Abstract
Objectives
To evaluate which patient and tumour characteristics are associated with remaining untreated in patients with potentially curable, non‐metastatic muscle‐invasive bladder cancer (MIBC), and to compare survival of untreated vs treated patients with similar characteristics.
Patients and methods
For this cohort study, 15 047 patients diagnosed with cT2–T4aN0/xM0/x urothelial MIBC between 2005 and 2019 were identified in the Netherlands Cancer Registry. Factors associated with remaining untreated were identified using logistic regression analyses. Interhospital variation was assessed using multilevel analysis. Using a propensity score, the median overall survival (mOS) of untreated and treated patients was evaluated. Analyses were stratified by age (<75 vs ≥75 years).
Results
One‐third of patients aged ≥75 years remained untreated; increasing age, worse performance status, worse renal function, cT4a stage and previous radiotherapy in the abdomen/pelvic area increased the odds of remaining untreated. One in 10 patients aged <75 years remained untreated; significant associations were only found for performance status, renal function and cT4a stage. Interhospital variation for remaining untreated was largest for patients aged ≥75 years, ranging from 37% to 69% (case‐mix‐adjusted). Irrespective of age, mOS was significantly worse for untreated patients: 6.4 months (95% confidence interval [CI] 5.1–7.3) vs 16.0 months (95% CI 13.5–19.1) for treated patients.
Conclusion
On average, one in five patients with non‐metastatic MIBC remained untreated. Untreated patients were generally older and had a more unfavourable prognostic profile. Untreated patients had significantly worse overall survival, regardless of age. Age alone should therefore not affect treatment decision‐making. Considering the large interhospital variation, a proportion of untreated patients might be wrongfully denied life‐prolonging treatment.
Keywords: muscle‐invasive bladder cancer (MIBC), patient and tumour characteristics, survival, treatment, urothelial bladder carcinoma, variation in healthcare, #BladderCancer, #blcsm
Abbreviations
- BMI
body mass index
- CCI
Charlson Comorbidity Index
- ECOG
Eastern Cooperative Oncology Group
- eGFR
estimated glomerular filtration rate
- MDTM
multidisciplinary team meeting
- MIBC
muscle‐invasive bladder cancer
- mOS
median overall survival
- NCR
Netherlands Cancer Registry
- RC
radical cystectomy
- TURBT
transurethral resection of bladder tumour
Introduction
Non‐metastatic muscle‐invasive bladder cancer (MIBC) is an aggressive disease with high risk of progression and death if left untreated [1]. The guideline‐recommended treatment is radical cystectomy (RC), preferably preceded by neoadjuvant chemotherapy in cisplatin‐eligible patients [2, 3]. Less aggressive treatment options for patients unfit or reluctant to undergo surgery are multimodality treatment, external beam radiotherapy, brachytherapy and chemotherapy. In recent years, only multimodality treatment has been considered to be a full alternative for RC in a selected patient group [4, 5, 6, 7, 8]. Despite these treatment options, clinical practice shows that a substantial proportion of potentially curable patients remains untreated.
This group of untreated patients with non‐metastatic MIBC is understudied. The same holds for the underlying factors and their effect on patient outcomes. In the few studies that included untreated patients, the proportion of untreated patients ranged between 13% and 34% [9, 10, 11, 12, 13, 14]. A recent UK cohort study reported that up to 47% of patients with localized MIBC did not receive treatment with curative intent [12]. This was associated with poor 1‐year survival: 55% for patients receiving palliative treatment and 32% for patients receiving no treatment. These studies did not elaborate on explanatory factors.
It is known that younger patients and patients with a more advanced disease stage are more likely to receive aggressive therapy [11]. Age, comorbidity [13, 15, 16, 17, 18, 19], performance status [13, 17], renal function [20], risk of treatment‐related morbidity/mortality [15], quality of life [15, 16] and patient preferences [20] are factors known to affect treatment decision‐making. It has not yet been studied whether these factors also play a role in deciding not to treat patients with non‐metastatic MIBC. More insight into the untreated patient population and underlying factors associated with being untreated is needed, as these insights may provide leads to improve bladder cancer care.
The aims of this study were to provide insight into the characteristics of the untreated patient population with non‐metastatic MIBC, to assess which patient and tumour characteristics are associated with remaining untreated, and to compare survival of untreated and treated patients with similar patient and tumour characteristics.
Patients and Methods
For this historic cohort study, data from the nationwide Netherlands Cancer Registry (NCR) were used. All patients diagnosed with primary non‐metastatic urothelial MIBC (cT2–T4aN0/xM0/x) between 2005 and 2019 were identified. Mixed histologies with urothelial carcinoma as the main component were classified as urothelial carcinoma [21]. Tumours with predominant non‐urothelial carcinoma were excluded. Patient and tumour characteristics and vital status were retrieved from the NCR. More detailed information is available from a subset of patients diagnosed between November 2017 and November 2019. These patients were included in the nationwide, prospective BlaZIB study, aiming to improve and provide insight into bladder cancer care in the Netherlands [22]. A detailed description of the patients and variables included can be found in Fig. S1.
Definitions
Patients were categorized into treatment groups: treated or untreated. Treatment consisted of upfront RC, neoadjuvant chemotherapy followed by RC, chemoradiotherapy, brachytherapy, external radiotherapy, or other (including partial cystectomy, systemic chemotherapy, immunotherapy and combination therapy). Patients with only transurethral resection of bladder tumour (TURBT) and/or bladder instillations were considered to be untreated. Age was dichotomized as <75 and ≥75 years. Body mass index (BMI) was categorized into <18.5 kg/m2 (underweight), 18.5–25 kg/m2 (normal weight), 25–30 kg/m2 (overweight) and ≥30 kg/m2 (obesity). Comorbidity was defined according to the 1987 weighted Charlson Comorbidity Index (CCI) [23] and categorized into a CCI score of 0, 1, 2 or ≥3. Performance status was defined according to the Eastern Cooperative Oncology Group (ECOG) performance score and categorized into 0, 1 and ≥2. Renal function was defined according to estimated GFR (eGFR) in mL/min/1.73 m2, which was measured before the first treatment. Socioeconomic status (SES) was derived from Statistics Netherlands (CBS), based on the patients' full six‐digit postal code.
Statistical analyses
Trends in treatment over time were evaluated stratified by age because, after the age of 75 years, the proportion of untreated patients showed a steep increase (Fig. S2). Descriptive analyses were performed to describe the untreated patient group over time, including P value for trend, and compared to treated patients, including ANOVA and chi‐squared tests. Missing data were imputed using single and multiple (n = 50) imputation [24]. Single imputed data were used to perform survival analyses and multilevel analyses, multiple imputed data were used for all other analyses.
Uni‐ and multivariable logistic regression analyses were performed in the BlaZIB subcohort stratified by age, to identify factors associated with not receiving bladder cancer‐related treatment. All variables univariably associated with remaining untreated were included in a multivariable model. To take into account the prognostic differences between untreated and treated patients due to different patient and tumour characteristics, a propensity score was calculated based on the multivariable logistic model, reflecting the patients' propensity for remaining untreated. Based on this propensity score, untreated and treated patients were matched on a 1:1 ratio in order to compare median overall survival (mOS) between treatment groups using the Kaplan–Meier method and the log‐rank test. A sensitivity analysis was performed excluding patients who died within 90 days after diagnosis to account for the unfavourable prognosis at diagnosis that would result in an anticipated timely death, logically depriving the patient of any chance of being treated. A Cox proportional hazards model including the propensity score was constructed to evaluate the effect of remaining untreated. Hospital variation in the proportion of untreated patients was evaluated using multilevel logistic regression analysis stratified by age, both unadjusted (i.e. observed probability) and adjusted for relevant case‐mix factors.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). P values < 0.05 were taken to indicate statistical significance. This study was approved by the Supervisory Committee of the NCR.
