Abstract
Objective
Sentinel node (SN) biopsy following lymphoscintography is recommended for high‐risk cutaneous malignancies. Herein, we investigate different lymphoscintography phases, focusing on the importance of the late static phase and the resultant discovery of distal echelon solitary positive sentinel nodes that would otherwise have been overlooked.
Methods
In this retrospective cohort study, conducted in a tertiary referral medical center, we assessed SN localization and time from tracer injection to SN identification on lymphoscintigraphy. Findings on scan were compared with SN found in the surgical field, and with the final pathological investigation.
Results
Seventy‐three patients, undergoing SN biopsy for head and neck skin malignancies, were investigated. Most patients were male (n = 50). The average age was 65.7 (±15.7) years and the average follow‐up time was 29.1 (±22.4) months. Overall, 101 SNs were histologically investigated, demonstrating 7 positive SN. Eleven patients (15%) benefited from the late lymphoscintigraphy phase. In four studies, an SN was identified only in the late static phase, one of which was positive for the disease. In seven patients, SN was identified in the early phase with additional, different, SN on the late phase, one of which was positive for the disease. Comparing the yield (positive SNs) of early versus late phases, demonstrated the same importance (p = 0.275).
Conclusions
The late lymphoscintigraphy phase has a crucial role in high‐risk HN cutaneous cancer.
Level of Evidence
3 Laryngoscope, 132:2164–2168, 2022
Keywords: cutaneous malignancies, head and neck cancer, lymphoscintigraphy, melanoma, sentinel lymph node biopsy
INTRODUCTION
Sentinel lymph nodes are regional nodes that directly receive lymph drainage from the primary tumor in the skin, 1 breast, 2 and other sites. 3 The risk of lymphatic spread in cutaneous malignancies depends on histological and clinical parameters, guiding the need for lymph node histological investigation. 4 , 5 Hence, lymphoscintigraphy followed by sentinel lymph node biopsy (SLNB) are indicated in high‐risk skin cancers since the first description by Donald L. Morton in 1992. 6 This technique evolved with time, reaching a high degree of accuracy. 7 Despite a lower success rate in the head and neck region in view of the more complex lymphatic drainage pathways 8 lymphoscintigraphy with SLNB is still useful. 9 Regional nodal status is the most powerful prognostic indicator for cutaneous melanoma 4 , 10 and cutaneous squamous cell carcinoma. 11
Lymphoscintigraphy consists of three phases. Imaging starting immediately after radiotracer injection, for the first 5–10 min, consists of the dynamic phase. Thereafter, serial static imaging conducted up to 60 min following radiotracer injection is defined as the early static phase, and further images are defined as the late static phase. 12 Current imaging protocols use SPECT CT in concert with the last imaging routine for more accurate localization of the SN. 13
While there is a general recommendation for having both early and late studies in lymphoscintigraphy for melanoma, 14 there are no specific recommendations in head and neck skin cancer elucidating which phases are important in identifying SLNs. Whereas, for comparison, the recommended lymphoscintigraphy protocol specifically for breast cancer includes only early phase imaging. 15
Herein, we investigate the role of lymphoscintigraphy for the identification of sentinel nodes in high‐risk skin malignancies of the head and neck region, focusing on the role of the late static phase.
MATERIALS AND METHODS
Patients and Study Design
This study is a retrospective analysis of head neck (HN) high‐risk cutaneous malignancies in patients treated in a tertiary referral medical center (case series). The Hadassah medical center local Internal Review Board (0891‐20‐HMO) approved the study with a waiver for the consent form. Data anonymization was rigorously maintained.
Primary Endpoints
Investigation of the additive information of the lymphoscintigraphy late phase, compared with early phase SN identification.
Comparison of the lymphoscintigraphy images (early and late phases) with the surgical findings using the gamma‐probe and the 10% role distinguishing sentinel nodes from background count.
Investigating the correspondence between sentinel nodes demonstrated on the different lymphoscintigraphy phases and the final histology result focusing on positive nodes with lymphatic spread.
Patients
Consecutive patients with cutaneous high‐risk head and neck malignancy undergoing SN biopsy at Hadassah Medical Center, between 1st of January 2014 and 31st of December 2020 were recruited. The medical and surgical records and pathological reports of all patients included in the study were surveyed. The following baseline parameters were retrieved: age, gender, co‐morbidities, tumor site and stage (American Joint Committee on Cancer Staging Manual, 8th edition), date of surgery, sentinel nodes' site as described in the surgical notes, and pathological reports for actual lymphatic spread. Dates of diagnosis, disease progression and recurrence, death and cause of death, and last follow‐up, were documented for the survival metrics. False‐negative SN biopsy was considered as a lymphatic spread on a site with previously negative SN.
