ABSTRACT
For the prevention of surgical site infection (SSI), continuous disinfection could be helpful. Short wavelength ultraviolet radiation C (UVC) is highly bactericidal but shows cytotoxicity. Radiation of UVC with a wavelength of 222 nm to the skin is considered to be safe because it only reaches the stratum corneum. However, the safety of 222 nm irradiation to the surgical field not covered with skin is unknown. The purpose of this study was to examine the safety of 222 nm UVC irradiation on a surgical field in a rabbit model. Five types of tissue were surgically exposed and irradiated with 222 or 254 nm UVC. Immunohistological assessment against cyclobutane pyrimidine dimer (CPD), an index of DNA damage by UVC, was performed. The CPD‐positive cell rate was significantly higher in the 254 nm group than in the other groups in all tissues. A 222 nm group showed significantly more CPD than control in fat tissue, but no significant difference in all other tissues. In fat tissue collected 24 h after irradiation, the 254 nm group showed higher CPD than the other groups, while the 222 nm group had reduced to the control level. These data suggest that 222 nm UVC irradiation could be a new method to safely prevent SSI.
To prevent surgical site infection, continuous disinfection could be helpful. Short wavelength UVC is highly bactericidal but shows cytotoxicity. Radiation of UVC with a wavelength of 222 nm to the skin is considered to be safe because it only reaches the stratum corneum. However, the safety of 222 nm irradiation to the surgical field not covered with skin is unknown. In this study, five types of tissue were exposed and irradiated with 222 nm or 254 nm UVC. An immunohistological assessment of DNA damage was performed. The results suggest that 222 nm UVC irradiation could be a new method to safely prevent surgical site infection.

INTRODUCTION
Surgical site infection (SSI) is one of the major perioperative complications, which in some cases could turn a surgery performed to benefit the patient into a long‐lasting condition requiring complicated treatment. The causative bacteria of SSI include endogenous species such as indigenous skin flora and exogenous species such as airborne bacteria (1, 2). It is difficult to completely prevent SSI solely using preoperative disinfection, and a novel method that could continuously sterilize and disinfect the surgical field would be desirable.
Ultraviolet radiation C (UVC) is a short wavelength UV band between 200 and 280 nm, and is known to be strongly bactericidal due to the high DNA absorption coefficient of these wavelengths (3). In particular, 254 nm UVC is widely used in germicidal lamps and water purification systems. One 254 nm UVC irradiation device is currently approved for the clinical treatment of infected pressure ulcers in the United States and Canada (4). However, UVC has been shown to be cytotoxic in vitro and UVC irradiation to the eyes could induce keratitis (5). When 254 nm UVC irradiation is delivered to the skin, it passes through the stratum corneum and reaches the basal epidermal cells, potentially leading to malignant tumors (6, 7).
On the other hand, UVC with a wavelength of 222 nm has a much higher protein absorption coefficient (8), and it reaches only the stratum corneum (9) and does not affect living skin cells, therefore it is considered to be a theoretically safe type of UVC. In one recently reported study, repeated long‐term irradiation with 222 nm UVC was performed on mice that were susceptible to carcinogenesis and no skin cancer or eye abnormalities were caused (10). We previously carried out a study on the biological effects of 222 nm UVC using a mouse model of an excisional skin wound infected with methicillin‐resistant Staphylococcus aureus (MRSA) (11). Irradiation with 222 nm UVC significantly reduced bacterial colony forming units (CFUs) on the skin surface compared with nonirradiated skin. Bacterial counts in wounds evaluated on days 3, 5, 8 and 12 after irradiation demonstrated that the bactericidal effect of 222 nm UVC was equal to or more effective than 254 nm UVC and was >2 logs of cell killing. Histological analysis revealed that migration of keratinocytes which is essential for the wound healing process was impaired in wounds irradiated with 254 nm UVC but was unaffected in 222 nm UVC irradiated wounds. No CPD‐expressing cells were detected in either epidermis or dermis of wounds irradiated with 222 nm UVC, whereas CPD‐expressing cells were found in both epidermis and dermis irradiation with 254 nm UVC.
Moreover, we also conducted a clinical trial to study the safety and bactericidal effect of 222 nm UVC on the skin in healthy human volunteers and found that 222 nm UVC at 500 mJ cm−2 was a safe irradiation dose while still showing bactericidal effects (12).
However, in practice the actual surgical field is usually not covered by skin. To realize the clinical application of 222 nm UVC as a new strategy for disinfection during surgery, it is necessary to examine the effects of irradiation on tissues and organs exposed during surgery.
Therefore, the purpose of the current study was to examine the safety of 222 nm UVC irradiation on a surgical field not covered with skin in a rabbit model.
MATERIALS AND METHODS
Animals
As experimental animals, 14‐week‐old female New Zealand white rabbits (Japan SLC, Inc., Hamamatsu, Japan) weighing 2.6–2.8 kg were used in this study.
All animal procedures and experimental protocols were in accordance with the ethical standards of the Animal Care and Use Committee of Kobe University Graduate School of Medicine and the study was approved by the Animal Care and Use Committee of Kobe University Graduate School of Medicine (approval no. P190805‐R1). Study protocols complied with Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines.