Results
In total, 15 047 patients diagnosed with non‐metastatic urothelial MIBC between 2005 and 2019 were identified from the NCR. On average, 9.9% (n = 777) of patients aged <75 years remained untreated vs 34.0% (n = 2459) in patients aged ≥75 years (Fig. 1). The proportion of untreated patients appears to decrease slightly over time. Use of neoadjuvant chemotherapy strongly increased over time and use of upfront RC decreased in patients aged <75 years. In the more recent years, use of chemoradiotherapy increased.
Fig. 1.
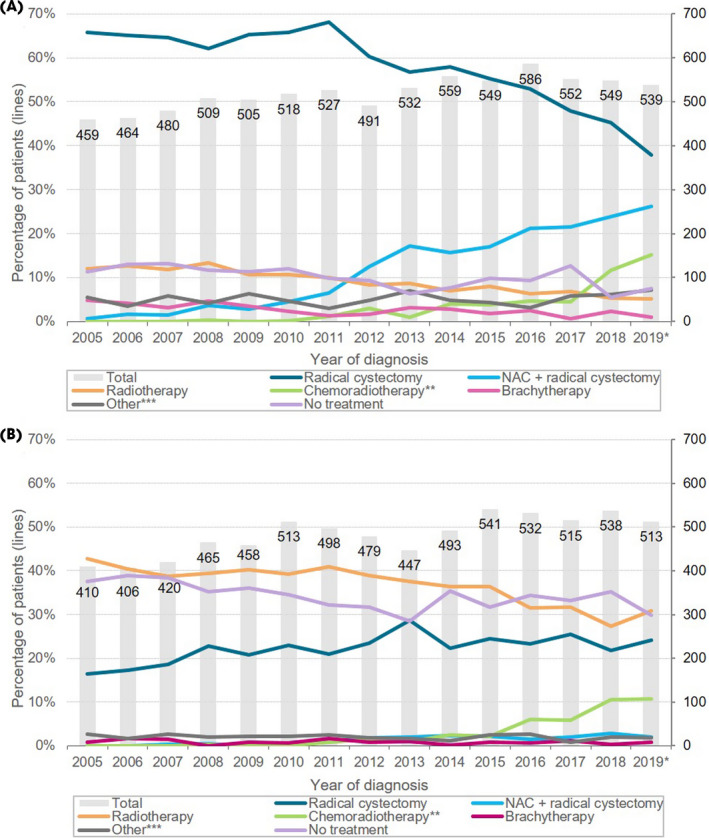
Treatment of patients younger than 75 years (A) and 75 years and older (B) diagnosed with non‐metastatic muscle‐invasive bladder cancer over time (2005–2019). * Data from 2019 are provisional (97% complete). ** Chemoradiotherapy was defined as concurrent treatment with chemotherapy and radiotherapy, i.e. both treatments should start at the same date or show overlap between treatment periods. *** Other includes: partial cystectomy, systemic chemotherapy, immunotherapy, combination therapy. NAC, neoadjuvant chemotherapy.
Table 1 shows patient and tumour characteristics of the 3236 (21.5%) untreated patients over time. An increase in the median age at diagnosis was observed, from 81 years in 2005–2007 to 83 years in 2017–2019 (P trend <0.05). Other trends were not as evident, although untreated patients appear to have become more fragile, i.e. they had a higher CCI score over time.
Table 1.
Patient, tumour and hospital characteristics of untreated patients diagnosed with non‐metastatic muscle‐invasive bladder cancer over time (2005–2019).
| Total | Year of diagnosis | ||||||
|---|---|---|---|---|---|---|---|
| 2005–2007 | 2008–2010 | 2011–2013 | 2014–2016 | 2017–2019 | P value for trend * | ||
| Total, N (%) | 3236 (100.0) | 648 (20.0) | 684 (21.1) | 571 (17.6) | 680 (21.0) | 653 (20.2) | |
| Patient characteristics | |||||||
| Gender, n (%) | 0.5378 | ||||||
| Male | 2282 (70.5) | 446 (68.8) | 491 (71.8) | 393 (68.8) | 494 (72.6) | 458 (70.1) | |
| Female | 954 (29.5) | 202 (31.2) | 193 (28.2) | 178 (31.2) | 186 (27.4) | 195 (29.9) | |
| Age at diagnosis (median, IQR), years |
82.0 75.0–86.0 |
81.0 74.0–85.0 |
81.0 74.0–86.0 |
82.0 75.0–86.0 |
83.0 76.0–87.0 |
83.0 76.0–87.0 |
<0.0001 |
| Age at diagnosis, n (%) | 0.0986 | ||||||
| <60 years | 138 (4.3) | 31 (4.8) | 35 (5.1) | 20 (3.5) | 32 (4.7) | 20 (3.1) | |
| 60–70 years | 319 (9.9) | 74 (11.4) | 70 (10.2) | 61 (10.7) | 58 (8.5) | 56 (8.6) | |
| 70–80 years | 842 (26.0) | 183 (28.2) | 191 (27.9) | 139 (24.3) | 169 (24.9) | 160 (24.5) | |
| ≥80 years | 1937 (59.9) | 360 (55.6) | 388 (56.7) | 351 (61.5) | 421 (61.9) | 417 (63.9) | |
| Age at diagnosis (dichotomous), n (%) | 0.0049 | ||||||
| <75 years | 777 (24.0) | 175 (27.0) | 178 (26.0) | 132 (23.1) | 152 (22.4) | 140 (21.4) | |
| ≥75 years | 2459 (76.0) | 473 (73.0) | 506 (74.0) | 439 (76.9) | 528 (77.6) | 513 (78.6) | |
| SES, n (%) | 0.1222 | ||||||
| Low | 469 (14.5) | 136 (21.0) | 83 (12.1) | 77 (13.5) | 97 (14.3) | 76 (11.6) | |
| Middle | 1291 (39.9) | 253 (39.0) | 299 (43.7) | 237 (41.5) | 263 (38.7) | 239 (36.6) | |
| High | 888 (27.4) | 149 (23.0) | 180 (26.3) | 161 (28.2) | 196 (28.8) | 202 (30.9) | |
| Unknown | 588 (18.2) | 110 (17.0) | 122 (17.8) | 96 (16.8) | 124 (18.2) | 136 (20.8) | |
| Weighted CCI score ** , n (%) | 0.0056 | ||||||
| 0 | 184 (23.7) | 33 (36.3) | 24 (31.6) | 13 (18.8) | 21 (23.1) | 93 (20.6) | |
| 1 | 213 (27.4) | 27 (29.7) | 19 (25.0) | 19 (27.5) | 26 (28.6) | 122 (27.1) | |
| 2 | 153 (19.7) | 17 (18.7) | 12 (15.8) | 17 (24.6) | 19 (20.9) | 88 (19.5) | |
| 3 or more | 166 (21.3) | 13 (14.3) | 20 (26.3) | 18 (26.1) | 16 (17.6) | 99 (22.0) | |
| Unknown | 62 (8.0) | 1 (1.1) | 1 (1.3) | 2 (2.9) | 9 (9.9) | 49 (10.9) | |
| Tumour characteristics, n (%) | |||||||
| cT stage (TNM) | 0.0007 | ||||||
| cT2 | 2525 (78.0) | 507 (78.2) | 566 (82.7) | 453 (79.3) | 520 (76.5) | 479 (73.4) | |
| cT3 | 402 (12.4) | 75 (11.6) | 58 (8.5) | 69 (12.1) | 88 (12.9) | 112 (17.2) | |
| cT4a | 309 (9.5) | 66 (10.2) | 60 (8.8) | 49 (8.6) | 72 (10.6) | 62 (9.5) | |
| Focality of the tumour | <0.0001 | ||||||
| Multifocal | 667 (20.6) | 116 (17.9) | 132 (19.3) | 112 (19.6) | 156 (22.9) | 151 (23.1) | |
| Unifocal | 2240 (69.2) | 423 (65.3) | 481 (70.3) | 415 (72.7) | 463 (68.1) | 458 (70.1) | |
| Unknown | 329 (10.2) | 109 (16.8) | 71 (10.4) | 44 (7.7) | 61 (9.0) | 44 (6.7) | |
| Localization of the tumour, n (%) | 0.0063 | ||||||
| Trigone | 256 (7.9) | 39 (6.0) | 61 (8.9) | 42 (7.4) | 47 (6.9) | 67 (10.3) | |
