Abstract
Background
Over 6 million Americans have Alzheimer's Disease or Related Dementia (ADRD) but whether spikes in spending surrounding a new diagnosis reflect pre‐diagnosis morbidity, diagnostic testing, or treatments for comorbidities is unknown.
Methods
We used the 1998–2018 Health and Retirement Study and linked Medicare claims from older (≥65) adults to assess incremental quarterly spending changes just before versus just after a clinical diagnosis (diagnosis cohort, n = 2779) and, for comparative purposes, for a cohort screened as impaired based on the validated Telephone Interview for Cognitive Status (TICS) (impairment cohort, n = 2318). Models were adjusted for sociodemographic and health characteristics. Spending patterns were examined separately by sex, race, education, dual eligibility, and geography.
Results
Among the diagnosis cohort, mean (SD) overall spending was $4773 ($9774) per quarter – 43% of which was spending on hospital care ($2048). In adjusted analyses, spending increased by $8400 (p < 0.001), or 156%, from $5394 in the quarter prior to $13,794 in the quarter including the diagnosis. Among the cohort in which impairment was incidentally detected using the TICS, adjusted spending did not change from just before to after detection of impairment, from $2986 before and $2962 after detection (p = 0.90). Incremental spending changes did not differ by sex, race, education, dual eligibility, or geography.
Conclusion
Large, transient spending increases accompany an ADRD diagnosis that may not be attributed to impairment or changes in functional status due to dementia. Further study may help reveal how treatment for comorbidities is associated with the clinical diagnosis of dementia, with potential implications for Medicare spending.
Keywords: dementia, diagnosis, impairment, Medicare, utilization
Key points
A dementia diagnosis is temporally associated with substantial increases in Medicare spending, primarily due to inpatient and skilled nursing facility treatment.
Cognitive impairment detected during routine screening is not associated with changes in Medicare spending.
Spending changes after a clinical diagnosis or first evidence of cognitive impairment do not vary by sex, race, education, dual eligibility, or geography
Why does this paper matter?
Rather than immediate care needs at the time of disease onset, the spending associated with a clinical diagnosis of dementia may reflect a health care crisis, during which the dementia is recognized as a contributing factor.
INTRODUCTION
Alzheimer's disease and related dementias (ADRD) affect as many as 6 million Americans, with care largely reflecting the acute, institutional, and home health care needs for the progressive behavioral and functional deficits of ADRD. 1 , 2 , 3 The costs of ADRD care are significant at both the societal and individual levels, with ADRD‐related spending expected to reach $205 billion annually by 2050; the medical expenditures also increase for individuals following a diagnosis. 4 , 5
A more nuanced examination of spending associated with an ADRD diagnosis may help uncover characteristics of the ADRD diagnosis period, including drivers of high spending levels. Spending might spike due to care needs for dementia itself, including limitations with functional status, injuries, and behavioral symptoms. Alternatively, it may reflect increased utilization related to the process of diagnosis (particularly if diagnoses are delayed), such as costly treatments including inpatient or rehabilitative care for common comorbidities 6 that result in a diagnosis. If so, health system factors – care for comorbidities or changes in decisions regarding expensive elective treatments following a diagnosis 7 – rather than costs due to underlying cognitive impairment could drive the high observed spending around a diagnosis.
While previous research has shown higher spending for individuals with ADRD, 4 , 5 with spending increases before and after disease onset 1 , 3 reflecting more preventable hospitalizations 8 and rehabilitation services, it is unclear if these spending spikes reflect underlying disease (i.e., the impairment associated with dementia) versus factors associated with clinical diagnosis. 9 , 10 , 11 , 12 We build on this work by exploring spending associated with a new ADRD diagnosis and, for comparative purposes, spending associated with the first detection of cognitive impairment at routine screening. Specifically, we compare spending before versus after each of an ADRD diagnosis and new evidence of cognitive impairment, overall and across sub‐groups of fee‐for‐service Medicare beneficiaries who differ in their baseline risk of ADRD spending. Through an examination of both clinical diagnosis and impairment, we can identify whether spending changes reflect underlying disease or other health or health system factors. With this approach, we aim to offer insights into spending changes and beneficiaries who could potentially benefit from greater surveillance and care management before and after an ADRD diagnosis.
METHODS
Data source and study population
Data for this study were from the 2000–2018 waves of the Health and Retirement Study (HRS), linked to fee‐for‐service Medicare claims. The nationally representative HRS contains longitudinal data on sociodemographic, health, employment and retirement, and health utilization characteristics of older Americans (ages 51 years and older). Conducted every other year since 1992, each survey wave provides information for approximately 20,000 respondents, with high survey response rates. 13 We used the HRS because of its robust set of sociodemographic, functional status, and health indicators and its linkage to Medicare claims for approximately 80% of respondents.
Our study population included 2779 older adults (ages ≥65) with incident ADRD who participated in the HRS and had linked Medicare fee‐for‐service claims in the years 1998–2018 (hereafter referred to as the “ADRD‐diagnosis” cohort); for which there were 33,348 person‐quarters. Incident ADRD diagnosis was defined as a first clinical diagnosis in the study period for the years 2000 and beyond, using a list of established ICD‐9 14 and ICD‐10 15 codes. Diagnoses were captured in both primary and secondary positions in the claims from inpatient and ambulatory records to identify ADRD diagnoses.
