Abstract
The prognosis for canine sinonasal tumors remains rather poor despite definitive‐intent radiotherapy (RT). Theoretical calculations predicted improved outcomes with simultaneously integrated boost (SIB) protocols. With the hypothesis of clinically detectable differences in outcome between groups, our retrospective study evaluated prognostic variables and outcome in dogs treated with regular versus SIB RT. Dogs with sinonasal tumors treated with either a regular (10 × 4.2 Gy) or new SIB protocol (10 × 4.83 Gy to macroscopic tumor) were included. Information regarding signalment, tumor stage, type, clinical signs, radiation toxicity, response, and outcome was collected. Forty‐nine dogs were included: 27 treated regularly and 22 treated with SIB RT. A total of 69.4% showed epistaxis, 6.1% showed epileptic seizures, 46.9% showed stage IV tumors, and 6.1% showed lymph node metastases. Early toxicity was mostly mild. Late grade 1 skin toxicity (alopecia/leucotrichia) was seen in 72.1% of dogs, and a possible grade 3 ocular toxicity (blindness) was seen in one dog. Complete/partial resolution of clinical signs was seen in 95.9% of patients as best clinical response and partial remission was seen as best imaging response in 34.7%. The median progression‐free survival (PFS) was 274 days (95% CI: 117–383) for regular and 300 days (95% CI: 143–451) for SIB RT, which was not significantly different (P = 0.42). Similarly, the median overall survival (OS) was 348 days (95% CI: 121–500) for regular and 381 days (95% CI: 295–634) for the SIB RT (P = 0.18). Stratified by protocol, the hazard ratio of stage IV versus stage I–III tumors was 2.29 (95% CI: 1.156‐4.551, P = 0.02) for OS but not PFS. All dogs showed acceptable toxicity. In contrast to theoretical predictions, however, we could not show a statistically significant better outcome with the new protocol.
Keywords: canine, IMRT, nasal tumor, ocular, SIB
Abbreviations
- CBCT
cone‐beam computed tomography
- CR
complete response
- CT
computed tomography
- CTV
clinical target volume
- GTV
gross tumor volume
- KCS
keratoconjunctivitis sicca
- n.s.
not significant
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- PTV
planning target volume
- RECIST
response evaluation criteria for solid tumors in dogs
- RT
radiation therapy
- SD
stable disease
- SIB
simultaneously integrated boost
- VRTOG
veterinary radiation therapy oncology group
1. INTRODUCTION
While radiation therapy (RT) is the standard of care for sinonasal tumors in dogs, the prognosis remains fair to poor. Prognosis depends in part on tumor stage, histologic type, and differentiation, but radiation dose and fractionation most likely also play a role. 1 , 2 , 3 , 4 Several definitive‐intent, moderately to severely hypofractionated RT protocols are used in dogs. 1 , 3 , 4 , 5 , 6 , 7 , 8 The term hypofractionation is defined as fraction size > 2 Gy in human radiation oncology, while regular irradiation protocols use 2 Gy per fraction. In veterinary radiation oncology, however, most or all fractionation schedules use larger fraction sizes. Definitive‐intent protocols usually use daily fractions of 2.5‐3 Gy with total dose > 40 Gy and are sometimes called finely fractionated protocols, whereas palliative protocols often use once‐ or twice‐weekly fractions of 5–8 Gy with total dose < 40 Gy and are termed coarsely fractionated protocols. 9
To date, it is unclear which total dose or fractionation is best to achieve long‐term control. The goal of a simultaneously integrated boost (SIB) radiotherapy protocol is to increase the dose to the part of the target volume with the highest tumor cell density (gross tumor volume, GTV) and possibly increased risk of failure, while treating the remaining volume (clinical target volume (CTV, microscopic disease) and planning target volume (PTV, setup uncertainties) with the regular dose during the same treatment session, thereby limiting radiation toxicity. 10 , 11 , 12 , 13 Compared to the newly emerging stereotactic protocols, a SIB protocol combines the benefit of a higher total dose with the advantage of fractionation. Reoxygenation of hypoxic tumor areas and redistribution of tumor cells to more sensitive cell cycle phases between fractions increase the antitumor effect. 14 , 15 An early fractionated SIB RT study showed severe (fatal) early radiation toxicity in dogs with sinonasal tumors, and such protocols were henceforth not further investigated prior to the advent of highly conformal radiation therapy. 10 Theoretical tumor control probability computations in a previous study foresaw an increase in the 1‐year tumor‐control probability from 29% (found as clinical outcome data by Lawrence et al. 2010) to 74% when using a SIB protocol of 10 × 4.83 Gy to the gross disease instead of the regular 10 × 4.2 Gy protocol and predicted acceptable toxicity with highly conformal RT. 12 A clinical pilot study used the same SIB protocol with intensity‐modulated radiation therapy (IMRT) and reported tolerable early toxicity. 11
In this study, we evaluated prognostic variables, progression‐free survival (PFS) and overall survival (OS) in dogs with our regular 10 × 4.2 Gy versus a SIB protocol; we hypothesized that dogs treated with the SIB protocol would show significantly longer PFS/OS and a higher progression‐free rate at 1 year.
2. MATERIALS AND METHODS
2.1. Case selection
Dogs were included under the following criteria: nonhematopoietic, cytologically or histologically confirmed, newly diagnosed sinonasal tumors of all stages, without pulmonary metastasis, and RT with either a definitive‐intent daily (Monday‐Friday) 10 × 4.2 Gy protocol (group 1) or SIB protocol with 10 × 4.2 Gy +20% to the GTV (group 2). Signalment, lymph node status, tumor type, location, stage, and clinical signs were documented. 3 The minimal pretreatment staging workup included hematology, serum biochemistry, bilateral mandibular lymph node cytology, CT of the head, and thoracic radiographs or CT.
This study was originally planned as a prospective, randomized, controlled clinical trial including client‐owned dogs presented to the Division of Radiation Oncology, Vetsuisse Faculty, University of Zurich for RT of a sinonasal tumor upon owner consent. Case accrual, however, was slower than anticipated, and an interim statistical evaluation of 28 randomized cases showed no significant difference between protocols in PFS at 1 year. For ethical reasons, we decided to include nonrandomized dogs treated with the regular protocol and dogs from a pilot study treated with the SIB protocol that fulfilled the abovementioned eligibility criteria. Both the pilot study and the prospective study were approved by the Animal Ethics Council of the Canton of Zurich, Switzerland (permit number: ZH075/17).
2.2. Treatment setup, contouring, planning
The CT characteristics and positioning are described in Appendix 1. Contouring of organs at risk (eyes, lenses, lacrimal glands, brain) and target volumes was performed as previously described. 11 , 16 All dogs were treated with a linear accelerator (Varian Clinac iX 6MV, Varian Medical Systems, Palo Alto, USA) with 120 multileaf collimators and a four‐degree‐of‐freedom couch, the treatment planning system (ECLIPSE version 10.0.28 or 15.1.25, Varian Oncology Systems, Palo Alto, USA), heterogeneity correction and the AAA algorithm. Computer‐based inverse treatment planning and dynamic, coplanar intensity‐modulated radiation therapy (IMRT) were used as previously described. 11 The delivered plan included two different dose levels: 42 Gy to the PTV and 48.3 Gy to the GTV with planned 98% GTV and 98% PTV coverage of at least 95% to 107% of the prescribed dose and maximum dose > 110% of the prescribed doses accepted if limited to a small volume (<2%) inside the PTV but not within an OAR. The dose to target volumes and OAR was reported as recommended by Keyerleber et al. and Rohrer Bley et al. 17 , 18 Tissue‐equivalent bolus was used at the discretion of the radiation oncologist for adequate dose build‐up. Each treatment plan was verified dosimetrically before treatment using a phantom (Octavius‐PTW, Freiburg, Germany) and approved by a medical physicist.
