Abstract
African Swine Fever Virus (ASFV) is a contagious pathogen that can cause severe acute haemorrhagic fever in pigs. The first occurrence of an ASF outbreak in Asia was reported in China in August 2018. The devastating impacts caused by ASF on the pig industry have strongly focused research on risk factors for the spread of ASFV. The purpose of this systematic review was to identify the potential knowledge gap in the English literature on risk factors for the spread of ASFV in outbreaks that occurred in China, 2018–2020. China National Knowledge Infrastructure (CNKI) was searched as the primary database. Sixty‐four records were screened, and 31 (48%) records were included in data extraction. These records were also assessed for quality of evidence. Frequency tables for reported risks were created, considering quality of evidence. A narrative summary of mortality rate and case fatality rate reported in a small number of records was made. Mortality rate was reported in seven studies, ranging from 3.7% to 84.0% (median 11.9%). Case fatality rate was reported in six studies, ranging from 20.6% to 100% (median 63.3%). Based on 31 reviewed records, live pig transport, swill feeding and vehicles were the three most important risks for spread contributing to the ASF epidemic in China. Bites of infected Ornithodoros ticks was stated in 12 low level of evidence records but only 1 high level of evidence record as a risk factor for transmission. Direct contact with wild pigs was reported to be a risk factor in 8 records with low level of evidence, and 1 record from the high level of evidence group. However, limited evidence was provided to support the tick–domestic pig or wild pig–domestic pig transmission routes in China. Lack of resources to obtain veterinary assistance and to improve husbandry and biosecurity was mentioned four times in the 31 records, especially in remote rural areas. In conclusion, to effectively control the spread of ASF, it is very important to reduce mechanical dissemination of ASFV by vehicles and live pig transport involved in the production cycle and to ensure that transported pigs are always subject to inspection and quarantine. Additionally, despite strict implementation of prohibition on swill feeding often being impractical or nearly impossible, ensuring the safety of pig feed can greatly contribute to disease prevention. Improvement in biosecurity management, specifically environment disinfection, carcass disposal, and decontamination of vehicles and personnel will be most effective in reducing the risk of infection in small‐scale pig farms.
Keywords: African Swine Fever, China, epidemiology, pig, spread, transmission risk
1. INTRODUCTION
African Swine Fever (ASF) is a contagious viral disease that causes haemorrhagic fever affecting domestic and wild pigs of all ages. Clinical signs of acute ASF include high fever, anorexia, vomiting, diarrhoea, skin haemorrhages, cyanosis and abortion in pregnant sows. The mortality rate of acute ASF is reported to be up to 100% (OIE, 2021). Subacute and chronic forms can be caused by lower virulent strains of the virus, which exhibit longer duration of clinical signs and lower mortality rates, ranging from 30% to 70% (OIE, 2021). Pigs infected with the chronic form of ASF can develop respiratory signs, chronic skin ulcers, intermittent fever, arthritis and show chronic weight loss (OIE, 2021).
ASF virus (ASFV) is an enveloped DNA virus of the Asfarviridae family, with 24 genotypes described for ASFV (Quembo et al., 2018). Other than wild and domestic pigs, the virus can also infect ticks of the genus Ornithodoros as biological transmission vectors (OIE, 2021). The major transmission routes of ASFV to domestic pigs include direct contact with infected pigs and indirectly via contact with contaminated feed, fomites and ticks. ASFV can tolerate pH values from 4–10, with certain strains being able to resist inactivation up to pH value of 13 (Fischer et al., 2020). It can also persist for variable but sometimes very long periods in organic material such as faeces, frozen meat and blood serum (Fischer et al., 2020).
ASF is listed as a notifiable disease by the World Organisation for Animal Health (OIE) because the disease is transboundary and contagious. It is responsible for serious production and economic losses in pig industries worldwide. The continued spread of ASF has had a substantial impact on supplies of pork products globally, and a devastating effect on food security, animal health and welfare (Ward et al., 2021). Between 2018 and 2019, the outbreak of ASF in China reportedly caused 850,000 live pigs to be culled (Li et al., 2019, p. 37). As of 21 April 2020, 176 ASF outbreaks had been reported in China (Gao et al., 2021) and disease spread continues. According to the OIE (2022), ASF has been reported in 35 countries, five different world regions (Africa, Americas, Asia, Europe and Oceania) since January 2020, involving more than 1,100,000 pigs with more than 1,800,000 animal losses (OIE, 2022). Although risk factors for ASFV transmission have been well studied in many countries, particularly in Europe and Africa, it is important to understand the risk factors involved in the spread of ASFV in China by studying the local situation.
The aim of this study was to gather evidence on the important factors that pose a risk for the spread of ASFV during the ASF epidemic in China via a systematic review of literature published in the Chinese language between 1 January 2018 and 31 December 2020. Since most articles are published in Chinese, there might potentially be a knowledge gap in the English literature concerning outbreaks in China. The primary objective of this study was to summarise the stated risk for transmission of ASF outbreaks in China, 2018−2020 and to evaluate the strength of the evidence reported in the Chinese literature. The secondary objective of the study was to analyse the reports to compare epidemiological characteristics – such as mortality rate, case fatality rate and suspected source of infection – in different outbreaks reported in China.
