Abstract
Background
Long‐term functional limitations are common after hip fractures. Exercise may alleviate these negative consequences but there is no consensus on an optimal training program. The objective was to study the effects of a 12‐month home‐based supervised, progressive exercise program on functioning, physical performance, and physical activity.
Methods
Secondary analysis of a randomized controlled trial targeting patients with surgical repair of a hip fracture, aged ≥60 years, Mini‐Mental State Examination (MMSE) score of ≥12. The participants were randomized into Exercise (n = 61) or Usual care (n = 60). Assessments at baseline, 3, 6, and 12 months included Lawton's Instrumental Activities of Daily Living (IADL), Short Physical Performance Battery (SPPB), handgrip strength, and self‐reported frequency of sessions of leisure‐time physical activity. Analyzed using mixed‐effects models.
Results
Participants' (n = 121) mean age was 81 years (SD 7), and 75% were women. The mean IADL score at baseline was 17.1 (SD 4.5) in the exercise group, and 17.4 (5.1) in the usual care group. The mean SPPB scores were 3.9 (1.6) and 4.2 (1.8), and handgrip strength was 17.7 (8.9) kg and 20.8 (8.0) kg, respectively. The age‐ and sex‐adjusted mean changes in IADL over 12 months were 3.7 (95% CI 2.8–4.7) in the exercise and 2.0 (1.0–3.0) in the usual care group (between‐group difference, p = 0.016); changes in SPPB 4.3 (3.6–4.9) and 2.1 (1.5–2.7) (p < 0.001); and changes in handgrip strength 1.2 kg (0.3–2.0) and 1.0 kg (−1.9 to −0.2) (p < 0.001), respectively. We found no between‐group differences in changes in the frequency of leisure‐time activity sessions.
Conclusion
A 12‐month home‐based supervised, progressive exercise program improved functioning and physical performance more than usual care among patients with hip fractures. However, the training did not increase leisure‐time physical activity.
Keywords: functioning, hip fracture, home‐based exercise, physical performance
Key points
A 12‐month home‐based supervised, progressive exercise program was more effective than usual care in improving functioning and physical performance after a hip fracture.
Why does this paper matter?
Providing 12‐month, home‐based progressive and supervised rehabilitation to patients after surgical repair of a hip fracture will help to improve functioning and to reduce dependence, both common consequences of hip fractures.
INTRODUCTION
Hip fracture is a major health problem among older people. 1 It often results in long‐term, sometimes persistent, functional impairments such as poor mobility and reduced independence in daily activities. 1 , 2 , 3 , 4 , 5 , 6 Sedentary behavior and low level of physical activity are also common among patients recovering from surgical repair of a hip fracture. 7 , 8 , 9
The usual care offered after discharge does not seem to meet the requirements of effective rehabilitation, 10 , 11 as many patients with hip fractures do not reach their pre‐fracture level of functioning. 12 , 13 There is growing evidence that multidisciplinary and well‐coordinated rehabilitation initiated at the hospital and continued after discharge enhances the recovery of patients with hip fractures. 14 , 15 Multicomponent rehabilitation in particular, which includes individualized and progressive resistance training, has improved functioning and mobility 11 , 16 , 17 , 18 , 19 and decreased dependency in activities of daily living, 11 , 18 , 20 in both outpatient and home settings. 15 , 21 Furthermore, exercise programs lasting from 6 to 12 months have reduced or reversed incident disability after hip fractures. 1 , 16 , 19 , 20 , 22 Even though the relatively low frequency of supervision has improved functioning in longer exercise interventions, 16 , 22 , 23 the evidence shows that the impact on functioning is associated with the amount of supervision received. 24 Therefore, more research is needed to define the aspects of an optimal post‐discharge exercise program.
The aim of these secondary analyses of our randomized controlled trial was to investigate the effects of a 12‐month home‐based physiotherapist‐supervised, progressive exercise program on the functioning, physical performance, and leisure‐time physical activities of home‐dwelling older adults recovering from surgical repair of a hip fracture, and to compare these with the effects of usual care.
METHODS
Design and settings
This study was a parallel‐group randomized controlled trial with a 1:1 allocation ratio to the Exercise and Usual care arms. The trial was conducted in South Karelia Social and Health Care District (131,000 inhabitants) in southern Finland between December 9, 2014 and December 31, 2019. Participation was voluntary and all participants signed informed consent. We conducted our trial in accordance with the Declaration of Helsinki and received approval from the regional ethics committee on November 12, 2014. We registered the trial with ClinicalTrials.gov (NCT02305433) on December 4, 2014.
The protocol of our trial 25 and the results of our main outcome, days lived at home over 24 months, and our secondary outcomes, the use and costs of social and health care services, and mortality over 24 months, 26 have been reported earlier. We found no differences between the groups' days lived at home and mortality, and the intervention was cost neutral. 26
Participants
The first evaluation of hip fracture patients' eligibility used the patient records after surgery. We contacted potentially suitable patients for the first time after they had been transferred from the operating hospital to either the adjacent rehabilitation hospital or home. If they were willing, a home visit after discharge was arranged to assess eligibility. Acceptance for the study was decided during this visit.
