Abstract
Root resorption is the loss of dental hard tissue because of odontoclastic action. In permanent teeth, it is undesirable and pathological in nature. Root resorption may occur on the inner aspect of the root canal (internal root resorption) or on the outer aspect of the root (external root resorption). Regardless of its location, root resorption is irreversible, and may result in discomfort for the patient, requires management and/or, in some cases, results in the premature loss of the affected tooth. Root resorption is often challenging to accurately diagnose and manage. The aim of this narrative review is to present the relevant literature on the aetiology, pathogenesis, diagnosis and management, as well as discuss the future directions of diagnosis and management of root resorption.
Keywords: external root resorption, internal root resorption
INTRODUCTION
Root resorption is the loss of dental hard tissue due to odontoclastic activity (Patel et al., 2018). In primary teeth, root resorption is usually physiological and desirable as it allows the underlying permanent successor to erupt. In permanent teeth, root resorption may occur within the root canal [internal root resorption (IRR)] or on the outer aspect of the root (external root resorption). In advanced cases, the resorptive defect may progress into the crown of the tooth. Regardless of its origins, it is irreversible and typically pathological in nature and may result in discomfort for the patient require treatment and/or, in some cases, the premature loss of the affected tooth (Figure 1).
FIGURE 1.
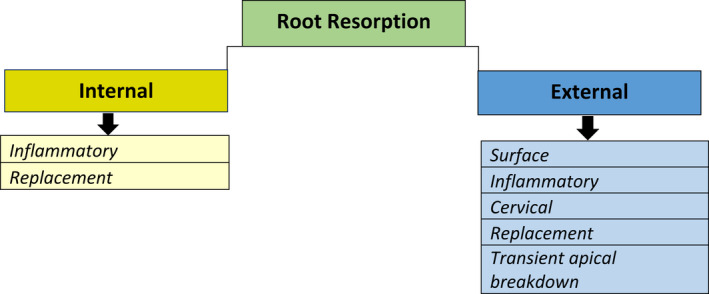
The classification of root resorptions based on their location in the root and the subsequent subclassification based on the pathogenesis
The noncollagenous organic outer aspect of the root canal wall (odontoblast layer and predentine) and root surface (precementum and periodontal ligament) protect the underlying dentine from internal and external root resorption, respectively (Wedenberg, 1987; Wedenberg & Lindskog, 1987). Once the noncollagenous layer is damaged or irritated, odontoclasts are recruited to the site of injury or irritation by the release of proinflammatory cytokines. Odontoclasts may bind to extracellular proteins containing the arginine–glycine–aspartic acid (RGD) sequence of amino acids present on the surface of mineralized tissues by means of integrins (Schaffner & Dard, 2003). From the perspective of molecular signalling, the osteoprotegerin (OPG)/receptor activator of factor kappa B (RANK)/RANK ligand (RANKL) transcription factor system, which are involved in bone remodelling, has also been identified in root resorption (Tyrovola et al., 2008). Studies have reported that periodontal ligament (PDL), cementum and dental pulp are able to express RANKL, which is essential for the differentiation of odontoclasts during root resorption (Diercke et al., 2012; Kikuta et al., 2015; Uchiyama et al., 2009; Yamaguchi et al., 2006).
Odontoclasts are smaller, have fewer nuclei and sealing zones than osteoclasts, and create resorption depressions, Howship lacunae, on the surface of the mineralized tissues (Pierce, 1989). Root resorption may occur in three phases: initiation, resorption and repair (Mavridou et al., 2016). It may be self‐limiting and may go undetected clinically; however, if the resorptive process is sustained, for example, by infection or pressure, then there will be further loss of dental hard tissue. If root resorption is not managed appropriately, it may result in the tooth becoming unsalvageable eventually leading to extraction. Root resorption can be challenging to accurately diagnose and manage. A classification based on the resorptive lesion's location on the root and its radiographic nature is suggested (Table 1).
TABLE 1.
Salient clinical and radiographic features of internal and external root resorptions, adapted from Patel and Saberi (2018)
| Type of resorption | Internal root resorption | External root resorption | |||||
|---|---|---|---|---|---|---|---|
| Internal inflammatory resorption | Internal replacement resorption | External surface resorption | External cervical resorption | External inflammatory resorption | External replacement resorption | Transient apical resorption | |
| Clinical features | Asymptomatic (early), symptoms of pulpitis and/or apical periodontitis (advanced) | Asymptomatic (early), symptoms of pulpitis and/or apical periodontitis (advanced) | None | Asymptomatic (early), symptoms of pulpitis and/or apical periodontitis (advanced), ± probeable periodontal defect with profuse bleeding | Symptoms of apical periodontitis | Variable, none or ankylosis and/or exhibit high‐pitched metallic sound on percussion in advanced cases. | None |
| Clinical appearance | Healthy (vital), discoloured (necrotic), pink spot (rare) | Healthy (vital), discoloured (necrotic), pink spot (rare) | Healthy | Healthy (vital), discoloured (necrotic), pink spot (rare) | Healthy (vital), discoloured (necrotic) | Healthy | Discoloured, usually resolve within 1 year |
| Location on root | Anywhere | Anywhere | Adjacent to impacted tooth/cyst/tumour, apical in orthodontically treated teeth | Cervical third (early) but can extend to middle or apical third (advanced) | Anywhere | Anywhere | Apical third |
| Pulp sensibility testing | +ve in (partially) vital cases, −ve in necrotic cases | +ve in (partially) vital cases, −ve in necrotic cases | +ve | Usually +ve, −ve in advanced necrotic cases | −ve | Usually +ve, may exhibit −ve/delayed response due to tertiary dentin formation | −ve or delayed response, usually returns to normal within 1 year |
| Radiographic features | Oval/round ballooning of root canal | Oval/round ballooning of root canal but with cloudy/mottled inclusions | Flattened/blunted root apex, asymmetrical loss of root, intact root canal | (A)symmetrical radiolucency in early cases, mottled radiopaque appearance in advanced cases. Perforation of root canal in advanced cases | Ragged saucer‐shaped indentations along the root surface, adjacent bone loss, periapical radiolucency, Perforation of root canal in advanced cases | Asymmetrical bony replacement of root surface, absence of PDL space, root appears 'fused' to adjacent bone. Intact root canal |
Widened PDL space, blurred/loss of apical lamina dura. Intact root canal |
There are insufficient data within meta‐analyses or systematic reviews on root resorption. Therefore, the aim of this review is to update the reader, based on the most relevant literature on the present status of the pathogenesis, aetiology, diagnosis and management of root resorption; where appropriate, the future directions of research will be suggested.
INTERNAL ROOT RESORPTION
Internal inflammatory and internal replacement root resorption
Introduction
Internal root resorption is initiated along the root canal wall and may result in the progressive destruction of the adjacent radicular dentine (Tronstad, 1988). IRR may consist of granulation tissue only (inflammatory internal root resorption) or a combination of granulation and bone‐like tissue (replacement internal root resorption; Patel et al., 2010). As these two subtypes of IRR share general similarities, they will be discussed together.
Aetiology and prevalence of internal root resorption
The cause of IRR is not fully understood. It is widely accepted that IRR initiation is dependent on damage to the odontoblasts and (unmineralized) predentine layer exposing the underlying mineralized tissue to odontoclasts (Wedenberg & Zetterqvist, 1987). Several factors have been implicated in the injury and destruction of the predentine such as trauma (Andreasen, 1970), periodontal infections (Rabinowitch, 1972), caries‐related pulpitis (Rabinowitch, 1972), excessive heat during restorative procedures in teeth with vital pulps (Rabinowitch, 1972), root resections in teeth with vital pulps (Allen & Gutmann, 1977), anachoresis (Penido et al., 1980), orthodontic treatment (Silveira et al., 2009), pulp amputation and calcium hydroxide capping (Cabrini & Manfredi, 1957; Sönmez & Durutürk, 2008), cracked teeth (Walton & Leonard, 1986) and idiopathic dystrophic changes in healthy pulps (Ashrafi & Sadeghi, 1980).
The prevalence of IRR has rarely been reported in the literature. Thoma (1935) observed only one case of internal resorption out of 1000 teeth studied. Another study noted eight out of 28 teeth (28%) having IRR after coronal pulp amputation and capping with calcium hydroxide (Cabrini & Manfredi, 1957). Ahlberg et al. (1983) identified 51.5% (17 out of 33 teeth) of IRR in autotransplanted maxillary canines. The diagnosis of IRR in these studies was based solely on 2‐dimensional radiographic findings, which could have underestimated the true prevalence of IRR (Patel et al., 2010).
Histopathology
It is well established that clastic cells are not capable of adhering to unmineralized collagen matrices (Wedenberg, 1987; Wedenberg & Lindskog, 1987). Initiation of IRR is therefore dependent on the removal and/or damage of the odontoblastic layer and unmineralized predentine (Wedenberg & Zetterqvist, 1987) and subsequent exposure of the underlying mineralised dentine (Trope, 1998; Wedenberg & Lindskog, 1985). Localized inflammation leads to an increase in clastic activity. Dental pulp tissue is able to express RANKL, which is part of the OPG/RANK/RANKL system that could trigger the differentiation of the clastic cells during IRR (Uchiyama et al., 2009).
Two phases of IRR (transient and progressive) have been reported (Wedenberg & Lindskog, 1985). The transient phase is self‐limiting as there is no inflammatory irritation and/or infection on the damaged root canal surface to stimulate and sustain the resorptive process. In the progressive phase, the IRR continues due to bacterial stimulation of the clastic cells and a viable blood supply within the root canal. The source of noxious stimuli is the necrotic pulp coronal to the resorptive lacunae with the clastic cells being sustained by nutrients from the vital pulp tissue apical to the resorption site (Tronstad, 1988).
Bacteria may gain access into the root canal through dentinal tubules, caries, cracks, fractures and lateral canals. IRR may advance and potentially result in perforation of the root leading to a lateral or periodontal lesion (Calişkan & Türkün, 1997) or apical periodontitis once the entire root canal system is infected by microorganisms. Complete removal of the pulp or pulp necrosis arrests the resorptive process.
The features of IRR in extracted primary and permanent teeth have been assessed using histology, scanning electron microscopy and enzyme histochemistry by Wedenberg and Zetterqvist (1987). The histochemical profile was similar in both groups; however, the resorptive process progressed more rapidly in primary teeth. The affected pulpal tissue was less vascular and infiltrated by inflammatory cells, and the resorption front was denuded of predentine and odontoblasts. The presence of odontoclasts in the resorption lacunae was evidence of progressive resorption. The root canal surface was partially lined with bone and cementum‐like mineralised tissue, suggesting internal replacement resorption (Wedenberg & Lindskog, 1987).
Internal inflammatory resorption only involves the loss of intraradicular dentine without subsequent deposition of hard tissue onto the resorptive defects. With internal replacement resorption, the resorption of intraradicular dentine is usually followed by deposition of metaplastic bone‐like or cementum‐like hard tissue instead of dentine in the resorption cavities (Figure 2), and this has been described as ‘frustrated repair’ (Patel et al., 2010). It has been hypothesized that postnatal dental pulp stem cells from the vital (apical) pulp tissue are responsible for the metaplastic hard tissue of internal replacement root resorption (Gronthos et al., 2002; Huang et al., 2009). A second hypothesis suggests that clastic cells originate from the periodontium (Yang et al., 2009).
FIGURE 2.
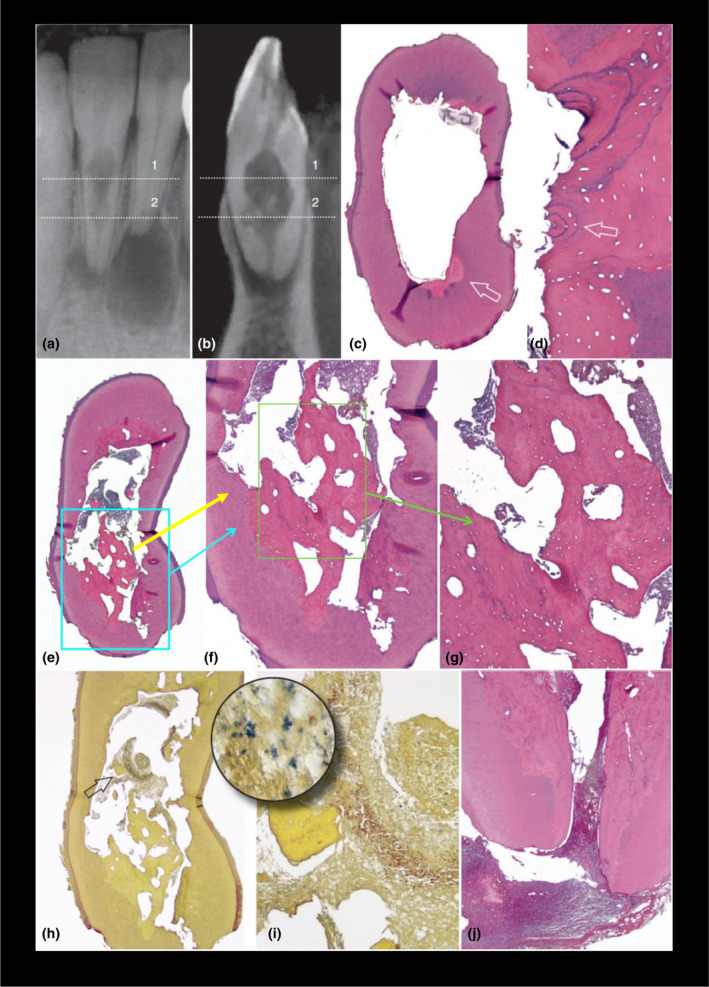
Internal replacement resorption. Light microscopy images of a variant of internal (root canal) replacement resorption with tunnelling resorption. The lower right lateral incisor belonged to a 39‐year‐old former boxer who had suffered a jaw fracture in a boxing match in his early twenties and was placed in intermaxillary fixation. The patient developed symptoms 20 years later and complained of pain associated with his lower incisors. (a) Radiograph of the mandibular right incisors. The lower right central incisor had asymptomatic apical periodontitis associated with a necrotic and infected pulp. The lower right lateral incisor showed a large area of internal root resorption. The tooth did not respond to sensitivity tests. (b) Sagittal CBCT slice shows some calcified tissue in the resorptive defect. C, Cross section taken at the level of line 1 in (a) and (b). The overview shows that the canal was apparently empty at this level. (H&E stain; ×6). (d), High magnification of the area indicated by the arrow. (c) Lamellar bone filling an area of previous resorption. Note the osteon structure (arrow). (H&E stain; ×100). (e) Cross section taken at the level of line 2 in (a) and (b). Overview shows that the canal lumen was partly occupied by necrotic remnants, partly by bonelike tissue (H&E stain; ×8). (f), High magnification of the lower part in (e; H&E stain; ×50). (g), Higher magnification of (f). Bone trabeculae are surrounded by necrotic debris. (H&E stain; ×100). (h), Cross section taken from the same area. (Taylor's modified Brown & Brenn [TBB] stain; ×16). (i), High magnification of the area indicated by the arrow in (h). A fragment of bonelike tissue can be seen surrounded by bacteria‐colonized necrotic tissues. (TBB stain; ×100; inset ×1000). (j), Longitudinal section passing approximately through the center of the root apex. Dentin walls have been resorbed and replaced by a bonelike tissue. (H&E stain; ×16). (From Patel S, Ricucci D, Durack C, Tay F: Internal root resorption: a review, Journal of Endodontics 36:1107, 2010)
Clinical features
Internal inflammatory and internal replacement types of IRR share similar clinical features. Teeth with IRR are often asymptomatic and detected as an incidental radiographic finding (Haapasalo & Endal, 2006; Patel et al., 2010). The symptoms and signs of acute or chronic pulpitis, if present, depend on the pulp status, the degree of hard tissue destruction and the position of the resorption in the tooth. The affected tooth with partially vital pulp tissue may be contaminated by bacteria and elicit symptoms and/or signs of acute pulpitis. In established IRR cases, the pulpal tissue may become necrotic and chronically infected resulting in symptoms and/or signs of acute or chronic apical periodontitis (Patel et al., 2010). Therefore, patients may present with an abscess or sinus, tenderness to percussion and/or discolouration. IRR in the coronal third of the root canal may present with a pink discolouration in the crown of the tooth due to resorption and replacement of the hard tissue by fibrovascular granulomatous tissue (Lyroudia et al., 2002). This pink discolouration of the crown may also be misdiagnosed as external cervical resorption (ECR) Patel et al., 2009).
Radiographic features
IRR may occur anywhere within the root canal system. Internal inflammatory root resorption (Figure 3a) typically presents as a symmetrical oval‐ or circular‐shaped extension of the root canal (ballooning) radiolucency (Gartner et al., 1976). Internal replacement root resorption (Figure 3b) usually presents as an irregularly shaped radiolucency with a mottled or clouded appearance due to bone‐like tissue deposits around the borders and within the resorptive defect (Patel et al., 2010).
FIGURE 3.