Surgical procedures
Each rabbit was anesthetized with inhalational anesthesia with 3–5% isoflurane (FUJIFILM Wako Pure Chemical, Osaka, Japan) in 1.5–4.0 L min−1 of 100% O2 using a mask, followed by intraperitoneal injection of 0.5 mg kg−1 of medetomidine (Nippon Zenyaku Kogyo, Koriyama, Japan), 2.0 mg kg−1 of midazolam (Astellas Pharma, Tokyo, Japan) and 0.5 mg kg−1 of butorphanol (Meiji Seika Pharma, Yokohama, Japan), and local injection around the incision of 30 mg kg−1 of lidocaine hydrochloride (Aspen Japan, Tokyo, Japan).
An H‐shaped skin incision after shaving hair was performed on the back to open the skin to make an irradiation area of 4.0 × 4.0 cm, where subcutaneous fat tissue and muscle fascia were exposed. The left thigh was incised on the lateral side to expose the muscle of vastus lateralis and subsequently femoral bone. After that, the left knee joint was opened, and the articular cartilage of the distal femur was exposed Fig. 1. The three irradiations were performed in sequence. The irradiated areas were marked by ink so that they could be identified when the wound was opened after temporary skin closure. Then 222 nm UVC irradiation was performed to each surgical field in sequence.
Figure 1.
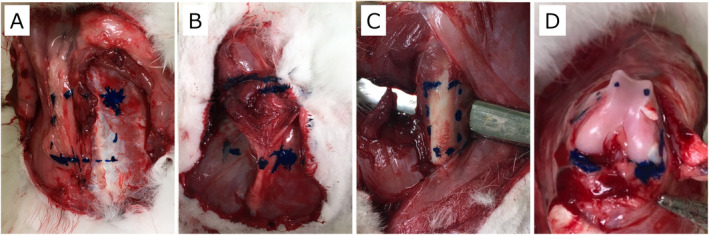
Examples of images of the irradiation area. The irradiation area was marked by blue ink. (A) Irradiation area on the back. The fat tissue and the fascia were exposed on the left and right sides, respectively. (B) Irradiation area on the left thigh. The vastus lateralis muscle was exposed by removing the fascia. (C) Irradiation area on the femoral bone in the left thigh. The bone was exposed by dividing the muscles sideways. (D) Irradiation area on the cartilage. The left knee joint was opened, and the articular cartilage of the distal femur was exposed in the knee flexion position
The tissues were collected from the irradiated areas either after 1 h or after 24 h following the irradiation for further assessment. Samples were identified by the tissue type and time of harvest.
UVC irradiation
The rabbits were randomly divided into three groups according to the type of UVC irradiation: the 222 nm group where 500 mJ cm−2 of 222 nm UVC was applied, the 254 nm group as a positive control where 200 mJ cm−2 of 254 nm UVC applied, and the nonirradiated control group as a negative control where no UV irradiation was performed (n = 5 in each group). For 222 nm UVC irradiation, krypton–chloride (Kr–Cl) excimer lamp and an optical filter that restricts the spectral emission band ranging from 200 to 230 nm, with the maximum output wavelength at 222 nm, were used. This 222 nm‐emitting SafeZoneUVC device (Ushio Inc. Tokyo, Japan) is composed of a lamp, air‐cooling fan, mirrors and a custom band‐pass filter. The filter is used for blocking almost all wavelengths, excluding the dominant 222 nm emission wavelength. The irradiance emitted by 222 nm light was measured using an S‐172/UIT250 accumulated UV meter (Ushio Inc.) and was found to be 3 mW cm−1 at the irradiated area. 254 nm UVC was irradiated by a low‐pressure mercury lamp (FL‐4W × 1, AS ONE, Osaka, Japan). Before the start of exposure, the irradiance was measured similarly to 222 nm UVC and was found to be 1 mW cm−2 at the irradiated area.
Immunohistochemical assessment for CPD
Cyclobutane pyrimidine dimers (CPDs) are formed as a result of DNA damage induced by ultraviolet light and are a common indicator of UV damage.
Harvested tissues were fixed in 4% paraformaldehyde at room temperature for 24 h. The samples of bone and cartilage were decalcified at room temperature with a decalcifying 10% formic acid solution for 1 week. Subsequently, each sample was embedded in paraffin wax. The samples were processed to obtain 4 μm sagittal sections using a microtome. The sections were deparaffinized in xylene and rehydrated in a graded alcohol series. Immunohistochemical staining for detection of monoclonal antibody against CPD protein (TDM‐2) (1:1,000 dilution with PBS containing 1% bovine serum albumin) was performed. After incubating the sections with Histofine MOUSESTAIN kit blocking reagent A (Nichirei, Tokyo, Japan) for 10 min at room temperature and being washed, the sections were incubated with Histofine simple stain MAX Po (M) (Nichirei), which was used as the secondary antibody. The sections were washed and then were stained with substrate‐chromogen AEC (Medac, Wedel, Germany). Afterward, the slides were washed with distilled water for 10 min, counterstained with and washed again in tap water, then mounted. Specimens were observed with a Biozero BZ‐X710 microscope (Keyence, Osaka, Japan), and the mean percentage of CPD‐positive cells in all cells from three random fields per section was calculated as the CPD‐positive rate.