To assess the first screening for impairment based on cognitive testing, we applied the Langa‐Weir Classification of Cognitive Function to the HRS' Telephone Interview Cognitive Status (TICS) survey and reports of cognition from proxy respondents. 16 The TICS is routinely conducted each survey wave for all HRS respondents ages 65 and older. It evaluates cognition based on questions that measure memory and mental processing. Because interviews for individuals who are unable to respond can be completed by family members or friends, the Langa‐Weir classification scheme also identifies cognitive status using a proxy. It accounts for the proxy's evaluations of the individual's memory and functional status, and the HRS interviewer's assessment of the individual's difficulty in completing the interview because of cognitive limitation. 17 , 18 The mean (SD) time between consecutive TICS surveys in the sample was 772.0 (111.3); the minimum is 394 and the maximum is 1188 days. We identified the date of the first detection of cognitive impairment from the routine screening using the date on which the TICS was administered to the respondent (or when a proxy answered questions about the respondent's cognitive status). Individuals first detected with cognitive impairment using the TICS are hereafter referred to as the “CI‐TICS” cohort).
Both cohorts, the ADRD‐diagnosis (based on claims data) and CI‐TICS (screened, or detected, as impaired based on TICS scores), were evaluated. The cohorts were not mutually exclusive; individuals with an incident ADRD diagnosis could, but were not required, also have cognitive impairment as measured by the TICS.
Specific to each respondent, we examined the three‐year period (12 quarters) surrounding incident impairment in the ADRD‐diagnosis and CI‐TICS cohorts. The 12 quarters entailed the 6 quarters prior to and the 6 quarters inclusive of and following incident impairment. The composition of individuals in “pre” and “post” periods of impairment incidence was the same.
Medicare spending (dependent variable)
The primary dependent variable was quarterly total Medicare spending. Claims data contain information about payments rendered by Medicare to providers, physicians, or suppliers for services rendered. For a comprehensive portrait of utilization, we examined overall spending plus sub‐categories: hospital, skilled nursing facility, outpatient physician, home health, and durable medical equipment. Spending was inflation‐adjusted to 2018 dollars. 19
Covariates
HRS survey data were extracted to account for beneficiary characteristics at baseline, prior to diagnosis or incident impairment. These included self‐reported (or in cases of proxy response, proxy‐reported) sociodemographic characteristics: age, sex, race/ethnicity (Non‐Hispanic White, Black, Hispanic, and the Other category that includes individuals of Native American, Alaskan Indian, and Asian backgrounds), education (less than high school, high school degree, some college, and college degree or more), and annual respondent income. We extracted health information, including self‐reported health status (fair/poor, good, very good/excellent), dichotomous indicators for eight chronic conditions (hypertension, diabetes, cancer, lung disease, heart failure, stroke, psychiatric conditions, and arthritis), vision (ranging from 1 to 6 with lower scores indicating better eyesight with glasses or corrective lenses) and hearing (ranging from 1 to 5 with lower scores indicating better hearing, including use of a hearing aid if applicable), and respondents' number of difficulties with each of activities of daily living (ADLs: bathing, dressing, eating, getting in and out of bed, and walking across a room) and instrumental ADLs (IADLs: using the phone, managing money, taking medications, shopping, and preparing a meal). These chronic conditions and sensory and physical functioning measures are each strongly associated with cognitive impairment. 6 Failure to account for differences prior to an ADRD diagnosis could confound ADRD spending estimates, potentially biasing them upwards.
Statistical analyses
We present descriptive statistics, including characteristics of both cohorts and unadjusted spending overall and according to the time period (pre versus post), with spending compared using paired t‐tests. Next, we assessed incremental changes in spending in the quarter before compared to the quarter of the ADRD diagnosis, adjusted for baseline patient differences that may reflect drivers of spending unrelated to ADRD (e.g., resources to access and use care, education, underlying health risks). Using two‐part regression models that account for the non‐normal distribution and a large number of zeroes, 20 we regressed spending on dummy variables indicating the specific quarters relative to the date of ADRD diagnosis indicated in the claims (i.e., 6 quarters before and 6 quarters after diagnosis) and baseline (i.e., prior to diagnosis) covariates. The first stage of each model involved a probit and the second stage was an ordinary least squares regression. 21 Standard errors were clustered by a person to account for within‐person correlations. We estimated adjusted spending for each quarter of the study period, then estimated the incremental spending change from the quarter prior to the quarter of the ADRD diagnosis or the first detection of impairment. Model outcomes included spending sub‐groups (e.g., hospital, skilled nursing facility, outpatient, home health) for the full sample of respondents. We also examined the leading (primary) ICD diagnoses (for all claims) and the leading DRGs (for hospitalizations) at the time of ADRD diagnosis.
