence was defined as histological confirmed PCa recurrence and International Society of Urological Pathology Grade Group (ISUP GG) ≥3 upgrading, and/or multifocal ISUP GG ≥2, not suitable for redo‐IRE or active surveillance. Patients without suspicion of metastatic disease, were offered salvage surgery or radiation therapy after agreement was reached in a multidisciplinary tumour board meeting. All patients had a life expectancy of ≥10 years and patients with any other prior or concurrent therapy (e.g., radiation therapy or hormonal treatment) of PCa were excluded.
The IRE Treatment Procedure
Transperineal IRE treatment with cognitive or software‐aided MRI/ultrasonography guidance was performed in the operating theatre of each participating centre using the Nanoknife® IRE system (AngioDynamics Inc., Queensbury, NY, USA) under general anaesthesia with deep muscle paralysis. IRE treatment was performed at ~1500 V/cm, delivering 90 pulses of 90 μs duration.
Follow‐Up after IRE
Follow‐up strategy after IRE treatment followed the consensus guidelines on follow‐up after FT [2]. See Table S1 for a detailed overview of the follow‐up schedule used by each participating centre. Prostate MRI was performed at 6 or 12 months after IRE, with consecutive standardised prostate template biopsies. Serial PSA levels were measured at least every 6 months after IRE, and in case of an increase diagnostic evaluation was performed for re‐staging purposes.
Salvage (Robot‐Assisted) Laparoscopic Prostatectomy Procedure
The sRP was performed by experienced surgeons across four participating centres, according to urologists’ insights using a standardised technique. Uni‐ or bilateral nerve‐sparing was performed in cases where it was deemed clinically safe. Pelvic lymph node dissection was conducted in case of a Briganti nomogram score for lymph node involvement of >7%.
Safety and Feasibility of sRP
Safety of sRP was evaluated according to the number of peri‐procedural and 30‐day post‐procedural adverse events according to the Clavien–Dindo classification of surgical complications [13]. Feasibility of sRP was evaluated by the amount of blood loss (mL) and the difficulty of sRP as reported by the surgeon (subjective scoring system on a 5‐point Likert scale).
Oncological Outcomes
Prostate specimen pathology was examined by dedicated uro‐pathologists following the standardised reporting of the ISUP. Extra parameters included the location of recurrent disease relative to the FT area, either inside (in‐field recurrence) or outside (out‐of‐field recurrence) the IRE‐ablation zone. Positive surgical margins (PSMs) were reported in absolute rates and rates of significant PSMs (>5 mm cancer core length and/or ISUP GG ≥2).
Follow‐up of patients after sRP was performed every 3–6 months with PSA measurements. The definition of PSA persistence was a PSA level of ≥0.1 ng/mL at 6 weeks after sRP. Biochemical recurrence (BCR) was defined as a confirmed PSA level of ≥0.2 ng/mL following sRP.
Functional Outcomes
Functional outcomes were evaluated by using patient‐reported outcomes, obtained by data managers. The international validated instruments used were the IPSS and five‐item version of the International Index of Erectile Function (IIEF‐5) questionnaires from baseline until the last follow‐up. Patients were considered continent when requiring 0–1 pads/day. Potency was described as erections firm enough for sexual intercourse with or without a phosphodiesterase type 5 (PDE‐5) inhibitor. Patients with a follow‐up duration of <3 months were not included in this analysis as they are considered to be in their functional recovery phase.
Statistical Analysis
Baseline and follow‐up characteristics were reported descriptively. No statistical tests were performed due to the limited cohort size.
Results
A total of 365 primary IRE patients were identified in the four participating centres. In all, 39 patients received sRP following initial IRE (Fig. 1). The median (interquartile range [IQR]) age was 64 (60–67) years at the time of IRE treatment.
Fig. 1.
