Abstract
Previous studies have reported that human vascular endothelial cells lack the membrane-bound lipopolysaccharide (LPS) receptor, CD14 (mCD14). By optimizing assay conditions, including the selection of anti-CD14 monoclonal antibody, we now demonstrate that human umbilical vein endothelial cells (HUVEC) express CD14 on the cell surface. Single-passage HUVEC showed approximately 20 times less expression of CD14 than monocytes. Interestingly, there was significant loss of surface CD14 expression with increasing numbers of culture passages. Evidence for synthesis of CD14 by HUVEC was provided by the finding that l-[35S]methionine was incorporated into CD14. In addition, the expression of CD14 on HUVEC was upregulated by LPS, lysophosphatidic acid, and tissue culture supplements, and this upregulation was dependent on protein synthesis. Furthermore, the results imply that mCD14 is required for LPS-induced activation of endothelial cells in the absence of serum and that it acts in concert with serum factors (soluble CD14). Our results provide evidence that CD14 is expressed by endothelial cells and suggest that the previous inability to observe expression of this molecule has been due to culture and staining conditions. This finding has important implications for the understanding of the mechanisms by which LPS stimulates endothelial cells and the management of sepsis caused by gram-negative bacteria.
CD14 was first described as a myeloid differentiation antigen in 1981 (8). It is a 55-kDa glycoprotein with multiple leucine-rich repeats and is encoded on chromosome 5 (5q) together with growth factors, such as granulocyte macrophage colony stimulating factor, and growth factor receptors, such as endothelial growth factor receptor and platelet-derived growth factor receptor (2, 3). CD14 has been identified as a receptor for complexes of lipopolysaccharide (LPS) and LPS-binding protein but it also binds to other bacterial products (8, 14). The LPS-binding region within the CD14 molecule is remarkably conserved across species with a high degree of gene sequence homology, and it has therefore been suggested that CD14 is a pattern recognition receptor (9). CD14 is linked to the cell membrane by a glycosylphosphatidylinositol anchor and possesses no transmembrane domain (25). It is thought to act as a lipid transfer protein, passing LPS on to a putative transmembrane signaling receptor (29). Recently two likely candidates for this receptor, human TOLL-like receptor 2 and moesin, have been described (20, 28). However, blockade of CD14 inhibits LPS-mediated effects almost completely, suggesting that CD14 is required for signaling by the transmembrane receptor.
Interestingly, while endothelial cells are sensitive to low concentrations of LPS, no evidence has been presented to suggest that CD14 is expressed by these cells and indeed it has been generally accepted that endothelial cells do not express CD14 (1). Soluble CD14 (sCD14), present in normal serum at 2 to 6 μg/ml, is thought to facilitate LPS-induced activation of endothelial cells (4). However, CD14-negative cells like Chinese hamster ovary fibroblasts (CHO) are unresponsive to LPS even in the presence of serum, and only when transfected with CD14 do these cells become responsive to LPS (5). Also, CD14-negative murine pre-B cells (70Z/3), which are unresponsive to low concentrations of LPS (0.1 ng/ml) even in the presence of serum, when transfected with CD14 show responses to LPS (15). Surprisingly, anti-CD14 antibodies block endothelial cell activation by LPS even in the absence of serum (24), an observation inconsistent with the concept that endothelial cells do not express CD14.
We now demonstrate that vascular endothelial cells synthesize and express CD14 on the cell surface and present evidence that the endothelial membrane-bound CD14 (mCD14) is functional in LPS-mediated cell activation.
MATERIALS AND METHODS
Anti-CD14 antibodies.
All anti-CD14 antibodies were monoclonal murine anti-human antibodies. MY4 was purchased from Coulter Corporation (Miami, Fla.), TÜK4 was from DAKO (Glostrup, Denmark), 2D-15C was from Silenus-AMRAD Biotech (Melbourne, Australia), Leu-M3 was from Becton Dickinson (San Jose, Calif.), and RMo52 was from Immunotech (Marseille, France). All antibodies achieved their optimal staining at a dilution of 1:100 and were used at a dilution of 1:50. Isotype-matched control antibodies were obtained from Becton Dickinson and DAKO, and all were used at a dilution of 1:50.
HUVEC.
