Abstract
Background
Dry eye disease (DED) is a common cause of ocular pain and discomfort. Dry eye disease (DED) stems from a loss‐of‐tear film homeostasis and is frequently seen in video display terminal (VDT) users. Video display terminal (VDT) use reduces blink rates and increases incomplete blinks, leading to tear film instability and ocular inflammation, promoting DED.
Purpose
To assess and evaluate the methods for preventing VDT‐associated DED and ocular discomfort.
Methods
Studies were found using PubMed and Embase with the search terms: (digital visual terminal* OR computer use OR screen use OR smartphone OR display OR visual display terminal* OR computer vision syndrome OR tablet OR phone OR screen time) AND (dry eye OR DED).
Results
Thirty‐one relevant articles were found. Ten described single‐visit studies, whereas 21 had a prolonged follow‐up. Most preventive measures of VDT‐associated DED aimed to increase blink rate or directly prevent tear film instability, ocular inflammation, mucin loss or ocular surface damage. Using an adjustable chair and ergonomic training, blink animations and omega‐3 supplementation improved signs and symptoms of VDT‐associated DED. Taking frequent breaks was associated with fewer symptoms, but no study assessed the commonly suggested 20‐20‐20 rule.
Conclusion
Preventive measures, such as blink animation programmes, oral intake of omega‐3 fatty acids and improved ergonomics act on different parts of the vicious cycle of dry eye and could supplement each other. A comparison of the efficacy of the different interventions as well as more evidence of the effect of increased humidity, VDT filters and ergonomic practices, are required.
Keywords: computer vision syndrome, dry eye disease, ergonomic practices, preventive measures, tear film stability, video display terminal
Introduction
Dry eye disease (DED) is a highly prevalent, multifactorial disorder characterized by the loss of homeostasis in the tear film and ocular surface (Bron et al. 2017). Dry eye disease (DED) is often presented as a vicious cycle, where tear film instability, excessive evaporation, inflammation and ocular surface damage exacerbate one another, leading to DED signs and symptoms, as illustrated in Fig. 1. In video display terminal (VDT) users, the vicious cycle is thought to begin with reduced blink rates and an increased rate of incomplete blinks, which over time diminish tear film stability and leave the ocular surface exposed (Stapleton et al. 2017). This process can become self‐perpetuating and worsen over years of VDT exposure.
Fig. 1.
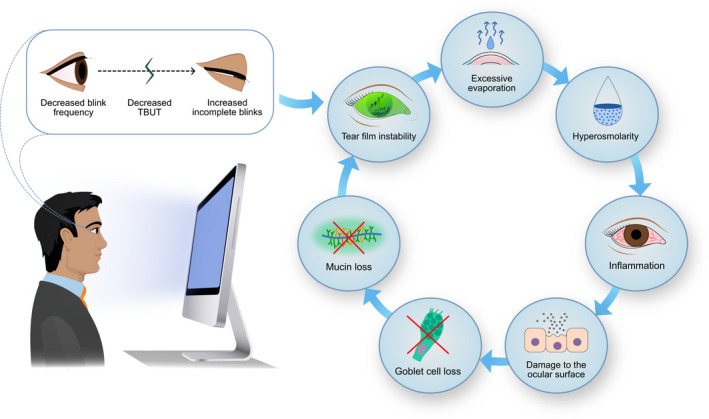
Vicious cycle of dry eye disease. Video display terminal (VDT) use leads to decreased blink rates and increased incomplete blinks, promoting tear film instability, tear evaporation rates and hyperosmolarity. Hyperosmolarity can increase inflammatory mediators that damage the ocular surface and goblet cells. The loss of goblet cells decreases mucin secretion and further reduces tear film stability, leading to a self‐perpetuating vicious cycle. Copyright Sara T. Nøland. [Colour figure can be viewed at wileyonlinelibrary.com]
Dry eye disease (DED) amongst VDT users has an estimated global prevalence of 26–70% (Fjaervoll et al. 2021). The cumulative and current daily VDT exposure increase DED risk (Yee et al. 2007; Uchino et al. 2013; Bron et al. 2017).
Despite frequently having clinically mixed presentations, DED is often categorized into two major etiological categories: (i) evaporative dry eye, where excessive tear evaporation promotes dry eye development and (ii) aqueous‐deficient dry eye, where tear production by the lacrimal gland is reduced (Schaumberg et al. 2011; Bron et al. 2017; Craig et al. 2017; Stapleton et al. 2017). Evaporative dry eye is most frequently caused by meibomian gland dysfunction (MGD) (Craig et al. 2017). MGD is characterized by altered meibum secretion and a defective lipid layer, which lead to reduced tear film stability and an unprotected ocular surface (Schaumberg et al. 2011; Craig et al. 2017; Stapleton et al. 2017). Evaporative dry eye and MGD appear to be particularly prevalent in office workers (Uchino et al. 2008; Bron et al. 2017). Aqueous‐deficient dry eye, on the other hand, is often caused by Sjogren syndrome, lacrimal deficiency, lacrimal gland duct obstruction, reflex block and several systemic drugs (Craig et al. 2017; Bjordal et al. 2020).
Computer vision syndrome (CVS) is a collection of visual and musculoskeletal symptoms that are the most frequently occurring manifestations amongst VDT users (Blehm et al. 2005; Gowrisankaran & Sheedy 2015). Computer vision syndrome (CVS) contains a wider array of symptoms than DED, including blurred vision, ocular dryness and burning as well as musculoskeletal symptoms (Blehm et al. 2005; Gowrisankaran & Sheedy 2015).
During VDT use, the interval between blinks increases (Patel et al. 1991; Tsubota & Nakamori 1993; Bentivoglio et al.1997; Tsubota 1998; Doughty 2001; Freudenthaler et al. 2003; Schlote et al. 2004), whereas the tear film break‐up time (TBUT) shortens (Yee et al. 2007; Yokoi et al. 2015; Bhargava et al. 2016; Shimazaki et al. 2017), which results in a reduced ocular protection index (OPI). As shown in Fig. 2, the unprotected ocular surface is exposed for longer periods. This shortening can lead to excessive evaporation and hyperosmolarity, triggering the vicious cycle of DED (Stapleton et al. 2017).
Fig. 2.
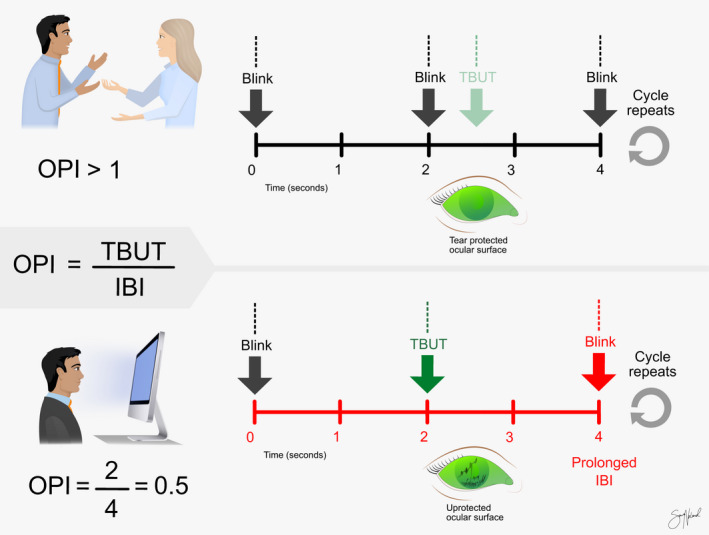
Illustration of the ocular protection index (OPI). The OPI is calculated by dividing the tear film break‐up time (TBUT) by the inter‐blink interval (IBI). In this illustration, two scenarios are presented: blinking during normal conversation (above) and during video display terminal (VDT) use (below). During a normal conversation, the IBI is generally shorter than, or close to, TBUT, due to high blink rates, making OPI closer to 1. During VDT use, IBI increases, and TBUT decreases, reducing the OPI and yielding an unprotected ocular surface. Copyright Sara T. Nøland. [Colour figure can be viewed at wileyonlinelibrary.com]
Dry eye disease (DED) is one of the most common reasons for ophthalmic visits (Stapleton et al. 2017) and is associated with a substantial financial burden. In the Osaka study, reduced work productivity caused by DED costs an office with approximately 600 workers 1.38 million USD annually (Uchino et al. 2014a). Historically, DED treatment mainly relied on artificial tears (Jones et al. 2017), which requires frequent instillation. Ergonomic practices and preventive measures could provide an opportunity to prevent DED development with VDT use (Rosenfield 2011).
This review aims to evaluate different preventive measures available today, assess their effectiveness and discuss future perspectives for VDT and ocular discomfort.
Methods
The search was conducted on PubMed and Embase on the 25th of November 2021, using the following search terms: (digital visual terminal* OR computer use OR screen use OR smartphone OR display OR visual display terminal* OR computer vision syndrome OR tablet OR phone OR screen time) AND (dry eye OR DED). All published articles available in English were included in the initial search results. Case reports, Letters‐to‐the‐Editor and review articles were excluded. The remaining full‐text articles were then evaluated first by title and then by abstract to ensure relevance to the topic. The studies were checked to meet the inclusion criteria: original, peer‐reviewed studies with available English, full text investigating the effect of preventive measures on either DED or CVS. An overview of the process is presented in Fig. 3.
