Abstract
African swine fever (ASF) and classical swine fever (CSF) are two major transboundary animal diseases of swine with important socioeconomic consequences at farm, subnational and national level. The objective of this study was to evaluate the direct cost of outbreaks and their control at country/regional level in four countries: namely CSF in Colombia in 2015–2016, the retrospective cost of ASF in the Philippines in 2019 and in a province of Vietnam in 2020 and a hypothetical ASF scenario in one region in North Macedonia, using the newly developed Outbreak Costing Tool (OutCosT). The tool calculates the costs of 106 different items, broken down by up to four types of farms, and by who assumes the cost (whether veterinary services, farmers or other stakeholders).
The total cost of CSF in Colombia was US$ 3.8 million, of which 88% represented the cost of the vaccination campaign. For ASF, there were wide differences between countries: US$ 8,26,911 in Lao Cai (Vietnam), US$ 33,19,666 in North Macedonia and over US$ 58 million in the Philippines. While in the Philippines and Vietnam, 96–98% of the cost occurred in the affected farms, the highest expenditure in North Macedonia scenario was the movement control of the neighbouring and at‐risk farms (77%). These important differences between countries depend on the spread of the disease, but also on the production systems affected and the measures applied. Apart from the financial cost, these diseases have other negative impacts, especially in the livelihoods of smallholder farms. The OutCosT tool also allows users to evaluate qualitatively other important aspects related to the epidemics, such as the impact on human health, the environment, animal welfare, socioeconomic vulnerability, trading and political response.
OutCosT, which is a FAO corporate tool (available online at: https://www.fao.org/fileadmin/user_upload/faoweb/animal‐health/OutCosT_PIG.xlsx), can be an important tool to support country authorities to rapidly respond to a swine disease outbreak by estimating the associated costs and for advocacy purposes to mobilize resources at national or international levels.
Keywords: African swine fever, classical swine fever, Colombia, economic cost, North Macedonia, the Philippines, tool, Vietnam
1. INTRODUCTION
Animal diseases, especially transboundary animal diseases (TADs), can have important economic consequences at farm, regional and national level due to livestock production losses and the high costs of prevention, control or eradication measures. Even if these costs are usually supported by governments, they still represent an important burden for livestock producers. The economic consequences of TADs present important differences across regions and countries (Marsh et al., 2017), which depend on the disease, its level of spread before being first diagnosed, the structure of the livestock industry, the control and/or eradication measures applied and the duration of the epidemic.
Besides the economic cost, the introduction of TADs has also an important social effect, especially in smallholder farms in developing countries, pushing families into poverty by reducing their purchasing capacity, their sources of protein and even their capacity to pay health and education expenses (Chenais et al., 2017; Cooper et al., 2019). In the long term, the occurrence of such diseases might shift those affected from livestock farming to other sources of income, change their social standing and reduce the public confidence on the authorities (Mohan et al., 2021). Small producers may have varied sources of income (i.e., diversified activities), which can partially compensate the losses (Nguyen‐Thi et al., 2021). The occurrence of such diseases may create concerns on food safety and drop the consumption of the affected species because of the fear of zoonotic transmission, which will affect not only farmers but also other actors along the value chain, such as traders, slaughterhouses and retailers (Nguyen‐Thi et al., 2021).
Knowing the cost of diseases outbreaks and applied control measures is critically important for veterinary services to prioritise resource allocation and to prepare and plan future possible events and interventions (Brown et al., 2021). Unfortunately, the success of control strategies depends on many aspects that may differ from one outbreak to another, making it difficult to provide a clear overview of their economic impact (Horst et al., 1999). Despite the importance of knowing the economic effects of the diseases, there are a few papers describing the cost of TADs.
African swine fever (ASF) and classical swine fever (CSF) are two of the most important TADs that affect swine production. ASF is caused by a DNA virus, member of the Asfarviridae family. The disease was first described in Africa in 1921, where it has remained endemic. In the mid‐20th century, ASF was first detected outside of this continent, in the Iberian Peninsula, with some limited spread throughout Europe and the Americas, but since 2007, ASF has spread at an unprecedented rate. Today, ASF is without doubt the most important and economically devastating disease of swine. Present in the five continents, the disease had never infected and killed so many animals as it does today, seriously becoming endemic in affected nations and threatening to continue spreading into still unaffected countries.
In Africa, the economic cost of ASF had been estimated at US$ 15,13,340 in Benin between 2014 and 2018 (Ohouko et al., 2020). The cost of an epidemic in Nigeria in 2001, just due to the high mortality (91%) in 306 farms, was US$ 9,41,492 (Babalobi et al., 2007). An epidemic in 219 households in Tanzania translated into a US$ 41,065 (Kivumbi et al., 2021). Outside of historically endemic regions, ASF caused devastating effects on the swine production in China, tripled the price of live finishers from about 13 yuan/kg to 38 yuan/kg (Huang et al., 2021). In Vietnam, 20% of pigs died or were culled within the first 5 months after the onset, with an economic impact only in 2019 of between US$ 880 million and 4.4 billion (Nguyen‐Thi et al., 2021). In India, the direct cost due to the loss of animals from April 2020 until June 2021 were estimated at US$ 37.32 million (Mohan et al., 2021).
Caused by a Pestivirus of the family Flaviviridae, CSF is in regression worldwide due to the extended campaigns based on vaccination, depopulation of infected farms, movement restrictions and surveillance. The World Organisation of Animal Health (OIE) recognises as disease‐free most countries from Europe and Oceania, Kazakhstan, the USA, Canada, Mexico, Costa Rica, Argentina, Chile, Paraguay, Uruguay and some areas of Brazil and Colombia (OIE, 2021). The CSF epidemics in the 1990s caused high economic losses to affected countries. In the Netherlands, 429 farms were affected between 1997 and 1998, with a total cost of US $ 2.3 billion and the destruction of 10 million pigs (Elbers et al., 1999). This wave also affected eight farms in Belgium, with a total cost for the country of €11 million (Mintiens et al., 2001). The estimated impact of animal mortality due to CSF in Mexico, Brazil and Dominican Republic between 1997 and 2001 ranged from US$ 1,40,000 in Brazil to US$ 1 million in Mexico (FAO, 2003).