2. MATERIAL AND METHODS
2.1. Data source and search strategy
China National Knowledge Infrastructure (CNKI) was searched as the primary database for this study (https://global.cnki.net/, assessed 3 March 2021). The research question was focused on risks for transmission of ASFV during ASF outbreaks reported in China. Initially, the following search terms were used for the mode of transmission:
title OR abstract OR keyword: ‘African swine fever’ OR ‘非洲猪瘟’
AND title or abstract or keyword = ‘mode of transmission’ OR ‘传播方式’
timeframe: ‘01/01/2018 – 31/12/2020’, language: ‘English OR Chinese’
Then, the following search terms were used to identify reports with information on risk factors:
title OR abstract OR keyword: ‘African swine fever’ OR ‘非洲猪瘟’
AND title OR abstract OR keyword: ‘risk factor’ OR ‘风险因素’
timeframe: ‘01/01‐2018 – 31/12/2020’, language: ‘English OR Chinese’
All records returned by CNKI using these search terms were exported to Endnote (https://endnote.com/, assessed 3 March 2021) for citation management.
2.2. Screening protocol and eligibility criteria
Duplicate records were identified and removed after combination of the search results. Selection of relevant records was conducted based on title and abstract. Full‐text records were assessed during the reviewing process for eligibility. Any records meeting the following exclusion criteria were removed:
The study population is outside of China, or
The study does not include discussion of risk factors for the transmission of ASFV, or
The study is conducted before the first incident of an ASF outbreak in China (1 August 2018), or
The study does not report a basic study design, methods or results. For example, any record that is a summary article, instruction, producers guide or news report.
2.3. Data items and charting process
A tabulated form was created using Excel for standardized information extraction. Topics of interest were extracted from each record; these included the year of publication, the type of article, whether the article is peer‐reviewed, the study population, the scale of the reported outbreak(s), the study period and study method, the stated mode of transmission of ASFV and the stated risk factors associated with the spread of ASFV. In cases of studies in which epidemiological investigations were conducted, epidemic curves and case fatality rates were extracted and recorded.
Eight reviewers (JC, AL, YT, CX, YC, CZ, KL, YY) with veterinary medical knowledge and fluent in Chinese were involved in the data extraction process. The reviewers were recruited on a voluntary basis from the cohort of veterinary medicine students at The University of Sydney. Each record that met the eligibility criteria was assessed by at least two reviewers. Differences of opinion were discussed between the two reviewers and consensus was determined before proceeding to qualitative and quantitative synthesis of the extracted data.
A level of evidence scoring system was developed based on Evidence‐based Veterinary Medicine described by Cockcroft (2003) and RCVS Knowledge (2020). Each record included in data extraction was given an evidence level score from 1 to 5 according to the type of study. Score 1 was given to any opinion orientated reviews; Score 2 indicates the study is mostly a narrative review with non‐specific search protocols; Score 3 implies the study is a descriptive case report in which a single outbreak of ASF was investigated and presented; Score 4 suggests the study has involved a collection of case reports and descriptive quantitative data were provided; and Score 5 was assigned to any observational studies including case control studies, and epidemiological investigation reports on single or multiple cases of outbreaks. Articles with Score 1 and Score 2 were classified as a low evidence group and articles with Scores 3–5 were classified as a high evidence group.
2.4. Synthesis of extracted data
The main topic of interest was the risk factors that contributed to the spread of ASFV in China. The extracted data stated in each record were listed. A list of categories for stated mode of transmission and reported risk factors was created, based on the extracted data. These categories of mode of transmission and risk factors were then combined to identify risks for transmission as the key variable of interest.
A numerical code was then given to each category to replace the text description of the combined risks for transmission of ASFV for the purposes of quantitative analysis. The frequency of each category reported in the studies was counted using Python and tabulated by categories in Excel. In cases of studies in which multiple risk factors were discussed, all were listed and counted. Tabulated summaries for the topics of interest were also created based on high versus low level of evidence group, to differentiate data originating from different sources and of different quality. A narrative summary of mortality rate and case fatality rate reported in a small number of studies was made.
3. RESULTS
3.1. Selection of relevant records
After removal of duplicates, 64 records were identified by searching the CNKI database (Figure 1). Twenty‐three records were excluded during screening based on title and abstract, due to the studied pig population being outside of China. The full text of 41 records were accessed and proceeded into eligibility assessment. During the eligibility assessment, nine records were removed because no information about risks for transmission was reported. One record was removed because the study was conducted prior to the index case of ASF in China. The remaining 31 records were eligible for data extraction and synthesis.
FIGURE 1.
