Abstract
Objective
To investigate the long‐term outcomes and prognosis of thyrotoxicosis in a large number of patients in a single UK county (Leicestershire).
Design
Retrospective cohort analysis of 56,741 thyroid function test (TFT) results, treatment modalities and outcomes in a well‐established virtual thyrotoxicosis clinic database.
Patients
One thousand four hundred and eighty‐nine patients were included with a median length of follow‐up of 10.9 years. The aetiology of thyrotoxicosis was autoimmune (85.9%), nodular (9.1%) and mixed (5.0%). Treatment modalities included antithyroid drugs (ATDs), radioiodine (RAI; 555 MBq fixed dose) and thyroidectomy.
Methods
We analysed both individual TFTs and groups of sequential TFTs on or after the same thyroid treatment(s), which we describe as 'phase of thyroid care' (POTC). Patients studied entered the virtual clinic between 1 January 1995 and 1 January 2010; we exported data on every TFT sample up to April 2020.
Results
ATD had been used in 99.2% (median 2, maximum seven courses) with long‐term ATD (>2 years) in 48%. RAI and thyroidectomy were used more commonly with nodular and mixed aetiology. Overall, T4 was more often controlled than thyroid‐stimulating hormone (TSH), and at the latest follow‐up, T4 was normal in >96%, TSH in >79% and both in >76% of different aetiologies. The mean percentage control of T4 was 85% and TSH 50%; in long‐term ATD courses, this improved to 89% and 62%, respectively. In the latest POTC, control of T4 and TSH was best in cases off treatment (95%/87%) and on T4 without ablative therapy (94%/72%), but was broadly similar in patients on long‐term ATD (90%/68%), after RAI (92%/60%) or after thyroidectomy (91%/58%). After the first course of ATD, remission or hypothyroidism was seen in 47.3% autoimmune, 20.9% nodular and 32.5% mixed, with 90% relapses seen within 4 years. Relapse was more common in patients with ophthalmopathy, but there was no difference between the sexes.
Conclusions
Thyrotoxicosis can be well controlled with minimal specialist clinic attendance using a software‐supported virtual shared‐care scheme. Long‐term ATD appears to be a valid patient choice achieving TFT control comparable to that seen after RAI or surgery. In patients with autoimmune disease, relapse is more common in patients with ophthalmopathy, and hypothyroidism is common after RAI. In nodular disease, we found that spontaneous remission may occur.
Keywords: carbimazole, hyperthyroidism, radioiodine, thyroid, thyrotoxicosis, virtual clinic
1. INTRODUCTION
Despite the fact that thyrotoxicosis is one of the most common conditions treated in a secondary care endocrine clinic, there is a relative lack of long‐term outcome studies in a large number of patients. UK NICE guidelines have given a comprehensive evidence‐based approach to the diagnosis, management and follow‐up of thyrotoxicosis 1 alongside the American Thyroid Association (ATA) Guidelines. 2 Such guidelines are largely based on the meta‐analysis of existing data and consensus opinion, rather than on long‐term observational cohort studies.
Endocrinologists have different ways of confirming the aetiology of thyrotoxicosis, as well as individual approaches to management, which have changed over time and therefore comparisons between different centres are difficult. A small single‐centre prospective study showed a remission rate of 30% after 5 years follow‐up, although this was seen only in 26 patients. 3 The ATA estimates the remission rate of thyrotoxicosis to be 20%–30% after 18 months of ATD treatment. 2 Relapse rates are thought to be higher in patients with significantly elevated fT4 and fT3 levels at presentation and in patients with strongly positive thyroid antibodies, large goitres, thyroid eye disease and persistently suppressed thyroid‐stimulating hormone (TSH) levels. 4
North America traditionally use early definitive treatment relative to their counterparts in Europe and Japan. 2 , 5 Definitive treatment for thyrotoxicosis has typically been considered preferable to long‐term antithyroid treatment to prevent long‐term complications of thyrotoxicosis. 1 Young women are advocated early definitive treatment to reduce the potential risk of congenital malformations in pregnant women on carbimazole (CBZ). 6 There is a relative lack of data regarding the safety of long‐term ATD treatment.