Only two tools have been described to calculate the cost of different human or zoonotic diseases at regional/country level including the Outbreak Costing Tool (OCT) developed by the US CDC (Bodenham et al., 2021) and a module of the Be‐FAST model that allows to calculate the cost of different swine diseases (Fernández‐Carrión et al., 2016). The objective of this work is to quantify the economic impact and qualitatively evaluate other consequences of ASF and CSF outbreaks in four scenarios in three continents: (a) CSF in the Atlantic Coast region of Colombia in 2015–2016; (b) ASF in the Philippines in 2019; (c) ASF in the province of Lao Cai in Vietnam in 2020 and (d) hypothetical ASF outbreak in North Macedonia. The calculations were made through the OUTbreak COSting Tool (OutCosT), a new developed spreadsheet tool that allows to quantify the cost of swine diseases and related control measures, whether at national or subnational level.
2. MATERIAL AND METHODS
2.1. Case studies
The main characteristics of the four cases evaluated in this study are described below and in Table 1. Tables S1, S3, S5 and S7 includes more detailed information of the cases.
TABLE 1.
Main characteristics of the epidemics for which the economic cost has been calculated
| Country | Colombia (Atlantic Coast) | North Macedonia | The Philippines | Vietnam (Lao Cai) |
|---|---|---|---|---|
| Disease | CSF | ASF | ASF | ASF |
| Year | 2015/2016 | Hypothetical scenario (2019) | 2019 | 2020 |
| Farms in affected area | ||||
| Affected farms (Total) | 96,606 | 2,889 | 8,62,200 | 55,647 |
| Population (sows and boars) | 1,51,785 | 17,451 | 23,65,780 | 43,999 |
| Population (fatteners) | 3,95,938 | 1,15,931 | 1,03,43,468 | 2,30,723 |
| Outbreaks | ||||
| Total confirmed | 91 | 18 | 18,221 | 976 |
| Population in outbreaks (sows and boars) | 706 | 151 | 28,913 | 833 |
| Population in outbreaks (fatteners) | 1,675 | 28 | 1,79,681 | 2697 |
| Affected animals (including deaths) | 1,141 | 150 | 2,08,595 | 3530 |
| Dead animals (total) | 961 | 36 | 10,708 | na |
| Stamping out | No | Yes | Yes | Yes |
| Neighbouring and at‐risk farms | ||||
| Investigated | 1,720 | 549 | na | na |
| Stamped out | No | No‐ | na | No |
| Immobilized | 1,720 | 549 | na | 976 |
| General population | ||||
| Increase of surveillance (farms) | 77 | 628 | 2,423 | N/A |
| Increase of surveillance (animals) | 215 | 10,982 | na | N/A |
| Surveillance in wildlife | No | Yes | No | No |
| Number of vaccinated farms | 2,64,778a | N/A | N/A | N/A |
| Number of vaccinated animals | 25,07,389a | N/A | N/A | N/A |
Abbreviations: N/A, not applicable; na, not available data.
aNumbers refer to the sum of the 2015 and 2016
2.1.1. CSF in the Atlantic Coast Region of Colombia, 2015–2016
The disease affected an economically deprived region at the north of the country with over 96,000 pig farms, mostly small family or backyard premises. The region has abundant waterbodies with an important population of free‐range animals. The disease appeared in 2013 and it remains endemic in the zone (Pineda et al., 2020). We calculated the cost for the region in the period 2015–2016, when 63 and 28 outbreaks were declared, respectively, mainly in backyard premises. The control of the disease was based on the immobilisation of affected and neighbouring farms, surveillance and vaccination of the whole region (Pineda et al., 2020). This case was used to validate the tool by comparing the results with those obtained by Pineda (2021), who calculates the cost for the period 2013–2020. Data from 2015 and 2016 were selected and grouped using the same headings as OutCosT. The differences between both methods are presented as a proportion.
2.1.2. ASF epidemic in the Philippines, 2019
The Philippines reported its first case of ASF in July 2019 in Rodriguez (Rizal Province) affecting several backyard farmers (i.e., raising pigs at a maximum of 20 fattener heads and/or 10 breeder pigs per household as defined by the Registry System for Basic Sectors in Agriculture under the Philippine Crop Insurance Corporation). By the end of 2019, ASF had spread to a total of 10 provinces from five regions, affecting more than 18,000 farms belonging mostly to the backyard sector. Cost estimates refer to the calendar year 2019.
2.1.3. ASF epidemic in Lao Cai Province of Vietnam, 2020
Lao Cai is a province of the mountainous Northwest region bordering China, covering an area of 6,384 square kilometres. The pig production in Lao Cai Province is dominant by small‐scale producers with less than 30 pigs per household. First confirmed in Lao Cai Province in June 2019, by the end of 2019, ASF was already reported in all nine districts of the province, causing the loss of 36,811 pigs (Animal Health from Lao Cai, 2019). In 2020, the disease was better controlled with only 3,530 pigs lost due to ASF (both direct deaths and the result of culling). We calculated the cost in the province for calendar year 2020.