Fig. 3.
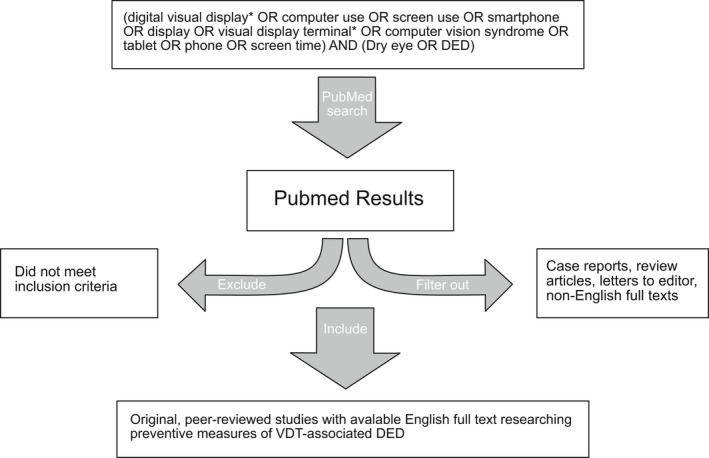
Overview of search strategy and selection of articles.
Results
The 31 articles included in this review were published between January 2004 (Guillon et al. 2004) and October 2021 (Ashwini et al. 2021) and referred to studies conducted in 19 different countries: Nepal (Kharel Sitaula & Khatri 2018), Siri Lanka (Ranasinghe et al. 2016), Saudi Arabia (Altalhi et al. 2020), India (Telles et al. 2006; Logaraj et al. 2014; Bhargava et al. 2015; Bhargava et al. 2016; Ashwini et al. 2021), Spain (Cardona et al. 2014; Ribelles et al. 2015), South Korea (Park et al. 2016), USA (Rempel et al. 2007; Yee et al. 2007; Amick et al. 2012; Menendez et al. 2012), Japan (Miyake‐Kashima et al. 2005; Hirayama et al. 2013; Shimazaki et al. 2017; Utsunomiya et al. 2017), China (Ren et al. 2018), Switzerland (Nosch et al. 2015), Portugal (Calvao‐Santos et al. 2011), Malaysia (Ang et al. 2014), United Kingdom (Guillon et al. 2004; Bilkhu et al. 2020), Jamaica (Mowatt et al. 2018), New Zealand (Wang et al. 2017), Brazil (Miura et al. 2013), Taiwan (Sun et al. 2020), Poland (Chlasta‐Twardzik et al. 2021) and Egypt (Zayed et al. 2021). Table 1 describes the nine included studies that only used subjective symptom scores for DED (Telles et al. 2006; Amick et al. 2012; Atreya et al. 2012; Menendez et al. 2012; Logaraj et al. 2014; Ranasinghe et al. 2016; Kharel Sitaula & Khatri 2018; Mowatt et al. 2018; Altalhi et al. 2020; Zayed et al. 2021). Table 2 describes the 12 prospective studies with a prolonged follow‐up that included objective measures of DED (Calvao‐Santos et al. 2011; Hirayama et al. 2013; Bhargava et al. 2015; Nosch et al. 2015; Ribelles et al. 2015; Bhargava et al. 2016; Park et al. 2016; Shimazaki et al. 2017; Utsunomiya et al. 2017; Sun et al. 2020; Ashwini et al. 2021; Chlasta‐Twardzik et al. 2021). The remaining 10 studies, featured in Table 3, were single‐visit studies measuring objective scores for DED (Guillon et al. 2004; Rempel et al. 2007; Yee et al. 2007; Miura et al. 2013; Ang et al. 2014; Cardona et al. 2014; Wang et al. 2017; Ren et al. 2018; Bilkhu et al. 2020). The number of participants in the studies varied from 11 (Cardona et al. 2014) to 2210 (Ranasinghe et al. 2016).
Table 1.
Studies with symptom scores only.
| Study (location) | Sample | Design | Preventive measure/protective effect | Follow‐Up | Outcome |
|---|---|---|---|---|---|
| Telles et al. 2006 (IN) | 281 computer workers | Single‐blinded RCT | 1 hr/day of yoga, 5 day/week | 60 days | Yoga increased visual comfort, whilst the control group decreased in visual comfort at the same time |
| Amick et al. 2011 (US) | 184 office workers | Prospective, controlled | Ergonomic chair + ergonomic training | 12 months | Those receiving both ergonomic chairs and training saw a greater improvement in their ocular symptoms than controls, and the group receiving ergonomic training only. Ergonomic training alone did not perform better than the control group |
| Menéndez et al. 2011 (US) | 154 office workers | Prospective, controlled | Ergonomic chair + ergonomic training | 12 months | The group receiving training only and the group receiving chair + ergonomic training had improved ocular symptoms after the intervention, compared to the control group |
| Sitaula et al. 2018 (NP) | 236 students (29% DED symptoms) | Cross‐sectional | <2 hr/day computer use and VDT below eye level | NA | VDT <2 hr/day was associated with substantially fewer CVS symptoms than >2 hr/day (31% versus 95%). Screen height below eye level was not found protective for CVS symptoms |
| Ranasinghe et al. 2016 (LK) | 2210 office workers (31% DED symptoms) | Cross‐sectional | VDT filter, distance to screen, ergonomics knowledge, breaks, background lighting, type of monitor | NA | Short occupational history and low daily VDT time, using a VDT filter and adjusting screen brightness to ambient light were associated with a lower prevalence of CVS. Type of VDT monitor, distance to screen, background lighting, and the number of breaks were not associated with CVS prevalence or severity |
| Altalhi et al. 2020 (SA) | 334 students (48% DED symptoms) | Cross‐sectional | Reduced glare on screen, frequent breaks, low angle of gaze | NA | Students with less screen glare on VDT were less likely to have CVS symptoms. Automatic screen brightness, taking breaks, angle of gaze, and < 6 hr/day of VDT time were not associated with fewer CVS symptoms |
| Logaraj, et al. 2014 (IN) | 416 students | Cross‐sectional | Breaks every hr | NA | Students taking breaks after 1 hr of computer had less dry eye symptoms than those breaking after 2 hr of continuous use |
| Mowatt et al. 2018 (JM) | 409 students (29% DED diagnosis) | Cross‐sectional | Angle of gaze, adjustable chair and frequency of brakes | NA | Ergonomic practices such as an adjustable chair and taking breaks at least every 3 hr were associated with reduced severity of dry eye symptoms. 43% of users holding their device in their hands had moderate dry eye symptoms, compared to only 22% using the device on a desk |
| Zayed et al. 2021 (EG) | 126 students | Cross‐sectional | Anti‐glare screen, AC, high relative humidity, adjusting screen brightness and distance to screen | NA | CVS users maintaining <51 cm to screen had less CVS symptoms. Use of AC, exposure to a windy environment and a low relative humidity was associated with ocular complaints. Using anti‐glare screens and adjusting screen brightness was associated with low CVS prevalence |
Dashed line: line splits prospective, interventional and cross‐sectional, observational studies.
AC = air‐condition, CVS = computer vision syndrome, DED = dry eye disease, DED = dry eye disease, NA = not applicable, RCT = randomized controlled trial, VDT = video display terminal.
Table 2.
Studies with prolonged follow‐up with objective measures.