Study inclusion criteria were (1) femoral neck (ICD code S72.0), pertrochanteric (S72.1) or subtrochanteric (S72.2) fracture, (2) ≥60 years of age, (3) Mini‐Mental State Examination (MMSE) 27 score of ≥12 points, (4) home‐dwelling, (5) ability to walk indoors (walking aid allowed), (6) ability to communicate in Finnish, and (7) no contraindications to physical exercise (e.g., severe cardiovascular disease with NYHA class of >II, or severe neurological disease). In April 2015, we modified the original inclusion criteria of age (≥65 years), and MMSE score (≥17) to intensify the recruitment of the participants, with no marked impact. Exclusion criteria were: (1) life expectancy of less than 2 years, (2) living in a 24‐hour nursing facility, (3) alcohol or drug abuse, or (4) severe problems with hearing or eyesight. For a detailed description of the recruitment and randomization process, see our previous article. 25
Outcomes
In this article, we report our secondary outcomes of functioning, physical performance, and physical activity. A trained research physiotherapist or a research nurse performed the assessments and measurements at the participant's home at baseline and at 3, 6, and 12 months. The assessors were not blinded to group allocation.
The person's functioning, such as doing laundry, grocery shopping, handling financial affairs, or using public transport was evaluated using Lawton's IADL (Instrumental Activities of Daily Living) scale. 28 We used the polytomous item scoring of 1–3, 1–4 or 1–5, resulting in a sum between 8 and 31, with a higher score indicating better ability. 29
Physical performance was assessed using a Short Physical Performance Battery (SPPB) and handgrip strength. SPPB has three components: standing balance, four‐meter habitual walking speed, and chair rise test. All components yield scores of 0–4, and the maximum summary score is 12. 30 If a four‐meter walking distance was not possible at the person's home, a 2.44‐m distance was used. To assess handgrip strength, we used the Saehan dynamometer (model Sh5001, South Korea). During the measurement, the participant was in a seated position with no arm support, with his/her upper arm next to the body, elbow in 90‐degree flexion, and the wrist in a neutral position. 31 We used the mean of the best values of three tries of both hands in the analyses to eliminate possible joint conditions in one hand, which could hinder maximal performance.
The frequency of weekly sessions of leisure‐time physical activity was queried using two slightly modified questions from the “Health Behaviour and Health among the Finnish Elderly” survey. 32 The questions were: (1) How many times per week have you walked outdoors for at least half an hour during the previous month, and (2) How many times per week have you pursued other physical activities for at least half an hour during the previous month? Both questions have seven response options from “daily” to “unable to walk/pursue physical activities due to illness or disability.” The participants assigned to the exercise group were instructed to leave out the intervention sessions from their responses.
Information on medication and physician‐diagnosed illnesses was queried and verified via medical records. Details of the surgical repair of a hip fracture and hospital stay were drawn from medical records. The Pain was assessed using the 100‐mm Visual Analogue Scale (VAS). 33 We gathered the information on the exercise sessions of the intervention from the physiotherapists' monthly reports.
Study groups
Exercise intervention
The detailed content of our 12‐month home‐based physiotherapist‐supervised, progressive exercise program has been described in Supplement 1 and by Soukkio et al. 25 Briefly, the participants assigned to the exercise group received both usual care according to the local guidelines and the exercise intervention. The intervention started on average within 2 weeks of discharge. All exercise sessions lasted 60 min and were held twice a week at the participant's home. The participants had the same physiotherapist during the 12‐month intervention. These physiotherapists had a minimum of 2 years of work experience, specifically with older people. They were trained by our research group to deliver a structured, and progressive exercise program, which included strength, balance, mobility, and functional components as well as brief counseling on physical activity and nutrition. The goal was to enhance participants' physical performance and overall functioning. Our exercises were mainly based on the Otago training program. 34 The physiotherapists modified the exercises to suit the participants' health and fitness status. To ensure progression and to define suitable resistance for training, the physiotherapists were instructed to periodically perform a multiple‐repetition maximum ‐test with ankle weights (0.5–10 kg). The targeted training intensity was based on subjective perceptions, ratings set at 12 to 17 RPE (Ratings of Perceived Exertion; range 6–20). 35 The mean cost of one exercise session was USD 98. The trial funders covered the costs of the intervention.
Usual care
The participants assigned to the usual care group received no intervention as part of the trial. In accordance with local guidelines, their need for home‐based rehabilitation was evaluated at the time of discharge, and if a need was found, the participants received brief guidance from home‐care personnel (practical nurses or physiotherapists) to get started. They were also instructed to perform home exercises on their own according to their exercise plan.
Participants in both groups were allowed to use any health care or social services (including rehabilitation) prescribed by their healthcare providers over the 24‐month study period.
Statistical analysis
Power calculations were made for our main outcome, days lived at home over 24 months. These calculations were based on the analysis of the national PERFECT study, from which data on the proportion of patients living at home 1 year after surgical repair of a hip fracture were available. 36 A sample size of 91 people per research arm was needed to detect the hypothesized 180‐day (SD 431) difference between the arms (α = 0.05, power = 80%). We estimated the discontinuation of the participants to be 15% over 12 months, and mortality to be 20% over 24 months, and therefore, the target was 300 participants. A computer‐generated, random sequence allocation program with randomly varying block sizes of two to ten, without stratification, was used. The program was constructed by a statistician at the university, who had no other role in the trial. Randomization was carried out by the project manager, who played no role in the implementation of the intervention.