To examine differences in spending by beneficiary characteristics, we estimated separate models stratified by biological sex, race (Non‐Hispanic White, Black), education (college versus non‐college educated), dual eligibility, and geography (rural/non‐rural).
For comparative purposes, we separately evaluated beneficiary characteristics and estimated all models for the time period surrounding the date of first detection of cognitive impairment as measured using the Langa‐Weir Classification of Cognitive Function (based on respondents' TICS score or on proxy responses).
Sensitivity analyses
Because the onset of cognitive impairment may have occurred prior to the TICS administration when impairment was first detected, we estimated spending for the impairment cohort using the mid‐point of the interview dates between the current and prior surveys. 22 To assess whether changes in spending were similar to other incident conditions, we examined hip fracture (using Diagnosis Related Group [DRG] codes 480–482) and heart failure cohorts (DRG codes 291–293).
For all analyses, two‐tailed p < 0.05 was considered statistically significant. This study was deemed exempt from review by the University of Michigan Institutional Review Board.
RESULTS
Characteristics of study population
Of the 2779 respondents in the ADRD‐diagnosis cohort, the mean (SD) age was 82.2 (6.9) years, 63% were female; 59.6% were Non‐Hispanic White, 13.0% Non‐Hispanic Black, and 26.2% Hispanic (Table 1). The mean (SD) numbers of reported difficulties with ADLs and IADLs were 1.3 (1.9) and 1.2 (1.4), respectively. In the comparison CI‐TICS cohort, the mean age, and gender distribution were similar; 46.4% were Non‐Hispanic White, 17.6% Non‐Hispanic Black, and 34.3% Hispanic (Table 1). The mean (SD) numbers of reported difficulties with ADLs and IADLs were 1.6 (2.0) and 1.7 (1.5), respectively.
TABLE 1.
Characteristics of study participants
| Cohort | ||
|---|---|---|
| Characteristic | ADRD‐Diagnosis | CI‐TICS |
| Sample, No. | 2779 | 2318 |
| Sociodemographic characteristics | ||
| Mean age in years (SD) | 82.2 (6.9) | 81.1 (7.7) |
| Sex, No. (%) | ||
| Male | 1024 (36.9) | 794 (34.3) |
| Female | 1755 (63.1) | 1524 (65.8) |
| Race/ethnicity, No. (%) | ||
| Non‐Hispanic White | 1655 (59.6) | 1075 (46.4) |
| Non‐Hispanic Black | 360 (13.0) | 407 (17.6) |
| Hispanic | 727 (26.2) | 794 (34.3) |
| Other | 37 (1.3) | 42 (1.8) |
| Mean annual income, $ (SD) a | 606.8 (10,298.2) | 376.9 (3591.0) |
| Education, No. (%) | ||
| High School or less | 950 (34.2) | 1167 (50.4) |
| Some college or associate's degree | 1340 (48.2) | 938 (40.5) |
| Bachelor's degree | 319 (11.5) | 138 (6.0) |
| Graduate or advanced degree | 170 (6.1) | 75 (3.2) |
| Health status | ||
| Self‐rated health, No. (%) | ||
| Very good/excellent | 574 (20.7) | 401 (17.3) |
| Good | 807 (29.0) | 559 (24.1) |
| Fair/poor | 1398 (50.3) | 1358 (58.6) |
| No. with hypertension diagnosis (%) | 1855 (66.8) | 1506 (65.0) |
| No. with mental health diagnosis (%) | 594 (21.4) | 577 (24.9) |
| No. with diabetes diagnosis (%) | 699 (25.2) | 538 (23.2) |
| No. with respiratory diagnosis (%) | 365 (13.1) | 296 (12.8) |
| No. with cardiovascular diagnosis b (%) | 1200 (43.2) | 913 (39.4) |
| No. with stroke diagnosis (%) | 522 (18.8) | 531 (22.9) |
| No. with cancer diagnosis (%) | 558 (20.1) | 350 (15.1) |
| No. with arthritis diagnosis (%) | 1995 (71.8) | 1630 (70.3) |
| Functional status | ||
| Mean No. of ADL limitations (SD) | 1.3 (1.9) | 1.6 (2.0) |
| Mean No. of IADL limitations (SD) | 1.2 (1.4) | 1.7 (1.5) |
Note: Characteristics of respondents at baseline. The ADRD‐Diagnosis cohort includes individuals with an incident ADRD diagnosis identified in the claims data. The CI‐TICS cohort includes individuals with the first detection of cognitive impairment using the TICS.
Most respondents in either sample reported no annual (wage and salary) income.
Includes heart failure and myocardial infarction.
Approximately one‐third (35%) of individuals in the ADRD‐diagnosis cohort also included evidence of cognitive impairment as assessed using the TICS. Of those overlapping individuals in the ADRD‐diagnosis cohort, the average time from the HRS assessment demonstrating cognitive impairment to a health care encounter with a dementia diagnosis was 1.3 years or 5 quarters. Within the CI‐TICS cohort, 618 (27%) had already been diagnosed with ADRD and within the ADRD‐diagnosis cohort, 597 (21%) had prior evidence of impairment based on prior assessment using the TICS.
