Abstract
Hyalomma ticks are important vectors of Crimean‐Congo haemorrhagic fever virus (CCHFV) and other pathogens. They are frequently carried as immatures from Africa, the Middle East and Mediterranean areas to temperate Europe via migratory birds and emergence of adults has been reported in many countries where it has so far been considered non‐endemic. This study aimed to implement the first steps of the DAMA (Document, Assess, Monitor, Act) protocol by monitoring the potential arrival of adult Hyalomma ticks in Hungary applying citizen‐science methods. Ticks were collected from April to December 2021 by asking volunteer participants through a self‐made website to look for large, quickly moving, striped‐legged hard ticks on themselves, their pets and livestock. Owing to an intensive media campaign, the project website had more than 31,000 visitors within 7 months; 137 specimens and several hundred photos of hard ticks were submitted by citizen scientists from all over the country. Beside Ixodes ricinus, Dermacentor reticulatus, Dermacentor marginatus and Haemaphysalis inermis, a specimen from a dog was morphologically identified as a male Hyalomma marginatum and another removed from a cow as a male Hyalomma rufipes. The dog and the cow had never been abroad, lived approximately 280 km apart, so the two Hyalomma observations can be considered separate introductions. Amplification of the partial mitochondrial cytochrome C oxidase subunit I gene was successfully run for both specimens. Sequencing confirmed the morphological identification for both ticks. Based on the phylogenetic analyses, the Hy. marginatum individual most likely belongs to the Eurasian population and the Hy. rufipes tick to a clade of mixed sequences from Europe and Africa. We summarize the scattered historical reports about the occurrence of Hyalomma ticks and CCHFV in Hungary. Our data highlight the effectiveness of citizens science programmes in the monitoring and risk assessment of CCHFV emergence and preparedness in the study area.
Keywords: citizen science, Crimean‐Congo haemorrhagic fever, DAMA protocol, emerging infectious diseases, Hyalomma adult , imported ticks
1. BACKGROUND
Emergence of pathogens and vectors in new hosts and geographical areas poses great threats for public and animal health (Jones et al., 2008). Ticks are responsible for the spread of numerous pathogens worldwide and species belonging to the genus Hyalomma have been shown to have a particular epidemiologic role (Estrada‐Peña et al., 2020; Hubálek et al., 2020). The tick species Hyalomma marginatum and Hyalomma rufipes are the main vectors of Crimean‐Congo haemorrhagic fever virus (CCHFV). This negative‐sense single‐stranded RNA virus, belonging to the Nairoviridae family (order Bunyavirales), is able to cause debilitating human disease with a fatality rate up to 30%, given the absence of any treatment or vaccine (Bente et al., 2013). The public health significance of Crimean‐Congo haemorrhagic fever is related to the fact that this infection is the most geographically widespread tick‐transmitted viral disease in humans. It is found in the southern parts of Asia, the Middle East, Europe and Africa. The emergence of the virus overlaps with the distribution of Hyalomma spp., as these ticks are responsible for the spread of the disease agent (Bente et al., 2013).
In contrast to the majority of hard ticks that have a three‐host life cycle, Hy. marginatum and Hy. rufipes are two‐host ticks (Estrada‐Peña et al., 2018). The larva moults into a nymph on the first host, feeding on that animal at both stages of development. For these two Hyalomma species, birds are known to be preferred first hosts. The fact that they do not change hosts before becoming a nymph greatly contributes to the successful geographical spread of these ticks, as it allows them to travel longer distances when attached to a (migratory) bird. The adults moult from the engorged nymphs that dropped off the first host and actively look for a second host, which is usually a large wild or domesticated mammal or human (Valcárcel et al., 2020; Walker et al., 2003).
Immature Hyalomma ticks arrive annually with migratory birds in temperate Europe but (Capek et al., 2014) emergence of adults has only been detected in higher numbers and wider distribution recently. Although adults of Hy. marginatum were found in Slovakia (Nosek et al., 1982) and Austria (Duscher et al., 2018), those of Hy. rufipes were retrieved in Hungary (Hornok & Horváth, 2012). The adults of both tick species have recently been detected the Czech Republic (Hubálek et al., 2020; Lesiczka et al., 2022; Rudolf et al., 2021), Germany (Chitimia‐Dobler et al., 2019), Sweden (Grandi et al., 2020), the United Kingdom (Hansford et al., 2019; McGinley et al., 2021) and the Netherlands (Uiterwijk et al., 2021).
The citizen science approach, or community research, is gaining in popularity and involvement in many research fields today. The basic idea is that the data are collected or generated with the help of non‐researchers but are analyzed by researchers specializing in the given topic (Tran et al., 2021). Citizen science benefits both researchers who evaluate the data and citizens who collect them. With the help of citizen science, for example, professionals have access to the data they need without having to pay for the work of hundreds of civilian researchers involved in the project, and the travel, accommodation and education that may be associated with it. It is also a great advantage that a project can be carried out over a large geographical area for many years (MacPhail & Colla, 2020). This approach has been successfully used in searching for the emergence of Hyalomma adults in several European countries (Grandi et al., 2020; Lesiczka et al., 2022; Uiterwijk et al., 2021).
In Hungary, we have limited published information about the occurrence of Hyalomma ticks. An engorged nymph of Hy. marginatum has been reported from a hedgehog (Erinaceus roumanicus) living in a Budapest city park (Földvári et al., 2011) and two larvae and a nymph were identified from robins (Erithacus rubecula) (Hornok et al., 2013) sampled to the south of the capital. Three Hy. rufipes nymphs were reported from a common whitethroat (Sylvia communis) in another study (Hornok et al., 2016). The presence of antibodies against CCHFV has also been detected in Hungarian hares (Lepus europaeus) (Németh et al., 2013), rodents (Földes et al., 2019) and recently in humans as well (Magyar et al., 2021).