All analyses were performed in accordance with the intention‐to‐treat principle. The characteristics of the participants were presented as means with standard deviations (SDs), medians with interquartile ranges (IQR), or counts with percentages. Repeated measures at different time points (0, 3, 6, and 12 months) were analyzed using mixed‐effects models, with an unstructured covariance structure (Kenward‐Roger method to calculate the degrees of freedom). The fixed effects were group, time, and group‐time interactions, using age and sex as covariates. Mixed models enabled analyses of unbalanced datasets without imputation; therefore, all available data were analyzed with the full analysis set. In case of violation of assumptions (non‐normality), a bootstrap‐type or a permutation test was used. Normal distributions were evaluated graphically and using the Shapiro–Wilk W test. Statistical package Stata 17.0 (StataCorp LP, College Station, TX, USA) was used for analyses.
RESULTS
During the recruitment period 541 patients with hip fractures underwent surgical repair, 338 people (living at home) were contacted, 144 underwent an eligibility assessment at home, and 121 were recruited for the trial and randomized into Exercise (n = 61) or Usual care (n = 60) (Figure 1). At baseline, the mean age of participants was 81 years (SD 7), 75% were women (Table 1), and all were Caucasian. The mean MMSE score at baseline was 22.9 (SD 4.5). Sixty‐one percent had femoral neck fractures. The median time from fracture to baseline assessments was 33 days (IQR 28, 50) in the exercise group and 37 days in the usual care group (Table 1). Over 12 months, the participants in the exercise group received 292 (95% CI 288–296) health care and social service visits (home care, outpatient primary care, and specialized care visits) per person‐year and the usual care group 279 (95% CI 275–283) visits (between‐group difference p = 0.86).
FIGURE 1.
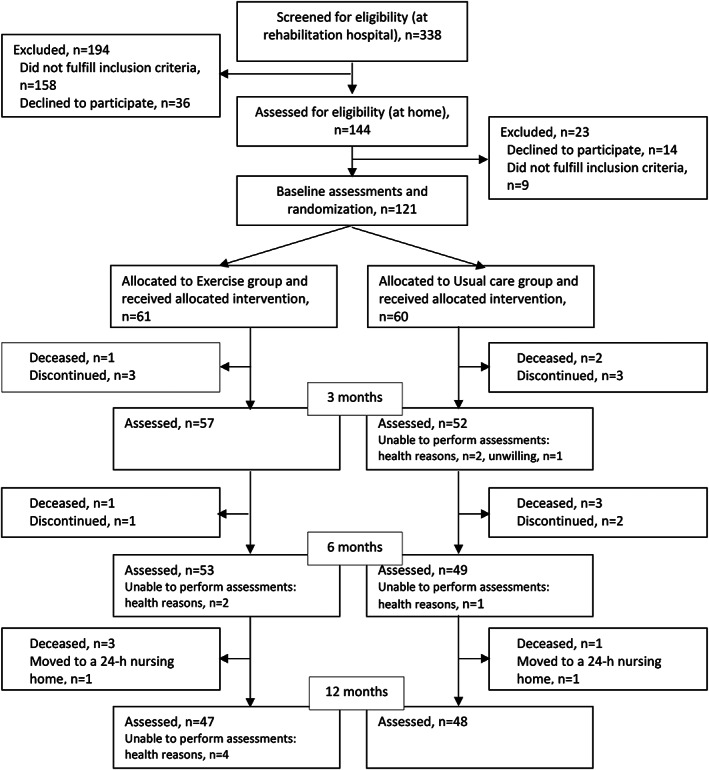
Flowchart of the study
TABLE 1.
Baseline characteristics of participants in the Exercise (n = 61) and Usual care (n = 60) groups.
| Characteristic | Exercise | Usual care |
|---|---|---|
| Age, years, mean (SD) | 83 (6) | 80 (7) |
| Women, n (%) | 50 (82) | 41 (68) |
| Number of regular medications, mean (SD) | 8.8 (3.4) | 8.7 (3.0) |
| Physician‐diagnosed diseases, n (%) | ||
| Coronary heart disease | 27 (44) | 27 (45) |
| Diabetes | 16 (26) | 12 (20) |
| MMSE, mean (SD) | 23.1 (4.7) | 22.7 (4.2) |
| Fracture type (ICD code), n (%) | ||
| Femoral neck fracture | 39 (64) | 35 (58) |
| Pertrochanteric fracture | 17 (28) | 21 (35) |
| Subtrochanteric fracture | 5 (8) | 4 (7) |
| From hospital admission to discharge, days, median (IQR) | 26 (20, 32) | 26 (21, 32) |
| From fracture to baseline assessment, days, median (IQR) | 33 (28, 50) | 37 (28, 44) |
| Pain, VAS, mean (SD) | 28 (24) | 32 (29) |
| Physical activity, sessions per week a , mean (SD) | ||
| Walking | 2.4 (2.7) | 3.0 (2.9) |
| Other physical activities | 1.1 (2.0) | 1.3 (1.9) |
Note: Frequencies (%), means (SD), or medians (IQR).