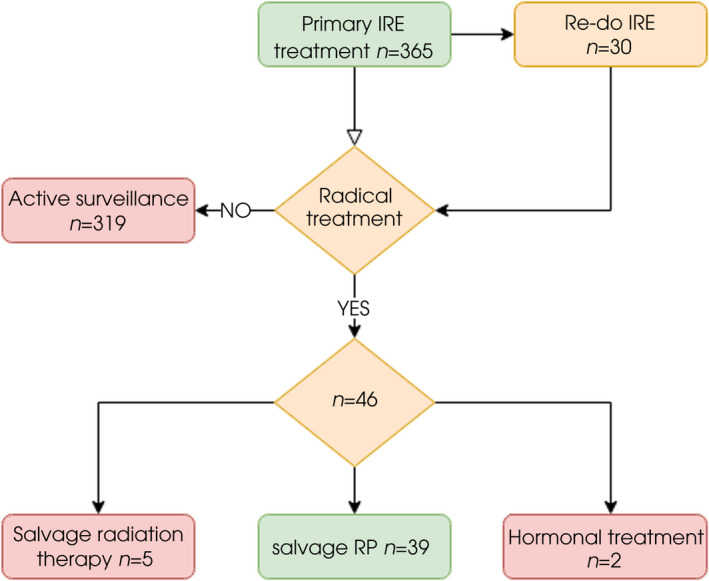
Flow chart of included patients who underwent sRP after initial IRE treatment.
Primary and Re‐Do IRE Treatment
At pre‐IRE staging, all patients had localised disease, with 10 patients (25.6%) ISUP GG 1, 25 (64.1%) ISUP GG 2, and four (10.3%) ISUP GG 3 following diagnostic biopsies. The median (IQR) pre‐IRE PSA level was 6.0 (4.9–8.1) ng/mL. The D’Amico risk classification resulted in seven (18.0%), 31 (79.5%), and one patient (2.6%, due to cT2c staging) being classified as having low‐, intermediate‐, and high‐risk disease, respectively. Treatment was performed unilaterally in 33 patients (84.6%). Re‐do IRE treatment was performed in seven patients (18.0%) after a median (IQR) of 29.8 (12.2–50.5) months. The median (IQR) time‐to‐recurrence after IRE treatment was 14.3 (9.1–38.8) months. The median (IQR) follow‐up duration after sRP was 17.7 (11.8–26.4) months. Table 1 shows patients’ characteristics at baseline (pre‐IRE).
Table 1.
Patients’ characteristics undergoing sRP before IRE treatment and IRE treatment characteristics.
| Characteristic | Value |
|---|---|
| Number of patients | 39 |
| Age at time of IRE, years, median (IQR) | 64 (60–67) |
| Pre‐IRE PSA levels, ng/mL, median (IQR) | 6.0 (4.9–8.1) |
| Prostate volume pre‐IRE, mL, mean (sd) | 45 (17.5) |
| ASA classification, n (%) | |
| I | 17 (43.6) |
| II | 20 (51.3) |
| III | 2 (5.1) |
| ISUP GG, n (%) | |
| 1 | 10 (25.6) |
| 2 | 25 (64.1) |
| 3 | 4 (10.3) |
| Number of biopsy cores, median (IQR) | 20 (12–30) |
| Number of positive cores, median (IQR) | 3 (2–4.5) |
| T‐staging, n (%) | |
| T1c | 24 (61.5) |
| T2a–b | 14 (35.9) |
| T2c | 1 (2.6) |
| D’Amico risk classification, n (%) | |
| Low‐risk disease | 7 (18.0) |
| Intermediate‐risk disease | 31 (79.5) |
| High‐risk disease | 1 (2.6) |
| IRE treatment site, n (%) | |
| Left apex | 11 (28.2) |
| Left base/mid | 7 (17.9) |
| Right apex | 7 (17.9) |
| Right base/mid | 10 (25.6) |
| Multifocal | 4 (10.3) |
| IRE treatment location, n (%) | |
| Unilateral | 33 (84.6) |
| Bilateral | 6 (15.4) |
| IRE needles used, median (IQR) | |
| During first treatment | 4 (3–4) |
| During second treatment (n = 7) | 4 (3–4) |
| Time to second IRE treatment, months, median (IQR) | 29.8 (12.2–50.5) |
ASA, American Society of Anesthesiology.