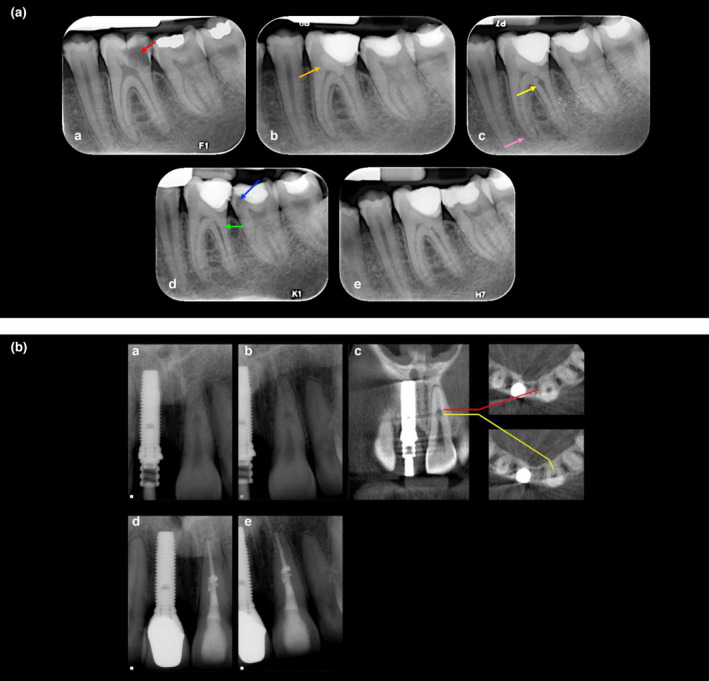
(a) Internal inflammatory resorption following pulpotomy. A 18‐year‐old male patient with an extensive carious lesion on tooth 36 with symptoms of irreversible pulpitis (a) preoperative periapical radiograph showing the deep carious lesion (red arrow), (b) a Biodentine pulpotomy (orange arrow) was carried out, (c) a 3‐month review confirmed that the tooth was asymptomatic; however, the radiographic examination revealed internal inflammatory root resorption (yellow arrow) on the distal canal, diffuse calcification on the mesial canals and apical widening of the periodontal ligament on the mesial root (pink arrow). After discussing the treatment options, as they were asymptomatic, the patient decided to have their tooth reviewed on a periodic basis. (d) A 15‐month review revealed that the tooth was asymptomatic, and there were no signs of endodontic or periodontal disease associated with the tooth, radiographic assessment confirmed that the IIR has spontaneously resolved and may have become IRR (green arrow), but did reveal secondary caries in tooth 37 (blue arrow), which was restored, (j) 24‐month review. (b) Internal replacement resorption. (a, b) Periapical radiographs of a maxillary left central incisor with radiographic signs of IRR; note the symmetrical nature of the defect, which remains centred with the parallax view, and the radio‐opaque nature of its coronal aspect. This patient sustained a dental traumatic injury 9 years previously, (c) CBCT slices through the same tooth reveals a calcified tissue in the coronal part of the lesion. (d, e) Obturated tooth and a 2‐year review radiograph demonstrating the irregular borders of the defect that have been obturated with thermoplastiicized gutta percha (Reprinted from Journal of Endodontics, Vol 36, Patel S, Ricucci D, Durak C, Tay F. Internal root resorption: a review. Journal of Endodontics pages 1107–1121, Copyright (2010) with permission from Elsevier)
It is well established that ECR and IRR share similar radiographic features on (2‐dimensional) radiographs and can be indistinguishable from each other resulting in misdiagnosis (Gulabivala & Searson, 1995; Tronstad, 1988). The parallax radiographic technique may be used to help confirm the nature of the resorption (Gartner et al., 1976); the IRR defect will not change position in relation to the root canal; however, ECR will move in the same or opposite direction of the second parallax radiograph, confirming it is on the external aspect of the lingual/palatal or buccal root respectively. In multirooted teeth, the IRR lesion in one root canal may be superimposed onto another unaffected root canal, thus causing confusion or even misdiagnosed as an ECR lesion (Gartner et al., 1976; Patel et al., 2010).
A high‐resolution, small field of view (FOV), cone beam‐computed tomography (CBCT) is recommended to investigate the exact nature of the IRR lesions, which appear to be potentially treatable (American Association of Endodontists/American Academy of Oral & Maxillofacial Radiology, 2015; European Society of Endodontology, 2019). CBCT will accurately distinguish IRR from ECR, as well as confirm the nature (i.e. extent and presence of any perforation of the root canal wall, Bhuva et al., 2011; Kamburoğlu et al., 2011; Lima et al., 2016). Several studies have confirmed that CBCT improves the diagnosis and treatment planning of root resorption when compared to radiographs (Chogle et al., 2020; Ee et al., 2014; Madani et al., 2016; Patel, Dawood, et al., 2009; Rodríguez et al., 2017).
Management
The management of inflammatory and replacement IRR are similar, it is essential to assess the extent of hard tissue destruction by taking a CBCT scan (European Society of Endodontology, 2019). If a perforation is detected, then irrigation must be limited to the coronal extent of the IRR to prevent an inadvertent hypochlorite accident (Bhuva et al., 2011). In advanced perforated cases, a combined nonsurgical/surgical may be warranted.
Endodontic treatment should be considered only if the tooth appears to be restorable. The main objective of the treatment is to remove the bacteria and disinfect the root canal system, at the same time eliminating any remaining apical vital tissue, which is sustaining the resorption. Profuse bleeding may occur due to the granulomatous nature being disturbed and will stop once the inflamed pulpal tissue and granulation tissue have been completely removed.
The canal should still be negotiable in cases of moderate Internal replacement root resorption; however, in more advanced cases, fine ultrasonic tips may be necessary to fragment the metaplastic bone‐like deposits to allow patency of the root canal to be established.
Sodium hypochlorite is the irrigant of choice, due to the irregular nature of the resorptive defect, the irrigant should be energized after which an intracanal calcium hydroxide medicament may be used to enhance the disinfection and dissolve pulp tissue remnants in the inaccessible parts of the root canal space, i.e. the resorptive defect (Burleson et al., 2007; Türkün & Cengiz, 1997).
Thermoplasticized canal filling techniques are indicated to improve the sealing of the irregular IRR defect. Warm vertical compaction has been shown to be a more effective root canal filling technique compared to lateral condensation and carrier‐based techniques such as Thermafil (Tulsa Dental Products, Tulsa, OK, USA; Gencoglu et al., 2008; Goldberg et al., 2000).
If the resorption defect has perforated the root canal wall, bioactive hydraulic silicate cements [i.e. mineral trioxide aggregate (MTA), DentsplySirona], Biodentine (Septodont) should be used to repair the perforation (Bhuva et al., 2011; Main et al., 2004; Regan et al., 2002). A hybrid root filling technique is indicated when the canal apical to the perforating resorptive defect is filled with gutta–percha (GP), and a bioactive silicate cement is used to seal the resorptive defect (Hsien et al., 2003; Jacobovitz & de Lima, 2008).
In cases where the perforating IRR defects are not amenable for internal repair, a surgical approach is indicated. The root canal system should first be accessed prior to the surgical repair and occluded with well‐fitting GP point(s) to maintain the patency of root canal(s) from unintentional blockage in the subsequent surgical repair. Surgical exposure and debridement of the perforation followed by repair using bioactive hydraulic silicate cements are recommended (Main et al., 2004). The root canal system can then be disinfected, instrumented and filled with thermoplasticized GP or with a hybrid of GP and bioactive materials.
Extraction is indicated in cases where IRR is too extensive to be managed effectively. These compromised teeth are likely to fracture when they are extracted as they have been weakened by the IRR, and therefore, the patient should be consented for a surgical extraction.
Recently, regenerative endodontic procedures (REP) have been used to treat perforating IRR (Arnold, 2021; Kaval et al., 2018; Saoud et al., 2016). Saoud et al. (2016) instrumented the entire root canal length and placed calcium hydroxide and triple antibiotic paste (metronidazole, ciprofloxacin and minocycline) in the root canal for 2 weeks each before sealing the root canal system with MTA. Kaval et al. (2018) debrided the entire root canal, followed by medicating the root canal with calcium hydroxide for 3 months before placing MTA over the blood clot. This treatment approach shows promising results with hard tissue formation in the perforated area and increased thickness of the root canal wall review after 2–3 years (Arnold, 2021; Kaval et al., 2018). REP appears to be able to arrest the resorption process and induce hard tissue formation, which leads to the reduction in the size of the resorptive defect and subsequently improve the restorability and longevity of IRR‐affected teeth, especially where there is a large perforating resorptive defect. However, more clinical studies are required to investigate the exact healing mechanism and to allow for a more standardized treatment protocol to improve the reproducibility of the result. Besides, tooth discolouration after REP should also be considered especially in anterior teeth, which are aesthetically demanding (Kahler & Rossi‐Fedele, 2016).
More research is needed to appreciate and confirm that these conditions (inflammatory and replacement IRR) are similar. There is minimal evidence on the long‐term outcome of IRR and its management due to heterogeneity in treatments and assessment methods. This may be due to the small number of IRR cases reported in the literature (Calişkan & Türkün, 1997; Haapasalo & Endal, 2006). Long‐term well‐designed clinical studies are required to assess the medium to long‐term outcomes of IRR.
EXTERNAL ROOT RESORPTION
External surface resorption
Introduction
External surface resorption (ESR) is pressure‐induced resorption and occurs on the external surface of the root, it is noninfective and self‐limiting, i.e. once the source of the pressure has been eliminated, cemental repair ensues and it has also been described as pressure resorption (Andreasen, 1981; Tronstad, 1988).
Aetiology and prevalence
Pressure exerted by impacted teeth, orthodontic treatment, cysts or tumours are common causes of ESR. To date, a few studies have assessed the prevalence of surface resorption. It has been reported that 1%–5% of orthodontically treated teeth have severe surface resorption (>4 mm or 1/3 of root loss; Killiany, 1999; Weltman et al., 2010). Teeth undergoing orthodontic treatment with a previous history of dental trauma are more prone to surface resorption (Levander & Malmgren, 1988; Malmgren et al., 1982).
Studies have suggested several potential predisposing factors associated with a greater risk of ESR as a consequence of orthodontic treatment. These factors may be classified into dental‐related, patient‐related and treatment‐related factors. Blunt and pipette‐shaped root apices (Levander & Malmgren, 1988), history of previous trauma (Linge & Linge, 1983; Malmgren et al., 1982) and Class III malocclusion (Kaley & Phillips, 1991) were among the potential dental‐related factors associated with higher rate of ESR in patients undergoing orthodontic treatment.
Patient‐related factors such as age and gender were found to be equivocal when it comes to higher risk of ESR (Baumrind et al., 1996; Kjaer, 1995; Linge & Linge, 1983; Sameshima & Sinclair, 2001). Sameshima and Sinclair (2001) reported a lower incidence rate of ESR in Asian compared to white or Hispanic patients. Systemic conditions such as asthma have been reported to be associated with a greater incidence of ESR (McNab et al., 1999) whilst increased thyroxine hormone level has been reported to be associated with lower ESR incidence (Shirazi et al., 1999). Harris et al. (1997) reported higher susceptibility to ESR among siblings. Nail‐biting habit was also found to be associated with a greater risk of ESR during orthodontic treatment (Odenrick & Brattström, 1985).
In terms of treatment‐related factors, application of intrusive forces was reported to significantly increase the risk of ESR (Han et al., 2005; Harris et al., 2006). There is limited evidence suggesting continuous force application carries a greater risk of ESR than interrupted force application (Acar et al., 1999; Weltman et al., 2010). Whilst clear aligners might not avoid ESR, they have been reported to be associated with a lower risk of ESR in terms of incidence and severity compared with fixed appliances (Fang et al., 2019). ESR is more likely to occur with fixed appliance orthodontic treatment compared with removable appliances (Linge & Linge, 1983; Yassir et al., 2021). Increased orthodontic forces, as well as increased treatment times, may result in an increased incidence of ESR (Roscoe et al., 2015; Weltman et al., 2010). It has been reported that orthodontic forces of 50–100 cN are optimal for orthodontic tooth movement and low risk of surface resorption (Theodorou et al., 2019).
Teo et al. (2021) determined that the incidence of surface resorption associated with cysts was 38% in an Asian population (Teo et al., 2021). The incidence of surface resorption caused by ameloblastomas and dentigerous cysts appears to be higher than with odontokeratocysts (OKC; Struthers & Shear, 1976; Teo et al., 2021). Ameloblastomas and dentigerous cysts are eight times and three times more likely to be associated with ESR in adjacent teeth compared with OKC, respectively. This may be due to increased expression of certain cytokines, e.g. RANK, RANKL and OPG that were involved in mediating odontoclastic activity (Da Silva et al., 2008; Teo et al., 2021).
Third molar and canine teeth are the most commonly impacted teeth and may result in ESR of the neighbouring teeth (Chu et al., 2003; Cooke & Wang, 2006). The incidence of ESR in the second molar due to impacted third molars when assessed with conventional radiography has been reported to be in the range of 0.3% to 24.2% (Nemcovsky et al., 1996; Yamaoka et al., 1999); however, with CBCT, the incidence of surface resorption in these types of cases has been reported to be as high as 54.9% (Matzen et al., 2017). Severe cases of ESR may result in significant damage and even loss of the resorbed tooth (Walker et al., 2005). One study reported the prevalence of ESR in incisor teeth due to impacted canines was 12.5% using conventional imaging technique (Ericson & Kurol, 1987), whilst more recent studies that used CBCT reported an incidence of 46%‐67.5% (Alemam et al., 2020; Rafflenbeul et al., 2019).
External surface resorption may also affect teeth, which have sustained a minor injury associated with dental trauma; however, in these cases, it is usually self‐limiting and will repair with normal cementum on its own accord in teeth, which maintain their vitality (Andreasen & Hjørting‐Hansen, 1966a). This type of ESR is very common, but not detected clinically and is based on histological findings (Andreasen & Hjørting‐Hansen, 1966a; Tronstad, 1988).
Histopathology
The pathogenesis of ESR is attributed to damage to the precementum due to the pressure from impacted teeth, cysts, tumours or orthodontic treatment. It has been reported that this pressure can compress and/or damage the blood vessels in the PDL, leading to hypoxia, anoxia and eventually the death of cementoblasts, odontoclasts then start to resorb the root surface (Martins et al., 2019). The resorptive process will stop with the removal of the source of pressure.
Clinical features
Surface resorption is usually asymptomatic and diagnosed as an incidental radiographic finding. The clinical findings are usually unremarkable, with no signs of endodontic disease. The affected teeth respond normally to pulp sensitivity tests.
Radiographic features
Asymmetric loss of external root surface adjacent to the source of pressure from an impacted tooth, cyst or tumour is a common radiographic presentation (Figure 4a–b). In advanced cases, this may result in perforation of the root canal. Surface resorption associated with orthodontic treatment may cause flattening or blunting of the root apices resulting in the affected teeth appearing to be shorter than neighbouring teeth, which have not been subjected to high orthodontic forces (Figure 4c–d). As described previously, the use of CBCT has resulted in increased detection and more accurate determination of the extent and nature of surface resorption (Marmulla et al., 2005; Moze et al., 2013; Sondeijker et al., 2020). It is important to appreciate that there is no ‘classical’ presentation of ESR. Active and stable (repaired) ESR may be differentiated by the disappearance and re‐establishment of the periodontal ligament respectively (Figure 4a–d).
FIGURE 4.