Abbreviations: ICD, International Classification of Diseases; MMSE, Mini Mental State Examination (range 0–30) 27 ; VAS, Visual Analogue Scale (range 0–100 mm). 33
Frequency of leisure‐time physical activity sessions of at least half an hour during the month preceding the fracture was included.
The baseline values of IADL, SPPB, and handgrip strength are presented in Table 2. Because there was a slight difference in age and proportion of women between the groups at baseline (Table 1), the analyses were adjusted for age and sex. The adjusted mean changes over 12 months in functioning and physical performance outcomes were significantly better in Exercise than in Usual care (Table 2, Figure 2A–C).
TABLE 2.
Instrumental activities of daily living (IADL), short physical performance battery (SPPB), and handgrip strength at baseline and their mean changes over 12 months in the exercise (n = 61) and usual care (n = 60) groups.
| Baseline | Change from baseline to 12 months a | p c | |||
|---|---|---|---|---|---|
| Exercise Mean (SD) | Usual care Mean (SD) | Exercise Mean (95% CI) b | Usual care Mean (95% CI) | ||
| IADL score | 17.1 (4.5) | 17.4 (5.1) | 3.7 (2.8 to 4.7) | 2.0 (1.0 to 3.0) | 0.016 |
| SPPB score | 3.9 (1.6) | 4.2 (1.8) | 4.3 (3.6 to 4.9) | 2.1 (1.5 to 2.7) | <0.001 |
| Handgrip strength, kg | 17.7 (8.9) | 20.8 (8.0) | 1.2 (0.3 to 2.0) | −1.0 (−1.9 to −0.2) | <0.001 |
Abbreviations: IADL, Instrumental Activities of Daily Living (range 8–31); SPPB, Short Physical Performance Battery (range 1–12).
Age‐ and sex‐adjusted mean changes over 12 months.
95% confidence interval.
Between‐group difference.
FIGURE 2.
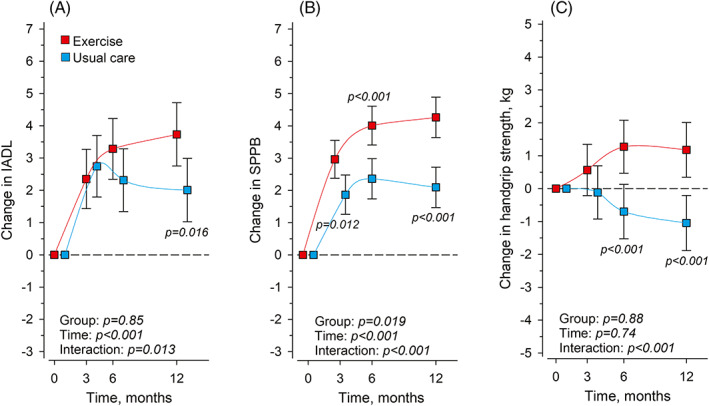
Mean changes in Instrumental activities of daily living (IADL) (A), Short Physical Performance Battery (SPPB) (B), and handgrip strength (C) in Exercise (n = 61) and Usual care (n = 60) at 3, 6 and 12 months from baseline. Adjusted for age and sex. Whiskers represent 95% confidence intervals.
The baseline values of the frequency of leisure‐time walking and other physical activity sessions are presented in Table 1. Over 12 months, the frequency of the walking sessions changed by −0.4 times per week (95% CI −1.1 to 0.2) and the frequency of other physical activity sessions by −0.2 times per week (95% CI −0.8 to 0.5) in Exercise and by −1.2 times (−1.8 to −0.6) and −0.3 times (−0.9 to 0.4) in Usual care, respectively. We found no differences between the groups in either of the physical activity forms over 12 months (Figure 3).
FIGURE 3.
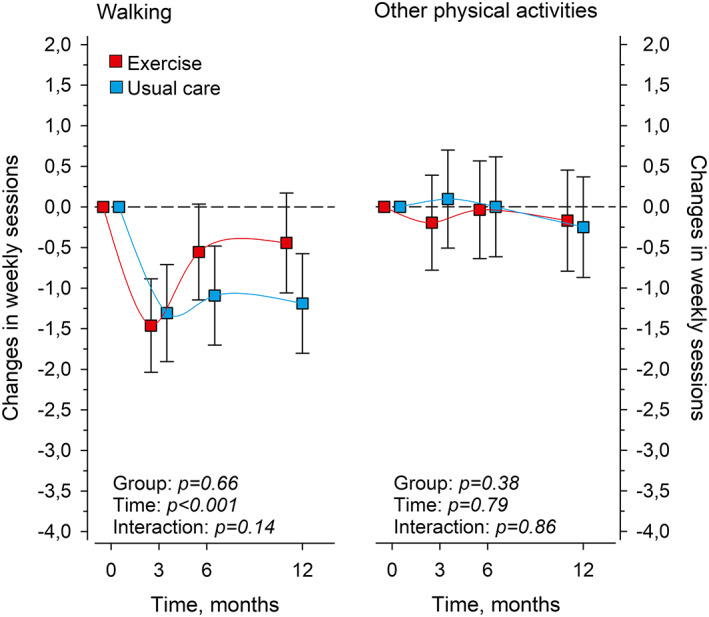
Mean changes in the weekly frequency of walking and other leisure‐time physical activities (for at least half an hour at a time) in Exercise (n = 61) and Usual care (n = 60) at 3, 6, and 12 months from baseline. Exercise intervention sessions are not included. Adjusted for age and sex. Whiskers denote 95% confidence intervals.