2.1.4. A hypothetical scenario of ASF in North Macedonia
The country has mainly family farms with low number of animals. In the hypothetical scenario, the outbreak occurred in the North‐eastern and Eastern regions, those with the highest pig population of the country (O'Hara et al., 2021), and it was eradicated before its spread to other regions. The scenario assumed that ASF was introduced into domestic pigs, affecting 18 farms: nine farrow‐to‐finish family farms (for a total of 25 sows and 226 fatteners) and nine backyard premises (for a total of 16 fatteners); while no wild boar were affected. The evaluation is based on population, costs and prices from calendar year 2019 as part of the follow‐up to the survey on ASF conducted in the country (O'Hara et al., 2021). Besides, the choice of North Macedonia as case study country was justified by the high risk for ASF introduction, given the presence of active outbreaks in two bordering countries, Serbia and Bulgaria. In fact, two ASF outbreaks were reported in North Macedonia in 2021, after the present economic evaluation was conducted.
The main control measures applied in the three ASF cases were depopulation, outbreak investigations, cleaning and disinfection, surveillance activities and public awareness campaigns.
2.2. The tool
The OUTbreak COSting Tool (OutCosT) was built in an Excel spreadsheet for the purpose of evaluating the financial cost of swine diseases, including the related control measures, both in a real situation or a scenario.
Calculations are based on a deterministic model, and the inputs required to run the model are listed below (while details about the formulas are included in the Supporting Information 2). The tool was developed to be applicable to all swine diseases, which is why it includes options not usually applied to ASF or CSF outbreaks, like treatment or partial stamping outs.
-
‐
Population: the number of farms and animals. The tool allows dividing the pig sector into up to four different types of farms, which can be defined by the number of animals and/or by the production system. As the characteristics of swine production vary greatly between and within countries, the farms’ classification is not predefined, and farm types are not fixed categories. By default, the tool suggests the following categories: (i) industrial; (ii) commercial; (iii) family; (iv) backyard (or any other outdoor or alternative production systems). Although they can be modified according to the characteristics of the pig sector, the country‐specific definitions, or the availability of data. For each farm type, the model considers two subtypes, either sows or fatteners. By sows, we refer to farms with adult animals, their piglets and the weaners (i.e., weaning units are included within this group to simplify the model). Farrow‐to‐finish farms should be considered twice: as sow farms and fattener units.
-
‐
Data about the epidemic: The number of suspected (by passive surveillance) and confirmed farms for each farm type are defined, including the total number of animals in each group. Data can be filled as total numbers in a real situation or as percentages of the population when calculating the cost of a hypothetical scenario.
-
‐
Control measures in the confirmed farms: The model allows for up to three stamping out strategies: all farms, only some farms and all farms but just some animals, for example, partial stamping out. If animals are not culled and some treatment is applied, the tool allows specifying up to two different drugs. For each treatment, collected information refers to the animals that were treated (e.g., all animals present in the farm, only affected animals, sows or fatteners, only), the price of the drug and that of its application. Cleaning and disinfection and insect control are other measures that can also be evaluated in affected farms.
-
‐
Control measures in neighbouring and at‐risk farms: This accounts for measures that can be applied to nonaffected farms that are at high risk of becoming infected, like those subjected to an outbreak investigation and testing, stamping out, immobilisation, disinfection and increased surveillance.
-
‐
Control measures applied in other farms beyond the affected or the neighbouring/at‐risk farms: Vaccination and other measures may be applied either at the whole population or just in a buffer zone, a region or a certain production system. In either case, the number of animals and farms should be specified to calculate the costs. Control measures applied to wildlife can also be included.
-
‐
Active surveillance: The tool allows to apply two different active surveillance strategies in a given number of farms and animals. Surveillance activities applied to wildlife or vectors are also considered.
-
‐
Production parameters: Duration of lactation, pigs weaned per sow, feed intake, mortality at the different ages are some parameters not directly related with the disease but required to calculate the value of sacrificed/died animals and costs related to the waiting time before resuming operations.
-
‐
Production losses due to the disease includes abortions, losses of body weight for fatteners (measured by the increase in days to reach the slaughtering weight, additional days in which sows remain open and an ‘other loses’ item if needed).
-
‐
Costs and prices: this section covers prices associated with the swine production (e.g., replacement animals, feed and fixed costs per animal), and the labour costs (time spent and salaries) of farmers and veterinarians for the extra work due to outbreak‐related activities (e.g., visiting farms, sampling and testing, culling and carcass disposal, disinfection, treatment of diseased animals, vaccination, surveillance, and so forth). Expenses for the organization of training and awareness campaigns are also included. Tables S2, S4, S6 and S8 includes the prices used to evaluate the four cases.
As model outputs, OutCosT returns the cost estimates of 106 different items (summarised by sections in Table 2). All costs are also broken down by farm type. An indication about who assumes each cost (veterinary services, farmers and other stakeholders, according to the information given by the user for each item) allows assessing how the total costs are allocated between sectors.
TABLE 2.
OutCosT results: Summary of the outputs given by the model
| Sections | Categories | Items/activities included (N) |
|---|---|---|
| Presence of the disease | Direct costs of the disease | 7 |
| Treatments costs | 4 | |
| Control activities in suspected outbreaks | Visits to suspected farms | 5 |
| Immobilisation of farms | 4 | |
| Eradication/control measures in outbreaks | Value of culled animals | 6 |
| Culling and carcass disposal | 8 | |
| Cleaning & disinfection/insect control | 6 | |
| Indirect costs due to loss of opportunity | 6 | |
| Measures in neighbouring and at‐risk farms | Visiting farms | 5 |
| Stamping out | 8 | |
| Cleaning and disinfection | 2 | |
| Immobilisation of farms | 5 | |
| Tests to confirm if farms are disease free | 2 | |
| Indirect costs due to loss of opportunity in case of culling animals | 6 | |
| Measures in nonaffected farms | Vaccination | 6 |
| Other measures | 2 | |
| Surveillance in domestic animals | Active surveillance – strategy 1 | 3 |
| Active surveillance – strategy 2 (if applied) | 3 | |
| Wildlife and vectors | Control measures in wildlife | 4 |
| Wildlife and vector surveillance | 6 | |
| Other costs | Coordination and bureaucratic tasks | 2 |
| Training and awareness campaigns | 6 |
Besides, the OutCosT dedicates a specific section on a simple qualitative assessment of indirect and nonmonetary costs providing a framework to evaluate the implications of a disease on human and animal welfare, the sociopolitical context and the environment. The qualitative assessment is adapted from the FMD impact calculator (available online at: https://www.eufmd.info/impactcalculator), and it includes 22 new items grouped into six main categories: socioeconomic vulnerability; human health; environment; animal welfare; social behaviour; trading and political response. For each item, the model returns a qualitative estimation of the level of concern (i.e., null, low, moderate, high and extreme). This is obtained by combining the scores attributed (by the user) to the importance of consequence and to the likelihood of occurrence through a semiquantitative method (from 0 to 4).