Daily positioning verification with kilovolt (kV) orthogonal radiographs was performed in all dogs with the on‐board imaging system (Varian On‐Board Imager, Varian, Palo Alto, USA) and matched by an experienced radiation therapist. Additional kV‐cone‐beam CTs (CBCTs) were performed four times in the prospective group and at the discretion of the radiation oncologist for the retrospective cases. Quality assurance of the onboard imager and linear accelerator was performed daily, weekly, monthly, and annually as required by institutional and federal guidelines. 19 , 20
2.3. Follow‐up and outcome
Dogs were evaluated for early (<3 months) or late radiation toxicity (≥3 months) according to the toxicity criteria of the veterinary radiation therapy oncology group (VRTOG) and as described by Wolf et al., and symptomatic treatment was prescribed at the discretion of the responsible radiation oncologist. 21 , 22 Early grade 3 skin/mucosal toxicity was defined as confluent dermatitis/mucositis > 2 cm in diameter or ulceration, hemorrhage, necrosis.
Clinical response was subjectively evaluated as complete/partial resolution, stable, or progression of clinical signs. The imaging response was graded according to visible tumor on CBCT (last fraction) or recheck CT. The contour‐based volume response determination by Nell et al. was used. 23 Recheck CT was recommended 6 and 12 months after the end of radiation therapy. Imaging time points varied due to owner preference; owner decision for or against euthanasia based on the quality of life and clinical signs also influenced outcome.
2.4. Statistical analysis
Descriptive statistical analysis was performed by two authors with 19 and 12 years of experience of clinical trials (CRB, VM). The remaining analysis was performed by our statistical consultant with a master's in biostatistics, and power analysis was performed by our former statistical consultant with a master's degree in mathematics and a PhD and venia legendiin biostatistics.
Data were coded in spreadsheet software (Excel Microsoft® Excel® for Mac 2011, Version 14.3.2) and analyzed with open‐source statistical analysis software (R http://www.R‐project.org/). Descriptive statistics such as absolute/relative frequencies and mean/median and standard deviation/IQR were computed. PFS was defined as the interval between first RT and either recurrence of clinical nasal signs or imaging PD (whichever occurred first) or death. 23 Dogs alive and free of progression at the time of data evaluation were censored. OS was defined as the interval between the first RT and death from any cause. Dogs still alive at the time of data evaluation or lost to follow‐up were censored. Follow‐up time was defined as the time from the first RT until death, loss to follow‐up, or time of data analysis. OS and PFS were coded and analyzed with Kaplan–Meier survival analysis accompanied by the log‐rank test. Survival estimates are reported with corresponding 95% confidence intervals (95% CI). Multivariate Cox survival analysis was used to determine whether covariate age, weight, stage, tumor type, tumor size, epistaxis, or protocol showed an influence on OS/PFS. The proportion of dogs free of progression and alive two years after RT was compared by a two‐sample test of proportions. The results of statistical analyses with p values < 0.05 were considered statistically significant.
This study was originally planned as a prospective, randomized, controlled clinical trial. A power analysis for the computation of the optimal sample size was therefore conducted. It was based on the outcome (percentage of patients free of progression at 1 year) of a previously published study where the same standard protocol and treatment technique (IG‐IMRT) was used. 4 Calculation was performed with a power of 80%, significance level (Alpha) set at 5% and dropout rate equal to 5% when applying a two‐sided log‐rank test using the formula of Schoenfeld. 24 We assumed that a simultaneously integrated boost of 20% would increase the tumor control probability from 29% to 74% at one year, as proposed by a theoretical planning study. 12 Given a drop‐out rate of 5%, for a power of 0.80, the sample size needed is 42/(1 − 0.05) = 44.2. Hence, a total of 46 dogs or 23 dogs each in the group (standard protocol versus boost protocol) would be needed to detect the predicted and clinically relevant difference in patients free of progression at 1 year.
3. RESULTS
3.1. Case selection
Forty‐nine dogs were included (Figure 1). In total, 27 dogs were treated with the regular 10 × 4.2 Gy, and 22 dogs were treated with the SIB protocol.
FIGURE 1.
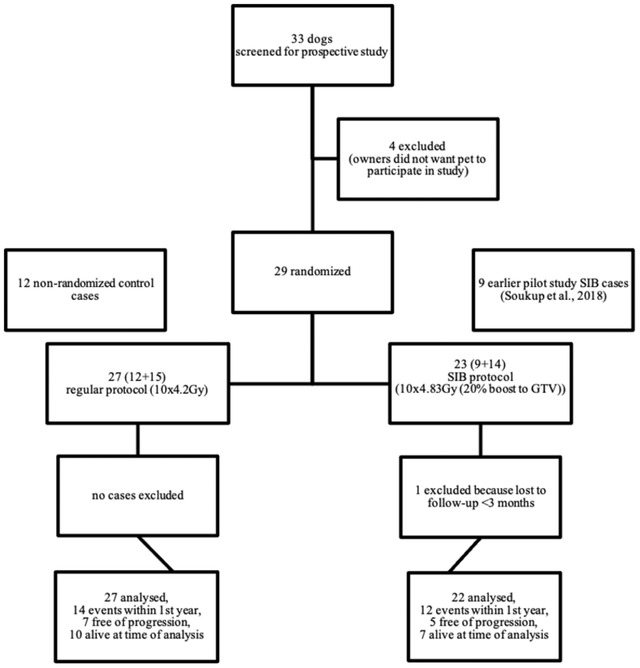
Box diagram showing all dogs included in the study and their protocols. Abbreviations: GTV, gross tumor volume; RT, radiation therapy; SIB, simultaneously integrated boost
3.2. Dog and tumor characteristics
Details regarding signalment, clinical signs, pretreatment, tumor type, location, and stage are described in Table 1 and Appendix 1. There were no statistically significant differences between groups 1 and 2 (P‐values > 0.05). The majority of dogs (57.1%) had carcinomas, and almost one‐third (30.6%) had sarcomas. Other tumor types were angiofibroma (n = 1), extensive polyp (n = 1), and esthesioneuroblastoma (n = 1), and nasal biopsies were nondiagnostic, but CT showed clear signs of bony lysis (n = 4). In one of the latter, esthesioneuroblastoma with distant metastasis was diagnosed at necropsy.
TABLE 1.
Dog characteristics, tumor characteristics, and clinical signs
| Control group (n = 27) | ||||
|---|---|---|---|---|
| Number of dogs (%) or mean (± SD) | SIB group (n = 22) | Total (n = 49) | P value | |
| Age (years) | 10.1 (± 2.6) | 10.7 (± 2.4) | 10.3 (± 2.5) | 0.49 |
| Weight (kg) | 21.6 (± 9.1) | 26.2 (± 16.5) | 23.7 (± 13.0) | 0.75 |
| Sex | 0.74 | |||
| Female | 4 (8.2%) | 3 (6.1%) | 7 (14.3%) | |
| Female spayed | 10 (20.4%) | 11 (22.4%) | 21 (42.9%) | |
| Male | 5 (10.2%) | 2 (4.1%) | 7 (14.3%) | |
| Male castrated | 8 (16.3%) | 6 (12.2%) | 14 (28.6%) | |
| Clinical signs | ||||
| Epistaxis | 20 (40.8%) | 14 (28.6%) | 34 (69.4%) | |
| Sneezing | 20 (40.8%) | 14 (28.6%) | 34 (69.4%) | |
| Nasal discharge | 13 (26.5%) | 17 (34.7%) | 30 (61.2%) | |
| Stridor | 14 (28.6%) | 8 (16.3%) | 22 (44.9%) | |
| Facial deformation | 11 (22.4%) | 7 (14.3%) | 18 (36.7%) | |
| Inappetence | 5 (10.2%) | 2 (4.1%) | 7 (14.3%) | |
| Epileptic seizures | 2 (4.1%) | 1 (2.0%) | 3 (6.1%) | |
| Head conformance | 1.0 | |||
| Brachycephalic | 2 (4.1%) | 2 (4.1%) | 4 (8.2%) | |
| Meso‐/dolichocephalic | 25 (51.0%) | 20 (40.8%) | 45 (91.8%) | |
| Tumor type | 0.28 | |||
| Malignant epithelial | 16 (32.7%) | 12 (24.5%) | 28 (57.1%) | |
| Malignant mesenchymal | 9 (18.4%) | 5 (10.2%) | 14 (28.6%) | |
| Other | 2 (4.1%) | 5 (10.2%) | 7 (14.3%) | |
| Tumor differentiation | 0.18 | |||
| Well‐differentiated | 14 (28.6%) | 15 (30.6%) | 29 (59.2%) | |
| Undifferentiated | 10 (20.4%) | 3 (6.1%) | 13 (26.5%) | |
| Not specified | 3 (6.1%) | 4 (8.2%) | 7 (14.3%) | |
| Tumor stage | 0.66 | |||
| I | 3 (6.1%) | 5 (10.2%) | 8 (16.3%) | |
| II | 5 (10.2%) | 3 (6.1%) | 8 (16.3%) | |
| III | 5 (10.2%) | 5 (10.2%) | 10 (20.4%) | |
| IV | 14 (28.6%) | 9 (18.4%) | 23 (46.9%) | |
| Lymph node status | 0.79 | |||
| Negative | 22 (44.9%) | 19 (38.8%) | 41 (83.7%) | |
| Metastatic | 2 (4.1%) | 1 (2.0%) | 3 (6.1%) | |
| Nondiagnostic | 1 (2.0%) | 0 | 1 (2.0%) | |
| Not sampled | 2 (4.1%) | 2 (4.1%) | 4 (8.2%) | |
Abbreviation: SIB, simultaneously integrated boost.