The median number of completed exercise sessions in the Exercise group was 96 (IQR 88, 98). Over the intervention year, 19 people (31%) suspended the training for at least 2 weeks, mostly for health reasons.
DISCUSSION
The secondary analyses of our randomized controlled trial among patients with surgical repair of a hip fracture showed that our 12‐month home‐based supervised, progressive exercise program improved functioning and physical performance to a greater extent than among those in Usual care. However, our intervention did not increase the frequency of leisure‐time physical activity sessions.
The IADL scores in our trial improved in both Exercise and in Usual care during the first 3 months after discharge, which mostly implies recuperation of bone and muscles after surgery. 37 , 38 After 3 months, the IADL scores of the participants in Usual care started to deteriorate but remained above the baseline level. The Exercise participants continued to improve their IADL scores up to 12 months, and at the 12‐month point, a significant difference emerged between the groups. Our 12‐month intervention thus appears to be sufficiently long to support the recovery of IADL functions, which can take up to 11 months after hip fractures. 37 , 38 Contrary to our results, one 12‐month randomized home‐based exercise trial 23 and two systematic reviews with different training durations, initiation of the intervention after discharge, and numbers of supervised home visits 15 , 24 observed no improvements in IADL functions compared with other modes of exercise or usual care. 15 , 23 , 24
The SPPB scores in our trial improved in Exercise and Usual care from baseline to 6 months and continued to improve from 6 to 12 months in Exercise. Both groups had clinically meaningful improvements over 12 months (mean change of 4.3 in Exercise, and 2.1 in Usual care), as a meaningful change among older adults is estimated to be from 0.4 to 1.5 points. 39 Studies with shorter home‐based exercise interventions have reported somewhat smaller changes in SPPB 22 , 40 than in our study. Sherrington et al. 41 in their low‐supervised, 12‐month exercise intervention found no improvement in SPPB scores. However, initiation of rehabilitation was delayed after discharge, whereas ours began immediately.
Although our exercise intervention mainly consisted of lower limb exercises, we also assessed handgrip strength, which is a strong predictor of mortality and may help identify people at an increased risk of health deterioration. 42 In our trial, handgrip strength improved in Exercise and deteriorated in Usual care over the 12 months. While there is no clear consensus on the clinically meaningful changes, Bohannon 43 proposed that changes of 5.0–6.5 kg may be a reasonable estimate among older adults. Viewed against this estimate, the attained mean changes in handgrip strength in our trial were not clinically meaningful. Similar to our results, Orwig et al. 44 did not find any effect of their 12‐month home‐based exercise trial on handgrip strength.
The brief physical activity guidance in our intervention did not affect leisure‐time physical activity. Contrary to our result, a supervised home‐based exercise program after a hip fracture increased the time used for physical activities in other randomized trials. 41 , 44 , 45 , 46 In these studies, as in ours, the amount of physical activity was evaluated with self‐reported questionnaires, and thus the results should be interpreted with caution. In the systematic review of Hulsbæk et al., 47 a small, but nonsignificant effect of exercise on physical activity was observed. A cohort study reported old age and frailty to be explanatory variables for unchanged physical activity levels (number of physical activity sessions per week or month) among home‐dwelling patients with hip fractures. 48 Although the significance of physical activity in recovery from hip fracture is unclear, it may enhance the functional recovery of patients with hip fractures. 49
Strengths of our trial were its rigorous randomized design, relatively low dropout rate, validated assessments, intention‐to‐treat analysis, and 12‐month supervised home‐based exercise program with good adherence and no serious adverse effects. 26 However, the study also had some limitations. First, we did not reach our targeted sample size (n = 300), as many patients declined to participate due to poor perceived health. However, the power of our sample (n = 121) was sufficiently strong to show statistical differences between the groups in these secondary outcomes. Second, there was a risk of assessment bias because the assessors were not blinded due to the lack of resources. However, they did not supervise the exercise intervention. Third, a minor difference was present in the distribution of women in the two randomized groups, as our randomization was not stratified by sex, which is why we adjusted the analyses with sex. Fourth, our sample mainly comprised those home‐dwelling patients with hip fractures who needed inpatient rehabilitation at the adjacent rehabilitation hospital after being discharged from the operating hospital and excluded those who were discharged directly home from the surgery ward. Although the number of patients who went straight home was very low, our sample is not completely representative of all patients with hip fractures, which may be considered a study limitation. Fifth, we used a self‐reported questionnaire to assess leisure‐time physical activity, which provides a possibility for cognitive bias. Also, these questions did not include an evaluation of the intensity of leisure‐time physical activities. Sixth, all of our participants were Caucasian, which may limit the generalizability of the results worldwide.
