Abstract
Objective
Ultrasound (US) is the pivotal procedure during the diagnostic work‐up of thyroid nodule and several US‐based risk stratification systems (RSSs) have been recently developed. Since the performance of RSSs in detecting medullary thyroid carcinoma (MTC) has been rarely investigated, the present systematic review aimed to achieve high evidence about (1) how MTC is classified according to RSSs; (2) if RSSs correctly classify MTC at high risk/suspicion, and (3) if MTC is classified as suspicious at US when RSSs are not used.
Design
The review was performed according to MOOSE. The online search was performed by specific algorithm on January 2022. A random‐effects model was used for statistical analysis.
Results
Twenty‐five papers were initially included and their risk of bias was generally low. According to ATA system, 65% of MTCs was assessed at high suspicion and 25% at intermediate suspicion. Considering all RSSs, a 54.8% of MTCs was put in a high‐risk/suspicion category. Pooling data from studies without data of RSS the prevalence of ultrasonographically suspicious MTCs was 60%.
Conclusions
As conclusion, MTC presentation according to RSSs is partially known and it is classified in a high‐risk/suspicion category of RSSs in just over a half of cases. This advises for further studies, ideally supported by international societies, to better define the US presentation of MTC.
Keywords: meta‐analysis, thyroid, TIRADS, ultrasound, ultrasound systems
1. INTRODUCTION
Ultrasound (US) examination is the pivotal procedure during the initial diagnostic workup of thyroid nodule. 1 Since using single US parameters to manage patients with thyroid nodule can have suboptimal efficacy, during the last years the major international societies in the thyroid field have developed and published specific systems to assess the risk for malignancy of thyroid lesions (risk stratification systems [RSSs], often reported as Thyroid Imaging Reporting And Data Systems [TIRADSs]). 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 With the advent of RSSs/TIRADSs most of us have experienced a general improvement of diagnostic performance and, importantly for clinical practice, have aligned to a common standard lexicon for descripting nodule and defining suspicious characteristics. 13 However, some shadows seem appearing alongside a number of lights. First, we must take into account that almost all knowledge about the US presentation of thyroid malignancy is based on papillary carcinoma (PTC). In fact, PTC represents the largest part of thyroid malignancies. In addition, since 1990s all of us have looked as at risk of malignancy those nodules with PTC‐like presentation and have mainly indicated biopsy accordingly. Nevertheless, while PTC can be detected on cytological preparation, follicular carcinoma (FTC) is invariably cytologically indeterminate, and medullary carcinoma (MTC) is diagnosed at cytology in about a half of cases. 14 , 15 As result, when we speak about US and/or RSSs/TIRADSs performance in detecting thyroid malignancy, we are really referring to PTC and we have sparse data about FTC and MTC. 16
In this context, the case of MTC is particularly challenging. Its diagnosis remains difficult during clinical practice, calcitonin is the most accurate diagnostic marker but its routine use in all patients with thyroid nodule is not universally accepted, and its US presentation is not fully known. The present systematic review was undertaken to achieve high evidence about the US presentation of MTC, considering or not its classification according to US‐RSSs/TIRADSs. Specifically, the present study aimed to evaluate (1) how MTC is classified according to RSSs/TIRADSs, (2) if RSSs/TIRADSs classify MTC at high risk/suspicion and indicate for biopsy, (3) if MTC is classified as suspicious at US when RSSs/TIRADSs are not used.
2. METHODS
2.1. Conduction of review
The systematic review was performed according to Meta‐analysis Of Observational Studies in Epidemiology (MOOSE). 17
2.2. Search strategy
A six‐step search strategy was planned; (1) sentinel studies were searched in PubMed; (2) keywords and MeSH terms were identified; (3) the terms medullary thyroid carcinoma, ultrasound, TIRADS were searched in PubMed; (4) PubMed and Cochrane were searched; (5) studies reporting US data of MTC were detected and studies with less than 10 MTCs were excluded from meta‐analysis; (6) references of included studies were screened for additional papers. The last search was performed on 5 January 2022. Articles in all languages were initially included and those not in English were translated, when appropriate. No publication year restriction was applied. Two investigators (G. F., P. T.) independently and in duplicate searched papers, screened titles and abstracts, reviewed the full‐texts and selected articles for inclusion.
2.3. Data extraction
Following information was extracted independently by the above two authors in a piloted form: (1) general information on the study (author, year of publication, country, study type, number of patients, number of MTC nodules, number of MTCs nodules classified as suspicious or not at US and/or according to RSSs/TIRADSs. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 The main paper and supplementary data were searched; if data was missing, authors were contacted via email. Data were cross‐checked and discrepancies were discussed.