The Endocrine Unit in Leicester (UK) has run a long‐established virtual thyrotoxicosis clinic since 1995. We are in a relatively unique position to look at a single‐centre outcome in a large number of patients over a long period of time. We were interested in attempting to answer some of the big questions regarding the long‐term follow‐up and prognosis of thyrotoxicosis. We have a unified approach in the management of thyrotoxicosis, making the comparison of the different groups more reliable than comparing different centres. Nevertheless, this is a real‐life retrospective observational study, and the methods of diagnosis and treatment of thyrotoxicosis have evolved over time as new investigation modalities have become available.
Since 1988, we have prospectively recorded diagnosis, treatment and major outcomes on all cases of thyrotoxicosis in our department in a comprehensive clinical information system, and since 1995 we have managed a majority of patients with thyrotoxicosis via a shared‐care scheme administered using the same software, with storage of the results of all thyroid function tests (TFTs) obtained during monitoring. We, therefore, reviewed these data to obtain insights on treatment choice, treatment outcomes and degree of control of TFTs on treatment and during long‐term follow‐up in routine clinical practice, comparing various treatment modalities and strategies and seeking markers that might predict outcomes for future patients. We were particularly interested to look at the remission rates and prognosis of patients with hyperthyroidism depending on the likely aetiology.
2. MATERIALS AND METHODS
2.1. Clinical information system and virtual clinic
The departmental clinical information system (Leicester Clinical Workstation, LCW) contains detailed information on diagnosis, clinical findings, investigations and treatment (encoded using the Clinical Terms v3 [Read Codes]) on all patients seen since 1988 in the Endocrinology service at the Leicester Royal Infirmary, United Kingdom, the main specialist endocrine provider for Leicestershire (serving a population of approximately 1.5 million people). Data are collected and maintained as part of routine clinical practice and the system is used as the primary source of clinical information during every clinical contact with the patient. The open ‘problem list’ data structure allows entry of any clinical concept that the clinician seeing the patient feels to be clinically relevant for the management of the patient and/or inclusion in correspondence. For example, the presence of thyroid‐associated ophthalmopathy or nodular goitre, results of thyroid antibody levels or imaging are typically added to the database to give a better idea about the aetiology of thyrotoxicosis. All current drugs and doses and dates of treatment with radioiodine (RAI) or surgery are recorded in the system and then stored for long‐term use.
2.2. Thyrotoxicosis shared‐care scheme
In 1995, we introduced a Leicestershire 'thyrotoxicosis shared‐care scheme' (TSC) administered via a software module in LCW, which stores TFT results together with information on current doses of ATD and levothyroxine, a qualitative description of the TFT result for the patient, which includes ‘normal’, 'overactive' and ‘underactive’. Information also includes the advice given on ongoing ATD and levothyroxine doses and the planned date for the next TFT blood test. The software algorithms prompt the clinician user to enter appropriate TFT descriptions and dose advice and then merge the relevant data into preformatted results and dose‐advice letters to the patient and to their primary care doctor (GP) with, in more recent years, the ability to create an advice email to patients who wish to receive advice in this way. Day‐to‐day reporting and advice are led by the endocrine specialist nurse, with support from consultant endocrinologists whenever required. Patients may be included in TSC from diagnosis at the point of referral to the clinic or after a variable length of routine follow‐up in the clinic. TSC was started when the responsible clinician considered that thyroid function could be successfully monitored and treatment adjusted via a virtual service to reduce the need for physical clinic visits. For uncomplicated cases with no current symptoms, the protocol involves annual clinic review for patients on ATD, and open appointments for patients who are on levothyroxine alone, who are off all thyroid‐related treatment or who are on ATD with a clearly documented plan to stop at the end of a planned course duration, with continued follow‐up of blood tests virtually after stopping treatment. TFT frequency is typically planned as every 8 weeks initially on ATD, with gradually increasing intervals when off ATD in remission, after RAI, when hypothyroid on levothyroxine or when stable on long‐term ATD; typically, when stable on levothyroxine or by 5 years of remission after ATD or RAI, TFTs are requested annually.