Based on these previous findings, the aim of the present research was to obtain a comprehensive, nationwide picture of the occurrence of adult Hyalomma ticks in Hungary through a citizen science approach. This kind of documentation is of great epidemiological importance because this is the first step for the prevention of emerging pathogens according to the DAMA (Document, Assess, Monitor, Act) protocol (Brooks et al., 2014). Based on this, we plan to continuously document the appearance of pathogens, their vectors and reservoir hosts; then, after appropriate scientific analyses (Assess), we will be able to identify the organisms that pose a threat and manage their targeted surveillance (Monitor). That will provide sufficient evidence for decision makers to take appropriate preventive measures (Act) (Brooks et al., 2019).
2. MATERIALS AND METHODS
2.1. Tick and data collection
Between April and December 2021, ticks were collected under the frame of a citizen‐science project (https://www.kullancsfigyelo.hu) for monitoring the emergence and possible establishment of Hyalomma spp. in Hungary. Participants were asked through the self‐made website to look for adult hard ticks on themselves, their pets and livestock. Attention was called to specimens larger than usual with visible stripes on their legs and with apparently quick movement. To enhance visibility, the project was intensively communicated in local and national on‐line media outlets, local and national television and radio channels and social media platforms.
Ticks were reported either by images sent by e‐mail or delivered to the Institute of Evolution, Centre for Ecological Research, personally or by mail. Information about safe removal, storage and postage of tick specimens was provided on the project website.
2.2. Morphological identification of ticks
Ticks received by mail or in person were kept at 4°C and examined alive. Specimens were identified with a Nikon SMZ800N stereomicroscope using appropriate morphological characters and identification keys (Estrada‐Peña et al., 2018; Walker et al., 2003). The species, sex, developmental stage, engorgement state, host and place of origin of the ticks were recorded.
2.3. Molecular identification of Hyalomma ticks
Hyalomma specimens were homogenized with Bertin Minilys (Bertin Instruments, Montigny‐le‐Bretonneux, France) after freeze‐thawing three times in liquid nitrogen. Multiple beads were used in various sizes with the addition of quartz sand after the freeze‐thaw cycles and before proceeding to homogenization (Lv et al., 2014). Five hundred microlitres Dulbecco's Modified Eagle Medium was added to samples and centrifugated on 8000 rpm for 5 min. One hundred microlitres supernatant was subjected to nucleic acid extraction performed with Quick‐DNA Miniprep Plus Kit (Zymo Research, Irvine, USA) following the manufacturers instruction. A fragment (800 bp) of the mitochondrial gene cytochrome C oxidase subunit I (cox1) was amplified using previously published Cox1F and Cox1R primers, PCR setup and cycling conditions (Lv et al., 2014). The PCR products were separated on 1.5% agarose gel and cleaned up with the usage of Monarch DNA Gel Extraction Kit (New England Biolabs, Ipswich, USA). Samples were sequenced at a commercial centre in both directions (Eurofins Genomics, Ebersberg, Germany).
All available cox1 sequence data from GenBankR database in relation to Hy. marginatum and Hy. rufipes species were collected (access date: 20 December 2021). Sequence data were subjected for phylogenetic analysis after evaluating the best fit model for the analysis, using the MEGA11 (Tamura et al., 2021). The visualization of final data was performed with iTOL v6 online tool.
3. RESULTS
Data used in the present study was gathered between 28 April and 7 December 2021 from the tick monitoring project, which is at time of writing still ongoing. During this time, the website https://www.kullancsfigyelo.hu/ had 31,325 individual visitors. A total of 137 hard tick specimens and several hundred photos were received. Some other arthropods, for example, soft ticks (two Argas reflexus adults), spiders, bugs and a beetle were also sent by participants as by‐catch. Most of the ticks were identified as Ixodes ricinus, but Dermacentor reticulatus, Dermacentor marginatus and Haemaphysalis inermis also occurred (Table 1). When participants sent only photos of the tick specimens found, a simple morphological identification at genus level was possible.
TABLE 1.
Species, stage, sex and host of hard tick specimens received within the tick monitoring citizen science project between 15 April and 7 December 2021
| Developmental stage | Host | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Female | Male | Nymph | Larva | Unknown | Human | Dog | Cat | Cattle | Unknown | Total | |
| Dermacentor | reticulatus | 2 | 5 | ─ | ─ | ─ | 2 | 5 | ─ | ─ | ─ | 7 |
| marginatus | 4 | 1 | ─ | ─ | ─ | 3 | ─ | ─ | ─ | 2 | 5 | |
| unspecified | 3 | 24 | ─ | ─ | 15 | 19 | 12 | ─ | ─ | 11 | 42 | |
| Ixodes | ricinus | 44 | 9 | 10 | 2 | 7 | 22 | 7 | 27 | ─ | 16 | 72 |
| Haemaphysalis | inermis | 4 | 5 | ─ | ─ | ─ | ─ | ─ | ─ | ─ | 9 | 9 |
| Hyalomma | marginatum | ─ | 1 | ─ | ─ | ─ | ─ | 1 | ─ | ─ | ─ | 1 |
| rufipes | ─ | 1 | ─ | ─ | ─ | ─ | ─ | ─ | 1 | ─ | 1 | |
| Total | 57 | 46 | 10 | 2 | 22 | 46 | 25 | 27 | 1 | 38 | 137 | |
Two specimens were morphologically identified as Hyalomma ticks. The first Hyalomma was discovered in a garden of the small town Bük (47°23′09.2"N 16°44′29.8″E) in Western Hungary (Vas County) on August 10. The tick was crawling on a dog's leg when the submitter noticed it. This tick was morphologically identified as a male Hy. marginatum. The second specimen was found feeding on a cow at a farm near Kiskunmajsa (46°26′09.6"N 19°41′13.4″E) in South‐Eastern Hungary (Bács‐Kiskun County) on September 10. This tick specimen was morphologically identified as a male Hy. rufipes. The dog and the cow had never been abroad. Following morphological identification, Hyalomma ticks were stored in liquid nitrogen until molecular analyses. Amplification of the cox1 gene was successfully run for both specimens. Sequencing confirmed the previous morphological identification as Hy. marginatum for the first specimen. Based on the phylogenetic analysis, this individual most likely belongs to the Eurasian population (Figure 1). Sequencing of the cox1 gene fragment of the second specimen confirmed its identity as Hy. rufipes with the highest sequence similarity to a clade of mixed sequences from Europe and Africa (Figure 2).