3. RESULTS
3.1. CSF in the Atlantic Coast Region, Colombia (2015–2016)
The total cost of CSF in Colombia in 2015/2016 was US$ 3.8 million. Approximately 86% of the total cost was attributed to the vaccination campaign, while the cost due to the disease and control measures implemented in the affected and connected farms was 4.3% and 2.3%, respectively, and the cost of surveillance represented 6.9% of the total (Table 3). The total cost was 11% higher than the cost obtained using a partial budgeting analysis (Pineda, 2021) (Table 4).
TABLE 3.
Cost of the outbreaks of African and classical swine fever (in US$)
| Country | CSF in Colombia (Atlantic Coast) | ASF in North Macedonia | ASF in The Philippines | ASF in Vietnam (Lao Cai) | ||||
|---|---|---|---|---|---|---|---|---|
| Year of reference | 2015/2016 | 2019 (Hypothetical scenario) | 2020 | 2020 | ||||
| Outbreaks | 166,171 | (4.3%) | 87,800 | (2.2%) | 57,616,000 | (98.1%) | 791,223 | (95.7%) |
| Disease cost a | 92,770 | (2.4%) | 17,533 | (0.7%) | 1,472,720 | (2.5%) | 0 b | |
| Outbreak investigation | 54,304 | (1.4%) | 16,220 | (0%) | 13,030,140 | (22.2%) | 18,303 | (2.2%) |
| Stamping out | 0 | 52,447 | (1.1%) | 31,976,638 | (54.5%) | 736,990 | (89.1%) | |
| Indirect costs c | 19,097 | (0.5%) | 1,601 | (0.3%) | 11,136,503 | (19%) | 35,930 | (4.3%) |
| Connected farms | 88,740 | (2.3%) | 5,851,038 | (83.9%) | na | 34,625 | (4.2%) | |
| Immobilisation | 49,518 | (1.3%) | 4,850,782 | (77%) | 34,625 | (4.2%) | ||
| Other | 39,223 | (1.0%) | 1,000,256 | (6.8%) | na | |||
| Vaccination /other | 3,358,176 | (86.3%) | N/A | N/A | N/A | |||
| Application | 2,710,957 | (69.7%) | ||||||
| Cost of vaccine | 647,220 | (16.6%) | ||||||
| Surveillance | 266,798 | (6.9%) | 131,071 | (10.2%) | 981,711 | (1.7%) | N/A | |
| Awareness | 9,269 | (0.2%) | 126,850 | (3.8%) | 119,136 | (0.2%) | 1,063 | (0.1%) |
| Total: | 3,889,155 | 6,196,760 | 58,716,847 | 826,911 | ||||
| Number of outbreaks (N) | 91 | 18 | 18,221 | 976 | ||||
| Average cost per outbreak: | 42,734 | 344,264 | 3,222 | 847 | ||||
Abbreviations: “N/A”, Not applicable;“na”, not available data.
The disease cost includes the cost of dead animals and losses of production, including abortion and suboptimal growth (i.e., extra days open in sows, and cost of extra days due to reach market weight).
Disease costs are included in the stamping out cost due to data limitations that do not allow to differentiate between culled and dead animals.
The indirect costs includes the cost of treatments and costs related to the waiting time before resuming operations (i.e., interrupted production). Cost of maintaining empty the farm.
TABLE 4.
Cost of the outbreaks of classical swine fever in Colombia estimated using OutCosT compared to the costs calculated by Pineda (2021)
| Type of cost | OutCosT (Total country cost) | Pineda (2021) | Difference (%) |
|---|---|---|---|
| Outbreaks | 1,77,273 | 1,32,194 | 25.7 |
| Connected farms | 88,740 | 85,997 | 3.2 |
| Vaccination/treatments | 33,58,176 | 30,16,125 | 11.3 |
| Surveillance (farms) | 1,89,163 | 2,50,538 | 6.5 |
| Wildlife | 0 | 0 | |
| Awareness | 9,269 | 3,219 | 188.0 |
| Total | 38,22,622 | 34,88,073 | 11.5 |
The mean cost of the disease and control activities per outbreak was US$ 2,923 (with US$ 975 of them for the activities in neighbouring and at‐risk farms). Most of the cost (78.7%) was covered by the pig industry through a national pig farming fund, which is financed by a tax that commercial farmers pay per each slaughtered animal. Affected farmers and the veterinary services assumed 14.6% and 6.7% of the total cost, respectively (Figure 1).
FIGURE 1.
