Abstract
In the case of African swine fever (ASF) outbreaks in pig farms, EU legislation requires a thorough epidemiological investigation to determine, among other tasks, the extent of infection in the affected farm. The main aim of this study was to implement a reliable sampling strategy to quickly obtain an overview of the extent of ASF virus spread in an affected pig farm. We developed and tested a three‐step approach: (i) identification of sub‐units within the affected farm, (ii) categorization of sub‐units, and (iii) targeted selection of animals for testing. We used commercially available lateral flow devices (LFDs) to detect ASF antigen and antibodies under field conditions and compared them with routinely performed laboratory tests (qPCR, ELISA, IPT). The study was conducted in three commercial farms in Latvia that were affected by ASF in July 2020. One of the affected farms was relatively small with only 31 pigs, whereas the other two were large with 1800 and 9800 animals, respectively. The approach proved to be helpful and practical for efficient and reliably assess the ASF situation on the farm and to identify sub‐units within a farm where infected animals are present and sub‐units which might (still) be free of infection. This important epidemiological information helps to better estimate the high‐risk period and to track the potential spread of infection outside the farm. It allows also to prioritize culling and, if appropriate, to pursue a partial culling strategy taking into account the absence of clinical signs, implemented biosecurity measures, quarantine and negative test results, among others. This might be of interest for large commercial farms where the infection was identified very early and has not yet spread widely. Due to its limited sensitivity, the antigen LFD test is useful for testing animals showing signs of disease.
Keywords: African swine fever, domestic pigs, lateral flow device, outbreak investigation, sampling strategy
1. INTRODUCTION
African swine fever (ASF) is a deadly viral animal disease that significantly affects domestic and wild suids (Sus scrofa) (Plowright et al., 1994; Chenais et al., 2019). The current epizootic is caused by ASF virus (ASFV) of genotype II and started in 2007 in Georgia, from where it spread to neighbouring Caucasian countries and the Russian Federation, reaching the European Union in 2014, including Latvia (EFSA, 2020; OIE, 2021; Viltrop et al., 2021). In 2018, China, the world's largest pig producer, reported its first ASF outbreaks, and by the end of 2019 China had lost more than half of its pig population (Berthe, 2020). During the summer of 2021, ASF arrived in the Dominican Republic and Haiti thus threatening the pig sectors in North and Latin America (Gonzales et al., 2021). The huge losses are caused not only by the infection itself, which kills the affected animals, but also by the trade restrictions and the culling of all animals on an affected farm.
For ASF outbreaks in pig farms, EU legislation requires a thorough outbreak investigation with the aim of identifying the likely origin of the virus and the high‐risk period (HRP), that is, the estimated time that ASF has been on the farm before detection as well as to obtain information on the likely spread of the disease to other farms (EC, 2002, 2016). To do this, it would be important to estimate the extent of an outbreak on a farm, that is, how far the virus has already spread within the farm, by identifying the affected pig units. For outbreak investigations after disease confirmation, EU legislation required (until 21 April 2021) the collection of random blood samples from pigs on the affected holding during the culling process for serological and virological testing. The minimum number of samples required should allow the detection of a seroprevalence of 10% in pigs in each sub‐unit of the holding with a 95% confidence level (EC, 2003). Analyses of domestic pig outbreaks in the current epizootic as well as experimental studies have shown that the contagiousness of ASF is rather low, leading to a slow spread within pig herds (Chenais et al., 2019). This in turn usually leads to low mortality on farms in the early phase, usually within 2 to 3 weeks after virus introduction, though after this period most of the animals in the same pen will be infected. To date, all ASF viruses detected in domestic pigs and the majority of wild boar in Latvia were virulent strains despite the finding of an attenuated strain in a hunted wild boar in 2017 (Gallardo, Soler, et al., 2019; Gallardo et al., 2021). Taking into account that the dominant ASFV genotype II strains currently circulating are causing a case‐fatality rate of >90%, seropositive healthy pigs are rarely found as majority of pigs do not survive the acute phase (Blome et al., 2020). Therefore, considering the circulation of virulent viruses, surveillance based on random serological sampling is no longer recommended for early detection of ASF or as a method to estimate the spread of the virus within an affected farm (EFSA, 2021). The targeted surveillance approach is based on the assumption that virulent viruses are still the ones infecting domestic pigs and therefore the majority of infected animals would get sick and die (Busch et al., 2021; Blome et al., 2020). Therefore, targeted sampling focuses on the selection of suspicious, sick, and dead animals to be tested for the presence of the virus (risk‐based surveillance). Nevertheless, antibody tests are important findings that provide additional information about the spread of the disease in a farm (EC, 2018).
Under field conditions, rapid detection of ASF‐infected animals can be increased with the help of lateral flow devices (LFDs) for ASF virus antigen (Ag‐LFD) and antibody (Ab‐LFD). Such pen‐side or point‐of‐care tests have been developed in recent years and are commercially available (Cappai et al., 2017; Gallardo, Fernandez‐Pinero, et al., 2019). While the sensitivity and specificity of the Ab‐LFD is over 90% compared to antibody ELISA tests, the sensitivity of the Ag‐LFD has been shown to be below standard tests routinely used in diagnostic laboratories, for example, qPCR (Carlson et al., 2017; Pikalo et al., 2021, 2020; Sastre et al., 2016).