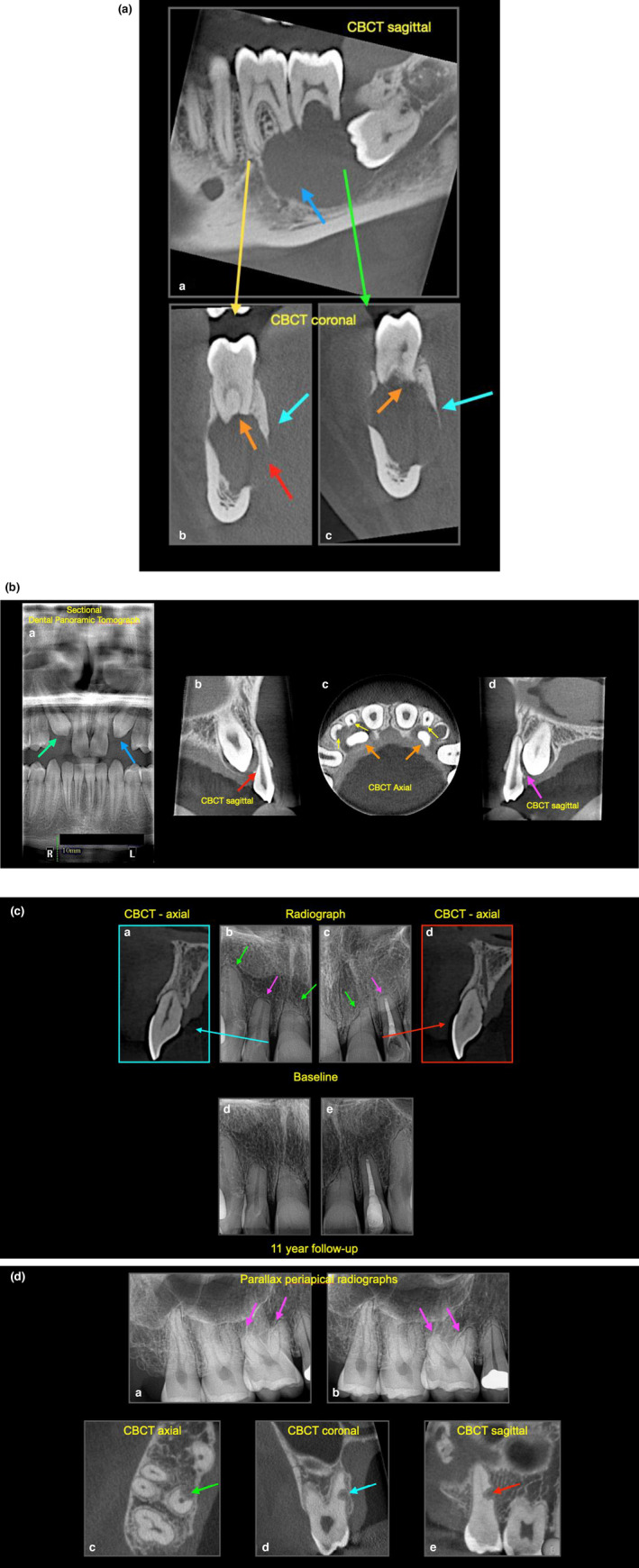
(a) External surface resorption. (a) Sagittal CBCT revealing radiographic large radiolucent lesion (blue arrow) associated with the lower left molar teeth, (b, c) coronal CBCT views revealing significant resorption of the apices of teeth 36 and 37 (orange arrows), expansion (cyan arrows) and perforation (red arrow) of the cortical plate. Note that the periodontal ligament space has disappeared. The lesion was surgically managed and subsequently confirmed to be an ameloblastoma after histological evaluation. (b) External surface resorption. (a) Sectional panoramic of a patient with unerupted, impacted canines (green and blue arrows), (b) sagittal CBCT reveals the 13 is impacted on the 12 resulting in ESR, and disappearance of the periodontal ligament in this region, (c) axial CBCT view reveals the extent of ESR (yellow arrows) associated with the impacted canines (orange arrow), (d) sagittal CBCT reveals the 23 is also impacted on 22. (c) External surface resorption (a– d) baseline radiographic images of a 45‐ year‐ old patient with history of 4‐ year fixed bracket orthodontic treatment. Radiographs show clear evidence of ESR, note the different presentations; teeth 12, 13, 22 have blunted apices, whereas teeth 11, 21 have more angular ESR, this may be due to the direction of orthodontic forces applied, (d–e) 11‐year follow up shows that all the teeth are heathy, note that the periodontal ligament space is visible. (d) External surface resorption and external replacement resorption. (a) Parallax periapical radiographs of upper right molars region taken to assess the degree of external surface resorption (pink arrow) on tooth 16 after a rapid course of orthodontics, fixed braces were placed from 16 to 26, the 27 and 28 were not used for orthodontic anchorage. (c–e) CBCT slices reveal an unusual presentation of external replacement resorption of the 17, the orthogonal views reveal the well‐defined resorptive defect, which is very close proximity to the root canal—no active treatment was advised, instead the patient was advised to attend for annual reviews (watchful waiting), including sensitivity testing and radiographic assessment. Note that the periodontal ligament space is visible
Management
ESR is managed by the appropriate elimination of the aetiological factor, i.e. excessive pressure, for example, removal of an impacted tooth or management of a cyst.
A temporary pause of 3 months in active orthodontic treatment has been suggested to allow the resorbed cementum to heal in the case of surface resorption associated with orthodontic treatment (Mehta et al., 2017; Roscoe et al., 2015). Termination of orthodontic treatment may be indicated when there is significant ESR. It has been reported that the ESR diagnosed after 6 to 12 months of orthodontic treatment with fixed appliances stabilizes resulting in minimal further ESR postorthodontic treatment (Artun et al., 2009; Levander & Malmgren, 1988). Therefore, it is good clinical practice to take a panoramic radiograph 6–12 months after placement of the fixed appliance (Sondeijker et al., 2020).
External cervical resorption
Introduction
ECR usually occurs in the cervical region of the tooth immediately below the epithelial attachment (Mavridou et al., 2016). It has the potential to invade the root dentine in any direction and to varying extent. In advanced cases, ECR can progress into the mid‐ and apical thirds of the root.
Aetiology and prevalence
The precise aetiology of ECR is poorly understood. Studies have shown that ECR could be multifactorial, with orthodontic treatment being the most commonly associated factor. Other factors frequently implicated are the history of trauma, parafunctional habits, poor oral hygiene, periodontal treatment etc (Mavridou et al., 2017). Orthodontic treatment and history of previous dental trauma or existing parafunctional habits are frequently seen combined in cases of ECR. Other factors that have been reported to contribute to ECR include extraction of adjacent teeth (Gunst et al., 2013), herpes zoster virus infection (Solomon et al., 1986), feline viruses (von Arx et al., 2009), playing wind instruments (Gunst et al., 2011), the use of bisphosphonates (Patel & Saberi, 2015) and intracoronal bleaching (Friedman et al., 1988). All the suggested aetiological factors are considered predisposing factors or association rather than causative, to date, there is no evidence of the cause‐and‐effect relationship (Patel, Mavridou, et al., 2018).
The prevalence of ECR is poorly reported. Some epidemiological and retrospective studies reported a 0.02%–2.3% prevalence rate for ECR (Gulsahi, 2014; Heithersay, 1999a; Irinakis et al., 2020). However, this rate might be underestimated due to the lack of evidence from well‐designed studies, and also the difficulty in the detection and diagnosis of ECR lesions.
Histopathology
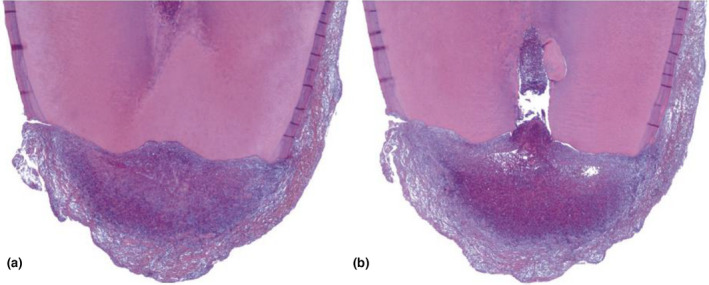
The pathogenesis of ECR is not fully understood. Damage to the protective unmineralized cementum allows the odontoclastic cells to resorb the underlying dentine. Pathogenesis involves a three‐stage process (Mavridou et al., 2016). Initially, the osteoclastic cells from the adjacent periodontium invade the exposed root surface through the breach in the cementum (initiation stage). The resorptive lesion resorbs the tooth structure and consists of fibrovascular tissue (resorption phase). The pulp space is protected from the ECR front by the pericanalar resorption‐resistant sheet, resulting in the lesion spreading circumferentially and apico‐coronally (Figure 5). Only in advanced cases does the ECR lesion perforate into the root canal. Bone‐like tissue is deposited into the resorption cavity in advanced cases of ECR (reparative phase; Figure 6).
FIGURE 5.
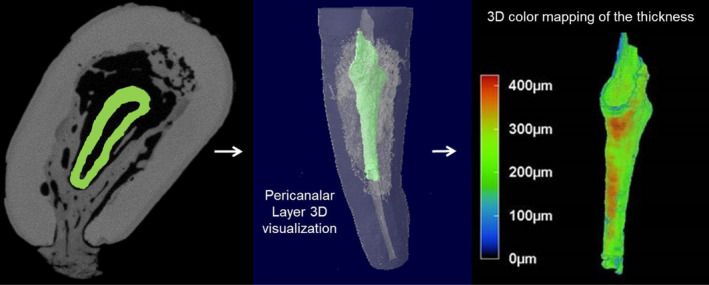
External cervical resorption. NanoCT imaging of tooth 13 and 3D modelling using CTan, CTvol and CTvox softwares, showing the thickness distribution of the pericanalar resorption resistant sheet (PRRS; Reprinted from International Endodontic Journal, Volume 51, Patel S, Mavridou A. M, Lambrechts P, Saberi N: External cervical resorption‐part 1: histopathology, distribution and presentation, pages, 1205–1223, Copyright (2018) with permission from Wiley)
FIGURE 6.
External cervical resorption (reparative phase). (a) Mandibular first and second molars in a 27‐year‐old female patient. The patient had symptoms of irreversible pulpitis associated with the lower right second molar; the lower right first molar was asymptomatic. (b) CBCT scan taken through the second molar at the level of the area indicated by the arrow in (a). Note massive resorption involving the crown and root. (c) Mesial portion of the crown of the second molar after clearing. (d) Section taken on a buccolingual plane. Overview shows resorption and replacement on the lingual side, corresponding to the area indicated by the arrow in (c) (H&E stain; original magnification ×6). (e) Detail of the area indicated by the arrow in (c). Several areas of resorption, with replacement by metaplastic tissue, can be seen (original magnification ×100). (f) Magnification of the area indicated by the right lower arrow in (e). The metaplastic tissue closely resembles bone (original magnification ×400). (g) High‐power view of the area indicated by the right upper arrow in (e). Note the lacuna in dentin occupied by a multinucleated clastic cell (original magnification ×1000). (h) High‐power view of the metaplastic bone tissue in the area indicated by the arrow in (f). A bone trabecula is being resorbed by a typical osteoclast (original magnification ×1000). Considerations: In this case, the metaplastic tissue was similar to normal lamellar bone. It is interesting that the bone tissue was undergoing remodeling, as evidenced by the presence of osteoclasts. Osteoclasts and odontoclasts can be observed in the same area and show morphologic similarity [Reprinted from Pathways of the Pulp (12th Edition), Patel S, Durack C, Riccuci D, Bakhash A, Root Resorption, Copyright (2020) with permission from Elsevier]
Clinical features
There is no ‘classic’ presentation of ECR (Figure 7a). The clinical findings can be variable depending on the severity and nature of the resorptive defect, tooth type and stage of ECR (Patel, Mavridou, et al., 2018). It is often asymptomatic in the early stage (Liang et al., 2003). Occasionally, a ‘pink spot’ may develop in the cervical region of the tooth, and it can be detected as an incidental finding if it occurs at the labial/buccal or lingual/palatal surface. The pink discolouration is due to the fibrovascular granulation tissue occupying the resorptive cavity, giving the tooth a pinkish hue (Figure 7b), through the thinned overlying enamel and dentine (Heithersay, 2004). Loss of periodontal attachment and profuse bleeding due to disturbing the vascular granulation tissue upon probing of the resorptive defect are among the other clinical features of ECR. Moreover, probing of ECR defects often gives a hard and scratchy tactile sensation, which helps to differentiate it from caries (Patel, Kanagasingam, et al., 2009).
FIGURE 7.
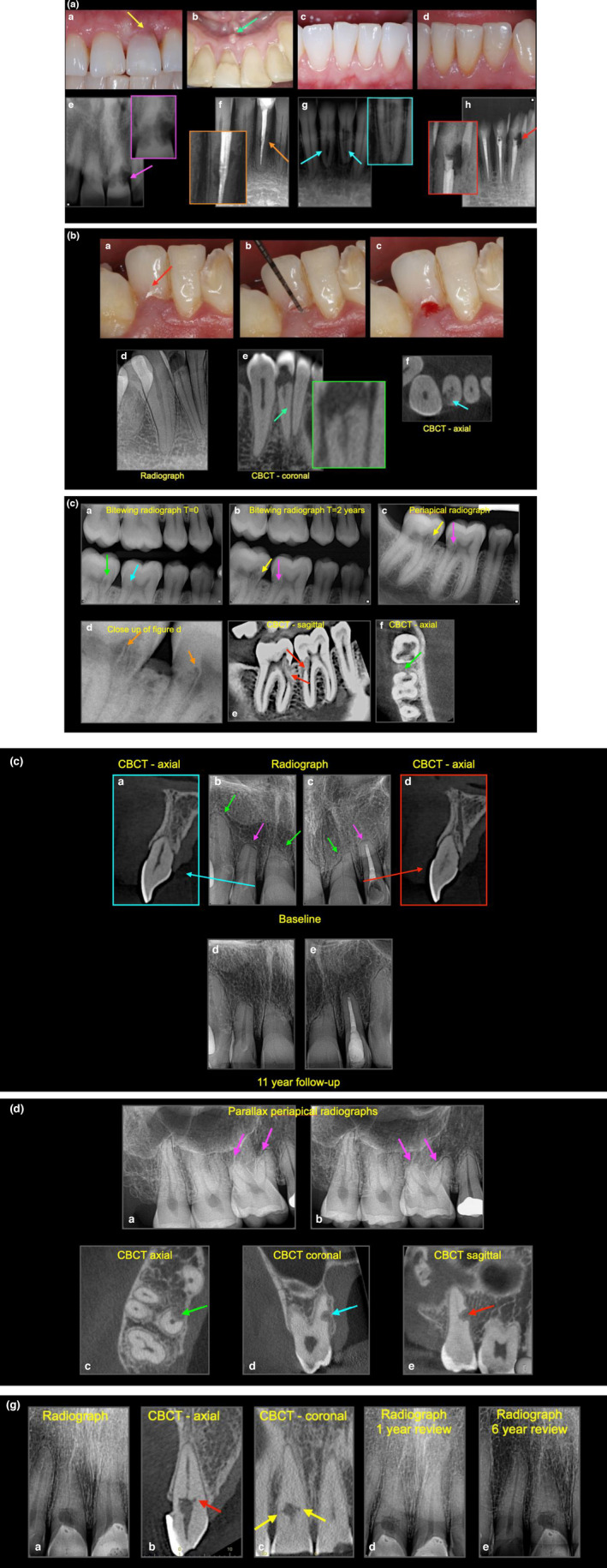
(a) External cervical resorption. Variable presentation of ECR, (a) pink spot (yellow arrow), (b) abscess (green arrow), (c–d) no signs, (e) moth eaten radiolucency with ragged margins (purple arrow), (f) irregular radiolucency with well‐defined margins (orange arrow), (g) radiolucency with radiopaque inclusions (radiolucency with well define margins (red arrow), the hard, glassy sensation to probing helps to differentiates it from caries. The potential predisposing factors for case (a) and (b) were unknown (idiopathic), case (c) was a history of orthodontic treatment (which has relapsed), case (d) was dental trauma, restorative (endodontic) treatment. (b) External cervical resorption. (a) Subtle pink hue on tooth 42, (b) probing (c) resulting in copious bleeding on probing, (d) well‐defined radiolucency (resorptive/destructive phase) appears to show that the ECR is confirmed to the coronal aspect of the tooth, (e) CBCT reveals the ECR extends into the coronal‐ third of the root, (f) and nearly up to 180° around the tooth (Patel 2Bp). The potential predisposing factors for this case was cricket ball injury sustained over 20 years ago. (c) External cervical resorption. (a) Routine bitewing radiograph reveals ECR in tooth 46 (cyan arrow) and 47 (green arrow)— this was missed by the clinician at the time. (b) Bitewing radiograph taken 2 years later confirms the presence and increased size of ECR on both teeth‐note the reparative nature of the ECR, (c) a periapical radiograph was then taken to help assess the nature of the ECR in both teeth (yellow and pink arrow), (d) close up of Figure 4c, note the intact root canal wall (orange arrow), (e, f) sagittal and axial CBCT views (tooth 46, Patel 2Ap, tooth 47 3Bp) confirm the extent and reparative nature of ECR (red arrows) and the portal of entry for the 46 (green arrow). Note, there are no signs of periapical pathology. The reparative nature of the ECR defect is also evident on the radiographs (a–c) and is indicated by the arrows in these images. The potential predisposing factor was parafunction. (d) Patel 3D external cervical resorption classification. (e) External cervical resorption. (a) Radiography, (b‐c) CBCT scans (cyan arrows) reveal nature of ECR in tooth 22 (Patel 1Ap), (d) post‐treatment, (e) 2‐year review, (f) envelope flap and isolation of ECR under rubber dam, (g) ECR lesion excavated, (h) instrumentation via ECR cavity, (i) irrigation, (j) obturation (red arrow) and (k) bonded restoration. Potential predisposing factor in this case was dental trauma sustained 16 years ago. (f) External cervical resorption. (a–c) Radiographic presentation ECR (Patel 2Ap), endodontic treatment and internal repair, (d) granulation and pulp tissue access, (e) ECR tag resulting in small bleeding point, (f) access cavity and root canal system prepared, (g) Biodentine to seal the access cavity, (h) post‐treatment, (i) 2‐year follow‐up. Potential predisposing factor in this case was a previous history of parafunction. (g) External cervical resorption. Untreatable, but asymptomatic ECR (Patel 2Cp), which was reviewed periodically and remained healthy and vital to sensitivity testing. The potential predisposing factor in this case was dental trauma and restorative treatment
In advanced cases, the resorption may eventually perforate the root canal wall and enter the pulp. Subsequent bacterial contamination of the pulp may result in symptoms and/or signs of pulpitis and/or periapical periodontitis. The affected tooth/teeth usually respond to pulp sensitivity tests, except if the ECR has perforated the pulp chamber and pulp necrosis has ensued (Frank & Torabinejad, 1998; Patel, Kanagasingam, et al., 2009).