Human umbilical vein endothelial cells (HUVEC) were prepared essentially as described previously (11). Human umbilical cords were collected immediately after delivery and were stored in sterile containers at 4°C. The veins were cannulated, washed with Hanks balanced salt solution (HBSS; Cell Image, Adelaide, Australia), and filled with collagenase (37°C; type II; 0.4 mg/ml; activity, 219 U/mg; Worthington, Freehold, N.J.). After incubation in a water bath (37°C, 2 min), the contents of each vein were collected, followed by one additional wash with HBSS to remove remaining cells. Cells were centrifuged (400 × g, 5 min), the supernatant was discarded, and the pellet was resuspended in RPMI 1640 culture medium (Cell Image) containing 20% pooled, heat-inactivated human group AB serum supplemented with penicillin (80 U/ml), streptomycin (80 μg/ml) and l-glutamine (3.2 mmol/liter) (all from ICN Pharmaceuticals, Costa Mesa, Calif.). Cells were grown to confluence in 75-cm2 culture flasks (Corning, Cambridge, Mass.) which were precoated with 0.2% gelatin (TRACE, Biosciences, Melbourne, Australia). Endothelial cells were identified by their characteristic monolayer cobblestone appearance and positive staining for factor VIII-related antigen using peroxidase-conjugated rabbit immunoglobulin G (IgG) antibody to human von Willebrand factor (DAKO, Carpinteria, Calif.) and 3,3′-diaminobenzidine. Prior to experiments, cells were harvested from culture flasks with trypsin (0.05 mg/ml; ICN)-EDTA (0.02 mg/ml; Sigma, St. Louis, Mo.).
Mononuclear leukocytes.
Mononuclear cells were isolated from the blood of healthy volunteers by centrifugation of blood on Hypaque-Ficoll medium of a density of 1.114 as described previously (11). The cells were >96% pure and >99% viable by trypan blue exclusion test. Cells were resuspended in RPMI 1640 and processed within 1 h of preparation.
Flow cytometric analysis.
Endothelial cells were plated at 4 × 105 cells per well in six-well plates (9.62 cm2; Linbro, ICN Biochemicals, Aurora, Ohio) or 2.5 × 106 per dish into 10-cm tissue culture dishes (Becton Dickinson). Cells were treated with varying concentrations of agonists and for the times indicated in the figure legends. LPS from Escherichia coli 0127:B8, chromatographically purified by gel filtration, phorbol-12-myristate 13-acetate (PMA), endothelial cell growth supplement (ECGS) and lysophosphatidic acid (LPA) were purchased from Sigma, St. Louis, Mo. Fetal calf serum (FCS) was purchased from TRACE, Biosciences. At the end of the treatment period the cells were washed twice with warm HBSS and were enzymatically removed with trypsin-EDTA. The cell suspension was washed with ISOTON II solution (2 ml, 4°C; Coulter Electronics, Brookvale, NSW, Australia) and was resuspended in 10 μl of human IgG for nonspecific blocking (Intragam [CSL, Parkville, Australia]; 60 mg/ml; 4°C, 20 min). Primary antibody solution (50 μl; dilution, 1:50) was added (4°C, 30 min), and after two washes with ISOTON II (2 ml, 4°C) the secondary antibody was added (rabbit anti-mouse antibody, R-phycoerythrin [PE] conjugated [DAKO]; 50 μl, 1:100, 4°C, 30 min). After two washes (ISOTON II; 2 ml, 4°C), cells were resuspended in ISOTON II (200 μl) and the fluorescence intensity of each cell population was analyzed immediately by flow cytometry on a FACScan (Becton Dickinson). Cells were assessed for viability according to their positive or negative staining with 7-amino-actinomycin D (7-AAD [Sigma]; 0.2%), and 10,000 events from within the viable, 7-AAD-unstained cell population per sample were counted. The data were processed using Lysis II software (Becton Dickinson), and the median fluorescence intensity (MFI) of isotype-matched negative controls was subtracted from the MFI of samples with anti-CD14.
In the case of assessing expression of CD14 on monocytes, the mononuclear cell fraction was subjected to flow cytometric analysis after treatment with anti-CD14 antibodies. Both lymphocytes and monocytes were identified on forward and side scatter. The lymphocyte population was negative for CD14 as expected, and the intensity of fluorescence of the brightly positive monocytes was used for comparison with HUVEC.
Immunofluorescence microscopy.
(i) For cells in culture, HUVEC were grown on chamber slides (Lab-Tek; Nunc, Naperville, Ill.), washed with phosphate-buffered saline (Cell Image), and fixed (37% formaldehyde in phosphate-buffered saline and 2% Triton X-100 from Ajax Chemicals, Auburn, NSW, Australia). After blocking (human IgG, 20 min), two-step staining was performed with MY4 (1:50) primary antibody and fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse polyclonal secondary antibody (1:100; Silenus, Melbourne, Australia). (ii) For cord tissue, a section of cord was snap-frozen in 20% ethanol (−170°C), cut into 10-μm-thick sections using a cryomicrotome, and transferred onto slides. After air drying, tissue samples were stained as described above. Samples were photographed with a UV fluorescence microscope (Leitz, Wetzlar, Germany) using Kodak 100 ASA slide film.
l-[35S]methionine incorporation studies.