The main aim of our study was to implement a reliable sampling strategy that would enable us to quickly obtain an overview of the extent of virus spread on an ASF‐affected pig farm. A fast and reliable overview would allow decision‐makers to quickly take further appropriate action in controlling the outbreak. The hypothesis we are pursuing is that ASF‐infected animals are most likely to be found in the group of sick animals, and LFDs may help in identifying such animals. Our study had two objectives: (i) to develop a targeted and efficient sampling strategy for ASF‐affected farms and (ii) test the suitability of LFDs under field conditions compared to standard laboratory tests (PCR, ELISA, and IPT). So far, LFDs have mainly been tested under experimental conditions and not as an additional tool on ASF‐affected farms.
In large commercial pig farms in endemic areas, consideration is being given to exempting animals not affected by the disease from culling, where legislation and biosecurity allow (Costard et al., 2022). On farms where the disease has not yet spread widely, unaffected sub‐units could be exempted from culling following an appropriate legal, biosecurity, and surveillance concept (Costard et al., 2022). In order to consider this partial culling approach, it is necessary to efficiently and carefully monitor the extent of virus spread on the farm after the disease has been notified.
2. MATERIALS AND METHODS
2.1. Outbreak information and farm characteristics
The study was conducted in three commercial farms in Latvia that were affected by ASF in July 2020. It was a purely random choice of farms that resulted from the epidemiological situation at the time. The farms were of different sizes and there was no epidemiological link between them. All three farms were located in regions with active ASF virus circulation in the wild boar population (Oļševskis et al., 2020). The following brief farm descriptions reflect the ASF situation on the farms when ASF was confirmed and before we started our study. The information was provided by the farm owners or the farm veterinarians.
Farm A was a small commercial pig farm with 31 pigs in two separate stables (A1 and A2) only a few metres apart. Stable A1 had 25 pigs in six pens: one gilt in a pen and 24 finishers (30–80 kg weight) in five pens. In stable A2, two sows and four piglets were kept. ASF was suspected and confirmed after four pigs had died in one pen of stable A1. All other pigs were clinically unsuspicious at the time the disease was notified.
On farm B, almost 1800 pigs were kept in two farm units (B1 and B2). Unit B1 consisted of three separate stables and unit B2 of one stable. The distance between the farm units was about 3 km. Breeding animals, piglets, and part of the fattening animals were kept in B1, the remaining finishers in B2. The units were epidemiologically linked through animal movements, shared farm equipment and vehicles, and farm workers who worked on both sites. ASF was confirmed after three pregnant sows from a pen in B1 had aborted and died.
Farm C, a farrow to finish farm, had almost 9800 pigs when ASF was confirmed. The pigs were kept in eight separate stables which were divided into sections or rooms according to pig categories: breeding animals, gilts, weaners, and finishers. Injured or sick pigs were isolated in a separate section.
The outbreak was confirmed in a breeding sow found dead. According to the farmer, several sows aborted, and there was increased mortality and morbidity in one of the stables where pregnant sows were kept.
2.2. Sampling strategy for assessing the virus spread on the farms
A three‐step sampling procedure was developed and applied as described in Figure 1.
FIGURE 1.
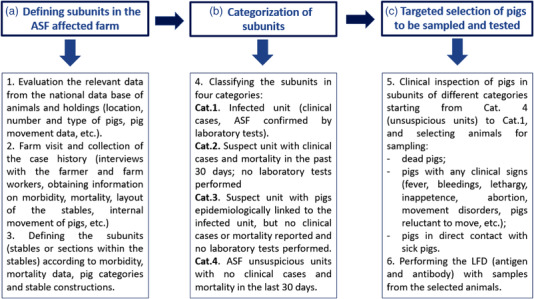
Sampling strategy of pigs in African swine fever (ASF)‐affected farms for the purpose of outbreak investigations
Our first step was to identify sub‐units for sampling, then categorize these units according to their likelihood of having ASF‐infected pigs, and finally select potentially infected pigs within the sampling units for testing. All pigs appearing sick during the investigation were sampled. Animals that had contact to sick appearing animals were also sampled (see below).
Blood was collected from the jugular vein or orbital sinus in serum and EDTA tubes. Tissue samples (spleen and lymph nodes) were taken from deceased pigs and packed in plastic bags.
2.2.1 Farm A
Stables A1 and A2 of farm A were considered as two sub‐units and were assigned to category 1 (Figure 2). Apart from five severely ill pigs found in A1, all other pigs were clinically inconspicuous. Due to the small number of pigs (n = 27) and the likelihood that all could be infected, all animals were sampled, starting in A1 (n = 21) and followed by A2 (n = 6).
FIGURE 2.