Unadjusted medicare spending
Overall mean (SD) spending across the study period was $4773 ($11,610) per quarter for the ADRD‐diagnosis cohort (Table 2). With $2048 ($7993) in spending, hospital care was the largest spending sub‐component, representing 43% of the quarterly total. Physician and SNF care represented 20% and 17% of total quarterly spending, with $940 ($1789) and $796 ($3612) in spending, respectively. Overall average quarterly spending increased from $3585 ($9408) in the pre‐period to $5960 ($13,352) after the ADRD clinical diagnosis or an average quarterly difference of $2375 (Table 2). Overall mean spending was lower in each of the pre and post periods for the CI‐TICS cohort (Table 2).
TABLE 2.
Unadjusted quarterly spending before and after a first ADRD diagnosis or screened as impaired for older adults, overall and by category
| Category | Overall ($) | Pre‐period, $ (% Total) | Post‐period, $ (% Total) | Difference, $ | p |
|---|---|---|---|---|---|
| Overall diagnosis cohort | 4772.8 | 3585.3 (100.0) | 5960.3 (100.0) | 2375.0 | <0.001 |
| Hospital | 2047.6 | 1433.6 (40.0) | 2661.5 (44.7) | 1227.9 | <0.001 |
| Outpatient | 458.6 | 422.3 (11.8) | 494.9 (8.3) | 72.6 | <0.001 |
| Physician | 939.6 | 828.3 (23.1) | 1051.0 (17.6) | 222.6 | <0.001 |
| SNF a | 796.2 | 437.7 (9.7) | 1154.7 (19.4) | 717.0 | <0.001 |
| Home health | 432.4 | 364.5 (10.1) | 500.4 (8.4) | 135.9 | <0.001 |
| DME b | 98.4 | 98.9 (2.8) | 97.9 (1.6) | −1.0 | 0.97 |
| Overall screened as impaired cohort | 3146.4 | 3138.3 (100.0) | 3154.5 (100.0) | −16.1 | 0.89 |
| Hospital | 1208.9 | 1240.9 (39.5) | 1176.8 (37.3) | 64.1 | 0.36 |
| Outpatient | 341.2 | 329.9 (10.5) | 352.4 (11.1) | −22.5 | 0.18 |
| Physician | 651.3 | 656.6 (20.9) | 646.1 (20.5) | 10.5 | 0.61 |
| SNF a | 465.3 | 421.4 (13.4) | 509.1 (16.1) | −87.7 | 0.02 |
| Home health | 373.4 | 392.3 (12.5) | 354.5 (11.3) | 37.7 | 0.13 |
| DME b | 106.4 | 97.2 (3.1) | 115.5 (3.7) | −18.3 | 0.01 |
Note: Specific to each respondent, we examined the three‐year period (12 quarters) surrounding incident impairment in the ADRD‐diagnosis and CI‐TICS cohorts. The 12 quarters entailed the 6 quarters prior to and the 6 quarters inclusive of and following incident impairment by the TICS screening.
SNF = Skilled Nursing Facility
DME = Durable Medical Equipment
The leading diagnoses during a hospitalization (the most costly component of incident ADRD spending) included heart failure, tracheostomy, bone marrow transplant, stroke, septicemia, and urinary tract infections (together representing ~20% of total diagnoses); ADRD (3.1%) and degenerative nervous system disorders (2.9%) were also among the leading diagnoses (see Table S1).
Adjusted medicare spending
Incremental quarterly spending change after ADRD diagnosis
Among all beneficiaries in the ADRD‐diagnosis cohort, adjusted spending increased by $8400 (p < 0.001), or 156%, from the quarter before to just after a first ADRD diagnosis (Figure 1). Spending increased from $5394 in the quarter prior to $13,794.4 in the quarter after the diagnosis (Table 3). Percentage increases in spending in the quarter after compared to before ADRD diagnosis were greatest in skilled nursing ($1868, p < 0.001; 222%), inpatient ($4858, p < 0.001; 199% increase), and home health ($388, p < 0.001; 86%) (Figure 1; Table S3). The overall difference in spending in the post‐ compared to the pre‐diagnosis period was $2497 (+71%, p < 0.001) (Table S2).
FIGURE 1.
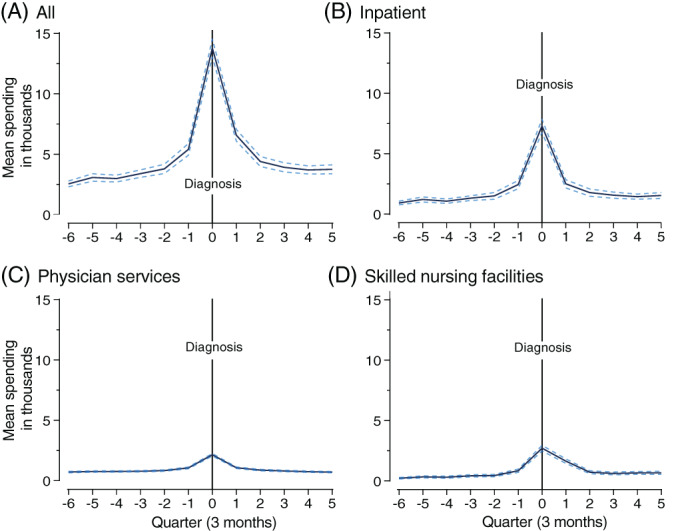
Caption: Spending changes, overall and by treatment setting, before and after an ADRD diagnosis for older (≥65) Medicare beneficiaries. Legend: Dotted lines indicate 95% confidence intervals. Quarter 7 (Q7) is the quarter in which ADRD was diagnosed clinically. Two‐part models were estimated, adjusting for sociodemographic, health, and functional status characteristics
TABLE 3.