| Study | Sample | Design | Preventive measure | Follow‐up | TBUT | OSS | Blink rate | Symptom score | Other study outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Bhargava et al. 2016 (IN) | 522 symptomatic VDT users | Double‐blinded RCT | Omega‐3 fatty acid | 45 days | ↑ | ↑1 | Oral omega‐3 fatty acids improved CIC, but not Schirmer score after 45 day. The group receiving omega‐3 fatty acids improved symptom scores, whilst the control group did not improve symptom scores. The improvement in symptom score for the intervention group was significant compared to the control group | ||
| Bhargava et al. 2015 (IN) | 456 with CVS symptoms | Double‐blinded RCT | Omega‐3 fatty acid | 3 months | ↑ | ↑1 | Oral omega‐3 for 3 months improved Schirmer and Nelson Grade significantly. 70% of patients receiving treatment were symptom‐free post‐intervention versus 15% in the control group | ||
| Ribelles et al. 2015 (ES) | 148 post‐menopausal women (46% with DED) | Open‐label RCT | Omega‐3 fatty acid | 45 days | ↑2 | Oral omega‐3 fatty acids reduced biomarkers of inflammation in tear fluid compared to controls. Almost 70% of the participants receiving omega‐3 improved their DED symptoms as compared to the group not receiving supplements | |||
| Park et al. 2016 (KR) | 50 healthy VDT users | Double‐blinded RCT | Oral intake of Vaccinium uliginosum | 4 weeks | — | ↑ | No change in high‐order wavefront aberration after oral intake of Vaccinium Uliginosum. Those receiving Vaccinium uliginosum had greater improvement in symptoms after VDT use than controls | ||
| Utsunomiya et al. 2017 (JP) | 63 DED patients | Prospective controlled | Diquafosol 6 times daily | 3 months | ↑ | ↑ | ↑3 | OSS and TBUT improved significantly more with diquafosol than in the control group. Tear volume and Schirmer score were not significantly increased at 3 months. Symptom score improved significantly in the intervention group but not compared to the control group. The effect was strongest during VDT use and reading | |
| Shimazaki et al. 2017 (JP) | 67 office workers | Open‐label RCT | Rebamipide and diquafosol | 8 weeks | ↑ | ↑* | ↑3 | Both mucin secretagogues improved symptoms at 8 weeks. There was no difference in symptom scores between the two groups. Both secretagogues improved TBUT after 2 and 4 weeks. Only rebamipide improved TBUT after 8 weeks | |
| Calvao‐Santos et al. 2011 (PT) | 27 symptomatic computer users | Open‐label RCT |
3 different AT Tears again®, Opticol® and Optive® |
30 days | — | ↑2 | Artificial tears decreased patients' OSDI score from baseline, but not compared to a control group receiving no treatment. The groups receiving Tears Again® and Opticol® had improved Schirmer's score compared with baseline, but not significantly when compared to control group | ||
| Hirayama et al. 2013 (JP) | 20 VDT workers with DED | Open‐label RCT | MCAD during VDT use | 5 days | ↑ | — | — | ↑4 | MCAD increased tear volume and functional VA improved. Standard VA did not improve. Blink rate increased during VDT use in the control group, but not with MCAD. Tear evaporation rate was steady with MCAD use |
| Nosch et al. 2015 (CH) | 24 computer users | Prospective controlled crossover | Blink animation programme during VDT use | 1 weeks | ↑ | ↑2 | Blink rate increased when using blink animation software compared to the placebo group. Symptom scores improved for both test and placebo groups | ||
| Sun et al. 2020 (TW) | 44 VDT user with DED symptoms | Single‐blinded RCT | 10 min EWS for 2 weeks | 2 weeks | ↑ | ↑ | ↑3 | TBUT increased from baseline in the study group, but not compared to the control group. There was no significant change in meibomian gland drop‐out score or meibum impressibility score after 2 weeks treatment | |
| Chlasta‐Twardzik et al. 2021 (PL) | 128 office and medical workers | Observational study | Various work environment factors | 1 year | ↑ | ↑2 | Low air humidity and rooms with AC increased dry eye symptoms. An increase of lighting intensity reduced the chance of an abnormal TBUT measurement | ||
| Ashwini et al. 2021 (IN) | 46 computer users with DED | Single‐blinded RCT | Blink animation programme during VDT use | 30 days | — | ↑ | ↑2 | Blink animation significantly improved OSDI score compared to baseline and control group after 15 and 30 days. There was no difference in blink rate after 15 days, but it improved significantly after 30 days compared to baseline |
1dry eye scoring system (DESS), 2ocular surface disease index (OSDI), 3dry eye related quality of life score (DEQS), 4visual analogue scale (VAS).
AC = air‐condition, AT = artificial tears, CIC = conjunctival impression cytology, CVS = computer vision syndrome, DED = dry eye disease, EWS = eyelid warming steamer, LLT = lipid‐layer thickness, MCAD = moist, cool air device, OSS = ocular surface staining, RCT = randomized controlled trial, TBUT = tear film break‐up time, TMH = tear meniscus height, VA = visual acuity, VDT = visual display terminal.
↑ significant improvement at p < 0.05, — no significant difference.
Increased in rebamipide group only.
Table 3.
Single visit studies with objective measures.
| Study | Sample | Design | Preventive measure | TBUT | OSS | Blink rate | LLT | Symptom score | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Yee et al. 2007 (US) | 40 computer users (50% DED) | Prospective, crossover design | MEGS + AT during computer use | ↑ | ↑* | ↑1 | MEGS + AT improved DED patients' comfort and prevented TBUT decrease with VDT use. No significant improvement was noted in the asymptomatic control group | ||
| Ang et al. 2014 (MY) | 26 visually healthy subjects | Prospective, controlled, crossover | Wink glasses during VDT use | — | ↑ | ↑1 | Use of wink glasses reduced ocular surface symptoms significantly compared to controls using plastic sheaths | ||
| Guillon et al. 2004 (UK) | 20 CL users | Controlled pilot study | 2% povidone preservative‐free lubricating AT | — | ↑ | AT improved visual acuity significantly. Dry eye symptoms decreased in all three installation modalities of eyedrops compared to no eyedrops | |||
| Bilkhu et al. 2020 (GB) | 40 healthy to mild dry eyes | Controlled experimental study | Heat chamber goggles and liposomal spray after VDT use | — | — | ↑ | ↑2 | LLT and TMH were unchanged during VDT use but improved with treatment. TBUT and blink rate and symptoms worsened during VDT use, but only symptoms improved with treatment | |
| Miura et al. 2013 (BR) | 15 with symptoms, 15 healthy subjects | Prospective, controlled trial | Light‐emitting power device during VDT use | — | — | ↑ | In symptomatic subjects, light‐emitting power device increased blink rates during VDT use both with and without air‐conditioning. In controls, improvement was only seen with exposure to both VDT and air‐conditioning. TBUT and OSS did not improve in either group | ||
| Wang et al. 2017 (NZ) | 44 computer workers | Prospective, controlled, crossover | USB‐desktop humidifier during VDT use | ↑ | — | ↑3 | Those using the humidifier had improved TBUT and subjective ocular comfort than the control group. The control group had a significant decrease in TBUT after VDT use. No change in TMH or lipid‐layer grade | ||
| Rempel et al. 2007 (US) | 24 healthy, young adults | Prospective, controlled, crossover | Screen viewing distance between 52 and 73 cm | ↑ | A viewing distance of between 52 and 73 cm was associated with significantly less dry eye symptoms and improved convergence recovery when compared to distances up to 84 cm and down to 44 cm | ||||
| Cardona et al. 2014 (ES) | 11 visually healthy subjects | Controlled experimental | Three different blink animation programmes during VDT use | ↑ | One of the three blink animations improved blink rate significantly from baseline with no animation. It was also the second most intrusive animation according to the subjects | ||||
| Ren et al. 2018 (CN) | 22 VDT workers with DED | Prospective crossover design | WMCG for 15 min | ↑ | ↑ | ↑4 | Use of WMCG increased TBUT, TMH and LLT compared to the control group receiving SH 0.1% eyedrops. The intervention group's improved visual comfort persisted after 60 min compared to 30 min in the control group | ||
| Miyake‐Kashima et al. 2005 (JP) | 7 visually healthy subjects | Prospective crossover design | VDT filter during screen use | — | Screen use without VDT filter resulted in a significantly worse asthenopic score, when compared to viewing with a VDT filter. Mean blink rate decreased significantly from baseline when not using a VDT filter. There was no such difference in blink rate when using with a VDT filter |
1ocular surface disease index (OSDI), 2symptom assessment in dry eye (SANDE), 3McMonnies dry eye questionnaire, 4visual analogue scale (VAS).
AT = artificial tears, CL = contact lens wear, DED = dry eye disease, LLT = lipid‐layer thickness, MEGS = microenvironment glasses, OSS = ocular surface staining, SH = sodium hyaluronate, TBUT = tear film break‐up time, TMH = tear meniscus height, VDT = video display terminal, WMCG = warming moist chamber goggles.
↑ Significant improvement at p < 0.05, — no significant difference.
Lissamine green staining showed significant results, not fluorescein staining.
Treatment interventions
Thirteen studies assessed treatment interventions and their impact on DED symptoms during VDT use (Guillon et al. 2004; Telles et al. 2006; Yee et al. 2007; Calvao‐Santos et al. 2011; Bhargava et al. 2015; Ribelles et al. 2015; Bhargava et al. 2016; Park et al. 2016; Shimazaki et al. 2017; Utsunomiya et al. 2017; Ren et al. 2018; Bilkhu et al. 2020; Sun et al. 2020). Four studies used topical treatment to alleviate VDT‐associated CVS symptoms (Guillon et al. 2004; Calvao‐Santos et al. 2011; Shimazaki et al. 2017; Utsunomiya et al. 2017). Both studies providing artificial tears to computer users found improved ocular symptoms with use, whereas clinical signs were less affected (Guillon et al. 2004; Calvao‐Santos et al. 2011). Topical secretagogue treatment with either diquafosol or rebamipide improved both symptoms and some clinical signs, such as ocular surface staining and TBUT in VDT users (Shimazaki et al. 2017; Utsunomiya et al. 2017).