Clinical signs are described in Table 1. The mean duration of clinical signs before diagnosis was 121 days (±157). The most common clinical signs were sneezing and epistaxis in 69.4% (each). Two of the dogs with epileptic seizures had stage IV tumors with marked brain invasion and were treated with antiepileptics, and the seizures stopped during RT. The third dog had a stage III tumor but mild thickening of the cerebral falx (possible early meningioma). Almost half (46.9%) had a stage IV tumor.
3.3. Treatment setup, contouring, planning
Delineation of GTV and PTV was performed as described in Section 2 in all dogs, delineation of CTV adhered to the rules in 41 dogs and deviated in eight dogs (less than the required 1.5 cm or 2 cm cranio‐caudal extension). Target coverage as specified in the methods section was adhered to in 42 dogs and differed in seven dogs: coverage of 98% of the PTV was <95% of the prescribed dose. The dose to target volumes and OAR is described in Appendix 2. Complete raw OAR and target volume and target volume dosimetry data are deposited in an open repository (Harvard Dataverse, https://doi.org/10.7910/DVN/VETMTP).
3.4. Follow‐up and response
Clinical and imaging responses are described in Appendix 3. More than half of the dogs (55.1%) showed a clinical benefit by the end of RT, even though the end‐of‐RT CBCT showed SD in the majority of dogs (79.6%). The majority of dogs (95.9%) showed a complete or partial resolution of clinical signs as the best clinical response. The most common best imaging response was SD (53.1%), followed by PR (34.7%). While few dogs showed almost complete disappearance of the tumor, none went into CR according to volume response criteria.
Two dogs showed imaging PD on the last day of RT according to the retrospective evaluation of the images (including contouring) for this study. One showed PR when reimaged 6 months afterward, and the other was not reimaged. Both showed partial resolution of clinical signs as the best clinical response but clear local PD: one according to clinical signs 259 days and according to CT 273 days post‐RT; the other 54 days post‐RT according to progressive facial asymmetry, it was euthanized 103 days after RT.
3.5. Radiation toxicity
Radiation toxicity is shown in Table 2. Early grade 3 moist desquamation was seen in two dogs, 4.1% (regular protocol). Early mucosal toxicity was mild, and only 10.2% showed grade 3 mucositis. There was no grade 3 early ocular radiation toxicity. Late skin toxicity was mild, with grade 1 alopecia and/or leucotrichia in 72.1% of dogs. Late grade 3 ocular toxicity consisting of bilateral blindness was seen in one dog (2.3%) with a stage IV tumor 18 months after RT (regular protocol). The dose to the eyes in this dog was as follows: the mean dose (D50), near‐maximum dose (D2), and dose to 60% of the eye were 11.11 Gy, 42.27 Gy, and 9.25 Gy for the left eye and 2.9 Gy, 4.26 Gy, and 2.73 Gy for the right eye, respectively.
TABLE 2.
Early and late radiation toxicity
| Highest early radiation toxicity (< 3 months) | |||
|---|---|---|---|
| Control group (n = 27) | SIB group (n = 22) | Total (n = 49) | |
| Skin grade | |||
| 0 | 6 (12.2%) | 1 (2.0%) | 7 (14.3%) |
| 1 | 9 (18.4%) | 12 (24.5%) | 21 (42.9%) |
| 2 | 10 (20.4%) | 9 (18.4%) | 19 (38.8%) |
| 3 | 2 (4.1%) | 0 | 2 (4.1%) |
| Unknown | 0 | 0 | 0 |
| Mucosa grade | |||
| 0 | 13 (26.5%) | 9 (18.4%) | 22 (44.9%) |
| 1 | 6 (12.2%) | 2 (4.1%) | 8 (16.3%) |
| 2 | 6 (12.2%) | 8 (16.3%) | 14 (28.6%) |
| 3 | 2 (4.1%) | 3 (6.1%) | 5 (10.2%) |
| Unknown | 0 | 0 | 0 |
| Ocular ipsilateral grade | |||
| 0 | 22 (44.9%) | 17 (34.7%) | 39 (79.6%) |
| 1 | 1 (2.0%) | 4 (8.2%) | 5 (10.2%) |
| 2 | 1 (2.0%) | 1 (2.0%) | 2 (4.1%) |
| 3 | 0 | 0 | 0 |
| Unknown | 3 (6.1%) | 0 | 3 (6.1%) |
| Ocular contralateral grade | |||
| 0 | 23 (46.9%) | 17 (34.7%) | 40 (81.6%) |
| 1 | 1 (2.0%) | 4 (8.2%) | 5 (10.2%) |
| 2 | 0 | 1 (2.0%) | 1 (2.0%) |
| 3 | 0 | 0 | 0 |
| Unknown | 3 (6.1%) | 0 | 3 (6.1%) |
| Highest late radiation toxicity (≥3 months) | |||
| Control group (n = 23) | SIB group (n = 20) | Total (n = 43) | |
| Any grade | |||
| 0 | 4 (9.3%) | 1 (2.3%) | 5 (11.6%) |
| 1 | 13 (30.2%) | 15 (34.8%) | 28 (65.1%) |
| 2 | 3 (7.0%) | 2 (4.7%) | 5 (11.6%) |
| 3 | 1 (2.3%) | 0 | 1 (2.3%) |
| Unknown | 2 (4.7%) | 2 (4.7%) | 4 (9.3%) |
| Skin grade | |||
| 0 | 6 (14.0%) | 1 (2.3%) | 7 (16.3%) |
| 1 | 14 (32.6%) | 17 (39.5%) | 31 (72.1%) |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 |
| Unknown | 3 (7.0%) | 2 (4.7%) | 5 (11.6%) |
| Mucosa grade | |||
| 0 | 17 (39.5%) | 15 (34.9%) | 32 (74.4%) |
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 |
| Unknown | 6 (14.0%) | 5 (11.6%) | 11 (25.6%) |
| Ocular ipsilateral grade | |||
| 0 | 9 (20.9%) | 10 (23.3%) | 19 (44.2%) |
| 1 | 3 (7.0%) | 3 (7.0%) | 6 (14.0%) |
| 2 | 3 (7.0%) | 2 (4.7%) | 5 (11.6%) |
| 3 | 1 (2.3%) | 0 | 1 (2.3%) |
| Unknown | 7 (16.3%) | 5 (11.6%) | 12 (27.9%) |
| Ocular contralateral grade | |||
| 0 | 11 (25.6%) | 11 (25.6%) | 22 (51.2%) |
| 1 | 3 (7.0%) | 2 (4.7%) | 5 (11.6%) |
| 2 | 1 (2.3%) | 2 (4.7%) | 3 (7.0%) |
| 3 | 1 (2.3%) | 0 | 1 (2.3%) |
| Unknown | 7 (16.3%) | 5 (11.6%) | 12 (27.9%) |
Abbreviation: SIB, simultaneously integrated boost.