TUNEL staining
The terminal transferase‐mediated dUTP nick end‐labeling (TUNEL) staining procedure was performed using MEBSTAIN Apoptosis TUNEL Kit III (Medical & Biochemical Laboratories, Nagoya, Japan) according to the manufacturer’s instructions. Tissue sections were deparaffinized with xylene, rehydrated in an ethanol series, exposed to proteinase K solution (20 μg ml−1) at 37°C for 30 min, and washed with distilled water. Then, they were incubated in terminal deoxynucleotidyl transferase (TdT) with biotinylated dUTP, immersed in TB buffer diluted with distilled water for 15 min, and washed. Afterward, they were incubated in Avidin‐DTAF solution for 60 min. The sections were counterstained with PI/RNase Staining Buffer (Phoenix Flow Systems, San Diego) and mounted. The quantification of apoptosis was determined by the mean percentage of the TUNEL‐positive cell nuclei in total cell nuclei from three random fields per section as a TUNEL‐positive rate.
Statistical analysis
The results were statistically analyzed using a software package (GraphPad Prism; MDF Software, Inc.). Columns and error bars indicate means and standard errors, respectively. Comparisons among the three groups were tested for significance via analysis of variance (ANOVA) followed by post hoc testing with a Tukey procedure. P values less than 0.05 were considered to be statistically significant.
RESULTS
Immunohistochemical assessment of DNA damage
Histological evaluation of DNA damage was carried out using immunohistochemical staining with an antibody for CPD on tissue samples collected at 1 h after the irradiation. In the 254 nm group, many CPD‐positive cells were observed in all five different types of tissues: fat, fascia, muscle, bone and articular cartilage. In the 222 nm group, only a very few CPD‐positive cells were detected in four kinds of tissues including, fat, fascia, muscle and bone, and no CPD‐positive cells were found in articular cartilage. No CPD‐positive cells were observed in any tissues from the nonirradiated control group. Representative images of all five types of tissues in all three groups are displayed in Fig. 2.
Figure 2.
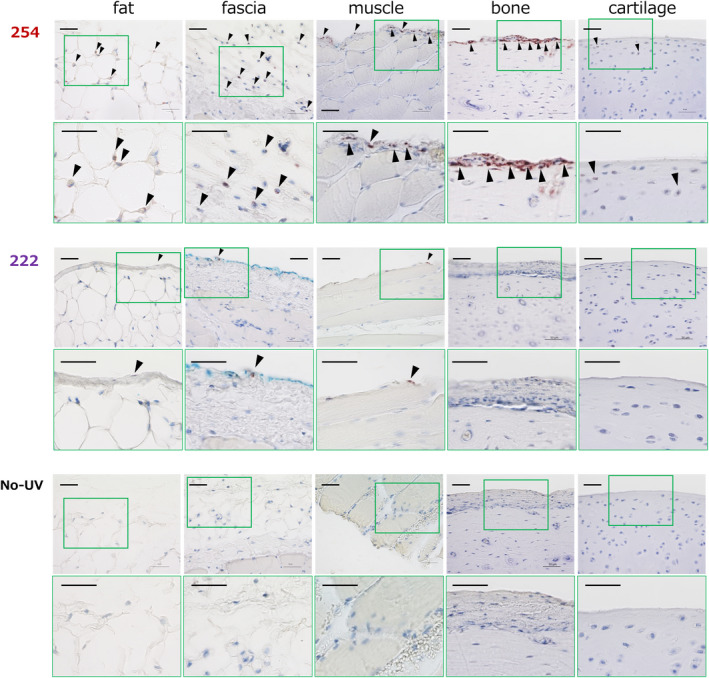
Representative images of histological CPD assessment in five types of tissues immediately after the irradiation in the 254 nm group (254), the 222 nm group (222) and the nonirradiated group (No‐UV). Images are shown as sets of lower‐ (above) and higher‐magnification figures (below) of the area surrounded by a green square in the lower ones. CPD stained cells are indicated with arrowheads. CPD‐positive cells were observed in all five kinds of tissues in the 254 nm group and no or few CPD‐positive cells in the 222 nm group. No CPD‐positive cells were found in the nonirradiated group. Scale bar = 50 µm
As a quantitative evaluation of DNA damage, the CPD‐positive rate was calculated Fig. 3. In fat tissue, the CPD‐positive rate was significantly higher in the 254 nm group compared with other two groups, and the CPD positive rate of the 222 nm group was significantly higher than the nonirradiation control group. (254, 47.5 ± 16.0%; 222, 5.6 ± 5.5%; nonirradiation, 0.0 ± 0.0; respectively, P < 0.001 for 254 vs 222 or nonirradiation group, P < 0.05 for 222 vs nonirradiation group). In the other four types of tissues, fascia, muscle, bone and articular cartilage, the CPD‐positive rate was significantly higher in the 254 nm group compared to the other two groups, and there was no significant difference between the 222 nm group and the nonirradiated control group. The values were Fascia: 254, 51.8 ± 17.0%; 222, 2.0 ± 1.9%; nonirradiation, 0.0 ± 0.0; respectively, P < 0.01 for 254 vs 222 group, P < 0.001 for 254 vs nonirradiation group, P = ns for 222 vs nonirradiation group. Muscle: 254, 36.5 ± 15.4%; 222, 2.0 ± 2.3%; nonirradiation, 0.0 ± 0.0; respectively, P < 0.01 for 254 vs 222 group, P < 0.001 for 254 vs nonirradiation group, P = ns for 222 vs nonirradiation group. Bone: 254, 42.4 ± 8.2%; 222, 0.5 ± 1.0%; nonirradiation, 0.0 ± 0.0; respectively, P < 0.001 for 254 or 222 vs nonirradiation group, P = ns for 222 vs nonirradiation group. Articular cartilage: 254, 17.3 ± 7.6%; 222, 0.0 ± 0.0%; nonirradiation, 0.0 ± 0.0; respectively, P < 0.001 for 254 or 222 vs nonirradiation group, P = ns for 222 vs nonirradiation group.