Incremental differences in spending after a first ADRD diagnosis or evidence of cognitive impairment among older adults, overall and by respondent characteristic
| Before ($) | After ($) | Difference ($) (% change) | p‐value | Group difference ($) | p | |
|---|---|---|---|---|---|---|
| Overall diagnosis cohort | 5393.8 | 13,794.4 | 8400.6 (156) | <0.001 | — | — |
| Sex | ||||||
| Women | 5119.7 | 13,018.2 | 7898.5 (154) | <0.001 | −1349.5 | 0.12 |
| Men | 5871.0 | 15,119.0 | 9248.0 (158) | <0.001 | ||
| Race | ||||||
| Non‐Hispanic Black | 5592.8 | 14,301.8 | 8709 (156) | <0.001 | 320.5 | 0.81 |
| Non‐Hispanic White | 5046.7 | 13,435.2 | 8388 (166) | <0.001 | ||
| Education | ||||||
| College | 5254.4 | 12,519.8 | 7265.4 (138) | <0.001 | −1373.3 | 0.14 |
| <College | 5428.0 | 14,066.7 | 8638.7 (159) | <0.001 | ||
| Dual eligibility | ||||||
| Dual | 6628.8 | 13,825.9 | 7197.1 (109) | <0.001 | −1468.5 | 0.23 |
| Non‐Dual | 5217.1 | 13,882.6 | 8665.5 (166) | <0.001 | ||
| Geography | ||||||
| Rural | 4662.0 | 13,511.1 | 8849.1 (190) | <0.001 | 507.4 | 0.68 |
| Non‐Rural | 5491.3 | 13,833.0 | 8341.7 (152) | <0.001 | ||
| Overall screened as impaired cohort | 2986.2 | 2962.2 | −24.0 (0) | 0.90 | — | — |
| Sex | ||||||
| Women | 2879.4 | 3127.1 | 247.7 (9) | 0.30 | −799.9 | 0.06 |
| Men | 3200.3 | 2648.1 | −552.3 (−17) | 0.11 | ||
| Race | ||||||
| Non‐Hispanic Black | 3393.6 | 3020.9 | −372.7 (−11) | 0.48 | −400.4 | 0.51 |
| Non‐Hispanic White | 2918.3 | 2946.1 | 27.8 (0) | 0.10 | ||
| Education | ||||||
| College | 3677.7 | 3173.3 | −504.4 (−14) | 0.52 | −530.3 | 0.51 |
| <College | 2905.8 | 2931.7 | 26.0 (0) | 0.89 | ||
| Dual eligibility | ||||||
| Dual | 3636.2 | 3196.6 | −439.6 (12) | 0.39 | −540.1 | 0.33 |
| Non‐Dual | 2803.5 | 2904.0 | 100.5 (4) | 0.63 | ||
| Geography | ||||||
| Rural | 3458.9 | 2266.8 | −1192.1 (34) | 0.03 | −1360.9 | 0.02 |
| Non‐Rural | 2909.4 | 3078.1 | 168.8 (6) | 0.42 | ||
Note: The spending differences reflect utilization changes in the quarter including and after compared to the quarter just before a first ADRD diagnosis. Results are from stratified models that each controlled for sociodemographic, health, and functional status characteristics (including those characteristics used to stratify other models, e.g., education, dual eligibility). Spending was standardized using 2018 dollars. The models stratified by race had a smaller total analytic sample (across sub‐groups) than other models, given the focus on only Non‐Hispanic White and Non‐Hispanic Black respondents. Those models included 24,180 quarters (4320 for Blacks and 19,860 Non‐Hispanic Whites) for 2015 (360 Blacks and 1655 Non‐Hispanic White) individuals.
Non‐Hispanic Blacks had a $320.5 greater increase than Non‐Hispanic Whites (p = 0.81); college graduates had a $1373.3 smaller increase than non‐college graduates (p = 0.14); dual eligibles had a $1468.5 smaller increase than non‐duals (p = 0.23); and those living in rural areas had a $507.4 greater increase than those in non‐rural areas (p = 0.68). However, relative differences in spending change from the quarter prior to the quarter after a first ADRD diagnosis did not differ by race, education, dual eligibility, or geography (Table 3).