HUVEC were grown to confluence in 10-cm tissue culture dishes. Cells were either maintained in normal culture medium (unstimulated) or in medium containing 40% FCS (stimulated). l-[35S]methionine (25 μCi) was added to each dish into a final volume of 10 ml (2.5 × 106 cells). After 24 h the cells were washed once on ice, mechanically removed from the dishes, and lysed in constant motion at 4°C for 4 h with 250 μl of lysis buffer (20 mM HEPES [pH 7.4], 0.5% [vol/vol] Nonidet P-40, 100 mM NaCl, 1 mM EDTA, 2 mM Na3VO4, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 10 μg each [per ml] of leupeptin, aprotinin, pepstatin A, and benzamidine). Triton X-100 at a concentration of 0.5 mg per mg of sample protein was added, and membranes were disrupted by sonication. The samples were centrifuged at 100,000 × g (4°C, 1 h) and then transferred onto 15 μl of protein A Sepharose beads (Sigma) for preclearance at constant motion (4°C, 45 min). Samples were centrifuged (30 s, 4°C, 10,000 × g), and the supernatants were transferred into two new tubes per sample. Either anti-IgG2b or anti-CD14 antibody (MY4) was added at 12 μl per tube. The samples were kept at gentle agitation (4°C, 2 h) and were subsequently transferred onto 12 μl of protein A Sepharose beads. Again, samples were incubated with gentle agitation (4°C, 1 h) and then centrifuged (4°C, 10,000 × g). For determination of the amount of radioactivity, the supernatant was discarded and the beads were resuspended in scintillation fluid (6 ml; Fisher Chemicals, Loughborough, England) and placed into scintillation vials. Radiation as counts per minute was recorded in a β-counter (LKB Wallac 1409-411 liquid scintillation counter; Turku, Finland) over a period of 10 min per sample, and the results of the IgG2b control precipitations were subtracted from the results obtained with MY4.
For identification of synthesized CD14, the molecular weight of the radiolabeled and immunoprecipitated protein was determined. The beads were resuspended in 40 μl of Laemmli buffer and boiled (100°C, 10 min). The samples were centrifuged (10,000 × g, 1 min), the supernatants were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE), and the protein was transferred to nitrocellulose (Schleicher & Schuell, Dassel, Germany).
Bands were visualized by treatment with an autoradiography enhancer (NEN Research Products, Boston, Mass.) and incubation with X-ray film (Kodak, Melbourne, Australia) for 24 h at −70°C. The molecular weight was determined by comparison with the position of unlabeled markers. The films were scanned using an ImageQuant scanner and ImageQuant software (version 3.3; Molecular Dynamics, Sunnyvale, Calif.).
Measurement of E-selectin.
HUVEC were plated into 96-well plates (gelatin precoated; 0.38 cm2; Linbro) at 5 × 104 cells per well. Human recombinant tumor necrosis factor alpha (TNF-α) was a gift from G. R. Adolf, Ernst Boehringer Institute, Vienna, Austria. The activity was 6 × 107 U/mg, and the preparation was >99% pure. The endotoxin contamination was less than 0.125 endotoxin U/ml as assessed by the Limulus lysate assay. After treatments, the wells were washed twice with 0.2 ml of 0.1% bovine serum albumin (BSA [CSL]) per well and were fixed overnight with 0.025% glutaraldehyde (0.2 ml/well; Probing and Structure, Queensland, Australia). The monolayers were washed twice with BSA and incubated with blocking buffer (0.1% BSA and 100 mM glycine) at 20°C for 2 h. The enzyme-linked immunosorbent assay (ELISA) was performed with three washes with 0.2 ml of 0.1% BSA per well between each step. HUVEC were incubated with 50 μl of primary monoclonal antibody (anti-CD62E MAb, 1:1,000 [Pharmingen] or IgG1 control MAb [DAKO]) per well and 70 μl of secondary horseradish peroxidase-conjugated antibody (rabbit anti-mouse polyclonal antibody, horseradish peroxidase-conjugated, 1:1,000 [DAKO]) per well 1 h each at 37°C. Finally, 100 μl per well of enzyme substrate (0.55 mg of 2,2′-azino-bis[3-ethylbenzthiazoline sulfonate]/ml and 0.012% hydrogen peroxide in citrate-phosphate buffer, pH 4.2) were added. Color was developed until cell-alone wells gave a standardized absorbance reading at 410 nm using an ELISA plate reader (Dynatech MR 7000).
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM). Statistical significance was assessed either by the Student t test or analysis of variance for multiple comparisons or by the nonparametric Kruskal-Wallis test with Dunne's posttest if the data were not normally distributed. P values of less than 0.05 were regarded as statistically significant.
RESULTS
Expression of CD14 on HUVEC.