In conclusion, the results of our randomized trial for patients with hip fractures confirm the effectiveness of exercise programs with progressive strength training in enhancing functioning and performance among patients with hip fractures after discharge. Moreover, our work confirms the findings of the importance of direct supervision of the effects of exercise on functioning and mobility among patients with hip fractures. 24 Altogether, the combination of a 12‐month, supervised, and progressive exercise intervention starting immediately after discharge in our trial might explain the better results than those of previous trials. 15 , 22 , 23 , 24 , 40 , 41 , 44
Future trials should include longer follow‐up periods to determine whether booster exercise sessions every few months, for example, would help maintain the effects on functioning achieved by exercise interventions. In addition, future trials should evaluate the effects of alternative home‐based exercise implementation strategies, such as remote rehabilitation or exercise programs, on functioning and physical performance in patients with surgical repair of hip fracture.
CONFLICT OF INTEREST
Authors Aartolahti, Kääriä, Pitkälä, Sipilä, and Kautiainen have no conflicts of interest. Authors Hupli, Kukkonen‐Harjula, Soukkio, and Suikkanen have been employed by the study implementer and financier, South Karelia Social and Health Care District (Eksote), Finland. As the principal investigator of the study and the head of the rehabilitation unit at the South Karelia Social and Health Care District, Hupli received funds for the study from The Social Insurance Institution of Finland and the State Research Funding for Academic Health Research (Ministry of Social Affairs and Health, through Helsinki University Hospital [HUS], Finland). Soukkio and Suikkanen also received personal research funds from Eksote.
AUTHOR CONTRIBUTIONS
Concept and design: Hupli, Kääriä, Kukkonen‐Harjula, Soukkio, Suikkanen, Pitkälä, Sipilä. Acquisition of participants and/or data: Soukkio, Suikkanen. Statistical analyses: Kautiainen, Soukkio, Suikkanen. Analysis and interpretation of data: All authors. Drafting of the manuscript: Soukkio. Critical revision of the manuscript and final approval of the version to be published: All authors. We thank Kaija Paajanen, RN, and Virpi Äärimaa, LPN for their contribution to participant recruitment and data collection, and the personnel of South Karelia Social and Health Care District for their help in recruiting the participants. We also thank our participants for taking part in our study and the physiotherapists of the private companies for implementing our intervention.
SPONSOR'S ROLE
The sponsors played no role in the design of the study, the choice of methods, participant recruitment, data collection, analysis, interpretation, or preparation of this manuscript.
Supporting information
Table S1 Contents of one physical exercise session.
Soukkio PK, Suikkanen SA, Kukkonen‐Harjula KT, et al. Effects of a 12‐month home‐based exercise program on functioning after hip fracture – Secondary analyses of an RCT . J Am Geriatr Soc. 2022;70(9):2561‐2570. doi: 10.1111/jgs.17824
Funding information South Karelia Social and Health Care District (Eksote), Finland (grant number 1236/00.01.05.01/2013); The Social Insurance Institution of Finland (Kela) (grant numbers 94/331/2013 and 17/26/2019), and the State Research Funding for Academic Health Research (Ministry of Social Affairs and Health, through Helsinki University Hospital [HUS]), Finland (grant numbers HUS 2016 [no grant number assigned], HUS/2931/2017, HUS/2571/2017, HUS/2631/2019, 864/2020).
An oral presentation in the online congress of the 25th Nordic Congress of Gerontology, June 2–4, 2021.