Recurrence of Localised Disease after Initial IRE Treatment
The median (IQR) PSA level at recurrence was 6.0 (3.4–8) ng/mL. All patients had localised disease at recurrence, with seven patients (18%) ISUP GG 1, 20 (51.3%) ISUP GG 2, eight (20.5%) ISUP GG 3, and four (10.3%) ISUP GG 4–5. The D’Amico risk classification at recurrence resulted in four (10.3%), 28 (71.8%) and seven patients (18.0%) being classified as having low‐, intermediate‐, and high‐risk disease, respectively. Table S1 shows patient characteristics at recurrence following IRE treatment(s).
Safety and Feasibility of sRP
Characteristics of sRP are shown in Table 2. All sRP procedures were performed using a laparoscopic or robot‐assisted approach. Bilateral nerve sparing was performed in 25 patients (64.1%), unilateral nerve sparing in 10 (25.6%) and non‐nerve sparing in three (7.7%). Pelvic lymph node dissection was performed in nine patients (23.1%). The mean (SD) intraoperative blood loss was 182 (61.6) mL. One case of self‐limiting gross haematuria occurred intraoperatively. No blood transfusions were needed. Feasibility of sRP reported by the surgeons was moderate to good without dissection difficulties. Specifically, no bladder or rectal injuries occurred. Although, fibrosis at the ablated site with adherence to the pelvic floor, neurovascular bundle or posterior plane was often noted. Surgeons reported that primary dissection of untreated tissue (e.g., prostatic pedicles, posterior plane, apex of prostate and nerve sparing) improved three‐dimensional visualisation of the prostate. This enabled determination of the surgical plane for dissection of the ablated site. The mean (SD) hospital admission duration was 1.9 (0.8) days. No serious (Grade ≥III) adverse events were observed within 30 days following sRP.
Table 2.
Salvage RP characteristics and follow‐up details.
| Variable | Value |
|---|---|
| Number of patients, n (%) | 39 (100) |
| sRP approach, n (%) | |
| Laparoscopic | 3 (7.7) |
| Robot‐assisted | 36 (92.3) |
| Nerve‐sparing surgery, n (%) | |
| Unilateral | 10 (25.6) |
| Bilateral | 25 (64.1) |
| None | 3 (7.7) |
| NA | 1 (2.6) |
| Pelvic lymph node dissection, n (%) | 9 (23.1) |
| Surgical feasibility, n (%) | |
| Good | 36 (92.3) |
| Moderately difficult | 3 (7.7) |
| Blood loss, mL, mean (sd) | 182 (61.6) |
| Post‐sRP hospitalisation, days, mean (sd) | 1.9 (0.8) |
| pT Stage, n (%) | |
| pT2a/b | 6 (15.4) |
| pT2c | 15 (38.5) |
| pT3a | 15 (38.5) |
| pT3b | 3 (7.7) |
| ISUP GG, n (%) | |
| 1 | 2 (5.1) |
| 2 | 21 (53.8) |
| 3 | 8 (20.5) |
| 4 | 4 (10.3) |
| 5 | 4 (10.3) |
| PSM rate, n (%) | 10 (25.6) |
| Lymph node involvement, n (%) | 0 (0) |
| Location of recurrence, n (%) | |
| In‐field only | 7 (18.0) |
| Out‐of‐field only | 16 (41.0) |
| In‐field and out‐of‐field | 16 (41.0) |
| Follow‐up duration, months, median (IQR) | 17.7 (11.8–26.4) |
Oncological Outcomes
Whole mount pathology of the RP specimen showed localised disease in 21 patients (53.8%) and locally‐advanced disease in 18 (46.2%), of which 15 (38.5%) had extracapsular extension (pathological T3a [pT3a]) and three (7.7%) had seminal vesicle invasion (pT3b). All patients with pT3a/b disease had out‐of‐field only or both in‐field and out‐of‐field recurrence. PSMs were found in 10 patients (25.6%), of which six (15.4%) had pT2, one (2.6%) pT3a, and three (7.6%) pT3b disease. Of the 10 patients with PSMs, six were considered significant (i.e., >5 mm PSM and/or ISUP GG ≥2). Two patients with pT2c disease had a significant PSM based on a PSM of 5 and 27 mm and ISUP GG 2 and 1, respectively. Three patients with pT3a/b disease had a significant PSM based on ISUP GG 2 with a PSM length of 2, 1.5 and 0.3 mm, respectively. The last patient with a significant PSM had pT2c disease with a PSM length of 4 mm and ISUP GG 2. The subgroup of nine patients that underwent a lymph node dissection showed no metastatic nodes.