Oral dietary supplements for alleviating dry eye symptoms in VDT users were assessed in four studies (Bhargava et al. 2015; Ribelles et al. 2015; Bhargava et al. 2016; Park et al. 2016). Three prospective studies found that interventions with oral omega‐3 fatty acids alleviated symptoms scores after 1.5 to 3 months (Bhargava et al. 2015; Ribelles et al. 2015; Bhargava et al. 2016). Additionally, both TBUT and Nelson grades improved at follow‐up in two of the studies (Bhargava et al. 2015; Bhargava et al. 2016). Omega‐3 use also decreased inflammation markers in the tear fluid, including interleukins (IL) IL‐1B and IL‐6 (Ribelles et al. 2015). Furthermore, one double‐blinded, randomized controlled trial (RCT) investigated the oral intake of Vaccinium uliginosum (bog bilberry) extract (Park et al. 2016). The authors found better symptom scores, but not clinical signs, in those receiving the supplement than in controls.
Devices, such as eye masks, glasses and goggles, were used to treat and prevent dry eyes in VDT users (Yee et al. 2007; Ren et al. 2018; Bilkhu et al. 2020; Sun et al. 2020). Three studies used devices that heated the eyelids (Ren et al. 2018; Bilkhu et al. 2020; Sun et al. 2020). All three found improved symptoms, and two observed increased tear film stability after use (Ren et al. 2018; Sun et al. 2020). Additionally, novel microenvironment glasses improved comfort scores, ocular surface staining and TBUT in VDT users (Yee et al. 2007). Collectively, using glasses or goggles designed to isolate the ocular surface, maintaining humidity and increasing temperature was shown to improve ocular comfort and patients' DED symptoms (Yee et al. 2007; Ren et al. 2018; Bilkhu et al. 2020; Sun et al. 2020).
Finally, one assessor‐blinded RCT found that performing 1‐hour daily yoga for 2 months led to a decrease in self‐rated visual discomfort (Telles et al. 2006).
Ergonomic and screen‐related preventive measures
Four cross‐sectional studies assessed the effect of the angle of gaze (between users' eyes and the screen) on dry eye symptoms, shown in Fig. 4 (Ranasinghe et al. 2016; Kharel Sitaula & Khatri 2018; Mowatt et al. 2018; Altalhi et al. 2020). Two of the studies found that the angle of gaze was associated with increased CVS symptoms (Kharel Sitaula & Khatri 2018; Mowatt et al. 2018). Although the authors found an association, they did not provide a conclusion regarding an ideal angle but rather indicated that an angle of gaze lower than eye level is beneficial (Kharel Sitaula & Khatri 2018; Mowatt et al. 2018). Despite this finding, the risk of dry eye symptoms with hand hand‐held VDTs, typically held lower than eye level, was greater than that with desktop VDTs (Mowatt et al. 2018). Two other studies found no correlation between the angle of gaze and CVS symptoms (Ranasinghe et al. 2016; Altalhi et al. 2020).
Fig. 4.
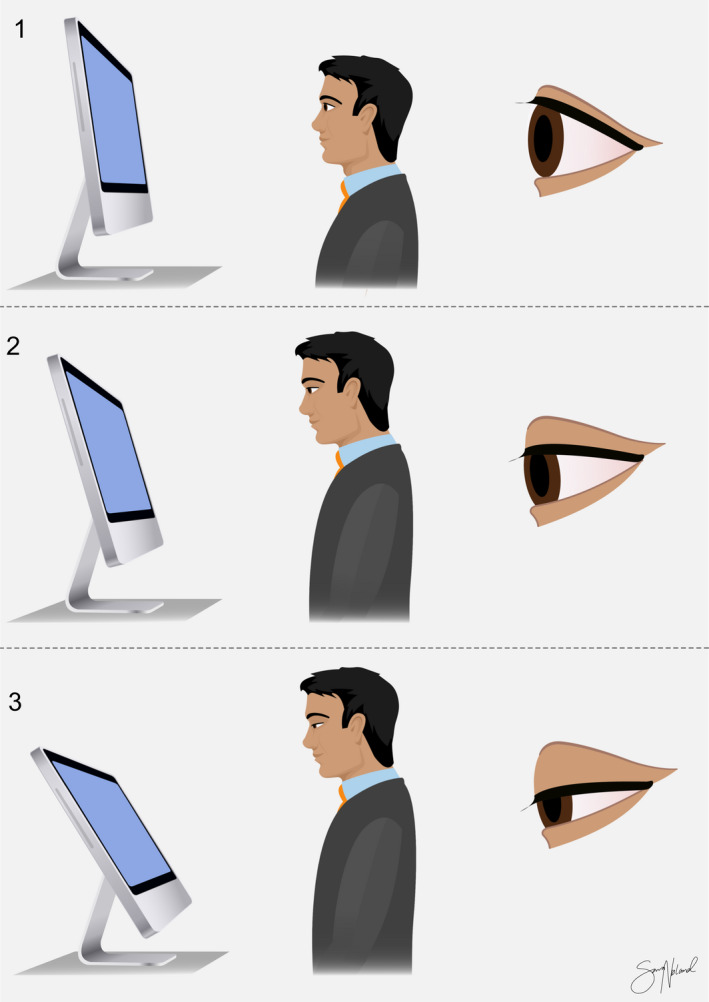
Illustrations of angle of gaze: The angle of gaze on the screen changes how exposed the ocular surface is between blinks. Copyright Sara T. Nøland. [Colour figure can be viewed at wileyonlinelibrary.com]
Three articles studied the impact of viewing distance on DED (Rempel et al. 2007; Ranasinghe et al. 2016; Zayed et al. 2021). The optimal viewing distance for reducing dry eye symptoms and CVS prevalence was found to be <51 cm in Egyptian students (Zayed et al. 2021) and 52–75 cm in a prospective study conducted in the United States(Rempel et al. 2007). In contrast, a cross‐sectional study conducted in Sri Lanka found no correlation between viewing distance and DED (Ranasinghe et al. 2016).
Four cross‐sectional (Ranasinghe et al. 2016; Mowatt et al. 2018; Altalhi et al. 2020; Zayed et al. 2021) and one prospective (Miyake‐Kashima et al. 2005) study assessed the impact of screen glare. Whereas two of the cross‐sectional studies found that CVS prevalence was higher in those not using a VDT filter or anti‐glare screen (Ranasinghe et al. 2016; Zayed et al. 2021), no significant association between DED severity and anti‐glare measures were found (Ranasinghe et al. 2016). It is reported that anti‐glare screens reduce eye strain (Miyake‐Kashima et al. 2005; Mowatt et al. 2018) and could prevent the decreased blink rate caused by VDT (Miyake‐Kashima et al. 2005). One study did not investigate the use of filters but found that glare on the screen was associated with a higher prevalence of CVS symptoms (Altalhi et al. 2020). All five studies found that reduced glare had a beneficial impact on CVS symptoms (Fig. 5).
Fig. 5.

Illustration of video display terminal (VDT) filter. Screen without VDT filter, illustrating glare (left). Screen with VDT filter, showing reduced glare (right). Copyright Sara T. Nøland. [Colour figure can be viewed at wileyonlinelibrary.com]
Other ergonomic practices have also been assessed. Two prospective works conducted in the United States with a 12‐month follow‐up investigated the use of highly adjustable chairs and office ergonomic training to reduce ocular symptoms in office workers (Amick et al. 2012; Menendez et al. 2012). Both studies found that the interventions reduced participants' visual symptoms. In one of the studies, ergonomic training alone also significantly improved visual symptoms (Menendez et al. 2012). Adjusting screen brightness with the ambient light was tied to reduced CVS prevalence (Ranasinghe et al. 2016; Zayed et al. 2021) but did not affect symptom severity (Ranasinghe et al. 2016). Another study found that increasing light intensity in the workplace reduced office workers' risk of short TBUT (Chlasta‐Twardzik et al. 2021). Air‐conditioned rooms and low air humidity in the workplace were shown to increase the risk of DED symptoms (Chlasta‐Twardzik et al. 2021) and intensify CVS symptom severity (Zayed et al. 2021). Two prospective studies found that desktop devices that increased air humidity improved patients' symptom scores and TBUT (Hirayama et al. 2013; Wang et al. 2017).
Blink animations
Blink animations were used in five studies to increase blink rate (Miura et al. 2013; Ang et al. 2014; Cardona et al. 2014; Nosch et al. 2015; Ashwini et al. 2021). Three studies used software designed to increase blink rate without interfering with the subject's concentration (Cardona et al. 2014; Nosch et al. 2015; Ashwini et al. 2021). One of the animations reminded the user to blink during VDT use by covering 20% of the screen for 0.6 seconds (Nosch et al. 2015). Blink rates recorded during VDT work improved compared to baseline and the control group. Dry eye symptoms also improved in the intervention group, and 21 out of the 24 participants decided to continue using the programme at the end of the study. Another study had a similar animation that could be customized by the user (Ashwini et al. 2021). The animation lasted for 1 second and covered 40% of the screen. Their animation significantly improved symptom scores compared to the control group and blink rate from baseline measurements. The other study assessing blink animation software also concluded that it improved the spontaneous eye blink rate (Cardona et al. 2014). The two studies aimed to increase the basal blink rate using wink glasses or a light‐emitting timer device (Miura et al. 2013; Ang et al. 2014). Wink glasses increased blink rate and improve dry eye symptom scores (Ang et al. 2014). The use of the light‐emitting timer device during VDT use increased blink rates but did not improve ocular surface staining and TBUT in DED patients (Miura et al. 2013). Although all five studies successfully increased blink rate during VDT use, only three found an improvement in patients' symptoms (Ang et al. 2014; Nosch et al. 2015; Ashwini et al. 2021).