FIGURE 1.

Phylogenetic tree of 22 partial gene sequences of the mitochondrial cytochrome oxidase C I gene of Hyalomma marginatum. The tree was constructed with the Neighbour‐Joining method using the Tamura 3‐parameter method to compute evolutionary distances. We applied the bootstrap test with 1000 replicates. Novel sequence data are highlighted with bold letters and an arrow
FIGURE 2.
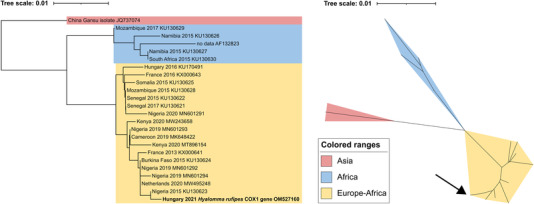
Phylogenetic tree of 24 partial gene sequences of the mitochondrial cytochrome oxidase C I gene of Hyalomma rufipes. The tree was constructed with the Neighbour‐Joining method using the Tamura 3‐parameter method to compute evolutionary distances. We applied the bootstrap test with 1000 replicates. Novel sequence data are highlighted with bold letters and an arrow
Ticks were sent by citizen scientists from all over the country, the largest number from Budapest and its surroundings. The least data were received from the south‐east of the country (Figure 3). Ticks were either collected from the environment or from hosts. Most ticks were removed by citizen scientists from humans, with a total of 45 such cases. The second most common hosts were dogs with 18 submissions (including Hy. marginatum specimen) and then cats with seven. A single specimen was received from a cow, this was the animal in which Hy. rufipes was discovered.
FIGURE 3.

Geographical distribution of the ticks received by the tick monitoring citizen science project
https://www.kullancsfigyelo.hu. Each dot represents a tick sent in by the civil participants of the research. 



4. DISCUSSION
Within 7 months from its start, the present tick monitoring project, via citizen science approach, revealed the emergence of two adult Hyalomma tick species in two different locations of Hungary. This project highlights the importance of public relations in the success of a citizen science project. We reached relatively high visibility (over 31,000 individual website visitors) within 7 months (data from Google Analytics not shown). The highest number of daily visitors, 5453 occurred after an extensive media release of the project. Participating civilians also benefit from initiatives that use citizen science. First, in the optimal case, all such research is intended to serve the interests of society directly or indirectly, so society in general should support the activities of citizen scientist researchers. In addition, the increased social awareness caused by various citizen science projects is a positive side effect. Due to their involvement in the survey, participants pay more attention to nature and to monitoring non‐endemic tick species in their environment and the relationship between participating citizens, scientists and public health stakeholders may also become closer. Participants received replies from us regarding their questions and samples within 24 h. This was important because some secondary media appearances, emphasizing the seriousness of Crimean‐Congo haemorrhagic fever, frightened some responders, and in the majority of the cases, they were comforted by the fact that the specimen they found was not a Hyalomma tick.
In addition to the advantages, limitations about the method herein followed should be highlighted. For example, at the beginning of any program, close attention should be paid to accurately describing the information requested from the public. Without this, it can be very time consuming to select relevant data from the set of information received. It may also be the case that, due to the incorrect or unverifiable data received, the decision‐making bodies may not take into account the results of such a project. It is particularly difficult to filter out or prevent the creation of such erroneous data, perhaps because citizen science is still often characterized by a lack of universal, reproducible methods. The introduction and strict adherence to these methods can easily dampen the enthusiasm of the participating civilians, which is why the high level of attention in project design is important (MacPhail & Colla, 2020).
The form of the requested data may also be crucial for setting up the research project. In monitoring the distribution of tick species, where, for example, citizen scientists may try to identify an animal found, the participants have three ways to submit the requested information. They can only send the name of the species identified, the photographic documentation of the individual, or the specimen itself. All these three options have advantages and disadvantages, the first case favours large amounts of incoming data, but there is no way to verify them. In the second case, a lot depends on whether the person making the shot is aware of what morphological characters make the identification possible, for example, from what angle and at what resolution it is worth taking pictures. The exact tick species identification is most secure when citizen scientists send the specimen to be identified by professionals. Although participants were advised to follow this third option, this procedure raised other issues, other than being time consuming. Submission might involve the destruction of the specimen, and on the other hand, it increases the risk of human‐tick contact (Eisen & Eisen, 2021). Therefore, we particularly made participants aware of the risks and suggested the safe removal, storage and postage of tick specimens.