Pathological analysis showed ISUP GG 1 in two patients (5.1%), ISUP GG 2 in 21 (53.8%), ISUP GG 3 in eight (20.5%), and ISUP GG 4–5 in eight (20.5%). ISUP on whole mount pathology, when compared to prostate biopsy at recurrence, was concordant in 22 cases (56.4%), led to upgrading in 14 (35.9%) and to downgrading in three (7.7%; see Table S1). Location of recurrence was in‐field for seven patients (18%), out‐of‐field for 16 (41%) and both in‐/out‐of‐field for 16 (41%). Figure 2 shows an example of in‐field recurrence on follow‐up MRI and the corresponding prostate specimen slide.
Fig. 2.
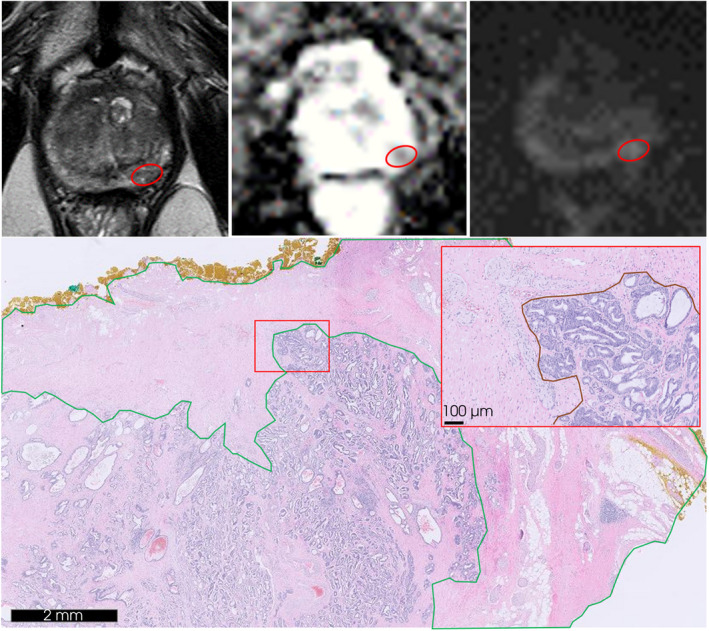
An example of in‐field recurrence at the left mid‐apex posterolateral peripheral zone on follow‐up MRI and corresponding haematoxylin and eosin stained (H&E) slide of the resected prostate specimen. Red circle demonstrates the aberrant lesion of 8 mm on MRI series: transverse T2‐weighted (upper left), apparent diffusion coefficient (upper mid) and diffusion weighted imaging of calculated b‐values (upper right). H&E slide obtained from the corresponding prostate specimen tissue (down) displaying IRE treatment‐induced inflammation and fibrosis (green) and recurrent disease of adenocarcinoma Gleason score 3 + 4 = 7 (down right; red).
A PSA persistence after sRP was detected in one patient, who did not require preoperative staging according to the Briganti nomogram. Postoperative prostate‐specific membrane antigen‐positron emission tomography/CT imaging detected metastatic disease in this patient for which hormonal treatment was given. BCR was detected in one patient after 6 months of follow‐up. Another patient received radiotherapy following sRP because of a significant PSM. Cancer‐specific survival and overall survival were 100% with a median (IQR) follow‐up of 17.7 (11.8–26.4) months following sRP.