2.4. Study quality assessment
The risk of bias of included studies was assessed independently by one author (L. S.). The National Heart, Lung, and Blood Institute Quality Assessment Tool was used. Following items were evaluated: study question; eligibility criteria; sample size calculation; description and delivering of intervention; definition of outcome measures; duration of follow‐up; blinding; loss to follow‐up; statistical methods. Each domain was assigned low, high or not reported. 18
2.5. Statistical analysis
The characteristics of studies were summarized and following data were extracted: (a) the proportion of MTC at high risk/suspicion; or (b) the proportion of MTC at high risk/suspicion in which FNA was indicated; or (c) the proportion of MTC in which FNA was indicated despite the RSSs classification. When at least four studies can be pooled, a proportion meta‐analysis was performed to calculate: (1) the pooled proportion of MTCs classified into RSSs/TIRADSs categories; (2) the proportion of MTCs classified as suspicious at US where studies did not classify them according to RSSs/TIRADSs. Heterogeneity was assessed by using I 2 and a value ≥50% mean high heterogeneity. A random‐effects model was used. Pooled data were presented with 95% confidence intervals (95% CI). Meta‐regression analysis was performed when heterogeneity was found. A p < 0.05 was regarded as significant. Statistical analyses were performed using OpenMeta[Analyst] (open‐source software developed by the Center for Evidence Synthesis in Health, Brown University).
3. RESULTS
3.1. Studies retrieved
A total of 740 records were found by the above search strategy and, after reviewing title and abstract; 38 papers were initially selected for retrieving their full‐text. Finally, 25 studies could be included in the systematic review (Figure 1). 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43
Figure 1.
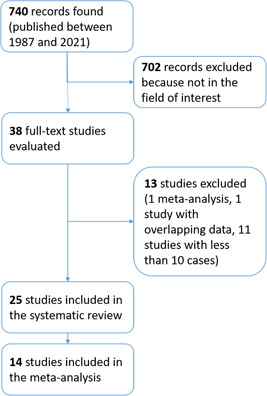
Flow of records searched according to the present systematic review. [Color figure can be viewed at wileyonlinelibrary.com].
3.2. Study quality assessment
The risk of bias of the included studies is shown in Supporting Information. Specifically, Table S2 summarizes the quality assessment of the 25 included studies. The risk of bias for each study could be judged as low in 12 of 14 items. By contrast, studies reported anything about power or sample size justification. Participation rate of eligible patients was not mentioned in any of the included studies.
3.3. Qualitative analysis (systematic review)
The 25 articles 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 found according to the criteria of the present systematic review were published between 2002 and 2021. The authors were from eastern countries in 18 papers, European in five, and American in two. Overall, there were 1698 MTCs. All of studies presented retrospective series of MTCs with a sample size ranging from 14 to 189 cases. The study period of the retrospective enrolment of MTC was between 2 and 20 years and generally started before the era of RSSs/TIRADSs (i.e., 2009 2 , 3 ). General characteristics of the 25 studies are illustrated in Table 1. Among the 25 articles, nine reported performance data of at least one RSS/TIRADS, other five classified MTCs as suspicious or not at US, and the remaining 11 did not reported US data to be pooled in a meta‐analysis (Table 2).
Table 1.
General characteristics of studies included in the present systematic review
| Ref. | First author | Country | Year | Journal | Case enrolment | Study period | MTC |
|---|---|---|---|---|---|---|---|
| [19] | Zhao | China | 2021 | Endocrine | Retrospective | 2010–2015 | 189 |
| [20] | Zhu | China | 2021 | BMC Cancer | Retrospective | 2009–2018 | 74 |
| [21] | Zhao | China | 2021 | Cancer Imaging | Retrospective | 2015–2017 | 78 |
| [22] | Ning | China, South Korea | 2021 | Asian J Surgery | Retrospective | 2006–2018 | 127 |
| [23] | Matrone | Italy | 2021 | Eur J Endocrinol | Retrospective | 2014–2020 | 152 |
| [24] | Hahn | South Korea | 2021 | Acta Radiol | Retrospective | 1999–2017 | 129 |
| [25] | de Oliveira | Brasil | 2021 | Arch Endocrinol Metab | Retrospective | 2013–2018 | 19 |
| [26] | Wang | China | 2020 | Technol Cancer Res Treat | Retrospective | 2008–2018 | 73 |
| [27] | Li | China | 2020 | Clin Endocrinol (Oxf) | Retrospective | 2010–2019 | 29 |
| [28] | Guo | China | 2019 | Chin Med J (Engl) | Retrospective | 2011–2016 | 71 |
| [29] | Yun | South Korea | 2018 | Endocrine | Retrospective | 2003–2016 | 57 |
| [30] | Wang | China | 2016 | Pak J Pharm Sci | Retrospective | 2014–2015 | 20 |
| [31] | Valderrabano | USA | 2016 | Thyroid | Retrospective | 1998–2014 | 30 |
| [32] | Cho | South Korea | 2016 | Asian Pac J Cancer Prev | Retrospective | 2007–2010 | 130 |
| [33] | Bao | China | 2016 | Zhonghua Yi Xue Za Zhi | Retrospective | 1993–2013 | 72 |
| [34] | Zhou | China | 2015 | J Ultrasound Med | Retrospective | 2008–2013 | 38 |
| [35] | Trimboli | Switzerland, Italy | 2014 | J Exp Clin Cancer Res | Retrospective | 2007–2013 | 134 |
| [36] | Sesti | Austria | 2014 | Anticancer Res | Retrospective | 2003–2009 | 28 |
| [37] | Choi | South Korea | 2011 | Acta Radiol | Retrospective | 2000–2008 | 36 |
| [38] | Lee | South Korea | 2010 | AJR Am J Roentgenol | Retrospective | 1997–2008 | 46 |
| [39] | Cai | China | 2010 | Chin Med J (Engl) | Retrospective | 1998–2009 | 35 |
| [40] | Kim | South Korea | 2009 | Korean J Radiol | Retrospective | 2002–2007 | 21 |
| [41] | Fukushima | Japan | 2009 | World J Surg | Retrospective | 1988–2007 | 77 |
| [42] | Boér | Hungary | 2003 | Eur J Surg Oncol | Retrospective | 1992–2000 | 14 |
| [43] | Saller | Germany | 2002 | Exp Clin Endocrinol Diabetes | Retrospective | 1995–2001 | 19 |
Note: Records are ordered according to their year of publication.