Lymphoscintigraphy
Lymphoscintigraphy was performed after the intradermal injection of 37 MBq of 99mTc‐nano‐colloid divided into 4 injections around the lesion. Imaging was performed on a dual‐head gamma camera with large field‐of‐view detectors (Discovery 670 NMCT, GE Healthcare) equipped with low‐energy high‐resolution collimators. Dynamic imaging was performed immediately following radiotracer injection for 10 min, one frame per minute, followed by static planar 5‐min images (early static images). Late 5‐min static images and whole‐body images were performed 1–3 h after radiotracer injection (or later in rare cases with no SNs identification). SPECT/CT was performed (low dose unenhanced CT) with 360° orbit, 3° angle step, with 25 sec/frame. SPECT/CT data were reconstructed with iterative reconstruction.
The lymphoscintigraphy studies were re‐evaluated (N.A.Q and S.B.H) for sentinel nodes location and time from 99mTc‐nanocolloid injections. We have categorized the lymphoscintigraphy phases into dynamic (0–10 min after radiotracer injection) and early static (11–60 min after injection) images, classified together as “early” phase. Late phase imaging was defined as 61 min or more following injection.
The late phase data was divided into two separate study groups. One group consisted of cases in whom only the late phase studies identified sentinel lymph nodes. The other group had detectable but different SNs in both the early and late phases. In our practice, all patients have both early and late phase studies the day before surgery.
Sentinel Node Biopsy
SLNB surgical technique, 16 and specifically in the head and neck region 9 , 17 was previously described. All patients underwent sentinel node localization and removal in the operating room by the same two experienced head and neck surgeons (N.H and J.W). In each case, we removed sentinel nodes as long the gamma probe count was 10% or more 18 compared with the ex‐vivo SNs.
Statistical Analysis
Statistical analyses were performed using R software, version 3.5.1 (R Development Core Team, 2018), and Microsoft Excel. All statistical analysis results and their interpretation were independently reviewed by a statistician. The statistics are descriptive in nature using average, median, range, and standard deviation as needed. Chi‐squared test was used for categorical parameters comparison, p‐value of 0.05 or less was considered statistically significant, applying the difference between investigated groups. Considering the relatively small study groups' size, we will further discuss the clinical importance and deliberately minimize the p‐value importance.
Results
Patients and Disease Characteristics
Overall, we have analyzed 73 lymphoscintigraphies in 20 females (27.4%) and 53 males (72.6%) with the majority having cutaneous melanoma (n = 68), three patients with high‐risk adnexal tumor, and two patients with high‐risk skin squamous cell carcinoma. (Table I). The average age at the operation day was 65.7 (±15.7) years. Eight patients (11%) had comorbidities or treatment with immune‐suppressants. Primary skin tumor site was in the auricle (n = 22), scalp (n = 22) and cheek (n = 21). Six patients had the tumor located on the neck skin and two had conjunctival melanoma. Patients were treated with curative intent and followed in the surgical oncology clinic for a median time of 26.7 (1–92) months. All patients are alive but 11 patients (15%) are alive with disease. The clinical T staging (8th edition AJCC staging system) distribution was relatively homogenous with T1, T2, T3, and T4 in 21.7%, 31.9%, 27.5%, and 18.9% of the patients, respectively (Table I).
TABLE I.
Demographic and Clinical Information of 73 Consecutive Head and Neck Cutaneous High‐Risk Malignancy Patients Who Underwent Sentinel Lymph Node Biopsy.