Incremental quarterly spending change after first detection of cognitive impairment
Among all beneficiaries in the CI‐TICS cohort, adjusted spending was not observed to change from the quarter before to just after the incident indication of impairment (Table 3, Figure 2). Spending was $2986 just before and $2962 just after detection, or a decrease of $24 (p = 0.90). Spending differences after impairment detection were observed by geography but not for other respondent characteristics. Spending in the quarter after compared to just before impairment detection decreased for rural (−$1192, p = 0.03) but not non‐rural respondents ($169, p = 0.42), or a $1361 relative decrease for rural versus non‐rural respondents (p = 0.02).
FIGURE 2.

Caption: Spending changes, overall and by treatment setting, before and after the first evidence of cognitive impairment for older (≥65) Medicare beneficiaries. Legend: Dotted lines indicate 95% confidence intervals. Quarter 7 (Q7) is the quarter in which ADRD was diagnosed clinically. Two‐part models were estimated, adjusting for sociodemographic, health, and functional status characteristics
Sensitivity analyses
When examining an alternative date for the first detection of TICS‐assessed cognitive impairment in the CI‐TICS cohort—the mid‐point of the time between the current and prior interview date—we found similar results (Table S3). Spending was $3408 just before and $3184 just after the midpoint between the first detection and the prior survey wave, or a decrease of $224 (p = 0.25).
For the hip fracture cohort, used as a comparison to the clinical ADRD diagnosis and indication of impairment cohorts, spending was $4402 just before and $30,183 just after onset, or an increase of $25,781 (p < 0.001) (Table S4). For this cohort, the overall difference in spending in the post compared to the pre‐event period was $6237 (+155%, p < 0.001). For the heart failure cohort, spending was $9573 just before and $25,154 just after onset, or an increase of $15,581 (p < 0.001) (Table S5). For this cohort, the overall difference in spending in the post compared to the pre‐event period was $4481 (+73%, p < 0.001).
DISCUSSION
In this study of older Medicare beneficiaries, large increases in spending occurred contemporaneously with an ADRD clinical diagnosis but not among a group in which cognitive impairment was identified from a validated assessment. Spending associated with an ADRD diagnosis primarily reflected increased hospital and skilled nursing facility care costs, which then subsided in the year‐and‐a‐half period following the diagnosis. An $8400 quarterly spending jump, or roughly 80% of annual per‐beneficiary Medicare spending, 23 was observed in the quarter following a diagnosis, but no differences were observed after the first detection of cognitive impairment, assessed using a routine cognitive and memory test. While no spending differences after diagnosis or first detection were observed by beneficiary sex, race, education, or dual eligibility, beneficiaries residing in rural locales had larger spending increases after detection. In contrast, spending changes were not seen with the first evidence of cognitive impairment, suggesting that, rather than immediate care needs around the time of disease onset, the spending associated with a clinical diagnosis of dementia may reflect costs associated with the diagnosis or a health care crisis, during which the dementia is recognized as a contributing factor.
Prior research has demonstrated higher incremental health care costs in the year following an Alzheimer's Disease diagnosis, as well as increased spending in the year before a diagnosis. 10 , 11 , 12 , 14 , 24 Similar to our study, Lin et al. (2016) found large spending increases emerged at the time of clinical diagnosis of ADRD, with changes primarily due to inpatient and post‐acute care services. 11 Our work confirms that changes in utilization reflect inpatient, skilled nursing facility, and physician service use changes, 9 , 10 , 11 , 12 while adding that the increases are restricted to new clinical diagnoses (and not observed after scheduled screening first detects impairment) and reflect non‐dementia diagnoses; the findings were common across all beneficiaries, regardless of sociodemographics or health care status. The spending patterns are also not unique to ADRD, but rather reflect broader spending phenomena for diagnoses common among older adults. In all, incremental spending changes involving ADRD are a phenomenon largely linked to diagnosis and not first detection and reflect costly hospital and post‐acute care largely for health issues for which dementia is part of but not the complete cause.
Researchers have posited several explanations for these temporary spending increases, including pre‐diagnosis morbidity and injury, diagnostic testing, and treatments for comorbidities. 1 , 9 , 11 , 12 Our findings suggest adverse events (e.g., suggested by heart failure, tracheostomy, stroke, septicemia) or functional limitations related to pre‐diagnosis impairment may lead to costly acute and post‐acute care, during which ADRD is tested for and diagnosed. Clinicians might make a diagnosis when cognitive impairment becomes severe enough that it noticeably interferes with and harms patients' self‐care, leading to acute exacerbations of treatable ambulatory conditions. Temporary health crises, as indicated by tracheostomy and bone marrow transplant procedures, may also result in opportunities for ADRD clinical diagnoses. It did not appear that beneficiaries who commonly have more health care needs (e.g., dual eligibles and lower‐socioeconomic status individuals) had differential spending patterns.