2.3. Investigation strategy
In routine clinical practice, we measured sensitive TSH throughout, together with total T4 until February 2001 and free T4 thereafter in line with UK practice, and requested T3 or free T3, where clinically relevant, for example, in cases where TSH was suppressed and fT4 in the normal range. In patients with a diffuse goitre or no palpable goitre, we sought and recorded other manifestations of Graves' disease and typically measured TPO antibody levels. Measurement of TSH‐receptor antibodies (TSHrAbs) was not routinely available during the dates of presentation under review here and was typically only requested during pregnancy for most of the study cohort. Measurement of TSHrAbs is now a routine part of our practice, but was not part of the diagnostic criteria used to define autoimmune thyrotoxicosis for the period of this study. We typically ordered ultrasound when a clinically nodular goitre or asymmetrical goitre was found on examination or in patients with negative TPO and no other features of Graves' disease, but rarely performed thyroid isotope imaging unless it was used to confirm thyroiditis or a single toxic nodule. We did not routinely attempt to assess the volume of the goitre either clinically or radiologically.
2.4. Treatment strategy
2.4.1. Medical versus definitive treatment
Once a biochemical diagnosis of thyrotoxicosis is secured, prompt ATD treatment is initiated and then patients are seen to discuss the three treatment options, offering a patient choice of ongoing treatment modality. We have typically recommended an 18‐month course of ATD for the first diagnosis of thyrotoxicosis without clinical evidence of nodular thyroid disease or thyroiditis and will advocate RAI as a good choice for patients who relapse after ATD, who are intolerant of ATD or who have evidence of multinodular or single nodule aetiology and offer thyroidectomy primarily only to patients who choose it. We have regarded active thyroid‐associated ophthalmopathy as a partial but not absolute contraindication to RAI, and consulted our ophthalmology colleagues regarding the need for pretreatment steroids. In patients who do not want definitive treatment, we offer repeated courses of ATD and consider 'long‐term' ATD for patients who relapse recurrently when ATD is stopped, or when the ATD dose is reduced.
2.5. Antithyroid regime
When commencing ATD for newly diagnosed thyrotoxicosis, we have typically recommended CBZ 40 mg once daily for 4 weeks and then reduced it to 20 mg once daily, based on the results of an earlier collaborative study. 7 Patients are frequently referred to the clinic having already started other dose regimes in primary care. At the start of the virtual clinic, in 1994, we planned to adopt a form of 'block and replace regime' (BRR) continuing with CBZ 20 mg daily and adding levothyroxine 50 or 100 μg daily when TSH rose above or T4 fell below normal, based on the perceived simplicity and stability of such a regime and the then prevailing evidence that BRR might increase the remission rate after ATD. 8 , 9 When we audited TSC data in 2001, 10 we found that relatively few patients had required levothyroxine despite continuing CBZ 20 mg daily, and with subsequent evidence that BRR did not enhance remission rate, 11 , 12 we thereafter adopted a CBZ titration regime as the default regime, reducing ATD dose if TSH rose or as the end of the planned course approached. We advised use of propylthiouracil (PTU) when the patient was intolerant of, or allergic to, CBZ or in women actively planning a pregnancy. 13
2.6. Definitive treatment
For RAI we have used a standard approach throughout the study period with a fixed treatment dose of 15 mCi/555 MBq; ATD pretreatment was given to patients to render them euthyroid in almost all cases. CBZ was stopped for 10–14 days and PTU or BBR regimes at least 21 days before RAI. ATDs were given post treatment only if patients were persistently thyrotoxic at >1month and a repeat dose of RAI was considered if the patient still required ATD after 1 year.
For surgery, the preferred operation in the earlier years of this study was subtotal thyroidectomy with increased use of near‐total thyroidectomy more recently in line with emerging surgical consensus.
2.7. Analysis of TFTs and treatment outcomes
To review a large consecutive cohort with long‐term follow‐up, we analysed data on all patients who were first seen in the clinic and enrolled in TSC after 1 January 1995 and before 1 January 2010 and who had been followed up in TSC for at least 750 days. For these patients, we exported data on every TFT sample up to April 2020 in the TSC database and used a series of software algorithms in LCW and subsequently in Microsoft Excel to link these TFTs with data, all stored encoded within LCW, on ATD and levothyroxine treatment, RAI, thyroidectomy, markers of aetiology, a variety of other relevant clinical features and TSC process and strategy terms.