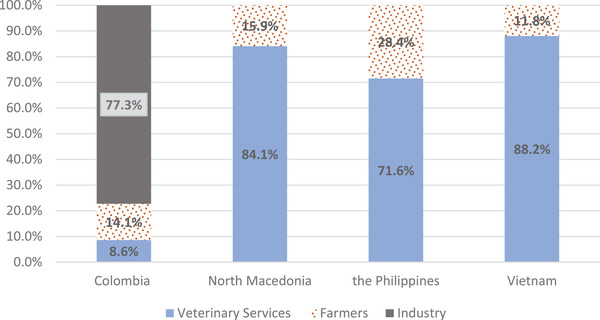
Proportion of costs assumed by each stakeholder (i.e., veterinary services, farmers and industry) by country
3.2. The ASF epidemics of the Philippines (2019) and Lao Cai, Vietnam (2020) and the hypothetical scenario in North Macedonia
The cost of ASF epidemics ranged widely from US$ 8,26,911 in Lao Cai (Vietnam) to more than US$ 58 million in the Philippines (Table 3). The average cost per outbreak varied also from US$ 1,84,426 in North Macedonia to US$ 847 in Vietnam.
Regarding the cost distribution, in the Philippines and Vietnam, more than 95% of the total cost was associated to affected farms (i.e., diseased animals and control activities). On the other hand, in North Macedonia, the cost attributed to affected farms was very low (about 2%), while 84% and 10% of the total cost were due to control measures implemented in connected farms and surveillance, respectively (Table 3).
In all ASF scenarios, most of the costs were paid by the veterinary services (ranging between 72% and 88%), while farmers covered the remaining part (between 12% and 28%) (Figure 1).
For ASF, the qualitative cost assessment indicated great concern about the effect of ASF on wildlife and about the increase in the price of pork (they were considered extreme in North Macedonia and Vietnam, respectively, and high in the other countries). Other points that were considered of high concern in at least two of the four countries were the possibility that people are forced to leave swine farming due to economic reasons (Vietnam and Colombia), the timing to pay compensation (the Philippines and Colombia) and the decreased confidence in the government (Vietnam and Colombia) (Table 5).
TABLE 5.
Main results of the qualitative estimation of other noneconomic costs evaluated using OutCosT
| Colombia (Atlantic Coast) | North Macedonia | Philippines | Vietnam (Lao Cai) | |
|---|---|---|---|---|
| Socioeconomic vulnerability: | ||||
| • People can be forced to leave the activity | Moderate | High | Null | High |
| Human health: | ||||
| • The disease or the control measures increases mental disturbances of farmers and related people | Moderate | Moderate | Moderate | High |
| Environment: | ||||
| • Disease can also affect wildlife animals | High | High | Extreme | Moderate |
| • Disease can modify the ecological balance or affect an endangered species | High | Null | Low | Low |
| • The control/eradication measures affect the environment | High | Null | Null | Null |
| Animal welfare: | ||||
| • The disease has an important effect on the welfare of affected animal | High | Low | Low | Moderate |
| • The control measures have an important effect on the welfare of affected animal | High | Moderate | Low | Moderate |
| Social behaviour: | ||||
| • Deficient management of public opinion related to response measures | High | Null | Low | Low |
| Political response: | ||||
| • Timing for paying the compensations for the carried out measures. | High | Moderate | Moderate | High |
| • Decrease in farmers confidence in relation to the administration | Moderate | High | Moderate | High |
| Trading: | ||||
| • Increase in prices due to a reduction of the production | High | Extreme | High | Moderate |
4. DISCUSSION
This work estimates the cost of different outbreaks of CSF and ASF using the OUTbreak COSting Tool (OutCosT), a novel tool which allows quantifying the financial cost of swine disease outbreaks and their control in a territory, whether national or subnational.
Despite the high burden of some TADs, the number of studies calculating the economic impact of swine diseases and their control is quite scarce. Rushton (2009) provided an exhaustive review of the cost of outbreaks, analysis of disease losses and cost‐benefit analyses of control strategies regarding the most relevant swine diseases (e.g., ASF, CSF, Aujeszky's disease and Porcine Reproductive and Respiratory Syndrome – PRRS).
More recently, Brown et al. (2021) published a scoping review on the economic impact of ASF, CSF and foot and mouth disease (FMD), examining scientific papers which included an analysis of outbreak costs/losses. The authors identified 14 studies for CSF and ASF. Eight papers reported an economic evaluation of CSF (one retrospective study from The Netherlands, and nine scenarios in Denmark, the Netherlands, Belgium and Australia) with costs ranging from US$ 58,338 in Australia to US$ 3.7 billion in the Netherlands. The economic impact of ASF has been assessed in six studies. Two used retrospective data (both from Nigeria) and four studies forecasted a hypothetical scenario (from Denmark, Nigeria, Spain and the USA). The costs ranged from US$ 6,49,000 of annual costs in Nigeria to US$ 94.5 million of total losses of a swine depopulation in Spain. Considering the significant number of outbreaks reported worldwide, the authors highlighted the low number of papers with retrospective economic studies on both diseases. Divergences in the economic impact of the diseases were attributed to methodological differences among studies, and especially to discrepancies in the items included in the analyses and a lack of consistency in definitions and descriptions of the used data. Besides, the observed variability can be due to the virus strains, epidemiology, location of outbreaks, trade implications, consumer reaction and especially to the disease management and control practices (Brown et al., 2021; Marsh et al., 2017).
In this paper, the economic impact of CSF and ASF was assessed using the same methodology. Besides, in the four analysed case studies, diseases mainly affected family and backyard farms; however, there were important differences in the total cost and its distribution. While in the Philippines (ASF) and Vietnam (ASF), 96–98% of the cost occurred in the affected farms, in Colombia (CSF) and North Macedonia (ASF) affected farms represented only a small proportion of the total costs (4.6% and 2.2%, respectively), and the major cost factors were the vaccination and measures applied in connected farms (especially the movement control), respectively. Such differences are mainly explained by the timeframe, the different magnitudes of the epidemics (i.e., number of outbreaks and/or affected animals), the measures applied and to a lesser extent by the production systems affected. Since the timeframe used for each case is different (as well as the disease in some cases), we cannot draw direct comparisons. However, our purpose here was to evaluate the tool's flexibility that allows its use under different situations rather than making direct comparisons between countries.