Radiographic features
ECR can have varying radiographic features depending on the location, severity and phase of the lesion, i.e. resorptive or reparative (Figure 7a–c,e–f). ECR often presents as a radiolucency in the resorptive phase; however, in moderate to advanced cases, the lesion may have mottled radiographic appearance as a result of the deposition of fibro‐osseous tissue within the resorptive as the body attempts to repair the resorptive defect (reparative phase; Gunst et al., 2013; Patel, Kanagasingam, et al., 2009). The border of the resorptive defect may be well defined or have a ragged, irregular appearance. There is no ‘classical’ radiographic appearance of ECR.
The root canal outline is visible as long as there is no perforation of the root canal wall. It can be difficult to distinguish ECR from IRR especially when the tooth is asymptomatic (Durack & Patel, 2016). As described in the IRR section of this paper, parallax radiographs aid in differentiating ECR from IIR, as well as determining the location of the ECR (Durack & Patel, 2016).
The 2‐dimensional Heithersay classification has been used to assess the extent of ECR detected on radiographs (Heithersay, 1999b). Four stages are described: class I lesion manifests as a shallow lesion in the coronal dentine; class II lesion extends deeper into the coronal dentine and close to the pulp; class III lesion penetrates the coronal third; and class IV penetrates beyond the coronal third of the root. Due to the limitations of (2‐dimensional) periapical radiographs, only the proximal surface (mesial and distal) extent of ECR can be appreciated, whilst the approximal (buccal and palatal/lingual) extent of ECR cannot be accurately determined. Vaz de Souza et al. (2017) compared the diagnostic accuracy of radiographs and CBCT in diagnosing the location with the Heithersay classification of simulated ECR lesions. The overall accuracy for correctly identifying ECR was 49% for periapical radiographs and 89% for CBCT. The ECR lesions were correctly classified according to the Heithersay classification in 32% and 70% of cases with periapical radiographs and CBCT respectively. An in vivo study assessed the accuracy of periapical radiographs and CBCT for the detection and management of different resorption lesions and revealed a significantly greater accuracy in the detection of resorption lesions by CBCT when compared to radiographs (Patel, Dawood, et al., 2009; Patel, Mannocci, et al., 2016). CBCT should be considered prior to managing ECR (ECR Position statement, European Society of Endodontology, 2018).
A 3D classification system (Figure 7d) has been proposed by taking into account the lesion height, circumferential spread and proximity to the root canal (Patel, Foschi, Mannocci, et al., 2018). The classification is based on radiographic findings of periapical radiographs and CBCT. It aims to ensure accurate diagnosis and facilitate communication between clinicians. In the future, it is anticipated that the classification will allow for an objective assessment of the treatment outcome in relation to the nature and extent of ECR (Patel, Foschi, Mannocci, et al., 2018).
Management
Management of ECR depends on the nature and accessibility of the lesion. Treatment aims include excavation of the resorptive lesion to arrest the resorptive process, restore the resorptive defect and monitor the affected tooth for recurrence. Prevention of ECR is not predictable as the cause of ECR is unknown. Preoperative CBCT is essential in the treatment planning and explanation of treatment options to the patient.
The treatment options of ECR include external repair with(out) root canal treatment (RCT), internal repair along with RCT, intentional replantation (IR), periodic review with sensitivity testing or extraction for untreatable ECR (Patel, Foschi, Condon, et al., 2018).
External repair involves surgical exposure of the resorptive defect, complete excavation of the defect and restoration of the defects with composite, glass ionomer cement or Biodentine (Figure 7e). Trichloroacetic acid (TCA) has been suggested to promote the coagulation necrosis of the resorptive tissue (Heithersay, 1999c). However, due to its potentially carcinogenic and genotoxic effects (Herren‐Freund et al., 1987; Varshney et al., 2014), it is not recommended by the authors of this paper. Furthermore, there are no studies comparing the prognosis of ECR managed with and without TCA. Sodium hypochlorite administered with a microbrush has been suggested to assist in thorough debridement of the resorptive cavity (Patel, Foschi, Condon, et al., 2018). RCT is indicated in the cases with (near) perforation of the root canal by ECR, and/or there are signs/symptoms of irreversible pulpitis, pulp necrosis or apical periodontitis. The root canal should first be accessed before external repair, a GP point should be placed in the root canal(s) to maintain its patency during the external repair. Once the external repair has been completed, the tooth may be root treated.
Internal repair is indicated when ECR is close to or has perforated the root canal system, and a surgical approach is not possible due to poor accessibility, or if surgical access will lead to an excessive amount of sound, tooth structure removal and/or the portal of entry cannot be located (Figure 7f). RCT is completed, and the access cavity is restored together with the resorptive defect. Long shank burs and ultrasonic tips are useful in removing the resorptive lesion under a dental operating microscope (Frank, 1981; Patel, Foschi, Condon, et al., 2018). Biodentine may be used to repair resorbed dentine, and its high pH may help to arrest the osteoclastic action of any residual osteoclastic remnants.
Heithersay (1999) reviewed the 3‐year outcome of 101 ECR cases managed with a specific treatment regimen (topical application of trichloroacetic acid, curettage and restoration) and reported 100% success rate for Heithersay class 1 and class 2 lesions (combined), 77.8% for class 3 lesions and only 12.5% for class 4 lesions. Irinakis et al. (2018) identified the location of ECR in the mouth and the Heithersay class awarded as two local determinants that could significantly affect the failure rate. Posterior teeth with ECR had 70% failure rate at up to a 10‐year follow‐up period, compared to below 30% in anterior teeth. The failure rates in ECR‐affected teeth with different Heithersay class in a 10‐year follow‐up period were 0%, 20%, 70% and 100% for class 1, class 2, class 3 and class 4 lesions respectively.
A retrospective assessment of 542 teeth diagnosed with ECR reported a mean 5‐ and 10‐year survival rate of 70.3% and 28.6%, respectively, using a 3‐step management strategy (Mavridou et al. (2022). Treatment options included monitoring, internal approach, external approach or the combination of both internal and external approach; the survival rate appeared to be related to the extent of the ECR lesion regardless of the treatment option selected.
Intentional replantation has been described in several case (series) reports to successfully repair ECR defects (Krug et al., 2019; Patel, Foschi, et al., 2016). This treatment option is indicated when ECR cannot be accessed and repaired by an external or internal approach, for example, ECR located interproximally in the middle or apical third of the root. Contemporary IR protocols (<15‐min extraoral time, restoration with bioactive material) have been reported to result in higher survival rates (Cho et al., 2016). Clinical procedures of IR have been described in detail in the position statement of the European Society of Endodontology (2021a). A recent systematic review reported a survival rate of between 88% and 95% for IR over 2‐year mean follow‐up (Torabinejad et al., 2015).
For untreatable ECR, if asymptomatic, the patient may choose to review the tooth periodically to monitor for any progression of the ECR and/or the development of symptoms. Irinakis (2018) concluded that there was no statistically significant difference between repairing ECR defects versus no active treatment. More research is needed to assess the relationship of the various management options on tooth retention. Currently, there are no guidelines on the recall interval for untreated ECR case, but annual review including sensitivity testing and radiographs after the initial examination has been suggested to be a pragmatic recall protocol (Patel, Foschi, Condon, et al., 2018). Vital pulp treatment rather than RCT may also be indicated in specific cases. In certain situations, i.e when the tooth is unrestorable in the developing dentition, decoronation and intentional submerging of the root may be indicated to preserve, and allow the alveolar bone to develop (Asgary et al., 2019).
Extraction is the treatment option for unrestorable, symptomatic ECR lesions. ECR tends to predispose the affected tooth to fracture during extraction due to the weakened and cavitated tooth structure and the infiltration of bone‐like tissue in the resorptive cavity, and therefore, this needs to be carefully considered when ECR affects teeth in the aesthetic zone, a multidisciplinary approach is recommended (Patel, Foschi, Condon, et al., 2018).
External inflammatory resorption (EIR)
External inflammatory resorption (EIR) is present on the external surface of the root of majority of the teeth diagnosed with chronic apical periodontitis (Laux et al., 2000; Tronstad, 1988; Vier & Figueiredo, 2002). EIR also affects teeth that suffer severe dental traumatic injury (for example, avulsion and luxation). In dental trauma injury (DTI) cases, EIR occurs as a result of injury to the root surface and adjacent periodontium. It is initially self‐limiting and only focussed on the damaged root surface. Consequently, the loss of pulp vitality and infection of the necrotic pulp can result in the progression of EIR.
Aetiology and prevalence
The root canal microbiome from infected necrotic root canals due to caries, microleakage and/or failed existing RCT results in EIR in the majority of teeth with radiographic signs of chronic apical periodontitis (Laux et al., 2000; Vier & Figueiredo, 2002). Pulp necrosis and subsequent infection of root canals of teeth affected by severe dental trauma (for example, avulsion and luxation) may result in bacteria traversing the dentinal tubules to the resorbed region of the external aspect of the root, resulting in EIR continuing.
It has been reported that EIR occurs in 5%–8% of teeth after luxation injuries and 30% of teeth after replantation of avulsed teeth (Crona‐Larsson et al., 1991). Andreasen and Pedersen (1985) reported 6% of extrusive luxation cases and 3% of lateral luxation cases developed EIR. In intrusion cases, EIR occurred in 38% of the teeth as a healing complication. EIR rarely occurs in mild dental trauma, with no EIR case seen in concussion injuries and only one EIR case was seen in subluxation injuries (Andreasen & Pedersen, 1985).
Histopathology
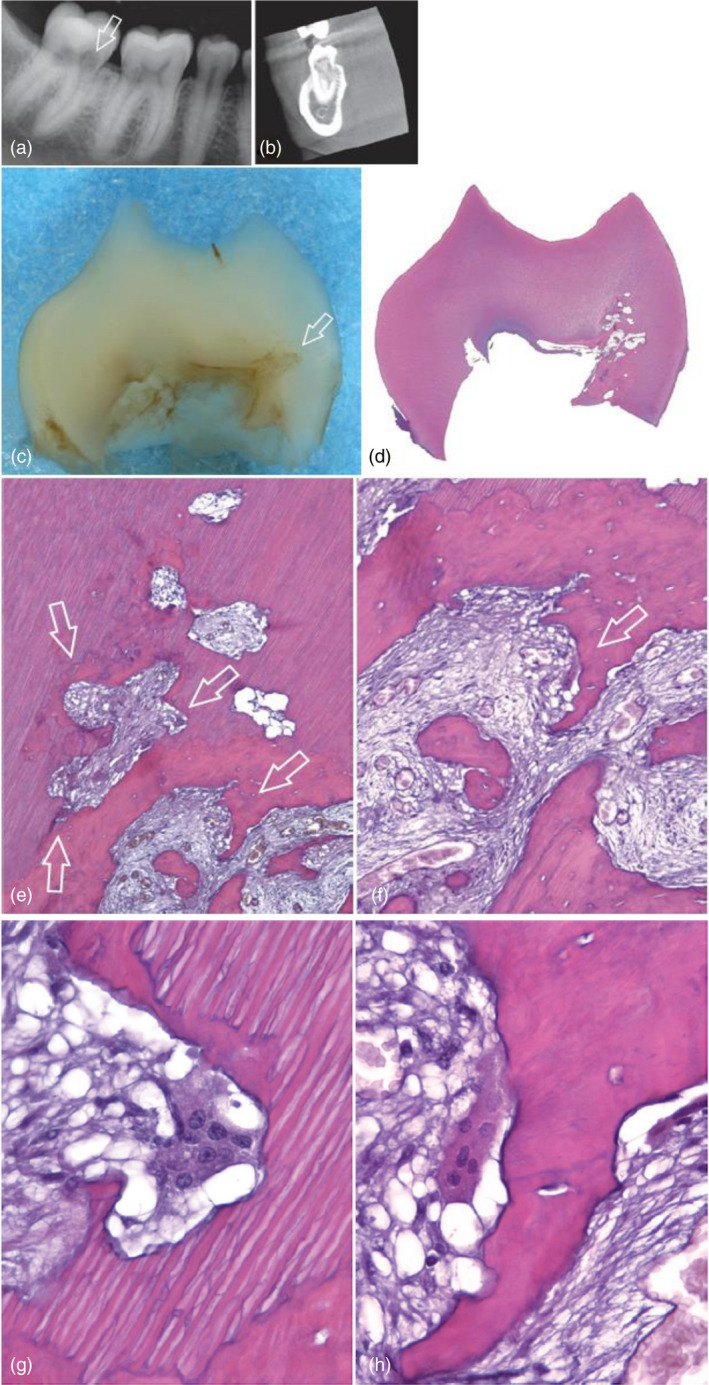
The pathogenesis of EIR after DTI commences following contusion injuries to the PDL, resulting in a breach in the protective nonmineralized precementum. Odontoclasts/osteoclasts and macrophages migrate to the injury site and bind to the underlying mineralized hard tissue and resorb the root surface (Fuss et al., 2003; Tronstad, 1988). The progression of the resorption, however, relies on microbial diffusion (bacteria and/or their byproducts) from the infected necrotic pulp (Andreasen & Hjørting‐Hansen, 1966b). Histologically, EIR appears as a saucer‐ or bowl‐shaped area of resorption, with associated inflammation in the adjacent PDL (Andreasen & Hjørting‐Hansen, 1966b). Resorption cavities contain Howship lacunae, which are occasionally occupied by odontoclasts. The inflamed area consists of mixed inflammatory cells infiltrate such as polymorphonuclear leukocytes, lymphocytes and plasma cells (Figure 8). The proliferation of capillaries is also a feature of inflammation (Andreasen & Hjørting‐Hansen, 1966b).
FIGURE 8.
External inflammatory resorption. (a) Mandibular second premolar extracted with the periapical lesion attached. This section, which did not pass through the canal, shows extensive apical resorption (H&E stain; original magnification ×25). (b) Section taken approximately 120 sections away encompasses the apical foramen. In addition to resorption, the opposite phenomenon can be observed; that is, a large calcification partly embedded in the right apical dentin wall (original magnification ×25). (Reprinted from Pathways of the Pulp (12th Edition), Patel S, Durack C, Riccuci D, Bakhash A, Root Resorption, Copyright (2020) with permission from Elsevier)
Clinical features
The clinical features of EIR are irreversible pulpitis and/or apical periodontitis such as pain, swelling, tenderness to percussion or palpation, sinus tract and discolouration. The affected tooth usually has a negative response to pulp sensitivity test (Andreasen & Hjørting‐Hansen, 1966a; Tronstad, 1988).
Radiographic features
The diagnosis of EIR is confirmed on radiographic findings. EIR due to solely infected necrotic pulp contents may appear to be shorter or stunted in appearance than normally expected and sometimes have a ragged margin at the root end and associated with periapical radiolucency adjacent to the affected root (Figure 9a,b). The root end may also have a ragged appearance.
FIGURE 9.
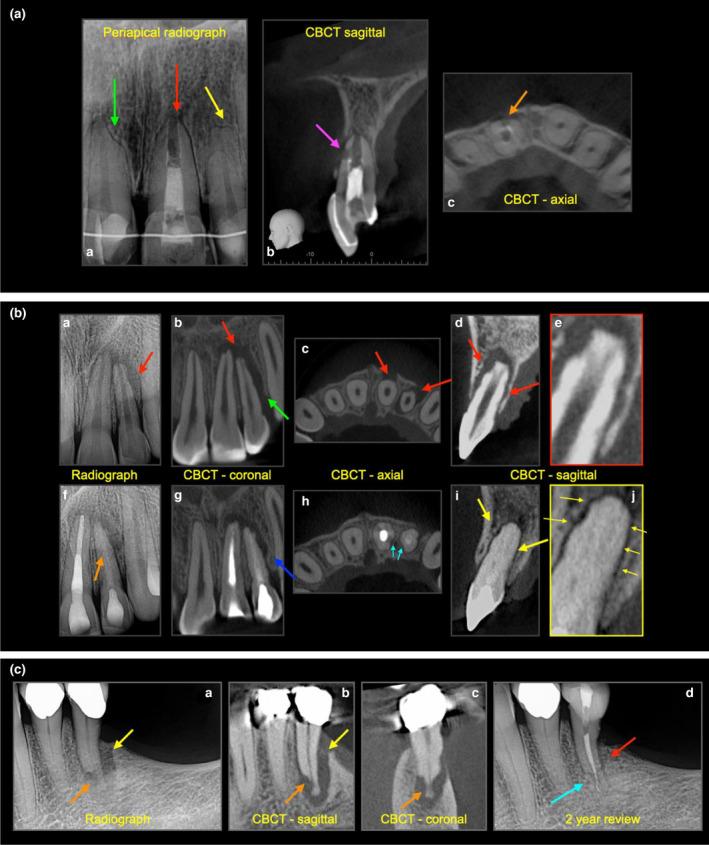
(a) External inflammatory resorption, external surface resorption. (a) Periapical radiographs of a maxillary incisor teeth with a history of dental trauma, extensive restorative and orthodontic treatment. RCT was attempted on tooth 21; however, the canal could not be dried and the apex locator reading was inconsistent. The tooth was partially dressed with calcium hydroxide paste. Tooth 11 has signs of external surface resorption (green arrow), tooth 21 (red arrow) and tooth 22 (yellow arrow) have signs of external surface resorption (purple & orange arrow), (b–c) the CBCT sagittal and axial views confirm the degree of external inflammatory resorption (purple and orange arrows) on the labial aspect of the 21. (b) External inflammatory resorption. (a) History of DTI on 21, 22, periapical radiograph reveals periradicular radiolucency associated with significant external inflammatory resorption on the lateral aspects on 22 and apical region of 21, (b–c) CBCT coronal and sagittal views reveal the significant bone loss, (d) CBCT sagittal view of 22 reveals resorption of the dentine, (e) close up figure, (f) 2‐year follow‐up after RCT, 21 has been obturated with gutta percha, the 22 has been obturated with Biodentine (orange arrow), hence the more radiolucent appearance. (g) Notice the bony infill (blue arrow), (h) cyan arrows reveal the bone apposition against the dimpled (resorbed dentine), (i) bony repair around tooth 22, (j) close up view, revealing bone apposition and intact periodontium around the 22. (c) External inflammatory resorption (a) A periapical radiograph, (b, c) sagittal and coronal CBCT scans revealing periradicular bone loss (yellow arrow) and periapical radiolucency associated with external inflammatory resorption (orange arrow) on the apical‐third of tooth 35. (d) Two‐year post‐treatment perirapical (cyan arrow) and periradicular healing
EIR associated with a history of moderate to severe DTI will usually have ragged bowl‐shaped indentation along the lateral border of root surface with an adjacent periradicular radiolucency (Andreasen & Hjørting‐Hansen, 1966a, 1966b). Loss of lamina dura can also be seen in the region affected by the EIR and may be detected as early as 3–4 weeks after DTI. The root canal outline should be intact in the earlier stage of EIR.
