Abstract
This study tested the hypothesis of gender bias in frequency of unconventional T cells. Unconventional T cells exist as minor subsets of T cells in peripheral blood. Despite their low number, they play a crucial role in various immune‐mediated diseases such as inflammation, autoimmunity, allergy, and cancer. Gender‐based frequency of these cells altogether on large number of healthy individuals are unestablished creating hurdles to manifest association with various immune‐mediated pathologic conditions. In this study, we used a multicolor flow cytometric panel to identify iNKT cells, γδ T cells, and MAIT cells altogether in the peripheral blood samples of 93 healthy adult males and 109 healthy adult females from the Caucasian population. The results revealed iNKT cell median value (% T cells) in females was higher: 0.114% ranging from 0.011 to 3.84%, than males: 0.076% (p value 0.0292), ranging from 0.007 to 0.816% and found to be negatively correlated with age in females (p value 0.0047). However, γδ T cell median value in males was higher: 2.52% ranging from 0.31 to 16.09%, than females: 1.79% (p value 0.0155), ranging from 0.078 to 12.49% and each gender was negatively correlated with age (male p value 0.0003 and female p value 0.0007). MAIT cell median values were 3.04% ranging from 0.11 to 10.75% in males and 2.67% ranging from 0.2 to 18.36% in females. MAIT cells did not show any statistically significant difference between genders and found to be negatively correlated with age (p value < 0.0001). Our results could be used for further gender‐wise investigations of various pathologic conditions such as cancer and their prognosis, autoimmune diseases, allergies, and their pathogenicity.
Keywords: frequency, gender dependency, iNKT cells, reference range, unconventional T cells, γδ T cells, MAIT cells
Graphical Abstract
Gender bias was investigated in frequency of unconventional T cells altogether, iNKT and γδ T cells showed gender dependent differences, unlike MAIT cells.
Abbreviations
- (γδ)
Gamma delta T cells
- (iNKT)
invariant NKT cells
- (MAIT)
mucosa‐associated invariant T cells
1. INTRODUCTION
The immune system is divided into an innate immune system comprised of monocytes, NK cells, and dendritic cells as cellular components, and the adaptive immune system mainly represented by T and B lymphocytes. 1 Both innate and adaptive immune system have humoral components. Descent of immune system is mainly mediated by age in healthy individuals, resulting in the fact that elderly people are more prone to be affected by infectious diseases, autoimmune disorders, and cancers due to immunosenescence. 2 Gender is also a variable affecting immune response to the infections and inflammations 3 ; and gender bias was found in both innate and adaptive immune responses. 4 In peripheral blood, males have higher number of NK cells than females, whereas females have higher number of B cells. Phytohemagglutinin‐stimulated PBMC resulted in significant increase in B and T cells in females and NK cells among males. 5 The main reason of gender differences in immune system is based on reproductive status, sex chromosome genes, sex hormones, and environmental factors. Due to these differences in the immune system, females are more susceptible to autoimmune disorders than males and males are more susceptible to nonreproductive cancers. 6 , 7 Immune response for the various bacterial and viral vaccines are often higher in females than males, the hormonal mediators are one of the major contributors for gender variance. Estradiol, overall enhances both cell‐mediated and humoral immunity by the production of IFNγ and proinflammatory cytokines such as IL‐1, IL‐6, and TNF. Progesterone receptors are found on various immune cells, such as NK cells, macrophages, dendritic cells, and T cells producing broad anti‐inflammatory effects upon exposure. 4
Unconventional T cells are non‐MHC‐restricted T cells, bearing αβ and γδ TCRs. These unconventional T cells are found as minor subsets of T cells in peripheral blood, yet they have been associated with various immune‐mediated disorders such as autoimmunity, inflammations, infections, and cancers. Unconventional T cell bearing αβ TCR recognizes nonpolymorphic antigen‐presenting molecules, and based on antigen recognition, they are characterized as CD1a, CD1b, CD1c, CD1d, HLA E, and MR1 cells. CD1d+ T cells are further characterized as type I and II. The type I CD1d restricted T cells are also called invariant NKT cells (iNKT). iNKT cells express an invariant TCRα chain variable region 10 (TRAV10) and joining region 18 (TRAJ18). MR1 is nonpolymorphic, MHC class I‐like antigen‐presenting molecule recognized by mucosa‐associated invariant T cells (MAIT). MAIT cells express semi‐invariant TCR chain Vα7.2‐Jα33 combined with β chain. Another unconventional T cell population collectively referred to as γδ T cell is originated as a result of somatic recombination during TCR generation through genes encoding V (variable) D (diversity) J (joining) segment. 8
iNKT cells recognize lipid antigens, which are being found on various bacterial pathogens, and malignant cells (hematologic and solid tumors). 9 Age‐related diseases such as obesity, asthma, diabetes, cancers, and various other pathologic conditions such as autoimmune disorders, tuberculosis, and allergies have been associated with either decrease in iNKT cell number or functional/phenotypical changes. 9 , 10 iNKT cells showed promising potential in cancer immunotherapy: a recent preclinical animal model showed iNKT cells can target cancer cells through various mechanism such as direct killing via CD1d + receptor, adjuvant killing via recruiting and activating other immune cells, and suppression of angiogenesis via inhibiting tumor associated macrophages. 11 γδ T cells recognize phosphate‐containing nonpeptide ligands called phospho‐antigens; these phospho‐antigens are being produced during microbial‐specific isoprenoid biosynthesis pathways and some synthetic analogues are also found called synthetic phospho‐antigens. 12 , 13 γδ TCR contain 8 variable (V) δ gene segments, which undergo rearrangements. The most frequent rearrangements are: Vδ1, Vδ2, and Vδ3. However, 5 less frequent gene rearrangements are also identified: Vδ4, Vδ5, Vδ6, Vδ7, and Vδ8. Seven Vγ gene segments are located within chromosome 7 and rearrangement of these genes are Vγ2, Vγ3, Vγ4, Vγ5, Vγ8, Vγ9, and Vγ11. 14 Majority (up to 95%) of adult human γδ T cells in peripheral blood are Vγ9Vδ2, in contrast Vδ1 and Vδ3 γδ T cells comprise only a minority of γδ T cells. The second most abundant γδ TCR is Vδ1 pairing with either of Vγ TCR chains. Vδ1+ T cells carry NK group 2 member D (NKG2D), NKp30, NKp44 receptor, although their antitumor and protumor relevance remains unclear. 15 , 16 Microbial and mammalian cells express nonpeptide ligands originated from phosphorylated metabolites of thymidine‐containing nucleotides that appear during salvage pathway. These nonpeptide ligands are overexpressed by damaged or stressed cells. MHC class‐I‐related molecule MIC‐A and MIC‐B are stress‐induced antigens and are expressed on various cancerous cells. 12 , 17 It was shown that γδ T cells are capable of killing these cells via NKG2D receptors. 18 Riboflavin‐derivative antigens presented by nonpolymorphic MHC class‐I like protein MR1 are recognized by MAIT cells. These antigens are produced by various riboflavin synthesizing pathogens and few nonriboflavin synthesizing pathogens (bacteria, fungi, and viruses). 19 , 20 , 21 MAIT cells are activated by TCR‐dependent or independent manner and upon activation they produce innate‐effector response. 22 MAIT cells are associated with various age‐related diseases and autoimmune/immune mediated disorders such as multiple sclerosis, coeliac disease, inflammatory bowel diseases, systemic lupus erythematosus, arthritis, Sjögren's syndrome, asthma, chronic obstructive pulmonary disease, psoriasis, type 1 diabetes, type 2 diabetes, obesity, and liver diseases. 23 , 24 Decrease in frequency and/or phenotypical change of MAIT cells has been showed in various cancers (solid tumors and hematologic malignancies), 25 , 26 , 27 highlighting their important role in immune system.