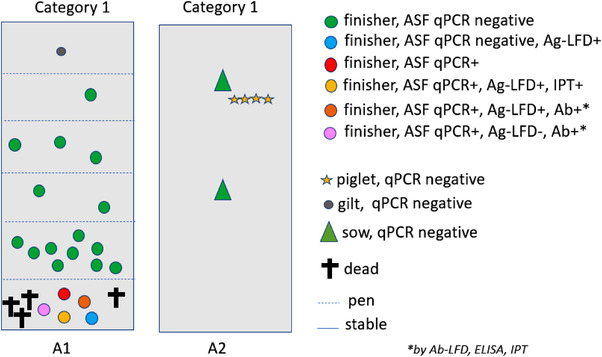
Schematic view of farm A with sampling units (A1 and A2) and results of testing
2.2.2 Farm B
Figure 3 shows the sub‐units and the number of samples taken at farm B. The assigned categories for the sub‐units and the sampled animals are shown in Table 1. In unit B2, 15 out of 447 pigs were sampled, including nine inconspicuous pigs which had direct contact with suspect animals and six pigs with mild clinical signs. In the different sub‐units of B1, a total of 20 pigs were sampled. Ten of these showed no clinical signs but had direct contact with suspect animals and six had mild signs. One sub‐unit of B1 was assigned to category 1 since several sows aborted and were severely sick. Four sick sows were sampled.
FIGURE 3.
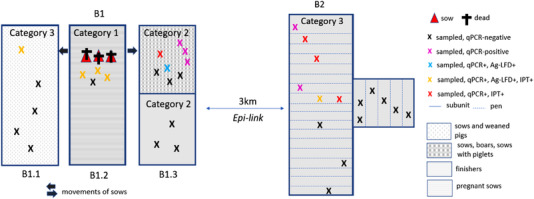
Schematic view of farm B with sampling units (B1.1, B1.2, B1.3, and B2) and results of testing
TABLE 1.
Assigned categories for sampling units and sampled animals on farm B
| Stable identification | No. of sub‐units per stable | Assigned category for sub‐unit | No. of pigs sampled |
|---|---|---|---|
| Farm unit B1 | |||
| B1.1 | 1 | 3 | 5 |
| B1.2 | 1 | 1 | 4 |
| B1.3 | 2 | 2 | 11 |
| Farm unit B2 | 1 | 3 | 15 |
| Total | 35 | ||
2.2.3 Farm C
The sampling strategy in farm C is shown in Figure 4. The assigned categories for the sampling units and the sampled animals are shown in Table 2. A total of 30 samples were taken from live pigs which were either sick or in contact with infected pigs. Clinically severe cases were found in C1, while the other samples were from inconspicuous pigs which were in contact with suspect pigs (n = 12) or pigs with mild clinical signs (n = 9). In addition, four pigs that were found dead were sampled (one in C8, one in C2 and two in C1). No samples were taken from sub‐units with only clinically healthy animals and no suspicion of having had contact with infected pigs.
FIGURE 4.
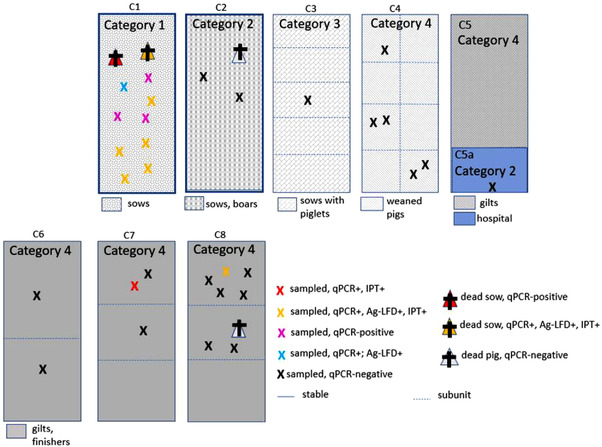
Schematic view of farm C with sampling units (C1, C2, C3, C4, C5, C5a, C6, C7, and C8) and results of testing
TABLE 2.
Assigned categories for sampling units and sampled animals (farm C)
| Stable identification | No. of sub‐units per stable | Assigned category for the sub‐unit | No. of pigs sampled |
|---|---|---|---|
| C 1 | 1 | 1 | 11 |
| C 2 | 1 | 2 | 3 |
| C 3 | 5 | 3 | 1 |
| C 4 | 8 | 4 | 5 |
|
C 5 C 5a |
1 1 |
4 2 |
0 1 |
| C 6 | 2 | 4 | 2 |
| C 7 | 3 | 4 | 3 |
| C 8 | 3 | 4 a | 8 |
| Total | 34 | ||
One dead pig was found during clinical inspection, thus after categorization of sub‐units according to Figure 1.
Stable 5 had two separate sections as shown in Figure 1; one for injured and sick pigs (C5a, hospital) and another for gilts and fattening pigs.
2.3. Scoring of clinical signs
The sampled animals were classified into three clinical categories: (i) inconspicuous: animals with no particular signs, apart from being less active; (ii) mild: animals with mild clinical signs, for example, with some visible skin lesions, but standing up when prompted; (iii) severe: moribund animals with high fever, with haemorrhagic lesions, not standing up when prompted. The dead animals are not included in any of three clinical categories.