Discussion
Video display terminal (VDT)‐associated DED is a substantial public health issue, which is likely to worsen in the coming years as the number of VDT users continues to increase. Effective treatment and evidence‐based guidelines for preventing this condition are urgently required but have been, thus far, understudied. A wide range of strategies have been explored, from more targeted measures, such as VDT filters (Ranasinghe et al. 2016; Mowatt et al. 2018; Altalhi et al. 2020), blink animations (Cardona et al. 2014; Nosch et al. 2015) and heat therapy goggles (Ren et al. 2018; Bilkhu et al. 2020) to lifestyle changes, such as 1 hr of daily yoga (Telles et al. 2006). Although varying substantially in design and intervention, nearly all the included articles noted an improvement in patients' comfort and symptoms with the intervention, indicating that VDT‐associated DED can be prevented, or at least symptoms can be reduced. As illustrated in Fig. 1, the pathophysiology of the disease can be explained through the vicious circle of DED (Bron et al. 2017). Most of the included studies aimed to improve tear film stability and reduce tear evaporation rate (Guillon et al. 2004; Yee et al. 2007; Hirayama et al. 2013; Miura et al. 2013; Ang et al. 2014; Cardona et al. 2014; Nosch et al. 2015; Wang et al. 2017; Ren et al. 2018; Bilkhu et al. 2020). Stimulation of endogenous mucin production (Shimazaki et al. 2017; Utsunomiya et al. 2017) successfully increased tear film stability and reduced tear evaporation rate during VDT use. Figure 6 highlights interventions used to address VDT‐associated dry eye and their mechanisms of action.
Fig. 6.
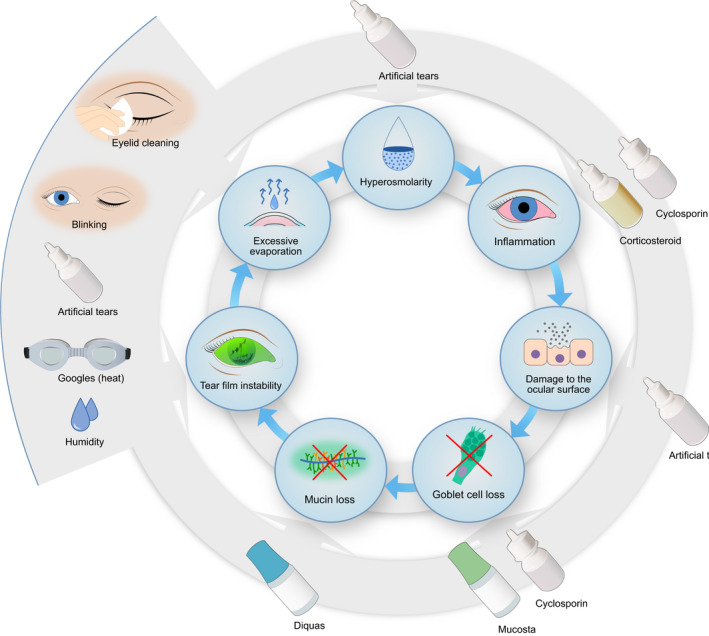
Illustration of the vicious circle with preventive measures. The outer circle shows the target of the different preventive measures. Copyright Sara T. Nøland. [Colour figure can be viewed at wileyonlinelibrary.com]
Measures aimed at improving blink rate and tear properties
Several of the included studies showed a substantial decrease in the blink rate during VDT use (Patel et al. 1991; Tsubota & Nakamori 1993; Bentivoglio et al. 1997; Tsubota 1998; Doughty 2001; Freudenthaler et al. 2003; Schlote et al. 2004; Cardona et al. 2014) and an increased number of incomplete blinks (Bilkhu et al. 2020). Blink frequency has been shown to fall from 26 blinks/min in conversation to 14.5 blinks/min during VDT use (Bentivoglio et al. 1997). Physiological blinking is essential for maintaining a healthy tear film and ocular surface (Pult et al. 2013; Bron et al. 2014). After a blink, water evaporates from the tear film, and the tear film eventually breaks up. The combination of a decreased blink rate and a shortened TBUT is a particularly unfortunate combination seen with VDT use (Ang et al. 2014).
Meibomian gland lipids are essential for maintaining a healthy and stable tear film. However, the lipids are temperature sensitive and thicken at lower temperatures, reducing tear film stability (Butovich et al. 2010). Devices that heated and insulated the eyelids were found to decrease VDT‐related ocular symptoms and stabilize the tear film (Yee et al. 2007; Ren et al. 2018; Bilkhu et al. 2020; Sun et al. 2020). Two studies increased tear film stability and subjective comfort using humidifier devices (Hirayama et al. 2013; Wang et al. 2017). In a study using a USB‐powered desktop humidifier, tear film stability and subjective comfort improved in computer users with use (Wang et al. 2017). Overall, the studies using goggles or desktop devices to decrease excessive evaporation and improve tear film stability showed promising results (Yee et al. 2007; Hirayama et al. 2013; Wang et al. 2017; Ren et al. 2018; Bilkhu et al. 2020; Sun et al. 2020). However, only two studies had a follow‐up period (Hirayama et al. 2013; Sun et al. 2020), whereas the remainder were single‐visit studies (Yee et al. 2007; Wang et al. 2017; Ren et al. 2018; Bilkhu et al. 2020). Larger, prospective investigations are required to assess the long‐term benefits of these preventive measures.
Artificial tears aim to enhance tear film stability by increasing the aqueous layer's volume and stabilizing the lipid layer (Pucker et al. 2016) In the included studies, eye drops effectively improved dry eye symptoms in VDT users (Guillon et al. 2004; Calvao‐Santos et al. 2011). However, in one of the studies, an RCT with 27 DED patients, the change in TBUT, Schirmer and comfort scores with artificial tears treatment were not significantly different from untreated controls (Calvao‐Santos et al. 2011). Behavioural changes due to study enrolment were mentioned as possible explanations of this finding. Because DED with tear film instability affects all tear film layers, a combination of artificial tears targeting each layer was suggested to be the best treatment in this patient group (Calvao‐Santos et al. 2011).
All five studies aiming to improve patients' blink rate were successful in doing so, using different tools (Miura et al. 2013; Ang et al. 2014; Cardona et al. 2014; Nosch et al. 2015; Ashwini et al. 2021). Three of them found that dry eye symptoms improved with increased blink rates (Ang et al. 2014; Nosch et al. 2015; Ashwini et al. 2021). However, only one study had a sample size larger than 30 participants (46 participants) (Ashwini et al. 2021). Additionally, none of the three studies measuring TBUT were able to find an association between increased blink rates and TBUT values (Miura et al. 2013; Ang et al. 2014; Ashwini et al. 2021). Nevertheless, the research using wink glasses showed results indicating that they can maintain tear film stability during VDT use (Ang et al. 2014). Because these glasses aimed to stimulate a blink rate of 12 blinks per minute by blocking the subject's view if no blink had occurred in 5 seconds, it is also important to consider these modalities´ invasiveness, which may affect patient compliance. Although the glasses were effective in improving both blink rate and symptom score after 20 min of reading on a computer, this method could present challenges in everyday life. Blink animations were also used to increase blink rate. The animations were able to improve blink rate with a low level of intrusiveness (Cardona et al. 2014; Nosch et al. 2015; Ashwini et al. 2021). It was noted that an animation covering 20% of the screen and lasting for 0.6 seconds did not interfere with the user's concentration (Nosch et al. 2015). The participants tolerated the animation well and had improved blink rates. In a later study, an updated version of the software where the user could adjust the coverage, frequency and duration, was used (Ashwini et al. 2021). With 8 animations/min, the DED symptoms remained improved up to 1 month after cessation, but no improvement in TBUT was observed. Spontaneous blinks are thought to be regulated by a central pacemaker, influenced by cognitive and visual tasks (Veltman & Gaillard 1998; Doughty 2001). Further, it is hypothesized that promoting spontaneous blinks is less intrusive than triggering reflexive blinking (Cardona et al. 2014). Animations designed to increase blink rates were overall successful (Cardona et al. 2014; Nosch et al. 2015; Ashwini et al. 2021). However, two of the trials had small sample sizes with only 11 (Cardona et al. 2014) and 24 (Nosch et al. 2015) participants, and the longest follow‐up time was only 1 month (Ashwini et al. 2021).
In sum, measures that aimed to increase blink rate and improve tear film stability successfully increased patients' subjective comfort and blink rates. These measures might, thus, play an important role in preventing VDT‐associated DED.