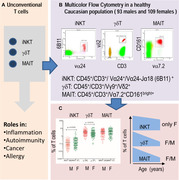
Nevertheless, various pathologic conditions have been associated with unconventional T cells (iNKT, γδ T, and MAIT) including infections (viral, fungal, and bacterial), allergies, immune‐mediated diseases, autoimmune diseases, inflammations, and malignancies (hematologic and solid tumor), and research is ongoing to discover more. 9 , 16 , 28 , 29 , 30 , 31 The utilization of unconventional T cells for cell‐based immunotherapy to target cancer cells was explored in various experiments in the last 2 decades. 11 , 32 , 33 , 34 , 35 However, there are very scanty studies done in previous decades for the evaluation of gender‐associated immunosenescence in iNKT cells, γδ T cells, and MAIT cells. To our knowledge, there is no study concerning the gender bias on these cells altogether, resulting in difficulties in their extensive use in research and routine laboratory diagnostics. In this cohort, we have established the gender‐based reference range on 93 male and 109 female peripheral blood samples of Caucasian population. The frequency of iNKT cells, γδ T cells, and MAIT cells was measured altogether using flow cytometry. Gender‐wise correlation of unconventional T cells with age was performed to see immunosenescence effects. Along with it, correlation between unconventional T cells was also performed.
2. MATERIAL AND METHOD
2.1. Exclusion criteria
Exclusion of the samples was defined as per the protocol of SENIEUR 36 with slight modifications due to pandemic situations, briefly, any infections (<6 weeks), inflammation (acute and chronic), autoimmune disorder, HIV infection, hepatitis B virus infection, hepatitis C virus infection, SARS CoV‐2 infection (<1 year), diabetes mellitus, immunosuppressive drugs, alcoholism and drug abuse, current pregnancy (including breastfeeding), malignancies (any form), immunomodulatory therapy, vaccination (<6 weeks), and other conditions that influence immune system were excluded from the study.
2.2. Study population
A prospective study was conducted to define the gender‐based reference range of unconventional T cells (iNKT cell, γδ T cell, and MAIT cell) in the healthy adult Caucasian population residing in central Europe mainly in Hungary. Samples were collected and categorized based on gender separately (Table 1). A total of 202 samples were included, in which 93 were males and 109 were females and 64 samples were excluded based on exclusion criteria.
TABLE 1.
Sample details included in this study
| Male | Female | |
|---|---|---|
| Total samples included (n) | 93 | 109 |
| Mean age (years, min–max) | 44 (18–77) | 45 (20–79) |
Mean age and minimum–maximum values are shown.
2.3. Blood sample collection and cell counting
Peripheral blood (3 ml) samples collected through venipuncture directly in BD vacutainer tube (Beckton Dickinson, San Jose, CA USA) containing tri‐potassium EDTA (K3‐EDTA) was obtained from the hematology division. A complete blood count (CBC) report was obtained from the 6‐part automated hematology analyzer Siemens ADVIA 2120i (Siemens Healthcare GmbH, Erlangen, Germany).
2.4. Flow cytometry
Multiparametric 8 color flow cytometric experiments were performed using the following pretitrated mouse antihuman monoclonal antibodies from Beckman Coulter/IOT (Brea, CA, USA), Dako (Glostrup, Denmark), Biolegend (San Diego, CA, USA), BD/Pharmingen (Franklin Lakes, New Jersey, USA), or Exbio (Vestec, Czech Republic): anti‐human CD16 (clone 3G8; BC/IOT), anti‐human TCR Vγ9 (clone B3; Biolegend), conjugated with FITC; anti‐human CD56 (clone MOC‐1; Dako), anti‐human CD7 (clone 8H8.1; BC/IOT), conjugated with PE; anti‐human TCR Vα24‐Jα18 (clone 6B11; Biolegend), anti‐human TCR Vδ2 (clone B6; Biolegend), anti‐human TCR Vα7.2 (clone 3C10; Biolegend), conjugated with peridinin chlorophyll protein/Cyanine 5.5 (PerCP/Cy5.5); anti‐human TCR Vα24 (clone C15; BC/IOT), anti‐human CD161 (clone 191B8; BC/IOT), conjugated with PE‐Cyanine 7 (PC7); anti‐human CD3 (clone SK7; BD), conjugated with allophycocyanin (APC); anti‐human CD8 (clone SK1; BD), conjugated with allophycocyanin cyanine 7 (APC‐H7); anti‐human CD4 (clone RPA‐T4; BD), conjugated with Pacific blue (PB); and anti‐human CD45 (clone HI30; Exbio), conjugated with Pacific orange (PO). These monoclonal antibodies were combined in 3 different tubes to quantify and characterize iNKT cells, γδ T cells, and MAIT cells. Cell surface staining was performed following stain‐lyse‐wash protocol briefly; 100 μl of peripheral blood was mixed with pretitrated cocktails of monoclonal antibodies in FACS tube and incubated for 15 min in dark at room temperature. Upon incubation cells were subjected to RBC lysing buffer (BD FACS™ lysing solution) for 10 min, then cells were washed with PBS using centrifugation (400 g for 5 min) and the pellet was finally resuspended in 400 μl of 1% paraformaldehyde for acquisition. Sample tube acquisition was performed using BD FACSCanto II™ (Franklin Lakes, NJ, USA) flow cytometer, using carousel setting with high acquisition of 300,000 events for each tube. For quality control, cytometer setup and tracking beads (CS&T) were measured on daily basis to keep performance tracking of equipment. An external quality control assessment was also evaluated by participating in the UK‐NEQAS Leukemia immunophenotyping program.