| Positive lymphatic spread | |||
|---|---|---|---|
| Gender (n = 73) | Female | 20 | 2 |
| Male | 53 | 5 | |
| Age (n = 73) years | Average ± STD | 65.7 ± 15.7 | |
| Median | 73.5 | ||
| Skin site (n = 73) | Cheek | 21 | 3 |
| Auricle | 22 | 1 | |
| Scalp | 22 | 3 | |
| Neck | 6 | ||
| Conjunctiva | 2 | ||
| Histology (n = 73) | Melanoma | 68 | 7 |
| Adnexal ca | 3 | ||
| SCC | 2 | ||
| Staging (n = 69) AJCC 8th edition | T1 | 15 | 1 |
| T2 | 22 | 1 | |
| T3 | 19 | 2 | |
| T4 | 13 | 3 | |
| Follow‐up (n = 73) months | Average ± STD | 29.1 ± 22.4 | |
| Median | 26.7 |
Lymphoscintigraphy Analyses
All patients underwent late lymphoscintigraphy studies (Fig. 1), 151 min (median) following the tracer injection (range 74–1580 min). In five patients (6.8%), no sentinel nodes were detected neither in the early nor late studies. This finding was more common among patients with skin tumors on the neck (2/6, 33.3%) and less in patients with tumors on the scalp (1/22, 4.5%), auricle (1/22, 4.5%), and cheek (1/21, 4.7%). Four studies revealed isolated sentinel nodes, only identified in the late phase. Seven other studies demonstrated late phase sentinel nodes different from those demonstrated in the early phase (Fig. 2). Overall, 11 patients (15%) benefited from the late lymphoscintigraphy phase.
Fig. 1.
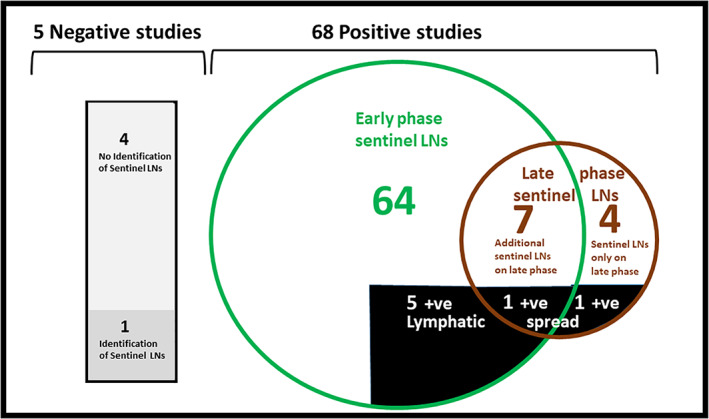
A scheme demonstrating the outcome of 73 patients undergoing lymphoscintigraphy and sentinel biopsy for head and neck skin malignancies. Presentation of early and late phase's findings and the association with positive lymphatic spread [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Fig. 2.
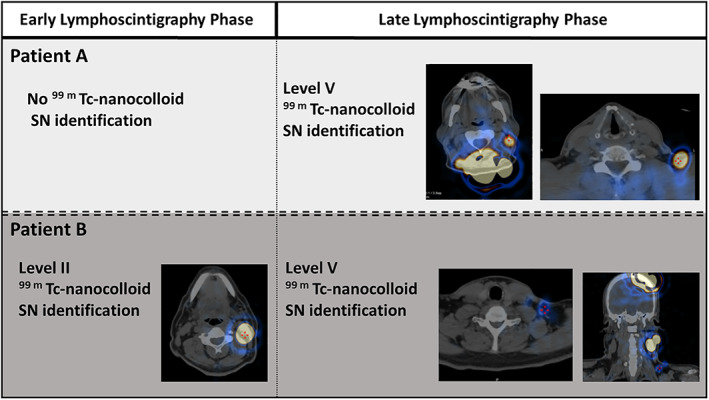
Representative patients with (A) SN identified in neck level V only on late phase scintigraphy; (B) SN level II demonstrated on the early phase and additional level IV neck SN demonstrated on late‐phase lymphoscintigraphy [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Lymphoscintigraphy Association with Surgical and Histological Parameters
One of the five patients without sentinel lymph node identification on lymphoscintigraphy had successful sentinel node removal (using a hand gamma probe following the required wide excision).
Overall, 101 sentinel nodes were excised and histologically investigated among 69 patients, demonstrating 7 positive sentinel nodes. All sentinel nodes demonstrated in the early and late phases were identified during surgery, using the gamma probe and 10% rule. Five of the positive lymph nodes (71%) were demonstrated in the early phase, whereas, 2 out of the 7 positive SN were demonstrated in the late phase and would have been missed had it not been performed. Of the 2 positive sentinel nodes identified in the late phase, one was a solitary SN, only identified on the late study and the second was an additional SN, different from the one identified in the early phase. Comparing the yield (positive SNs) of early 5/64 versus late phases 2/11, demonstrates the same importance (p = 0.275) for both phases.