Perforation of the root canal wall can occur in advanced stages where the EIR is diagnosed and treated late. EIR may be aggressive in nature, and the progression of the root resorption can be rapid after onset, for example, resorption of an entire root can occur within months. Therefore, it is essential to manage EIR as soon as possible and carry out RCT to arrest the progression of the resorption.
Conventional radiography is the initial imaging technique of choice for diagnosis of root resorption after DTI. However, it is well accepted that conventional radiography is unable to provide sufficient information due to its 2‐dimensional nature; therefore, it will only detect EIR on the proximal aspects of the root; the extent of EIR on buccal or palatal/lingual aspect cannot be accurately determined. Radiographs are not sensitive enough to detect small‐sized resorptive defects, CBCT was more accurate at detecting small simulated EIR lesions compared to parallax radiographs (Durack et al., 2011; Estrela et al., 2009). Root canal perforation in root resorption cases may not be diagnosed on a radiograph even if the root resorption is extensive, but the perforating resorption defect can be clearly visible on CBCT images (Bhuva et al., 2011). Therefore, CBCT is now considered a valid and reliable method for the assessment of moderate to advanced EIR defects.
Management
The objective in the management of EIR cases is the disinfection to eliminate the aetiological factor, therefore RCT for the treatable cases and extraction for the unsalvageable cases (Figure 9c).
Root canal treatment will eliminate the stimulating factors (microbes and their toxins) and arrest the resorptive process, thus preventing further damage on the root, at the same time allowing hard tissue repair of the damaged root surface (Fuss et al., 2003; Tronstad, 1988).
When EIR is associated with DTI it is important to commence RCT as soon as possible due to the potentially rapidly progressing nature of EIR. In avulsion injuries of teeth with closed apices, the replanted tooth should be root treated 7–10 days after the reimplantation, even when there are no radiographic signs of EIR due to the small chance of pulp vitality being maintained and a high risk of EIR (Fouad et al., 2020). Failure to do so may result in the affected tooth being extracted due to a severely resorbed root surface that is not possible to repair.
Enhanced protocols for root canal disinfection using intra‐appointment medicaments have been suggested for the management of EIR associated with DTI (European Society of Endodontology, 2021b; Krastl et al., 2021). An intracanal calcium hydroxide dressing for 4 weeks to several months has been suggested (Haapasalo & Endal, 2006; Mohammadi & Dummer, 2011; Trope, 2002). It has also been suggested to place calcium hydroxide dressing until the resorption process is under control radiographically; however, there is limited evidence to support the use of intracanal medicaments (Patel et al., 2020).
An alternative to calcium hydroxide dressing is the use of antibiotic‐corticosteroid paste such as Ledermix (Riemser) or Odontopaste (Australian Dental Manufacturing) followed by calcium hydroxide (Krastl et al., 2021). The combination of antibiotic‐corticosteroid intracanal medication aims to reduce the inflammation in the periodontal membrane by directly inhibiting odontoclasts and detaching the resorptive cells from the damaged root surface (Heithersay, 2007; Pierce et al., 1988; Pierce & Lindskog, 1987). In a randomized controlled trial, Day et al. (2012) reported a larger number of replanted teeth were associated with periodontal healing when treated with Ledermix compared with calcium hydroxide, although the difference between the two groups was not significant. To date, there is no strong evidence to support the superior efficacy of antibiotic‐corticosteroid dressings over calcium hydroxide in the management of EIR, nor of single versus multiple visit treatment protocols. Healing of existing the periapical lesion and re‐establishment of the periodontal ligament around the previously resorbed root is a clinically successful outcome.
Inadvertent overextrusion of the root filling is possible, therefore great care must be taken to ensure there is a good cone fit if gutta–percha is being used. Root filling with calcium silicate bioactive cements may be beneficial, due to their excellent biocompatibility (Camilleri et al., 2005; Saidon et al., 2003), good sealing ability (El Sayed & Saeed, 2012; Shahi et al., 2011), the potential to repair and restore the periodontal ligament/cementum (Zhou et al., 2013), creating an environment more conducive to hard tissue repair (Zanini et al., 2012) and inhibition of clastic activity (Arnett, 2008; Narita et al., 2010). REP has been reported in case reports to be one of the potential alternative treatment options to arrest EIR in teeth suffering from DTI (Tzanetakis, 2018; Yoshpe et al., 2020). However, there are limited evidence long‐term data on this treatment option.
External replacement resorption (ERR)
ERR refers to the resorption on the root surface and subsequent replacement by bone tissue, which may result in ankylosis.
Aetiology and prevalence
ERR is associated with severe luxation such as intrusion and avulsion injuries. A retrospective study by Soares et al. (2015) revealed that replacement resorption was seen more frequently in cases of avulsion (87.2%), followed by intrusive luxation (57.1%). A meta‐analysis revealed 51% of replacement root resorption in avulsion cases (Souza et al., 2018).
Histopathology
Depending on the nature of the injury, the periodontal ligament may tear, become crushed and/or degenerate due to desiccation resulting in the periodontal ligament cells undergoing necrosis and, together with damaged cementum and dentine, become resorbed via osteoclastic action and then ultimately replaced with alveolar bone laid down by osteoblasts as part of the repair process (Andersson et al., 1984). The osteoblastic activity may then gradually replace the radicular dentine with bone in the process of remodelling. ERR may be self‐limiting and/or localized. Andreasen (1980) reported total mineralization of PDL as the most common finding in ERR after tooth replantation in monkeys. Deposition of bone tissue was found on the root surface and socket wall with soft connective tissue zone in early ankylosed area (Andreasen, 1980).
Clinical features
Clinical features of ERR include a lack of physiological mobility (Andersson et al., 1984; Andreasen, 1975). A study by Andersson et al. (1984) concluded that the tooth may lose its physiological mobility and produce a high‐pitched or metallic sound to percussion if ERR involves more than 20% of the root surface (Andersson et al., 1984). This finding is possibly an underestimation as it was based on conventional radiograph only, and therefore, there was no appreciation of the presence and extent of ERR on the labial and lingual/palatal aspects of the root, which are not readily detected on conventional radiographs.
The tooth may also be infraoccluded if ERR occurs in developing dentition, especially before the pubertal growth spurt of the patient. The tooth should respond normally to pulp sensitivity testing unless there is tertiary dentine formation, which delays or masks the response to sensitivity testing. A lack of response to sensitivity testing is not an indication for RCT in the affected tooth, as this pathological entity is entirely driven by the affected periodontium.
Radiographic features
Conventional radiographic examination will reveal the absence of periodontal ligament space where the resorbed root surface appears to fuse with the surrounding bone (Figure 10). The root dentine will have an irregular or ‘moth‐eaten’ appearance as the dentine is replaced by bone (Andreasen & Hjørting‐Hansen, 1966b).
FIGURE 10.
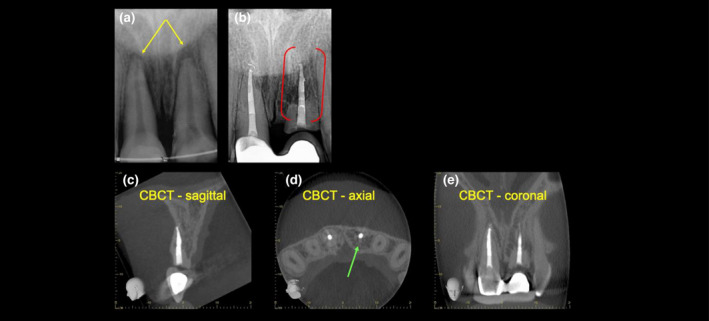
External replacement resorption. (a) A periapical radiograph of the maxillary central incisors with late presentation following severe luxation injury and complicated crown root fracture of the maxillary left central incisor. Note periapical radiolucency and blunting of the root ends (yellow arrows). (b) A 5‐year review radiograph following RCT of both teeth reveals direct bone replacement of the root dentine on the maxillary left central incisor (red brackets). Note the left central incisor has been decoronated and a restored with a temporary resin‐bonded bridge. Note the periapical radiolucency on both teeth have healed. (c–e) Sagittal, axial and coronal CBCT slices through the same tooth confirm an almost complete bony replacement of root dentine associated with ERR (green arrows). Reprinted from British Dental Journal, Volume 224, Patel S, Saberi N: The ins and out of root resorption. 691–699 Copyright (2018) with permission from Springer Nature
Radiographs will only reveal the extent of ERR on the proximal aspects of the root. As with the types of root resorption described above, CBCT may be indicated to accurately assess the true nature and extent of ERR (Durack & Patel, 2016). Conventional radiography underestimates the extent and even prevalence of ERR as the resorption occurring on the buccal and palatal aspects will not be detected.
Management
Presently, there is no treatment to arrest ERR. It may be self‐limiting or continue to resorb the root and replace it with bone‐like tissue for years, eventually resorbing the entire root. ERR may be reviewed periodically (Finucane & Kinirons, 2003). In the older patient, the progression of ERR may be slow, and the tooth can remain functional for many years without the need for any active intervention (Andersson et al., 1989).
The early detection and management of ERR and ankylosis are crucial in children and adolescents before or during their pubertal growth spurt. This is because ankylosed teeth will arrest the development of the alveolar ridge on that region whilst the adjacent alveolar ridge continues to grow, causing the affected tooth to become infraoccluded and the alveolar ridge to underdeveloped (Andersson & Malmgren, 1999). This will compromise the aesthetics, phonetics, function of the patient and further complicate future restorative or prosthetic treatment (Malmgren, 2013; Malmgren et al., 2006).
Malmgren (2013) has advocated that ankylosed, infraoccluded tooth is intentionally decoronated below the level of cementoenamel junction in children and adolescents. In this technique, a mucoperiosteal flap is raised, and the tooth is decoronated to 2 mm below the marginal bone level, the decoronated root is allowed to fill with a blood clot and sealed with the mucoperiosteal flap. This allows the root to be covered with attached mucosa. The decoronation aims to preserve the buccal‐palatal dimension of the alveolar ridge, to allow for vertical growth and facilitate new bone formation above the decoronated root. Once the patient is an adult, in their early 20’s, permanent restorative treatment such as dental implant treatment may be considered.
Other possible management strategies include composite build‐up on the infraoccluded tooth to maintain a satisfactory appearance. This option is a short‐ to medium‐term solution to improve the aesthetics. However, as the dentoalveolar process develops the tooth may look unacceptably long (Andersson & Malmgren, 1999).
Autotransplantation with a premolar has been suggested as a treatment option (Andreasen, Paulsen, Yu, Ahlquist, et al., 1990; Andreasen, Paulsen, Yu, Bayer, et al., 1990). The donor tooth may require additional treatment procedures including RCT (depending on maturation status of the root apex) and reshaping the premolar to incisor shape (Abella et al., 2018; Tsukiboshi et al., 2019). Surgical repositioning involves extraction that breaks the bony contact with the root and replantation into the socket at a correct vertical and horizontal relationship with the adjacent teeth (Takahashi et al., 2005). However, there is limited evidence supporting this treatment option (Andersson & Malmgren, 1999).
Distraction osteogenesis has also been used to restore the correct vertical position and aesthetics of the infraoccluded tooth and the associated bone (Isaacson et al., 2001). Filippi et al. (2006) treated 15 ankylosed teeth (early stage) with IR after extraction and application of Emdogain (Straumann; gel containing enamel matrix derivative aimed to induce the development of periodontium) on the root surface. Their 6‐year recall revealed success in only seven out of the 15 teeth (Filippi et al., 2006). Another clinical study reported failures with all cases when Emdogain was used (Schjøtt & Andreasen, 2005).
Extraction of the tooth with ERR is indicated if a pathological root fracture occurs or is likely to occur. It is also indicated in extremely compromised aesthetics. Extraction of an ankylosed tooth often requires a surgical approach and can result in a considerable amount of bone loss, complicating future implant placement.
A case series by Yoshpe et al. (2020) demonstrated the management of three ERR case with REP. They used platelet‐rich fibrin instead of induced blood clot as a scaffold to promote stem cells differentiation and then placed Biodentine on top of the scaffold. These cases were followed up to 3 years, and the ERR were arrested and even reversed in some cases (Yoshpe et al., 2020). The success of REP offered a promising potential solution for ERR to avoid decoronation or extraction. However, more clinical trials are required to provide definitive evidence.
In general, the management of ERR depends on the growth status of the patient. ERR in adult should be managed conservatively. Periodic review and/or composite build‐up to restore the aesthetic appearance is usually sufficient. If the affected tooth is extensively resorbed, extraction and replacement with prosthesis are recommended. However, the ERR in children/adolescents require more active intervention such as decoronation or REP. If the tooth is severely resorbed and extraction is unavoidable, autotransplantation or orthodontic space closure could be more relevant as permanent restorative or implant treatment is contraindicated in growing patient. To date, there is no clear guideline on the treatment protocol for ERR due to the lack of evidence‐based clinical trials. Most of the available treatment options were mainly based on case series or individual case report. Therefore, the treatment plan should be based on clinical judgement of individual case and often requires multidisciplinary approach from different specialties (orthodontics/restorative/endodontics etc).
Transient apical breakdown
Transient apical breakdown is resorption of the apical portion of the root in healthy teeth with a recent history of DTI (Boyd, 1995). TAB appears to be related to the type of injury and the stage of root development (Andreasen, 1986).
Aetiology and prevalence
The prevalence of transient apical breakdown is rarely reported, and to date, there is only one comprehensive study on TAB, which reported a 4.2% prevalence in 637 teeth affected by DTI (Andreasen, 1986). TAB was associated with moderate DTI, such as extrusion and lateral luxation and rarely associated with minor DTI (such as concussion and subluxation) and usually absent in severe dental traumatic injuries (intrusive luxation; Andreasen, 1986). TAB has only been reported in teeth with fully formed roots with closed or half‐closed apices. The reason TAB is not seen in teeth with incomplete root length may be due to the eruption periapical radiolucency associated with developing root apices masking signs of apical pathology.
Andreasen (1986) retrospectively assessed the three parallax periapical radiographs of 637 luxated teeth. A diagnosis of TAB was reached when an existing periapical radiolucency attributed to the dental injury resolved during the review appointment without any intervention. TAB was diagnosed based on the transient change in the size of apical PDL space ranging from a minimum of twice the normal width of PDL space up to semicircular radiolucency in the radiograph. The colour change and the sensitivity testing of the teeth with TAB were then recorded. TAB was found in 2.2% of subluxated teeth, 11.3% of extruded teeth and 12.3% of laterally luxated teeth. Most of the transient changes in radiograph, colour and sensitivity were resolved by the 1‐year follow‐up.
Histopathology
The exact pathogenesis of TAB is unknown. It has been suggested that TAB is a sequela of moderate dental traumatic injuries where the necrotic or damaged tissue undergoes a repair process; the injured tissue is removed and then is replaced with healthy tissue after some time. It has been speculated that a transient bacterial infection may also be the cause of TAB. It usually has no permanent effect on the pulp and tends to resolve within a year. The histological features of TAB are unknown as there are no human or animal studies that have examined the histological healing events after luxation injuries (Andreasen, 1986).
Clinical features
Clinical features of TAB include mild tooth discolouration and delayed or no response to pulp sensitivity testing. A return to a positive response to sensitivity testing occurs within 12 months of TAB being diagnosed. Delayed or the absence of response to sensitivity testing can be observed if there is pulp obliteration secondary to tertiary dentin formation. The discolouration in TAB usually resolves within a year (Andreasen, 1986; Boyd, 1995).