Monoclonal antibody (Vα24‐Jα18), clone 6B11 specifically recognizes CDR3 loop of invariant human canonical Vα24Jα18 TCR α chain of iNKT cells. 37 These cells were defined as CD45+/CD3+/ Vα24+/Vα24‐Jα18 (6B11) +. Since the majority (up to 95%) of γδ T cells express Vγ9Vδ2 receptors, we identified this specific population using different sets of antibodies for each receptor (Vγ9 and Vδ2) in our study, and they were defined as CD45+/CD3+/Vγ9+/Vδ2+ cells, referred to as γδ T cells throughout the manuscript. MAIT cells were defined as CD45+/CD3+/Vα7.2+/CD161bright+ (Figure S1). Flow cytometric data files (.fcs 3.0) were analyzed using FACSDiva version 6.1.3 software (BD Biosciences, San Jose, CA, USA). The following percentages were calculated: lymphocytes (among white blood cells); T cells (among lymphocytes and among WBC); iNKT cells, γδ T cells, and MAIT cells (among T cells and among lymphocytes). Absolute count of total iNKT cells, γδ T cells, and MAIT cells were calculated from the absolute WBC and lymphocyte counts (×109/L) obtained from CBC report of hematology analyzer using dual‐platform method and values were expressed as cells/μl.
2.5. Statistical analysis
Data were analyzed by GraphPad Prism statistical software v5.0 (GraphPad Software, San Diego, USA). Kolmogorov–Smirnov (SK) and Shapiro–Wilk normality tests were used to test normality of data distribution. The following data were assessed for gender‐wise normal distribution: an absolute number of white blood cells, lymphocytes, percentage of T cells, iNKT cells, γδ T cells, and MAIT cells. Absolute WBC count, lymphocyte count, and percentage of T cells in male and female both were accepted as a normal distribution; rest of the data including female absolute WBC count, percentage and absolute number of iNKT cells, γδ T cells, and MAIT cells in male and female both rejected the hypothesis of data normalization, and failed to achieve normal distribution. For nonparametric, Mann–Whitney test and for parametric, Student's t‐test was used to compare continuous variables of 2 related and independent samples. In addition, correlation and linear regression analysis were performed between 2 continuous variables and the statistical significance of the findings was set at a p value of less than 0.05.
3. RESULTS
3.1. Population statistics
A total of 202 peripheral blood samples from 93 male and 109 female healthy Caucasian adults were obtained between the period of December 2020 to May 2021 and 64 samples were excluded based on exclusion criteria. The distribution of age was normal and there was no significant difference between the mean age in both genders (p = 0.6582). The mean age in males was 44 years (18–77), whereas in females, it was 45 years (20–79) (Table 1).
3.2. Gender‐based analysis
The percentage of T cells showed significant difference between the genders: p value 0.0044 (Figure 1(A)), iNKT cells and γδ T cells percentage also showed a significant difference between the genders, p values 0.0292 and 0.0155 respectively (Figure 1(B)). Moreover, absolute numbers of iNKT cells and γδ T cells also showed statistically significant difference between the genders: p value 0.0189 and 0.0419, respectively (Figure 1(C)). Both percentage and absolute numbers of WBC, lymphocytes, and MAIT cells showed no significant difference (p value > 0.05 in all cases). The mean ± SD of females had dominance in percentage and absolute numbers of WBC, T cells, iNKT cells, and MAIT cells, compared with males, whereas males dominated γδ T cells over females (Table 2).
FIGURE 1.
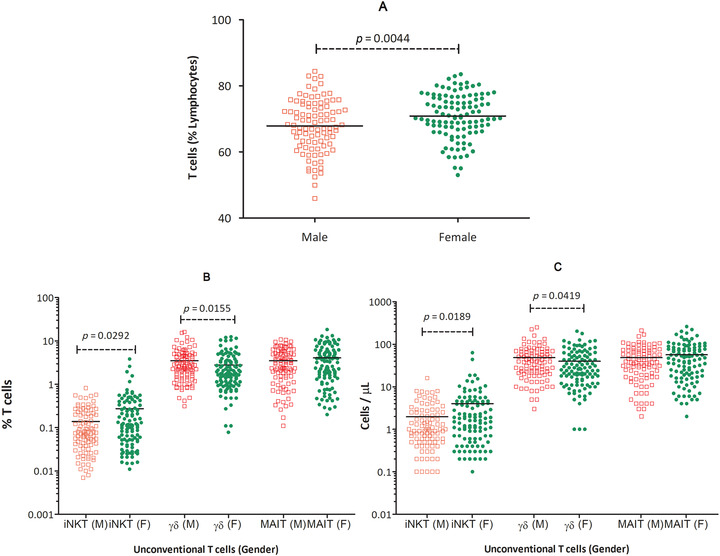
T cell percentages among lymphocytes in males and females (A) percentages of iNKT cells, γδ T cells, and MAIT cells (B) and absolute numbers of iNKT cells, γδ T cells, and MAIT cells (C), in peripheral blood of healthy adult males, compared with healthy adult females. Statistically significant p values are indicated for each of the comparisons inside the graphs
TABLE 2.