We developed procedures in Excel, using VBA, a standard programing language to allow program‐driven data, which ran through all sequential TFTs in date order in the same patient, and grouped TFTs together when all aspects of thyroid therapy remained the same. We refer to these groups of TFTs as a ‘phase of thyroid care' (POTC). The software procedure created a new POTC when any of the following changed: starting or stopping ATD, starting or stopping levothyroxine and RAI treatment or thyroidectomy. Each POTC was automatically given a descriptive label to reflect these changes (e.g., 'On ATD', 'Off Treatment', 'Post RAI—Off Treatment', 'Post Thyroidectomy—on T4'). Each POTC on ATD was analysed as a distinct 'course' of ATD. For the purposes of analysis, we regarded any course of ATD lasting over 2 completed years as the ‘long term’.
Although technology changed, all TFT samples were assayed in a single district laboratory and the reference ranges for fT4, tT4 and TSH remained constant throughout the study period: tT4 (60–160 nmol/L), fT4 (9–25 pmol/L) and TSH (0.3–5.0 mIU/L). The software algorithms calculated, for each TFT sample, whether fT4 or tT4 (hereafter referred to as T4), TSH and both T4 + TSH were normal.
We used every available clinical data item in the exported data to allocate the clinically most likely aetiology of thyrotoxicosis to each sample, patient and POTC on the following basis:
-
a.
‘Autoimmune’—Diffuse goitre (or no goitre) with no record of another cause (thyroiditis, amiodarone) and no record of the nodular disease. Considered ‘proven’ in the presence of positive thyroid antibodies and/or clinical features of Graves’ disease and otherwise ‘presumed’.
-
b.
‘Nodular’—negative thyroid antibodies (or none recorded), no clinical features of Graves' disease, and a diagnosis or scan result confirming multinodular goitre or thyroid nodule.
-
c.
‘Mixed’—evidence for both autoimmune and nodular disease, for example, positive antibodies and nodular scan appearances, making it difficult to be certain about the predominant lesion
When studying remission after ATD, we analysed any POTC on no medication (with ATD or levothyroxine), which followed a course of ATD treatment in patients who had not previously received RAI or thyroidectomy. In classifying outcome, ‘remission’ indicates that the patient remained off treatment or had become hypothyroid, at the latest TFT; ‘relapse’ indicates initiation of a new POTC with ATD, RAI or thyroidectomy. Hypothyroidism was identified by the initiation of a new POTC with levothyroxine.
For the main analysis of treatment outcomes in the 66,655 sets of TFTs in the export, we excluded the following patients:
Patients who had received amiodarone or a diagnosis of amiodarone‐induced thyrotoxicosis.
Patients with definite or possible thyroiditis.
Patients with T3 toxicosis (defined by fT3 above reference range).
Patients with only subclinical thyroid disease.
2.8. Statistical analysis
Data were initially analysed manually in Excel, initially using pivot table analysis and then more formal statistical analysis where possible clinically relevant differences or trends were apparent. Proportions of patients in different treatment and aetiological groups were compared by χ 2 with Yates correction. The probability of relapse over time was later investigated using the Kaplan–Meier estimate, stratified by aetiology of thyrotoxicosis.
3. RESULTS
3.1. Patients excluded
The exported data set comprised 66,555 sets of TFTs in 1784 patients. We excluded 50 patients due to amiodarone, 56 due to thyroiditis, 79 with forms of T3 toxicosis, and 110 with borderline thyroid states, as well as any TFTs in the database that dated before TSC monitoring was started.
3.2. Study cohort
The data set represented 55% of all patients with any form of thyrotoxicosis, who were first seen in the department between 1995 and 2009—both before and after the relevant exclusions. Patients not suitable for TSC included those living in areas where blood tests are sent to laboratories outside our region, patients moving area, unsuitable to comply with a virtual clinic, for example, lack of ability to organize test with GP, no stable address, illiteracy or poor English, and those lost to follow‐up. During that time, total TFTs reported via TSC rose from 898/year in 1995 to 4597/year in 2005 and over 7000/year from 2015 onwards. This increase in number is in parallel with the increase in the number of patients referred over this period of time.