2.4. Testing
On the farms for antigen detection in whole blood samples, the INgezim PPA CROM Ag LFD (11.ASF.K.42, Ingenasa, Madrid, Spain) was used. For ASF‐specific antibodies, we used the corresponding INgezim PPA CROM Ab LFD (11.PPA.K.41, Ingenasa, Madrid, Spain). Both tests were performed according to the manufacturer's instructions.
The samples were also tested at the National Reference Laboratory. For genome detection, quantitative PCR (qPCR) was performed with all EDTA and tissue samples after extraction of the viral DNA using the Nuclisens® Easymag® assay (bioMérieux, France) according to the producer's manual. The qPCR was performed according to the protocol of the European Union Reference Laboratory for ASF (Fernández‐Pinero et al., 2013). A heterologous control DNA—intype IC‐DNA (QIAGEN, Germany) was used to assess the quality of extracted DNA as described earlier (Hoffmann et al., 2006). Results were recorded as quantification cycle (Ct) values and further interpreted as positive or negative according to the obtained value.
For antibody detection, the commercially available Ingezim PPA Compac blocking ELISA kit (11.PPA.K.3, Ingenasa, Madrid, Spain) was used. In farm A, all sampled animals were tested for ASFV‐specific antibodies, while in farms B and C only the qPCR‐positive animals were tested. The test and the interpretation of the results were carried out according to the manufacturer's instructions.
Antibody tests were also carried out on all qPCR‐positive samples using IPT. The IPT was performed according to the protocol of the European Union Reference Laboratory for ASF (European Union Reference Laboratory for ASF, 2018). The reason for the additional use of the IPT was to detect low antibody titres that were not detected with the ELISA.
All results were plotted in simple sketches of the layout of the farms (Figures 2, 3, 4).
2.5. Statistical analysis
Sensitivity and specificity were calculated for qPCR and antigen LFD separately for each farm. Sensitivity was calculated as true positive/(true positive + false negative). Specificity was calculated as true negative/(true negative + false positive). Confidence intervals for sensitivity and specificity estimates were calculated using Epitools Epidemiological Calculators (Sergeant, 2018).
Spearman correlation analysis was performed using XLSTAT (Addinsoft, 2019) to assess the correlation between the results of different tests and the clinical score.
3. RESULTS
3.1. Test results
Table 3 gives an overview of the results by different categories, and Figures 2, 3, 4 show the recorded results for all three farms.
TABLE 3.
Overview of testing results by categories of farm sub‐units
| Category of farm sub‐unit | No. of tested animals | Ag‐LFD+ | qPCR+ Ct<18 | qPCR+ Ct>18 | Ab‐LFD+ | ELISA+ | IPT+ |
|---|---|---|---|---|---|---|---|
| Farm A | |||||||
| 1 | 21 | 3 | 2 | 3 | 2 | 2 | 3 |
| 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Farm B, unit B1 | |||||||
| 1 | 4 | 3 | 3 | 0 | 0 | 0 | 0 |
| 2 | 11 | 1 | 1 | 4 | 0 | 0 | 1 |
| 3 | 5 | 1 | 1 | 0 | 0 | 0 | 1 |
| Farm B, unit B2 | |||||||
| 3 | 15 | 1 | 2 | 4 | 0 | 0 | 4 |
| Farm C | |||||||
| 1 | 11 | 7 | 7 | 4 | 0 | 0 | 6 |
| 2 | 4 | 0 | 0 | 0 | 0 | nd | nd |
| 3 | 1 | 0 | 0 | 0 | 0 | nd | nd |
| 4 | 18 | 1 | 1 | 1 | 0 | 0 | 0 |
3.1.1 Farm A
A total of five pigs from A1 were found qPCR positive (Figure 2, Table 3). In A2, no qPCR positive pigs were detected despite the epidemiological link. Three of the qPCR positive pigs also reacted positively with the Ag‐LFD.
Three pigs were seropositive with the IPT, two of them were also positive with the Ab‐LFD and ELISA. One of these pigs was qPCR positive but negative with the Ag‐LFD (Table 3).
3.1.2 Farm B
In farm B, 15 pigs were found qPCR positive. Six of these pigs with Ct values below 15 also reacted positively with Ag‐LFD (Figure 3). There were no pigs that tested positive with ELISA or Ab‐LFD, while six animals tested IPT positive (Table 3).
According to the results, all stables and both farm units were affected.
3.1.3 Farm C
A total of 13 pigs tested qPCR positive. Eight of these pigs also tested positive with Ag‐LFD (Figure 4). There were no pigs that tested positive with ELISA or Ab‐LFD, while six animals tested IPT positive (Table 3).
According to the results, ASF‐infected pigs were located in C1 (category 1), C7, and C8 (both category 4).