The most commonly submitted tick species was I. ricinus. This is in line with the widespread distribution of this species, although participants are frequently not able to recognize this common tick species. Dermacentor ticks were also submitted in large numbers. This is again partly due to the frequent occurrence of these tick species throughout the country (Földvári et al., 2007), however the on‐line media also biased the study in this aspect. Unfortunately, several news portals used images that featured a Dermacentor species instead of a Hyalomma tick. As a consequence, several citizen scientists, especially dog owners, sent Dermacentor specimens that are larger and quicker compared to I. ricinus and, in addition, matched the mistakenly included photograph of some online portals. We archived all tick specimens and data for further analyses; thus, this by‐catch might provide useful epidemiological information in the future. A recent study involving citizen scientists also reported frequent occurrence of Dermacentor ticks on hunters and their hunting dogs in Italy (Sgroi et al., 2022). In contrast to Hungary, where D. reticulatus is the most common species of this genus (Földvári et al., 2016), the above study detected only D. marginatus.
Mitochondrial gene‐based genetic analysis is relevant to reveal dispersal patterns and introduction routes of Hyalomma ticks (Capek et al., 2014; Wallménius et al., 2014). Novel mitochondrial sequence data for Hyalomma spp. is increasingly reported (Ciloglu et al., 2021; Lang et al., 2022). The importance of providing sequence data of these vectors is getting more relevant in the light of disease emergence, especially the geographic expansion of CCHFV during the last decades (Akyildiz et al., 2021; Arteaga et al., 2021; Moraga‐Fernández et al., 2021; Sánchez‐Seco et al., 2021). We further highlight the importance of sequence data by integrating this activity into the citizens science programme and also into the risk assessment of CCHFV emergence and preparedness in the study area. The two sites were Hyalomma ticks were found in the study are approximately 280 kilometres apart, with the first specimen discovered on August 10 and the second on September 10, so exactly one month apart. Due to the large geographical distance and the time difference, the two specimens must have arrived in the country separately as a result of two different introductions. Although much more published sequence data would be necessary, we suspect that the probable source of introduction was a Eurasian population for the detected Hy. marginatum and a population along the Africa‐Europe bird migration route for Hy. rufipes.
Another important issue is whether the specimens found entered the country in the same year, for example, as engorged nymphs dropping off from migratory birds, or overwintered before finding them in the next year, or even already hatched here as larvae in the previous year. The latter possibility is of greatest concern because it would indicate the establishment of a local population, making this species able to successfully complete its entire life cycle in Hungary. This seems unlikely currently because a critical number of conspecific Hyalomma adults would be needed to yield egg‐laying fertilized females. The moulting into adult in the year of drop‐off, among many other factors, is affected mainly by temperature. On the one hand, hot springs are preferred by Hyalomma ticks, where the average daily temperature above 8°C for Hy. marginatum ticks can be determined as a criterion for moulting from nymph to adult (Gale et al., 2012). Due to the colder‐than‐average April weather in Hungary in 2021, this was only achieved in mid‐May (National Meteorological Service). Only adults found before this could have unambiguously overwintered in the country, giving the opportunity to develop a local population. However, during the present project, both specimens of Hyalomma were discovered well after May, so these adults were presumably introduced the same year. The tick monitoring herein performed was not running during the spring, and there is a possibility for overwintering adult Hyalomma in Hungary, as it has recently been reported in the Czech Republic (Rudolf et al., 2021). Unfed adult Hyalomma ticks tolerate the cold months well, with the most critical period being late summer and autumn due to the heat sensitivity of engorged nymphs (Uiterwijk et al., 2021; Valcárcel et al., 2020). Thus, it is not the cold winter but the cold autumn that is critical in shaping the geographical spread of Hyalomma species. Climate change might easily enhance establishment of new local Hyalomma populations with elevating autumn temperatures in temperate Europe as exemplified by the rising presence of the tick Hy. marginatum in southern France (Vial et al., 2016). Once imported, establishment of Hy. marginatum on common local hosts, wild and domesticated mammals and even urban ones like hedgehog has been detected in Hungary (Földvári et al., 2011) and also recently in Bulgaria (Arnaudov et al., 2022).
The ultimate reason for monitoring the emergence of adult Hyalomma ticks is the possible introduction of tick‐borne pathogens, mainly CCHFV. There are scattered historical data about the occurrence of Hyalomma ticks and CCHFV in Hungary as summarized in Figure 4. Immature Hy. marginatum from a hedgehog in a city park (Földvári et al., 2011), immature Hy. rufipes and Hy. marginatum from songbirds (Hornok et al., 2013; Hornok et al., 2016) and two adult Hy. rufipes from cattle were previously detected in the country. Reported occurrence of CCHFV spans from isolation from I. ricinus ticks (Molnár, 1982), to seropositive cattle, sheep (S. Horváth, 1974), brown hares (Németh et al., 2013) and wild rodents (Földes et al., 2019). Most interestingly, humans seropositive for CCHFV were reported well before the first observation of adult Hyalomma ticks (Horváth, 1976). There was also a reported human case of CCHF with unknown origin in 2004 (Országos Epidemiológiai Központ, 2008) and CCHFV seropositives were detected in 12 healthy blood donors collected between 2008 and 2017 (Magyar et al., 2021). All these data indicate that CCHFV might be transmitted by tick species other than Hyalomma spp. and that the earlier emergence of adult Hyalomma ticks might have been overlooked in Hungary.
FIGURE 4.