Abbreviation: MTC, medullary thyroid carcinoma.
Table 2.
Available data to perform a meta‐analysis
| Ref | First author | Suspicious/unsuspicious US | Modified TIRADS | Horvath TIRADS 3 | ACR‐TIRADS 9 | EU‐TIRADS 11 | K‐TIRADS 12 | ATA 10 | AACE 5 , 8 |
|---|---|---|---|---|---|---|---|---|---|
| [19] | Zhao | x (incomplete) | |||||||
| [21] | Zhao | x | |||||||
| [22] | Ning | x (incomplete) | |||||||
| [23] | Matrone | x | x | x | x | x | |||
| [24] | Hahn | x | x | ||||||
| [25] | de Oliveira | x | |||||||
| [26] | Wang | x | |||||||
| [27] | Li | x | x | ||||||
| [29] | Yun | x | |||||||
| [31] | Valderrabano | x | |||||||
| [32] | Cho | x | |||||||
| [35] | Trimboli | x | |||||||
| [41] | Fukushima | x | |||||||
| [42] | Boér | x |
Abbreviations: AACE, American Association of Clinical Endocrinologists; ACR, American College of Radiologists; ATA, American Thyroid Association; EU, European; K, Korean; TIRADS, Thyroid Imaging Reporting And Data System; US, ultrasound.
3.4. Quantitative analysis (meta‐analysis)
According to the available data retrieved in the articles, among all RSSs/TIRADSs it was possible to perform only a meta‐analysis pooling ATA data. There were four studies reporting the distribution of a total of 340 MTCs over the ATA categories 23 , 24 , 27 , 31 (Reference [24] classified according ATA system 120/129 cases). The pooled prevalence of MTCs in the five categories of American Thyroid Association (ATA) is reported in Table 3. Heterogeneity was found only in intermediate‐ and high‐suspicion category. The heterogeneity was explored with a meta‐regression using the sample size (< or > 100 MTCs) as covariate but it was not solved.
Table 3.
Distribution of MTCs over the five classes of ATA risk stratification
| Pooled proportion (95% CI) | I 2 | |
|---|---|---|
| ATA benign (n = 1) | 0.006 (−0.002 to 0.014) | 0% |
| ATA very low suspicion (n = 5) | 0.012 (−0.002 to 0.026) | 21.2% |
| ATA low suspicion (n = 22) | 0.057 (0.025 to 0.088) | 28.9% |
| ATA intermediate suspicion (n = 98) | 0.249 (0.122 to 0.376) | 84.7% |
| ATA high risk (n = 205) | 0.651 (0.501 to 0.801) | 86.9% |
Abbreviations: ATA, American Thyroid Association; CI, confidence interval; MTC, medullary thyroid carcinoma.
Since the high‐risk/suspicion categories of the RSSs/TIRADSs are generally associated to similar risk of malignancy, 44 the pooled prevalence of MTCs classified at high risk according to any RSSs/TIRADSs were calculated. There were six studies 22 , 23 , 24 , 25 , 27 , 29 , 31 reporting MTCs classified according to several RSSs/TIRADSs, with some series of them classified according to two or more systems. When these series were pooled, a 54.8% of MTCs was put in a high‐risk/suspicion category with significant heterogeneity. The heterogeneity was explored with a meta‐regression using RSS/TIRADS as covariate but inconsistency was not deleted (Figure 2).
Figure 2.