Radiographic features
Radiographic examination of the affected tooth may reveal widening of the periodontal ligament space and blurry appearance or loss of apical lamina dura. The radiographic appearance of the periodontal ligament and lamina dura may return to a normal state within a year (Andreasen, 1986; Cohenca et al., 2003). Surface resorption and pulp canal obliteration almost consistently occur after the resolution of TAB (Andreasen, 1986). The resolution of the radiolucency may also be in part due to the affected tooth being subtly displaced at the time of the dental injury, and over time as it seats down completely into the socket, the radiolucency disappears (Figure 11).
FIGURE 11.
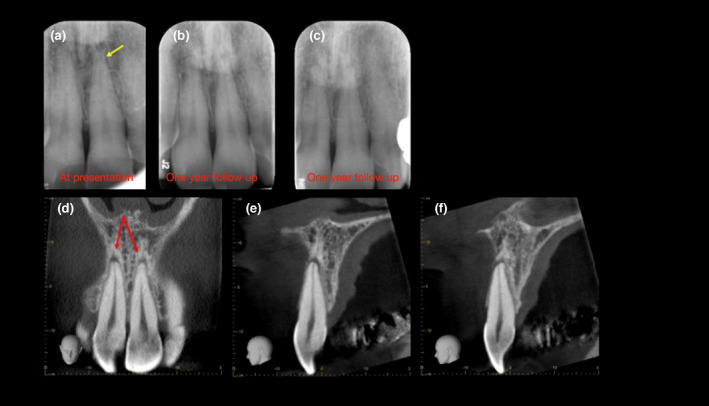
Transient apical breakdown (a) A periapical radiograph revealing widening of the periodontal ligament space and loss of apical lamina dura following subluxation injury to the maxillary anterior teeth (yellow arrow). (b) One‐year follow‐up radiograph demonstrates an improvement, and (c) a 10‐degree vertical shift eliminates the presence of the lesion altogether, confirming a diagnosis of transient apical breakdown. (d–f) Coronal and sagittal CBCT slices of the same teeth, however, reveal well‐defined periapical radiolucencies associated with the maxillary right and left central incisors (red arrows); therefore, the diagnosis was changed to chronic periapical periodontitis associated with infected necrotic pulp. This case highlights anatomical noise and subtle geometrical distortion associated with conventional radiographs, which may be overcome with CBCT (Reprinted from British Dental Journal, Volume 224, Patel S, Saberi N: The ins and out of root resorption. 691–699 Copyright (2018) with permission from Springer Nature)
It is well established that conventional radiographs, even with a beam aiming device, have limited capabilities to accurately replicate the same angulation and view (Rudolph & White, 1988). Any minor alteration in angulation may result in the disappearance of the periapical or PDL changes; therefore, TAB may be a radiographic artefact due to the limitation of the radiographic technique (Patel & Saberi, 2018). An extrusive luxation can appear as a uniform expansion of the PDL space, which will then be misdiagnosed as TAB (Andreasen, 1986). Intraoral radiographic examination at different angulations (parallax technique) or with bisecting angle technique may create more confusion and complicate the diagnosis (Brynolf, 1970).
Management
Understanding the aetiology and clinical features of TAB in traumatized or orthodontically treated teeth is important. If the risk of pulpal necrosis is low (concussion, subluxation and orthodontic forces), a no‐treatment and wait approach may be indicated. Reversal of signs and symptoms should be observed within 1 year.
The disappearance of initial radiographic changes in the periapical region and PDL does not confirm the initial diagnosis of TAB as a slight change in angulation during radiographic examination could mask minor periapical radiolucency or widening of PDL space.
It is critical to evaluate the risk of pulp necrosis for traumatized teeth. Andreasen (1985) reported that with more extensive injuries (intrusion and lateral luxation), the risk of pulp necrosis increased, whereas concussion and subluxation had the best prognosis. The discolouration, delayed response to pulp sensitivity testing and radiographic changes reported in TAB should not serve solely as proxies for the determination of pulp necrosis. Tenderness to percussion or on buccal palpation are more frequently associated with pulp necrosis than sensitivity tests (Andreasen, 1988). Doppler flowmetry or pulse oximetry can assess pulpal blood flow and thus be used to determine the vitality following trauma, more accurately than cold or electric tests (Cohenca et al., 2003; Roeykens et al., 2002; Schnettler & Wallace, 1991). Research into more objective sensitivity testing is needed.
CONCLUSION
Robust clinical research is required to gain a deeper knowledge of the aetiology and pathogenesis of the various types of root resorption. The diagnosis and/or management of root resorption can be challenging for clinicians resulting in misdiagnosis, and a greater appreciation of this area of Endodontology is required.
The importance of a thorough and systematic clinical and radiographic examination is paramount to ensure appropriate management. The prognosis of root resorption is dependent on an accurate and early diagnosis. Increasingly, CBCT is being used to confirm the diagnosis and/or aid management.
CONFLICT OF INTEREST
The authors deny any conflicts of interest.
AUTHOR CONTRIBUTION
Shanon Patel, conceptualization, writing, review and editing (lead). Peng‐Hui Teng, Navid Saberi and Tiago Pimental writing, review and editing.
ETHICAL APPROVAL
This has been answered when submitting manuscript.
Patel, S. , Saberi, N. , Pimental, T. & Teng, P.‐H. (2022) Present status and future directions: Root resorption. International Endodontic Journal, 55(Suppl. 4), 892–921. Available from: 10.1111/iej.13715
REFERENCES
- Abella, F. , Ribas, F. , Roig, M. , González Sánchez, J.A. & Durán‐Sindreu, F. (2018) Outcome of autotransplantation of mature third molars using 3‐dimensional‐printed guiding templates and donor tooth replicas. Journal of Endodontics, 44, 1567–1574. [DOI] [PubMed] [Google Scholar]
- Acar, A. , Canyürek, U. , Kocaaga, M. & Erverdi, N. (1999) Continuous vs. discontinuous force application and root resorption. The Angle Orthodontist, 69, 159–163, discussion 163–154. [DOI] [PubMed] [Google Scholar]
- Ahlberg, K. , Bystedt, H. , Eliasson, S. & Odenrick, L. (1983) Long‐term evaluation of autotransplanted maxillary canines with completed root formation. Acta Odontologica Scandinavica, 41, 23–31. [DOI] [PubMed] [Google Scholar]
- Alemam, A.A. , Abu Alhaija, E.S. , Mortaja, K. & AlTawachi, A. (2020) Incisor root resorption associated with palatally displaced maxillary canines: analysis and prediction using discriminant function analysis. American Journal of Orthodontics & Dentofacial Orthopedics, 157, 80–90. [DOI] [PubMed] [Google Scholar]
- Allen, A.L. & Gutmann, J.L. (1977) Internal root resorption after vital root resection. Journal of Endodontics, 3, 438–440. [DOI] [PubMed] [Google Scholar]
- American Association of Endodontists/American Academy of Oral & Maxillofacial Radiology . (2015) AAE and AAOMR joint position statement: use of cone beam computed tomography in endodontics 2015 update. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 120, 508–512. [DOI] [PubMed] [Google Scholar]
- Andersson, L. , Blomlöf, L. , Lindskog, S. , Feiglin, B. & Hammarström, L. (1984) Tooth ankylosis: clinical, radiographic and histological assessments. International Journal of Oral Surgery, 13, 423–431. [DOI] [PubMed] [Google Scholar]
- Andersson, L. , Bodin, I. & Sörensen, S. (1989) Progression of root resorption following replantation of human teeth after extended extraoral storage. Endodontics & Dental Traumatology, 5, 38–47. [DOI] [PubMed] [Google Scholar]
- Andersson, L. & Malmgren, B. (1999) The problem of dentoalveolar ankylosis and subsequent replacement resorption in the growing patient. Australian Endodontic Journal, 25, 57–61. [DOI] [PubMed] [Google Scholar]
- Andreasen, F.M. (1986) Transient apical breakdown and its relation to color and sensibility changes after luxation injuries to teeth. Dental Traumatology, 2, 9–19. [DOI] [PubMed] [Google Scholar]
- Andreasen, F.M. (1988) Histological and bacteriological study of pulps extirpated after luxation injuries. Endodontics & Dental Traumatology, 4, 170–181. [DOI] [PubMed] [Google Scholar]
- Andreasen, F.M. & Pedersen, B.V. (1985) Prognosis of luxated permanent teeth—the development of pulp necrosis. Endodontics & Dental Traumatology, 1, 207–220. [DOI] [PubMed] [Google Scholar]
- Andreasen, J.O. (1970) Luxation of permanent teeth due to trauma. A clinical and radiographic follow‐up study of 189 injured teeth. Scandinavian Journal of Dental Research, 78, 273–286. [DOI] [PubMed] [Google Scholar]
- Andreasen, J.O. (1975) Periodontal healing after replantation of traumatically avulsed human teeth: assessment by mobility testing and radiography. Acta Odontologica Scandinavica, 33, 325–335. [Google Scholar]
- Andreasen, J.O. (1980) Analysis of pathogenesis and topography of replacement root resorption (ankylosis) after replantation of mature permanent incisors in monkeys. Swedish Dental Journal, 4, 231–240. [PubMed] [Google Scholar]
- Andreasen, J.O. (1981) Relationship between surface and inflammatory resorption and changes in the pulp after replantation of permanent incisors in monkeys. Journal of Endodontics, 7, 294–301. [DOI] [PubMed] [Google Scholar]
- Andreasen, J.O. & Hjørting‐Hansen, E. (1966a) Replantation of teeth. I. Radiographic and clinical study of 110 human teeth replanted after accidental loss. Acta Odontologica Scandinavica, 24, 263–286. [DOI] [PubMed] [Google Scholar]
- Andreasen, J.O. & Hjørting‐Hansen, E. (1966b) Replantation of teeth. II. Histological study of 22 replanted anterior teeth in humans. Acta Odontologica Scandinavica, 24, 287–306. [DOI] [PubMed] [Google Scholar]
- Andreasen, J.O. , Paulsen, H.U. , Yu, Z. , Ahlquist, R. , Bayer, T. & Schwartz, O. (1990a) A long‐term study of 370 autotransplanted premolars. Part I. Surgical procedures and standardized techniques for monitoring healing. European Journal of Orthodontics, 12, 3–13. [DOI] [PubMed] [Google Scholar]
- Andreasen, J.O. , Paulsen, H.U. , Yu, Z. , Bayer, T. & Schwartz, O. (1990b) A long‐term study of 370 autotransplanted premolars. Part II. Tooth survival and pulp healing subsequent to transplantation. European Journal of Orthodontics, 12, 14–24. [DOI] [PubMed] [Google Scholar]
- Arnett, T.R. (2008) Extracellular pH regulates bone cell function. The Journal of Nutrition, 138, 415S–418S. [DOI] [PubMed] [Google Scholar]
- Arnold, M. (2021) Reparative endodontic treatment of a perforating internal inflammatory root resorption: a case report. Journal of Endodontics, 47, 146–155. [DOI] [PubMed] [Google Scholar]
- Årtun, J. , Van ‘t Hullenaar, R. , Doppel, D. & Kuijpers‐Jagtman, A.M. (2009) Identification of orthodontic patients at risk of severe apical root resorption. American Journal of Orthodontics & Dentofacial Orthopedics, 135, 448–455. [DOI] [PubMed] [Google Scholar]
- von Arx, T. , Schawalder, P. , Ackermann, M. & Bosshardt, D.D. (2009) Human and feline invasive cervical resorptions: the missing link?—Presentation of four cases. Journal of Endodontics, 35, 904–913. [DOI] [PubMed] [Google Scholar]
- Asgary, S. , Nourzadeh, M. , Verma, P. , Hicks, M.L. & Nosrat, A. (2019) Vital pulp therapy as a conservative approach for management of invasive cervical root resorption: a case series. Journal of Endodontics, 45, 1161–1167. [DOI] [PubMed] [Google Scholar]
- Ashrafi, M.H. & Sadeghi, E.M. (1980) Idiopathic multiple internal resorption: report of case. ASDC Journal of Dentistry for Children, 47, 196–199. [PubMed] [Google Scholar]
- Baumrind, S. , Korn, E.L. & Boyd, R.L. (1996) Apical root resorption in orthodontically treated adults. American Journal of Orthodontics & Dentofacial Orthopedics, 110, 311–320. [DOI] [PubMed] [Google Scholar]
- Bhuva, B. , Barnes, J.J. & Patel, S. (2011) The use of limited cone beam computed tomography in the diagnosis and management of a case of perforating internal root resorption. International Endodontic Journal, 44, 777–786. [DOI] [PubMed] [Google Scholar]
- Boyd, K.S. (1995) Transient apical breakdown following subluxation injury: a case report. Endodontics & Dental Traumatology, 11, 37–40. [DOI] [PubMed] [Google Scholar]
- Brynolf, I. (1970) Roentgenologic periapical diagnosis. 3. The more roentgenograms—the better the information? Svensk Tandlakare Tidskrift, 63, 409–413. [PubMed] [Google Scholar]
- Burleson, A. , Nusstein, J. , Reader, A. & Beck, M. (2007) The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. Journal of Endodontics, 33, 782–787. [DOI] [PubMed] [Google Scholar]
- Cabrini, R.L. & Manfredi, E.E. (1957) Internal resorption of dentine; histopathologic control of eight cases after pulp amputation and capping with calcium hydroxide. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 10, 90–96. [DOI] [PubMed] [Google Scholar]
- Calişkan, M.K. & Türkün, M. (1997) Prognosis of permanent teeth with internal resorption: a clinical review. Endodontics & Dental Traumatology, 13, 75–81. [DOI] [PubMed] [Google Scholar]
- Camilleri, J. , Montesin, F.E. , Di Silvio, L. & Pitt Ford, T.R. (2005) The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. International Endodontic Journal, 38, 834–842. [DOI] [PubMed] [Google Scholar]
- Cho, S.‐Y. , Lee, Y. , Shin, S.‐J. , Kim, E. , Jung, I.‐Y. , Friedman, S. et al. (2016) Retention and healing outcomes after intentional replantation. Journal of Endodontics, 42, 909–915. [DOI] [PubMed] [Google Scholar]
- Chogle, S. , Zuaitar, M. , Sarkis, R. , Saadoun, M. , Mecham, A. & Zhao, Y. (2020) The recommendation of cone‐beam computed tomography and its effect on endodontic diagnosis and treatment planning. Journal of Endodontics, 46, 162–168. [DOI] [PubMed] [Google Scholar]
- Chu, F.C. , Li, T.K. , Lui, V.K. , Newsome, P.R. , Chow, R.L. & Cheung, L.K. (2003) Prevalence of impacted teeth and associated pathologies—a radiographic study of the Hong Kong Chinese population. Hong Kong Medical Journal, 9, 158–163. [PubMed] [Google Scholar]
- Cohenca, N. , Karni, S. & Rotstein, I. (2003) Transient apical breakdown following tooth luxation. Dental Traumatology, 19, 289–291. [DOI] [PubMed] [Google Scholar]
- Cooke, J. & Wang, H.L. (2006) Canine impactions: incidence and management. The International Journal of Periodontics & Restorative Dentistry, 26, 483–491. [PubMed] [Google Scholar]
- Crona‐Larsson, G. , Bjarnason, S. & Norén, J.G. (1991) Effect of luxation injuries on permanent teeth. Endodontics & Dental Traumatology, 7, 199–206. [DOI] [PubMed] [Google Scholar]
- Da Silva, T.A. , Batista, A.C. , Mendonça, E.F. , Leles, C.R. , Fukada, S. & Cunha, F.Q. (2008) Comparative expression of RANK, RANKL, and OPG in keratocystic odontogenic tumors, ameloblastomas, and dentigerous cysts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, 105, 333–341. [DOI] [PubMed] [Google Scholar]
- Day, P.F. , Gregg, T.A. , Ashley, P. , Welbury, R.R. , Cole, B.O. , High, A.S. et al. (2012) Periodontal healing following avulsion and replantation of teeth: a multi‐centre randomized controlled trial to compare two root canal medicaments. Dental Traumatology, 28, 55–64. [DOI] [PubMed] [Google Scholar]
- Diercke, K. , Kohl, A. , Lux, C.J. & Erber, R. (2012) IL‐1β and compressive forces lead to a significant induction of RANKL‐expression in primary human cementoblasts. Journal of Orofacial Orthopedics, 73, 397–412. [DOI] [PubMed] [Google Scholar]
- Durack, C. , Patel, S. , Davies, J. , Wilson, R. & Mannocci, F. (2011) Diagnostic accuracy of small volume cone beam computed tomography and intraoral periapical radiography for the detection of simulated external inflammatory root resorption. International Endodontic Journal, 44, 136–147. [DOI] [PubMed] [Google Scholar]
- Durack, C. & Patel, S. (2016) Root resorption. In: Patel, S. , Harvey, S. , Shemesh, H. & Durack, C. (Eds.) Cone beam computed tomography in endodontics, 1st edition. Berlin, Germany: Quintessence Publishing Co., Ltd, pp. 119–131. [Google Scholar]