Historical published data about the occurrence of Hyalomma ticks and Crimean‐Congo haemorrhagic fever virus (CCHFV) in Hungary in chronological order. 1, CCHFV isolation in 1972 from two I. ricinus ticks in Veszprém county (Molnár, 1982). 2, CCHFV seropositive cattle and sheep in 1973 in Hajdú‐Bihar county (Horváth, 1974). 3, CCHFV antibody in 17 human sera. One from a slaughterhouse worker in Budapest, all others from Hajdú‐Bihar county (Horváth, 1976). 4, Human CCHF infection from unknown source in Baranya county in 2004 (Országos Epidemiológiai Központ, 2008). 5, CCHFV seropositive brown hares collected between 2008 and 2009 from near Dévaványa village (Németh et al., 2013). 6, An engorged Hyalomma marginatum nymph found on a Northern white‐breasted hedgehog in 2009 in an urban park of Budapest (Földvári et al., 2011). 7, Two Hy. rufipes males found on two cows in September 2011 in south‐western Hungary (Hornok & Horváth, 2012). 8, CCHFV seropositive wild rodents collected between 2011 and 2013 in the Mecsek Mountain region (Földes et al., 2019). 9, Three Hy. marginatum (two larvae and a nymph) collected from a European robin at the Ócsa Ringing Station in 2011 and three Hy. rufipes nymphs from a common whitethroat (Sylvia communis) in 2014 (Hornok et al., 2013; Hornok et al., 2016). Red crosses indicate origin of CCHFV seropositive blood donors collected between 2008 and 2017 (Magyar et al., 2021). Red dots indicate the origin of the two Hyalomma specimens of the present study
5. CONCLUSIONS
In the present survey, the occurrence of non‐endemic Hyalomma ticks in Hungary, by using citizen science methods, was investigated. The use of community research was successful in terms of both website traffic and number of submitters. Participants reacted positively; people took the program noticeably seriously. Due to the importance of the topic and the good reception, our goal is to continue the research next spring, so the first years’ experience is also useful for fine‐tuning the project. One striking result of this research was that one of the two Hyalomma found was discovered in the only cow involved in the study. Hyalomma often parasitize large domesticated mammals in their adult stages, as was previously known (Hornok & Horváth, 2012; Uiterwijk et al., 2021). It would therefore be worthwhile next year for the program to target inspections of establishments affected in this respect. This could be done, for example, by finding journals, online platforms and social media groups for animal husbandry, horse riding, or by looking for farms specializing in these. Data were received from across the country, but most submissions are clearly from the capital or a narrower neighbourhood. In the future, greater emphasis should be placed on rural areas, especially livestock farms, villages and towns. As media presence has proven to be key to this study, in the future we will try to focus on the print and electronic media presence also in rural areas.
In addition, it would be worthwhile to launch a media campaign for the project as early as the end of the winter, ensuring that people take pictures of the ticks already discovered in the spring and send them via mail. This would be important because in such cases, there would probably only be individuals of the potentially overwintering Hyalomma ticks in the country, so such an observation would indicate that the group may be able to form local populations in the future. It is of utmost importance to continue systematic monitoring of Hyalomma ticks in the future. We will analyze these and future Hyalomma specimens for the occurrence of CCHFV and other pathogens to be able to monitor and prepare for the introduction of transboundary and emerging diseases.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
G. F. conceptualized, designed and coordinated the study and drafted the original manuscript. G. F. and É. Sz. coordinated the citizen science project, morphologically identified ticks. É. Sz. designed and managed the project website https://www.kullancsfigyelo.hu/ . G. E. T., Z. S. L. and Z. S. V. performed laboratory work and sequencing. B. Z. and G. K. performed phylogenetic analysis. Z. S. L. did data visualization. G. K. supervised the molecular and sequencing works. G. E. T. and G. K. edited and reviewed the manuscript and carried out data analysis. All authors read and approved the final manuscript.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. All relevant guidelines for the use of animals in scientific studies were followed.
ACKNOWLEDGEMENTS
We are grateful to all citizen scientists who took part in the project and helped with submitting photos, ticks or data. We would like to acknowledge Kornél Baráth and Attila Ádám for providing the Hyalomma specimens. We thank Zoltán Soltész for his advice on the citizen science project and the excellent work of Eszter Draskóczy who played a crucial role in reaching society through the appropriate media appearances. The help of Sonia A. Olmeda and Félix Valcárcel in confirming our morphological identification of the two Hyalomma specimens is much appreciated. We are indebted to Daniel R. Brooks who gave useful advice on the revised version of the manuscript.