Figure 3.
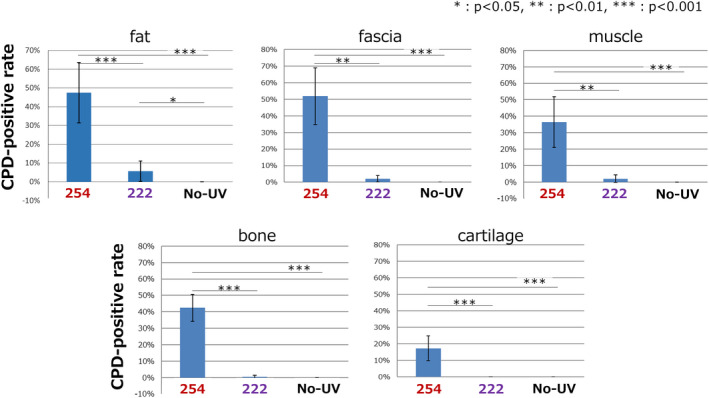
The quantitative CPD‐positive rate in all five types of tissues immediately after the irradiation. In fat tissues, the CPD‐positive rate was significantly higher in the 254 nm group compared with the other two groups and that of the 222 nm group was significantly higher than the nonirradiated group. In the other four types of tissues, fascia, muscle, bone and articular cartilage, the CPD‐positive rate was significantly higher in the 254 nm group compared with the other two groups and there was no significant difference between the 222 nm group and the nonirradiated group. Error bars represent standard deviation. P values are shown in the figure (n = 5 in each group) [Color figure can be viewed at wileyonlinelibrary.com]
Because the CPD‐positive rate in the fat tissue samples collected immediately after the irradiation was significantly higher in the 222 nm group compared with the nonirradiated group, we also assessed the rate in the fat tissue samples that were collected at 24 h after the irradiation Fig. 4. The CPD‐positive rate was significantly higher in the 254 nm group compared with the other two groups, and there was no significant difference between the 222 nm group and the nonirradiation group. (254, 15.2 ± 5.5%; 222, 2.0 ± 2.3%; nonirradiation, 0.0 ± 0.0; respectively, P < 0.01 for 254 vs 222 group, P < 0.001 for 254 vs nonirradiation group, P = ns for 222 vs nonirradiation group).
Figure 4.
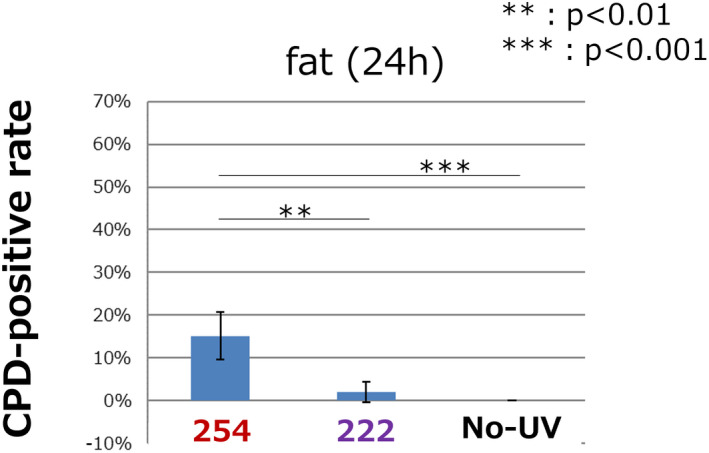
The quantitative CPD‐positive rate in fat tissue 24 h after the irradiation. The CPD‐positive rate was significantly higher in the 254 nm group compared with the other two groups and there was no significant difference between the 222 nm group and the nonirradiated group. Error bars represent standard deviation. P values are shown in the figure (n = 5 in each group) [Color figure can be viewed at wileyonlinelibrary.com]
Evaluation of apoptosis after the irradiations
To evaluate apoptosis after the irradiation, TUNEL staining was performed on fat tissue samples collected at 24 h after the irradiation, because the significantly increased CPD‐positive rate in the 222 nm group compared with the nonirradiation group was detected only in the fat tissue. TUNEL staining is shown as green and nuclei were stained as red, and the spots colored yellow by overlapping of green and red within a 200 µm square were counted as TUNEL positive apoptotic cells. TUNEL‐positive cells were detected in the 254 nm and the 222 nm groups, while more cells were observed in the 254 nm group. There were no TUNEL‐positive cells in the nonirradiated group Fig. 5. As a quantitative evaluation of cell apoptosis, the TUNEL‐positive rate was calculated Fig. 6. The TUNEL‐positive rate was significantly higher in the 254 nm group than the nonirradiated group, while there was no significant difference between the 254 nm group and the 222 nm group, or between the 222 nm group and the nonirradiated group. (254, 4.2 ± 5.9%; 222, 1.2 ± 2.6%; nonirradiation, 0.0 ± 0.0; respectively, P < 0.05 for 254 vs nonirradiation group, P = ns for 222 vs 254 or nonirradiated group.)