| Dome | 113 (3.5) | 19 (2.9) | 25 (3.7) | 23 (4.0) | 23 (3.4) | 23 (3.5) | |
| Right or left wall | 693 (21.4) | 140 (21.6) | 146 (21.3) | 120 (21.0) | 135 (19.9) | 152 (23.3) | |
| Anterior wall | 85 (2.6) | 22 (3.4) | 18 (2.6) | 13 (2.3) | 12 (1.8) | 20 (3.1) | |
| Posterior wall | 191 (5.9) | 57 (8.8) | 25 (3.7) | 35 (6.1) | 42 (6.2) | 32 (4.9) | |
| Bladder neck | 134 (4.1) | 27 (4.2) | 20 (2.9) | 19 (3.3) | 39 (5.7) | 29 (4.4) | |
| Left or right ureteric orifice | 167 (5.2) | 34 (5.2) | 41 (6.0) | 31 (5.4) | 27 (4.0) | 34 (5.2) | |
| Overlapping localizations | 1161 (35.9) | 227 (35.0) | 268 (39.2) | 215 (37.7) | 249 (36.6) | 202 (30.9) | |
| Unknown | 436 (13.5) | 83 (12.8) | 80 (11.7) | 73 (12.8) | 106 (15.6) | 94 (14.4) | |
| Hospital characteristics | |||||||
| Type of hospital (diagnosis), n (%) | 0.4210 | ||||||
| Community hospital | 1408 (43.5) | 269 (41.5) | 294 (43.0) | 264 (46.2) | 286 (42.1) | 295 (45.2) | |
| Non‐university referral hospital | 1688 (52.2) | 354 (54.6) | 357 (52.2) | 277 (48.5) | 364 (53.5) | 336 (51.5) | |
| University hospital | 140 (4.3) | 25 (3.9) | 33 (4.8) | 30 (5.3) | 30 (4.4) | 22 (3.4) | |
CCI, Charlson Comorbidity Index; IQR, interquartile range; SES, socioeconomic status.
P value for trend (two‐sided) was calculated using linear regression for parametric continuous variables, Cochran–Armitage trend test for binary variables and Cochran–Mantel–Haenszel test for categorical variables with more than two categories.
Before November 2017, CCI score was only available for the southern region of the Netherlands.
The BlaZIB subcohort (November 2017–November 2019) included 2116 patients, of whom 19.4% (n = 410) were not treated. Table 2 presents the patient, tumour and hospital characteristics for this subcohort, overall and stratified by treatment. The subcohort was comparable to the entire cohort of 2005–2019 (Table S1). Untreated patients were older, and had a lower BMI and SES, worse renal function and performance status, higher CCI score, and more often stage cT4a bladder carcinoma. Also, untreated patients were less often discussed in a multidisciplinary team meeting (MDTM) compared to treated patients. The most important reasons for remaining untreated, as noted in the medical files, were poor functional status (46.1%, n = 189) and patients' own preference (27.8%, n = 114), followed by expected fast progression of the disease or expected timely death (13.9%, n = 57) and no complaints or low tumour load (1.7%, n = 7). Of 33 patients (10.5%), the reason for remaining untreated was not documented.
Table 2.
Patient, tumour and hospital characteristics of patients diagnosed with non‐metastatic muscle‐invasive bladder cancer between 1 November 2017 and 31 October 2019 included in the BlaZIB study, by treatment.
| Treatment | ||||
|---|---|---|---|---|
| Total | Untreated | Treated | P * | |
| Total, N (%) | 2116 (100.0) | 410 (19.4) | 1706 (80.6) | |
| Patient characteristics | ||||
| Gender, n (%) | 0.1895 | |||
| Male | 1506 (71.2) | 281 (68.5) | 1225 (71.8) | |
| Female | 610 (28.8) | 129 (31.5) | 481 (28.2) | |
|
Median age at diagnosis, years IQR |
74.0 67.0–81.0 |
83.0 77.0–87.0 |
72.0 65.0–78.0 |
<0.0001 |
| Age at diagnosis, n (%) | <0.0001 | |||
| <75 years | 1089 (51.5) | 73 (17.8) | 1016 (59.6) | |
| ≥75 years | 1027 (48.5) | 337 (82.2) | 690 (40.4) | |
|
Median BMI, kg/m 2 IQR (missing %) |
25.7 23.2–28.7 (8.3%) |
24.6 22.2–27.2 (17.8%) |
25.9 23.5–29.0 (6.0%) |
0.0002 |
| BMI, n (%) | <0.0001 | |||
| <18.5 kg/m2, underweight | 41 (1.9) | 10 (2.4) | 31 (1.8) | |
| 18.5–25 kg/m2, normal weight | 810 (38.3) | 174 (42.4) | 636 (37.3) | |
| 25–30 kg/m2, overweight | 775 (36.6) | 109 (26.6) | 666 (39.0) | |
| ≥30 kg/m2, obesity | 315 (14.9) | 44 (10.7) | 271 (15.9) | |
| Unknown | 175 (8.3) | 73 (17.8) | 102 (6.0) | |
| Weighted CCI score, n (%) | <0.0001 | |||
| 0 | 766 (36.2) | 89 (21.7) | 677 (39.7) | |
| 1 | 594 (28.1) | 120 (29.3) | 474 (27.8) | |
| 2 | 335 (15.8) | 84 (20.5) | 251 (14.7) | |
| 3 or more | 299 (14.1) | 94 (22.9) | 205 (12.0) | |
| Unknown | 122 (5.8) | 23 (5.6) | 99 (5.8) | |
| Type of comorbidity ** , n (%) | ||||
| Diabetes | 383 (31.2) | 99 (33.2) | 284 (30.5) | 0.3841 |
| Chronic pulmonary disease | 345 (28.1) | 82 (27.5) | 263 (28.3) | 0.7988 |
| Myocardial infarct | 197 (16.0) | 36 (12.1) | 161 (17.3) | 0.0322 |
| Peripheral vascular disease | 218 (17.8) | 57 (19.1) | 161 (17.3) | 0.4753 |
| Any tumour | 200 (16.3) | 55 (18.5) | 145 (15.6) | 0.2438 |
| Cerebrovascular disease | 237 (19.3) | 71 (23.8) | 166 (17.8) | 0.0229 |