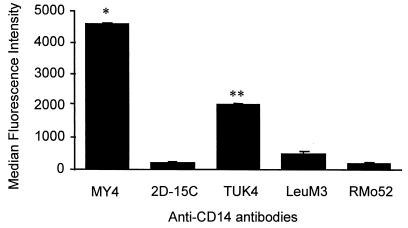
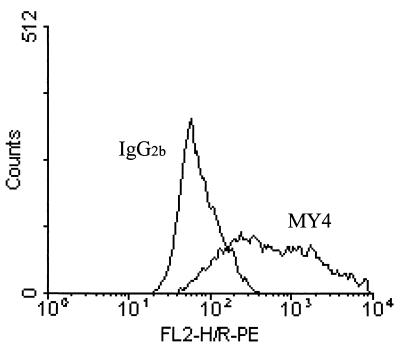
Assay conditions for detecting CD14 expression were examined using monocytes, since high expression of CD14 on these cells has been well established. To standardize the assay, five different anti-CD14 MAb were tested for their degree of staining of monocytes using PE-conjugated secondary antibody (Fig. 1). With optimal concentrations of these antibodies it was evident that MY4 was the most effective MAb for detection of CD14 expression, and it was used in all subsequent experiments unless stated otherwise. Endothelial cells treated with MY4 displayed a median fluorescence intensity (MFI) distinct from that of cells treated with the isotype-matched control antibody (Fig. 2). HUVEC grown to confluence on slides stained positive by indirect immunofluorescence microscopy using MY4 (Fig. 3), compared to isotype-matched controls, as did the endothelial layer of umbilical veins in situ (Fig. 3).
FIG. 1.
Monocytes were stained for indirect flow cytometry using different primary MAbs against CD14, and the secondary antibody was a rabbit anti-mouse R-PE-conjugated polyclonal antibody (1:100). The data represent the means ± SEM of duplicates of three independent experiments. Statistical analysis: ∗, MY4 versus 2D-15C, TÜK4, LeuM3, and RMo52, P < 0.001; ∗∗, TÜK4 versus 2D-15C, LeuM3, and RMo52, P < 0.001.
FIG. 2.
Expression of CD14 on endothelial cells. A typical histogram of HUVEC stained by the indirect fluorescence method illustrates the shift between cell populations incubated either with isotype control antibody (IgG2b) or anti-CD14 antibody (MY4).
FIG. 3.
MY4 stains HUVEC in culture and in situ. HUVEC were grown on slides and then fixed. Staining was performed with MY4 primary antibody (A) or the isotype-matched control (B) and FITC-conjugated rabbit anti-mouse polyclonal secondary antibody (magnification, ×100). (C) Cords were snap-frozen, cut, transferred onto slides, and stained as described above. The endothelial layer of the partially collapsed umbilical vein displayed bright fluorescence (magnification, ×10). Similarly, the negative control antibody for cord sections showed no staining of blood vessel lining, and no cell outline was visible under UV.
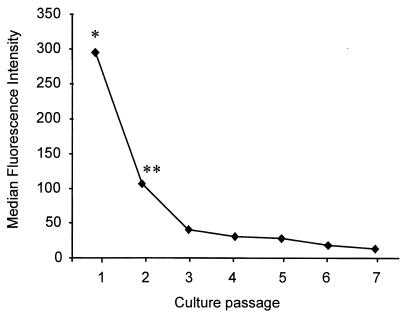
Loss of cell surface expression of CD14 during subculturing.
Further studies demonstrated that the ability of HUVEC to express CD14 on the surface was dependent on the number of passages the endothelial cells had been subjected to. When HUVEC were grown in culture medium containing FCS (10%), heparin (2.5%), and ECGS (150 μg/ml) and were passaged every 3 days, the cells rapidly lost CD14 with subculturing. By passage 3, CD14 expression was less than 14% of the expression of passage 1 cells. By passage 7, less than 7% was observed (Fig. 4). Thus, in subsequent experiments passage 1 cells were used unless otherwise stated.
FIG. 4.
Effect of passaging HUVEC on CD14 expression. HUVEC were grown in RPMI 1640 medium supplemented with FCS, heparin, and ECGS. Every 3 days the cells were passaged, at which point one-third of the cells were prepared for flow cytometric analysis. The data represent the means of duplicates of three experiments, each conducted with cells from a different cord. Statistical analysis: ∗, passage 1 versus all other passages, P < 0.01; ∗∗, passage 2 versus passage 3 to 7, P < 0.05.
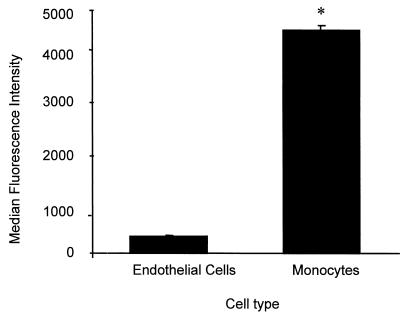
Comparison of the expression of CD14 on HUVEC and monocytes.
Peripheral blood monocytes from 12 healthy donors were examined under the same conditions as those for endothelial cells obtained from 12 different umbilical cords. These cells were then analyzed using identical flow cytometry settings. The mean difference (± the SEM) in MFI between the cells treated with anti-CD14 MAb and those treated with isotype-matched control antibody was 333 ± 28 for endothelial cells and 4,568 ± 121 for monocytes (Fig. 5).
FIG. 5.