3.2. Sensitivity and specificity of LFD compared to qPCR
Out of 87 animals tested with Ag‐LFD, 17 reacted positive. By qPCR, 34 of 87 pigs tested positive. All animals showed mild to severe clinical signs. The median Ct value was 17. The Ct values of the samples that were positive for Ag‐LFD ranged from Ct 14 to 18 (Figure 5).
FIGURE 5.
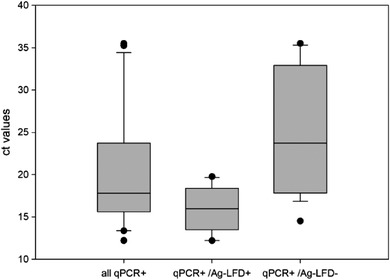
Comparison of Ct‐values for all tested blood samples, samples that were qPCR and Ag‐LFD positive and samples that were qPCR positive but Ag‐LFD negative. The boundaries of the boxes indicate the 25th and 75th percentiles, and the line within the box marks the median
The relative sensitivity of Ag‐LFD compared to PCR was 50% (CI 34.66%).
None of the qPCR‐positive samples with a Ct value of >20 was detected as positive by the Ag‐LFD (Figure 5). Samples that gave negative results in the qPCR were also negative in the Ag‐LFD. The relative specificity was thus 100%.
With a single exception (sampling unit C7), all sampling units that were positive by qPCR (n = 9) were also positive by Ag‐LFD (n = 8).
Two animals tested seropositive with the Ab‐LFD. The results were confirmed in the laboratory with the ELISA test. Thirteen samples which reacted positive in the IPT were not detected with the Ab‐LFD and the ELISA. At the same time, all antibody positive animals were also qPCR positive. Since the number of samples showing ASFV‐specific antibodies was low (n = 2), relative specificity and sensitivity could not be calculated for the Ab‐LFD.
Whisker boundaries indicate minimum and maximum values.
3.3. Ag‐LFD / Ab‐LFD and laboratory test results versus clinical signs
Comparison of the Ag‐LFD results with the clinical score of the sampled pigs showed that most animals with advanced or severe clinical signs were tested positive. However, three pigs with mild clinical signs were also Ag‐LFD positive. Neither Ag‐LFD nor qPCR gave positive results in inconspicuous pigs (Tables S1 and S2, Figure 6). Positive antibody results were only found in pigs with clinical signs (Table S1). The results of correlation analysis for the different ASF tests and the ASF clinical scores showed significant correlations with Ab‐LFD and ELISA, Ag‐LFD and clinical score, and qPCR and clinical score (Table 4).
FIGURE 6.
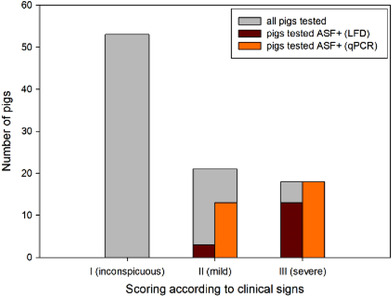
Number of pigs tested positive for African swine fever (ASF) (qPCR, Ag‐LFD) depending on clinical score of sampled pigs
TABLE 4.
Spearman correlation matrix for different African swine fever (ASF) test results and ASF clinical score
| Variables | Ag‐LFD | Ab‐LFD | qPCR | ELISA | IPT | Clinical score |
| Ag‐LFD | 1 | 0.008 | 0.246 | 0.008 | 0.271 | 0.531 |
| Ab‐LFD | x | 1 | 0.065 | 1.000 | 0.296 | 0.227 |
| qPCR | x | x | 1 | 0.065 | 0.218 | 0.469 |
| ELISA | x | x | x | 1 | 0.296 | 0.227 |
| IPT | x | x | x | x | 1 | 0.201 |
| Clinical score | x | x | x | x | x | 1 |
Note: Values in bold are different from 0 with a significance level p < .05.
4. DISCUSSION
The measures to be taken following confirmation of ASF in domestic pig holdings are quite explicit according EU legislation. After confirmation of the disease outbreak, investigations must be carried out, obtaining information on the movement of animals, persons, products, vehicles, and so on that may have spread the pathogen during the period in question prior to notification. Some ASF farms are detected at a very early stage of infection when only few animals are infected, while other farms are found ASF positive after weeks of virus circulation within the farm with many pens and sub‐units affected (Lamberga et al., 2020). To assess the virus spread within the holding and to other holdings, it is important to have an overview of virus circulation within the farm. Assessing the spread of the virus within a farm gives also reliable information about the high‐risk period, that is, the estimated time that ASF has been on the farm before detection. For that purpose, sampling and testing of animals from different sub‐units of the affected farm are necessary. This is a challenging task, especially under the time pressure of mandatory control measures.
Targeted sampling of sick or dead animals instead of random sampling is nowadays recommended to detect ASF‐infected animals (Busch et al. 2021; EFSA, 2021). We used a simple and straightforward approach to divide farms into sub‐units and clearly categorize them for targeted sampling in order to identify the units where ASF virus is likely circulating and units that are likely not to be infected. However, on small farms, where a categorization is difficult due to closer contact of the pigs among each other, testing all individual pig is possible, as we did on farm A.