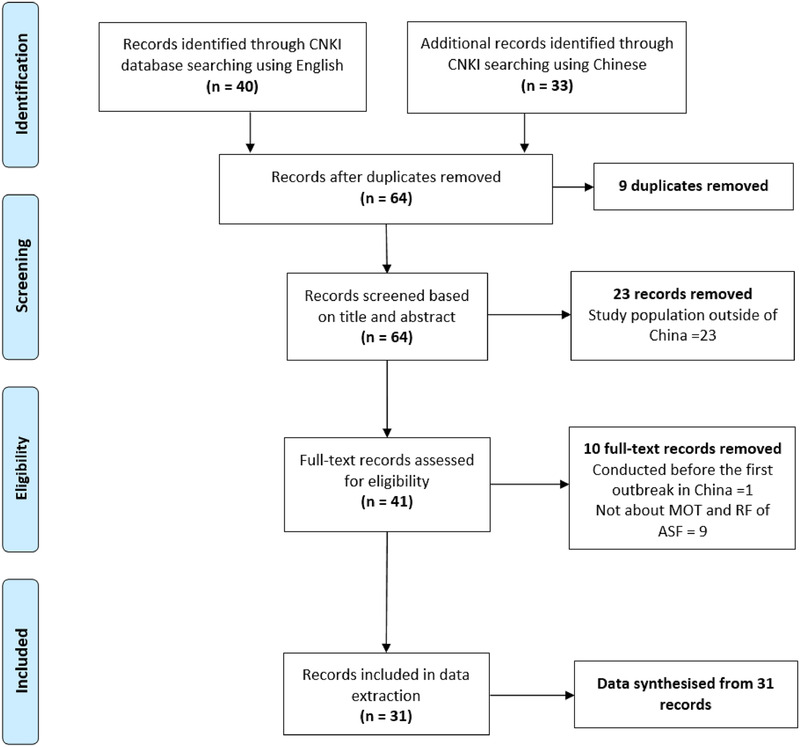
Flow chart of record selection in a systematic review of African Swine Fever reports in the China National Knowledge Infrastructure (CNKI) database
3.2. Data charting and synthesis
The majority of the 31 records were published in 2019 and 2020 (n = 29, Figure 2a) in peer‐reviewed journals (n = 27), with 2 records sourced from industry publications (Figure 2b) and 4 records from non‐peer reviewed journals (Figure 2c). The 31 records consisted of 10 opinion articles, 6 narrative reviews, 1 case report, 7 case series and 7 observational studies (Figure 2d). Overall, 48.4 % of the records (n = 15) were classified as high level of evidence (evidence level Scores 3–5) and 51.6% (n = 17) were identified as low level of evidence (Scores 1 and 2).
FIGURE 2.
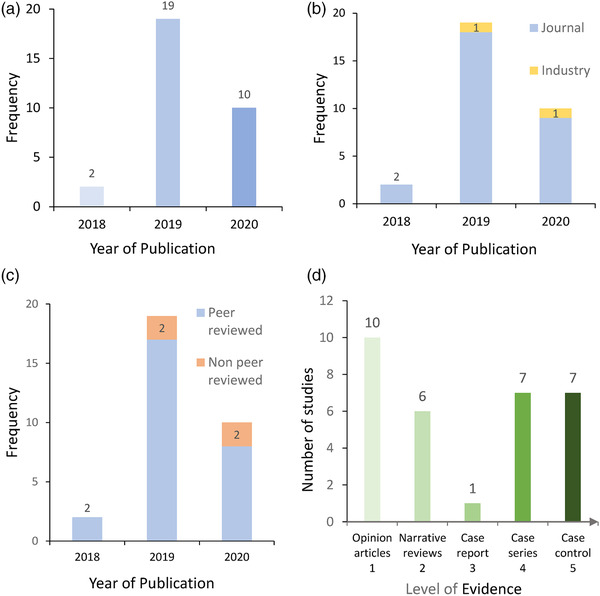
Distribution by year of publication (a), publication type (b), peer‐reviewed status (c) and level of evidence (d) of 31 records in a systemic review of risk factors associated with African Swine Fever outbreaks in China, reported in Chinese literature
Of the 31 records, all the stated issues that pose a risk for the transmission of ASFV were identified. These risk factors were grouped into 30 categories to generate a frequency table (Figure 3). Live pig transport (20), swill feeding (17), vehicle transmission (15), personnel transmission (13) and contaminated feed (12; Figure 4) were the most frequent risk factors reported from the 31 records.
FIGURE 3.