One of the 62 patients with negative sentinel node investigation (1.6%) had cervical lymph node spread (false negative) a month following the SN biopsy. The diseased lymph node was in the contralateral neck, away from the sentinel surgical field.
DISCUSSION
The importance of SLNB in treatment decision making, follow‐up plan, and survival is well known in melanoma 4 , 19 and other high‐risk skin malignancies. 20 While general lymphoscintigraphy protocols for melanoma 14 recommend both early and late studies, the role of late‐phase lymphoscintigraphy specifically in head and neck skin malignancies has not been investigated yet. The possibility of conducting only early studies derived from different research groups demonstrating no advantage in having late‐phase lymphoscintigraphy in breast cancer. 15 , 21
In the present study, we have demonstrated the clinical importance of late‐phase lymphoscintigraphy in head and neck high‐risk skin malignancies when SLNB is indicated. Overall, eleven patients (15%) had sentinel nodes identified only in the late phase and 2/7 (29%) of the diseased lymphatic spread was identified in the late phase. All patients are alive during study follow‐up.
The neck has a dense complex lymphatic drainage system, with several 22 potential lymphatic routes to various levels, 23 and possibly traversing even to the contralateral neck or other non‐traditional nodal regions. 24 Late‐phase lymphoscintigraphy may enable the identification of these unpredictable pathways.
In one‐third of patients with neck skin malignancies lymphoscintigraphy failed to identify SN, significantly higher compared to other HN skin tumors. This relatively high failure rate of lymphoscintigraphy and gamma probe identification may be due to the proximity between the injection site and the drainage basin, causing a “shine‐through” masking effect.
The limitations of the present study include the retrospective nature of this single‐center study and the small study group, which may preclude firm conclusions. Nevertheless, the data suggest an added clinical value of late‐phase lymphoscintigraphy compared to early phase lymphoscintigraphy alone with successful recognition of nodal disease.
CONCLUSION
Our data suggest that late‐phase lymphoscintigraphy has an added clinical value and improved outcomes and should be performed in all head and neck skin SLNB.
Further studies in larger patient cohorts and meta‐analyses are needed.
Editor's Note: This Manuscript was accepted for publication on February 14, 2022.
Nir Hirshoren and Narmeen abd el Qadir contributed equally to this study.
This material has not been published and is not currently under evaluation in any other peer‐reviewed publication. There is no conflict of interest or financial support.
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. Bello DM, Faries MB. The landmark series: MSLT‐1, MSLT‐2 and DeCOG (Management of Lymph Nodes). Ann Surg Oncol 2020;27:15–21. 10.1245/s10434-019-07830-w. [DOI] [PubMed] [Google Scholar]
- 2. Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early‐stage breast cancer. J Clin Oncol 2005;23:7703–7720. 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3. Garrel R, Poissonnet G, Moyà Plana A, et al. Equivalence randomized trial to compare treatment on the basis of sentinel node biopsy versus neck node dissection in operable T1‐T2N0 Oral and oropharyngeal cancer. J Clin Oncol 2020;38:4010–4018. 10.1200/JCO.20.01661. [DOI] [PubMed] [Google Scholar]
- 4. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel‐node biopsy versus nodal observation in melanoma. N Engl J Med 2014;370:599–609. 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ross AS, Schmults CD. Sentinel lymph node biopsy in cutaneous squamous cell carcinoma: a systematic review of the English literature. Dermatol Surg 2006;32:1309–1321. 10.1111/j.1524-4725.2006.32300.x. [DOI] [PubMed] [Google Scholar]
- 6. Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392–399. 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 7. Sabel MS, Gibbs JF, Cheney R, McKinley BP, Lee JS, Kraybill WG. Evolution of sentinel lymph node biopsy for melanoma at a National Cancer Institute‐designated cancer center. Surgery 2000;128:556–563. 10.1067/msy.2000.108053. [DOI] [PubMed] [Google Scholar]