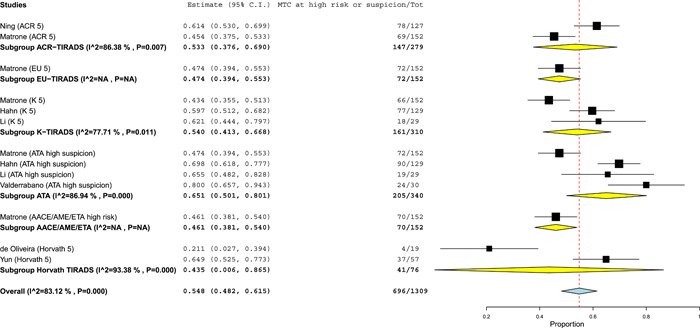
Pooled proportion of medullary thyroid carcinomas (MTCs) classified at high risk/suspicion according to risk stratification systems/thyroid Imaging Reporting And Data Systems (RSSs/TIRADSs). Diamond (yellow for subgroup, blue for the whole group) indicates the pooled proportion and its wideness indicates 95% confidence interval (CI). Square indicates the study sample size and line indicates 95% CI. The studies by Matrone et al., 23 Hahn et al. 24 and Li et al. 27 classified their series of MTCs according to five, two, and two different RSSs/TIRADSs, respectively. [Color figure can be viewed at wileyonlinelibrary.com].
When the indication for biopsy according to RSSs/TIRADSs was searched, this data was found only in the study by Matrone et al. 23 Then, a meta‐analysis was not performed.
There were five studies with no RSSs/TIRADSs data. 26 , 32 , 35 , 41 , 42 Overall, they reported 428 MTCs classified as suspicious or not. The procedure to classify MTCs as suspicious or not is reported in Table 4. As illustrated in Figure 3, the prevalence of ultrasonographically suspicious MTCs ranged from 37.7% to 75.3% and the pooled value was 60.5% with significant heterogeneity. The last one was explored with a meta‐regression using the sample size (< or >100 MTCs) as covariate but inconsistency was not solved.
Table 4.
Modality of evaluation and classification of MTCs adopted by those five studies in which no RSS/TIRADS was used
| Ref. | First author | Procedure to re‐evaluate US images | Procedure to assess MTCs as suspicious or not |
|---|---|---|---|
| [26] | Wang | 2 sonographers with more than 10 years of experience reviewed US images blinded to clinical data, other imaging findings, and pathology results. | Malignant US features were irregular margin, marked hypoechogenicity, microcalcifications, and taller‐than‐wide shape. |
| [32] | Cho | Retrospective review of US images was performed by 2 radiologists with 5 and 15 years of experience blinded to pathologic results. | Malignant features were marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller than wide shape. |
| [35] | Trimboli | US images were reviewed by 4 reviewers with more than 10 years of experience in thyroid US. | MTCs were classified according to a 5‐class system. 45 Nodules with ≥3.5 were regarded as suspicious. |
| [41] | Fukushima | Not detailed | MTCs were classified according to a 5‐class system. 45 Nodules with ≥3.5 were classified as suspicious. |
| [42] | Boér | Not detailed | Not detailed |
Abbreviations: MTC, medullary thyroid carcinoma; RSS, risk stratification system; TIRADS, Thyroid Imaging Reporting And Data System; US, ultrasound.
Figure 3.
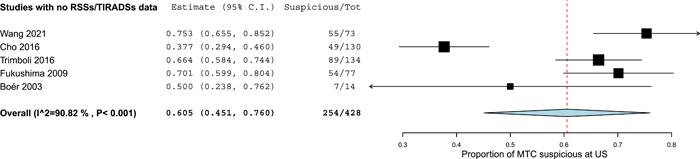
Pooled prevalence of medullary thyroid carcinomas (MTCs) classified as suspicious at ultrasound (US). Diamond indicates the pooled proportion and its wideness indicates 95% confidence interval (CI). Square size indicates the sample and its line indicates 95% CI. [Color figure can be viewed at wileyonlinelibrary.com].
4. DISCUSSION
To diagnose MTC is still a challenge. Even if calcitonin represents the most accurate diagnostic tool to detect MTC, its measurement is affected by several technical factors 46 thus limiting its routine use in clinical practice in the enormous number of patients with thyroid nodule. In this context, a discrepancy between the major international societies exists: 2006 European Thyroid Association (ETA) consensus recommended in favour of testing for calcitonin in all patients during their initial workup, 47 2010 ETA, American Association of Clinical Endocrinologists (AACE) and Associazione Medici Endocrinologi (AME) guidelines recommended to measure calcitonin in some specific clinical conditions, 5 and 2015 ATA guidelines do not recommend for nor against the routine calcitonin measurement. 10 With this premises, achieving the highest evidence about the accuracy of US, and RSSs/TIRADSs in particular, has major interest for our clinical practice.