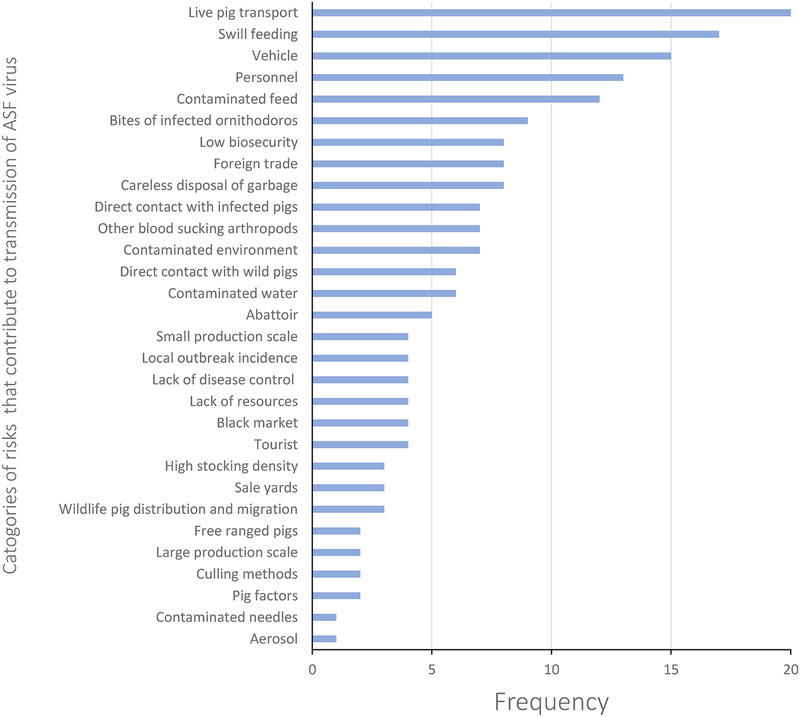
Frequency of reported risk factors for transmission of African Swine Fever virus in 31 records identified within a systematic review of the China National Knowledge Infrastructure (CNKI) database
FIGURE 4.
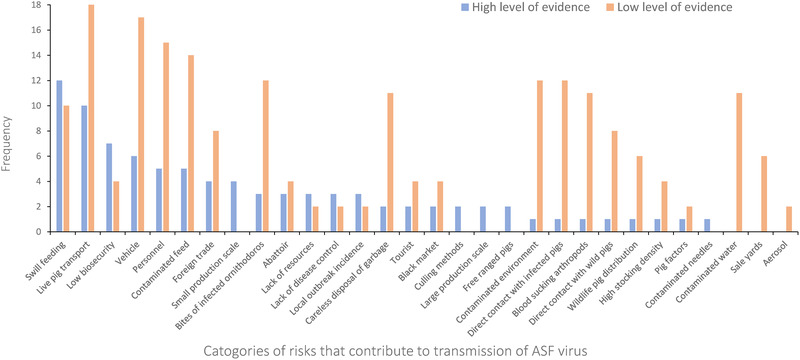
Distribution by level of evidence groups of stated risk factors for transmission in African Swine Fever outbreaks in China, identified within a systematic review of the China National Knowledge Infrastructure (CNKI) database
Within 15 articles from the high level of evidence group, the most commonly stated risk factors were swill feeding (12), live pig transport (10) and low biosecurity (7; Figure 4). Comparatively, a range of risk categories were mentioned frequently in the low level of evidence group, including live pig transport, vehicle and personnel transmission, transmission through contaminated feed, contaminated environment, contaminated water, bites of infected Ornithodoros ticks, direct contact with infected pigs, careless dispose of garbage and blood sucking arthropods (Figure 4). Additionally, there were five risk factors that were only mentioned by the high level of evidence group: small production scale, large production scale, culling methods, free‐range pigs and contaminated needles. Three categories of risk factors were stated by the low evidence group but not reported by the high evidence group. Those were contaminated water, sale yards and aerosol transmission. Spearman's rank correlation coefficient for the frequency of stated risk factors in high and low evidence group was .209 (p = .267). There was a significant higher frequency of responses in the low evidence group than the high evidence group (Mann–Whitney U test, U = 625.5, p = .009).
Mortality rate was reported in seven records and ranged from 3.7% to 84% with a median mortality rate of 11.9% (Ding et al., 2019; Gu et al., 2020; Liu et al., 2020; Ma et al., 2019; Tang et al., 2019; Wei et al., 2018; Zhou, Yang & Xiao, 2018). Case fatality rate was reported in six records, ranging from 20.6% to 100%, with a median case fatality rate of 63.3% (Ding et al., 2019; Gu et al., 2020; Ma et al., 2019; Tang et al., 2019; Wei et al., 2018; Zhou, Yang & Xiao, 2018).