Five dogs were euthanized due to epileptic seizures. In all dogs, local progressive disease rather than radiation toxicity was suspected. Four dogs had stage 4 tumors, two of which had marked intracranial extension and epileptic seizures as the cause of initial presentation. Both dogs showed recurrent epileptic seizures 36 days and 97 days after radiation therapy with the regular and the new SIB protocol, respectively. One of the four dogs showed bilateral blindness and progressive signs of dementia before euthanasia due to epileptic seizures of its stage 4 sinonasal tumor 20 months after the end of radiation therapy with the regular protocol. One dog showed marked clinical disease progression with a large mass visible in the region of the frontal sinus 9 months after radiation therapy with the SIB protocol; because he suffered from a stage 4 tumor at initial presentation and tumor progression was evident on the outside, intracranial progression as a cause of epileptic seizure was suspected. One dog with a stage 3 sinonasal tumor was suspected to have an early falx meningioma with one questionable episode of epileptic seizures before starting radiation therapy (thickened meninges visible on initial CT). Because this was not treated at all, progression of this lesion was suspected to be the cause of epileptic seizures, and the dog was euthanized 80 days after the end of RT with the SIB protocol due to this and his concurrent diseases (hepatopathy, among others). Although PD was suspected, central nervous system toxicity could not be ruled out in those five dogs and was therefore graded as follows: grade 2 central nervous system early (n = 2) or late (n = 3) toxicity.
3.6. Outcome
The mean overall follow‐up time was 407 days, with a median of 376 days (95% CI: 296–457). Mean follow‐up for the group with the regular protocol was 347 days, median 348 days (95% CI: 121–500) and for the SIB group 480 days and 381 days (95% CI: 295–634), respectively.
Tumor progression was suspected or confirmed in 34 dogs (69.4%). The median PFS was 279 days (95% CI: 178–379) for all cases, 274 days (95% CI: 117–383) for the control group, and 300 days (95% CI: 143–451) for the SIB group (Figure 2). There was no significant difference in PFS between protocols (P = 0.42). The median PFS of dogs with stage IV tumors was significantly shorter, with 196 days (95% CI: 103–371) versus 358 days (95% CI: 178–465) for stage I‐III tumors (P = 0.02), as shown in Figure 3.
FIGURE 2.
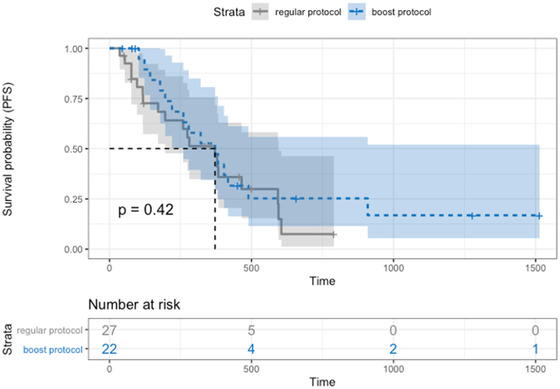
Progression‐free survival Kaplan–Meier curve of dogs split by protocol: SIB protocol (blue dotted line) with median PFS 300 days (95% CI: 143–451), control protocol (gray line) with median PFS 274 days (95% CI: 117–383). There was no significant difference between protocols (P = 0.42) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
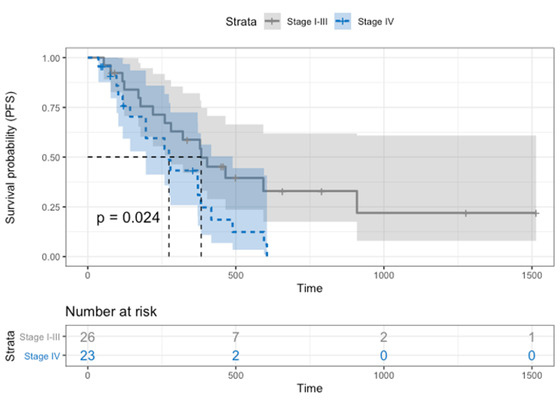
Progression‐free survival Kaplan–Meier curve of dogs depending on tumor stage: dogs with stage IV tumors (blue dotted line) with a median PFS of 196 days (95% CI: 103–371), dogs with stage I‐III tumors (gray line) with a median PFS of 358 days (95% CI: 178–465). Dogs with stage IV tumors showed a significantly shorter PFS (P = 0.02) [Colour figure can be viewed at wileyonlinelibrary.com]
The proportion of dogs without progression at one year was 40.7% (95% CI: 23.0‐61.0) for the control group and 45.5% (95% CI: 25.1‐67.3) for the SIB group. At the 2‐year mark, the proportion of dogs without progression was 3.7% (95% CI: 0.2‐20.9) for the control and 13.6% (95% CI: 3.6‐36) for the SIB group. There was no statistically significant difference (P = 0.46).
At the time of analysis, 39/49 (79.6%) dogs were dead. The median OS was 376 days (95% CI: 295–457) for all dogs, 348 days (95% CI: 121–500) for the control group, and 381 days (95% CI: 295–634) for the SIB group (Figure 4), without a significant difference between protocols (P = 0.18). Dogs with stage IV tumors had a significantly (P = 0.01) shorter median OS (330 days [95% CI: 121–382]) than dogs with stage I–III tumors (454 days (95% CI: 309–524)) (Figure 5).
FIGURE 4.
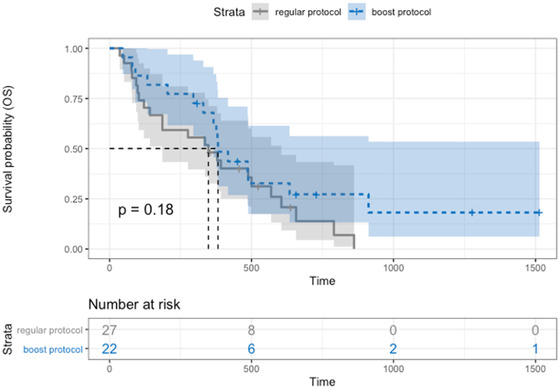
Overall survival Kaplan–Meier curve of dogs split by protocol: SIB protocol (blue dotted line) with median OS 381 days (95% CI: 295–634), control protocol (gray line) with median OS 348 days (95% CI: 121–500). There was no significant difference between protocols (P = 0.18) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
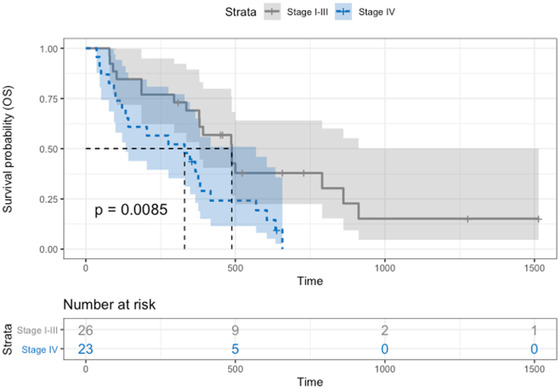
Overall survival Kaplan–Meier curve of dogs depending on the protocol: dogs with stage IV tumors (blue dotted line) with a median OS of 330 days (95% CI: 121–382) and dogs with stage I‐III tumors (gray line) with a median OS of 454 days (95% CI: 309–524). Dogs with stage IV tumors showed a significantly shorter OS (P = 0.01) [Colour figure can be viewed at wileyonlinelibrary.com]
The proportion of dogs alive at one year was 44.4% (95% CI: 26.0–64.4) for the control and 63.6% (95% CI: 40.8–82.0) for the SIB group. At the two‐year mark, 7.4% were alive (95% CI: 1.3–25.8) in the control group and 13.6% (95% CI: 3.6–36) in the SIB group. There was no statistically significant difference (P = 0.81).