In Colombia (2015/2016), the vaccination campaigns against CSF had a cost of US$ 3.3 million (out of a total of US$ 3.8 million). The campaign was mainly financed (79%) by the national pig farming fund, through a tax paid by commercial pig producers and managed by the Colombian Pork Producers Association (Porkcolombia). In addition, the cost of time spent by animal owners was evaluated in US$ 3,80,133.
The presented results have been validated with the results of the study from Pineda (2021). Estimations from both studies (Table 4) show that results obtained with both methods differ only by 11.5%. Main discrepancies were observed in the evaluation of the impact of CSF in the outbreaks, the vaccination campaign and the surveillance. The higher costs attributed by the OutCosT to outbreaks (difference of US$ 33,977) and vaccination (difference of US$ 3,42,051) can be explained by a lower discrimination of OutCosT between the cost of salaries of the farmers and workers from the veterinary services. The main advantage of this tool in comparison with the more detailed approach proposed by Pineda is its ease of use (i.e., how easily and quickly it can be performed).
The cost of ASF in the Philippines was the highest of the evaluated ASF epidemics due to the high number of affected premises (18,221) and the application of stamping out measure in all these farms, which represented 55% of the total cost. Unfortunately, we could not include the cost in neighbouring (noninfected) farms, because reliable estimates were not available in the Philippines, which implies that the total cost would be even higher.
In Lao Cai Province (Vietnam), the cost was mainly incurred in affected farms (96%), especially due to the cost of culling and destroying the animals (89% of the total cost). Due to the unavailability of reliable data, we included the number of dead pigs within the number of compensated pigs; therefore, the cost of mortality was covered by the stamping out cost. This indicates that the proportion for the cost of culling and destroying should be lower, since it also includes some direct cost of disease. The same pattern of cost distribution was observed in Vietnam and the Philippines: the vast majority of costs were related to the direct costs due to ASF and the control measures in the outbreaks, whereas very few costs were attributed to surveillance activities (1.7% or less) and almost none to trainings and awareness campaigns (0.2% or less).
Finally, in the hypothetical scenario of an ASF epidemic in North Macedonia, most costs were related to the activities and measures applied in neighbouring farms (84% of the total cost). This was a consequence of the defined scenario, which considered the region with highest number of farms in the country. As a result, the occurrence of 18 outbreaks implied the immobilisation of 549 connected farms, which translated into a cost of US$ 2.5 million. The cost pattern differed also in the proportion attributed to surveillance strategies and awareness campaigns, which represented 10% and 4% of the total cost, respectively.
OutCosT is to become a corporate tool of the Food and Agriculture Organization (FAO), and it is available online at: https://www.fao.org/fileadmin/user_upload/faoweb/animal‐health/OutCosT_PIG.xlsx. Through this validation process, OutCosT was demonstrated to be a useful, powerful, flexible, yet simple tool to rapidly evaluate the financial cost of epidemic and endemic swine diseases in different situations, and at subnational or national level. The tool allows users to consider different elements involved in the management of swine diseases. It is flexible by allowing the evaluation of real cases or hypothetical scenarios, the inclusion of input data in alternative ways and not introducing some data items in case of lack of information. OutCosT evaluates the costs of diseases and the cost of activities associated with their control, including measures applied at farm level, in neighbouring and at‐risk farms, but also in wildlife. Moreover, the cost of applying prevention and control measures in the general population (i.e., vaccination, awareness campaigns, and surveillance in domestic species, wildlife and vectors) are also considered. As with any simulation model, the results will depend only on the inputs (provided by the users), which should therefore be defined carefully.
Depending on the scenarios, some data may be unknown or present a high uncertainty. In these cases, users can insert average values or use different scenarios to calculate an approximate range of the costs.
Another strength of OutCosT beyond its ease of use is that it allows a detailed evaluation of the cost of each activity, differentiating who pays for them (famers, veterinary services and other stakeholders), and breaking down these costs by up to four types of farms, which can be defined differently depending on the swine production characteristics and the country‐specific definitions. The differentiation between types of farms allows a more accurate evaluation of the total cost and can provide relevant information for the decision makers at country level. Although due to the different definitions used for each case study, the cost estimate by type of farms is not comparable. The knowledge about which groups bear the burden of the costs and which groups receive the benefits is especially important to plan compensation programs (Marsh et al., 2017). Besides, the tool also allows to make a qualitative evaluation of other important aspects related with the epidemics, such as the effect of the epidemics on the human health, environment, animal welfare, socioeconomic vulnerability and trading and political response.
OutCosT focuses on materialized costs due to ASF outbreaks to be bear by the pig sector or the government (central, regional and local). On the other hand, it is not designed to conduct complex analyses; in fact, its simplicity is one of its key advantages. OutCosT does not address macroeconomic impacts. Indirect costs due to trade restrictions or modification in the consumer habits were out of the scope. Other indirect costs such as the restrictions in movements of veterinarians between farms and the stricter measures for animal transportation outside the affected zone have not been calculated either. These indirect costs can indeed have an important impact. For example, the reduction in pig meat price in Vietnam reduced from US$ 1.61/kg of live weight to 1.39/kg after the first outbreaks in 2019 (Nguyen‐Thi et al., 2021). In North Eastern India, the foregone export revenue after the irruption of ASF has been estimated in US$ 2.47 million (April 2020 to June 2021) (Mohan et al., 2021).
The main purpose of OutCosT is to support authorities to rapidly respond to ASF outbreaks by estimating the associated costs. Depending on how responsibilities are distributed between central and local authorities, an adequate level of resources must be allocated for high‐risk areas to ensure that the response to outbreaks is not delayed. Authorities can use the tool to create scenarios to estimate the budgetary needs for an imminent disease incursion or in case the disease spreads to other regions. The budgetary needs coming out from scenarios can be compared with the costs of implementing more radical measures to contain the outbreak to estimate the benefits of doing so and advocate in case the benefits outweigh the costs.