| Moderate or severe renal disease | 208 (16.9) | 60 (20.1) | 148 (15.9) | 0.0910 |
| Congestive heart failure | 93 (7.6) | 35 (11.7) | 58 (6.2) | 0.0018 |
| Ulcer disease | 41 (3.3) | 11 (3.7) | 30 (3.2) | 0.6971 |
| Connective tissue disease | 55 (4.5) | 14 (4.7) | 41 (4.4) | 0.8335 |
| Dementia | 34 (2.8) | 22 (7.4) | 12 (1.3) | <0.0001 |
| Metastatic solid tumour (other than bladder cancer) | 24 (2.0) | 11 (3.7) | 13 (1.4) | 0.0128 |
| Mild liver disease | 19 (1.5) | 3 (1.0) | 16 (1.7) | 0.3850 |
| Diabetes with end‐organ damage | 27 (2.2) | 10 (3.4) | 17 (1.8) | 0.1176 |
| Hemiplegia or paraplegia | 11 (0.9) | 4 (1.3) | 7 (0.8) | 0.3472 |
| HIV | 3 (0.2) | ‐ | 3 (0.3) | 0.3263 |
| ECOG performance status, n (%) | <0.0001 | |||
| 0 | 654 (30.9) | 32 (7.8) | 622 (36.5) | |
| 1 | 438 (20.7) | 53 (12.9) | 385 (22.6) | |
| ≥2 | 231 (10.9) | 78 (19.0) | 153 (9.0) | |
| Unknown | 793 (37.5) | 247 (60.2) | 546 (32.0) | |
| Renal function: eGFR, mL/min/1.73m 2 (missing %) |
63.0 47.0–81.0 (25.1%) |
48.0 32.0–65.0 (18.3%) |
67.8 52.0–83.0 (26.7%) |
<0.0001 |
| SES, n (%) | 0.0087 | |||
| Low | 634 (30.0) | 147 (35.9) | 487 (28.5) | |
| Middle | 729 (34.5) | 128 (31.2) | 601 (35.2) | |
| High | 537 (25.4) | 91 (22.2) | 446 (26.1) | |
| Unknown | 216 (10.2) | 44 (10.7) | 172 (10.1) | |
| Previous surgery, n (%) | 0.0531 | |||
| Yes | 545 (25.8) | 108 (26.3) | 437 (25.6) | |
| No | 1514 (71.6) | 284 (69.3) | 1230 (72.1) | |
| Unknown | 57 (2.7) | 18 (4.4) | 39 (2.3) | |
| Previous radiation, n (%) | 0.0221 | |||
| Yes | 84 (4.0) | 26 (6.3) | 58 (3.4) | |
| No | 1979 (93.5) | 373 (91.0) | 1606 (94.1) | |
| Unknown | 53 (2.5) | 11 (2.7) | 42 (2.5) | |
| Tumour characteristics | ||||
| cT stage (TNM) , n (%) | 0.0286 | |||
| cT2 | 1477 (69.8) | 292 (71.2) | 1185 (69.5) | |
| cT3 | 506 (23.9) | 83 (20.2) | 423 (24.8) | |
| cT4a | 133 (6.3) | 35 (8.5) | 98 (5.7) | |
| Focality of the tumour, n (%) | 0.0016 | |||
| Multifocal | 508 (24.0) | 104 (25.4) | 404 (23.7) | |
| Unifocal | 1533 (72.4) | 280 (68.3) | 1253 (73.4) | |
| Unknown | 75 (3.5) | 26 (6.3) | 49 (2.9) | |
| Localization of the tumour, n (%) | 0.4045 | |||
| Trigone | 173 (8.2) | 39 (9.5) | 134 (7.9) | |
| Dome | 91 (4.3) | 12 (2.9) | 79 (4.6) | |
| Right or left wall | 566 (26.7) | 100 (24.4) | 466 (27.3) | |
| Anterior wall | 59 (2.8) | 12 (2.9) | 47 (2.8) | |
| Posterior wall | 109 (5.2) | 21 (5.1) | 88 (5.2) | |
| Bladder neck | 78 (3.7) | 14 (3.4) | 64 (3.8) | |
| Left or right ureteric orifice | 103 (4.9) | 22 (5.4) | 81 (4.7) | |
| Overlapping localisations | 724 (34.2) | 138 (33.7) | 586 (34.3) | |
| Unknown | 213 (10.1) | 52 (12.7) | 161 (9.4) | |
| Hospital characteristics | ||||
| Type of hospital, n (%) | 0.0019 | |||
| Community | 910 (43.0) | 190 (46.3) | 720 (42.2) | |
| Non‐university referral | 1115 (52.7) | 215 (52.4) | 900 (52.8) | |
| University | 91 (4.3) | 5 (1.2) | 86 (5.0) | |
| Discussed in MDTM, n (%) | <0.0001 | |||
| Yes, discussed in MDTM | 1963 (92.8) | 314 (76.6) | 1649 (96.7) | |
| No MDTM documented | 153 (7.2) | 96 (23.4) | 57 (3.3) | |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; eGFR: estimated glomerular filtration rate; IQR, interquartile range; MDTM, multidisciplinary team meeting; SES, socioeconomic status.
P value was calculated using Chi‐square for categorical variables and ANOVA for continuous variables.
Type of comorbidity was only considered for patients with a Charlson Comorbidity Index score of 1 or higher.
Multivariable logistic regression analysis (Table 3) showed that in both age groups (<75 and ≥75 years), ECOG performance status ≥2 vs 0 and cT4a vs cT2 stage were associated with an increased odds of remaining untreated. After stratification by age group, increasing age still increased the odds of being untreated in patients aged ≥75 years. In these patients, previous radiation in the abdomen/pelvic area was also associated with being untreated. These latter associations were not found in patients aged <75 years. With regard to hospital characteristics, being diagnosed in a university hospital decreased the odds of being untreated for patients aged ≥75 years. In case no MDTM was documented, increased odds were observed in both age groups.
Table 3.
Uni‐ and multivariable logistic regression analysis on the association between patient, tumour and hospital characteristics and receiving no treatment, in patients diagnosed with non‐metastatic muscle‐invasive bladder cancer between 1 November 2017 and 31 October 2019, included in the BlaZIB study.