The present study was undertaken to achieve a comprehensive reappraisal of the US presentation of MTC, according and not to RSSs/TIRADSs. A number of 25 studies were retrieved by systematic review and 1698 MTCs with US description were included. As initial results, a meta‐analysis of the distribution of MTCs over the RSSs/TIRADSs categories could be performed only for ATA system. Data from those 11 articles which did not meet the criteria to be included in the meta‐analysis was reported in Table 5; as detailed in this table, the presentation of MTC was quite heterogeneous. This intrinsically confirmed that the MTC presentation at US has not been enough explored in terms of RSSs/TIRADSs performance. When considering the ATA system, we could find that a 65% of cases was put into the high‐suspicion category. Unfortunately, the available data in the studies did not allow to evaluate the reliability of RSSs/TIRADSs in indicating for biopsy. Finally, a percentage of 54.8% among MTCs is at high risk/suspicion according to RSSs/TIRADSs. Lastly, a 60% of MTCs was classified as suspicious when RSSs/TIRADSs were not used. The herein reported results should be discussed taking into account one previous meta‐analysis on this topic. 31 That study investigated the frequency of single US features (i.e., solid composition, hypoechogenicity, irregular margins, taller‐than‐wide shape, micro‐ and macrocalcifications) in MTC, and assumed that, based on pooled findings, >95% of MTCs would be classified at least in the intermediate‐suspicion pattern according to ATA, 10 warranting the lowest‐size threshold for biopsy. Considering that the paper by Valderrabano et al. 31 was published just after the publication of ATA guidelines and no data about RSSs/TIRADSs were available, the present study represents an important advancement in the field of MTC presentation. Indeed, these findings extend the information about the US, and allows us to know what is the performance of current RSSs/TIRADSs, especially that of ATA. 10
Table 5.
Summary of findings reported in the 11 papers included in the systematic review but not in the meta‐analyses
| Ref | First author | Main findings |
|---|---|---|
| [20] | Zhu | Tumour size >40 mm, capsular invasion, metastatic cervical lymph nodes positively correlated with the risk of postoperative recurrence of MTC. |
| [28] | Guo | In presence of cystic change, circumscribed margin, regular shape, no calcification, no rich vascularity, and normal cervical lymph nodes, MTC is easily misdiagnosed as benign by US. |
| [30] | Wang | The higher frequency US presentation of MTC was the low‐echo tumour in round or quasi‐circular shape, with obscure boundary and often combined with rough calcification. |
| [33] | Bao | The common US findings for MTC were solid composition, hypoechogenicity, regular sharp, well‐defined margin, and calcifications. |
| [34] | Zhou | Sonographic features of MTC are similar to those of small papillary carcinomas but greatly different from those of large papillary ones. |
| [36] | Sesti | US can detect recurrent MTCs with an accuracy positively correlated with calcitonin levels. |
| [37] | Choi | The predominant US findings of MTC included solid internal content, round‐to‐oval shape, smooth margins, hypoechogenicity, and micro‐ or macro‐calcifications. |
| [38] | Lee | MTCs differ from PTCs in size, presence of a cystic change, and echotexture. |
| [39] | Cai | The typical sonographic features of MTC are hypoechogenicity, predominantly solid, irregularly shaped with intranodular micro‐ or macro‐calcifications. |
| [40] | Kim | US findings for MTC are not greatly different from PTC except for the prevalence of an ovoid‐to‐round shape. |
| [43] | Saller | Conventional US reveals a combination of hypoechogenicity, intranodular calcifications, and absence of ‘halo sign’ in the vast majority of MTC. |
Abbreviations: MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma; US, ultrasound.
The present findings prompted us to discuss their clinical implications. Ultrasound is the pivotal in‐office procedure to stratify the risk of malignancy of thyroid nodules of our patients. In fact, we generally indicate for biopsy, clinical follow‐up or other options according to US presentation of nodules. Even if this is a largely‐proven careful strategy to detect the majority of cancers (i.e., PTC), we are aware that the less common malignancies might be overlooked. Among the latter ones, the detection of MTC has to be considered. As above, the routine testing for calcitonin in all thyroid nodule patients is not universally accepted and some societies are neither in favour neither against it. Thus, on one hand we have no rule to identify patients in whom the calcitonin evaluation is needed (with the exception of specific situation as familiarity for MTC), on the other hand, when we face patients with high calcitonin levels and multinodular goiter, we have no rule to select nodule(s) for biopsy. Furthermore, the accuracy of cytological examination is recognized as poor 14 while the measurement of calcitonin in needle washout is near to excellent but not routinely performed. 15 In the context of multinodular goiter, further imaging procedures (e.g., molecular imaging procedures) could be of value in identifying both primary MTC and its regional and distant metastatic involvement. 48 , 49 Basically, we need some strategies to measure calcitonin appropriately in our patients, use appropriate imaging procedures (ultrasound and other) according to calcitonin levels to localize MTC, and perform biopsy (with calcitonin measurement in needle fluids) when indicated. An MTC‐specific/adapted US RSS/TIRADS should be developed.