Estimation of the number of CD14 on HUVEC by direct comparison to monocytes. The MFI of passage 1 endothelial cells from 12 different cords was compared to the MFI of monocytes (12 different preparations, each from a different healthy donor). Statistical analysis: ∗, P < 0.0001.
Modulation of CD14 expression on HUVEC.
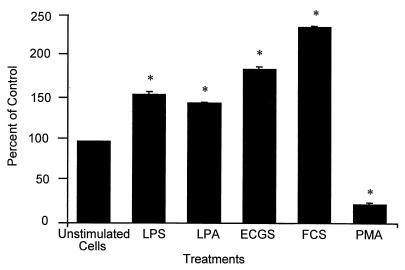
In further studies we examined whether CD14 expression on endothelial cells was amenable to alteration by cell agonists and tissue culture media supplements. The expression of CD14 was increased by LPS (1 ng/ml), LPA (2.5 μM), ECGS (150 μg/ml), and FCS (40%), whereas PMA (10 nM) reduced the expression (Fig. 6). When human AB-group serum at concentrations from 10 to 60% was used instead of FCS, there was no change in CD14 expression (data not shown). TNF-α and gamma interferon, which increase CD14 expression in neutrophils (19), had no effect on the expression of this molecule in endothelial cells (data not shown).
FIG. 6.
Modulation of surface expression of CD14 by endothelial cells by agonists, medium, and medium supplements. HUVEC were treated for 24 h with LPS (1 ng/ml), 2.5 μM LPA, 150 μg of ECGS/ml, FCS (40%), and 10 nM PMA and then were examined for CD14 expression by flow cytometry. The results are derived from three independent experiments and are expressed as the percentage of control; unstimulated cells with an MFI of 333 were defined as 100%. Statistical analysis: ∗, comparison with unstimulated cells, P < 0.001.
Effects of protein synthesis inhibition on CD14 expression.
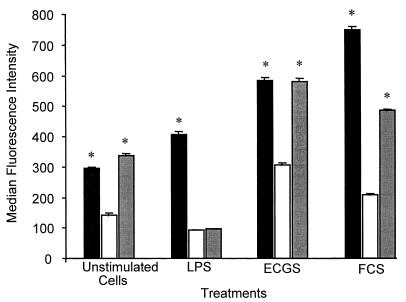
To exclude the possibility that CD14 expressed on endothelial cells originated from an exogenous source, HUVEC were incubated with various mediators in the presence or absence of the protein synthesis inhibitor cycloheximide (CHX). When cells were analyzed after 24 h, CHX (5 μg/ml) had caused a reduction in the expression of CD14 in unstimulated cells and in LPS-, ECGS-, and FCS-treated HUVEC (Fig. 7). After the cells were washed and maintained for a further 72 h in CHX-free culture medium, these effects were reversed with the exception of cells treated with LPS and CHX (Fig. 7). Control cells and LPS- or FCS-treated cells were >85% viable at the time of fluorescence-activated cell sorter analysis, and ECGS-treated cells were >95% viable as assessed by 7-AAD staining.
FIG. 7.
Effects of the protein inhibitor CHX on CD14 expression. Cells were treated with either medium only (black bars) or medium with CHX (white bars), stimulated with LPS, ECGS, or FCS (same concentrations as above), and then tested for expression of CD14 or were washed to remove CHX and recultured for 72 h (grey bars) and were then examined for CD14 expression. Statistical analysis: ∗, CHX versus non-CHX-treated or CHX-treated and washed cells, P < 0.001.
CD14 is synthesized by endothelial cells.
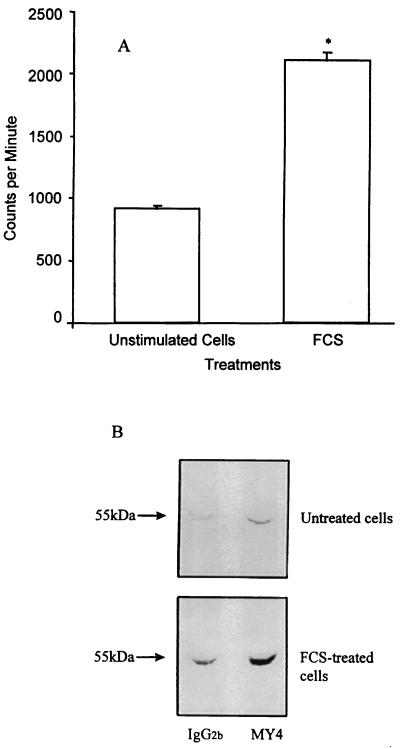
Evidence that HUVEC synthesize CD14 was derived from radiolabeling studies. Human CD14 consists of 356 amino acids, 6 of which are methionine (3). After incubation with l-[35S]methionine for 24 h, there was incorporation of label into CD14 in unstimulated HUVEC. Cells grown in FCS incorporated 2.3-fold more label (Fig. 8A), which corresponded to the 2.3-fold increase seen in upregulation of CD14 surface expression by FCS (Fig. 6 and 7). Immunoprecipitates were also subjected to SDS-PAGE, and radioactive bands of a molecular mass of 55 kDa were observed. The density of the bands was increased in cells stimulated with FCS (Fig. 8B).