Functional Outcomes
All patients were continent before IRE treatment. Urinary continence was preserved at recurrence following initial IRE treatment in 36 patients (92.3%) and it was preserved at last follow‐up after sRP in 34 (94.4%). Two patients needed a urinary sphincter prosthesis following sRP. Erectile dysfunction before IRE treatment was reported by three patients (7.7%) and thus they were excluded from the erectile function analysis. Erectile function was examined in a total of 36 patients with a median (IQR) age of 63 (60–67) years. Erectile function was preserved at recurrence after IRE in 23 patients (63.9%) and it was preserved at last follow‐up in 18 (52.9%; Table 3). The median (IQR) IPSS reduced from 16 (7.5–21) at baseline to 7.5 (5–9.8) at recurrence and to 7.5 (3–7.5) at last follow‐up, while the median (IQR) IPSS quality‐of‐life score remained stable at 2 (1–2). The median (IQR) IIEF‐5 score reduced substantially from 23 (18.5–24) at baseline to 14 (5–22.5) at recurrence and to 3 (0.8–6.5) at last follow‐up.
Table 3.
Overview of (significant) PSMs, and continence and potency preserved at last follow‐up per participating centre and in total.
| St. Vincent’s Prostate Cancer Centre | Instituto Valenciano de Oncologia | St. Antonius Hospital | Amsterdam University Medical Centres | Total | Missing, n (%) | |
|---|---|---|---|---|---|---|
| Primary IRE treatments performed, n | 248 | 58 | 30 | 29 | 365 | – |
| sRP performed, n (%) | 22/248 (8.9) | 4/58 (6.9) | 5/30 (16.7) | 8/29 (27.6) | 39/365 (10.7) | – |
| PSMs, n (%) | 2/22 (9.1) | 2/4 (50) | 2/5 (40) | 4/8 (50) | 10/39 (25.6) | 0 (0) |
| Significant PSMs, n (%) | 0/22 (0) | 2/4 (50) | 0/5 (0) | 4/8 (50) | 6/39 (15.4) | 0 (0) |
| Continence, n (%) | 20/20 (100) | 2/4 (50) | 4/4 (100) | 8/8 (100) | 34/36 (94.4) | 3 (7.7) |
| Potency, n (%) | 11/18 (61.1) | 3/4 (75) | 1/5 (20) | 3/7 (42.9) | 18/34 (52.9) | 2 (5.1) |
| Follow‐up, months, median (IQR) | 19.6 (17.1–28.5) | 15.8 (12.2–21.1) | 17.7 (12–20.3) | 19.7 (11.5–38.6) | 17.7 (11.8–26.4) | – |
A significant PSM is considered as >5 mm cancer core length and/or ISUP GG ≥2. Patients were considered continent when requiring 0–1 pads/day and potency was described as erections firm enough for sexual intercourse with or without PDE‐5‐inhibitor.
Discussion
For carefully selected patients with PCa FT is an increasingly applied alternative, preferably within a clinical trial setting. However, the inherent risk of recurrence within, but mostly outside the ablation zone raises the question whether initial FT might jeopardise sequential radical therapies. Earlier studies have shown safety of sRP following different primary FT modalities, and comparable functional and oncological outcomes when compared to primary RP [14, 15, 16, 17, 18, 19, 20]. Yet, there is also evidence that surgical dissection of the prostate is arguably more difficult after FT, which could also apply for IRE [21, 22]. To our knowledge, evidence on outcomes after sRP following initial IRE treatment is lacking. This study is the first international multicentre analysis on safety, feasibility, medium‐term oncological and functional outcomes of sRP for recurrent localised PCa after initial IRE treatment.
We showed that, in a relatively large cohort, sRP following IRE treatment, after a median (IQR) of 14.3 (9.1–38.8) months, could be performed safely, without major intra‐ and postoperative serious adverse events. Yet, a systematic review by Marra et al. [23] reporting on 67 cases of sRP following initial failure of mainly focal HIFU and focal cryotherapy, found eight high‐grade complications. Surgical feasibility was overall not compromised and bilateral nerve‐sparing surgery could be performed in most cases (61.4%).
This study confirms the reported high number of PSMs and locally advanced stage following other FT modalities. Although sRP could be performed safely and was feasible, there was a relatively high rate of upgraded pathology, including locally advanced tumours (pT3a [38.5%], pT3b [7.7%]) and a PSM rate of 25.6%. This confirms findings after treatment with other FT modalities [23]. However, the significant PSM rate was 15.4%, differing among centres, and is similar to the mean (range) PSM rate of 15% (6.5%–32%) seen after RPs in the primary setting [24]. The long‐term outcomes remain to be evaluated. No mortality occurred during medium‐term follow‐up in this study.