Limitations and strengths of the present review should be discussed. First, all the included studies were retrospective. Second, the sample size of the studies was often small. Indeed, the prevalence of MTCs among all malignancies was unknown through the studies thus limiting the reliability of the selected samples. Third, sparse data about calcitonin levels were reported; as a consequence, the indication for biopsy and/or thyroidectomy was not explored thus introducing a selection bias. Fourth, the available data in the included studies did not allow to further explore the heterogeneity, when found. Lastly, one paper, 33 non‐included in the meta‐analyses, was written in Chinese language and was translated in English with potential interpretation bias.
Based on the present systematic review, we can conclude that: (1) MTC presentation according to RSSs/TIRADSs is partially known because of the sparse literature about; (2) MTC is classified in a high‐risk/suspicion category of RSSs/TIRADSs in just over a half of cases; (3) MTC is suspicious at ultrasound in 60% of cases when RSSs/TIRADSs are not used for clinical practice. We advise for further studies, ideally supported by international societies, to better define the US presentation of MTC and then improve the accuracy of RSSs/TIRADSs.
AUTHOR CONTRIBUTIONS
Pierpaolo Trimboli conceived the meta‐analysis. Pierpaolo Trimboli and Giulia Ferrarazzo developed the search strategy and extracted data. Pierpaolo Trimboli performed statistical analysis. Lorenzo Scappaticcio performed the quality assessment. Pierpaolo Trimboli drafted the manuscript. All Authors read, provided feedback, and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
These systematic review and meta‐analysis were in accordance with the principles of the Declaration of Helsinki. Analyses were performed on data extracted from published papers.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Open access funding provided by Universita della Svizzera italiana.
Ferrarazzo G, Camponovo C, Deandrea M, Piccardo A, Scappaticcio L, Trimboli P. Suboptimal accuracy of ultrasound and ultrasound‐based risk stratification systems in detecting medullary thyroid carcinoma should not be overlooked. Findings from a systematic review with meta‐analysis. Clin Endocrinol (Oxf). 2022;97:532‐540. 10.1111/cen.14739
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
REFERENCES
- 1. Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: a review. JAMA. 2018;319(9):914‐924. 10.1001/jama.2018.0898 [DOI] [PubMed] [Google Scholar]
- 2. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer , Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167‐1214. 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3. Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94(5):1748‐1751. 10.1210/jc.2008-1724 [DOI] [PubMed] [Google Scholar]
- 4. Park JY, Lee HJ, Jang HW, et al. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009;19(11):1257‐1264. 10.1089/thy.2008.0021 [DOI] [PubMed] [Google Scholar]
- 5. Gharib H, Papini E, Paschke R, et al. AACE/AME/ETA Task Force on Thyroid Nodules American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract. 2010;16(3):468‐475. 10.4158/EP.16.3.468 [DOI] [PubMed] [Google Scholar]
- 6. Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260(3):892‐899. 10.1148/radiol.11110206 [DOI] [PubMed] [Google Scholar]
- 7. Perros P, Boelaert K, Colley S, et al. British Thyroid Association Guidelines for the management of thyroid cancer. Clin Endocrinol. 2014;81(suppl 1):1‐122. 10.1111/cen.12515 [DOI] [PubMed] [Google Scholar]
- 8. Gharib H, Papini E, Garber JR, et al. AACE/ACE/AME Task Force on Thyroid Nodules American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr Pract. 2016;22(5):622‐639. 10.4158/EP161208.GL [DOI] [PubMed] [Google Scholar]
- 9. Tessler FN, Middleton WD, Grant EG, et al. ACR thyroid imaging, reporting and data system (TI‐RADS): white paper of the ACR TI‐RADS Committee. J Am Coll Radiol. 2017;14(5):587‐595. 10.1016/j.jacr.2017.01.046 [DOI] [PubMed] [Google Scholar]
- 10. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1‐133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU‐TIRADS. Eur Thyroid J. 2017;6(5):225‐237. 10.1159/000478927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shin JH, Baek JH, Chung J, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology Ultrasonography diagnosis and imaging‐based management of thyroid nodules: revised Korean Society of thyroid radiology consensus statement and Recommendations. Korean J Radiol. 2016;17(3):370‐395. 10.3348/kjr.2016.17.3.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trimboli P, Durante C. Ultrasound risk stratification systems for thyroid nodule: between lights and shadows, we are moving towards a new era. Endocrine. 2020;69:1‐4. 10.1007/s12020-020-02196-6 [DOI] [PubMed] [Google Scholar]