Full analysis was then performed on 54,617 TFTs in 1489 patients (median age 47 years [13–92 years] at diagnosis; 77% ♀; median 32, maximum 124 TFTs per patient) covering 16,181 patient‐years of observation (median 10.9 years, maximum 24.7 years). Patients had been seen in the clinic a median of five times, and at the time of the latest TFT, a median of 5.2 years after last clinic attendance. TSC had been stopped before the time of analysis in 71% and the patient was referred back to primary care; 11.5% were known to have died at the point of analysis.
3.3. Aetiology of thyrotoxicosis
The aetiology was classified as 'Autoimmune' in 85.9% (46.9% 'proven'; 39.0% 'likely'), 'Nodular' in 9.1% and 'Mixed' in 5.0%. Overall, 80% of the nodular disease was multinodular goitre, and nodularity was an ultrasound finding only in 55%.
3.4. Treatment of thyrotoxicosis
ATD had been used in 99.2% (98.1% had received CBZ, 14.0% PTU), RAI in 26.5% and thyroidectomy in 8.2% of patients. Treatment choices and outcomes for each aetiology are summarized in Table 1. Fewer patients with a nodular disease received ATD, and as would be expected, more patients with the nodular and mixed disease received RAI and thyroidectomy. Patients had started a median of two courses of ATD (maximum seven courses), and the distribution of the number of ATD courses by aetiology is shown in Figure 1. Long‐term ATD had been used during management in 48% of patients.
Table 1.
Summary of patient treatment choices and outcomes by aetiology
| Autoimmune | Nodular | Mixed | |
|---|---|---|---|
| Number of patients | 1279 (85.9%) | 136 (9.1%) | 74 (5.0%) |
| Had ATD | 99.3% | 97.8% | 100% |
| Had carbimazole | 98.2% | 96.3% | 100% |
| Had PTU | 14.4% | 10.3% | 14.9% |
| ATD courses [mean (maximum)] | 1.9 (7) | 1.7 (4) | 1.9 (4) |
| On ATD at latest TFTs | 17% | 30% | 9% |
| Had long‐term ATD | 47% | 57% | 49% |
| RAI | 24% | 46% | 38% |
| Thyroidectomy | 7% | 10% | 18% |
| On levothyroxine at latest TFTs | 29% | 25% | 41% |
| Median follow‐up from TSC start [years (range)] | 11.0 (2.1–24.7) | 9.6 (2.1–23.7) | 10.7 (2.4–23.4) |
| Median interval since last clinic attendance [years (range)] | 5.2 (0–23.4) | 4.3 (0–20.5) | 6.2 (0–18.1) |
| Median number of TFTs per patient | 32 | 32.5 | 36 |
| Result of latest TFTs: normal T4 | 97% | 96% | 97% |
| Normal TSH | 82% | 79% | 84% |
| Normal T4 + TSH | 80% | 76% | 84% |
| Low TSH | 12% | 17% | 16% |
| High TSH | 6% | 4% | 0% |
Abbreviations: ATD, antithyroid drug; PTU, propylthiouracil; RAI, radioiodine; TFT, thyroid function test; TSC, thyrotoxicosis shared‐care scheme; TSH, thyroid‐stimulating hormone.
Figure 1.
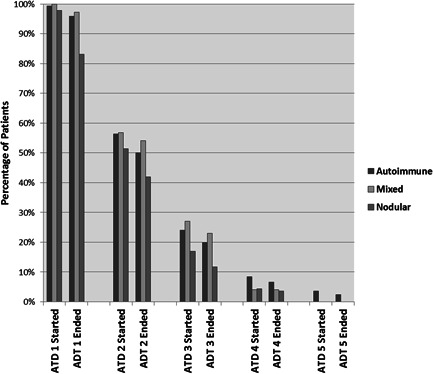
Percentage of patients who started and ended courses of antithyroid drugs (ATDs). The proportion of cases are shown, divided by aetiology, who started (and ended), ATD1–5 indicates separate courses of ATD.