FIG. 8.
Incorporation of radiolabeled amino acid into CD14. (A) HUVEC (2.5 × 106/assay) were incubated for 24 h with l-[35S]methionine, and the extent of incorporation into CD14 was determined by immunoprecipitation. The results of three experiments, each with cells from a different cord, are expressed as counts per minute (counts per minute values of isotype-matched controls, 1,400 for AB serum and 2,300 for FCS, have been subtracted). (B) The SDS-PAGE profile of the immunoprecipitates from l-[35S]methionine-labeled endothelial cell lysates confirmed their molecular mass to be ∼55 kDa. Statistical analysis: ∗, FCS-stimulated cells versus unstimulated cells, P < 0.0001.
Endothelial cell-associated CD14 has a functional role.
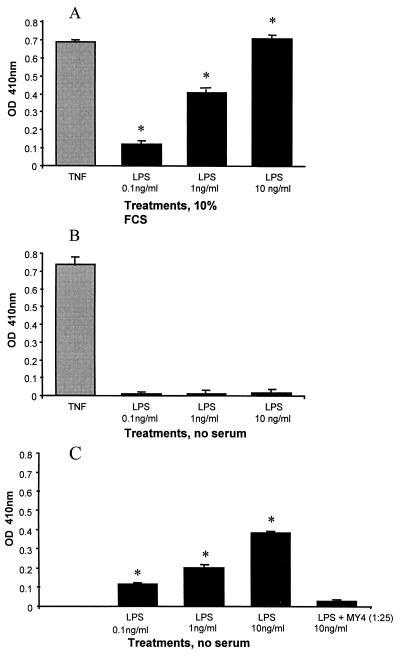
To examine the functional relevance of CD14 expressed by HUVEC in terms of cell activation, cultures were passaged for a total of seven times. When the MFI for CD14 had dropped to undetectable levels, these cells were grown to confluence in 96-well plates. After washing the plates three times, one half of the cells were kept in culture medium containing 10% serum, whereas the other half was kept in serum-free RPMI 1640 media. HUVEC were stimulated with either TNF (5 U/well) or LPS (0.1 to 10 ng/ml) for 6 h, and E-selectin expression was assessed by ELISA. Only the cells kept in 10% serum responded to LPS, which could be abrogated by pretreatment with MY4 (data not shown). In contrast, TNF-α caused a similar upregulation of E-selectin in these cells with and without serum present (Fig. 9A and B). In comparison, passage 1 HUVEC (with normal expression of CD14) showed upregulation of E-selectin in response to LPS in the absence of serum (Fig. 9C). This response was enhanced by the addition of serum (data not shown) and could be inhibited by the prior addition of MY4 (1:25) (Fig. 9C).
FIG. 9.
Activation of HUVEC by LPS via cell-associated CD14. As a measure of cell activation, upregulation of the adhesion molecule E-selectin was assessed by ELISA after 6 h of treatment. Results are expressed as the optical density (OD) at 410 nm. The data represent the means ± SEM of quadruplicates of three experiments, each conducted with cells from a different cord. (A, B) Passage 7 HUVEC, now lacking expression of CD14, were treated with TNF (5 U/well) or LPS at the concentrations indicated. Cells were treated either in medium containing 10% serum or under serum-free conditions. (C) Passage 1 HUVEC were treated with LPS under serum-free conditions. In addition, some cells were pretreated with MY4 antibody (dilution, 1:25) for 10 min before LPS was added. Statistical analysis: ∗, LPS treatment versus nontreated controls, P < 0.001.
DISCUSSION
Contrary to the generally held view that endothelial cells are CD14 negative, our data show that human endothelial cells express this molecule on their surface, both in vitro and in vivo. However, compared to monocytes, the number of molecules expressed on HUVEC was small. Assuming that each monocyte expresses between 29,000 and 42,000 CD14 molecules (22, 23), then the calculated number of CD14 per HUVEC (mean ± SEM) ranged from 2,114 ± 400 to 3,061 ± 400. Secondly, duplicates of the monocytes and HUVEC described above were stained with FITC-conjugated secondary antibody to enable their MFI to be compared to a normogram generated by analyzing FITC Quantum 26 beads (Flow Cytometry Standards Corp., San Juan, P.R.). These consist of five populations of beads of identical size, each of which is coated with different, defined numbers of fluorochromes. The estimates for the number of fluorochromes per cell for endothelial cells ranged from 2,000 to 3,000 (data not shown).