Földvári, G. , Szabó, É. , Tóth, G. E. , Lanszki, Z. , Zana, B. , Varga, Z. , & Kemenesi, G. (2022). Emergence of Hyalomma marginatum and Hyalomma rufipes adults revealed by citizen science tick monitoring in Hungary. Transboundary and Emerging Diseases, 69, e2240–e2248. 10.1111/tbed.14563
DATA AVAILABILITY STATEMENT
Sequence data are available in NCBI GenBank database under the accession numbers: OM527159‐OM527160. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akyildiz, G. , Bente, D. , Keles, A. G. , Vatansever, Z. , & Kar, S. (2021). High prevalence and different genotypes of Crimean‐Congo hemorrhagic fever virus genome in questing unfed adult Hyalomma marginatum in Thrace, Turkey. Ticks and Tick‐Borne Diseases, 12(2), 101622. [DOI] [PubMed] [Google Scholar]
- Arnaudov, A. , Mikov, A. , & Georgiev, D. (2022). Infestation of the road‐killed Eastern European hedgehogs (Erinaceus roumanicus) with Ixodidae ticks in some parts of Upper Thracian Plain (Bulgaria). ZooNotes, 192, 1–4. [Google Scholar]
- Arteaga, L. M. , Bellido, J. L. M. , Negredo, A. I. , Criado, J. G. , Lista, M. C. V. , Serrano, J. Á. S. , Santiago, M. B. V. , Bernús, A. L. , de Ory Manchón, F. , Seco, M. P. S. , Leralta, N. , Sardón, M. A. , Muro, A. , & Belhassen‐García, M. (2021). New circulation of genotype V of Crimean‐Congo haemorrhagic fever virus in humans from Spain. PLOS Neglected Tropical Diseases, 15(2), e0009197. 10.1371/journal.pntd.0009197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente, D. A. , Forrester, N. L. , Watts, D. M. , McAuley, A. J. , Whitehouse, C. A. , & Bray, M. (2013). Crimean‐Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Research, 100(1), 159–189. 10.1016/j.antiviral.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Brooks, D. R. , Hoberg, E. P. , & Boeger, W. A. (2019). The Stockholm Paradigm: Climate Change and Emerging Disease. Chicago: University of Chicago Press. [Google Scholar]
- Brooks, D. R. , Hoberg, E. P. , Boeger, W. A. , Gardner, S. L. , Galbreath, K. E. , Herczeg, D. , Mejía‐Madrid, H. H. , Rácz, S. , & Dursahinhan, A. T. (2014). Finding them before they find us: Informatics, parasites, and environments in accelerating climate change. Comparative Parasitology, 81(2), 155–164. 10.1654/4724b.1 [DOI] [Google Scholar]
- Capek, M. , Literak, I. , Kocianova, E. , Sychra, O. , Najer, T. , Trnka, A. , & Kverek, P. (2014). Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks and Tick‐Borne Diseases, 5(5), 489–493. 10.1016/j.ttbdis.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Chitimia‐Dobler, L. , Schaper, S. , Rieß, R. , Bitterwolf, K. , Frangoulidis, D. , Bestehorn, M. , Springer, A. , Oehme, R. , Drehmann, M. , Lindau, A. , Mackenstedt, U. , Strube, C. , & Dobler, G. (2019). Imported Hyalomma ticks in Germany in 2018. Parasites & Vectors, 12(1), 134. 10.1186/s13071-019-3380-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciloglu, A. , Ibis, O. , Yildirim, A. , Aktas, M. , Duzlu, O. , Onder, Z. , Simsek, E. , Yetismis, G. , Ellis, V. A. , & Inci, A. (2021). Complete mitochondrial genome characterization and phylogenetic analyses of the main vector of Crimean‐Congo haemorrhagic fever virus: Hyalomma marginatum Koch, 1844. Ticks and Tick‐Borne Diseases, 12(5), 101736. 10.1016/j.ttbdis.2021.101736 [DOI] [PubMed] [Google Scholar]
- Duscher, G. G. , Hodžić, A. , Hufnagl, P. , Wille‐Piazzai, W. , Schötta, A.‐M. , Markowicz, M. A. , Estrada‐Peña, A. , Stanek, G. , & Allerberger, F. (2018). Adult Hyalomma marginatum tick positive for Rickettsia aeschlimannii in Austria, October 2018. Eurosurveillance, 23(48), 1800595. 10.2807/1560-7917.ES.2018.23.48.1800595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen, L. , & Eisen, R. J. (2021). Benefits and drawbacks of citizen science to complement traditional data gathering approaches for medically important hard ticks (Acari: Ixodidae) in the United States. Journal of Medical Entomology, 58(1), 1–9. 10.1093/jme/tjaa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada‐Peña, A. , D́Amico, G. , & Fernández‐Ruiz, N. (2020). Modelling the potential spread of Hyalomma marginatum ticks in Europe by migratory birds. International Journal for Parasitology, 10.1016/j.ijpara.2020.08.004 [DOI] [PubMed] [Google Scholar]
- Estrada‐Peña, A. , Mihalca, A. D. , & Petney, T. N. (2018). Ticks of Europe and North Africa: A Guide to Species Identification (1st edn. 2017 edn.). New York, NY: Springer. [Google Scholar]
- Földes, F. , Madai, M. , Németh, V. , Zana, B. , Papp, H. , Kemenesi, G. , Bock‐Marquette, I. , Horváth, G. , Herczeg, R. , & Jakab, F. (2019). Serologic survey of the Crimean‐Congo haemorrhagic fever virus infection among wild rodents in Hungary. Ticks and Tick‐Borne Diseases, 10(6), 101258. 10.1016/j.ttbdis.2019.07.002 [DOI] [PubMed] [Google Scholar]
- Földvári, G. , Márialigeti, M. , Solymosi, N. , Lukács, Z. , Majoros, G. , Kósa, J. P. , & Farkas, R. (2007). Hard ticks infesting dogs in hungary and their infection with Babesia and Borrelia species. Parasitology Research, 101(S1), 25–34. 10.1007/s00436-007-0608-6 17252272 [DOI] [Google Scholar]