| Univariable model | Multivariable model 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | <75 years | 75 years and older | Overall | <75 years | 75 years and older | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Patient characteristics | ||||||||||||
| Gender | ||||||||||||
| Male | ref. | ref. | ref. | ref. | ref. | ref. | ||||||
| Female | 1.17 | 0.93–1.48 | 1.25 | 0.75–2.08 | 1.15 | 0.87–1.53 | ||||||
| Age at diagnosis (per year increase) | 1.14 | 1.12–1.16 | 1.04 | 1.00–1.08 | 1.19 | 1.16–1.23 | 1.09 | 1.07–1.12 | 1.01 | 0.97–1.06 | 1.15 | 1.11–1.19 |
| BMI (per kg/m 2 increase) | 0.95 | 0.92–0.98 | 0.97 | 0.91–1.02 | 0.96 | 0.92–0.99 | 0.97 | 0.93–1.00 | 0.98 | 0.92–1.03 | 0.97 | 0.93–1.02 |
| Weighted CCI score | ||||||||||||
| 0 | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| 1 | 1.94 | 1.44–2.62 | 2.05 | 1.10–3.82 | 1.48 | 1.03–2.13 | 1.25 | 0.86–1.82 | 1.48 | 0.71–3.09 | 1.22 | 0.77–1.93 |
| 2 | 2.56 | 1.84–3.56 | 2.63 | 1.27–5.45 | 1.66 | 1.12–2.47 | 1.29 | 0.84–1.97 | 1.54 | 0.65–3.66 | 1.24 | 0.74–2.07 |
| ≥3 | 3.58 | 2.57–4.97 | 3.49 | 1.62–7.51 | 2.11 | 1.43–3.10 | 1.19 | 0.76–1.86 | 1.05 | 0.39–2.83 | 1.26 | 0.75–2.13 |
| ECOG performance status | ||||||||||||
| 0 | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| 1 | 2.35 | 1.48–3.73 | 1.56 | 0.69–3.51 | 1.83 | 1.05–3.20 | 1.47 | 0.90–2.40 | 1.29 | 0.55–3.01 | 1.44 | 0.79–2.62 |
| ≥2 | 14.84 | 9.37–23.49 | 15.67 | 7.41–33.13 | 8.07 | 4.70–13.86 | 6.32 | 3.63–11.01 | 12.16 | 5.10–28.95 | 4.90 | 2.53–9.47 |
| Renal function (eGFR, mL/min/1.73m 2 ) | 0.97 | 0.96–0.97 | 0.97 | 0.96–0.99 | 0.97 | 0.97–0.98 | 0.99 | 0.98–0.99 | 0.99 | 0.97–1.00 | 0.99 | 0.98–1.00 |
| SES | ||||||||||||
| Low | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| Middle | 0.71 | 0.54–0.92 | 0.49 | 0.27–0.89 | 0.86 | 0.63–1.18 | 1.13 | 0.79–1.61 | 0.62 | 0.31–1.26 | 1.44 | 0.96–2.16 |
| High | 0.67 | 0.50–0.89 | 0.45 | 0.24–0.86 | 0.96 | 0.67–1.37 | 1.11 | 0.75–1.64 | 0.76 | 0.36–1.60 | 1.30 | 0.82–2.06 |
| Previous surgery | ||||||||||||
| No | ref. | ref. | ref. | ref. | ref. | ref. | ||||||
| Yes | 1.06 | 0.83–1.36 | 0.88 | 0.49–1.58 | 0.95 | 0.71–1.27 | ||||||
| Previous radiation | ||||||||||||
| No | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| Yes | 1.92 | 1.19–3.08 | 1.05 | 0.25–4.54 | 1.57 | 0.91–2.71 | 2.17 | 1.20–3.90 | 0.92 | 0.18–4.65 | 2.77 | 1.42–5.41 |
| Tumour characteristics | ||||||||||||
| cT stage (TNM) | ||||||||||||
| cT2 | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| cT3 | 0.80 | 0.61–1.04 | 0.97 | 0.54–1.72 | 0.89 | 0.64–1.24 | 0.90 | 0.64–1.28 | 0.92 | 0.48–1.77 | 0.85 | 0.56–1.30 |
| cT4a | 1.45 | 0.97–2.18 | 3.05 | 1.53–6.08 | 1.26 | 0.73–2.17 | 2.49 | 1.46–4.23 | 3.23 | 1.36–7.66 | 2.23 | 1.12–4.42 |
| Focality of the tumour | ||||||||||||
| Unifocal | ref. | ref. | ref. | ref. | ref. | ref. | ||||||
| Multifocal | 1.14 | 0.88–1.46 | 1.48 | 0.87–2.50 | 1.03 | 0.76–1.39 | ||||||
| Hospital characteristics | ||||||||||||
| Type of hospital (diagnosis) | ||||||||||||
| Community hospital | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| Non‐university referral hospital | 0.91 | 0.73–1.13 | 0.80 | 0.49–1.30 | 0.93 | 0.72–1.21 | 0.98 | 0.73–1.30 | 0.73 | 0.41–1.30 | 1.04 | 0.74–1.46 |
| University hospital | 0.22 | 0.09–0.55 | 0.71 | 0.21–2.38 | 0.11 | 0.03–0.47 | 0.28 | 0.10–0.76 | 0.53 | 0.13–2.09 | 0.15 | 0.03–0.71 |
| Discussed in MDTM | ||||||||||||
| Yes, discussed in MDTM | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| No MDTM documented | 8.85 | 6.24–12.54 | 4.83 | 2.35–9.95 | 10.74 | 6.52–17.69 | 5.40 | 3.20–9.10 | 3.56 | 1.28–9.86 | 6.80 | 3.63–12.75 |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; MDTM, multidisciplinary team meeting; OR, odds ratio (for remaining untreated); SES, socioeconomic status.
The multivariable model includes age, BMI, weighted CCI, performance status, renal function, SES, tumour stage and previous radiation in abdomen/pelvic area, type of hospital of diagnosis and whether the patient was discussed in an MDTM.
The proportion of untreated patients ranged between hospitals, from 0–27% for patients aged <75 years and 0–72% for patients aged ≥75 years (Fig. S3a–e). After adjustment for case‐mix factors, namely, age at diagnosis, BMI, performance status, renal function, disease stage and previous radiation, interhospital variation decreased to 37–69% for patients aged ≥75 years. For patients aged <75 years, multilevel analysis was not performed due to limited variation within this patient group (Fig. S3e).
To compare the overall survival of treated and untreated patients, 337 untreated patients (82%) were matched to treated patients by age at diagnosis, BMI, renal function, performance status, disease stage, and previous radiation in the abdomen/pelvic area, thereby reducing the imbalance regarding these variables between treatment groups (Table S1). The mOS of untreated patients was 6.4 months vs 16.0 months for treated patients (P < 0.0001; Fig. 2a). After excluding patients who died <90 days after diagnosis, the mOS of untreated patients improved to 10.4 months but was still significantly worse compared to treated patients, whose mOS was then 17.5 months (P < 0.0001; Fig. 2b). After stratification by age, mOS remained worse for untreated patients (Fig. 2c). Multivariable Cox regression analyses showed a fourfold increased risk for untreated patients aged <75 years and an over twofold increased risk for untreated patients aged ≥75 years (Table S1).
Fig. 2.
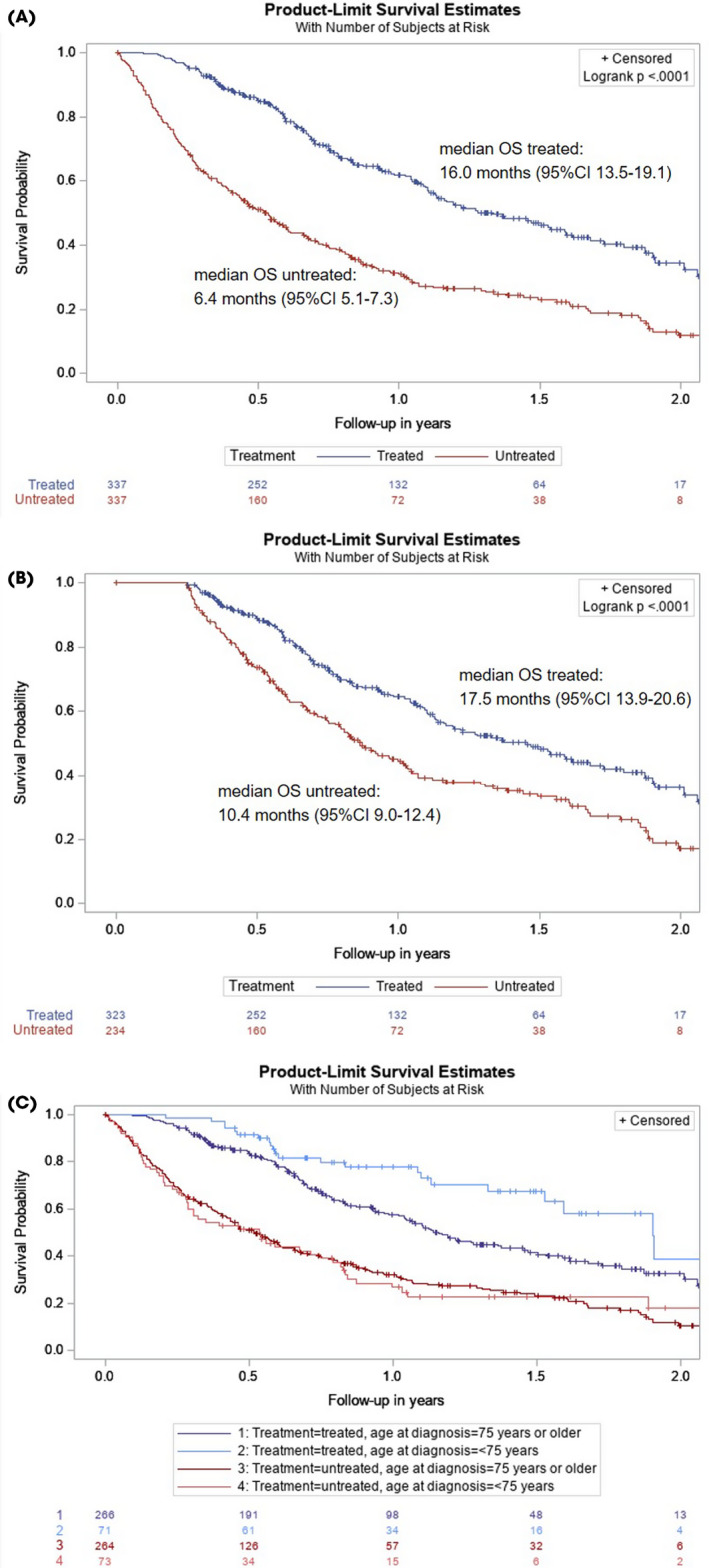
Overall survival (OS) of untreated patients vs treated patients with non‐metastatic muscle‐invasive bladder cancer (MIBC), matched on age, body mass index, renal function, performance status, tumour stage, and previous radiation in abdomen/pelvic area (A), with patients who deceased within 90 days after diagnosis excluded (B), and stratified by age (C). Median OS of treated patients aged ≥75 years: 13.9 months (95% CI 12.3–17.5). Median OS of treated patients aged <75 years: 22.8 months (95% CI 18.3–26.0). Log‐rank P = 0.007. Median OS of untreated patients aged ≥75 years: 6.2 months (95% CI 5.1–7.4). Median OS of untreated patients aged <75 years: 6.5 months (95% CI 3.4–9.5). Log‐rank P = 0.908.