- Ee, J. , Fayad, M.I. & Johnson, B.R. (2014) Comparison of endodontic diagnosis and treatment planning decisions using cone‐beam volumetric tomography versus periapical radiography. Journal of Endodontics, 40, 910–916. [DOI] [PubMed] [Google Scholar]
- El Sayed, M. & Saeed, M. (2012) In vitro comparative study of sealing ability of Diadent BioAggregate and other root‐end filling materials. Journal of Conservative Dentistry, 15, 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, S. & Kurol, J. (1987) Radiographic examination of ectopically erupting maxillary canines. American Journal of Orthodontics & Dentofacial Orthopedics, 91, 483–492. [DOI] [PubMed] [Google Scholar]
- Estrela, C. , Bueno, M.R. , De Alencar, A.H.G. , Mattar, R. , Valladares Neto, J. , Azevedo, B.C. et al. (2009) Method to evaluate inflammatory root resorption by using cone beam computed tomography. Journal of Endodontics, 35, 1491–1497. [DOI] [PubMed] [Google Scholar]
- European Society of Endodontology . (2018) European Society of Endodontology position statement: external cervical resorption. International Endodontic Journal, 51, 1323–1326. [DOI] [PubMed] [Google Scholar]
- European Society of Endodontology . (2019) European Society of Endodontology position statement: use of cone beam computed tomography in Endodontics: European Society of Endodontology (ESE) developed by. International Endodontic Journal, 52, 1675–1678. [DOI] [PubMed] [Google Scholar]
- European Society of Endodontology . (2021a) European Society of Endodontology position statement: surgical extrusion, intentional replantation and tooth autotransplantation. International Endodontic Journal, 54, 655–659. [DOI] [PubMed] [Google Scholar]
- European Society of Endodontology . (2021b) European Society of Endodontology position statement: endodontic management of traumatized permanent teeth. International Endodontic Journal, 54, 1473–1481. [DOI] [PubMed] [Google Scholar]
- Fang, X. , Qi, R. & Liu, C. (2019) Root resorption in orthodontic treatment with clear aligners: a systematic review and meta‐analysis. Orthodontics & Craniofacial Research, 22, 259–269. [DOI] [PubMed] [Google Scholar]
- Filippi, A. , Pohl, Y. & Von Arx, T. (2006) Treatment of replacement resorption by intentional replantation, resection of the ankylosed sites, and Emdogain®—results of a 6‐year survey. Dental Traumatology, 22, 307–311. [DOI] [PubMed] [Google Scholar]
- Finucane, D. & Kinirons, M.J. (2003) External inflammatory and replacement resorption of luxated, and avulsed replanted permanent incisors: a review and case presentation. Dental Traumatology, 19, 170–174. [DOI] [PubMed] [Google Scholar]
- Fouad, A.F. , Abbott, P.V. , Tsilingaridis, G. , Cohenca, N. , Lauridsen, E. , Bourguignon, C. et al. (2020) International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dental Traumatology, 36, 331–342. [DOI] [PubMed] [Google Scholar]
- Frank, A.L. (1981) External‐internal progressive resorption and its nonsurgical correction. Journal of Endodontics, 7, 473–476. [DOI] [PubMed] [Google Scholar]
- Frank, A.L. & Torabinejad, M. (1998) Diagnosis and treatment of extracanal invasive resorption. Journal of Endodontics, 24, 500–504. [DOI] [PubMed] [Google Scholar]
- Friedman, S. , Rotstein, I. , Libfeld, H. , Stabholz, A. & Heling, I. (1988) Incidence of external root resorption and esthetic results in 58 bleached pulpless teeth. Endodontics & Dental Traumatology, 4, 23–26. [DOI] [PubMed] [Google Scholar]
- Fuss, Z. , Tsesis, I. & Lin, S. (2003) Root resorption–diagnosis, classification and treatment choices based on stimulation factors. Dental Traumatology, 19, 175–182. [DOI] [PubMed] [Google Scholar]
- Gartner, A.H. , Mack, T. , Somerlott, R.G. & Walsh, L.C. (1976) Differential diagnosis of internal and external root resorption. Journal of Endodontics, 2, 329–334. [DOI] [PubMed] [Google Scholar]
- Gencoglu, N. , Yildirim, T. , Garip, Y. , Karagenc, B. & Yilmaz, H. (2008) Effectiveness of different gutta–percha techniques when filling experimental internal resorptive cavities. International Endodontic Journal, 41, 836–842. [DOI] [PubMed] [Google Scholar]
- Goldberg, F. , Massone, E.J. , Esmoris, M. & Alfie, D. (2000) Comparison of different techniques for obturating experimental internal resorptive cavities. Endodontics & Dental Traumatology, 16, 116–121. [DOI] [PubMed] [Google Scholar]
- Gronthos, S. , Brahim, J. , Li, W. , Fisher, L.W. , Cherman, N. , Boyde, A. et al. (2002) Stem cell properties of human dental pulp stem cells. Journal of Dental Research, 81, 531–535. [DOI] [PubMed] [Google Scholar]
- Gulabivala, K. & Searson, L.J. (1995) Clinical diagnosis of internal resorption: an exception to the rule. International Endodontic Journal, 28, 255–260. [DOI] [PubMed] [Google Scholar]
- Gulsahi, A. (2014) Clinical and radiologic appearances of invasive cervical resorption. Oral Health and Dental Management, 13, 934–939. [Google Scholar]
- Gunst, V. , Huybrechts, B. , De Almeida, N.A. , Bergmans, L. , Van Meerbeek, B. & Lambrechts, P. (2011) Playing wind instruments as a potential aetiologic cofactor in external cervical resorption: two case reports. International Endodontic Journal, 44, 268–282. [DOI] [PubMed] [Google Scholar]
- Gunst, V. , Mavridou, A. , Huybrechts, B. , Van Gorp, G. , Bergmans, L. & Lambrechts, P. (2013) External cervical resorption: an analysis using cone beam and microfocus computed tomography and scanning electron microscopy. International Endodontic Journal, 46, 877–887. [DOI] [PubMed] [Google Scholar]
- Haapasalo, M. & Endal, U. (2006) Internal inflammatory root resorption: the unknown resorption of the tooth. Endodontic Topics, 14, 60–79. [Google Scholar]
- Han, G. , Huang, S. , Von den Hoff, J.W. , Zeng, X. & Kuijpers‐Jagtman, A.M. (2005) Root resorption after orthodontic intrusion and extrusion: an intraindividual study. The Angle Orthodontist, 75, 912–918. [DOI] [PubMed] [Google Scholar]
- Harris, D.A. , Jones, A.S. & Darendeliler, M.A. (2006) Physical properties of root cementum: part 8. Volumetric analysis of root resorption craters after application of controlled intrusive light and heavy orthodontic forces: a microcomputed tomography scan study. American Journal of Orthodontics & Dentofacial Orthopedics, 130, 639–647. [DOI] [PubMed] [Google Scholar]
- Harris, E.F. , Kineret, S.E. & Tolley, E.A. (1997) A heritable component for external apical root resorption in patients treated orthodontically. American Journal of Orthodontics & Dentofacial Orthopedics, 111, 301–309. [DOI] [PubMed] [Google Scholar]
- Heithersay, G.S. (1999a) Invasive cervical resorption: an analysis of potential predisposing factors. Quintessence International, 30, 83–95. [PubMed] [Google Scholar]
- Heithersay, G.S. (1999b) Clinical, radiologic, and histopathologic features of invasive cervical resorption. Quintessence International, 30, 27–37. [PubMed] [Google Scholar]
- Heithersay, G.S. (1999c) Treatment of invasive cervical resorption: an analysis of results using topical application of trichloracetic acid, curettage, and restoration. Quintessence International, 30, 96–110. [PubMed] [Google Scholar]
- Heithersay, G.S. (2004) Invasive cervical resorption. Endodontic Topics, 7, 73–92. [Google Scholar]
- Heithersay, G.S. (2007) Management of tooth resorption. Australian Dental Journal, 52, S105–121. [DOI] [PubMed] [Google Scholar]
- Herren‐Freund, S.L. , Pereira, M.A. , Khoury, M.D. & Olson, G. (1987) The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicology and Applied Pharmacology, 90, 183–189. [DOI] [PubMed] [Google Scholar]
- Hsien, H.C. , Cheng, Y.A. , Lee, Y.L. , Lan, W.H. & Lin, C.P. (2003) Repair of perforating internal resorption with mineral trioxide aggregate: a case report. Journal of Endodontics, 29, 538–539. [DOI] [PubMed] [Google Scholar]
- Huang, G.T.J. , Gronthos, S. & Shi, S. (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of Dental Research, 88, 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irinakis, E. (2018) External cervical root resorption: determinants and treatment outcomes. British Columbia: University of British Columbia. [Google Scholar]
- Irinakis, E. , Aleksejuniene, J. , Shen, Y. & Haapasalo, M. (2020) External cervical resorption: a retrospective case‐control study. Journal of Endodontics, 46, 1420–1427. [DOI] [PubMed] [Google Scholar]
- Isaacson, R.J. , Strauss, R.A. , Bridges‐Poquis, A. , Peluso, A.R. & Lindauer, S.J. (2001) Moving an ankylosed central incisor using orthodontics, surgery and distraction osteogenesis. The Angle Orthodontist, 71, 411–418. [DOI] [PubMed] [Google Scholar]
- Jacobovitz, M. & de Lima, R.K. (2008) Treatment of inflammatory internal root resorption with mineral trioxide aggregate: a case report. International Endodontic Journal, 41, 905–912. [DOI] [PubMed] [Google Scholar]
- Kahler, B. & Rossi‐Fedele, G. (2016) A review of tooth discoloration after regenerative endodontic therapy. Journal of Endodontics, 42, 563–569. [DOI] [PubMed] [Google Scholar]
- Kaley, J. & Phillips, C. (1991) Factors related to root resorption in edgewise practice. The Angle Orthodontist, 61, 125–132. [DOI] [PubMed] [Google Scholar]
- Kamburoğlu, K. , Kolsuz, E. , Kurt, H. , Kiliç, C. , Özen, T. & Paksoy, C.S. (2011) Accuracy of CBCT measurements of a human skull. Journal of Digital Imaging, 24, 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaval, M.E. , Güneri, P. & Çalışkan, M.K. (2018) Regenerative endodontic treatment of perforated internal root resorption: a case report. International Endodontic Journal, 51, 128–137. [DOI] [PubMed] [Google Scholar]
- Kikuta, J. , Yamaguchi, M. , Shimizu, M. , Yoshino, T. & Kasai, K. (2015) Notch signaling induces root resorption via RANKL and IL‐6 from hPDL cells. Journal of Dental Research, 94, 140–147. [DOI] [PubMed] [Google Scholar]
- Killiany, D.M. (1999) Root resorption caused by orthodontic treatment: an evidence‐based review of literature. Seminars in Orthodontics, 5, 128–133. [DOI] [PubMed] [Google Scholar]
- Kjaer, I. (1995) Morphological characteristics of dentitions developing excessive root resorption during orthodontic treatment. European Journal of Orthodontics, 17, 25–34. [DOI] [PubMed] [Google Scholar]
- Krastl, G. , Weiger, R. , Filippi, A. , Van Waes, H. , Ebeleseder, K. , Ree, M. et al. (2021) Endodontic management of traumatized permanent teeth: a comprehensive review. International Endodontic Journal, 54, 1221–1245. [DOI] [PubMed] [Google Scholar]
- Krug, R. , Soliman, S. & Krastl, G. (2019) Intentional replantation with an atraumatic extraction system in teeth with extensive cervical resorption. Journal of Endodontics, 45, 1390–1396. [DOI] [PubMed] [Google Scholar]
- Laux, M. , Abbott, P.V. , Pajarola, G. & Nair, P.N. (2000) Apical inflammatory root resorption: a correlative radiographic and histological assessment. International Endodontic Journal, 33, 483–493. [DOI] [PubMed] [Google Scholar]
- Levander, E. & Malmgren, O. (1988) Evaluation of the risk of root resorption during orthodontic treatment: a study of upper incisors. European Journal of Orthodontics, 10, 30–38. [DOI] [PubMed] [Google Scholar]
- Liang, H. , Burkes, E.J. & Frederiksen, N.L. (2003) Multiple idiopathic cervical root resorption: systematic review and report of four cases. Dentomaxillofacial Radiology, 32, 150–155. [DOI] [PubMed] [Google Scholar]
- Lima, T.F. , Gamba, T.O. , Zaia, A.A. & Soares, A.J. (2016) Evaluation of cone beam computed tomography and periapical radiography in the diagnosis of root resorption. Australian Dental Journal, 61, 425–431. [DOI] [PubMed] [Google Scholar]
- Linge, B.O. & Linge, L. (1983) Apical root resorption in upper anterior teeth. European Journal of Orthodontics, 5, 173–183. [DOI] [PubMed] [Google Scholar]
- Lyroudia, K.M. , Dourou, V.I. , Pantelidou, O.C. , Labrianidis, T. & Pitas, I.K. (2002) Internal root resorption studied by radiography, stereomicroscope, scanning electron microscope and computerized 3D reconstructive method. Dental Traumatology, 18, 148–152. [DOI] [PubMed] [Google Scholar]
- Madani, Z. , Moudi, E. , Bijani, A. & Mahmoudi, E. (2016) Diagnostic accuracy of cone‐beam computed tomography and periapical radiography in internal root resorption. Iranian Endodontic Journal, 11, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main, C. , Mirzayan, N. , Shabahang, S. & Torabinejad, M. (2004) Repair of root perforations using mineral trioxide aggregate: a long‐term study. Journal of Endodontics, 30, 80–83. [DOI] [PubMed] [Google Scholar]
- Malmgren, B. (2013) Ridge preservation/decoronation. Journal of Endodontics, 39, S67–S72. [DOI] [PubMed] [Google Scholar]
- Malmgren, B. , Malmgren, O. & Andreasen, J.O. (2006) Alveolar bone development after decoronation of ankylosed teeth. Endodontic Topics, 14, 35–40. [Google Scholar]
- Malmgren, O. , Goldson, L. , Hill, C. , Orwin, A. , Petrini, L. & Lundberg, M. (1982) Root resorption after orthodontic treatment of traumatized teeth. American Journal of Orthodontics, 82, 487–491. [DOI] [PubMed] [Google Scholar]
- Marmulla, R. , Wörtche, R. , Mühling, J. & Hassfeld, S. (2005) Geometric accuracy of the NewTom 9000 Cone Beam CT. Dentomaxillofacial Radiology, 34, 28–31. [DOI] [PubMed] [Google Scholar]
- Martins, G.G. , Oliveira, I.A. & Consolaro, A. (2019) The mechanism: how dental resorptions occur in ameloblastoma. Dental Press Journal of Orthodontics, 24, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzen, L.H. , Schropp, L. , Spin‐Neto, R. & Wenzel, A. (2017) Radiographic signs of pathology determining removal of an impacted mandibular third molar assessed in a panoramic image or CBCT. Dentomaxillofacial Radiology, 46, 20160330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavridou, A.M. , Bergmans, L. , Barendregt, D. & Lambrechts, P. (2017) Descriptive analysis of factors associated with external cervical resorption. Journal of Endodontics, 43, 1602–1610. [DOI] [PubMed] [Google Scholar]
- Mavridou, A.M. , Hauben, E. , Wevers, M. , Schepers, E. , Bergmans, L. & Lambrechts, P. (2016) Understanding external cervical resorption in vital teeth. Journal of Endodontics, 42, 1737–1751. [DOI] [PubMed] [Google Scholar]
- Mavridou, A.‐M. , Rubbers, E. , Schryvers, A. , Maes, A. , Linssen, M. , Barendregt, D.S. et al. (2022) A clinical approach strategy for the diagnosis, treatment and evaluation of external cervical resorption. International Endodontic Journal. Available from: 10.1111/iej.13680 [DOI] [PubMed] [Google Scholar]
- McNab, S. , Battistutta, D. , Taverne, A. & Symons, A.L. (1999) External apical root resorption of posterior teeth in asthmatics after orthodontic treatment. American Journal of Orthodontics & Dentofacial Orthopedics, 116, 545–551. [DOI] [PubMed] [Google Scholar]
- Mehta, S.A. , Deshmukh, S.V. , Sable, R.B. & Patil, A.S. (2017) Comparison of 4 and 6 weeks of rest period for repair of root resorption. Progress in Orthodontics, 18, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi, Z. & Dummer, P.M.H. (2011) Properties and applications of calcium hydroxide in endodontics and dental traumatology. International Endodontic Journal, 44, 697–730. [DOI] [PubMed] [Google Scholar]
- Moze, G. , Seehra, J. , Fanshawe, T. , Davies, J. , McDonald, F. & Bister, D. (2013) In vitro comparison of contemporary radiographic imaging techniques for measurement of tooth length: reliability and radiation dose. Journal of Orthodontics, 40, 225–233. [DOI] [PubMed] [Google Scholar]