Figure 5.
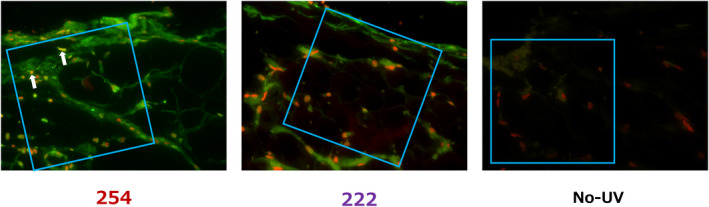
Representative images of TUNEL staining of fat tissue 24 h after irradiation in the 254 nm group (254), the 222 nm group (222) and the nonirradiated group (No‐UV). The red signal highlights the nucleus, and the green signal indicates positive for apoptosis. TUNEL‐positive cells are shown as yellow (overlap of red and green) and indicated with arrows. TUNEL‐positive cells were observed in the 254 nm group and no or few TUNEL‐positive cells in the 222 nm group. No TUNEL‐positive cells were found in the nonirradiated group. The blue squares are 200 × 200 µm where TUNEL‐positive rates were evaluated in the areas of interest
Figure 6.
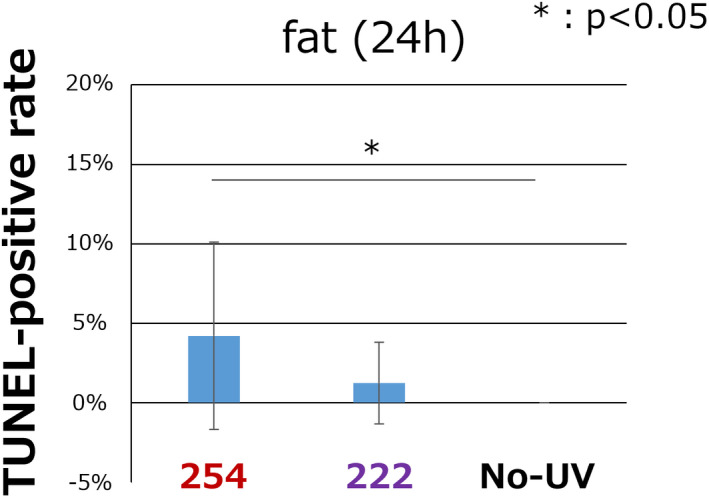
The quantitative TUNEL‐positive rate in fat tissue 24 h after the irradiation. The TUNEL‐positive rate was significantly higher in the 254 nm group compared with the nonirradiated group and there was no significant difference between the 222 nm group and the other two groups. Error bars represent standard deviation. P value are shown in the figure (n = 5 in each group) [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
In our previous study, we demonstrated that 500 mJ cm−2 of 222 nm UVC showed a good bactericidal effect and was safe when irradiating human skin (12). In that study, the bactericidal effect was evaluated by quantifying the skin flora. Recently, 222 nm UVC has also been shown to be effective not only against various bacteria, but also against viruses, including severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), the cause of the coronavirus disease 2019 (COVID‐19), and has therefore been attracting much attention (13, 14, 15, 16). Therefore, it is likely that 222 nm UVC is promising in terms of effectiveness for sterilization and disinfection of human tissues. However, before 222 nm UVC can be used to prevent SSI in a clinical setting, it was necessary to examine the safety of irradiation on the tissue in a surgical site not covered with skin.
We used 254 nm UVC and a nonirradiated control as comparisons for 222 nm UVC. The dose of 254 nm UVC was decided with reference to a report by Ritter et al., where UVC irradiation was safely performed during almost 6000 total joint replacement surgeries, and it significantly decreased the postoperative infection rate. In that study, a 254 nm UVC irradiator was installed on the ceiling of the operating room and produced 25 µW cm−2 at the operating table height. Based on this information, the dose of UVC at the surgical site was calculated as 90 mJ cm−2 over 1 h (16, 17). It is supposed that a total joint replacement generally takes about 2 h, therefore we set the dose of 254 nm UVC as 200 mJ cm−2. As for the dose of 222 nm UVC, 500 mJ cm−2 was employed according to the result of our previous clinical trial (12).