The clinical score helped to better understand and interpret the LFD test results. In particular, we could correlate the test results to the clinical categories (inconspicuous, mild and severe). None of the inconspicuous pigs (n = 53) that were tested because they had contact to suspicious pigs, reacted positively neither in Ag‐LFD nor qPCR, whereas severely diseased animals had a significantly high chance of being detected by Ag‐LFD. The progression of clinical disease correlates with the viral load in the blood. Samples from mildly diseased pigs with qPCR Ct values above 20 were negative when tested with Ag‐LFD. These field results are in line with experimental studies (Pikalo et al., 2021). A recent study found a high positivity rate for Ag‐LFD during the acute phase of disease between days 4 and 7 post‐infection (dpi) when testing EDTA‐blood as sample matrix or up to 10 days when serum is used as the sample matrix (Deutschmann et al., 2022; Pikalo et al., 2021). Therefore, it should be kept in mind that the sensitivity of Ag‐LFD limits its use to acutely sick animals 4–10 dpi. The same authors also found that at 10 dpi antibodies can be detected by IPT, ELISA, or Ab‐LFD. In our study, IPT results showed a higher positivity rate than ELISA and Ab‐LFD, with the latter two showing similar sensitivity and a significant high correlation. Ag‐LFD can help to quickly identify infected pigs; consequently, stables or sub‐units with infected pigs can be immediately confined. However, due to the lower sensitivity compared to standard laboratory tests, LFDs can only be a complementary tool in outbreak investigations on farms where ASF has already been confirmed, but not for outbreak confirmation or for demonstration of the disease freedom.
According to EU legal requirements, all pigs on the affected holding must be culled, although derogations for culling are theoretically possible (EC, 2019). Application of derogation of killing of animals is a very complex question, where several epidemiological and also financial aspects have to be evaluated. A veterinary risk management that takes into account the characteristics of the disease, the husbandry system, biosecurity, and contacts between animal groups has to be considered within other measures (Costard et al., 2022). An important pre‐condition is to obtain a clear picture of virus circulation within the farm. If the disease is detected early, there is a probability that large parts of the farm are not yet affected. In this study, we have demonstrated how it might be feasible to get an overview of the ASF virus spread in affected farms. Farm A, due to its small size and categorization results would not be a farm where partial culling could be envisaged. In farm B, derogation from culling could not be applied as all stables were affected. In farm C, several stables appeared not to be affected yet by ASF and therefore partial culling of the affected units only and closely monitoring the situation in the other units by constantly testing sick animals might have been an option, if biosecurity and legal requirements were met to the best of knowledge.
5. CONCLUSIONS
The targeted selection of pigs to be sampled based on a careful clinical inspection followed by LFD testing, as well as plotting the results in the sketch of the farm is efficient and gives an initial rapid overview of the spread of the virus within the farm.
Targeted sampling of sick and dead animals is highly effective for identifying infected animals. The sampling strategy we have presented allows efficient and targeted identification of units where ASF virus is actively circulating.
The LFD test proved to be suitable for use in the field as part of outbreak investigations when samples from clinically suspect and dead animals are examined. Due to its limited sensitivity, the LFD test neither can be used as a diagnostic tool for primary detection and confirmation of disease nor for demonstrating freedom from infection.
It should be further investigated and discussed how the derogation from culling could be implemented in practice to save the animals in non‐infected units of large farms and to continue operation of the business.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Kristīne Lamberga, Klaus Depner, and Laura Zani. Methodology: Kristīne Lamberga, Klaus Depner, Edvīns Oļševskis, Mārtiņš Seržants, Anja Globig, and Sandra Blome. Farm investigations: Kristīne Lamberga, Edvīns Oļševskis, and Mārtiņš Seržants. Laboratory investigations: Santa Ansonska and Žanete Šteingolde. Formal analysis, data curation, and graphs: Kristīne Lamberga, Anja Globig, Laura Zani, and Arvo Viltrop. Writing—original draft preparation: Kristīne Lamberga. Writing and editing: Anja Globig, Klaus Depner, Laura Zani, and Santa Ansonska. Review: Sandra Blome, Edvīns Oļševskis, Mārtiņš Seržants. Supervision: Arvo Viltrop, Aivars Bērziņš, and Klaus Depner. All authors have read and agreed to the published version of the manuscript.
ETHICS STATEMENT
The outbreak investigation was based on the national and EU legislation and all ethical requirements were followed in the study.
Supporting information
Supplementary table 1: Number of positive test results in relation to the categorization according clinical signs.
Supplementary table 2. Proportion agreement of Ag‐LFD test results with qPCR test results among animals with different clinical status
ACKNOWLEDGEMENTS
We thank the farm owners, their veterinarians, and workers from the investigated farms for their support and collaboration during the outbreak investigation. The authors acknowledge Svetlana Cvetkova and Laura Krivko for the careful laboratory work. Furthermore, we are grateful for the critical and careful review by autonomous reviewer. LFD and publication were funded by the FLI ASF research network.