Interestingly, pT Stage and ISUP GG were substantially upgraded at recurrence in our series. Also, location of recurrence was observed in‐field only or both out‐of‐field and in‐field of the initial IRE ablation zone in 23 patients (59%). Furthermore, all patients with locally‐advanced disease (pT3a/b) had out‐of‐field or both in‐field and out‐of‐field recurrence. This might be due to inadequate initial staging and/or patient selection, as mainly low‐, or favourable intermediate‐risk patients are candidates for this treatment modality. This is probably as a result of the heterogeneity and multifocal growth of PCa combined with current diagnostic limitations, which is supported by the short interval to recurrence. Another possibility is that inadequate or incomplete ablation induces biological changes in the untreated tissue leading to pro‐oncogenic effects in some patients [25]. This reinforces the need that FT should only be performed after proper patient selection and with rigorous follow‐up after FT, which should not be primarily imaging based but should also rely on repeat systematic biopsies [26]. There is a need for consensus guidelines on how prostate MRIs should be interpreted following FT and how FT failure is defined on MRI.
The medium‐term continence and potency at last follow‐up, at a median (IQR) of 17.7 (11.8–26.4) months, were preserved in 94.4% and 52.9%, respectively. The systematic review by Marra et al. [23] showed that 56.7% maintained continence, while 5.9% maintained erectile function, albeit that information on erectile function was missing in 62.8% of their cases. Also, these functional outcomes are comparable to the Prostate Cancer Outcome Study by Penson et al. [27], which reported on 1288 primary RP patients, and showed a continence rate of 90% and potency rate of 22% at 24 months.
Interestingly, notable differences in both oncological and functional outcomes were found between centres (Table 3). This could reflect local expertise and treatment volume regarding IRE and sRP procedures, impacting patient selection and follow‐up. Moreover, a standardised follow‐up after IRE is crucial for early detection of recurrence. Currently, follow‐up is mainly based on high‐resolution MRI and systematic biopsies. However, this also requires a high level of local expertise of radiologists, biopsy operators and pathologists. These factors combined enable early detection of organ‐confined recurrent disease, and therefore, a higher chance of improved functional and oncological outcomes with salvage surgery.
The limitations of this study include its retrospective design and heterogeneity. Furthermore, this report does not provide an accurate image of all patients that recur after initial IRE treatment due to selection bias. Patients were selected for sRP based on pathology outcomes, and less aggressive tumours were more likely to be treated by re‐do IRE or radiotherapy, and therefore not included in this study. However, the international and multicentre design allowed demonstration of real‐world data from multiple urologists with different backgrounds and levels of experience. Moreover, follow‐up after FT can be standardised with a core outcome set, as suggested in the literature [2, 28].
In conclusion, sRP is a safe and feasible option for patients with recurrent localised PCa after initial IRE treatment. Medium‐term functional and oncological outcomes after sRP are comparable to primary RP and should, therefore, be considered a suitable treatment option for selected patients with recurrent disease following initial IRE treatment. Stringent patient selection for FT and standardised follow‐up, including systematic biopsies, are crucial as some patients develop locally‐advanced or high‐grade disease.
Funding
This research received no external funding. L.A.M.J.G. van Riel receives a research grant from the ‘Cure for Cancer’ foundation.
Disclosures of Interest
P.D. Stricker, M.J. Scheltema and J.L. Domínguez‐Ecrig received consulting fees from Angiodynamics. All other authors have no disclosures of interest to declare.