The second objective of the tool is raising awareness about the importance of implementing control efforts to prevent further spread. Being able to provide such monetary figures can prove extremely useful for advocacy purposes to mobilize resources at national or international levels (e.g., donors, development agencies). The estimated cost for producers can be used to raise awareness and promote collaborations (including resource mobilization) with the private sector for controlling the outbreaks.
As a conclusion, the application of OutCosT allowed the evaluation of the financial cost of swine diseases in different situations and at different levels. Three retrospective economic analyses (CSF in Colombia, ASF in the Philippines and Vietnam) and a simulation of a hypothetical ASF epidemic in North Macedonia were conducted. The financial cost of the four case studies showed big differences depending on the type of disease, incidence and epidemiology of the disease, the density of farms and the measures applied in the country. The tool can be applied following the introduction of a TAD, as in the ASF cases, or for endemic diseases, as the CSF in Colombia. In summary, OutCosT can be used (1) to estimate the cost of an outbreak that already occurred; (2) to estimate the potential cost of a future outbreak (with best and worst case scenarios) and alternative response strategies and (3) to estimate the cost of an endemic disease. Moreover, by comparing different scenarios (e.g., with or without vaccination, or different stamping out strategies), the tool can inform decisions around control options and guide decision makers on the optimal strategy. However, this requires consultation with local experts to make the disease‐spread scenarios realistic and in alignment with the different strategies assessed. Countries that decide to institutionalize the use of this tool will generate substantial data over the years that can then be used as inputs for realistic scenarios.
CONFLICT OF INTEREST
None. The views expressed in this publication are those of the author(s) and do not necessarily reflect the views of FAO.
ETHICAL APPROVAL
No ethical approval was required as this is a research employing secondary data.
Supporting information
Table 1: Number of farms and animals considered for the calculations in Colombia
Table 2: Prices and costs in Colombian Pesos applied for the calculations in Colombia
Table 3: Number of farms and animals considered for the calculations in Philippines
Table 4: Prices and costs in Philippine pesos applied for the calculations in Philippines
Table 5: Number of farms and animals considered for the calculations in Vietnam
Table 6: Prices and costs in Vietnamese dong used for the evaluation of ASF cost in Lao Cai (Vietnam)
Table 7: Number of farms and animals considered for the calculations in North Macedonia
Table 8: Prices and costs in Euros of the swine applied for the calculations in North Macedonia
Supporting Information
ACKNOWLEDGEMENTS
The authors acknowledge the national agencies of Colombia, North Macedonia, the Philippines and Vietnam for providing the outbreak and cost data. Acknowledgements also go to the Food and Agriculture Organization of the United Nations (FAO), which financed the study through the Technical Cooperation Project (TCP/RER/3704) on ‘African swine fever emergency preparedness in the Balkans’. United States Agency for International Development (USAID) grant (720FDA19IO00092).
Casal, J. , Tago, D. , Pineda, P. , Tabakovski, B. , Santos, I. , Benigno, C. , Huynh, T. , Ciaravino, G. , & Beltran‐Alcrudo, D. (2022). Evaluation of the economic impact of classical and African swine fever epidemics using OutCosT, a new spreadsheet‐based tool. Transboundary and Emerging Diseases, 69, e2474–e2484. 10.1111/tbed.14590
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- Animal Health from Lao Cai . (2019). Results of livestock and veterinary work in 2019. Orientation and tasks in 2020. Lao Cai Sub‐department of Livestock and Animal Health. [Google Scholar]
- Babalobi, O. O. , Olugasa, B. O. , Oluwayelu, D. O. , Ijagbone, I. F. , Ayoade, G. O. , & Agbede, S. A. (2007). Analysis and evaluation of mortality losses of the 2001 African swine fever outbreak, Ibadan, Nigeria. Tropical Animal Health and Production, 39, 533–542. 10.1007/s11250-007-9038-9 [DOI] [PubMed] [Google Scholar]
- Bodenham, R. F. , Mtui‐Malamsha, N. , Gatei, W. , Woldetsadik, M. A. , Cassell, C. H. , Salyer, S. J. , Halliday, J. E. B. , Nonga, H. E. , Swai, E. S. , Makungu, S. , Mwakapeje, E. , Bernard, J. , Bebay, C. , Makonnen, Y. J. , & Fasina, F. O. (2021). Multisectoral cost analysis of a human and livestock anthrax outbreak in Songwe Region, Tanzania (December 2018‐January 2019), using a novel Outbreak Costing Tool. One Health, 30(13), 100259. 10.1016/j.onehlt.2021.100259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, V. R. , Miller, R. S. , McKee, S. C. , Ernst, K. H. , Didero, N. M. , Maison, R. M. , Grady, M. J. , & Shwiff, S. A. (2021). Risks of introduction and economic consequences associated with African swine fever, classical swine fever and foot‐and‐mouth disease: A review of the literature. Transboundary and Emerging Diseases, 68, 1910–1965. 10.1111/tbed.13919 [DOI] [PubMed] [Google Scholar]