3.5. Control of thyroid function
Amongst all individual TFTs in the data set, T4 was more often in the normal range (80%–98% in different treatment contexts) than TSH (39%–76%) or both (36%–75%), reflecting the fact that often patients had persistently suppressed TSH levels after treatment of thyrotoxicosis despite improvement of symptoms and normalization of T4.
To compare the control of thyroid function throughout the duration of different treatment regimes, we identified 4947 individual POTC and calculated the length of each POTC, as well as the percentage control of each TFT parameter in that time. Table 2 summarizes the treatment type, length of treatment and mean percentage control of TFT parameters. Table 3 shows the same parameters during the latest POTC in each of the 1489 patients.
Table 2.
Analysis of TFT control in 4947 phases of thyroid care in different clinical contexts
| Mean of percentage control of TFT parameters in POTC | ||||||
|---|---|---|---|---|---|---|
| Phase description | Number of POTCs (patients) | Mean POTC length (years) | Mean number of TFTs in POTC | T4 (%) | TSH (%) | T4+ TSH (%) |
| ATD treatment courses | ||||||
| All ATD Rx courses | 2447 | 2.4 | 12.1 | 83 | 49 | 46 |
| All ATD courses as primarya treatment | 2231 | 2.4 | 12.2 | 85 | 50 | 47 |
| First‐course ATD | 1111 | 2.1 | 11.0 | 86 | 52 | 49 |
| Long‐term ATD > 2 years | 607 (528) | 6.1 | 26.8 | 89 | 62 | 58 |
| Long‐term ATD > 5 years | 277 (275) | 9.6 | 38.6 | 89 | 66 | 62 |
| Off Rx | 1546 | 3.4 | 8.5 | 78 | 56 | 54 |
| RAI | ||||||
| Post‐RAI on ATD | 173 | 2.2 | 9.6 | 63 | 32 | 27 |
| Post‐RAI off Rx | 411 | 1.2 | 4.6 | 54 | 23 | 21 |
| Post‐RAI on T4 | 279 | 8.1 | 22.4 | 93 | 60 | 57 |
| Thyroidectomy | ||||||
| Post surgery on T4 | 79 | 7.7 | 21.5 | 91 | 56 | 51 |
| Post surgery off Rx | 67 | 1.3 | 4.4 | 70 | 34 | 30 |
| Post surgery on ATD | 42 | 3.1 | 14.9 | 90 | 47 | 43 |
| Other on T4 | 94 (70) | 5.9 | 15.7 | 87 | 66 | 62 |
Note: The percentage of TFT parameters controlled was calculated for each POTC and the mean of these percentages is presented in the table for each parameter in each phase group.
Abbreviations: ATD, antithyroid drug; POTC, phase of thyroid care; RAI, radioiodine; Rx, treatment; TFT, thyroid function test; TSH, thyroid‐stimulating hormone.
Primary treatment signifies any use of ATD before RAI or thyroidectomy.
Table 3.
Treatment status and TFT control during the latest phase of thyroid care in 1489 patients
| Result of latest TFTs | Mean of percentage control of TFTs in POTC | ||||||
|---|---|---|---|---|---|---|---|
| Treatment status during the latest POC | Number of patients (%) | Mean current POTC length (years) | T4 normal (%) | TSH normal (%) | T4 (%) | TSH | T4 + TSH (%) |
| Off treatment | 562 (38) | 6.9 | 99 | 90 | 95 | 87 | 83 |
| On ATD | 356 (24) | 6.9 | 96 | 76 | 89 | 64 | 59 |
| Long‐term ATD > 2 years | 294 (20) | 8.1 | 97 | 80 | 90 | 68 | 64 |
| Long‐term ATD > 5 years | 207 (14) | 10.1 | 97 | 81 | 90 | 70 | 66 |
| On T4 (without ablative therapy) | 77 (5) | 6.7 | 100 | 82 | 94 | 72 | 68 |
| After RAI (+ surgery) | 393 (26) | 7.5 | 98 | 79 | 92 | 60 | 57 |
| After thyroidectomy (without RAI) | 101 (7) | 7.5 | 96 | 66 | 91 | 58 | 53 |
Note: Patients who had been treated for both RAI and thyroidectomy are analysed in the RAI totals.