Inflammation and mucin loss
Inflammation and hyperosmolarity are key factors in DED (Bron et al. 2017), and it is important to implement solutions that reduce ocular inflammation in VDT users. The inflammatory cascade causes a loss of goblet cells and mucins, leading to damage to the conjunctival epithelium (Bron et al. 2017).
Across the three studies assessing omega‐3 supplements, omega‐3 was found to be effective at improving symptoms and reducing markers of ocular inflammation (Bhargava et al. 2015; Ribelles et al. 2015; Bhargava et al. 2016). Omega‐3 is an essential fatty acid that can act as the substrate for the production of pro‐resolving mediators such as resolvins and block the synthesis of proinflammatory mediators such as IL‐1 and tumour necrosis factor (TNF)‐α, which are elevated in the tear film of DED patients (Calder 2003; Rosenberg & Asbell 2010; Spite et al. 2014). Oral supplements reduced inflammation markers in the tear film of VDT users (Ribelles et al. 2015), improved patients' dry eye symptoms (Bhargava et al. 2015; Bhargava et al. 2016) and increased tear film stability (Bhargava et al. 2015). Further, high doses of omega‐3 fatty acids were shown to prevent changes to the meibum fatty acid composition, potentially reducing inflammation from blocked meibomian gland ducts (Bhargava et al. 2016). All three studies had low drop‐out rates and increased patient comfort, making oral omega‐3 a potential option for tackling VDT‐associated DED (Bhargava et al. 2015; Ribelles et al. 2015; Bhargava et al. 2016).
Mucins play an essential role in preventing desiccation of the ocular surface by holding water and protecting the eye from environmental conditions (O'Neil et al. 2019). The two included studies aiming to prevent mucin loss showed that topical secretagogues were effective in alleviating DED symptoms in VDT users (Shimazaki et al. 2017; Utsunomiya et al. 2017). Office workers spending prolonged periods of time using VDTs have been found to have lower mucin concentrations in their tears than those with fewer hours on VDTs (Uchino et al. 2014b). In a Japanese study comparing rebamipide and diquafosol, some participants preferred using diquafosol over rebamipide due to the opacity of the rebamipide emulsion (Shimazaki et al. 2017). Ensuring patient comfort is essential because DED often requires long‐term treatment. The other prospective study found that diquafosol improved ocular surface staining, TBUT and THM in office workers with DED (Utsunomiya et al. 2017). Due to the fact that VDT use has been shown to decrease mucin concentration (Uchino et al. 2014b), measures promoting mucin secretion could be important in treating or preventing VDT‐associated DED. The treatments aiming to reduce inflammation and mucin loss all had little drop‐out in the included studies. These measures could supplement each other, helping reduce the burden of VDT‐related ocular complaints.
Occupational health
The 20–20‐20 rule is recommended for preventing DED and CVS during VDT use by the American Optometric Association, American Academy of Ophthalmology and Canadian Association of Optometrists (Reddy et al. 2013). The rule dictates that every 20 min the user should take a 20‐second break and focus their eyes on something 20 feet away. Despite being frequently recommended, there have been no peer‐reviewed studies validating the effects of this approach. However, some studies have shown that regular breaks during VDT use were associated with fewer DED symptoms (Logaraj et al. 2014; Ranasinghe et al. 2016; Kharel Sitaula & Khatri 2018; Zayed et al. 2021). These studies were all cross‐sectional and found breaks every 30–60 min of VDT used to be sufficient.
Other ergonomic practices, such as adjusting viewing distance, positively impacted dry eye symptoms (Rempel et al. 2007). There is no consensus on the optimal eye‐to‐screen distance. The results varied from a recommended distance of <51 cm (Zayed et al. 2021) to a distance between 52–73 cm (Rempel et al. 2007), and one study found no correlation between viewing distance and DED (Ranasinghe et al. 2016). It is, thus, difficult to assess the clinical relevance of the results related to eye‐to‐screen distance. More research is necessary to determine the optimal distance to a screen and the effect of screen quality and font size on this distance.
The exposed area of the eye is proportional to the angle of opening, which is determined by the angle of gaze. A study by Tsubota and Nakamori found that the tear evaporation rate was proportional to the exposed area of the ocular surface (Tsubota & Nakamori 1995). When viewing computers, users tended to adopt an angle of gaze that exposes an increased area of the ocular surface compared to reading a book (Gowrisankaran & Sheedy 2015). The studies assessing angle of gaze had conflicting results (Ranasinghe et al. 2016; Kharel Sitaula & Khatri 2018; Mowatt et al. 2018; Altalhi et al. 2020). Two studies found an association between elevated gaze angle (above eye level) and increased CVS symptoms (Kharel Sitaula & Khatri 2018; Mowatt et al. 2018). However, two other studies found no significant correlation between different gaze angles and CVS (Ranasinghe et al. 2016; Altalhi et al. 2020), despite a non‐significant higher mean angle of gaze amongst those with CVS compared to those without (Ranasinghe et al. 2016; Mowatt et al. 2018). None of the included studies assessed ergonomic practices for DED specifically, but rather the broader definition of CVS. Common CVS symptoms, including neck pain and blurred vision, have been reported to increase when looking upwards to a VDT (Rosenfield 2011; Mowatt et al. 2018). All the included studies assessing the angle of gaze and its effectiveness as a preventive measure for screen‐related DED were cross‐sectional (Ranasinghe et al. 2016; Kharel Sitaula & Khatri 2018; Mowatt et al. 2018; Altalhi et al. 2020). The results of the included studies seem to support that adopting an ideal angle of gaze could be an effective preventive measure, particularly when avoiding CVS symptoms such as neck pain and blurred vision, but more evidence is still required to provide effective guidelines.
Highly adjustable chairs and ergonomic training alleviated CVS symptoms and DED (Amick et al. 2012; Menendez et al. 2012). Highly adjustable chairs might give users a better opportunity to adjust their eye location, thereby minimizing glare. Height adjustability was thought to offer a better viewing angle for the individual user. The positive effect on visual symptoms persisted 12 months after the initial intervention in the groups receiving ergonomic training and adjustable chairs (Amick et al. 2012; Menendez et al. 2012), potentially because the participants adopted long‐term behaviour changes (Menendez et al. 2012). The preventive measures used in these studies are relatively user friendly, and after 12 months, only 15% of the participants had dropped out of the study (Menendez et al. 2012). Both studies showed promising results in preventing VDT‐associated DED and increasing the productivity of office workers (Amick et al. 2012; Menendez et al. 2012).
Anti‐glare measures are easy and cheap measures that could help VDT users. More glare and screen reflections increase eye strain (Mork et al. 2016), and VDT filters reduce or eliminate screen glare and reflection (Zunjic et al. 2012). Glare‐reducing filters have been shown to increase blink rate during VDT use (Miyake‐Kashima et al. 2005). All studies found that VDT filters or low glare improved symptoms or decreased CVS severity (Ranasinghe et al. 2016; Mowatt et al. 2018; Altalhi et al. 2020). None of the studies investigated the connection between VDT filters and DED but rather focused on CVS prevalence and severity. All three were cross‐sectional and found that glare on screens was associated with worsening of CVS measures. Furthermore, Ranasinghe et al. found that automatic adjustment of screen brightness to ambient light conditions reduced the CVS prevalence (Ranasinghe et al. 2016). More evidence on the efficacy of VDT filters for the prevention of DED is required, particularly from longitudinal interventional studies. Still, filters may be beneficial for many, especially considering the low cost and ease of use.
A high drop‐out rate can signify that the preventive measure is difficult to adopt. Forty per cent of participants dropped out when instructed to perform yoga for 1 hr per day, 5 days a week, for 60 days (Telles et al. 2006). Although the 174 participants who finished the 60‐day trial had improved symptom scores, the high drop‐out rate illustrates the importance of easy‐to‐use preventive measures for VDT‐associated DED. Simple behaviour‐related preventive measures such as ergonomic chairs and training were effective for extended periods (Amick et al. 2012; Menendez et al. 2012) and could be important for preventing VDT‐related ocular discomfort.
Limitations and future directions
Only English full‐text articles were included in the literature search. This approach was necessary to ensure that correct information was included in this review. Furthermore, these included studies had limitations that are important to consider. A limitation of the prospective studies was the short follow‐up time. The longest follow‐up time was 12 months, and the shortest 5 days (Amick et al. 2012; Menendez et al. 2012; Hirayama et al. 2013). Several of the prospective studies had a sample size under 30 subjects (Guillon et al. 2004; Miyake‐Kashima et al. 2005; Rempel et al. 2007; Calvao‐Santos et al. 2011; Rosenfield et al. 2012; Hirayama et al. 2013; Miura et al. 2013; Ang et al. 2014; Cardona et al. 2014; Nosch et al. 2015; Ren et al. 2018). In addition, several studies did not disclose what kind of VDT the participants were using or for how many hours per day (Rempel et al. 2007; Miura et al. 2013; Ang et al. 2014; Cardona et al. 2014; Bhargava et al. 2016; Utsunomiya et al. 2017; Wang et al. 2017; Bilkhu et al. 2020; Sun et al. 2020), making direct comparisons challenging (Hirayama et al. 2013; Bhargava et al. 2016; Ren et al. 2018; Sun et al. 2020). Of the studies included, few used validated questionnaires to assess symptoms scores. Furthermore, nine studies only assessed symptoms when measuring the preventive measures´ efficacy (Telles et al. 2006; Amick et al. 2012; Menendez et al. 2012; Logaraj et al. 2014; Ranasinghe et al. 2016; Kharel Sitaula & Khatri 2018; Mowatt et al. 2018; Altalhi et al. 2020), whereas two studies did not include subjective symptom scores (Miura et al. 2013; Cardona et al. 2014).