Author Contributions
Conceptualisation: L.A.M.J.G. van Riel, T.M. de Reijke, M.J. Scheltema, J.R. Oddens; Methodology: L.A.M.J.G. van Riel, T.M. de Reijke, M.J. Scheltema, J.R. Oddens; Investigation: L.A.M.J.G. van Riel, B. Geboers, E. Kabaktepe; Data curation: L.A.M.J.G. van Riel, B. Geboers, E. Kabaktepe, A. Blazevski, D.J. Reesink, J.L. Domínguez‐Escrig; Formal analysis: L.A.M.J.G. van Riel; Visualisation: L.A.M.J.G. van Riel; Writing – original draft: L.A.M.J.G. van Riel; Writing – review and editing: L.A.M.J.G. van Riel, B. Geboers, E. Kabaktepe, A. Blazevski, D.J. Reesink, P. Stijns, P.D. Stricker, J. Casanova, J.L. Domínguez‐Escrig, T.M. de Reijke, M.J. Scheltema, J.R. Oddens; Supervision, T.M. de Reijke, M.J. Scheltema, J.R. Oddens. All authors have read and agreed to the current version of the manuscript.
Ethical Approval Statement/Patient Consent Statement
The Medical Ethics Review Committee of the Amsterdam University Medical Centers confirmed that the Medical Research Involving Human Subjects Act (in Dutch: WMO) does not apply to this study. Reference number: W20_291. Therefore, written informed consent was not required.
Abbreviations
- BCR
biochemical recurrence
- FT
focal therapy
- HIFU
high‐intensity focussed ultrasound
- IIEF‐5
International Index of Erectile Function
- IQR
interquartile range
- IRE
irreversible electroporation
- ISUP GG
International Society of Urological Pathology Grade Group
- PCa
prostate cancer
- PDE‐5
phosphodiesterase type 5
- PSM
positive surgical margin
- (s)RP
(salvage) radical prostatectomy
Supporting information
Table S1 . Patients’ characteristics at time of sRP following IRE treatment.
Acknowledgements
The authors acknowledge the prostate biobank and database CANSTO, which are funded and maintained by Cancer Institute NSW Grant and St Vincent’s Prostate Cancer Centre, and specifically Anne‐Maree Haynes and Shikha Agrawal for data management.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Mottet N, van den Bergh RCN, Briers E et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG guidelines on prostate cancer‐2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2021; 79: 243–62 [DOI] [PubMed] [Google Scholar]
- 2. Muller BG, van den Bos W, Brausi M et al. Follow‐up modalities in focal therapy for prostate cancer: results from a Delphi consensus project. World J Urol 2015; 33: 1503–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganzer R, Hadaschik B, Pahernik S et al. Prospective multicenter phase II study on focal therapy (Hemiablation) of the prostate with high intensity focused ultrasound. J Urol 2018; 199: 983–9 [DOI] [PubMed] [Google Scholar]
- 4. Lebdai S, Bigot P, Leroux PA, Berthelot LP, Maulaz P, Azzouzi AR. Vascular targeted photodynamic therapy with padeliporfin for low risk prostate cancer treatment: midterm oncologic outcomes. J Urol 2017; 198: 335–44 [DOI] [PubMed] [Google Scholar]
- 5. Shah TT, Peters M, Eldred‐Evans D et al. Early‐medium‐term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol 2019; 76: 98–105 [DOI] [PubMed] [Google Scholar]
- 6. van Luijtelaar A, Greenwood BM, Ahmed HU et al. Focal laser ablation as clinical treatment of prostate cancer: report from a Delphi consensus project. World J Urol 2019; 37: 2147–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guillaumier S, Peters M, Arya M et al. A multicentre study of 5‐year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol 2018; 74: 422–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albisinni S, Melot C, Aoun F et al. Focal treatment for unilateral prostate cancer using high‐intensity focal ultrasound: a comprehensive study of pooled data. J Endourol 2018; 32: 797–804 [DOI] [PubMed] [Google Scholar]
- 9. Thomson KR, Cheung W, Ellis SJ et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011; 22: 611–21 [DOI] [PubMed] [Google Scholar]
- 10. Blazevski A, Scheltema MJ, Yuen B et al. Oncological and quality‐of‐life outcomes following focal irreversible electroporation as primary treatment for localised prostate cancer: a biopsy‐monitored prospective cohort. Eur Urol Oncol 2020; 3: 283–90 [DOI] [PubMed] [Google Scholar]