Prior work suggests that such transient spending increases are not uncommon. One study found that only 7% of beneficiaries with Alzheimer's disease had persistently high expenditures over a three‐year period, compared to 43% of beneficiaries with heart failure and 54% of individuals with chronic kidney disease; while fall injuries were associated with large spending increases, 25 just 2% of beneficiaries with hip fracture had persistently high spending. 26 We observed transient spikes in spending for three conditions – ADRD, hip fracture, and heart failure – but overall spending (both before and after diagnosis) was substantially higher for the heart failure compared to the other cohorts. Thus, a broader health system phenomenon is apparent, in which health events lead to transient spikes in treatment (150% to 600% spending increases in the quarter before compared to the quarter of diagnosis) that later resolve (spending only 70%–150% higher in the 6 quarters after compared to the 6 quarters before a diagnosis). That spending for ADRD does not appear to uniquely reflect factors related to impairment detection may be cause for concern if this spending reflects potentially preventable diagnoses that co‐occur with dementia but which can be addressed earlier on.
Finally, for some readers, the study might raise concerns about the accuracy of ADRD clinical diagnoses, as just over one‐third of those diagnosed with the clinical diagnosis of ADRD had evidence of survey‐based detection of cognitive impairment. Prior evidence also suggests limited congruence between clinical diagnoses identified in claims data and cognitive impairment as identified by validated and widely used survey screenings or clinician assessment, 27 , 28 so it is to be expected that clinicians would potentially diagnose dementia that is not identified by the TICS‐based classification. It may be that claims are picking up delirium or rule‐out diagnoses.
Limitations
This study was subject to several potential limitations. First, studies using claims to identify ADRD are subject to misclassification bias. False‐positive cases are among the costliest in terms of Medicare spending, which can lead to underestimates of ADRD spending when comparing those with and without ADRD. 14 We used a sensitive and specific algorithm and adjusted for health characteristics related to ADRD that are likely factors in high spending for false‐positive cases but cannot rule out some bias. We also move beyond earlier work using only claims‐based ADRD status, by examining spending changes around incident impairment detection (measured using the TICS).
Second, we did not include a comparison group of individuals without ADRD, to control for potential secular trends that could explain incremental spending differences. However, short‐term spending trends for Medicare beneficiaries are flat, 3 , 11 reducing the benefit of a comparison group. Third, by focusing on fee‐for‐service Medicare beneficiaries, we cannot generalize findings to the ~40% of beneficiaries currently enrolled in Medicare Advantage.
Fourth, our measurement of impairment detection during routine screening may misclassify individuals by using the date of the HRS interview (whereas impairment may have begun earlier); however, use of an alternative measure – the mid‐point in time between the current and prior wave – did not change results; moreover, individuals in this cohort may have had symptoms of impairment that are not from ADRD. Fifth, we did not measure Medicaid costs, which could reflect a substantial fraction of total spending. Sixth, we used individual rather than household income as a covariate. Finally, the results could be subject to unmeasured confounding if individuals developed new conditions over time. Because our main results involved the quarterly change in spending from just before to just after a diagnosis, large health or functional changes during that period would likely be limited.
These limitations notwithstanding, the findings have important implications. The 156% spike in spending (during the quarter of the diagnosis) plus the lag in diagnosis after the first detection of impairment may be suggestive of missed opportunities for earlier intervention. Individuals with cognitive impairment rely largely on an unpaid caregiver workforce consisting of family and friends, who may be unable to diagnose the presence of disease. 29 This suggests that care needs may be missed, leading to emergent issues (health crises or preventable treatment for ambulatory conditions). Earlier identification of patients with mild impairment and broader adoption of family‐centered interventions might benefit individuals whose care needs are met as they arise.
CONCLUSION
In this study of older Medicare beneficiaries, after an ADRD diagnosis, large spending increases driven by the hospital and skilled nursing utilization were followed by spending decreases. Spending did not change after the first screen indicated impairment or vary across sex, race, education, or dual eligibility status. The findings suggest that ADRD diagnosis may be delayed, reflecting opportunities for diagnosis arising during treatment for comorbidities. This raises questions about the timeliness of health system assessments of older adult cognition as well as broader concerns about the costs of inpatient care and the extent to which such care effectively addresses underlying patient needs.
AUTHOR CONTRIBUTIONS
Study concept and design: Hoffman, Maust, Davis. Acquisition of subjects and/or data: Hoffman, Ha. Analysis and interpretation of data: Hoffman, Ha, Maust, Davis, Harris. Preparation of manuscript: Hoffman, Maust, Davis, Harris.
CONFLICT OF INTEREST
Dr. Hoffman, Dr. Maust, and Dr. Davis received support for this manuscript from the Center to Accelerate Population Research in Alzheimer's (CAPRA, NIA P30 AG066582). Dr. Harris has no conflicts to report.
SPONSOR'S ROLE
The funders had no role in the study design, data collection, management, and analysis, nor any participation in the preparation, review, and approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Supporting information
Data S1 Supporting information.
ACKNOWLEDGMENTS
I have listed everyone who contributed significantly to the work.