In the current study, five different types of tissues that could be exposed during orthopaedic surgery were examined. CPD generation by 222 nm UVC was observed but was not significantly increased compared with nonirradiated control, and was significantly lower when compared with 254 nm UVC in four kinds of tissue, fascia, muscle, bone and articular cartilage. This would suggest the safety of 500 mJ cm−2 of 222 nm UVC on those tissues. On the other hand, significantly more CPD generation was detected in fat tissue collected immediately after the 222 nm UVC irradiation compared with nonirradiated control. However, we suggest that this result does not directly demonstrate that 222 nm UVC is harmful. Following the result of fat tissue collected immediately after the irradiation, fat tissues were also studied when collected at 24 h after the irradiation. The CPD‐positive rates decreased by 32.0% and 35.7% in 24 h after the irradiation in the 254 nm and the 222 nm groups, respectively. In previous reports, the CPD‐positive rate in mouse skin irradiated with 254 nm UVC decreased by 35% in 24 h (18), and in an in vivo experiment in which human primary fibroblasts were irradiated with 254 nm UVC, the CPD rate decreased by 50% in 24 h (19). The decrease of CPD‐positive rate in the current study is in agreement with these reports. The CPD‐positive rate with 222 nm UVC irradiation at 24 h was only <2% and there was no significant difference from the nonirradiated control, while the CPD‐positive rate at 24 h with 254 nm UVC at almost 5% was significantly higher compared with the other two groups. These results would indicate that CPD generation in fat tissue by 222 nm UVC can be repaired in a short time and is therefore not harmful.
In general, it is supposed that accumulation of DNA damage in humans by repeated UV irradiation prolonged over years or even decades, could eventually induce carcinogenesis, depending on the Fitzpatrick skin phototype. In an in vivo study where about 25 mJ cm−2 of 254 nm UVC was irradiated on a mouse skin abrasion, CPD generation that was detected immediately after the irradiation was found to be extensively repaired within 48 h (20). A previous study using albino hairless mice who received repeated sessions of 254 nm UVC irradiation showed that malignant tumors were not induced, even with a high dose of 700 mJ cm−2, unless at least more than 50 daily irradiation sessions were performed (7). Another in vitro study demonstrated that unrepaired DNA damage can cause cell death or permanent cell‐cycle arrest (21). Therefore, the possibility of malignant tumor formation by a single UVC irradiation (regardless of the DNA damage which was observed to be repaired after 24 h) in the current study seems extremely low. Moreover, we used a dosage of 254 nm UVC of 200 mJ cm−2, which is lower than 225 mJ cm−2, the dosage of 254 nm UVC clinically applied to infected pressure ulcers in the United States and Canada (4). As far as we have been able to search, there have been no reports of significant adverse events caused by 254 nm UVC treatment of infected pressure ulcers. However, previously reported adverse effects on the skin and cornea by 254 nm UVC cannot be ignored. In addition, the tissues exposed to pressure ulcers and the actual surgical field are different; in this study, CPD was generated even in tissues (e.g. bones) that are not normally exposed to pressure ulcers. Therefore, the adverse effects of 254 nm UVC irradiation on a surgical field cannot be ruled out. For these reasons, we used 222 nm UVC because it is safer than 254 nm UVC. The results indicated that 222 nm UVC irradiation is harmless compared with 254 nm UVC irradiation used at a lower dosage than the clinically approved safe dosage. These results could be indirect but powerful reasons to suggest that the temporary CPD positive cells found in adipose tissue do not pose a risk of carcinogenesis. The reason why the CPDs were found in fat tissue to a greater extent compared to other cellular tissues, such as muscle, is probably due to the lack of competing chromophores in fat, while the muscle is rich in hemoglobin and myoglobin, which could prevent the DNA from being damaged by UVC. It is likely considered that 500 mJ cm−2 of 222 nm UVC is highly safe even if it is irradiated to a surgical field that is not covered with skin. This is consistent with the result of a study using mice with a photocarcinogenic phenotype conducted by Yamano et al. who found that no tumor formation was observed after long‐term 222 nm UVC irradiation at 500 mJ cm−2 on dorsal skin wounds caused by scratches and biting from fighting with the other mice in the same cage (10).
The assessment for apoptosis by TUNEL staining in the current study showed that apoptosis induced by 254 nm UVC irradiation was significantly higher compared with nonirradiated control and there were no significant differences between the 222 nm group and the other two groups. It is well‐known that UV irradiation induces CPD formation and apoptosis as shown in previous reports. (22, 23, 24) The current results show a similar trend of the CPD‐positive rate in fat tissue although the statistically significant differences were not the same. It is supposed that DNA damage in fat tissue can be repaired within 24 h without leading to apoptosis. It is well known that the CPDs produced in the skin by the action of UVB light reach a maximum at 1 h postirradiation, and are largely repaired at 24 h and completely repaired at 48 h (25). Not surprisingly the action of 222 nm UVC on the formation and repair of CPDs in fat cells has not been studied, but the biological mechanisms are likely to be similar.