Both dogs with benign tumors showed large tumor extension and would have been euthanized due to complete obstruction of the nasal pathways if not irradiated. The dog with angiofibroma showed local progression of disease according to the recheck CT performed 220 days after the end of RT. The dog with the extensive polyp showed partial imaging remission 6 months after the end of RT and was humanely euthanized due to breathing difficulties 500 days after RT. This was most likely due to the previously known heart disease according to the owner (but no imaging or information from the private veterinarian was available).
Three dogs had metastatic mandibular lymph nodes at presentation (included in the treated volume): one dog with stage IV tumors was euthanized 36 days after RT due to (ongoing) epileptic seizures; one was euthanized 80 days after RT due to epileptic seizures as mentioned above (stage III, thickened cerebral falx). The third dog was euthanized 379 days after RT due to new tumor formation or metastasis on the leg with the same cytological features as the nasal tumor (myxosarcoma) without nasal signs. Only one study dog underwent necropsy at the time of death, and it was diagnosed with distant metastasis of its esthesioneuroblastoma.
Univariate analysis stratified by protocol showed evidence of a correlation between tumor stage (IV) and GTV size (cm3) with OS. The hazard ratio of stage IV versus I–III tumors was 2.29 (95% CI: 1.156–4.551) and was significantly higher for OS (P = 0.02); dogs with stage IV tumors were 2.29 times more likely to experience death than dogs with stage I‐III tumors. The difference between dogs with stage IV and dogs with stage I–III tumors was not statistically significant for PFS (HR = 1.88, 95% CI: 0.9322–3.778, P = 0.08). Similarly, GTV size had a mild association with survival (HR = 1.01, 95% CI: 1.002‐1.015, P = 0.02); the risk of death increased by 1% for every 1 cm3 increase in GTV. There was no statistically significant influence of GTV size on progression (HR = 1.00, 95% CI: 0.9936–1.011, P = 0.62). There was no effect on OS for age (P = 0.74), weight (P = 0.07), epistaxis (P = 0.63), tumor differentiation (P = 0.12 for undifferentiated versus well‐differentiated tumors, P = 0.67 for undifferentiated differentiation versus differentiated tumors), or tumor type (P = 0.37 for mesenchymal versus epithelial, P = 0.59 for other tumors versus epithelial). None of the following variables investigated showed a significant association with PFS: age (P = 0.49), weight (P = 0.29), stage I‐III versus stage IV tumors (P = 0.78), presence of epistaxis (P = 0.15), tumor differentiation (P = 0.18, P = 0.68), tumor type (P = 0.68, P = 0.82), and GTV (P = 0.62).
Multivariate analysis adjusting for age, weight, tumor stage, and GTV showed no significant difference for PFS and OS between the regular and the SIB protocol: hazard ratio for the SIB protocol was 0.69 (95% CI: 0.3310–1.419, P = 0.31) for PFS and 0.69 (95% CI: 0.3436–1.397, P = 0.31) for OS. The full results of the Cox model are shown in Appendices 4 and 5.
In summary, tumor stage (IV) was the only significant predictor of OS and PFS in our study after adjusting for protocol, age, weight, epistaxis, and GTV. For OS, the hazard ratio of stage IV tumors was 2.16 (95% CI: 1.0828–4.319, P = 0.03), and for PFS, the hazard ratio of stage IV tumors was 2.06 (95% CI: 1.0153–4.191, P = 0.05).
4. DISCUSSION
In contrast to theoretical predictions, neither median progression‐free survival nor overall survival at 1 year was significantly longer in dogs treated with the SIB protocol than in those treated with our regular protocol. While radiation toxicity has decreased with more conformal techniques in recent years, prognosis remains fair at most in sinonasal tumors after definitive‐intent conventional or stereotactic body RT (SBRT), with OS ranging from 10.8–21 months 1 , 2 , 3 , 4 , 25 , 26 and 8.5–19.5 months. 5 , 6 , 7 , 8 , 27 respectively.
Most dogs seem to show local failure, 1 , 2 , 28 , 29 and the reasons can be multifold and include inadequate PTV dose coverage, total radiation dose, margins (clinical and/or planning target volume), and long overall treatment time (allowing for tumor cell repopulation). 26 With conformal avoidance due to the advances of radiation equipment and techniques, toxicity was successfully decreased. 4 This led to the possibility of total dose escalation to either the whole PTV with conventional fractionation, severe hypofractionation with SBRT, or dose escalation to a subvolume only with SIB RT. 5 , 11 Limiting the dose received by CTV/PTV to the regular (tolerated) dose in an SIB plan should minimize unwanted toxicity. Not only is the toxicity risk minimized, but dose escalation to the GTV increases tumor control probability and at the same time exploits the advantages of fractionation. 11 , 12
Most studies reported OS after RT in the past, and only a few reported progression‐free‐interval or ‐survival. In the current study, we chose PFS as a conservative measure (as the endpoint is either progression or death) but also described the 1‐year progression‐free rate, which was 40.7% with the regular protocol and 45.5% with the SIB protocol. This is higher than the 29% reported by Forrest et al. 30 Despite theoretical calculations by Gutierrez et al. 12 predicting a marked increase in tumor control probability with an increase in GTV dose to 48.3 Gy, our study could not demonstrate improved outcome with the SIB protocol, in line with prior findings of Bradshaw et al. 31 Multivariate analysis showed no significant difference in PFS or OS between the different protocols. While hazard ratios of <1 suggested a possible protective effect of the SIB protocol, the confidence intervals contain 1; therefore, there was no statistical significance between the two protocols.
Our study included a high percentage of dogs with epistaxis (69.4%), stage IV tumors (46.9%), and stage IV tumors with marked intracranial invasion (14.3%), all of which were found to be negative prognostic factors in previous studies. 2 , 3 , 4 , 8 , 32 , 33 This might point toward a population with advanced disease and could explain the rather poor outcome, even though those factors were not found to be of importance in our study. Predictions based on theoretical calculations depend on assumptions made and can therefore be flawed. In the study by Gutierrez et al., for example, computations were made using human data (due to lack of appropriate dog data). 12 This could be a possible reason why we could not detect a difference between the two protocols. Another possibility would be insufficient dose escalation, as the theoretical tumor control probability at one year was higher (86%) when the dose to the GTV was escalated to 51.3 Gy according to the abovementioned calculations.
We used a fractionated approach to total dose escalation with a subvolume (GTV) receiving a higher dose. Dogs treated with the dose‐escalated SIB protocol showed similar early and late radiation toxicity compared to the regular protocol (descriptive statistics only, due to small groups), and toxicity was clinically acceptable in all dogs. This is different from SBRT protocols, where severe (grade 3) presumed late toxicity was seen in 12–38% of dogs. 5 , 6 With the protocol reported here, the infrequent adverse effects of radiation are likely to be the result of increased fractionation when compared to stereotactic radiosurgery (SRT) protocols and thus might represent an important base protocol for future SIB investigations.
Early/late grade 2 CNS toxicity could not be ruled out in five dogs that showed epileptic seizures at the time of death. However, PD was deemed most likely in all dogs, as 4/5 had stage IV tumors before RT (2/4 even showed marked intracranial tumor invasion), and all had epileptic seizures at initial presentation. The fifth dog had a stage III tumor extending up to the cribriform plate at diagnosis and initially presented with seizures and a thickening of the cerebral falx suspected to be an early meningioma. Because of the small size/unclear tumor diagnosis, no antitumor treatment was administered. CNS toxicity as a reason for seizures could therefore not be ruled out in those five dogs but was considered less likely than PD. None of the dogs had concurrent MRI to improve treatment planning of the intracranial or cribriform tumor extension at the beginning. It is possible that intracranial tumor extension was therefore underestimated. Another possible reason for epileptic seizures could be suboptimal medical antiepileptic treatment. As most dogs with nasal tumors show visible remaining tumors after radiation therapy, this is most likely true for the intracranial part as well. As long as there is a space‐occupying lesion in the region of the olfactory bulb/frontal lobe, seizures can occur.