- 14. Trimboli P, Treglia G, Guidobaldi L, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta‐analysis. Clin Endocrinol. 2015;82(2):280‐285. 10.1111/cen.12563 [DOI] [PubMed] [Google Scholar]
- 15. Trimboli P, Giannelli J, Marques B, Piccardo A, Crescenzi A, Deandrea M. Head‐to‐head comparison of FNA cytology vs. calcitonin measurement in FNA washout fluids (FNA‐CT) to diagnose medullary thyroid carcinoma. A systematic review and meta‐analysis. Endocrine. 2021;75:33‐39. 10.1007/s12020-021-02892-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trimboli P, Castellana M, Piccardo A, et al. The ultrasound risk stratification systems for thyroid nodule have been evaluated against papillary carcinoma. A meta‐analysis. Rev Endocr Metab Disord. 2021;22(2):453‐460. 10.1007/s11154-020-09592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eusebi P. Diagnostic accuracy measures. Cerebrovasc Dis. 2013;36(4):267‐272. 10.1159/000353863 [DOI] [PubMed] [Google Scholar]
- 18. National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Accessed March 1, 2020. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 19. Zhao J, Yang F, Wei X, et al. Ultrasound features value in the diagnosis and prognosis of medullary thyroid. Carcinoma. 2021;72(3):727‐734. 10.1007/s12020-020-02510-2 [DOI] [PubMed] [Google Scholar]
- 20. Zhu Q, Xu D. Correlation between preoperative ultrasonic features of medullary thyroid carcinoma and postoperative recurrence. BMC Cancer. 2021;21(1):344. 10.1186/s12885-021-07953-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao J, Zheng X, Gao M, et al. Ultrasound features of medullary thyroid cancer as predictors of biological behavior. Cancer Imaging. 2021;21(1):33. 10.1186/s40644-021-00402-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ning CP, Kim EK. Sonographic risk factors of aggressive behaviors in medullary thyroid carcinomas. Asian J Surg. 2022;45(1):291‐298. 10.1016/j.asjsur.2021.05.036 [DOI] [PubMed] [Google Scholar]
- 23. Matrone A, Gambale C, Biagini M, Prete A, Vitti P, Elisei R. Ultrasound features and risk stratification systems to identify medullary thyroid carcinoma. Eur J Endocrinol. 2021;185(2):193‐200. 10.1530/EJE-21-0313 [DOI] [PubMed] [Google Scholar]
- 24. Hahn SY, Shin JH, Oh YL, Park KW. Characteristics of medullary thyroid carcinoma according to nodule size: application of the Korean Thyroid Imaging Reporting and Data System and American Thyroid Association Guidelines. Acta Radiol. 2021;62(4):474‐482. 10.1177/0284185120929699 [DOI] [PubMed] [Google Scholar]
- 25. De Oliveira DHA, Huning LP, Belim MC, Rodrigues PF, Nagai HM, Graf H. Is there a place for measuring serum calcitonin prior to thyroidectomy in patients with a non‐diagnostic thyroid nodule biopsy? Arch. Endocrinol Metab. 2021;65(1):40‐48. 10.20945/2359-3997000000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Kou H, Chen W, Lu M, Zhou L, Zou C. The diagnostic value of ultrasound in medullary thyroid carcinoma: a comparison with computed tomography. Technol Cancer Res Treat. 2020;19:1533033820905832. 10.1177/1533033820905832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J, Li H, Yang Y, Zhang X, Qian L. The KWAK TI‐RADS and 2015 ATA guidelines for medullary thyroid carcinoma: combined with cell block‐assisted ultrasound‐guided thyroid fine‐needle aspiration. Clin Endocrinol (Oxf). 2020;92(5):450‐460. 10.1111/cen.14121 [DOI] [PubMed] [Google Scholar]
- 28. Guo QQ, Zhang SH, Niu LJ, Zhang YK, Li ZJ, Chang Q. Comprehensive evaluation of medullary thyroid carcinoma before surgery. Chin Med J. 2019;132(7):834‐841. 10.1097/CM9.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yun G, Kim YK, Choi SI, Kim JH. Medullary thyroid carcinoma: application of Thyroid Imaging Reporting and Data System (TI‐RADS) Classification. Endocrine. 2018;61(2):285‐292. 10.1007/s12020-018-1594-4 [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Liu M, Yang J, Song Y. High frequency ultrasound features and pathological characteristics of medullary thyroid carcinoma. Pak J Pharm Sci. 2016;29(6 Suppl):2269‐2271. [PubMed] [Google Scholar]