Descriptive statistics of unconventional T cells
| Gender | Method (equipment) | Parameter | Median (min–max) | 25–75% | Mean ± sd | 2.5–97.5% |
|---|---|---|---|---|---|---|
| Male | Hematology analyzer | WBC (×109/L) | 6.6 (3.7–11) | 5.8–7.5 | 6.7 ± 1.3 | 4.5–9.8 |
| Lymphocytes (×109/L) | 2 (1–3.7) | 1.7–2.4 | 2 ± 0.49 | 1.1–3.2 | ||
| Flow cytometer | T cell (% Lymphocytes) | 68 (45.9–84.4) | 62.2–73.9 | 67.8 ± 7.9 | 50.8–82.9 | |
| iNKT (% T cell) | 0.076 (0.007–0.816) | 0.038–0.203 | 0.18 ± 0.51 | 0.0087–0.56 | ||
| γδ T cell (% T cell) | 2.52 (0.31–16.09) | 1.4–4.6 | 3.5 ± 3 | 0.43–14.2 | ||
| MAIT (% T cell) | 3.04 (0.11–10.75) | 1.4–5 | 3.4 ± 2.6 | 0.20–10.2 | ||
| Dual platform | iNKT (cells/μl) | 1 (0.1–16) | 0.5–2.5 | 2.5 ± 6.4 | 0.1–7.9 | |
| γδ T cell (cells/μl) | 38 (3 −249) | 20–67 | 48.9 ± 42 | 5–202 | ||
| MAIT (cells/μl) | 37.5 (2–211) | 18‐71 | 48.5 ± 38 | 3–162 | ||
| Female | Hematology analyzer | WBC (×109/L) | 6.7 (4.1–12.3) | 5.8–7.8 | 7 ± 1.6 | 4.6–11 |
| Lymphocytes (×109/L) | 2 (0.97–4.91) | 1.6–2.4 | 2 ± 0.62 | 1–3.6 | ||
| Flow cytometer | T cell (% Lymphocytes) | 70.6 (53–83.5) | 66.8–76.5 | 70.8 ± 6.7 | 55.1–82.3 | |
| iNKT (% T cell) | 0.114 (0.011–3.84) | 0.048–0.306 | 0.27 ± 0.5 | 0.015–2.1 | ||
| γδ T cell (% T cell) | 1.79 (0.078–12.49) | 1–3.4 | 2.7 ± 2.6 | 0.11–11.6 | ||
| MAIT (% T cell) | 2.67 (0.2–18.36) | 1.4–5.8 | 4 ± 3.5 | 0.285–13 | ||
| Dual platform | iNKT (cells/μl) | 1.6 (0.1–63.5) | 0.6‐4.4 | 4 ± 7.9 | 0.2–25 | |
| γδ T cell (cells/μl) | 26 (1–203) | 13–55 | 39.7 ± 37 | 1–139 | ||
| MAIT (cells/μl) | 45 (2–261) | 18–78 | 56.5 ± 49.8 | 4‐206 |
Gender‐dependent PB unconventional T cells (iNKT, γδ, MAIT) along with WBC and lymphocyte counts were analyzed. Median, minimum, maximum, 25−75% and 2.5–97.5% (reference range) of hematologic counts, percentages, and their absolute numbers are also shown in Table 2. In males, the median percentage of iNKT cells among T cells was 0.076%, ranging from 0.007 to 0.816% and the median absolute number of iNKT cells was 1 cells/μl, ranging from 0.1 to 16 cells/μl. The median percentage of γδ T cells among T cells was 2.52%, ranging from 0.31 to 16.09% and the median absolute number was 38 cells/μl, ranging from 3 to 249 cells/μl. MAIT cell ratio among T cells was 3.04%, ranging from 0.11 to 10.75% and the absolute number of MAIT cells was 37.5 cells/μl, ranging from 2 to 211 cells/μl (Table 2). Reference range (2.5–97.5%) for percentages of unconventional T cells (iNKT, γδ T, and MAIT cells) among T cells was 0.0087–0.56, 0.43–14.2, and 0.20–10.2%, respectively.
In females, percentage of iNKT cells among T cells was 0.114%, ranging from 0.011 to 3.84% and the median absolute number of iNKT cells was 1.6 cells/μl, ranging from 0.1 to 63.5 cells/μl. The median percentage of γδ T cells among T cells was 1.79%, ranging from 0.078 to 12.49% and the median absolute number was 26 cells/μl, ranging from 1 to 203 cells/μl. MAIT cell ratio among T cells was 2.67%, ranging from 0.2 to 18.36% and the absolute number of MAIT cells was 45 cells/μl, ranging from 2 to 261 cells/μl (Table 2). Reference range (2.5–97.5%) for percentages of unconventional T cells (iNKT, γδ T, and MAIT cells) among T cells was 0.015–2.1, 0.11–11.6, and 0.28–13%, respectively.
3.3. Combined analysis of age and gender
When age and gender were compared together, we found that iNKT cells in males; percentage and absolute number showed no correlation with age and remained constant throughout: p value 0.6614 and 0.7740, respectively (Figures 2 and 3). Interestingly, iNKT cells (percentage and absolute number) in females showed a negative correlation with age: p value 0.0047 and 0.0016, respectively. A negative correlation with age was found in γδ T cells: percentage and absolute numbers in males: p value 0.0003 and 0.0002 as well as in females: p value 0.0007 and 0.0003, respectively. A very strong correlation was observed between MAIT cells and age for both males and females: p value < 0.0001 for all cases. Linear regression analysis of percentage and absolute numbers revealed that γδ T cells and MAIT cells drop strikingly by age in both males and females (Figures 2 and 3).
FIGURE 2.
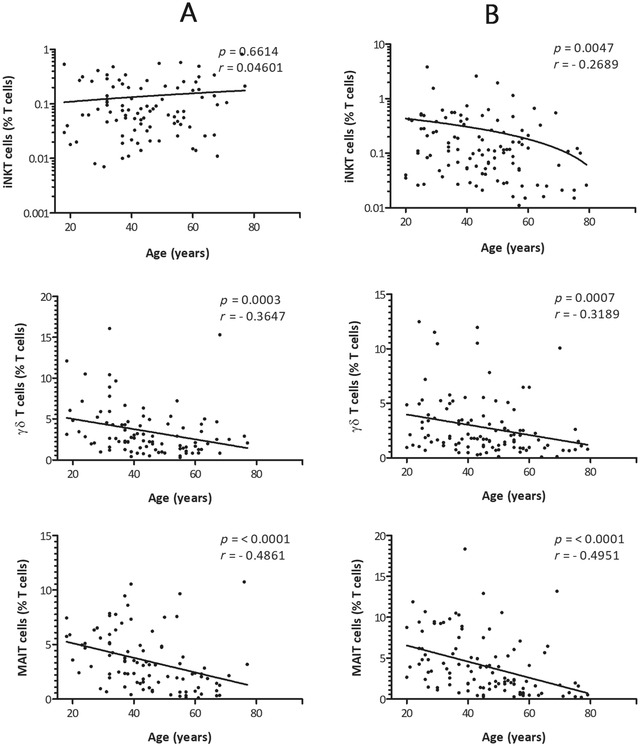
Correlation between age and the percentages of iNKT cells, γδ T cells, and MAIT cells in the study population of healthy adult males (A) and females (B). x‐axis represents age (years) and y‐axis represents unconventional T cells respectively. The p values and Spearman's rank correlation coefficients for each of the comparisons are indicated within the graphics. The statistical significance was set at a p value of less than 0.05
FIGURE 3.