One (2.3%) dog with bilateral blindness was scored as grade 3 late ocular radiation toxicity. This might have been caused by age‐ or radiation‐related cataracts or PD (the dog was euthanized due to progressive neurological signs 20 months post‐RT of his stage IV nasal tumor) but was scored as late toxicity, as this could not be ruled out completely. The dose to the contralateral eye was negligibly low in this dog (2.9 Gy) and therefore unlikely to cause blindness. The dose to the ipsilateral eye was higher, with a mean dose of 11.11 Gy. A previous study reported no severe ocular radiation effects if the dose to 60% of the eye was not higher than 15 Gy, which was the case in our dog (9.25 Gy). In the same study, the mean dose to eyes turning blind was reported to be 39 Gy. 4
A recent study evaluated the relationship between keratoconjunctivitis sicca (KCS) and lacrimal gland dose in dogs treated with 10 × 4.2 Gy, with 33% (5/15) developing KCS after a median of 111 days. The mean lacrimal gland dose was 33.08 Gy for dogs developing KCS versus 10.33 Gy for healthy dogs. 34 The mean dose to the lacrimal gland was 14.72 (±8.65) on the left and 15.10 (±9.91) on the right in our study. Approximately half had thorough ophthalmologic examinations at regular time points, and most others had Schirmer tear tests performed (data not shown). Keratoconjunctivitis sicca was not documented in any of our dogs.
Dogs with stage IV tumors were 2.3 times more likely to die than those with stage I–III tumors. A recent study reported improved outcomes of stage IV sinonasal tumors treated with 10 × 4.2 Gy (no control group), with a median PFS and OS of 6 months and 10.6 months, respectively. The authors discussed that older nonconformal RT techniques might have led to underdosage of PTV in the region of the cribriform plate in previous studies, which was eliminated in their study (and ours) with adequate target dose coverage. 29 The question of whether GTV/CTV was adequate in our study remains unanswered, as mentioned below.
End of RT imaging response is rarely reported in dogs. 35 Our population showed a clinical benefit in most (55.1%), despite stable disease in the majority (79.6%) of dogs. While this important information influences quality of life, it could also influence radiation dosimetry in cases of remission. This was dismissed by a recent cadaver study: replanning was only necessary in cases of near‐complete GTV resolution, which was not the case in our study. 36 The finding of SD in half and PR in a third of dogs as the best imaging response is most likely influenced by the time point of imaging. Reimaging was recommended but not always performed 6 and 12 months after RT. Those fixed time points were chosen with the intent of evaluating progression‐free time in a standardized manner. To detect the best response (smallest tumor size) after radiation therapy, a sooner time point should most likely be chosen. With PFS of <1 year or reimaging because of recurrent nasal signs, the imaging response in our study probably does not represent the true best response.
We have to address several limitations: (1) This study was originally planned as a prospective, randomized clinical trial. Even though case numbers were sufficient according to power analysis, the deviation from randomization after an intermediate analysis clearly weakens the quality of the (nonrandomized) results. (2) Similar to earlier studies, rather than treating a homogeneous group of dogs, different tumor histological diagnoses, differentiations, and stages—factors that influenced outcome in the past—were included, and the numbers per subgroup were small. 1 , 3 , 6 , 37 (3) We decided to include two dogs with benign tumors due to life‐threatening initial clinical signs. These dogs could potentially have influenced outcomes in a different way than malignant tumors. (4) CTV extension and target volume coverage were less than originally planned in almost one‐fifth of the cases, which might have influenced the outcome. (5) The extent of disease and/or stage (intracranial extension) might have been underestimated in our study (no magnetic resonance imaging) and could have led to inadequately small margins. This could also explain possible PD in the region of the cribriform plate/olfactory bulb in dogs with epileptic seizures at the time of death.
In conclusion, dogs treated with dose‐escalated SIB RT showed clinically acceptable toxicity. In contrast to theoretical predictions, however, there was no significant difference in outcome compared to the regular protocol. Given the well‐tolerated side effects, a higher boost dose/other protocol adjustments should be considered.
LIST OF AUTHOR CONTRIBUTIONS
Category 1
-
(a)
Conception and Design: Rohrer Bley, Meier
-
(b)
Acquisition of Data: Soukup, Rohrer Bley, Staudinger, Körner, Meier
-
(c)
Analysis and Interpretation of Data: Rohrer Bley, Staudinger, Meier
Category 2
-
(a)
Drafting the Article: Rohrer Bley, Meier
-
(b)
Revising Article for Intellectual Content: Soukup, Rohrer Bley, Staudinger, Körner, Meier
Category 3
-
(a)
Final Approval of the Completed Article: Soukup, Rohrer Bley, Staudinger, Körner, Meier
Category 4
-
(a)
Agreement to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work appropriately investigated and resolved: Soukup, Rohrer Bley, Staudinger, Körner, Meier
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
ACKNOWLEDGMENTS
Open Access Funding provided by Universitat Zurich.
APPENDIX 1.
DOG BREEDS, TUMOR LOCATION, TYPE, PRETREATMENT, CT INFORMATION
| Control group (n = 27) | SIB group (n = 22) | Total (n = 49) | |
|---|---|---|---|
| Number of dogs (%) or mean (± SD) | |||
| Breed |
Mixed breed: 12 (24.5%) Golden Retriever: 4 (8.2%) Labrador Retriever: 4 (8.2%) Doberman Pinscher: 2 (4.1%) Fox Terrier: 2 (4.1%) French Bulldog: 2 (4.1%) Jack Russel Terrier: 2 (4.1%) Beagle, Border Collie, Boxer, Cairn Terrier, Chihuahua, Cocker Spaniel, Collie, Dutch Shepherd, Flat Coated Retriever, Irish Setter, Irish Wolfhound, Malinois, Podenco, Poitevin, Pug, Rottweiler, Shar Pei, Spanish Water Dog, Welsh Corgi, Weimaraner, West Highland White Terrier: 1 (2.0%) each |
||
| Tumor location | |||
| Unilateral | 19 (38.8%) | 18 (36.7%) | 37 (75.5%) |
| Bilateral | 6 (12.2%) | 3 (6.1%) | 9 (18.4%) |
| Mainly frontal sinus | 2 (4.1%) | 1 (2.0%) | 3 (6.1%) |
| Pretreatment | |||
| None | 2 (4.1%) | 5 (10.2%) | 7 (14.3%) |
| NSAIDs | 23 (46.9%) | 11 (22.4%) | 34 (69.4%) |
| Surgical biopsy | 1 (2.0%) | 0 | 1 (2.0%) |
| RT | 0 | 0 | 0 |
| Unknown | 1 (2.0%) | 6 (12.2%) | 7 (14.3%) |
| CT characteristics | |||
|
General Anesthesia Position Equipment Slice thickness |
Native and postcontrast CT scan of the head General anesthesia Sternal recumbency with thorax and front legs immobilized in a custom‐made, deflatable vacuum cushion (BlueBag BodyFix, Elekta AB, Stockholm, Sweden) and a custom‐made maxillary dental mold bite block (President The Original, Putty Soft, Coltène, Whaledent AG, Altstaetten, Switzerland) 16 multidetector CT unit [Brilliance CT 16‐slice, Philips Health Care Ltd, Best, the Netherlands] 2 mm, contiguous slices |
||
Abbreviaitons: CT, computed tomography; NSAIDs, nonsteroidal anti‐inflammatory drugs; RT, radiation therapy; SIB, simultaneously integrated boost.
APPENDIX 2.