4. DISCUSSION
4.1. Live pig transport
In this review, live pig transport was the most commonly reported risk factor contributing to the transmission of ASFV from combined level of evidence groups, as well as the high and low level of evidence groups (10 and 18, respectively; Figure 4). Transportation of live pigs is an essential component in the pig production industry, whether it is from the finisher farms to the abattoir or from breeder farms to finisher farms. The transportation of live, infected pigs can directly result in the mechanical transmission of ASFV, if the contaminated vehicle is not disinfected properly before carrying the next batch of pigs or used to transport feed and equipment. Whereas in scenarios that an infected pig is transported to a disease‐free region without being detected and making direct contact with other pigs to spread the virus, it can be attributed to the non‐detection of infected live pigs. In China, cross‐regional live pig transport can happen very frequently and in very large scale to supply fresh meat for consumption in non‐pig producing provinces. For example, according to Ma et al. (2020a), 1.88 million head of live pigs and 10.1 billion tonne of pork product were transported from 7 to 12 provinces to Qingdao city during the first half of 2019. With transport of such large scale, wide range of origin and long distance, mixing of pigs from different farms is often inevitable. Moreover, lack of traceability and cross‐regional identification systems for animals from different farms were described by Zhang et al. (2019), contributing to further difficulties in disease control and investigation of the route of transmission. In Qingdao city, even after the start of the ASF outbreak, diagnostic tests for ASF in live pig transport were only performed at the place of departure but not at the place of arrival, potentially increasing the risk of missed diagnoses in subclinical pigs (Ma et al., 2020a). The potential proportion of unidentified cases due to this testing regime was not reported. Instead, ASF testing was performed on a large scale (samples randomly collected from abattoirs, finisher farms, carcass decontamination yards and food processing plants) to mitigate the risk arising from live pig transport (Ma et al., 2020a). Furthermore, existence of unregulated illegal live pig transport to avoid quarantine and inspection further exacerbated the potential risk of disease transmission by direct contact with sick pigs (Shi et al., 2020). It was reported by Chen et al. (2019) that in 68 ASF outbreaks, which occurred in the early phase of disease transmission during 2019, 19.1% were introduced by live pig or pork product transport. In terms of the role that pig transportation plays in long‐distance spread of ASFV, a case control study of the first 18 ASF cases in Liaoning province during August 2018 to July 2020 described that the 24 farms involved in the 18 cases were all located near 3 national highways and clustered in 3 provincial highways (Cui et al., 2020). The authors proposed that the spatial distribution characteristics in this case can be seen as evidence of pig transportation‐related transmission. Apart from domestic live pig transport, imported live pigs and pork products as risks for transmission were also described. Imports of live pig and pork products from Russia have been prohibited by the Chinese government since 2008, due to the incidence of ASF outbreaks in Russia. While ASF has become an endemic disease in Russia, incidents of illegal live pig purchases in cross‐border regions between China and Russia have been reported (Zhang et al., 2019), and potentially contributed to the spread of ASFV in China.
4.2. Vehicle
Mechanical dissemination via vehicles was another risk factor described very frequently in both high and low level of evidence groups (6 and 17, respectively; Figure 4), and it is closely related to other risk factors including live pig transport and contaminated feed. For vehicle transmission, statements about its importance in ASFV transmission include that the movement of vehicles contributed to the cross‐regional spread of ASFV in China (Cui et al., 2020), vehicles associated with abattoir and meat processing plants played a vital role in ASFV transmission (Ma et al., 2020a) and vehicles involved in feed transport, faeces processing, carcases disposal and decontamination processes were a transmission route of very high risk (Tang et al., 2019). According to Gu et al. (2020), in more than 40% of ASF outbreaks that occurred in pig farms in China regardless of production scale, the virus was introduced by movement of personnel or vehicles; in contrast, in outbreaks that occurred in pig farms with more than 2000 pigs, almost all were caused by movement of personnel or vehicles. A greater role of vehicle transmission in large pig production was suggested to be associated with the larger number of sows that need to be culled in large‐scale farms, thus more frequent loading and transport of sows to abattoir or sale yards (Gu et al., 2020). This can necessitate increased contact between on‐farm personnel or vehicles and off‐farm personnel or vehicles, posing a higher risk of ASFV exposure (Gu et al, 2020). For transmission route inside the large‐scale farm, ineffective decontamination of vehicles due to lack of facilities was proposed to be a potential cause that leads to environmental contamination (Gu et al., 2020; Ma et al., 2019). Other issues include unspecified registration requirements for live pig transport vehicles that make regulation and identification of vehicles more difficult and lack of available equipment for vehicle decontamination (Ma et al., 2019).
4.3. Soft shell ticks and wild pigs
Disease transmission caused by bites of infected Ornithodoros (soft shell ticks) was reported very frequently in the low level of evidence group (12) but not as often in the high level of evidence group (3; Figure 4). In the reviewed records, the relationship between the distribution of Ornithodoros ticks and location of ASF outbreaks were not fully analysed or compared, providing limited evidence to support the domestic pig–tick transmission route. In the three records from the high level of evidence group that did report soft shell ticks as a risk factor, it was stated as low risk in the ASF outbreaks in China (Liu et al., 2020), briefly listed as a potential route of transmission in disease investigation reports (Zhang et al., 2019), or an extrapolation from previous outbreaks in different countries (Ma et al., 2020b). Similarly, direct contact with wild pigs was also reported regularly in the low level of evidence group (12) but infrequently in the high level of evidence group (1). Insufficient analysis was conducted in the wild pig population to determine the level of risk carried by the distribution and migration of wild pigs and evidence of direct contact with wild pigs causing disease transmission.