Some of the simplest preventive measures for screen‐related DED, such as VDT filters, angle of gaze and screen brightness, were only included in cross‐sectional studies (Ranasinghe et al. 2016; Kharel Sitaula & Khatri 2018; Mowatt et al. 2018; Altalhi et al. 2020; Zayed et al. 2021). There may be several confounding factors not accounted for, and these studies do not assess long‐term effects. Additionally, none of the studies aimed to individualize the preventive measures assessed. DED is highly complex and the ocular surface hosts a wide variety of microorganisms (Lu & Liu 2016). More knowledge about the ocular surface microbiome may, therefore, unlock exciting new opportunities to individualize the treatment and prevention of DED (Borroni et al. 2019). Furthermore, faecal microbial transplants have been shown to improve DED symptoms in 50% of patients with immune‐mediated DED in a recent study (Watane et al. 2022).
High‐quality, placebo‐controlled trials with larger sample sizes are required to validate the effect of the preventive measures discussed in the current review. Of particular importance are studies assessing ergonomic interventions and preventive measures that are cheap and easily accessible for the general population.
Conclusion
Several different strategies can be implemented to improve tear film stability and decrease tear evaporation, mucin loss and ocular surface inflammation in VDT users. Based on the current literature, blink animation programmes, oral intake of omega‐3 fatty acids and adjustable chairs with ergonomic training appear to be effective at alleviating DED symptoms. These approaches are simple to use and have high patient compliance. Because they act on different parts of the vicious cycle of dry eye, these measures could complement each other. More research is necessary to assess the effect of the 20‐20‐20 rule, optimal angle of gaze, VDT filters and devices aiming to increase humidity and provide heat to the ocular surface. Thus, this review highlights the need for robust, randomized, double‐blinded clinical trials assessing these measures because VDT‐associated DED is likely to increase in the coming decades.
Irrespective of potential conflicts of interest, for the sake of transparency: Tor Paaske Utheim is the co‐founder and co‐owner of The Norwegian dry eye clinic and the Clinic of eye health, Oslo, Norway, which delivers talks for and/or receives financial support from the following: ABIGO, Alcon, Allergan, AMWO, Bausch&Lomb, Bayer, European school for advanced studies in ophthalmology, InnZ Medical, Medilens Nordic, Medistim, Novartis, Santen, Specsavers, Shire Pharmaceuticals and Thea Laboratories. He has served on the global scientific advisory board for Novartis and Alcon as well as the European advisory board for Shire Pharmaceuticals. Utheim is the Norwegian Global Ambassador for Tear Film and Ocular Surface Society (TFOS), a Board Member of the International Ocular Surface Society, a Consultant at the Norwegian Association for the Blind and Partially Sighted, and the Editor‐in‐Chief of Oftalmolog, an eye journal distributed to all eye doctors in the Nordic region since 1980.
References
- Altalhi A, Khayyat W, Khojah O, Alsalmi M & Almarzouki H (2020): Computer vision syndrome among health sciences students in Saudi Arabia: prevalence and risk factors. Cureus 12: e7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amick BC 3rd, Menendez CC, Bazzani L, Robertson M, DeRango K, Rooney T & Moore A (2012): A field intervention examining the impact of an office ergonomics training and a highly adjustable chair on visual symptoms in a public sector organization. Appl Ergon 43: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CK, Mohidin N & Chung KM (2014): Effects of wink glass on blink rate, nibut and ocular surface symptoms during visual display unit use. Curr Eye Res 39: 879–884. [DOI] [PubMed] [Google Scholar]
- Ashwini DL, Ve RS, Nosch D & Wilmot N (2021): Efficacy of blink software in improving the blink rate and dry eye symptoms in visual display terminal users ‐ a single‐blinded randomized control trial. Indian J Ophthalmol 69: 2643–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya K, Sitaula BK, Overgaard H, Bajracharya RM & Sharma S (2012): Knowledge, attitude and practices of pesticide use and acetylcholinesterase depression among farm workers in Nepal. Int J Environ Health Res 22: 401–415. [DOI] [PubMed] [Google Scholar]
- Bentivoglio AR, Bressman SB, Cassetta E, Carretta D, Tonali P & Albanese A (1997): Analysis of blink rate patterns in normal subjects. Mov Disord 12: 1028–1034. [DOI] [PubMed] [Google Scholar]
- Bhargava R, Kumar P & Arora Y (2016): Short‐term omega 3 fatty acids treatment for dry eye in young and middle‐aged visual display terminal users. Eye Contact Lens 42: 231–236. [DOI] [PubMed] [Google Scholar]
- Bhargava R, Kumar P, Phogat H, Kaur A & Kumar M (2015): Oral omega‐3 fatty acids treatment in computer vision syndrome related dry eye. Cont Lens Anterior Eye 38: 206–210. [DOI] [PubMed] [Google Scholar]
- Bilkhu P, Wolffsohn J & Purslow C (2020): Provocation of the ocular surface to investigate the evaporative pathophysiology of dry eye disease. Cont Lens Anterior Eye 44: 24–29. [DOI] [PubMed] [Google Scholar]
- Bjordal O, Norheim KB, Rodahl E, Jonsson R & Omdal R (2020): Primary Sjogren's syndrome and the eye. Surv Ophthalmol 65: 119–132. [DOI] [PubMed] [Google Scholar]
- Blehm C, Vishnu S, Khattak A, Mitra S & Yee RW (2005): Computer vision syndrome: a review. Surv Ophthalmol 50: 253–262. [DOI] [PubMed] [Google Scholar]
- Borroni D, Romano V, Kaye SB et al. (2019): Metagenomics in ophthalmology: current findings and future prospectives. BMJ Open Ophthalmol 4: e000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, de Paiva CS, Chauhan SK et al. (2017): TFOS DEWS II pathophysiology report. Ocul Surf 15: 438–510. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tomlinson A, Foulks GN, Pepose JS, Baudouin C, Geerling G, Nichols KK & Lemp MA (2014): Rethinking dry eye disease: a perspective on clinical implications. Ocul Surf 12: S1–S31. [DOI] [PubMed] [Google Scholar]
- Butovich IA, Arciniega JC & Wojtowicz JC (2010): Meibomian lipid films and the impact of temperature. Invest Ophthalmol Vis Sci 51: 5508–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC (2003): N‐3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids 38: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvao‐Santos G, Borges C, Nunes S, Salgado‐Borges J & Duarte L (2011): Efficacy of 3 different artificial tears for the treatment of dry eye in frequent computer users and/or contact lens users. Eur J Ophthalmol 21: 538–544. [DOI] [PubMed] [Google Scholar]
- Cardona G, Gomez M, Quevedo L & Gispets J (2014): Effects of transient blur and VDT screen luminance changes on eyeblink rate. Cont Lens Anterior Eye 37: 363–367. [DOI] [PubMed] [Google Scholar]
- Chlasta‐Twardzik E, Gorecka‐Niton A, Nowinska A & Wylegala E (2021): The influence of work environment factors on the ocularsurface in a one‐year follow‐up prospective clinical study. Diagnostics (Basel) 11: article number: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JP, Nichols KK, Akpek EK et al. (2017): TFOS DEWS II definition and classification report. Ocul Surf 15: 276–283. [DOI] [PubMed] [Google Scholar]
- Doughty MJ (2001): Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optom Vis Sci 78: 712–725. [DOI] [PubMed] [Google Scholar]
- Fjaervoll H, Fjaervoll K, Magno M, Moschowits E, Vehof J, Dartt DA & Utheim TP (2021): The association between visual display terminal use and dry eye: a review. Acta Ophthalmol. [DOI] [PubMed] [Google Scholar]
- Freudenthaler N, Neuf H, Kadner G & Schlote T (2003): Characteristics of spontaneous eyeblink activity during video display terminal use in healthy volunteers. Graefes Arch Clin Exp Ophthalmol 241: 914–920. [DOI] [PubMed] [Google Scholar]
- Gowrisankaran S & Sheedy JE (2015): Computer vision syndrome: a review. Work 52: 303–314. [DOI] [PubMed] [Google Scholar]
- Guillon M, Maissa C, Pouliquen P & Delval L (2004): Effect of povidone 2% preservative‐free eyedrops on contact lens wearers with computer visual syndrome: pilot study. Eye Contact Lens 30: 34–39. [DOI] [PubMed] [Google Scholar]
- Hirayama M, Murat D, Liu Y, Kojima T, Kawakita T & Tsubota K (2013): Efficacy of a novel moist cool air device in office workers with dry eye disease. Acta Ophthalmol 91: 756–762. [DOI] [PubMed] [Google Scholar]
- Jones L, Downie LE, Korb D et al. (2017): TFOS DEWS II management and therapy report. Ocul Surf 15: 575–628. [DOI] [PubMed] [Google Scholar]