The CD14 molecules were of endothelial cell origin and were not passively acquired from serum. Firstly, their surface expression was up- and downregulated by various cell agonists. Interestingly, while there was a significant upregulation of cell surface CD14 by FCS, human serum had no effect on the expression of the molecule. ECGS, a bovine pituitary extract, was also capable of upregulating CD14 expression. Both FCS and ECGS were LPS-free and did not stimulate E-selectin expression in HUVEC. This suggests that FCS and ECGS may contain a bovine growth factor(s) that stimulates the synthesis of CD14 and is absent from human serum. Secondly, the expression of CD14 molecules was inhibited by CHX, the protein synthesis inhibitor. This inhibition was reversible under all conditions examined except when cells were treated with LPS and CHX. LPS- and CHX-treated cells displayed the same viability as control cells and grew in culture for an additional 72 h without detachment from the culture dish or from each other, and the reason for the inability of these cells to reexpress CD14 is currently not clear but is obviously of major interest. The fact that the cells do not recover even to basal expression suggests that exposure to LPS in the presence of protein synthesis inhibition leads to a long-term inhibition of expression of CD14. Whether this is at the level of transcription, mRNA stability, translation, or posttranslation events is at present being investigated. Thirdly, endothelial cells synthesized CD14, as shown by the incorporation of radiolabeled amino acid into the protein.
To address the discrepancy between our findings and previously published data, we examined the influence of culture conditions on the expression of CD14. Routine passaging of primary cultures of HUVEC or purchasing HUVEC from tissue culture laboratories at passages 3 to 5 is widely practiced. When we subjected cells to multiple passaging, these cells were indistinguishable from passage 1 HUVEC in a number of properties. The passaged cells displayed normal morphology and viability and responded to TNF to the same extent as passage 1 cells. However, unlike passage 1 cells, HUVEC that had undergone multiple passaging expressed extremely low amounts of CD14. While the reason for this reduction in CD14 is not clear, it is likely that this reduction constitutes the main explanation for the previously reported lack of CD14 on the endothelial cell surface. The choice of MAb against CD14 for the flow cytometric analysis may have been an additional limitation to the detection of CD14. Although we did not conduct comparisons between all available anti-CD14 antibodies, it is surprising how differently the five antibodies tested performed; only MY4 and TÜK4 produced a positive stain in passage 1 HUVEC.
Another distinction between passage 1 cells and cells that had undergone multiple passaging was the responsiveness to LPS under serum-free conditions. Cells subjected to several passages, which expressed very low amounts of CD14, failed to respond to LPS but their responsiveness could be restored by the addition of serum. Passage 1 cells responded to LPS in the absence of serum in a CD14-dependent manner, and this response could be augmented by serum. These data demonstrate that, despite the low numbers of CD14 on HUVEC compared to monocytes, mCD14 on HUVEC is functional. Furthermore, mCD14 is required for the response of HUVEC to LPS in the absence of serum and acts in concert with serum factors in the presence of serum. Previous studies in macrophages and neutrophils showed that sCD14 facilitates the rapid transfer and efficient presentation of LPS to mCD14 (9). This is likely to account for the augmentation of the LPS response in passage 1 mCD14-bearing endothelial cells. Taken together our data suggest that in LPS-induced activation of cells in vivo, mCD14 is essential and sCD14 promotes the response by presenting LPS to mCD14. This concept is further supported by results from transfection studies in which CD14-deficient cells, which were LPS unresponsive (to low and high LPS concentrations for CHO cells and to low concentrations for 70Z/3 cells) in the presence of serum factors, became highly sensitive to LPS when these cells were transfected with CD14 (5, 15).
As a pattern recognition receptor for bacterial products, CD14 plays an important part in innate immunity to bacteria (10, 17, 26). There is evidence that CD14 not only binds to LPS molecules but also to bacterial cell wall fragments and to whole bacteria (12, 13, 16). The recent report that CD14-deficient mice infected with gram-negative bacteria had a reduced dissemination of bacteria compared to normal mice is consistent with CD14 having a major role in the invasion of endothelial cells by bacteria (10). In the setting of sepsis with bacteremia there are direct contacts between bacteria and endothelial cells, and the presence of mCD14 on endothelial cells is likely to greatly enhance the activation of this tissue. Activated endothelial cells upregulate adhesion molecules, secrete proinflammatory, procoagulatory mediators and vasoactive substances, such as eicosanoids, nitric oxide, and endothelin (21). Bacterial infections caused by gram-negative organisms often result in sepsis, multiple organ failure, and death and remain an important clinical problem. In the face of emerging antibiotic resistance, advancing the understanding of the interaction of host cells with bacteria is pivotal for identifying new therapeutic strategies (18).
ACKNOWLEDGMENTS
We thank Mary Carli and Angie Pollard for technical assistance in the preparation of cords for immunofluorescence microscopy, Ian Bates from the Red Cross, South Australia, for the generous provision of AB serum, and the mothers and midwives of the Women's and Children's Hospital labor ward for providing umbilical cords.
This work was supported by the Australian Heart Foundation. Hubertus P. A. Jersmann is a recipient of the Reginald Walker scholarship of the University of Adelaide.