- 31. Valderrabano P, Klippenstein DL, Tourtelot JB, et al. New American Thyroid Association Sonographic Patterns for Thyroid Nodules perform well in medullary thyroid carcinoma: institutional experience, systematic review, and meta‐analysis. Thyroid. 2016;26(8):1093‐1100. 10.1089/thy.2016.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho KE, Gweon HM, Park AY, et al. Ultrasonographic features of medullary thyroid carcinoma: do they correlate with pre and postoperative calcitonin levels? Asian Pac J Cancer Prev. 2016;17(7):3357‐3362. [PubMed] [Google Scholar]
- 33. Bao SD, Pang P, Zang L, et al. Predictive value of sonographic features in preoperative evaluation of medullary thyroid carcinoma. Zhonghua Yi Xue Za Zhi. 2016;96(31):2482‐2486. 10.3760/cma.j.issn.0376-2491.2016.31.009 [DOI] [PubMed] [Google Scholar]
- 34. Zhou L, Chen B, Zhao M, Zhang H, Liang B. Sonographic features of medullary thyroid carcinomas according to tumor size: comparison with papillary thyroid carcinomas. J Ultrasound Med. 2015;34(6):1003‐1009. 10.7863/ultra.34.6.1003 [DOI] [PubMed] [Google Scholar]
- 35. Trimboli P, Giovanella L, Valabrega S, et al. Ultrasound features of medullary thyroid carcinoma correlate with cancer aggressiveness: a retrospective multicenter study. J Exp Clin Cancer Res. 2014;33(1):87. 10.1186/s13046-014-0087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sesti A, Mayerhoefer M, Weber M, et al. Relevance of calcitonin cut‐off in the follow‐up of medullary thyroid carcinoma for conventional imaging and 18‐fluorine‐fluorodihydroxyphenylalanine PET. Anticancer Res. 2014;34(11):6647‐6654. [PubMed] [Google Scholar]
- 37. Choi N, Moon WJ, Lee JH, Baek JH, Kim DW, Park SW. Ultrasonographic findings of medullary thyroid cancer: differences according to tumor size and correlation with fine needle aspiration results. Acta Radiol. 2011;52(3):312‐316. 10.1258/ar.2010.100247 [DOI] [PubMed] [Google Scholar]
- 38. Lee S, Shin JH, Han BK, Ko EY. Medullary thyroid carcinoma: comparison with papillary thyroid carcinoma and application of current sonographic criteria. AJR Am J Roentgenol. 2010;194(4):1090‐1094. 10.2214/AJR.09.3276 [DOI] [PubMed] [Google Scholar]
- 39. Cai S, Liu H, Li WB, et al. Ultrasonographic features of medullary thyroid carcinoma and their diagnostic values. Chin Med J. 2010;123(21):3074‐3078. [PubMed] [Google Scholar]
- 40. Kim SH, Kim BS, Jung SL, et al. Ultrasonographic findings of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Korean J Radiol. 2009;10(2):101‐105. 10.3348/kjr.2009.10.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukushima M, Ito Y, Hirokawa M, et al. Excellent prognosis of patients with nonhereditary medullary thyroid carcinoma with ultrasonographic findings of follicular tumor or benign nodule. World J Surg. 2009;33(5):963‐968. 10.1007/s00268-009-9939-z [DOI] [PubMed] [Google Scholar]
- 42. Boér A, Szakáll Jr., S , Klein I, et al. FDG PET imaging in hereditary thyroid cancer. Eur J Surg Oncol. 2003;29(10):922‐928. 10.1016/s0748-7983(03)00137-9 [DOI] [PubMed] [Google Scholar]
- 43. Saller B, Moeller L, Görges R, Janssen OE, Mann K. Role of conventional ultrasound and color Doppler sonography in the diagnosis of medullary thyroid carcinoma. Exp Clin Endocrinol Diabetes. 2002;110(8):403‐407. 10.1055/s-2002-36546 [DOI] [PubMed] [Google Scholar]
- 44. Castellana M, Castellana C, Treglia G, et al. Performance of five ultrasound risk stratification systems in selecting thyroid nodules for FNA. J Clin Endocrinol Metab. 2020;105(5):dgz170‐dgz1669. 10.1210/clinem/dgz170 [DOI] [PubMed] [Google Scholar]
- 45. Ito Y, Amino N, Yokozawa T, et al. Ultrasonographic evaluation of thyroid nodules in 900 patients: comparison among ultrasonographic, cytological, and histological findings. Thyroid. 2007;17(12):1269‐1276. 10.1089/thy.2007.0014 [DOI] [PubMed] [Google Scholar]
- 46. Trimboli P, Giovanella L, Crescenzi A, et al. Medullary thyroid cancer diagnosis: an appraisal. Head Neck. 2014;36(8):1216‐1223. 10.1002/hed.23449 [DOI] [PubMed] [Google Scholar]
- 47. Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JWA. 2012 European Thyroid Association guidelines for metastatic medullary thyroid cancer. Eur Thyroid J. 2012;2012(1):5‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rasul S, Hartenbach S, Rebhan K, et al. [18F]DOPA PET/ceCT in diagnosis and staging of primary medullary thyroid carcinoma prior to surgery. Eur J Nucl Med Mol Imaging. 2018;45(12):2159‐2169. 10.1007/s00259-018-4045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Archier A, Heimburger C, Guerin C, et al. (18)F‐DOPA PET/CT in the diagnosis and localization of persistent medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2016;43(6):1027‐1033. 10.1007/s00259-015-3227-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data sets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.