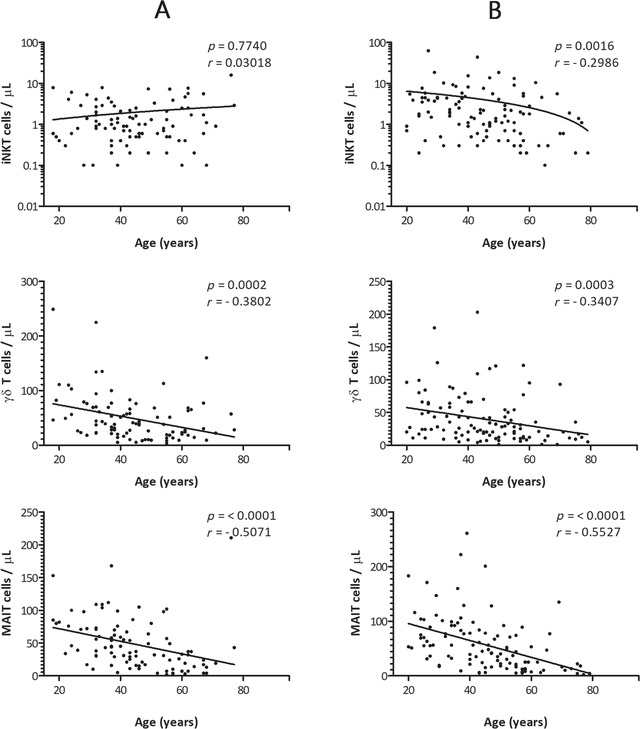
Correlation between age and the absolute numbers of iNKT cells, γδ T cells, and MAIT cells in the study population of healthy adult males (A) and females (B). x‐axis represents age (years) and y‐axis represents unconventional T cells, respectively. The p values and Spearman's rank correlation coefficients for each of the comparisons are indicated within the graphics. The statistical significance was set at a p value of less than 0.05
3.4. Gender‐based unconventional T cell correlation
We correlated gender‐specific unconventional T cells with each other separately, and found a significant positive correlation with each other except, the iNKT cells with γδ T cells in males: p value 0.4017. iNKT cells showed positive correlation with MAIT cells: p value 0.0027 and γδ T cells also showed significant correlation with MAIT cells: p value 0.0134. In females, unconventional T cells showed positive correlation with each other. iNKT cells correlated with γδ T cells and MAIT cells: p value 0.0074 and 0.0017 respectively, and γδ T cells also positively correlated with MAIT cells: p value 0.0427 (Figure 4). The overall total population of unconventional T cells, irrespective of gender, also correlated with each other and a significant positive correlation was found: iNKT cell with γδ T cell and MAIT cell; p value 0.0262, r = 0.1568 and p value < 0.0001, r = 0.3071, respectively. γδ T cell with MAIT cell; p value 0.0020, r = 0.2164 (Figure 5).
FIGURE 4.
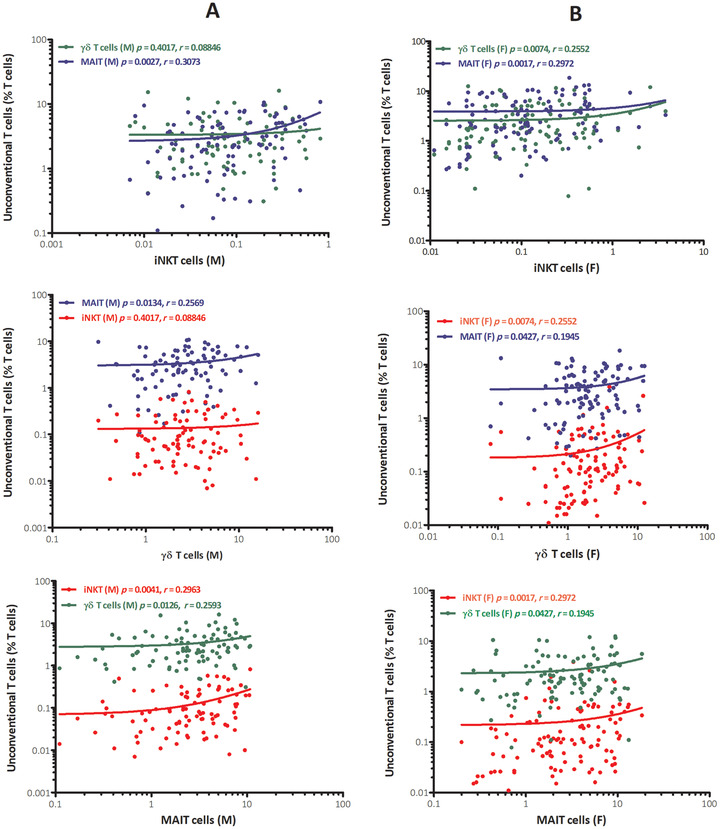
Gender‐based correlation between percentages of iNKT cells, γδ T cells, and MAIT cells in peripheral blood of healthy adult males (A) and females (B). x‐axis represents unconventional T cells and y‐axis represents % of unconventional T cells among T cells. The p values and Spearman's rank correlation coefficients of each correlation are indicated within the graphics separately
FIGURE 5.