Discussion
In this population‐based cohort study, we aimed to provide insight into the characteristics of the untreated patient population with non‐metastatic MIBC, the factors associated with remaining untreated, and the survival of untreated vs treated patients matched on prognostic characteristics. A substantial proportion of patients, especially elderly patients, remained untreated. Next to age, several other factors affected the probability of remaining untreated. There was large variation in the proportion of untreated, elderly patients among hospitals, even after adjusting for case‐mix factors. In addition, untreated patients fared significantly worse compared to treated patients with a similar prognostic profile. This study therefore provides a rationale to re‐evaluate whether we should treat a larger proportion of these patients in the near future.
One‐fifth of patients with non‐metastatic MIBC was not treated. This is largely consistent with the limited number of earlier studies evaluating untreated patients with non‐metastatic MIBC [9, 10, 11, 12, 13]. In our study, the proportion of untreated patients decreased slightly over time. A US study by Fletcher et al. showed a larger trend over time; between 2004 and 2013, the proportion of untreated patients decreased from 47% to 34% [14]. Furthermore, the median age at diagnosis and comorbidity of untreated patients increased over time, indicating that, over time, more older and fragile patients have been treated. Use of chemoradiotherapy, often applied in the context of trimodality therapy as an alternative to RC, increased over time. It should be noted that the application of trimodality therapy can differ among countries, which may affect the generalizability of our results: in countries applying trimodality therapy more often, the proportion of untreated patients might be smaller since the characteristics of patients undergoing trimodality therapy resemble, in part, the characteristics of the untreated patient group in our cohort.
Even though international guidelines state that chronological age is of less importance than biological age with regard to treatment decisions [2], chronological age still appeared to be an important factor associated with remaining untreated. On average, 10% of patients aged <75 years remained untreated (other than best supportive care, i.e. no anticancer treatment, but radiotherapy, for example, to control haematuria or pain). However, this percentage steeply increased to 34% for patients aged ≥75 years. Even after stratification by age, age remained significantly associated with remaining untreated in patients aged ≥75 years. This indicates that age and/or its associated characteristics such as comorbidity and performance status play an important role in being untreated. It could be questioned whether the weight given to age as a determinant of treatment candidacy is appropriate. Because international guidelines do not exclude patients for curative treatment based on age and explicitly state that chronological age is of limited relevance, we feel that disease stage, comorbidity, disease‐related complaints and life expectancy, next to patient preference, should be the determinants of treatment decisions. It seems that in current clinical practice, chronological age is an important determinant in treatment decision‐making, but chronological age may differ significantly from biological age. This should be emphasized in the guidelines. The focus should shift from chronological age to the biological age of the patient, which could for instance be assessed using the frailty index or by consulting a geriatrician.
Previous studies, although mostly not focusing specifically on the untreated patient population, showed that elderly patients less often receive curative treatment, probably due to the presence of multiple or severe comorbidities [16, 17, 25]. Leliveld et al. examined the association between patient and tumour characteristics and receiving RC, and showed that comorbidity was associated with receiving RC in univariable analysis. However, when adjusting for age, this association was no longer present [10]. Likewise in our study, comorbidity was univariably associated with remaining untreated, but was no longer associated with this in multivariable analysis. This could possibly be explained by the strong association between age and treatment, and several other patient and tumour characteristics also associated with comorbidity but even more so with remaining untreated.
In contrast to comorbidity, performance status remained significantly associated with being untreated throughout all of our analyses, even after stratification by age. We also observed that in patients aged ≥75 years, a more advanced disease stage and previously having received radiotherapy in the abdomen or pelvic area (not bladder cancer‐related) were associated with not receiving treatment. Better renal function showed a borderline significant inverse association in both age groups. Even though inferior renal function and previous radiotherapy are a contraindication for treatment with (neoadjuvant) chemotherapy or radiotherapy, respectively, this should not be a contraindication for receiving any type of treatment [2, 27].
Next to patient and tumour characteristics, hospital‐related factors might also affect treatment decision‐making. We observed large interhospital variation in the proportion of untreated patients, especially in patients aged ≥75 years, even after adjustment for case‐mix factors such as age. This indicates differences in hospital policy and an interplay of doctors' advice and patient preferences, since patient preferences partly reflect the doctor's advice. In our study, we found that 25% of patients remained untreated as a result of patient preference. This is probably an underestimate as only one reason to abstain from treatment could be documented and patient preferences often go hand in hand with the patient's condition and (quality of) life expectancy [28]. However, it is unlikely that the large hospital variation can be completely explained by differences in patient preferences. Therefore, our results suggest that there is room for improvement regarding treatment of patients with non‐metastatic MIBC. Re‐evaluation of the guidelines, that is, improved selection of patients with appropriate treatment candidacy, is warranted. This will hopefully decrease interhospital variation and potential under‐treatment, which in turn will increase the consistency in quality of care for each patient independent of the hospital providing treatment.
In order to compare OS, untreated patients were matched to treated patients with similar characteristics. It is important to note that the matched patients receiving treatment represent a subgroup of older patients with worse condition as compared to the overall MIBC patient population. Therefore, the mOS of matched, treated patients does not reflect the survival of the total population of treated MIBC patients and was only 16 months. The mOS of untreated patients was 6 months. In addition, we observed that the mOS of untreated patients was similar in the younger and older age groups, implying that treatment, and not age, is crucial for better survival.