- Földvári, G. , Rigó, K. , Jablonszky, M. , Biró, N. , Majoros, G. , Molnár, V. , & Tóth, M. (2011). Ticks and the city: Ectoparasites of the Northern white‐breasted hedgehog (Erinaceus roumanicus) in an urban park. Ticks and Tick‐Borne Diseases, 2(4), 231–234. 10.1016/j.ttbdis.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Földvári, G. , Široký, P. , Szekeres, S. , Majoros, G. , & Sprong, H. (2016). Dermacentor reticulatus: A vector on the rise. Parasites & Vectors, 9(1), 314. 10.1186/s13071-016-1599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, P. , Stephenson, B. , Brouwer, A. , Martinez, M. , de la Torre, A. , Bosch, J. , Foley‐Fisher, M. , Bonilauri, P. , Lindström, A. , Ulrich, R. G. , de Vos, C. J. , Scremin, M. , Liu, Z. , Kelly, L. , & Muñoz, M. j. (2012). Impact of climate change on risk of incursion of Crimean‐Congo haemorrhagic fever virus in livestock in Europe through migratory birds. Journal of Applied Microbiology, 112(2), 246–257. 10.1111/j.1365-2672.2011.05203.x [DOI] [PubMed] [Google Scholar]
- Grandi, G. , Chitimia‐Dobler, L. , Choklikitumnuey, P. , Strube, C. , Springer, A. , Albihn, A. , Jaenson, T. G. T. , & Omazic, A. (2020). First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks and Tick‐Borne Diseases, 101403. 10.1016/j.ttbdis.2020.101403 [DOI] [PubMed] [Google Scholar]
- Hansford, K. M. , Carter, D. , Gillingham, E. L. , Hernandez‐Triana, L. M. , Chamberlain, J. , Cull, B. , McGinley, L. , Phipps, L. P. , & Medlock, J. M. (2019). Hyalomma rufipes on an untraveled horse: Is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom?. Ticks and Tick‐Borne Diseases, 10(3), 704–708. 10.1016/j.ttbdis.2019.03.003 [DOI] [PubMed] [Google Scholar]
- Hornok, S. , Csörgő, T. , de la Fuente, J. , Gyuranecz, M. , Privigyei, C. , Meli, M. L. , Kreizinger, Z. , Gönczi, E. , Fernández de Mera, I. G. , & Hofmann‐Lehmann, R. (2013). Synanthropic birds associated with high prevalence of tick‐borne rickettsiae and with the first detection of Rickettsia aeschlimannii in Hungary. Vector Borne and Zoonotic Diseases (Larchmont, N.Y.), 13(2), 77–83. 10.1089/vbz.2012.1032 [DOI] [PubMed] [Google Scholar]
- Hornok, S. , Flaisz, B. , Takács, N. , Kontschán, J. , Csörgő, T. , Csipak, Á. , Jaksa, B. R. , & Kováts, D. (2016). Bird ticks in Hungary reflect western, southern, eastern flyway connections and two genetic lineages of Ixodes frontalis and Haemaphysalis concinna . Parasites & Vectors, 9, 101. 10.1186/s13071-016-1365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok, S. , & Horváth, G. (2012). First report of adult Hyalomma marginatum rufipes (vector of Crimean‐Congo haemorrhagic fever virus) on cattle under a continental climate in Hungary. Parasites & Vectors, 5, 170. 10.1186/1756-3305-5-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth, L. B. (1976). Precipitating antibodies to Crimean haemorrhagic fever virus in human sera collected in Hungary. Acta Microbiologica Academiae Scientiarum Hungaricae, 23(4), 331–335. [PubMed] [Google Scholar]
- Horváth, S. (1974). [Crimean hemorrhagic fever antibodies in Hungary]. Orvosi Hetilap, 115(21), 1214. [PubMed] [Google Scholar]
- Hubálek, Z. , Sedláček, P. , Estrada‐Peña, A. , Vojtíšek, J. , & Rudolf, I. (2020). First record of Hyalomma rufipes in the Czech Republic, with a review of relevant cases in other parts of Europe. Ticks and Tick‐Borne Diseases, 11(4), 101421. 10.1016/j.ttbdis.2020.101421 [DOI] [PubMed] [Google Scholar]
- Jones, K. E. , Patel, N. G. , Levy, M. A. , Storeygard, A. , Balk, D. , Gittleman, J. L. , & Daszak, P. (2008). Global trends in emerging infectious diseases. Nature, 451(7181), 990–993. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, J. , Shan, Y. , Zhang, M. , Liu, J. , & Wang, F. (2022). The complete mitochondrial genome of Hyalomma rufipes (Acari: Ixodidae) from China and comparative analysis of mitogenomes in genus Hyalomma . International Journal of Acarology, 0(0), 1–11. 10.1080/01647954.2022.2030794 [DOI] [Google Scholar]
- Lesiczka, P. M. , Daněk, O. , Modrý, D. , Hrazdilová, K. , Votýpka, J. , & Zurek, L. (2022). A new report of adult Hyalomma marginatum and Hyalomma rufipes in the Czech Republic. Ticks and Tick‐Borne Diseases, 13(2), 101894. 10.1016/j.ttbdis.2021.101894 [DOI] [PubMed] [Google Scholar]
- Lv, J. , Wu, S. , Zhang, Y. , Chen, Y. , Feng, C. , Yuan, X. , Jia, G. , Deng, J. , Wang, C. , Wang, Q. , Mei, L. , & Lin, X. (2014). Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida). Parasites & Vectors, 7(1), 93. 10.1186/1756-3305-7-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail, V. J. , & Colla, S. R. (2020). Power of the people: A review of citizen science programs for conservation. Biological Conservation, 249, 108739. 10.1016/j.biocon.2020.108739 [DOI] [Google Scholar]
- Magyar, N. , Kis, Z. , Barabás, É. , Nagy, A. , Henczkó, J. , Damjanova, I. , Takács, M. , & Pályi, B. (2021). New geographical area on the map of Crimean‐Congo hemorrhagic fever virus: First serological evidence in the Hungarian population. Ticks and Tick‐Borne Diseases, 12(1), 101555. 10.1016/j.ttbdis.2020.101555 [DOI] [PubMed] [Google Scholar]