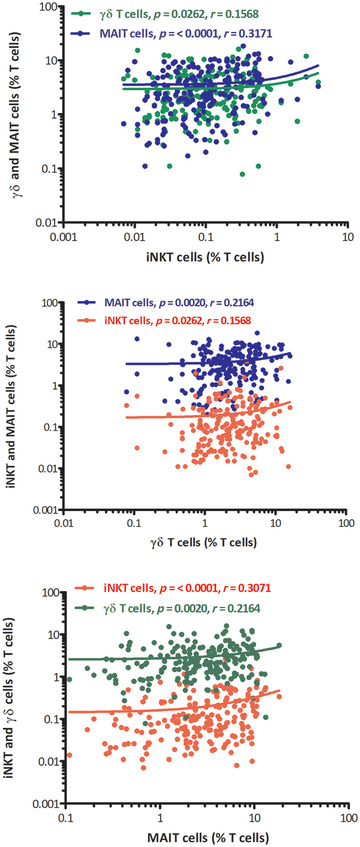
Correlation between percentages of iNKT cells, γδ T cells, and MAIT cells altogether in peripheral blood of healthy adult samples. x‐axis represents unconventional T cells and y‐axis represents % of unconventional T cells among T cells. The p values and Spearman's rank correlation coefficients of each correlation are indicated within the graphics separately
4. DISCUSSION
Unconventional T cells play important roles in various immune‐mediated diseases and their potential is investigated in cell‐based immunotherapy through various animal and clinical studies. Gender bias in frequency is important to know for the accurate and specific evaluation of cell populations. In case of unconventional T cells altogether, gender‐based frequency is largely unestablished leading to various challenges for the researches in this field. In our study, gender bias was investigated in unconventional T cells. Percentage of CD3+ T cells (% of lymphocytes) was found to be significantly higher in females compared to males. Similar observations were reported by several studies, where female‐specific higher percentage of T cells were noted compared to males, 38 and the possible reason for these differences in immune cells is due to the sex hormones, sex chromosomes, reproductive status, and environmental factors. 4 iNKT cells (percentage and absolute number) were found to be significantly affected by genders. This fact highlights the importance of iNKT cells to be studied in more detail in various immunologic disorders, despite their low number in peripheral blood. Furthermore, iNKT cells in females were significantly higher than in males. Fereidouni et al. 39 found no statistically significant difference in the frequency of iNKT cells between genders on 42 healthy adult volunteers (24 male, 16 female). However, Montoya et al. 37 observed that modest higher frequency in iNKT cells trend in females compared with males, yet the data were not statistically significant. The explanation, which resolves the discrepancy between our results and previously published results of Fereidouni et al., 39 lies in the size of the populations studied. Sample size of their study was not comparable to our study, which provided us the advantage of correct statistical calculation. The advantage of our study is that it is a single center, prospective study covering iNKT cells, γδ T cells, and MAIT cells altogether, including single population (Caucasian), large number of gender‐balanced samples, and intensive exclusion criteria. The gender‐based significant difference was also found in the percentage and absolute number of γδ T cells. In contrast to iNKT cells, γδ T cells showed that males have higher numbers than females. Similar results were reported by Michishita et al. 40 (42 males and 42 females), who showed that males have higher numbers of γδ T cells than females. In the figure of the paper recently published by Schultze‐Florey et al., 41 it is clearly shown that γδ T cell numbers are higher in males, especially in younger age. In a study by Caccamo et al. 42 on gender‐based differences between different age groups: 60 male and 67 female samples from the age group of 20–30 years, a statistically significant difference was found. In the age group 30–60 years, including 51 male and 47 female samples, significant difference was also detected between genders. However, in the same study, females had a higher percentage of γδ T cells than males. 42 Fonseca et al. 43 (18 males and 12 females) have not found any statistically significant difference between the genders. Gender‐based difference was not found in MAIT cells for both percentage and absolute numbers; however, mean ± sd was higher in females than males. The studies published by Novak et al. 44 also found similar results, showing no statistically significant differences between males and females in MAIT cells.
Gender‐wise correlation of unconventional T cells with age revealed interesting results for iNKT cells in males; it did not show a statistically significant correlation with age both in percentage and absolute numbers. However, female iNKT cells (percentage and absolute numbers) showed a significant negative correlation with age. The immunosenescence of iNKT cells in females poses more threat for an immune‐mediated disorder such as cancer and autoimmunity 45 , 46 than males. Jing et al. 47 found that iNKT cells negatively correlated with age both in males and females. γδ T cells showed a significant negative correlation with age in males and females altogether, for the percentage as well as absolute numbers. Similar results were obtained by Fonseca et al., 43 who reported a similar negative correlation of age with γδ T cells in males and females both, whereas females had a higher striking slope of linear regression compared with males. γδ T cells in both males and females support the immunosenescence that poses the risk of immune‐mediated disorders in the elderly population. Percentage and absolute numbers of MAIT cells in males and females both showed a statistically significant negative correlation with age. Similar results were obtained by Chen et al. 48 who showed a negative correlation of the percentage of MAIT cells in young to elderly males and females.
Unconventional T cells were correlated altogether and showed significant positive correlation in both genders except for iNKT cells with γδ T cells in males (Figure 4). The total population of unconventional T cells, irrespective of gender, also showed a statistically significant correlation with each other (Figure 5). Notably, iNKT cells correlation with MAIT cells was always stronger irrespective of gender. Similar results were found by Gherardin et al. 49 on 27 healthy adult peripheral blood samples, showing a positive correlation between the percentage of iNKT cells with the percentage of MAIT cells and percentage of γδ T cells with the percentage of MAIT cells. MAIT cells and iNKT cells are different populations; MAIT cells show a semi‐invariant α chain (Vα7.2‐Jα33) combined with a limited repertoire of β chain of TCR, whereas iNKT cells bear semi‐invariant αβTCR (Vα24/Jα18) combined with Vβ11. The antigen recognition mechanisms of these cells are also distinctly different. However, both share the same transcriptional factors: T‐bet, RORγt, and PLZF 50 ; and common genes were also found for their intra‐thymic development such as members of the signaling lymphocyte activation molecule family receptor. 51 Another experiment suggests the relation of iNKT cells and MAIT cells when CD1d knockout mice showed an increased number of MAIT cells and MR‐1‐deficient mice showed increasing number of iNKT cells. 52 Altogether it has been shown that iNKT cells and MAIT cells are complementary to each other, despite the huge difference in frequency. 53
In conclusion, this study investigates the frequency of unconventional T cell differences between genders in the Caucasian population, highlighting that the iNKT cells were higher and found to be negatively correlated with age in females, while γδ T cells were higher in males than females and each gender was negatively correlating with age. MAIT cells did not show any difference in genders and showed negative correlation with age in both genders. A positive correlation between the unconventional T cells was an interesting finding and it could be used as a foundational study to build upon further, to investigate immune‐mediated diseases and their relation to unconventional T cells together.