This large, population‐based, nationwide cohort study provides detailed and relevant insight into the group of untreated patients with non‐metastatic bladder cancer, which, to our knowledge, has not previously been described. Nevertheless, the retrospective data collection and observational character of this study have to be recognized as limitations. Missing values, which are inherent to this study design using administrative data, were addressed by employing imputation [24]. For this study we also collected information on the reason why a patient was not treated, for example, patient preference. Unfortunately, this was documented poorly in the electronic medical files: information was missing in two‐thirds of patients; therefore, we could not take this into account in our analyses. However, we do not expect patient populations to differ much among hospitals with regard to patient preference. Therefore, the large interhospital variation we observed in this study is unlikely to be fully explained by patient preference. The results of this study are based on observational data collected from the electronic health records and therefore the results depend on the completeness of reporting, which might be considered to be a limitation. Nevertheless, the data collected in the NCR are collected in a standardized manner by well‐trained data managers and are subject to regular quality controls, thereby guaranteeing high quality. For our study we used CCI score as a summary score of the patients' comorbidity status [23]. Using CCI score as a measure of treatment candidacy has some limitations, as shown by Austin et al. [26]. One limitation is that if patients, based on their characteristics, have an almost 100% chance of (not) being assigned to a treatment arm, the CCI score might not control for confounding by comorbidity as well as it should [26]. However, we have shown that, even within our study population, untreated patients are a heterogenous population and could potentially have been considered treatment‐eligible. To avoid selection bias occurring from the systematic baseline differences between treatment groups, propensity‐score matching was performed before evaluating OS. We assume that after employing propensity‐score matching, any confounding by treatment indication, if present, would be minimal. Despite the detailed information that was collected, it is possible that residual bias remained because of unmeasured confounding factors. Patients treated with (re)TURBT only were categorized in the untreated patient group. However, maximal TURBT could be regarded a curative treatment in a small minority of patients [29] and these patients were thus wrongfully classified as being untreated. We estimate that the effect of the potential misclassification would be minimal.
The insight gained from the results of our study could aid doctors and patients in the decision‐making process regarding whether or not to treat patients with non‐metastatic MIBC, potentially improving patient outcomes. Whereas the untreated patients were mostly elderly, survival of patients treated with any type of treatment was better compared to that of untreated patients, regardless of age. Therefore, treatment decision‐making should not be solely based on chronological age. This is also supported by multiple studies, reviews [16, 28, 30, 31, 32] and international guidelines [2]. From our analysis it is clear that elderly patients are still undertreated even though treatment possibilities, for example, with trimodality therapy or immune checkpoint inhibition, are expanding and are quite well endured by elderly patients [5, 33]. Therefore, clinicians should consider treating elderly patients with curative intent if no other contraindications are present. For untreated patients, often no documentation was found in the medical file regarding an MDTM. Discussing these patients in an MDTM could be a useful aid in deciding on (abstaining from) treatment. If it is unclear whether an elderly patient could opt for RC, or any kind of treatment, a geriatrician could be consulted. Further centralization of bladder cancer care could also positively influence the treatment decision‐making process as this might alleviate any doubt on whether an (elderly) patient should, for instance, undergo surgery. Furthermore, the results of our study highlight the need for improved selection of patients with appropriate treatment candidacy, as well as for better predictors of response to treatment. For this, alternative treatment modalities should also be taken into account because they may also result in cure, or delay progression or time of death in elderly patients. This could be addressed in the guidelines.
In conclusion, one‐fifth of patients with non‐metastatic MIBC remained untreated. Untreated patients were generally older and had a more unfavourable prognostic profile. Untreated patients showed significantly worse OS compared to treated patients with similar characteristics, regardless of age. Chronological age alone should, therefore, not affect treatment decision‐making. Considering the difference in survival of untreated vs treated patients with similar characteristics and, given the large, case‐mix‐adjusted interhospital variation, a proportion of untreated patients might be wrongfully denied life‐prolonging treatment.
Funding
The BlaZIB study is supported by the Dutch Cancer Society (KWF; IKNL 2015–7914).The funding agency had no further role in this study.
Disclosure of Interests
The authors declare that they have no conflicts of interest.
Data Availability
All data used for this study can be requested from the NCR. All data requests are reviewed by the supervisory committee of the NCR for compliance with the NCR objectives and (inter)national (privacy) regulation and legislation (https://iknl.nl/en/ncr/apply‐for‐data).
Supporting information
Fig. S1 Inclusion of patients with cT2‐T4aN0/xM0/x urothelial MIBC in the Netherlands and available variables per cohort.
Fig. S2 Percentage of untreated patients with non‐metastatic MIBC between 2005–2019 per age group.
Fig. S3 Observed and case‐mix adjusted variation between hospitals of multidisciplinary meeting in the propotion of untreated patients of all ages (a,b) 75 years and older (c, d) and younger than 75 years (e, f) diagnosed with cT2‐4aN0/xM0/x urothelial bladder carcinoma between 1 November 2017 and 31 October 2019.
Table S1 Patient, tumour and hospital characteristics of patients diagnosed with non‐metastatic MIBC between 2005 and 2019, by treatment.
Table S2 Patient and tumour characteristics of untreated and treated patients in the BlaZIB cohort after propensity‐score matching.
Table S3 Uni‐ and multivariable Cox proportional hazards regression analyses on the association between patient, tumour and hospital characteristics and survival, in patients diagnosed with non‐metastatic MIBC between 1 November 2017 and 31 October 2019, included in the BlaZIB study.
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the NCR.
The members of the BlaZIB study group (in addition to the authors) are as follows: Joost Boormans, MD, PhD (Erasmus Medical Centre), Theo M. de Reijke, MD, PhD (Amsterdam University Medical Centres), Catharina A. Goossens‐Laan, MD, PhD (Alrijne hospital), Sipke Helder (Patient association ‘Leven met blaas‐ of nierkanker’), Maarten C.C.M. Hulshof, MD, PhD (Amsterdam University Medical Centres), Geert J.L.H. van Leenders, MD, PhD (Erasmus Medical Centre), Anna M. Leliveld, MD, PhD (University Medical Centre Groningen), Sasja F. Mulder, MD, PhD (Radboud University Medical Centre), Juus L. Noteboom, MD, PhD (University Medical Centre Utrecht), Jorg R. Oddens, MD, PhD (Amsterdam University Medical Centres), Tineke J. Smilde, MD, PhD (Jeroen Bosch ziekenhuis), Guus W.J. Venderbosch (Patient association ‘Leven met blaas‐ of nierkanker’), Antoine G. van der Heijden, MD, PhD (Radboud University Medical Centre), Michiel S. van der Heijden, MD, PhD (Netherlands Cancer Institute), Reindert J.A. van Moorselaar, MD, PhD, Prof (Amsterdam University Medical Centres), Bas W.G. van Rhijn, MD, PhD, FEBU (Netherlands Cancer Institute – Antoni van Leeuwenhoek Hospital), Joep G.H. van Roermund, MD, PhD (Maastricht University Medical Centre), Bart P. Wijsman, MD, PhD (Elisabeth‐TweeSteden Ziekenhuis).
BlaZIB study group members are listed in the Acknowledgements section.