The safety of 222 nm UVC is proposed to be due to the fact that UVC does not penetrate the stratum corneum. However, this theory cannot be the reason why 222 nm UVC irradiation is safe for tissues that are not covered by skin. An in vivo study by Kochevar et al. demonstrated that human fibroblasts irradiated by 193 nm UVC did not show DNA damage because the 193 nm UVC could not reach the nuclear DNA as it was absorbed by protein in the cytoplasm, however, this was not the case for 254 nm UVC which did cause DNA damage (26). Buonannno et al. (27) conducted an in vitro study where 207 nm UVC was irradiated to human fibroblasts and MRSA, and reported that 207 nm UVC killed MRSA efficiently but produced little human cell killing. In the study by Narita et al. 750 mJ cm−2 of 222 nm UVC or 150 mJ cm−2 of 254 nm UVC was irradiated onto a full‐thickness wound made by a skin biopsy punch. CPD were generated in the fibroblasts by the 254 nm UVC but not by the 222 nm UVC (11). We previously carried out a study on mice, which were genetically susceptible to UV carcinogenesis and skin damage caused by the lack of the xeroderma pigmentosum complementation group A (Xpa) gene, which is involved in the repair of CPDs in the skin (10). The fact that no tumors were caused in these genetically damaged Xpa‐knockout mice, even after repetitive irradiation with 222 nm UVC, using the same protocol which had been shown to produce tumors when irradiated with broad‐band UVB, is additional evidence of the safety of 222 nm UVC. Furthermore, no erythema or ear swelling was observed in mice with either genotype following 222 nm UVC exposure. In a recent clinical study, 540 mJ cm−2 of 222 nm UVC was irradiated onto sacral or gluteal pressure ulcers in human patients, and there was no evidence of complications or side effects during the 2 weeks of intermittent irradiation sessions (28). Based on this evidence, it is proposed that UVC with a shorter wavelength (e.g. 222 nm) will also be harmless on human cells, which are not protected by the stratum corneum. This is because the absorbance coefficient of tissue depends on wavelength (8). Shorter wavelength UVC as 222 nm UVC is more easily absorbed by proteins in the cytoplasm and is reduced to 50% by only about 0.3 µm of tissue, in contrast to UVC with a longer wavelength as 254 nm which is reduced to 50% by about 3 µm of tissue (29, 30). Bacteria are generally smaller than 1 µm in diameter while human cells are 10–25 µm in diameter, so the difference in cell size can be an explanation that 222 nm UVC shows a germicidal effect but is not harmful to human cells. Further experimental studies are required to confirm the amount of bacterial cell killing in the complex environment of an exposed surgical field as might be found during an orthopaedic joint replacement, as described in the present report.
In summary, a surgical field not covered by skin was irradiated with 222 nm UVC in a rabbit model and found to be a highly safe procedure. A 222 nm UVC could be a new method for preventing perioperative infections.
Acknowledgements
The authors of this manuscript have the following competing interests: A. N., R. K., H. O., M. S. and T. I. have received support in the form of salaries from USHIO Inc., Tokyo, Japan. M. R. H. is a consultant for USHIO Inc. We declare no other potential conflicts of interest, including employment, consultancy and patents. No further intellectual property relating to this paper will be developed.
REFERENCES
- 1. Jolivet, S. and Lucet J. C. (2019) Surgical field and skin preparation. Orthop. Traumatol. Surg. Res. 105, S1–S6. [DOI] [PubMed] [Google Scholar]
- 2. Bitkover, C. Y. , Marcusson E. and Ransjo U. (2000) Spread of coagulase‐negative staphylococci during cardiac operations in a modern operating room. Ann. Thorac. Surg. 69, 1110–1115. [DOI] [PubMed] [Google Scholar]
- 3. Bintsis, T. , Litopoulou‐Tzanetaki E. and Robinson R. K. (2000) Existing and potential applications of ultraviolet light in the food industry – a critical review. J. Sci. Food Agric. 80, 637–645. [DOI] [PubMed] [Google Scholar]
- 4. Nussbaum, E. L. , Flett H., Hitzig S. L., McGillivray C., Leber D., Morris H. and Jing F. (2013) Ultraviolet‐C irradiation in the management of pressure ulcers in people with spinal cord injury: A randomized, placebo‐controlled trial. Arch. Phys. Med. Rehabil. 94, 650–659. [DOI] [PubMed] [Google Scholar]
- 5. Sliney, D. (2013) Balancing the risk of eye irritation from UV‐C with infection from bioaerosols. Photochem. Photobiol. 89, 770–776. [DOI] [PubMed] [Google Scholar]
- 6. Kowalczuk, C. I. , Priestner M. C., Pearson A. J., Saunders R. D. and Bouffler S. D. (2006) Wavelength dependence of cellular responses in human melanocytes and melanoma cells following exposure to ultraviolet radiation. Int. J. Radiat. Biol. 82, 781–792. [DOI] [PubMed] [Google Scholar]
- 7. Sterenborg, H. J. , van der Putte S. C. and van der Leun J. C. (1988) The dose‐response relationship of tumorigenesis by ultraviolet radiation of 254 nm. Photochem. Photobiol. 47, 245–253. [DOI] [PubMed] [Google Scholar]
- 8. Kreusch, S. , Schwedler S., Tautkus B., Cumme G. A. and Horn A. (2003) UV measurements in microplates suitable for high‐throughput protein determination. Anal. Biochem. 313, 208–215. [DOI] [PubMed] [Google Scholar]