- Chenais, E. , Boqvist, S. , Emanuelson, U. , von Brömssen, C. , Ouma, E. , Aliro, T. , Masembe, C. , Ståhl, K. , & Sternberg‐Lewerin, S. (2017). Quantitative assessment of social and economic impact of African swine fever outbreaks in northern Uganda. Preventive Veterinary Medicine, 1(144), 134–148. 10.1016/j.prevetmed.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Cooper, D. S. T. , Pereira, A. , & da Costa Jong, J. B. (2019). Counting the cost: The potential impact of African Swine Fever on smallholders in Timor‐Leste. One Health, 18(8), 100109. 10.1016/j.onehlt.2019.100109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers, A. R. , Stegeman, A. , Moser, H. , Ekker, H. M. , Smak, J. A. , & Pluimers, F. H. (1999). The classical swine fever epidemic 1997–1998 in the Netherlands: Descriptive epidemiology. Preventive Veterinary Medicine, 42, 157–184. 10.1016/S0167-5877(99)00074-4 [DOI] [PubMed] [Google Scholar]
- FAO . (2003). Estimación del impacto de la Peste Porcina Clásica en sistemas productivos porcinos en América Latina: Estudios de casos en tres países latinoamericanos – Plan Continental para la Erradicación de la Peste Porcina Clásica en las Américas. [Google Scholar]
- Fernández‐Carrión, E. , Ivorra, B. , Martínez‐López, B. , Ramos, A. M. , & Sánchez‐Vizcaíno, J. M. (2016). Implementation and validation of an economic module in the Be‐FAST model to predict costs generated by livestock disease epidemics: Application to classical swine fever epidemics in Spain. Preventive Veterinary Medicine, 126, 66–73. 10.1016/j.prevetmed.2016.01.015 [DOI] [PubMed] [Google Scholar]
- Horst, H. S. , de Vos, C. J. , Tomassen, F. H. M. , & Stelwagen, J. (1999). The economic evaluation of control and eradication of epidemic livestock diseases. Revue scientifique et technique (OIE), 18, 367–379. 10.20506/rst.18.2.1169 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Li, J. , Zhang, J. , & Jin, Z. (2021). Dynamical analysis of the spread of African swine fever with the live pig price in China. Mathematical Biosciences and Engineering, 18, 8123–8148. 10.3934/mbe.2021403 [DOI] [PubMed] [Google Scholar]
- Kivumbi, C. C. , Yona, C. , Hakizimana, J. N. , & Misinzo, G. (2021). An assessment of the epidemiology and socioeconomic impact of the 2019 African swine fever outbreak in Ngara district, western Tanzania. Veterinary and Animal Science, 24, 14,100198. 10.1016/j.vas.2021.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, T. L. , Pendell, D. , & Knippenberg, R. (2017). Animal health economics: An aid to decision making on animal health interventions – Case studies in the United States of America. Revue Scientifique et Technique (OIE), 36, 137–145. [DOI] [PubMed] [Google Scholar]
- Mintiens, K. , Deluyker, H. , Laevens, H. , Koenen, F. , Dewulf, J. , & De Kruif, A. (2001). Descriptive epidemiology of a Classical Swine Fever outbreak in the Limburg Province of Belgium in 1997. Journal of Veterinary Medicine, Series B, 48, 143–149. 10.1046/j.1439-0450.2001.00429.x [DOI] [PubMed] [Google Scholar]
- Mohan, N. H. , Misha, M. M. , & Gupta, V. K. (2021). Consequences of African swine fever in India: Beyond economic implications. Transboundary and Emerging Diseases, 68, 3009–3011. 10.1111/tbed.14318 [DOI] [PubMed] [Google Scholar]
- Nguyen‐Thi, T. , Pham‐Thi‐Ngoc, L. , Nguyen‐Ngoc, Q. , Dang‐Xuan, S. , Lee, H. S. , Nguyen‐Viet, H. , Padungtod, P. , Nguyen‐Thu, T. , Nguyen‐Thi, T. , Tran‐Cong, T. , & Rich, K. M. (2021). An assessment of the economic impacts of the 2019 African Swine Fever Outbreaks in Vietnam. Frontiers in Veterinary Science, 8, 686038. 10.3389/fvets.2021.686038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara, K. C. , Beltrán‐Alcrudo, D. , Hovari, M. , Tabakovski, B. , & Martínez‐López, B. (2021). Descriptive and multivariate analysis of the pig sector in North Macedonia and its implications for African Swine Fever Transmission. Frontiers in Veterinary Science, 8, 733157. 10.3389/fvets.2021.733157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohouko, O. F. H. , Koudouvo, K. , Dougnon, T. J. , Agbonon, A. , Karim, I. Y. A. , Farougou, S. , & Gbeassor, M. (2020). African swine fever in Benin and prevalence of the disease in Southern Benin: A retrospective study (2014‐2018). Journal of Advanced Veterinary and Animal Research, 7, 464–470. 10.5455/javar.2020.g442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . (2021). https://www.oie.int/en/disease/classical‐swine‐fever/#ui‐id‐2 (last accessed 1st December 2021).
- Pineda, P. , Deluque, A. , Peña, M. , Diaz, O. L. , Allepuz, A. , & Casal, J. (2020). Descriptive epidemiology of classical swine fever outbreaks in the period 2013–2018 in Colombia. Plos One, 15, e0234490. 10.1371/journal.pone.0234490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda, P. (2021). Epidemiology of classical swine fever in Colombia. Description of outbreaks, economic impact and epidemiological surveillance in free zones [PhD thesis, Universitat Autònoma de Barcelona]. [Google Scholar]
- Rushton, J. (2009). The economics of animal health and production . Cabi, . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Number of farms and animals considered for the calculations in Colombia
Table 2: Prices and costs in Colombian Pesos applied for the calculations in Colombia
Table 3: Number of farms and animals considered for the calculations in Philippines
Table 4: Prices and costs in Philippine pesos applied for the calculations in Philippines
Table 5: Number of farms and animals considered for the calculations in Vietnam
Table 6: Prices and costs in Vietnamese dong used for the evaluation of ASF cost in Lao Cai (Vietnam)
Table 7: Number of farms and animals considered for the calculations in North Macedonia
Table 8: Prices and costs in Euros of the swine applied for the calculations in North Macedonia
Supporting Information
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