- 8. Passmore‐Webb B, Gurney B, Yuen HM, et al. Sentinel lymph node biopsy for melanoma of the head and neck: a multicentre study to examine safety, efficacy, and prognostic value. Br J Oral Maxillofac Surg 2019;57:891–897. 10.1016/j.bjoms.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 9. Carlson GW, Murray DR, Lyles RH, Hestley A, Cohen C. Sentinel lymph node biopsy in the management of cutaneous head and neck melanoma. Plast Reconstr Surg 2005;115:721–728. 10.1097/01.prs.0000152429.06593.c1. [DOI] [PubMed] [Google Scholar]
- 10. Balch CM, Soong SJ, Atkins MB, et al. An evidence‐based staging system for cutaneous melanoma. CA Cancer J Clin 2004. May‐Jun;54:131–149; quiz 182‐4. 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 11. Durham AB, Lowe L, Malloy KM, et al. Sentinel lymph node biopsy for cutaneous squamous cell carcinoma on the head and neck. JAMA Otolaryngol Head Neck Surg 2016;142:1171–1176. 10.1001/jamaoto.2016.1927. [DOI] [PubMed] [Google Scholar]
- 12. Moncayo VM, Aarsvold JN, Alazraki NP. Lymphoscintigraphy and sentinel nodes. J Nucl Med 2015;56:901–907. 10.2967/jnumed.114.141432. [DOI] [PubMed] [Google Scholar]
- 13. van der Ploeg IM, Valdés Olmos RA, Nieweg OE, Rutgers EJ, Kroon BB, Hoefnagel CA. The additional value of SPECT/CT in lymphatic mapping in breast cancer and melanoma. J Nucl Med 2007;48:1756–1760. 10.2967/jnumed.107.043372. [DOI] [PubMed] [Google Scholar]
- 14. Bluemel C, Herrmann K, Giammarile F, et al. EANM practice guidelines for lymphoscintigraphy and sentinel lymph node biopsy in melanoma. Eur J Nucl Med Mol Imaging 2015;42:1750–1766. 10.1007/s00259-015-3135-1. [DOI] [PubMed] [Google Scholar]
- 15. Sadeghi R, Forghani MN, Memar B, et al. How long the lymphoscintigraphy imaging should be continued for sentinel lymph node mapping? Ann Nucl Med 2009;23:507–510. 10.1007/s12149-009-0284-y. [DOI] [PubMed] [Google Scholar]
- 16. Bagaria SP, Faries MB, Morton DL. Sentinel node biopsy in melanoma: technical considerations of the procedure as performed at the John Wayne cancer institute. J Surg Oncol 2010;101:669–676. 10.1002/jso.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morton DL, Wen DR, Foshag LJ, Essner R, Cochran A. Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early‐stage melanomas of the head and neck. J Clin Oncol 1993;11:1751–1756. 10.1200/JCO.1993.11.9.1751. [DOI] [PubMed] [Google Scholar]
- 18. McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann Surg Oncol 2001;8:192–197. 10.1007/s10434-001-0192-4. [DOI] [PubMed] [Google Scholar]
- 19. Leiter U, Eigentler TK, Häfner HM, et al. Sentinel lymph node dissection in head and neck melanoma has prognostic impact on disease‐free and overall survival. Ann Surg Oncol 2015;22:4073–4080. 10.1245/s10434-015-4439-x. [DOI] [PubMed] [Google Scholar]
- 20. Takahashi A, Imafuku S, Nakayama J, Nakaura J, Ito K, Shibayama Y. Sentinel node biopsy for high‐risk cutaneous squamous cell carcinoma. Eur J Surg Oncol 2014;40:1256–1262. 10.1016/j.ejso.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 21. Jangjoo A, Forghani MN, Mehrabibahar M, et al. Comparison of early and delayed lymphoscintigraphy images of early breast cancer patients undergoing sentinel node mapping. Nucl Med Commun 2010;31:521–525. [PubMed] [Google Scholar]
- 22. Kaveh AH, Seminara NM, Barnes MA, et al. Aberrant lymphatic drainage and risk for melanoma recurrence after negative sentinel node biopsy in middle‐aged and older men. Head Neck 2016;38:E754–E760. 10.1002/hed.24094. [DOI] [PubMed] [Google Scholar]
- 23. Stewart CL, Gleisner A, Kwak J, et al. Implications of sentinel lymph node drainage to multiple basins in head and neck melanoma. Ann Surg Oncol 2017;24:1386–1391. 10.1245/s10434-016-5744-8. [DOI] [PubMed] [Google Scholar]
- 24. Creighton F, Bergmark R, Emerick K. Drainage patterns to nontraditional nodal regions and level IIB in cutaneous head and neck malignancy. Otolaryngol Head Neck Surg 2016;155:1005–1011. 10.1177/0194599816662864. [DOI] [PubMed] [Google Scholar]