AUTHORSHIP
P. S. was involved in cell immunophenotyping, sample acquisition, preliminary flow cytometry data analysis, interpretation, and writing the manuscript; Z. M. and M. S.‐S. contributed to donor recruitment, procurement of laboratory materials, and management of patients’ data; S. B. and Z. H. are equally contributed to flow cytometry data analysis and interpretation, sample selection procedures, designed the experiments, performed flow cytometry data analysis and interpretation, collected and interpreted the data, analyzed statistics, reviewed the literatures. Sandor Barath and Zsuzsanna Hevessy share equal senior authorship.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Supplementary figure 1: Dot plots and gating strategies obtained from multicolor flow cytometric panel of a healthy adult donor's peripheral blood, which includes iNKT cells, γδ T cells and MAIT cells; Universally doublets were excluded via gating singlets on FSC‐A vs FSC‐H dot plot, then WBCs were gated on FSC vs SSC dot plot, and later lymphocytes were gated on CD45 vs SSC.
ACKNOWLEDGMENTS
The authors would like to thank blood donors for agreeing to participate in the study, to nurses of the phlebotomy and technical officers of the hematology department and department of flow cytometry laboratory for support concerning the routine laboratory tests.
Singh P, Szaraz‐Szeles M, Mezei Z, Barath S, Hevessy Z. Gender‐dependent frequency of unconventional T cells in a healthy adult Caucasian population: A combinational study of invariant NKT cells, γδ T cells, and mucosa‐associated invariant T cells. J Leukoc Biol. 2022;112:1155–1165. 10.1002/JLB.5A1121-583RR
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1. Weiskopf D, Weinberger B, Grubeck‐Loebenstein B. The aging of the immune system. Transpl Int. 2009;22:1041‐1050. 10.1111/j.1432-2277.2009.00927.x [DOI] [PubMed] [Google Scholar]
- 2. Mousset CM, Hobo W, Woestenenk R, Preijers F, Dolstra H, van der Waart AB. Comprehensive phenotyping of t cells using flow cytometry. Cytom Part A. 2019;95:647‐654. 10.1002/cyto.a.23724 [DOI] [PubMed] [Google Scholar]
- 3. Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8:380‐383. 10.1177/096120339900800510 [DOI] [PubMed] [Google Scholar]
- 4. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626‐638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 5. Abdullah M, Chai PS, Chong MY, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272:214‐219. 10.1016/j.cellimm.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 6. Jacobson DL, Gange SJ, Rose NR, Graham NMH. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223‐243. 10.1006/clin.1997.4412 [DOI] [PubMed] [Google Scholar]
- 7. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174‐1182. 10.1158/1055-9965.EPI-08-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godfrey DI, Uldrich AP, Mccluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114‐1124. 10.1038/ni.3298.1114 [DOI] [PubMed] [Google Scholar]
- 9. Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131‐142. 10.1038/nri2904 [DOI] [PubMed] [Google Scholar]
- 10. Lam PY, Nissen MD, Mattarollo SR. Invariant natural killer T cells in immune regulation of blood cancers: harnessing their potential in immunotherapies. Front Immunol. 2017;8:1‐12. 10.3389/fimmu.2017.01355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Y, Smith DJ, Zhou Y, et al. Development of hematopoietic stem cell‐engineered invariant natural killer T cell therapy for cancer. Cell Stem Cell. 2019;25:542‐557.e9. 10.1016/j.stem.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Constant P, Davodeau F, Peyrat MA, et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science (80‐). 1994;264:267‐270. 10.1126/science.8146660 [DOI] [PubMed] [Google Scholar]
- 13. Belmant C, Espinosa E, Halary F, et al. A chemical basis for recognition of nonpeptide antigens by human γδ T cells*. FASEB J. 2000;14:1669‐1670. 10.1096/fj.99-0909fje [DOI] [PubMed] [Google Scholar]
- 14. Adams ErinJ, Gu S, Luoma AM. Human gamma delta T cells: evolution and ligand recognition. Cell Immunol. 2015;296:31‐40. 10.1016/j.cellimm.2015.04.008.Human [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Araújo ND, Gama FM, De Souza Barros M, et al. Translating unconventional T cells and their roles in leukemia antitumor immunity. J Immunol Res. 2021;2021. 10.1155/2021/6633824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva‐Santos B, Serre K, Norell H. γδT cells in cancer. Nat Rev Immunol. 2015;15:683‐691. 10.1038/nri3904 [DOI] [PubMed] [Google Scholar]
- 17. Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor‐associated expression and recognition by tumor‐derived γδ T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879‐6884. 10.1073/pnas.96.12.6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bahram S, Inoko H, Shiina T, Radosavljevic M. MIC and other NKG2D ligands: from none to too many. Curr Opin Immunol. 2005;17:505‐509. 10.1016/j.coi.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 19. Salou M, Franciszkiewicz K, Lantz O. MAIT cells in infectious diseases. Curr Opin Immunol. 2017;48:7‐14. 10.1016/j.coi.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 20. Sattler A, Thiel LG, Ruhm AH, et al. Mucosal associated invariant T cells are differentially impaired in tolerant and immunosuppressed liver transplant recipients. Am J Transplant. 2020. 10.1111/ajt.16122 [DOI] [PubMed] [Google Scholar]
- 21. Ussher JE, Willberg CB, Klenerman P. MAIT cells and viruses. Immunol Cell Biol. 2018;96:630‐641. 10.1111/imcb.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat Immunol. 2019;20:1110‐1128. 10.1038/s41590-019-0444-8 [DOI] [PubMed] [Google Scholar]
- 23. Rouxel O, Lehuen A. Mucosal‐associated invariant T cells in autoimmune and immune‐mediated diseases. Immunol Cell Biol. 2018;96:618‐629. 10.1111/imcb.12011 [DOI] [PubMed] [Google Scholar]