- McGinley, L. , Hansford, K. M. , Cull, B. , Gillingham, E. L. , Carter, D. P. , Chamberlain, J. F. , Hernandez‐Triana, L. M. , Phipps, L. P. , & Medlock, J. M. (2021). First report of human exposure to Hyalomma marginatum in England: Further evidence of a Hyalomma moulting event in north‐western Europe?. Ticks and Tick‐Borne Diseases, 12(1), 101541. 10.1016/j.ttbdis.2020.101541 [DOI] [PubMed] [Google Scholar]
- Molnár, E. (1982). Occurrence of tick‐borne encephalitis and other arboviruses in Hungary. Geographica Medicina, 12, 78. [PubMed] [Google Scholar]
- Moraga‐Fernández, A. , Ruiz‐Fons, F. , Habela, M. A. , Royo‐Hernández, L. , Calero‐Bernal, R. , Gortazar, C. , de la Fuente, J. , & Fernández de Mera, I. G. (2021). Detection of new Crimean–Congo haemorrhagic fever virus genotypes in ticks feeding on deer and wild boar, Spain. Transboundary and Emerging Diseases, 68(3), 993–1000. 10.1111/tbed.13756 [DOI] [PubMed] [Google Scholar]
- Németh, V. , Oldal, M. , Egyed, L. , Gyuranecz, M. , Erdélyi, K. , Kvell, K. , Kalvatchev, N. , Zeller, H. , Bányai, K. , & Jakab, F. (2013). Serologic evidence of Crimean‐Congo hemorrhagic fever virus infection in Hungary. Vector Borne and Zoonotic Diseases (Larchmont, N.Y.), 13(4), 270–272. 10.1089/vbz.2012.1011 [DOI] [PubMed] [Google Scholar]
- Nosek, J. , Kožuch, O. , & Lysý, J. (1982). The finding of the female Hyalomma marginatum Koch, 1844 in southern Slovakia. Folia Parasitol, 29, 251. [Google Scholar]
- Országos Epidemiológiai Központ . (2008). Krími‐Kongó haemorrhagiás láz Görögországban. Epinfo, 15(27), 313–315. [Google Scholar]
- Rudolf, I. , Kejíková, R. , Vojtíšek, J. , Mendel, J. , Peňázziová, K. , Hubálek, Z. , Šikutová, S. , & Estrada‐Peña, A. (2021). Probable overwintering of adult Hyalomma rufipes in Central Europe. Ticks and Tick‐Borne Diseases, 12(4), 101718. 10.1016/j.ttbdis.2021.101718 [DOI] [PubMed] [Google Scholar]
- Sánchez‐Seco, M. P. , Sierra, M. J. , Estrada‐Peña, A. , Valcárcel, F. , Molina, R. , de Arellano, E. R. , Olmeda, A. S. , Miguel, L. G. S. , Jiménez, M. , Romero, L. J. , & Negredo, A. (2021). Widespread detection of multiple strains of Crimean‐Congo hemorrhagic fever virus in ticks, Spain. Emerging Infectious Diseases, 28(2), 394–402. 10.3201/eid2802.211308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi, G. , Iatta, R. , Lia, R. P. , Napoli, E. , Buono, F. , Bezerra‐Santos, M. A. , Veneziano, V. , & Otranto, D. (2022). Tick exposure and risk of tick‐borne pathogens infection in hunters and hunting dogs: A citizen science approach. Transboundary and Emerging Diseases, 69(4), e386–e393. 10.1111/tbed.14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Stecher, G. , & Kumar, S. (2021). MEGA11: Molecular evolutionary genetics analysis Version 11. Molecular Biology and Evolution, 38(7), 3022–3027. 10.1093/molbev/msab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, T. , Porter, W. T. , Salkeld, D. J. , Prusinski, M. A. , Jensen, S. T. , & Brisson, D. (2021). Estimating disease vector population size from citizen science data. Journal of The Royal Society Interface, 18(184), 20210610. 10.1098/rsif.2021.0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiterwijk, M. , Ibáñez‐Justicia, A. , van de Vossenberg, B. , Jacobs, F. , Overgaauw, P. , Nijsse, R. , Dabekaussen, C. , Stroo, A. , & Sprong, H. (2021). Imported Hyalomma ticks in the Netherlands 2018–2020. Parasites & Vectors, 14(1), 244. 10.1186/s13071-021-04738-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcárcel, F. , González, J. , González, M. G. , Sánchez, M. , Tercero, J. M. , Elhachimi, L. , Carbonell, J. D. , & Olmeda, A. S. (2020). Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects, 11(5), 303. 10.3390/insects11050303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial, L. , Stachurski, F. , Leblond, A. , Huber, K. , Vourc'h, G. , René‐Martellet, M. , Desjardins, I. , Balança, G. , Grosbois, V. , Pradier, S. , Gély, M. , Appelgren, A. , & Estrada‐Peña, A. (2016). Strong evidence for the presence of the tick Hyalomma marginatum Koch, 1844 in southern continental France. Ticks and Tick‐Borne Diseases, 7(6), 1162–1167. 10.1016/j.ttbdis.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Walker, A. , Bouattour, A. , Camicas, J. L. , Estrada‐Peña, A. , Horak, I. , Latif, A. , Pegram, R. , & Preston, P. M. (2003). Ticks of Domestic Animals in Africa: A guide to identification of species. Edinburgh: Bioscience Reports. [Google Scholar]
- Wallménius, K. , Barboutis, C. , Fransson, T. , Jaenson, T. G. , Lindgren, P.‐E. , Nyström, F. , Olsen, B. , Salaneck, E. , & Nilsson, K. (2014). Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasites & Vectors, 7(1), 318. 10.1186/1756-3305-7-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data are available in NCBI GenBank database under the accession numbers: OM527159‐OM527160. The data that support the findings of this study are available from the corresponding author upon reasonable request.