TARGET VOLUME AND OAR DOSIMETRY DATA
| Mean (± SD), median (IQR) | |
|---|---|
| Gross tumor volume (cm3) | 47.64 (± 46.0), 34.20 (47.80) |
| Clinical target volume (cm3) | 99.76 (± 78.96), 75.30 (100.70) |
| Planning target volume (cm3) | 135.80 (± 96.45), 114.20 (130.20) |
| PTV mean dose (D50) (Gy) | 43.46 (± 1.72), 42.45 (2.88) |
| PTV near minimum dose (D98) (Gy) | 39.42 (± 1.53), 39.93 (1.06) |
| PTV near maximum dose (D2) (Gy) | 46.34 (± 2.87), 44.66 (5.77) |
| Left eye mean dose (D50) (Gy) | 14.73 (± 6.49), 15.92 (6.41) |
| Left eye near maximum dose (D2) (Gy) | 29.54 (± 11.50), 33.50 (13.35) |
| Left eye 60% dose (D60) (Gy) | 13.48 (± 6.27), 15.10 (9.01) |
| Left lacrimal gland dose | 14.72 (± 8.65), 14.23 (10.46) |
| Right eye mean dose (D50) (Gy) | 15.44 (± 6.71), 17.36 (6.82) |
| Right eye near maximum dose (D2) (Gy) | 30.08 (± 11.77), 34.15 (14.37) |
| Right eye 60% dose (D60) (Gy) | 14.21 (± 6.36), 15.88 (6.39) |
| Right lacrimal gland dose | 15.10 (± 9.91), 15.38 (10.68) |
| Brain mean dose (D50) (Gy) | 6.81 (± 9.94), 3.08 (7.00) |
| Brain near maximum dose (D2) (Gy) | 33.64 (± 13.21), 39.52 (7.01) |
| Brain near maximum dose (Dmax) (Gy) | 42.81 (± 9.87), 44.36 (4.25) |
Abbreviation: OAR contours included target volumes in case these structures overlapped.
APPENDIX 3.
CLINICAL AND IMAGING RESPONSE
| Control group (n = 27) | SIB group (n = 22) | Total (n = 49) | |
|---|---|---|---|
| Clinical response at end of RT | |||
| CR | 5 (10.2%) | 6 (12.2%) | 11 (22.4%) |
| PR | 9 (18.4%) | 7 (14.3%) | 16 (32.7%) |
| SD | 13 (26.5%) | 9 (18.4%) | 22 (44.9%) |
| PD | 0 | 0 | 0 |
| Imaging (CBCT) response at end of RT | |||
| CR | 0 | 0 | 0 |
| PR | 2 (4.1%) | 0 | 2 (4.1%) |
| SD | 23 (46.9%) | 16 (32.7%) | 39 (79.6%) |
| PD | 0 | 2 (4.1%) | 2 (4.1%) |
| Unknown | 2 (4.1%) | 4 (8.2%) | 6 (12.2%) |
| Best clinical response | |||
| CR | 14 (28.6%) | 18 (36.7%) | 32 (65.3%) |
| PR | 12 (24.5%) | 3 (6.1%) | 15 (30.6%) |
| SD | 1 (2.0%) | 1 (2.0%) | 2 (4.1%) |
| PD | 0 | 0 | 0 |
| Best imaging response | |||
| CR | 0 | 0 | 0 |
| PR | 9 (18.4%) | 8 (16.3%) | 17 (34.7%) |
| SD | 17 (34.7%) | 9 (18.4%) | 26 (53.1%) |
| PD | 0 | 3 (6.1%) | 3 (6.1%) |
| Unknown | 1 (2.0%) | 2 (4.1%) | 3 (6.1%) |
Abbreviaitons: CBCT, cone‐beam computed tomography; CR, complete remission; PD, progressive disease; PR, partial remission; RT, radiation therapy; SD, stable disease; SIB, simultaneously integrated boost.
APPENDIX 4.
SUMMARY RESULTS OF MULTIVARIATE COX MODEL FOR PROGRESSION‐FREE SURVIVAL
| Progression‐free survival | Hazard ratio | P value | Raw p values |
|---|---|---|---|
| Protocol (SIB versus regular) | 0.69 | 0.31 | 0.30904839 |
| Stage IV (versus stage I‐III) | 2.06 | < 0.05* | 0.04529261* |
| Age | 0.94 | 0.44 | 0.44053209 |
| Weight | 1.00 | 0.9 | 0.89334119 |
| Presence of epistaxis | 0.63 | 0.23 | 0.23042982 |
| GTV | 1.00 | 1.00 | 0.99485189 |
*Statistically significant.
APPENDIX 5.
SUMMARY RESULTS OF THE MULTIVARIATE COX MODEL FOR OVERALL SURVIVAL
| Overall survival | Hazard ratio | P value | Raw p values |
|---|---|---|---|
| Protocol (SIB versus regular) | 0.69 | 0.31 | 0.30534124 |
| Stage IV (versus stage I‐III) | 2.16 | < 0.05* | 0.02885318* |
| Age | 1.02 | 0.77 | 0.76943503 |
| Weight | 1.01 | 0.68 | 0.67327002 |
| Epistaxis (Yes) | 0.76 | 0.44 | 0.44196149 |
| GTV | 1.01 | 0.17 | 0.16818191 |
*Statistically significant.
Meier V, Staudinger C, Körner M, Soukup A, Bley CR. The dose‐escalated simultaneously integrated boost radiation protocol fails to result in a survival advantage for sinonasal tumors in dogs. Vet Radiol Ultrasound. 2022;63:633–648. 10.1111/vru.13086
[Correction added on 14 May 2022, after first online publication: CSAL funding statement has been added.]
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are openly available in the Harvard Dataverse at [https://doi.org/10.7910/DVN/VETMTP].