- 24. Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunol. 2016;5:e98. 10.1038/cti.2016.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling L, Lin Y, Zheng W, et al. Circulating and tumor‐infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep. 2016;6:1‐10. 10.1038/srep20358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zabijak L, Attencourt C, Guignant C, et al. Increased tumor infiltration by mucosal‐associated invariant T cells correlates with poor survival in colorectal cancer patients. Cancer Immunol Immunother. 2015;64:1601‐1608. 10.1007/s00262-015-1764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mérédis F, Koen V, Sylvia F, et al. Both mucosal‐associated invariant and natural killer T‐cell deficiency in multiple myeloma can be countered by PD‐1 inhibition. Haematologica. 2016;23:471‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayday AC. γδ Cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975‐1026. 10.1146/annurev.immunol.18.1.975 [DOI] [PubMed] [Google Scholar]
- 29. Rajoriya N, Fergusson J, Leithead JA, Klenerman P. Gamma delta T‐lymphocytes in hepatitis C and chronic liver disease. Front Immunol. 2014;5:1‐9. 10.3389/fimmu.2014.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal‐associated invariant T cells and disease. Nat Rev Immunol. 2019;19:643‐657. 10.1038/s41577-019-0191-y [DOI] [PubMed] [Google Scholar]
- 31. Meermeier EW, Harriff MJ, Karamooz E, Lewinsohn DM. MAIT cells and microbial immunity. Immunol Cell Biol. 2018;96:607‐617. 10.1111/imcb.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heczey A, Courtney AN, Montalbano A, et al. Anti‐GD2 CAR‐NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26:1686‐1690. 10.1038/s41591-020-1074-2 [DOI] [PubMed] [Google Scholar]
- 33. Deniger DC, Moyes JS, Cooper LJN. Clinical applications of gamma delta T cells with multivalent immunity. Front Immunol. 2014;5:1‐10. 10.3389/fimmu.2014.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gogoi D, Chiplunkar SV. Targeting gamma delta T cells for cancer immunotherapy: bench to bedside. Indian J Med Res. 2013;138:755‐761. [PMC free article] [PubMed] [Google Scholar]
- 35. Qin VM, Souza CD, Neeson PJ, Zhu JJ, Chimeric Antigen Receptor beyond CAR‐T Cells, (2021) 1‐17. [DOI] [PMC free article] [PubMed]
- 36. Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the senieur protocol. Mech Ageing Dev. 1984;28:47‐55. 10.1016/0047-6374(84)90152-0 [DOI] [PubMed] [Google Scholar]
- 37. Montoya CJ, Pollard D, Martinson J, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell‐clonotypic monoclonal antibody, 6B11. Immunology. 2007;122:1‐14. 10.1111/j.1365-2567.2007.02647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahnstedt H, McCullough LD. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell Immunol. 2019:345. 10.1016/j.cellimm.2019.103960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fereidouni M, Hosseini RF, Azad FJ, Schenkel J, Varasteh A, Mahmoudi M. Frequency of circulating iNKT cells among Iranian healthy adults. Cytom Part B ‐ Clin Cytom. 2010;78:65‐69. 10.1002/cyto.b.20489 [DOI] [PubMed] [Google Scholar]
- 40. Michishita Y, Hirokawa M, Guo YM, et al. Age‐associated alteration of γδ T‐cell repertoire and different profiles of activation‐induced death of Vδ1 and Vδ2 T cells. Int J Hematol. 2011;94:230‐240. 10.1007/s12185-011-0907-7 [DOI] [PubMed] [Google Scholar]
- 41. Schultze‐Florey CR, Chukhno E, Goudeva L, et al. Distribution of major lymphocyte subsets and memory T‐cell subpopulations in healthy adults employing GLP‐conforming multicolor flow cytometry. Leukemia. 2021;35:3021‐3025. 10.1038/s41375-021-01348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caccamo N, Dieli F, Wesch D, Jomaa H, Eber M. Sex‐specific phenotypical and functional differences in peripheral human V 9/V 2 T cells. J Leukoc Biol. 2006;79:663‐666. 10.1189/jlb.1105640 [DOI] [PubMed] [Google Scholar]
- 43. Fonseca S, Pereira V, Lau C, dos A Teixeira M, Bini‐Antunes M, Lima M. Human peripheral blood gamma delta T cells: report on a series of healthy caucasian portuguese adults and comprehensive review of the literature. Cells. 2020;9:729. 10.3390/cells9030729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Novak J, Dobrovolny J, Novakova L, Kozak T. The decrease in number and change in phenotype of mucosal‐associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol. 2014;80:271‐275. 10.1111/sji.12193 [DOI] [PubMed] [Google Scholar]
- 45. Molling JW, Kölgen W, Van Der Vliet HJJ, et al. Peripheral blood IFN‐γ‐secreting Vα24+Vβ11 + NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87‐93. 10.1002/ijc.20998 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Sedimbi S, Löfbom L, Singh AK, Porcelli SA, Cardell SL. Unique invariant natural killer T cells promote intestinal polyps by suppressing TH1 immunity and promoting regulatory T cells. Mucosal Immunol. 2018;11:131‐143. 10.1038/mi.2017.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jing Y, Gravenstein S, Rao Chaganty N, et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d‐restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42:719‐732. 10.1016/j.exger.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 48. Chen P, Deng W, Li D, et al. Circulating mucosal‐associated invariant T Cells in a large cohort of chiniese individuals from newborn to elderly. Front Immunol. 2019;10:1‐12. 10.3389/fimmu.2019.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gherardin NA, Souter MNT, Koay HF, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol. 2018;96:507‐525. 10.1111/imcb.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim EY, Lynch L, Brennan PJ, et al. The transcriptional programs of iNKT cells. 2019;27:26‐32. doi: 10.1016/j.smim.2015.02.005.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koay HF, Su S, Amann‐Zalcenstein D, et al. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci Immunol. 2019;4:1‐17. 10.1126/sciimmunol.aay6039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koay HF, Gherardin NA, Enders A, et al. A three‐stage intrathymic development pathway for the mucosal‐associated invariant T cell lineage. Nat Immunol. 2016;17:1300‐1311. 10.1038/ni.3565 [DOI] [PubMed] [Google Scholar]
- 53. Lukasik Z, Elewaut D, Venken K. Mait cells come to the rescue in cancer immunotherapy? Cancers (Basel). 2020;12:1‐18. 10.3390/cancers12020413 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Dot plots and gating strategies obtained from multicolor flow cytometric panel of a healthy adult donor's peripheral blood, which includes iNKT cells, γδ T cells and MAIT cells; Universally doublets were excluded via gating singlets on FSC‐A vs FSC‐H dot plot, then WBCs were gated on FSC vs SSC dot plot, and later lymphocytes were gated on CD45 vs SSC.