4.4. Swill feeding
In the current review, swill feeding was often reported to be an extremely high‐risk factor for the transmission of ASFV in outbreaks. Swill feeding is an important risk factor that is closely associated with contaminated feed, water and the environment. In the theoretical study based on an ASF risk assessment model conducted by Wang et al. (2020), swill feeding was determined to be one of the most important risk factors in China. It was suggested that swill feeding caused 34% of the first 68 ASF outbreaks in China (Zhang et al., 2019). According to Hua et al. (2019), whether prohibition of swill feeding was implemented strictly was a key factor in the success of outbreak control and prevention of disease transmission. Yet difficulties in eliminating swill feeding were indicated by Ma et al. (2019) in the investigation report for the first two outbreaks in Henan province (118 diseased pigs and 64 dead pigs). First, it was reported that the lack of an established swill collection and disposal system in commercial restaurants led to inadequate processing and regulation in the source from where the swill came (Ma et al., 2019). Second, in cases in which swill feeding was found in pig farms, especially in small backyard farms, producers were only warned, and no penalties were imposed. The consequences of breaching regulations were minimal due to lack of enforcement and financial penalties, yet the potential impact was substantial (Ma et al., 2019). Interestingly, despite swill feeding being classified as an extremely high‐risk factor in the early phase of the ASFV transmission in China during 2018 (Ma et al., 2020b) and the difficulties in implementation of control, the reported proportion of outbreaks in the epidemic directly associated with swill feeding has decreased since 2019. Within the 31 included articles, there has been no outbreak reported to be directly caused by swill feeding during the second half of 2019. This might reflect the success of implementation of prohibition of swill feeding to certain degree.
4.5. Low biosecurity
Low biosecurity was described in multiple outbreak farms, including open pig shed or free‐range practice (Cong et al., 2019; Wang et al., 2020), poor movement control of personnel and vehicles (Gu et al., 2020), underequipped quarantine facility and insufficient disinfection and decontamination (Ma et al., 2020a). The prevalence of poor management of biosecurity was believed to be linked to a substantial portion of the pig production being small‐ to medium‐scale producers. In 2018, small backyard farms with a production scale of 1–99 pigs made up 33.1% of the live pig production in China, and medium producers with 100–999 pigs made up a further 28.6% (Chen, 2021). It was stated that small‐scale pig producers with free‐range and open shed practices were much more prone to inadequate biosecurity (Cong et al., 2019). Increased risk of contact between domestic pigs and wild pigs and ticks was also reported in free‐range and open shed practice (Zhang et al., 2019). However, neither ticks nor wild pigs have been confirmed to play a role in the transmission of ASF outbreaks in domestic pigs in China to date.
4.6. Lack of resources
Another risk factor regularly reported in the high level of evidence group was lack of resources to improve husbandry and biosecurity, especially in small pig producers located in remote rural areas. Deficiencies reported include failure to obtain veterinary assistance and diagnostic laboratory tests, lack of training for disease observation and funding to improve biosecurity, and insufficient government subsidy to farmers in the culling process (Cong et al., 2019). Deficiency in disease control resources was also reported to negatively impact ASF disease surveillance in the long term and improvement of biosecurity management after the initial outbreaks (Cong et al., 2019).
4.7. Pig factors
Despite pigs of all breeds and ages and both sexes being susceptible to ASFV infection, there were two records that reported certain pig populations to be at higher risk of becoming infected during the ASF outbreaks. Cui et al. (2020) reported 24 ASF cases in Liaoning province during August 2018 to July 2020. Among the 24 cases, sows were the first group to show clinical signs in 10 cases (42%), and fattener pigs were the first group to show clinical signs in 9 cases (37%). It was suspected that older pigs and those with heavier body weight are more susceptible to ASFV transmission due to increased feed intake and hence greater chance of virus exposure (Cui et al., 2020). Additionally, it was suggested that weaner and grower pigs have a higher fatality rate due to other acquired and congenital diseases other than ASF, thus the clinical signs of ASF in these pigs are more likely to be missed (Cui et al., 2020). Another record (Peng et al., 2020) from the low level of evidence group proposed that perinatal sows are the highest risk group due to increased water intake thus exposure to contaminated water, and increased contact with on‐farm personnel during feeding, vaccination, farrowing monitoring and weaning processes (Peng et al., 2020). However, those risk factors described in different pig groups are largely associated with management, instead of the animal group.
4.8. Sources of infection
For the ASF outbreak that started in August 2018 in China, the source of ASFV was speculated in several studies. According to Zhang et al. (2019), the genotype of ASFV involved in the 2018 ASF outbreak in China shares almost identical nucleotide identity with strains isolated from ASFV outbreaks in Georgia, Russia and Estonia. This statement is also supported by a genomic report from Zhou et al. (2018). They reported that genotype II strain ASFV‐SY18, which was isolated from organ samples in three ASF cases in China, shares 100% nucleotide identity with strains isolated from Russia, Georgia and Estonia (Estonia 2014, Georgia 2007/1, Krasnodar 2012, Irkutsk 2017). As for the potential method of introduction, speculation includes illegal live pig or pork product imports, contaminated products carried by tourists and inappropriate waste disposal from international airports and ports (Ma et al., 2020b; Wu et al., 2019; Wei et al., 2018).
4.9. Comparison with previous studies
The findings from this review that small pig farms are more at‐risk for disease transmission because of low biosecurity is supported by the study of Gao et al. (2021). Based on 176 outbreaks reported from 3 August 2018 to 21 April 2020, small farms had significantly higher outbreak rates than medium and large farms (Gao et al., 2021). The same study also reported a significant increased odds of infection associated with dissemination through vehicles and personnel (OR = 2.7), and swill feeding (OR = 2.5). These results are consistent with the findings of the current review.