References
- 1. Martini A, Sfakianos JP, Renström‐Koskela L et al. The natural history of untreated muscle‐invasive bladder cancer. BJU Int 2020; 125: 270–5 [DOI] [PubMed] [Google Scholar]
- 2. Witjes JA, Bruins HM, Cathomas R et al. European association of urology guidelines on muscle‐invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 2021; 79: 82–104 [DOI] [PubMed] [Google Scholar]
- 3. Chang SS, Bochner BH, Chou R et al. Treatment of non‐metastatic muscle‐invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol 2017; 198: 552–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fahmy O, Khairul‐Asri MG, Schubert T et al. A systematic review and meta‐analysis on the oncological long‐term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle‐invasive bladder cancer. Urol Oncol 2018; 36: 43–53 [DOI] [PubMed] [Google Scholar]
- 5. Giacalone NJ, Shipley WU, Clayman RH et al. Long‐term outcomes after bladder‐preserving tri‐modality therapy for patients with muscle‐invasive bladder cancer: an updated analysis of the massachusetts general hospital experience. Eur Urol 2017; 71: 952–60 [DOI] [PubMed] [Google Scholar]
- 6. Smelser WW, Austenfeld MA, Holzbeierlein JM, Lee EK. Where are we with bladder preservation for muscle‐invasive bladder cancer in 2017? Indian J Urol 2017; 33: 111–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ploussard G, Daneshmand S, Efstathiou JA et al. Critical analysis of bladder sparing with trimodal therapy in muscle‐invasive bladder cancer: a systematic review. Eur Urol 2014; 66: 120–37 [DOI] [PubMed] [Google Scholar]
- 8. Efstathiou JA, Spiegel DY, Shipley WU et al. Long‐term outcomes of selective bladder preservation by combined‐modality therapy for invasive bladder cancer: the MGH experience. Eur Urol 2012; 61: 705–11 [DOI] [PubMed] [Google Scholar]
- 9. Washington SL 3rd, Neuhaus J, Meng MV, Porten SP. Social determinants of appropriate treatment for muscle‐invasive bladder cancer. Cancer Epidemiol Biomarkers Prev 2019; 28: 1339–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leliveld AM, Doornweerd BH, Bastiaannet E, Schaapveld M, de Jong IJ. Treatment and outcome in muscle invasive bladder cancer: a population‐based survey. World J Urol 2010; 28: 439–44 [DOI] [PubMed] [Google Scholar]
- 11. Gray PJ, Fedewa SA, Shipley WU et al. Use of potentially curative therapies for muscle‐invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol 2013; 63: 823–9 [DOI] [PubMed] [Google Scholar]
- 12. John JB, Varughese MA, Cooper N et al. Treatment allocation and survival in patients diagnosed with nonmetastatic muscle‐invasive bladder cancer: an analysis of a national patient cohort in England. Eur Urol Focus 2021; 7: 359–65 [DOI] [PubMed] [Google Scholar]
- 13. Ogawa K, Shimizu Y, Uketa S, Utsunomiya N, Kanamaru S. Prognosis of patients with muscle invasive bladder cancer who are intolerable to receive any anti‐cancer treatment. Cancer Treat Res Commun 2020; 24: 100195 [DOI] [PubMed] [Google Scholar]
- 14. Fletcher SA, Harmouch SS, Krimphove MJ et al. Characterizing trends in treatment modalities for localized muscle‐invasive bladder cancer in the pre‐immunotherapy era. World J Urol 2018; 36: 1767–74 [DOI] [PubMed] [Google Scholar]
- 15. Fischer‐Valuck BW, Rao YJ, Rudra S et al. Treatment patterns and overall survival outcomes of octogenarians with muscle invasive cancer of the bladder: an analysis of the national cancer database. J Urol 2018; 199: 416–23 [DOI] [PubMed] [Google Scholar]
- 16. Erlich A, Zlotta AR. Treatment of bladder cancer in the elderly. Investig Clin Urol 2016;57 Suppl 1(Suppl 1):S26‐35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guancial EA, Roussel B, Bergsma DP et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging 2015; 10: 939–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Booth CM, Siemens DR, Peng Y, Mackillop WJ. Patterns of referral for perioperative chemotherapy among patients with muscle‐invasive bladder cancer: a population‐based study. Urol Oncol 2014; 32: 1200–8 [DOI] [PubMed] [Google Scholar]
- 19. Koppie TM, Serio AM, Vickers AJ et al. Age‐adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer 2008; 112: 2384–92 [DOI] [PubMed] [Google Scholar]
- 20. Mohamed N, Leung TM, Shah QN et al. Involving patients in the development and evaluation of an educational and training experiential intervention (ETEI) to improve muscle invasive bladder cancer treatment decision‐making and post‐operative self‐care: a mixed methods approach. J Cancer Educ 2020; 35: 808–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fritz A, Percy C, Jack A et al. International Classification of Diseases for Oncology/Editors, April Fritz … [et al.]. 3rd edn. Geneva: World Health Organization, 2000. [Google Scholar]
- 22. Ripping TM, Kiemeney LA, van Hoogstraten LMC, Witjes JA, Aben KKH. Insight into bladder cancer care: study protocol of a large nationwide prospective cohort study (BlaZIB). BMC Cancer 2020; 20: 455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83 [DOI] [PubMed] [Google Scholar]
- 24. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation and Updating. Chapter 7: Missing Values. 2nd edn. New York: Springer, 2009. [Google Scholar]
- 25. Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JW. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer 2013; 108: 1534–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the charlson comorbidity index and elixhauser score work. Med Care 2015; 53: e65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. James ND, Hussain SA, Hall E et al. Radiotherapy with or without chemotherapy in muscle‐invasive bladder cancer. N Engl J Med 2012; 366: 1477–88 [DOI] [PubMed] [Google Scholar]
- 28. Grubmueller B, Seitz C, Shariat SF. The treatment of muscle‐invasive bladder cancer in geriatric patients. Curr Opin Urol 2016; 26: 160–4 [DOI] [PubMed] [Google Scholar]
- 29. Solsona E, Iborra I, Collado A, Rubio‐Briones J, Casanova J, Calatrava A. Feasibility of radical transurethral resection as monotherapy for selected patients with muscle invasive bladder cancer. J Urol 2010; 184: 475–80 [DOI] [PubMed] [Google Scholar]
- 30. Tanaka H, Fukushima H, Kijima T et al. Feasibility and outcomes of selective tetramodal bladder‐preservation therapy in elderly patients with muscle‐invasive bladder cancer. Int J Urol 2020; 27: 236–43 [DOI] [PubMed] [Google Scholar]
- 31. Ploussard G, Albrand G, Rozet F, Lang H, Paillaud E, Mongiat‐Artus P. Challenging treatment decision‐making in older urologic cancer patients. World J Urol 2014; 32: 299–308 [DOI] [PubMed] [Google Scholar]
- 32. Soria F, Moschini M, Korn S, Shariat SF. How to optimally manage elderly bladder cancer patients? Transl Androl Urol 2016; 5: 683–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jodon G, Fischer SM, Kessler ER. Treatment of urothelial cancer in elderly patients: focus on immune checkpoint inhibitors. Drugs Aging 2018; 35: 409–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Inclusion of patients with cT2‐T4aN0/xM0/x urothelial MIBC in the Netherlands and available variables per cohort.
Fig. S2 Percentage of untreated patients with non‐metastatic MIBC between 2005–2019 per age group.
Fig. S3 Observed and case‐mix adjusted variation between hospitals of multidisciplinary meeting in the propotion of untreated patients of all ages (a,b) 75 years and older (c, d) and younger than 75 years (e, f) diagnosed with cT2‐4aN0/xM0/x urothelial bladder carcinoma between 1 November 2017 and 31 October 2019.
Table S1 Patient, tumour and hospital characteristics of patients diagnosed with non‐metastatic MIBC between 2005 and 2019, by treatment.
Table S2 Patient and tumour characteristics of untreated and treated patients in the BlaZIB cohort after propensity‐score matching.
Table S3 Uni‐ and multivariable Cox proportional hazards regression analyses on the association between patient, tumour and hospital characteristics and survival, in patients diagnosed with non‐metastatic MIBC between 1 November 2017 and 31 October 2019, included in the BlaZIB study.
Data Availability Statement
All data used for this study can be requested from the NCR. All data requests are reviewed by the supervisory committee of the NCR for compliance with the NCR objectives and (inter)national (privacy) regulation and legislation (https://iknl.nl/en/ncr/apply‐for‐data).