REFERENCES
- 1. Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long‐term disability outcomes following hip fracture. BMC Geriatr. 2016;16:158. doi: 10.1186/s12877-016-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bertram M, Norman R, Kemp L, Vos T. Review of the long‐term disability associated with hip fractures. Inj Prev. 2011;17:365‐370. doi: 10.1136/ip.2010.029579 [DOI] [PubMed] [Google Scholar]
- 3. Vochteloo AJ, Borger van der Burg BL, et al. Do clinical characteristics and outcome in nonagenarians with a hip fracture differ from younger patients? Geriatr Gerontol Int. 2013;13:190‐197. doi: 10.1111/j.1447-0594.2012.00885.x [DOI] [PubMed] [Google Scholar]
- 4. Pajulammi HM, Pihlajamäki HK, Luukkaala TH, Nuotio MS. Pre‐ and perioperative predictors of changes in mobility and living arrangements after hip fracture. A population‐based study. Arch Gerontol Geriatr. 2015;61:182‐189. doi: 10.1016/j.archger.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 5. Vergara I, Vrotsou K, Orive M, Gonzales N, Garcia S, Quintana JM. Factors related to functional prognosis in elderly patients after accidental hip fractures: a prospective cohort study. BMC Geriatr. 2014;26:124. doi: 10.1186/1471-2318-14-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganczak M, Chrobrowski K, Korzén M. Predictors of a change and correlation in activities of daily living after hip fracture in elderly patients in a community hospital in Poland: a six‐month prospective cohort study. Int J Environ Res Public Health. 2018;15:95. doi: 10.3390/ijerph15010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Resnick B, Galik E, Boltz M, et al. Physical activity in the post‐hip‐fracture period. J Aging Phys Act. 2011;19:373‐387. doi: 10.1123/japa.19.4.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fleig L, McAllister MM, Brasher P, et al. Sedentary behavior and physical activity patterns in older adults after hip fracture: a call to action. J Aging Phys Act. 2016;24:79‐84. doi: 10.1123/japa.2015-0013 [DOI] [PubMed] [Google Scholar]
- 9. Zusman EZ, Dawes MG, Ashe N, Ashe MC. A systematic review of evidence for older adults' sedentary behaviour and physical activity after hip fracture. Clin Rehabil. 2018;31:679‐691. doi: 10.1177/0269215517741665 [DOI] [PubMed] [Google Scholar]
- 10. Beaupre LA, Binder EF, Cameron ID, et al. Maximising functional recovery following hip fracture in frail seniors. Best Pract Res Clin Rheumatol. 2013;27:771‐788. doi: 10.1016/j.berh.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Binder EF, Brown M, Sinacore DR, Stager‐May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture. A randomized controlled trial. JAMA. 2004;292:837‐846. doi: 10.1001/jama.292.7.837 [DOI] [PubMed] [Google Scholar]
- 12. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community‐dwelling aged. Am J Epidemiol. 2003;157:1023‐1031. doi: 10.1093/aje/kwg081 [DOI] [PubMed] [Google Scholar]
- 13. Vl T, Sudore R, Cencer IS, et al. Rates of recovery to pre‐fracture function in older persons with hip fracture: an observational study. J Gen Intern Med. 2017;32:153‐158. doi: 10.1007/s11606-016-3848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dyer SM, Perracini MR, Smith T, et al. Rehabilitation following hip fracture. In: Falacshi P, Marsh D, eds. Orthogeriatrics, Practical Issues in Geriatrics. The Management of Older Patients with Fragility Fractures. 2nd ed. Springer Nature Switzerland; 2021. doi: 10.1007/978-3-030-48126-12 [DOI] [Google Scholar]
- 15. Auais MA, Eilayyan O, Mayo NE. Extended exercise rehabilitation after hip fracture improves patients' physical function: a systematic review and meta‐analysis. Phys Ther. 2012;92:1437‐1451. doi: 10.2522/ptj.20110274 [DOI] [PubMed] [Google Scholar]
- 16. Salpakoski A, Törmäkangas T, Edgren J, et al. Effects of a multicomponent home‐based physical rehabilitation program on mobility recovery after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2014;15:361‐368. doi: 10.1016/j.jamda.2013.12.083 [DOI] [PubMed] [Google Scholar]
- 17. Diong J, Allen N, Sherrington C. Structured exercise improves mobility after hip fracture: a meta‐analysis with meta‐regression. Br J Sports Med. 2016;50:346‐355. doi: 10.1136/bjsports-2014-094465 [DOI] [PubMed] [Google Scholar]
- 18. Lee SY, Yoon BH, Beom J, Ha YC, Lim JY. Effect of lower‐limb progressive resistance exercise after hip fracture surgery: a systematic review and meta‐analysis of randomized controlled studies. J Am Med Dir Assoc. 2017;18:1096.e19‐1096.e26. doi: 10.1016/j.jamda.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Butts WJ, You T. Exercise interventions, physical function, and mobility after hip fracture: a systematic review and meta‐analysis. Disabil Rehabil. 2021;8:1‐11. doi: 10.1080/09638288.2021.1924299 [DOI] [PubMed] [Google Scholar]
- 20. Singh NA, Quine S, Clemson LM, et al. Effects of high‐intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2012;13:24‐30. doi: 10.1016/j.jamda.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 21. Kuijlaars IAR, Sweerts L, Nijhuis‐van der Sanden MWG, et al. Effectiveness of supervised home‐based exercise therapy compared to a control intervention on functions, activities, and participation in older patients after hip fracture: a systematic review and meta‐analysis. Arch Phys Med Rehab. 2019;100:101‐114.e6. doi: 10.1016/j.apmr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 22. Latham NK, Harris BA, Bean JF, et al. Effect of a home‐based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. 2014;311:700‐708. doi: 10.1001/jama.2014.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edgren J, Salpakoski A, Sihvonen SE, et al. Effects of a home‐based physical rehabilitation program on physical disability after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2015;16(350):e1‐e7. doi: 10.1016/j.jamda.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 24. Chen B, Hu N, Jin‐Hai T. Efficacy of home‐based exercise programme on physical function after hip fracture: a systematic review and meta‐analysis of randomised controlled trials. Int Wound J. 2020;17:45‐54. doi: 10.1111/iwj.13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soukkio P, Suikkanen S, Kääriä S, et al. Effects of 12‐month home‐based physiotherapy on duration of living at home and functional capacity among older persons with signs of frailty or with a recent hip fracture ‐ protocol of a randomized controlled trial (HIPFRA study). BMC Geriatr. 2018;18:232. doi: 10.1186/s12877-018-0916-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soukkio P, Suikkanen S, Aartolahti E, et al. Effects of home‐based physical exercise on days at home, healthcare utilization and functional independence among patients with hip fractures: a randomized controlled trial. Arch Phys Med Rehabil. 2021;102:1692‐1699. doi: 10.1016/j.apmr.2021.04.004 [DOI] [PubMed] [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 28. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179‐186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 29. Vittengl JR, White CN, McGovern RJ, Morton BJ. Comparative validity of seven scoring systems for the instrumental activities of daily living scale in rural elders. Aging Ment Health. 2006;10:40‐47. doi: 10.1080/13607860500307944 [DOI] [PubMed] [Google Scholar]
- 30. Guralnik J, Simonsick E, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:85‐94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 31. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of handgrip strength in clinical and epidemiological studies: towards a standardized approach. Age Aging. 2011;40:423‐429. doi: 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 32. Helldán A, Helakorpi S. Health behaviour and health among the Finnish elderly, Spring 2013, with trends 1993–2013. National Institute for Health and Welfare (THL), Report 15/2014. http://urn.fi/URN:ISBN:978-952-302-188-4. In Finnish, with abstract and tables in English. Accessed Jan 30, 2022.