REFERENCES
- 1. Theon AP, Madewell BR, Harb MF, Dungworth DL. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J Am Vet Med Assoc. 1993; 202: 1469‐1475. [PubMed] [Google Scholar]
- 2. Adams WM, Miller PE, Vail DM, Forrest LJ, MacEwen EG. An accelerated technique for irradiation of malignant canine nasal and paranasal sinus tumors. Vet Radiol Ultrasound. 1998; 39: 475‐481. [DOI] [PubMed] [Google Scholar]
- 3. Adams WM, Kleiter MM, Thrall DE, et al. Prognostic significance of tumor histology and computed tomographic staging for radiation treatment response of canine nasal tumors. Vet Radiol Ultrasound. 2009; 50: 330‐335. [DOI] [PubMed] [Google Scholar]
- 4. Lawrence JA, Forrest LJ, Turek MM, et al. Proof of principle of ocular sparing in dogs with sinonasal tumors treated with intensity‐modulated radiation therapy. Vet Radiol Ultrasound. 2010; 51: 561‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox‐Alvarez S, Shiomitsu K, Lejeune AT, Szivek A, Kubicek L. Outcome of intensity‐modulated radiation therapy‐based stereotactic radiation therapy for treatment of canine nasal carcinomas. Vet Radiol Ultrasound. 2020; 61: 370‐378. [DOI] [PubMed] [Google Scholar]
- 6. Kubicek L, Milner R, An Q, Kow K, Chang M, Cooke K, et al. Outcomes and prognostic factors associated with canine sinonasal tumors treated with curative intent cone‐based stereotactic radiosurgery (1999‐2013). Vet Radiol Ultrasound. 2016; 57: 331‐340. [DOI] [PubMed] [Google Scholar]
- 7. Gieger TL, Nolan MW. Linac‐based stereotactic radiation therapy for canine nonlymphomatous nasal tumors: 29 cases (2013‐2016). Vet Comp Oncol. 2018; 16: E68‐E75. [DOI] [PubMed] [Google Scholar]
- 8. Mayer MN, DeWalt JO, Sidhu N, Mauldin GN, Waldner CL. Outcomes and adverse effects associated with stereotactic body radiation therapy in dogs with nasal tumors: 28 cases (2011–2016). J Am Vet Med Assoc. 2019; 254: 602‐612. [DOI] [PubMed] [Google Scholar]
- 9. Nolan MW, Dobson JM. The future of radiotherapy in small animals ‐ should the fractions be coarse or fine?. J Small Anim Pract. 2018; 59: 521‐530. [DOI] [PubMed] [Google Scholar]
- 10. Thrall D, McEntee MC, Novotney CA. A boost technique for irradiation of malignant canine nasal tumors. Vet Radiol Ultrasound. 1993; 34: 295‐300. [Google Scholar]
- 11. Soukup A, Meier V, Pot S, Voelter K, Rohrer Bley C. A prospective pilot study on early toxicity from a simultaneously integrated boost technique for canine sinonasal tumors using image‐guided intensity‐modulated radiation therapy. Vet Comp Oncol. 2018; 16: 441‐449. [DOI] [PubMed] [Google Scholar]
- 12. Gutierrez AN, Deveau M, Forrest LJ, Tome WA, Mackie TR. Radiobiological and treatment planning study of a simultaneously integrated boost for canine nasal tumors using helical tomotherapy. Vet Radiol Ultrasound. 2007; 48: 594‐602. [DOI] [PubMed] [Google Scholar]
- 13. Orlandi E, Palazzi M, Pignoli E, Fallai C, Giostra A, Olmi P. Radiobiological basis and clinical results of the simultaneous integrated boost (SIB) in intensity modulated radiotherapy (IMRT) for head and neck cancer: a review. Crit Rev Oncol Hematol. 2010; 73: 111‐125. [DOI] [PubMed] [Google Scholar]
- 14. Horsman MR, Wouters BG, Joiner MC, Overgaard J. The oxygen effect and fractionated radiotherapy. In: Joiner MC, van der Kogel A, eds. Basic Clinical Radiobiology. Hodder Arnold; 2009; 207‐216. [Google Scholar]
- 15. Zips D. Tumor growth and response to radiation. In: Joiner MC, van der Kogel A, eds. Basic Clinical Radiobiology. Hodder Arnold; 2009; 78‐101. [Google Scholar]
- 16. Wolf F, Rohrer Bley C, Besserer J, Meier V. Estimation of planning organ at risk volumes for ocular structures in dogs undergoing three‐dimensional image‐guided periocular radiotherapy with rigid bite block immobilization. Vet Radiol Ultrasound. 2021; 62: 246‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rohrer Bley C, Meier VS, Besserer J, Schneider U. Intensity‐modulated radiation therapy dose prescription and reporting: sum and substance of the International Commission on Radiation Units and Measurements Report 83 for veterinary medicine. Vet Radiol Ultrasound. 2019; 60: 255‐264. [DOI] [PubMed] [Google Scholar]
- 18. Keyerleber MA, McEntee MC, Farrelly J, Podgorsak M. Completeness of reporting of radiation therapy planning, dose, and delivery in veterinary radiation oncology manuscripts from 2005 to 2010. Vet Radiol Ultrasound. 2012; 53: 221‐230. [DOI] [PubMed] [Google Scholar]
- 19.Quality control for intensity‐modulated radiation therapy. http://www.ssrpm.ch/old/recrep‐m.htm#rec: Swiss Society for Radiobiology and Medical Physics, 2007;
- 20.Quality assurance of gantry‐mounted image‐guided radiotherapy systems. http://www.ssrpm.ch/old/recrep‐m.htm#rec: Swiss Society of Radiobiology and Medical Physics; 2010;1‐16.
- 21. Ladue T, Klein MK, Veterinary Radiation Therapy Oncology G . Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound. 2001; 42: 475‐476. [DOI] [PubMed] [Google Scholar]
- 22. Wolf F, Meier VS, Pot SA, Rohrer Bley C. Ocular and periocular radiation toxicity in dogs treated for sinonasal tumors: a critical review. Vet Ophthalmol. 2020; 23: 596‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nell E, Ober C, Rendahl A, Forrest L, Lawrence J. Volumetric tumor response assessment is inefficient without overt clinical benefit compared to conventional, manual veterinary response assessment in canine nasal tumors. Vet Radiol Ultrasound. 2020; 61: 592‐603. [DOI] [PubMed] [Google Scholar]
- 24. Schoenfeld DA. Sample‐size formula for the proportional‐hazards regression model. Biometrics. 1983; 39: 499‐503. [PubMed] [Google Scholar]
- 25. Adams WM, Bjorling DE, McAnulty JE, Green EM, Forrest LJ, Vail DM. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1990‐2002). J Am Vet Med Assoc. 2005; 227: 936‐941. [DOI] [PubMed] [Google Scholar]
- 26. Sones E, Smith A, Schleis S, et al. Survival times for canine intranasal sarcomas treated with radiation therapy: 86 cases (1996‐2011). Vet Radiol Ultrasound. 2013; 54: 194‐201. [DOI] [PubMed] [Google Scholar]
- 27. Glasser SA, Charney S, Dervisis NG, et al. Use of an image‐guided robotic radiosurgery system for the treatment of canine nonlymphomatous nasal tumors. J Am Anim Hosp Assoc. 2014; 50: 96‐104. [DOI] [PubMed] [Google Scholar]
- 28. Thrall DE, Heidner GL, Novotney CA, McEntee MC, Page RL. Failure patterns following cobalt irradiation in dogs with nasal carcinoma. Veterinary Radiology and Ultrasound. 1993; 34: 126‐133. [Google Scholar]
- 29. Stevens A, Turek M, Vail D, Christensen N, Forrest L. Definitive‐intent intensity modulated radiotherapy for modified‐Adams' stage 4 canine sinonasal cancer: a retrospective study of 29 cases (2011‐2017). Vet Radiol Ultrasound. 2020. [DOI] [PubMed] [Google Scholar]
- 30. Forrest L, Lawrence J, Miller P. Ocular sparing using image‐guided helical tomotherapy (IGHT) in spontaneous sino‐nasal tumors in dogs. Int J Radiat Oncol Biol Phys. 2006; 66: S425. [Google Scholar]
- 31. Bradshaw TJ, Bowen SR, Deveau MA, et al. Molecular imaging biomarkers of resistance to radiation therapy for spontaneous nasal tumors in canines. Int J Radiat Oncol Biol Phys. 2015; 91: 787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rassnick KM, Goldkamp CE, Erb HN, et al. Evaluation of factors associated with survival in dogs with untreated nasal carcinomas: 139 cases (1993‐2003). J Am Vet Med Assoc. 2006; 229: 401‐406. [DOI] [PubMed] [Google Scholar]
- 33. Wada Y, Noguchi S, Sasaki H, et al. Prognostic significance of midline shift of the olfactory or frontal lobes of the brain in canine nasal carcinomas treated by palliative radiotherapy: a pilot study. J Vet Med Sci. 2018; 80: 1724‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poirier VJ, Matsuyama A, Kim C, Darko J, Fleck A. Clinical‐dosimetric relationship between lacrimal gland dose and keratoconjunctivitis sicca in dogs with sinonasal tumors treated with radiation therapy. J Vet Intern Med. 2020; 34: 867‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morgan MJ, Lurie DM, Villamil AJ. Evaluation of tumor volume reduction of nasal carcinomas versus sarcomas in dogs treated with definitive fractionated megavoltage radiation: 15 cases (2010–2016). BMC Res Notes. 2018; 11: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoshikawa H, Nolan MW. Changes in target volume during irradiation of canine intranasal tumors can significantly impact radiation dosimetry. Vet Radiol Ultrasound. 2019; 60: 594‐604. [DOI] [PubMed] [Google Scholar]
- 37. Adams WM, Withrow SJ, Walshaw R, et al. Radiotherapy of malignant nasal tumors in 67 dogs. J Am Vet Med Assoc. 1987; 191: 311‐315. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are openly available in the Harvard Dataverse at [https://doi.org/10.7910/DVN/VETMTP].