- 9. Pfeifer, G. P. and Besaratinia A. (2012) UV wavelength‐dependent DNA damage and human non‐melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 11, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamano, N. , Kunisada M., Kaidzu S., Sugihara K., Nishiaki‐Sawada A., Ohashi H., Yoshioka A., Igarashi T., Ohira A., Tanito M. and Nishigori C. (2020) Long‐term effects of 222‐nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation. Photochem. Photobiol. 96, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Narita, K. , Asano K., Morimoto Y., Igarashi T., Hamblin M. R., Dai T. and Nakane A. (2018) Disinfection and healing effects of 222‐nm UVC light on methicillin‐resistant Staphylococcus aureus infection in mouse wounds. J. Photochem. Photobiol. B Biol. 178, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukui, T. , Niikura T., Oda T., Kumabe Y., Ohashi H., Sasaki M., Igarashi T., Kunisada M., Yamano N., Oe K., Matsumoto T., Matsushita T., Hayashi S., Nishigori C. and Kuroda R. (2020) Exploratory clinical trial on the safety and bactericidal effect of 222‐nm ultraviolet C irradiation in healthy humans. PLoS One 15, e0235948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buonanno, M. , Welch D., Shuryak I. and Brenner D. J. (2020) Far‐UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 10, 10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitagawa, H. , Nomura T., Nazmul T., Omori K., Shigemoto N., Sakaguchi T. and Ohge H. (2021) Effectiveness of 222‐nm ultraviolet light on disinfecting SARS‐CoV‐2 surface contamination. Am. J. Infect. Control 49, 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Welch, D. , Buonanno M., Grilj V., Shuryak I., Crickmore C., Bigelow A. W., Randers‐Pehrson G., Johnson G. W. and Brenner D. J. (2018) Far‐UVC light: A new tool to control the spread of airborne‐mediated microbial diseases. Sci. Rep. 8, 2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritter, M. A. , Olberding E. M. and Malinzak R. A. (2007) Ultraviolet lighting during orthopaedic surgery and the rate of infection. J. Bone Joint Surg. Am. 89, 1935–1940. [DOI] [PubMed] [Google Scholar]
- 17. Kowalski, W. J. (2009) Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection. Springer‐Verlag, Heidelberg, New York. [Google Scholar]
- 18. Narita, K. , Asano K., Morimoto Y., Igarashi T. and Nakane A. (2018) Chronic irradiation with 222‐nm UVC light induces neither DNA damage nor epidermal lesions in mouse skin, even at high doses. PLoS One 13, e0201259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakagawa, A. , Kobayashi N., Muramatsu T., Yamashina Y., Shirai T., Hashimoto M. W., Ikenaga M. and Mori T. (1998) Three‐dimensional visualization of ultraviolet‐induced DNA damage and its repair in human cell nuclei. J. Invest. Dermatol. 110, 143–148. [DOI] [PubMed] [Google Scholar]
- 20. Dai, T. , Garcia B., Murray C. K., Vrahas M. S. and Hamblin M. R. (2012) UVC light prophylaxis for cutaneous wound infections in mice. Antimicrob. Agents Chemother. 56, 3841–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lisby, S. , Gniadecki R. and Wulf H. C. (2005) UV‐induced DNA damage in human keratinocytes: quantitation and correlation with long‐term survival. Exp. Dermatol. 14, 349–355. [DOI] [PubMed] [Google Scholar]
- 22. de Gruijl, F. R. , van Kranen H. J. and Mullenders L. H. (2001) UV‐induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J. Photochem. Photobiol. B Biol. 63, 19–27. [DOI] [PubMed] [Google Scholar]
- 23. Godar, D. E. , Miller S. A. and Thomas D. P. (1994) Immediate and delayed apoptotic cell death mechanisms: UVA versus UVB and UVC radiation. Cell Death Differ. 1, 59–66. [PubMed] [Google Scholar]
- 24. Takasawa, R. , Nakamura H., Mori T. and Tanuma S. (2005) Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis 10, 1121–1130. [DOI] [PubMed] [Google Scholar]
- 25. Toriyama, E. , Masuda H., Torii K., Ikumi K. and Morita A. (2021) Time kinetics of cyclobutane pyrimidine dimer formation by narrowband and broadband UVB irradiation. J. Dermatol. Sci. 103(3), 151–155. [DOI] [PubMed] [Google Scholar]
- 26. Kochevar, I. E. , Walsh A. A., Green H. A., Sherwood M., Shih A. G. and Sutherland B. M. (1991) DNA damage induced by 193‐nm radiation in mammalian cells. Can. Res. 51, 288–293. [PubMed] [Google Scholar]
- 27. Buonanno, M. , Randers‐Pehrson G., Bigelow A. W., Trivedi S., Lowy F. D., Spotnitz H. M., Hammer S. M. and Brenner D. J. (2013) 207‐nm UV light – a promising tool for safe low‐cost reduction of surgical site infections. I: in vitro studies. PLoS One 8, e76968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goh, J. C. , Fisher D., Hing E. C. H., Hanjing L., Lin Y. Y., Lim J., Chen O. W. and Chye L. T. (2021) Disinfection capabilities of a 222 nm wavelength ultraviolet lighting device: A pilot study. J. Wound Care 30, 96–104. [DOI] [PubMed] [Google Scholar]
- 29. Coohill, T. P. (1986) Virus‐cell interactions as probes for vacuum‐ultraviolet radiation damage and repair. Photochem. Photobiol. 44, 359–363. [DOI] [PubMed] [Google Scholar]
- 30. Green, H. , Boll J., Parrish J. A., Kochevar I. E. and Oseroff A. R. (1987) Cytotoxicity and mutagenicity of low intensity, 248 and 193 nm excimer laser radiation in mammalian cells. Can. Res. 47, 410f413. [PubMed] [Google Scholar]