Lamberga, K. , Depner, K. , Zani, L. , Oļševskis, E. , Seržants, M. , Ansonska, S. , Šteingolde, Ž. , Bērziņš, A. , Viltrop, A. , Blome, S. , & Globig, A. (2022). A practical guide for strategic and efficient sampling in African swine fever‐affected pig farms. Transboundary and Emerging Diseases, 69, e2408–e2417. 10.1111/tbed.14582
DATA AVAILABILITY STATEMENT
The original data used for the analysis can be obtained from the author after approval from the responsible institution in Latvia.
REFERENCES
- Addinsoft . (2019). Xlstat statistical and data analysis solution, Addinsoft. [Google Scholar]
- Berthe, F. (2020). The global impact of ASF. OIE Bull 10.20506/bull.2020.1.3119 [DOI]
- Blome, S. , Franzke, K. , & Beer, M. (2020). African swine fever—A review of current knowledge. Virus Research, 287, 198099. 10.1016/j.virusres.2020.198099 [DOI] [PubMed] [Google Scholar]
- Busch, F. , Haumont, C. , Penrith, M.‐L. , Laddomada, A. , Dietze, K. , Globig, A. , Guberti, V. , Zani, L. , & Depner, K. (2021). Evidence‐based African swine fever policies: Do we address virus and host adequately? Frontiers in Veterinary Science, 8, 637487. 10.3389/fvets.2021.637487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai, S. , Loi, F. , Coccollone, A. , Cocco, M. , Falconi, C. , Dettori, G. , Feliziani, F. , Sanna, M. L. , Oggiano, A. , & Rolesu, S. (2017). Evaluation of a commercial field test to detect african swine fever. Journal of Wildlife Diseases. 10.7589/2016-05-112 [DOI] [PubMed]
- Carlson, J. , Zani, L. , Schwaiger, T. , Nurmoja, I. , Viltrop, A. , Vilem, A. , Beer, M. , & Blome, S. (2017). Simplifying sampling for African swine fever surveillance: Assessment of antibody and pathogen detection from blood swabs. Transboundary and Emerging Diseases, 65, e165–e172. 10.1111/tbed.12706 [DOI] [PubMed] [Google Scholar]
- Chenais, E. , Depner, K. , Guberti, V. , Dietze, K. , Viltrop, A. , & Ståhl, K. (2019). Epidemiological considerations on African swine fever in Europe 2014–2018. Porcine Health Management, 5(1), 6. 10.1186/s40813-018-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costard, S. , Perez, A. M. , Zagmutt, F. J. , Pouzou, J. G. , & Groenendaal, H. (2022). Partitioning, a novel approach to mitigate the risk and impact of African Swine Fever in affected areas. Frontiers in Veterinary Science, 8, 812876. 10.3389/fvets.2021.812876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschmann, P. , Pikalo, J. , Beer, M. , & Blome, S. (2022). Lateral flow assays for the detection of African swine fever virus antigen are not fit for field diagnosis of wild boar carcasses. Transboundary and Emerging Diseases, 69, 2344–2348. 10.1111/tbed.14248 [DOI] [PubMed] [Google Scholar]
- EC. (2002). “Council directive 2002/60/EC of 27 June 2002 laying down specific provisions for the control of African swine fever and amending Directive 92/119/EEC as regards Teschen disease and African swine fever.” Council of the European Union. [Google Scholar]
- EC. (2003). “Commission decision of 26 May 2003 approving an African swine fever diagnostic manual (2003/422/EC).” The Commission of the European Communities. [Google Scholar]
- EC. (2016). “Regulation (EU) 2016/429 OF the european parliament and of the council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’) Article 57.” Council and Parliament of the European Union. [Google Scholar]
- EC. (2018). “Working document Strategic approach to the management of African Swine Fever for the EU”.
- EC. (2019). “Commission delegated regulation (EU) 2020/687 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and the Council, as regards rules for the prevention and control of certain listed diseases.” The European Commission. [Google Scholar]
- EFSA . (2020). Epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019), European Food Safety Authority, 18(1), e05996. 10.2903/j.efsa.2020.5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA . (2021). Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: African Swine Fever. EFSA Journal, 19(1), e06402. 10.2903/j.efsa.2021.6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Union Reference Laboratory for ASF. (2018). “Standard operating procedure for the detection of antibodies against African swine fever by indirect immunoperoxidase technique”.