- Narita, H. , Itoh, S. , Imazato, S. , Yoshitake, F. & Ebisu, S. (2010) An explanation of the mineralization mechanism in osteoblasts induced by calcium hydroxide. Acta Biomaterialia, 6, 586–590. [DOI] [PubMed] [Google Scholar]
- Nemcovsky, C.E. , Libfeld, H. & Zubery, Y. (1996) Effect of non‐erupted 3rd molars on distal roots and supporting structures of approximal teeth. A radiographic survey of 202 cases. Journal of Clinical Periodontology, 23, 810–815. [DOI] [PubMed] [Google Scholar]
- Odenrick, L. & Brattström, V. (1985) Nailbiting: frequency and association with root resorption during orthodontic treatment. British Journal of Orthodontics, 12, 78–81. [DOI] [PubMed] [Google Scholar]
- Patel, K. , Foschi, F. , Pop, I. , Patel, S. & Mannocci, F. (2016b) The use of intentional replantation to repair an external cervical resorptive lesion not amenable to conventional surgical repair. Primary Dental Journal, 5, 78–83. [DOI] [PubMed] [Google Scholar]
- Patel, K. , Mannocci, F. & Patel, S. (2016a) The assessment and management of external cervical resorption with periapical radiographs and cone‐beam computed tomography: a clinical study. Journal of Endodontics, 42, 1435–1440. [DOI] [PubMed] [Google Scholar]
- Patel, S. , Dawood, A. , Wilson, R. , Horner, K. & Mannocci, F. (2009b) The detection and management of root resorption lesions using intraoral radiography and cone beam computed tomography—an in vivo investigation. International Endodontic Journal, 42, 831–838. [DOI] [PubMed] [Google Scholar]
- Patel, S. , Durack, C. , Ricucci, D. & Bakhsh, A. (2020) Root resorption. In: Hargreaves, K.M. & Berman, L.H. (Eds.) Cohen's pathways of the pulp, 12th edition. St. Louis, MO: Elsevier, pp. 711–736. [Google Scholar]
- Patel, S. , Foschi, F. , Condon, R. , Pimentel, T. & Bhuva, B. (2018b) External cervical resorption: part 2 – management. International Endodontic Journal, 51, 1224–1238. [DOI] [PubMed] [Google Scholar]
- Patel, S. , Foschi, F. , Mannocci, F. & Patel, K. (2018c) External cervical resorption: a three‐dimensional classification. International Endodontic Journal, 51, 206–214. [DOI] [PubMed] [Google Scholar]
- Patel, S. , Kanagasingam, S. & Pitt Ford, T. (2009a) External cervical resorption: a review. Journal of Endodontics, 35, 616–625. [DOI] [PubMed] [Google Scholar]
- Patel, S. , Mavridou, A.M. , Lambrechts, P. & Saberi, N. (2018a) External cervical resorption‐part 1: histopathology, distribution and presentation. International Endodontic Journal, 51, 1205–1223. [DOI] [PubMed] [Google Scholar]
- Patel, S. , Ricucci, D. , Durak, C. & Tay, F. (2010) Internal root resorption: a review. Journal of Endodontics, 36, 1107–1121. [DOI] [PubMed] [Google Scholar]
- Patel, S. & Saberi, N. (2015) External cervical resorption associated with the use of bisphosphonates: a case series. Journal of Endodontics, 41, 742–748. [DOI] [PubMed] [Google Scholar]
- Patel, S. & Saberi, N. (2018) The ins and outs of root resorption. British Dental Journal, 224, 691–699. [DOI] [PubMed] [Google Scholar]
- Penido, R.S. , Carrel, R. & Chialastri, A.J. (1980) The anachoretic effect in root resorption: report of case. The Journal of Pedodontics, 5, 85–88. [PubMed] [Google Scholar]
- Pierce, A.M. (1989) Experimental basis for the management of dental resorption. Endodontics & Dental Traumatology, 5, 255–265. [DOI] [PubMed] [Google Scholar]
- Pierce, A. , Berg, J.O. & Lindskog, S. (1988) Calcitonin as an alternative therapy in the treatment of root resorption. Journal of Endodontics, 14, 459–464. [DOI] [PubMed] [Google Scholar]
- Pierce, A. & Lindskog, S. (1987) The effect of an antibiotic/corticosteroid paste on inflammatory root resorption in vivo. Oral Surgery, Oral Medicine, Oral Pathology, 64, 216–220. [DOI] [PubMed] [Google Scholar]
- Rabinowitch, B.Z. (1972) Internal resorption. Oral Surgery, Oral Medicine, Oral Pathology, 33, 263–282. [DOI] [PubMed] [Google Scholar]
- Rafflenbeul, F. , Gros, C.I. , Lefebvre, F. , Bahi‐Gross, S. , Maizeray, R. & Bolender, Y. (2019) Prevalence and risk factors of root resorption of adjacent teeth in maxillary canine impaction, among untreated children and adolescents. European Journal of Orthodontics, 41, 447–453. [DOI] [PubMed] [Google Scholar]
- Regan, J.D. , Gutmann, J.L. & Witherspoon, D.E. (2002) Comparison of Diaket and MTA when used as root‐end filling materials to support regeneration of the periradicular tissues. International Endodontic Journal, 35, 840–847. [DOI] [PubMed] [Google Scholar]
- Rodríguez, G. , Abella, F. , Durán‐Sindreu, F. , Patel, S. & Roig, M. (2017) Influence of cone‐beam computed tomography in clinical decision making among specialists. Journal of Endodontics, 43, 194–199. [DOI] [PubMed] [Google Scholar]
- Roeykens, H. , Van Maele, G. , Martens, L. & De Moor, R. (2002) A two‐probe laser Doppler flowmetry assessment as an exclusive diagnostic device in a long‐term follow‐up of traumatised teeth: a case report. Dental Traumatology, 18, 86–91. [DOI] [PubMed] [Google Scholar]
- Roscoe, M.G. , Meira, J.B. & Cattaneo, P.M. (2015) Association of orthodontic force system and root resorption: a systematic review. American Journal of Orthodontics & Dentofacial Orthopedics, 147, 610–626. [DOI] [PubMed] [Google Scholar]
- Rudolph, D.J. & White, S.C. (1988) Film‐holding instruments for intraoral subtraction radiography. Oral Surgery, Oral Medicine, Oral Pathology, 65, 767–772. [DOI] [PubMed] [Google Scholar]
- Saidon, J. , He, J. , Zhu, Q. , Safavi, K. & Spångberg, L.S. (2003) Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, 95, 483–489. [DOI] [PubMed] [Google Scholar]
- Sameshima, G.T. & Sinclair, P.M. (2001) Predicting and preventing root resorption: part I. Diagnostic factors. American Journal of Orthodontics & Dentofacial Orthopedics, 119, 505–510. [DOI] [PubMed] [Google Scholar]
- Saoud, T.M. , Mistry, S. , Kahler, B. , Sigurdsson, A. & Lin, L.M. (2016) Regenerative endodontic procedures for traumatized teeth after horizontal root fracture, avulsion, and perforating root resorption. Journal of Endodontics, 42, 1476–1482. [DOI] [PubMed] [Google Scholar]
- Schaffner, P. & Dard, M. (2003) Structure and function of RGD peptides involved in bone biology. Cellular and Molecular Life Sciences, 60, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjøtt, M. & Andreasen, J.O. (2005) Emdogain does not prevent progressive root resorption after replantation of avulsed teeth: a clinical study. Dental Traumatology, 21, 46–50. [DOI] [PubMed] [Google Scholar]
- Schnettler, J.M. & Wallace, J.A. (1991) Pulse oximetry as a diagnostic tool of pulpal vitality. Journal of Endodontics, 17, 488–490. [DOI] [PubMed] [Google Scholar]
- Shahi, S. , Yavari, H.R. , Rahimi, S. , Eskandarinezhad, M. , Shakouei, S. & Unchi, M. (2011) Comparison of the sealing ability of mineral trioxide aggregate and Portland cement used as root‐end filling materials. Journal of Oral Science, 53, 517–522. [DOI] [PubMed] [Google Scholar]
- Shirazi, M. , Dehpour, A.R. & Jafari, F. (1999) The effect of thyroid hormone on orthodontic tooth movement in rats. Journal of Clinical Pediatric Dentistry, 23, 259–264. [PubMed] [Google Scholar]
- Silveira, F.F. , Nunes, E. , Soares, J.A. , Ferreira, C.L. & Rotstein, I. (2009) Double ‘pink tooth’ associated with extensive internal root resorption after orthodontic treatment: a case report. Dental Traumatology, 25, e43–e47. [DOI] [PubMed] [Google Scholar]
- Soares, A.J. , Souza, G.A. , Pereira, A.C. , Vargas‐Neto, J. , Zaia, A.A. & Silva, E.J. (2015) Frequency of root resorption following trauma to permanent teeth. Journal of Oral Science, 57, 73–78. [DOI] [PubMed] [Google Scholar]
- Solomon, C.S. , Coffiner, M.O. & Chalfin, H.E. (1986) Herpes zoster revisited: implicated in root resorption. Journal of Endodontics, 12, 210–213. [DOI] [PubMed] [Google Scholar]
- Sondeijker, C.F.W. , Lamberts, A.A. , Beckmann, S.H. , Kuitert, R.B. , van Westing, K. , Persoon, S. et al. (2020) Development of a clinical practice guideline for orthodontically induced external apical root resorption. European Journal of Orthodontics, 42, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönmez, D. & Durutürk, L. (2008) Ca(OH)2 pulpotomy in primary teeth. Part I: internal resorption as a complication following pulpotomy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, 106, e94–e98. [DOI] [PubMed] [Google Scholar]
- Souza, B.D.M. , Dutra, K.L. , Kuntze, M.M. , Bortoluzzi, E.A. , Flores‐Mir, C. , Reyes‐Carmona, J. et al. (2018) Incidence of root resorption after the replantation of avulsed teeth: a meta‐analysis. Journal of Endodontics, 44, 1216–1227. [DOI] [PubMed] [Google Scholar]
- Struthers, P. & Shear, M. (1976) Root resorption by ameloblastomas and cysts of the jaws. International Journal of Oral Surgery, 5, 128–132. [DOI] [PubMed] [Google Scholar]
- Takahashi, T. , Takagi, T. & Moriyama, K. (2005) Orthodontic treatment of a traumatically intruded tooth with ankylosis by traction after surgical luxation. American Journal of Orthodontics & Dentofacial Orthopedics, 127, 233–241. [DOI] [PubMed] [Google Scholar]
- Teo, K.W. , Shi, A.H. , Teh, L.Y. & Lee, A.M.H. (2021) External root resorption in common odontogenic cysts and ameloblastomas of the jaw: a retrospective radiographic study in an Asian population. Oral Surgery, 14, 335–341. [Google Scholar]
- Theodorou, C.I. , Kuijpers‐Jagtman, A.M. , Bronkhorst, E.M. & Wagener, F. (2019) Optimal force magnitude for bodily orthodontic tooth movement with fixed appliances: a systematic review. American Journal of Orthodontics & Dentofacial Orthopedics, 156, 582–592. [DOI] [PubMed] [Google Scholar]
- Thoma, K.H. (1935) Central osteoclastic resorption of dentine and complete repair with osteo‐dentine in the permanent tooth of an adult. Dental Items of Interest, 57, 28. [Google Scholar]
- Torabinejad, M. , Dinsbach, N.A. , Turman, M. , Handysides, R. , Bahjri, K. & White, S.N. (2015) Survival of intentionally replanted teeth and implant‐supported single crowns: a systematic review. Journal of Endodontics, 41, 992–998. [DOI] [PubMed] [Google Scholar]
- Tronstad, L. (1988) Root resorption—etiology, terminology and clinical manifestations. Endodontics & Dental Traumatology, 4, 241–252. [DOI] [PubMed] [Google Scholar]
- Trope, M. (1998) Root resorption of dental and traumatic origin: classification based on etiology. Practical Periodontics and Aesthetic Dentistry, 10, 515–522. [PubMed] [Google Scholar]
- Trope, M. (2002) Root resorption due to dental trauma. Endodontic Topics, 1, 79–100. [Google Scholar]
- Tsukiboshi, M. , Yamauchi, N. & Tsukiboshi, Y. (2019) Long‐term outcomes of autotransplantation of teeth: a case series. Dental Traumatology, 35, 358–367. [DOI] [PubMed] [Google Scholar]
- Türkün, M. & Cengiz, T. (1997) The effects of sodium hypochlorite and calcium hydroxide on tissue dissolution and root canal cleanliness. International Endodontic Journal, 30, 335–342. [DOI] [PubMed] [Google Scholar]
- Tyrovola, J.B. , Spyropoulos, M.N. , Makou, M. & Perrea, D. (2008) Root resorption and the OPG/RANKL/RANK system: a mini review. Journal of Oral Science, 50, 367–376. [DOI] [PubMed] [Google Scholar]
- Tzanetakis, G.N. (2018) Management of intruded immature maxillary central incisor with pulp necrosis and severe external resorption by regenerative approach. Journal of Endodontics, 44, 245–249. [DOI] [PubMed] [Google Scholar]
- Uchiyama, M. , Nakamichi, Y. , Nakamura, M. , Kinugawa, S. , Yamada, H. , Udagawa, N. et al. (2009) Dental pulp and periodontal ligament cells support osteoclastic differentiation. Journal of Dental Research, 88, 609–614. [DOI] [PubMed] [Google Scholar]
- Varshney, M. , Chandra, A. , Chauhan, L.K. & Goel, S.K. (2014) In vitro cytogenetic assessment of trichloroacetic acid in human peripheral blood lymphocytes. Environmental Science and Pollution Research, 21, 843–850. [DOI] [PubMed] [Google Scholar]
- Vaz de Souza, D. , Schirru, E. , Mannocci, F. , Foschi, F. & Patel, S. (2017) External cervical resorption: a comparison of the diagnostic efficacy using 2 different cone‐beam computed tomographic units and periapical radiographs. Journal of Endodontics, 43, 121–125. [DOI] [PubMed] [Google Scholar]
- Vier, F.V. & Figueiredo, J.A. (2002) Prevalence of different periapical lesions associated with human teeth and their correlation with the presence and extension of apical external root resorption. International Endodontic Journal, 35, 710–719. [DOI] [PubMed] [Google Scholar]
- Walker, L. , Enciso, R. & Mah, J. (2005) Three‐dimensional localization of maxillary canines with cone‐beam computed tomography. American Journal of Orthodontics & Dentofacial Orthopedics, 128, 418–423. [DOI] [PubMed] [Google Scholar]
- Walton, R.E. & Leonard, L.A. (1986) Cracked tooth: an etiology for "idiopathic" internal resorption? Journal of Endodontics, 12, 167–169. [DOI] [PubMed] [Google Scholar]
- Wedenberg, C. (1987) Evidence for a dentin‐derived inhibitor of macrophage spreading. Scandinavian Journal of Dental Research, 95, 381–388. [DOI] [PubMed] [Google Scholar]
- Wedenberg, C. & Lindskog, S. (1985) Experimental internal resorption in monkey teeth. Endodontics & Dental Traumatology, 1, 221–227. [DOI] [PubMed] [Google Scholar]
- Wedenberg, C. & Lindskog, S. (1987) Evidence for a resorption inhibitor in dentin. Scandinavian Journal of Dental Research, 95, 205–211. [DOI] [PubMed] [Google Scholar]
- Wedenberg, C. & Zetterqvist, L. (1987) Internal resorption in human teeth—a histological, scanning electron microscopic, and enzyme histochemical study. Journal of Endodontics, 13, 255–259. [DOI] [PubMed] [Google Scholar]
- Weltman, B. , Vig, K.W. , Fields, H.W. , Shanker, S. & Kaizar, E.E. (2010) Root resorption associated with orthodontic tooth movement: a systematic review. American Journal of Orthodontics & Dentofacial Orthopedics, 137, 462–476; discussion 412A. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M. , Aihara, N. , Kojima, T. & Kasai, K. (2006) RANKL increase in compressed periodontal ligament cells from root resorption. Journal of Dental Research, 85, 751–756. [DOI] [PubMed] [Google Scholar]
- Yamaoka, M. , Furusawa, K. , Ikeda, M. & Hasegawa, T. (1999) Root resorption of mandibular second molar teeth associated with the presence of the third molars. Australian Dental Journal, 44, 112–116. [DOI] [PubMed] [Google Scholar]
- Yang, X. , van der Kraan, P.M. , Bian, Z. , Fan, M. , Walboomers, X.F. & Jansen, J.A. (2009) Mineralized tissue formation by BMP2‐transfected pulp stem cells. Journal of Dental Research, 88, 1020–1025. [DOI] [PubMed] [Google Scholar]
- Yassir, Y.A. , McIntyre, G.T. & Bearn, D.R. (2021) Orthodontic treatment and root resorption: an overview of systematic reviews. European Journal of Orthodontics, 43, 442–456. [DOI] [PubMed] [Google Scholar]
- Yoshpe, M. , Einy, S. , Ruparel, N. , Lin, S. & Kaufman, A.Y. (2020) Regenerative endodontics: a potential solution for external root resorption (case series). Journal of Endodontics, 46, 192–199. [DOI] [PubMed] [Google Scholar]
- Zanini, M. , Sautier, J.M. , Berdal, A. & Simon, S. (2012) Biodentine induces immortalized murine pulp cell differentiation into odontoblast‐like cells and stimulates biomineralization. Journal of Endodontics, 38, 1220–1226. [DOI] [PubMed] [Google Scholar]
- Zhou, H.M. , Shen, Y. , Wang, Z.J. et al. (2013) In vitro cytotoxicity evaluation of a novel root repair material. Journal of Endodontics, 39, 478–483. [DOI] [PubMed] [Google Scholar]