Hoffman GJ, Maust DT, Harris M, Ha J, Davis MA. Medicare spending associated with a dementia diagnosis among older adults. J Am Geriatr Soc. 2022;70(9):2592‐2601. doi: 10.1111/jgs.17835
Funding information Center to Accelerate Population Research in Alzheimer's, Grant/Award Number: P30 AG066582
REFERENCES
- 1. Zhu CW, Cosentino S, Ornstein K, et al. Medicare utilization and expenditures around incident dementia in a multiethnic cohort. J Gerontol A Biol Sci Med Sci. 2015;70(11):1448‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisele M, van den Bussche H, Koller D, et al. Utilization patterns of ambulatory medical care before and after the diagnosis of dementia in Germany–results of a case‐control study. Dement Geriatr Cogn Disord. 2010;29(6):475‐483. [DOI] [PubMed] [Google Scholar]
- 4. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurd MD, Martorell P, Langa K. Future monetary costs of dementia in the United States under alternative dementia prevalence scenarios. J Popul Ageing 2015;8(1–2):101–112, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox C, Smith T, Maidment I, et al. The importance of detecting and managing comorbidities in people with dementia? Age Ageing. 2014;43(6):741‐743. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman GJ, Nuliyalu U, Bynum J, Ryan AM. Alzheimer's disease and related dementias and episode spending under Medicare's bundled payment for care improvements advanced (BPCI‐A). J Gen Intern Med. 2020;36:2499‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187‐194. 10.1111/j.1532-5415.2004.52054.x [DOI] [PubMed] [Google Scholar]
- 9. Association As . 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13(4):325‐373. [Google Scholar]
- 10. Geldmacher DS, Kirson NY, Birnbaum HG, et al. Pre‐diagnosis excess acute care costs in Alzheimer's patients among a US Medicaid population. Appl Health Econ Health Policy. 2013;11(4):407‐413. [DOI] [PubMed] [Google Scholar]
- 11. Lin P‐J, Zhong Y, Fillit HM, Chen E, Neumann PJ. Medicare expenditures of individuals with Alzheimer's disease and related dementias or mild cognitive impairment before and after diagnosis. J Am Geriatr Soc. 2016;64(8):1549‐1557. 10.1111/jgs.14227 [DOI] [PubMed] [Google Scholar]
- 12. Suehs BT, Davis CD, Alvir J, et al. The clinical and economic burden of newly diagnosed Alzheimer's disease in a medicare advantage population. Am J Alzheim Dis Other Demen. 2013;28(4):384‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. HRS Staff . Sample sizes and response rates. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2017. [Google Scholar]
- 14. Taylor DH, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheim Dis. 2009;17(4):807‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maust DT, Strominger J, Kim HM, et al. Prevalence of central nervous system–active polypharmacy among older adults with dementia in the US. JAMA. 2021;325(10):952‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162‐i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: insights from linked survey and administrative claims data. Alzheim Dement. 2019;5:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu Y, Chen Y, Crimmins EM, Zissimopoulos JM. Sex, race, and age differences in prevalence of dementia in Medicare claims and survey data. J Gerontol B Psychol Sci Soc Sci. 2020;76:596‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Medical Expenditure Panel Survey . Using appropriate price indices for analyses of health care expenditures or income across multiple years. Agency for Heatlhcare Research and Qualtiy. https://meps.ahrq.gov/about_meps/Price_Index.shtml. Published 2021. Accessed September 24, 2021, 2021.
- 20. Afifi AA, Kotlerman JB, Ettner SL, Cowan M. Methods for improving regression analysis for skewed continuous or counted responses. Annu Rev Public Health. 2007;28(1):95‐111. [DOI] [PubMed] [Google Scholar]
- 21. Johnston KJ, Joynt Maddox KE. The role of social, cognitive, and functional risk factors in Medicare spending for dual and nondual enrollees. Health Aff. 2019;38(4):569‐576. [DOI] [PubMed] [Google Scholar]
- 22. Ahmadi‐Abhari S, Guzman‐Castillo M, Bandosz P, et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. BMJ. 2017;358:j2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. KFF . Medicare Spending Per Enrollee. Kaiser Family Foundation. spending. Published 2019. Accessed June 30, 2021.
- 24. Albert SM, Glied S, Andrews H, Stern Y, Mayeux R. Primary care expenditures before the onset of Alzheimer's disease. Neurology. 2002;59(4):573‐578. [DOI] [PubMed] [Google Scholar]
- 25. Hoffman GJ, Hays RD, Shapiro MF, Wallace SP, Ettner SL. The costs of fall‐related injuries among older adults: annual per‐faller, service component, and patient out‐of‐pocket costs. Health Serv Res. 2017;52(5):1794‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Figueroa JF, Zhou X, Jha AK. Characteristics and spending patterns of persistently high‐cost Medicare patients. Health Aff. 2019;38(1):107‐114. [DOI] [PubMed] [Google Scholar]
- 27. Gianattasio KZ, Wu Q, Glymour MM, Power MC. Comparison of Methods for algorithmic classification of dementia status in the health and retirement study. Epidemiology. 2019;30(2):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCarthy EP, Chang CH, Tilton N, Kabeto MU, Langa KM, Bynum JPW. Validation of claims algorithms to identify Alzheimer's disease and related dementias. J Gerontol A Biol Sci Med Sci. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Alliance for caregiving. Caregiving in the U.S.: 2015 Report. National Alliance for Caregiving; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information.