4.10. Bias and limitations
CNKI was the only database searched in this review. Selection bias and incomplete collection of available data can result from the use of a single source of records. However, databases in the Chinese language that include animal diseases are limited. Although data extraction was conducted by at least two reviewers, the screening process and eligibility assessment were performed by the lead author, which might have led to potential bias. Limited observational studies (7) were included in this review, and effect measures (such as odds ratios) were not reported by any of the 31 records for any of the potential risk factors. The level of evidence system used in this review was based only on the type of study, which might not accurately capture the quality of analysis.
5. CONCLUSION
In summary, based on a systematic review of the CNKI, live pig transport, swill feeding and vehicle transmission were the most commonly cited risk factors contributing to transmission during the ASF epidemic in China. Low biosecurity is the most significant risk, especially in small‐scale producers. Therefore, to effectively control the spread of ASF, it is very important to reduce mechanical dissemination of ASFV by vehicles involved in the production cycle and to ensure that transported pigs are always subject to inspection and quarantine. Additionally, strict implementation of prohibition of swill feeding can greatly contribute to disease prevention. Improvement in biosecurity management in aspects of environment disinfection, carcass disposal and decontamination of vehicles and personnel will be most effective in reducing the risk of infection in small‐scale pig farms.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICS APPROVAL
No ethical approval was required for this study since this is a review of published studies.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance of the following veterinary students who reviewed articles in this study: Alice Lao, Yilin Tang, Yui Kiu Chan, Cynthia Zhou, Kwan Kei Leung and Yifei Yu. Special thanks to Chutian Xiang who reviewed articles in this study and created a custom Python script to facilitate data extraction and analysis. This work was completed in partial fulfilment for the requirements of the Doctor of Veterinary Medicine degree, The University of Sydney (JC). This research was funded by the Sydney School of Veterinary Science Research & Enquiry Unit of Study 2019 fund.
Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
Cheng, J. , & Ward, M. P. (2022). Risk factors for the spread of African Swine Fever in China: A Systematic Review of Chinese‐language literature. Transboundary and Emerging Diseases, 69, e1289–e1298. 10.1111/tbed.14573
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Chen, F. , Zhu, Z. , Huang, J. , Yu, Q. , Du, H. , & Tan, H. (2019). Jiating Nongchang Fangkong Feizhou Zhuwen Zhi Maizhu Guanli. Animal Science Abroad‐Pigs and Poultry, 39(07), 86–89. [Google Scholar]
- Chen, L. (2021). Shengzhu Guimohua Yangzhi Jiancheng Qushi. China Investment, 7(13‐14), 19–23. [Google Scholar]
- Cockcroft, P. D. , & Holmes, M. A. (2003). Research studies. Handbook of evidence‐based veterinary medicine. Blackwell Publishing Ltd. [Google Scholar]
- Cong, L. , Hang, L. U. , Feng, L. , & Wei, Z. (2019). Difficulties in prevention and control of African Swine Fever in Hebei Province and relevant countermeasures. Chinese Journal of Animal Health Inspection, 36(09), 44–47. [Google Scholar]
- Cui, J. . , Yu, B. , Gu, G. , Li, F. , Lan, D. , & Yu, X. (2020). Epidemiological characteristics of the outbreak of African Swine Fever in pig production in Liaoning Province. Chinese Journal of Animal Health Inspection, 37(09), 30–34. [Google Scholar]
- Ding, M. , Yang, A. , Shu, S. , & Shu, X. (2019). An outbreak investigation on African Swine Fever in Hunan Province. Chinese Journal of Animal Health Inspection, 36(08), 1–5. [Google Scholar]
- Fischer, M. , Hühr, J. , Blome, S. , Conraths, F. J. , & Probst, C. (2020). Stability of African Swine Fever virus in carcasses of domestic pigs and wild boar experimentally infected with the ASFV “Estonia 2014” isolate. Viruses, 12(10), 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, L. , Sun, X. , Yang, H. , Xu, Q. , Li, J. , Kang, J. , Liu, P. , Zhang, Y. , Wang, Y. , & Huang, B. (2021). Epidemic situation and control measures of African Swine Fever outbreaks in China. 2018–2020. Transboundary and Emerging Diseases, 68(5), 2676–2686. [DOI] [PubMed] [Google Scholar]
- Gu, G. , Cui, J. , Lan, D. , Tan, L. , Liu, H. , & Yu, B. (2020). Investigation on the bio‐safety status of large‐scale swine farms in Liaoning Province. Chinese Journal of Animal Health Inspection, 37(05), 28–32. [Google Scholar]
- Hua, L. , Feng, Z. , Zhang, Y. , Hao, F. , Ge, S. , Wang, J. , & Shao, G. (2019). Prevention and control of African Swine Fever in China: Lessons from past outbreaks. Chinese Journal of Animal Infectious Disease, 27(02), 96–104. [Google Scholar]
- Li, B. , Li, X. , Wang, Y. , Tang, Y. , & Wang, Y. (2019). International and domestic disease status of African swine fever and mode of transmission. The Chinese Livestock and Poultry Breeding, 4, 37–39. [Google Scholar]
- Liu, Y. , Lu, P. , Liu, J. , Li, W. , & Liang, Z. (2020). Epidemiological investigation on an outbreak of African Swine Fever in Tianjing city. Chinese Journal of Animal Health Inspection, 37(02), 10–14. [Google Scholar]
- Ma, Q. , Liu, Q. , Jiang, Q. , Tai, Y. , He, Y. , Bing, Q. , & Duan, X. (2020a). Risk assessment on African Swine Fever introduced into Qingdao City of Shandong Province. Chinese Journal of Animal Health Inspection, 37(02), 19–23. [Google Scholar]
- Ma, W. , Meng, W. , Yu, H. , Chang, J. , & Li, Z. (2019). Analysis on problems in prevention and control of African Swine Fever in Henan Province and relevant countermeasures. Chinese Journal of Animal Health Inspection, 36(09), 48–51. [Google Scholar]
- Ma, Y. , Wang, B. , Sun, D., & Shi, X. (2020b). Feizhou Zhuwen Chuanru ZHongguo De Fengxian Lujing Tongji Diaocha Fenxi. Xiandai Nongye Keji, 12, 224–225.