REFERENCES
- 1.Beekhuizen H, Blokland I, Corsel van Tilburg A J, Koning F, van-Furth R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J Immunol. 1991;147:3761–3767. [PubMed] [Google Scholar]
- 2.Ferrero E, Goyert S M. Nucleotide sequence of the gene encoding the monocyte differentiation antigen, CD14. Nucleic Acids Res. 1988;16:4173. doi: 10.1093/nar/16.9.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrero E, Hsieh C L, Francke U, Goyert S M. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J Immunol. 1990;145:331–336. [PubMed] [Google Scholar]
- 4.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golenbock D T, Liu Y, Millham F H, Freeman M W, Zoeller R A. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 6.Griffin J D, Ritz J, Nadler L M, Schlossman S F. Expression of myeloid differentiation antigens on normal and myeloid cells. J Clin Investig. 1981;68:932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grünwald U, Krueger C, Schütt C. Endotoxin-neutralizing capacity of soluble CD14 is a highly conserved specific function. Circ Shock. 1993;39:220–225. [PubMed] [Google Scholar]
- 8.Hailman E, Lichenstein H S, Wurfel M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hailman E, Vasselon T, Kelley M, Busse L A, Hu M C-T, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 10.Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 11.Huang Z H, Bates E J, Ferrante J V, Hii C S T, Poulos A, Robinson B S, Ferrante A. Inhibition of stimulus-induced endothelial cell intercellular adhesion molecule-1, E-selectin and vascular cellular adhesion molecule-1 expression by arachidonic acid and its hydroxy and hydroperoxy derivatives. Circ Res. 1997;80:149–158. doi: 10.1161/01.res.80.2.149. [DOI] [PubMed] [Google Scholar]
- 12.Jack R S, Grünwald U, Stelter F, Workelamahu G, Schütt C. Both membrane-bound and soluble forms of CD14 bind to gram-negative bacteria. Eur J Immunol. 1995;25:1436–1441. doi: 10.1002/eji.1830250545. [DOI] [PubMed] [Google Scholar]
- 13.Katz S S, Chen K, Chen S, Doerfler M E, Elsbach P, Weiss J. Potent CD14-mediated signalling of human leukocytes by Escherichia coli can be mediated by interaction of whole bacteria and host cells without extensive prior release of endotoxin. Infect Immun. 1996;64:3592–3600. doi: 10.1128/iai.64.9.3592-3600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusunoki T, Hailman E, Juan T S-C, Lichenstein H S, Wright S D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J-D, Kato K, Tobias P S, Kirkland T N, Ulevitch R J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1703. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel R F, Jr, Sato T T, Mendez C, Johnson M C, Pohlman T H. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect Immun. 1995;63:4046–4053. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schimke J, Mathison J, Morgiewicz J, Ulevitch R J. Anti-CD14 mAb treatment provides therapeutic benefit after in vivo exposure to endotoxin. Proc Natl Acad Sci USA. 1998;95:13875–13880. doi: 10.1073/pnas.95.23.13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone R. Search for sepsis drugs goes on despite past failures. Science. 1994;264:365–367. doi: 10.1126/science.8153620. [DOI] [PubMed] [Google Scholar]
- 19.Takeshita S, Nakatani N, Takata Y, Kawase H, Sekine I, Yoshioka S. Interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) enhance lipopolysaccharide binding to neutrohils via CD14. Inflamm Res. 1998;47:101–103. doi: 10.1007/s000110050290. [DOI] [PubMed] [Google Scholar]
- 20.Tohme Z N, Amar S, van Dyke T E. Moesin functions as a lipopolysaccharide receptor on human monocytes. Infect Immun. 1999;67:3215–3220. doi: 10.1128/iai.67.7.3215-3220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vane J R, Ånggård E E, Botting R M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–34. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 22.Van Voorhis W C, Steinman R M, Hair L S, Luban J, Witmer M D, Koide M D, Cohn Z A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue location of macrophages. J Exp Med. 1983;158:126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasselon T, Pironkova R, Detmers P. Sensitive responses of leukocytes to lipopolysaccharide require a protein distinct from CD14 at the cell surface. J Immunol. 1997;159:4498–4505. [PubMed] [Google Scholar]
- 24.Von Asmuth E J U, Dentener M A, Bazil V, Bouma M G, Leeuwenberg J F M, Buurman W A. Anti-CD14 antibodies reduce responses of cultured human endothelial cells to endotoxin. Immunology. 1993;80:78–83. [PMC free article] [PubMed] [Google Scholar]
- 25.Wright S D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991;3:83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]
- 26.Wright S D. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- 27.Wright S D, Ramos R A, Hermanowski-Vosatka A, Rockwell P, Detmers P. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang R-B, Mark M R, Gray M A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 29.Yu B, Hailman E, Wright S D. Lipopolysaccharide-binding protein and soluble CD14 catalyze exchange of phospholipids. J Clin Investig. 1997;99:315–324. doi: 10.1172/JCI119160. [DOI] [PMC free article] [PubMed] [Google Scholar]