- 11. Ahdoot M, Lebastchi AH, Turkbey B, Wood B, Pinto PA. Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol 2019; 31: 200–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Bos W, de Bruin DM, Jurhill RR et al. The correlation between the electrode configuration and histopathology of irreversible electroporation ablations in prostate cancer patients. World J Urol 2016; 34: 657–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pierrard V, Lebdai S, Terrier JE et al. Radical prostatectomy after vascular targeted photodynamic therapy tookad soluble: feasability, short and long term results. Eur Urol Suppl 2017; 16: e2089–90 [Google Scholar]
- 15. Peretsman S, Brooks J. Salvage robotic prostatectomy following whole gland high‐intensity focused ultrasound with a Sonablate 500 device: technical feasibility and safety. J Robot Surg 2017; 11: 217–21 [DOI] [PubMed] [Google Scholar]
- 16. Onol FF, Bhat S, Moschovas M et al. Comparison of outcomes of salvage robot‐assisted laparoscopic prostatectomy for post‐primary radiation vs focal therapy. BJU Int 2020; 125: 103–11 [DOI] [PubMed] [Google Scholar]
- 17. Marconi L, Stonier T, Tourinho‐Barbosa R et al. Robot‐assisted radical prostatectomy after focal therapy: oncological, functional outcomes and predictors of recurrence. Eur Urol 2019; 76: 27–30 [DOI] [PubMed] [Google Scholar]
- 18. Leonardo C, Franco G, De Nunzio C et al. Salvage laparoscopic radical prostatectomy following high‐intensity focused ultrasound for treatment of prostate cancer. Urology 2012; 80: 130–3 [DOI] [PubMed] [Google Scholar]
- 19. Lawrentschuk N, Finelli A, Van Der Kwast TH et al. Salvage radical prostatectomy following primary high intensity focused ultrasound for treatment of prostate cancer. J Urol 2011; 185: 862–8 [DOI] [PubMed] [Google Scholar]
- 20. Herrera‐Caceres JO, Nason GJ, Salgado‐Sanmamed N et al. Salvage radical prostatectomy following focal therapy: functional and oncological outcomes. BJU Int 2020; 125: 525–30 [DOI] [PubMed] [Google Scholar]
- 21. Ogaya‐Pinies G, Linares‐Espinos E, Sanchez‐Salas R, Hernandez‐Cardona E, Cathelineau X, Patel V. Salvage robotic‐assisted radical prostatectomy: oncologic and functional outcomes from two high‐volume institutions. J Urol 2017; 197(4 Suppl. 1): e630–1 [DOI] [PubMed] [Google Scholar]
- 22. Nunes‐Silva I, Barret E, Srougi V et al. Effect of prior focal therapy on perioperative, oncologic and functional outcomes of salvage robotic assisted radical prostatectomy. J Urol 2017; 198: 1069–76 [DOI] [PubMed] [Google Scholar]
- 23. Marra G, Gontero P, Walz JC et al. Complications, oncological and functional outcomes of salvage treatment options following focal therapy for localized prostate cancer: a systematic review and a comprehensive narrative review. World J Urol 2019; 37: 1517–34 [DOI] [PubMed] [Google Scholar]
- 24. Yossepowitch O, Briganti A, Eastham JA et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 2014; 65: 303–13 [DOI] [PubMed] [Google Scholar]
- 25. Geboers B, Scheffer HJ, Graybill PM et al. High‐voltage electrical pulses in oncology: irreversible electroporation, electrochemotherapy, gene Electrotransfer, electrofusion, and Electroimmunotherapy. Radiology 2020; 295: 254–72 [DOI] [PubMed] [Google Scholar]
- 26. Scheltema MJ, Chang JI, van den Bos W et al. Preliminary diagnostic accuracy of multiparametric magnetic resonance imaging to detect residual prostate cancer following focal therapy with irreversible electroporation. Eur Urol Focus 2019; 5: 585–91 [DOI] [PubMed] [Google Scholar]
- 27. Penson DF, McLerran D, Feng Z et al. 5‐year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol 2005; 173: 1701–5 [DOI] [PubMed] [Google Scholar]
- 28. van den Bos W, Muller BG, Ahmed H et al. Focal therapy in prostate cancer: International multidisciplinary consensus on trial design. Eur Urol 2014; 65: 1078–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 . Patients’ characteristics at time of sRP following IRE treatment.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