- 33. Scott J, Huskisson E. Graphic representation of pain. Pain. 1976;2:175‐184. [PubMed] [Google Scholar]
- 34. Gardner MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise‐based falls prevention programme. Age Aging. 2001;30:77‐83. doi: 10.1093/ageing/30.1.77 [DOI] [PubMed] [Google Scholar]
- 35. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377‐381. doi: 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 36. Sund R, Juntunen M, Lüthje P, Huusko T, Häkkinen U. Monitoring the performance of hip fracture treatment in Finland. Ann Med. 2011;43:39‐46. doi: 10.3109/07853890.2011.586360 [DOI] [PubMed] [Google Scholar]
- 37. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55:M498‐M507. doi: 10.1093/gerona/55.9.m498 [DOI] [PubMed] [Google Scholar]
- 38. Magaziner J, Chiles N, Orwig D. Recovery after hip fracture: interventions and their timing to address deficits and desired outcomes – evidence from the Baltimore hip studies. Nestle Nutr Inst Workshop Ser. 2015;83:71‐81. doi: 10.1159/000382064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE‐P study). J Nutr Health Aging. 2009;13:538‐544. doi: 10.1007/s12603-009-0104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taraldsen K, Thingstad P, Døhl Ø, et al. Short and long‐term clinical effectiveness and cost‐effectiveness of a late‐phase community‐based balance and gait exercise program following hip fracture. The EVA‐hip randomised controlled trial. PLoS One. 2019;14:e0224971. doi: 10.1371/journal.pone.0224971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherrington C, Fairhall N, Kirkham C, et al. Exercise to reduce mobility disability and prevent falls after fall‐related leg or pelvic fracture: RESTORE randomized controlled trial. J Gen Intern Med. 2020;35:2907‐2916. doi: 10.1007/s11606-020-05666-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rantanen T, Volpato S, Ferrucini L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause‐specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636‐641. doi: 10.1034/j.1600-0579.2003.00207.x [DOI] [PubMed] [Google Scholar]
- 43. Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci. 2019;31:75‐78. doi: 10.1589/jpts.31.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Orwig DL, Hochberg M, Yu‐Yahiro J, et al. Delivery and outcomes of a yearlong home exercise program after hip fracture: a randomized controlled trial. Arch Intern Med. 2011;171:323‐331. doi: 10.1001/archinternmed.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Resnick B, Orwig D, Yu‐Yahiro J, et al. Testing the effectiveness of the exercise plus program in older women post‐hip fracture. Ann Behav Med. 2007;34:67‐76. doi: 10.1007/BF02879922 [DOI] [PubMed] [Google Scholar]
- 46. Turunen K, Salpakoski A, Edgren J, et al. Physical activity after a hip fracture: effect of a multicomponent home‐based rehabilitation program‐a secondary analysis of a randomized controlled trial. Arch Phys Med Rehabil. 2017;98:981‐988. doi: 10.1016/j.apmr.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 47. Hulsbæk S, Juhl C, Røpke A, Bandholm T, Kristensen MT. Exercise therapy is effective at improving short‐ and long‐term mobility, activities of daily living, and balance in older patients following hip fracture: a systematic review and meta‐analysis. J Gerontol A Biol Sci Med Sci. 2021;glab236;77:861‐871. doi: 10.1093/gerona/glab236 [DOI] [PubMed] [Google Scholar]
- 48. Aboelmagd T, Dainty JR, MacGregor A, Smith TO. Trajectory of physical activity after hip fracture: an analysis of community‐dwelling individuals from the english longitudinal study of ageing. Injury. 2018;49:697‐701. doi: 10.1016/j.injury.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 49. Talkowski JB, Lenze EJ, Munin MC, Harrison C, Brach JS. Patient participation and physical activity during rehabilitation and future functional outcomes in patients after hip fracture. Arch Phys Med Rehabil. 2009;90:618‐622. doi: 10.1016/j.apmr.2008.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Contents of one physical exercise session.