- Fernández‐Pinero, J. , Gallardo, C. , Elizalde, M. , Robles, A. , Gómez, C. , Bishop, R. , Heath, L. , Couacy‐Hymann, E. , Fasina, F. O. , Pelayo, V. , Soler, A. , & Arias, M. (2013). Molecular diagnosis of African Swine Fever by a new real‐time PCR using universal probe library. Transboundary and Emerging Diseases, 60(1), 48–58. 10.1111/j.1865-1682.2012.01317.x [DOI] [PubMed] [Google Scholar]
- Gallardo, C. , Fernandez‐Pinero, J. , & Arias, M. (2019). African swine fever (ASF) diagnosis, an essential tool in the epidemiological investigation. Virus Research, 271, 197676. 10.1016/j.virusres.2019.197676 [DOI] [PubMed] [Google Scholar]
- Gallardo, C. , Soler, A. , Nurmoja, I. , Cano‐Gómez, C. , Cvetkova, S. , Frant, M. , Woźniakowski, G. , Simón, A. , Pérez, C. , Nieto, R. , & Arias, M. (2021). Dynamics of African swine fever virus (ASFV) infection in domestic pigs infected with virulent, moderate virulent and attenuated genotype II ASFV European isolates, Transboundary and Emerging Diseases, 58, 2826–2841. 10.1111/tbed.14222 [DOI] [PubMed] [Google Scholar]
- Gallardo, C. , Soler, A. , Rodze, I. , Nieto, R. , Cano‐Gómez, C. , Fernandez‐Pinero, J. , & Arias, M. (2019). Attenuated and non‐haemadsorbing (non‐HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transboundary and Emerging Diseases, 66, 1399–1404. 10.1111/tbed.13132 [DOI] [PubMed] [Google Scholar]
- Gonzales, W. , Moreno, C. , Duran, U. , Henao, N. , Bencosme, M. , Lora, P. , Reyes, R. , Núñez, R. , De Gracia, A. , & Perez, A. M. (2021). African swine fever in the Dominican Republic. Transboundary and Emerging Diseases, 68, 3018–3019. [DOI] [PubMed] [Google Scholar]
- Hoffmann, B. , Depner, K. , Schirrmeier, H. , & Beer, M. (2006). A universal heterologous internal control system for duplex real‐time RT‐PCR assays used in a detection system for pestiviruses. Journal of Virological Methods, 136(1–2), 200–209. 10.1016/j.jviromet.2006.05.020 [DOI] [PubMed] [Google Scholar]
- Lamberga, K. , Oļševskis, E. , Seržants, M. , Bērziņš, A. , Viltrop, A. , & Depner, K. (2020). African swine fever in two large commercial pig farms in Latvia—Estimation of the high risk period and virus spread within the farm. Veterinary Sciences, 7(3), 105. 10.3390/vetsci7030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oļševskis, E. , Schulz, K. , Staubach, C. , Seržants, M. , Lamberga, K. , Pūle, D. , Ozoliņš, J. , Conraths, F. J. , & Sauter‐Louis, C. (2020). African swine fever in Latvian wild boar—A step closer to elimination. Transboundary and Emerging Diseases, 67(6), 2615–2629. 10.1111/tbed.13611 [DOI] [PubMed] [Google Scholar]
- Pikalo, J. , Deutschmann, P. , Fischer, M. , Roszyk, H. , Beer, M. , & Blome, S. (2021). African swine fever laboratory diagnosis—Lessons learned from recent animal trials. Pathogens, 10(2), 177. 10.3390/pathogens10020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikalo, J. , Schoder, M. E. , Sehl, J. , Breithaupt, A. , Tignon, M. , Cay, A. B. , Gager, A. M. , Fischer, M. , Beer, M. , & Blome, S. (2020). The African swine fever virus isolate Belgium 2018/1 shows high virulence in European wild boar. Transboundary and Emerging Diseases, 67(4), 1654–1659. 10.1111/tbed.13503 [DOI] [PubMed] [Google Scholar]
- Plowright, W. , Thomson, G. R. , & Neser, J. A. (1994). African swine fever. In J. A. W., Coetzer , G. R., Thomson , & Tustin R. C. (Eds.), Infectious diseases of livestock, with special reference to southern Africa (vol. 1, pp. 568–599). Oxford University Press. [Google Scholar]
- Sastre, P. , Gallardo, C. , Monedero, A. , Ruiz, T. , Arias, M. , Sanz, A. , & Rueda, P. (2016). Development of a novel lateral flow assay for detection of African swine fever in blood. BMC Veterinary Research, 12, 206. 10.1186/s12917-016-0831-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant, E. S. G. (2018). Epitools epidemiological calculators. Ausvet. http://epitools.ausvet.com.au
- Viltrop, A. , Boinas, F. , Depner, K. , Jori, F. , Kolbasov, D. , Laddomada, A. , Ståhl, K. , & Chenais, E. (2021). African swine fever epidemiology, surveillance and control. In Iacolina, L. M.‐L. Penrith, S. Bellini, E. Chenais, F. Jori, M. Montoya, K. Stahl, & D. Gavier‐Widen (Eds.), Understanding and combatting African swine fever. Wageningen Academic Publishers, 230‐263. 10.3920/978-90-8686-910-7 [DOI] [Google Scholar]
- World Organization for Animal Health (OIE). (2021). Global Situation of African swine fever. Report No. 47: 2016 –2020 [https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/ASF/Report_47_Global_situation_ASF.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1: Number of positive test results in relation to the categorization according clinical signs.
Supplementary table 2. Proportion agreement of Ag‐LFD test results with qPCR test results among animals with different clinical status
Data Availability Statement
The original data used for the analysis can be obtained from the author after approval from the responsible institution in Latvia.