- Kharel Sitaula R & Khatri A (2018): Knowledge, attitude and practice of computer vision syndrome among medical students and its impact on ocular morbidity. J Nepal Health Res Counc 16: 291–296. [PubMed] [Google Scholar]
- Logaraj M, Madhupriya V & Hegde S (2014): Computer vision syndrome and associated factors among medical and engineering students in chennai. Ann Med Health Sci Res 4: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LJ & Liu J (2016): Human microbiota and ophthalmic disease. Yale J Biol Med 89: 325–330. [PMC free article] [PubMed] [Google Scholar]
- Menendez CC, Amick BC 3rd, Robertson M, Bazzani L, DeRango K, Rooney T & Moore A (2012): A replicated field intervention study evaluating the impact of a highly adjustable chair and office ergonomics training on visual symptoms. Appl Ergon 43: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura DL, Hazarbassanov RM, Yamasato CK, Bandeira e Silva F, Godinho CJ & Gomes JA (2013): Effect of a light‐emitting timer device on the blink rate of non‐dry eye individuals and dry eye patients. Br J Ophthalmol 97: 965–967. [DOI] [PubMed] [Google Scholar]
- Miyake‐Kashima M, Dogru M, Nojima T, Murase M, Matsumoto Y & Tsubota K (2005): The effect of antireflection film use on blink rate and asthenopic symptoms during visual display terminal work. Cornea 24: 567–570. [DOI] [PubMed] [Google Scholar]
- Mork R, Bruenech JR & Thorud HM (2016): Effect of direct glare on orbicularis oculi and trapezius during computer reading. Optom Vis Sci 93: 738–749. [DOI] [PubMed] [Google Scholar]
- Mowatt L, Gordon C, Santosh ABR & Jones T (2018): Computer vision syndrome and ergonomic practices among undergraduate university students. Int J Clin Pract 72: e13035. [DOI] [PubMed] [Google Scholar]
- Nosch DS, Foppa C, Toth M & Joos RE (2015): Blink animation software to improve blinking and dry eye symptoms. Optom Vis Sci 92: e310–e315. [DOI] [PubMed] [Google Scholar]
- O'Neil EC, Henderson M, Massaro‐Giordano M & Bunya VY (2019): Advances in dry eye disease treatment. Curr Opin Ophthalmol 30: 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Gu N, Lim CY, Oh JH, Chang M, Kim M & Rhee MY (2016): The effect of Vaccinium uliginosum extract on tablet computer‐induced asthenopia: randomized placebo‐controlled study. BMC Complement Altern Med 16: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Henderson R, Bradley L, Galloway B & Hunter L (1991): Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci 68: 888–892. [DOI] [PubMed] [Google Scholar]
- Pucker AD, Ng SM & Nichols JJ (2016): Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev 2: CD009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pult H, Riede‐Pult BH & Murphy PJ (2013): The relation between blinking and conjunctival folds and dry eye symptoms. Optom Vis Sci 90: 1034–1039. [DOI] [PubMed] [Google Scholar]
- Ranasinghe P, Wathurapatha WS, Perera YS, Lamabadusuriya DA, Kulatunga S, Jayawardana N & Katulanda P (2016): Computer vision syndrome among computer office workers in a developing country: an evaluation of prevalence and risk factors. BMC Res Notes 9: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SC, Low CK, Lim YP, Low LL, Mardina F & Nursaleha MP (2013): Computer vision syndrome: a study of knowledge and practices in university students. Nepal J Ophthalmol 5: 161–168. [DOI] [PubMed] [Google Scholar]
- Rempel D, Willms K, Anshel J, Jaschinski W & Sheedy J (2007): The effects of visual display distance on eye accommodation, head posture, and vision and neck symptoms. Hum Factors 49: 830–838. [DOI] [PubMed] [Google Scholar]
- Ren Y, Chen J, Zheng Q & Chen W (2018): Short‐term effect of a developed warming moist chamber goggle for video display terminal‐associated dry eye. BMC Ophthalmol 18: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelles A, Galbis‐Estrada C, Parras MA, Vivar‐Llopis B, Marco‐Ramirez C & Diaz‐Llopis M (2015): Ocular surface and tear film changes in older women working with computers. Biomed Res Int 2015: 467039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES & Asbell PA (2010): Essential fatty acids in the treatment of dry eye. Ocul Surf 8: 18–28. [DOI] [PubMed] [Google Scholar]
- Rosenfield M (2011): Computer vision syndrome: a review of ocular causes and potential treatments. Ophthalmic Physiol Opt 31: 502–515. [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Hue JE, Huang RR & Bababekova Y (2012): The effects of induced oblique astigmatism on symptoms and reading performance while viewing a computer screen. Ophthalmic Physiol Opt 32: 142–148. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M & Nichols KK (2011): The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 52: 1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlote T, Kadner G & Freudenthaler N (2004): Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol 242: 306–312. [DOI] [PubMed] [Google Scholar]
- Shimazaki J, Seika D, Saga M, Fukagawa K, Sakata M, Iwasaki M & Okano T (2017): A prospective, randomized trial of two mucin secretogogues for the treatment of dry eye syndrome in office workers. Sci Rep 7: 15210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Claria J & Serhan CN (2014): Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 19: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Alves M, Bunya VY et al. (2017): TFOS DEWS II epidemiology report. Ocul Surf 15: 334–365. [DOI] [PubMed] [Google Scholar]
- Sun CC, Lee CY, Hwang YS, Michihito I, Tagami K & Hsiao CH (2020): Effect of warming eyelids on tear film stability and quality of life in visual display terminal users: a randomized controlled trial. Sci Rep 10: 16919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telles S, Naveen KV, Dash M, Deginal R & Manjunath NK (2006): Effect of yoga on self‐rated visual discomfort in computer users. Head Face Med 2: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota K (1998): Tear dynamics and dry eye. Prog Retin Eye Res 17: 565–596. [DOI] [PubMed] [Google Scholar]
- Tsubota K & Nakamori K (1993): Dry eyes and video display terminals. N Engl J Med 328: 584. [DOI] [PubMed] [Google Scholar]
- Tsubota K & Nakamori K (1995): Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol 113: 155–158. [DOI] [PubMed] [Google Scholar]
- Uchino M, Schaumberg DA, Dogru M et al. (2008): Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 115: 1982–1988. [DOI] [PubMed] [Google Scholar]
- Uchino M, Uchino Y, Dogru M et al. (2014a): Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol 157: 294–300. [DOI] [PubMed] [Google Scholar]
- Uchino M, Yokoi N, Uchino Y et al. (2013): Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol 156: 759–766. [DOI] [PubMed] [Google Scholar]
- Uchino Y, Uchino M, Yokoi N et al. (2014b): Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol 132: 985–992. [DOI] [PubMed] [Google Scholar]
- Utsunomiya T, Kawahara A, Hanada K & Yoshida A (2017): Effects of diquafosol ophthalmic solution on quality of life in dry eye assessed using the dry eye‐related quality‐of‐life score questionnaire: effectiveness in patients while reading and using visual display terminals. Cornea 36: 908–914. [DOI] [PubMed] [Google Scholar]
- Veltman JA & Gaillard AW (1998): Physiological workload reactions to increasing levels of task difficulty. Ergonomics 41: 656–669. [DOI] [PubMed] [Google Scholar]
- Wang MTM, Chan E, Ea L, Kam C, Lu Y, Misra SL & Craig JP (2017): Randomized trial of desktop humidifier for dry eye relief in computer users. Optom Vis Sci 94: 1052–1057. [DOI] [PubMed] [Google Scholar]
- Watane A, Cavuoto KM, Rojas M, Dermer H, Day JO, Banerjee S & Galor A (2022): Fecal microbial transplant in individuals with immune‐mediated dry eye. Am J Ophthalmol 233: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee RW, Sperling HG, Kattek A, Paukert MT, Dawson K, Garcia M & Hilsenbeck S (2007): Isolation of the ocular surface to treat dysfunctional tear syndrome associated with computer use. Ocul Surf 5: 308–315. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Uchino M, Uchino Y et al. (2015): Importance of tear film instability in dry eye disease in office workers using visual display terminals: the Osaka study. Am J Ophthalmol 159: 748–754. [DOI] [PubMed] [Google Scholar]
- Zayed HAM, Saied SM, Younis EA & Atlam SA (2021): Digital eye strain: prevalence and associated factors among information technology professionals. Environ Sci Pollut Res Int: Egypt. [DOI] [PubMed] [Google Scholar]
- Zunjic A, Ristic L & Milanovic DD (2012): Effects of screen filter on visibility of alphanumeric presentation on CRT and LCD monitors. Work 41(Suppl 1): 3553–3559. [DOI] [PubMed] [Google Scholar]