- OIE World Organisation for Animal Health (2021). African Swine Fever, viewed 19 September 2020, https://www.oie.int/en/disease/african‐swine‐fever/
- OIE World Organisation for Animal Health, African Swine Fever (ASF)—Situation Report 8, 2022. Viewed 3 April (2022), https://www.oie.int/app/uploads/2022/03/asf‐report8.pdf
- Peng, Y. , Liu, Z. , & Zhu, Z. (2020). Risk management of prenatal period under the background of African Swine Fever in pig farm. Swine Industry Science, 37(01), 112–114. [Google Scholar]
- Quembo, C. J. , Jori, F. , Vosloo, W. , & Heath, L. (2018). Genetic characterization of African Swine Fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transboundary and Emerging Diseases, 65(2), 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCVS Knowledge (2020). EBVM Toolkit 3 – Introduction to “levels of evidence” and study design, viewed 17 September 2020, https://knowledge.rcvs.org.uk/document‐library/ebvm‐toolkit‐3‐introduction‐to‐levels‐of‐evidence‐and‐study/
- Shi, X. , Liu, H. , Dou, S. , & Lin, Q. (2020). Research progress on etiology and epidemiology of African Swine Fever. Chinese Journal of Animal Health Inspection, 37(05), 63–67. [Google Scholar]
- Tang, M. , Xie, Z. , Zhang, J. , Sun, J. , & Leng, D. (2019). Yiyangshi Guimohua Zhuchang Feizhou Zhuwen Fangkong Fengxian Dian Ji Duice. Swine Industry Science, 36(10), 42–44. [Google Scholar]
- Wang, X. , Feng, P. , Tian, Q. , Wu, M. , Shi, L. , Wu, M. , Zhang, J. , & Chen, R. (2020). Fuzzy analytical hierarchy process based potential‐risk assessment model for African Swine Fever. Progress in Veterinary Medicine, 41(12), 13–17. [Google Scholar]
- Wang, Y. , Wang, X. , Ma, L. , Yin, C. , Zhou, H. , Li, J. , Song, Q. , & LI, L. (2020). An emergency epidemiological investigation on the first case of African Swine Fever in Ningxia. Chinese Journal of Animal Health Inspection, 37(03), 17–22. [Google Scholar]
- Ward, M. P. , Tian, K. , & Nowotny, N. (2021). African Swine Fever, the forgotten pandemic. Transboundary and Emerging Diseases, 68, 2640–2642. [DOI] [PubMed] [Google Scholar]
- Wei, S. , Duan, Y. , Li, S. , Cui, J. , Zhou, C. , & Jiang, B. (2018). Epidemiological investigation of the first African Swine Fever case in China. Modern Journal of Animal Husbandry and Veterinary Medicine, 10, 48–50.
- Wu, J. , Bao, Y. , & Du, J. (2019). Feizhou Zhuwen Liuxingbingxue Jiqi Fengxian Yinsu Fenxi. Chinese Journal of Veterinary Medicine, 55(03), 123–126. [Google Scholar]
- Zhang, R. , Huang, Y. , Bao, C. , Jung, Y. , Xu, J. , Qian, Y. , & Dai, J. (2019). Epidemiology of African Swine Fever and analysis of risk factors of its spread in China: An overview. Chinese Journal of Virology, 35(03), 512–522. [Google Scholar]
- Zhou, J. , Yan, J. , & Xiao, C. (2018). Tonglingshi Yianqu Feizhou zhuwen Quezhen Bingli Liuxing Bingxue Diaochao Baogao. Chinese Journal of Animal Husbandry and Veterinary Medicine, 12, 25–26. [Google Scholar]
- Zhou, X. , Li, N. , Luo, Y. , Liu, Y. , Miao, F. , Chen, T. , Zhang, S. , Cao, P. , Li, X. , Tian, K. , Qiu, H.‐J. , & Hu, R. (2018). Emergence of African Swine Fever in China, 2018. Transboundary and Emerging Diseases, 65(6), 1482–1484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