Abbreviations: ATD, antithyroid drug; POTC, phase of thyroid care; RAI, radioiodine; TFT, thyroid function test; TSH, thyroid‐stimulating hormone.
In the latest treatment regime, 38% were off all treatment, 26% had RAI, 7% were post thyroidectomy and 5% were on levothyroxine without prior ablative therapy. The remaining 24% of patients remained on ATD, and in 20% this treatment was long term (20% >2 years; 14% >5 years). Control of TFT parameters in this latest POTC was best seen in patients who were off treatment, followed by those on T4 without ablative therapy, those on long‐term ATD and those on ATD after RAI or thyroidectomy (in descending order of control of both T4 + TSH). Overall, in clinical terms, TFT control on all three long‐term treatment options (ATD, RAI and thyroidectomy) were broadly similar, although the highest percentage of normal TSH levels were seen in those patients on long‐term ATD.
3.6. Remission and relapse rates
We identified 1398 post‐ATD remission phases, of which 831 remission phases followed the first course of ATD. The mean interval from the start of this post‐ATD phase to the date of the latest TFTs was 9.0 years. Overall, 57.2% of these phases ended in relapse (55.5% after the first course, 59.6% after subsequent courses); in the 1257 phases where the length of the preceding course of ATD was at least 180 days, the equivalent relapse rates were 56.2% (54.9% and 58.2%).
The detailed outcomes of these 1257 phases are summarized in Table 4. In autoimmune aetiology, the relapse rate was 52.7% after the first course of ATD occurring at a mean of 542 days. Relapse rate was higher in nodular disease (79.1% after a first course), but it was notable that a significant minority of cases remained in remission off treatment at prolonged follow‐up (20.9% at a mean follow‐up of 7.8 years).
Table 4.
Crude rates of remission, relapse and hypothyroidism after first and subsequent ATD treatment courses in different aetiologies
| Aetiology and course number | Number of periods of care | Relapse (%) (mean F/U to the outcome) | Remission (all) (%) (mean F/U to the outcome) | Remission becoming hypothyroid (mean F/U to the outcome) | Mean ATD course length (days) |
|---|---|---|---|---|---|
| Autoimmune | |||||
| First ATD course | 698 | 52.7% (542) | 47.3% (2956) | 5.0% (816) | 663 |
| Other courses | 437 | 58.8% (386) | 41.2% (1917) | 5.0% (1048) | 796 |
| Nodular | |||||
| First ATD course | 43 | 79.1% (431) | 20.9% (2839) | 2.3% | 713 |
| Other courses | 21 | 52.4% (209) | 47.6% (1681) | 4.8% | 968 |
| Mixed | |||||
| First ATD course | 40 | 67.5% (417) | 32.5% (2977) | 5.0% (2388) | 623 |
| Other courses | 18 | 50.0% (204) | 50.0% (1607) | 11.1% (1205) | 630 |
Note: Outcome of periods of thyroid care monitoring TFTs off treatment following any ATD treatment course of at least 180 days duration. Percentages show the proportion with each outcome at the end of the POTC (identified by the start of treatment for relapse or hypothyroidism or by the latest follow‐up TFT for ongoing remission)—mean follow‐up in days to these outcomes is shown within parentheses. The mean follow‐up from the start of post‐ATD monitoring to the latest TFTs was 9.1 years.
Abbreviations: ATD, antithyroid drug; F/U, follow‐up; POTC, phase of thyroid care; TFT, thyroid function test.
Over 60% of relapses occurred within the first year and over 90% within 4 years, with relapse occurring slightly earlier in patients with the nodular disease and after repeat courses of ATD in autoimmune disease.
Overall, in patients who relapsed, the median time to that diagnosis was 213 days, but the range was extremely wide from 0 (i.e., relapse identified on the first posttreatment blood) to 20.6 years. The median time to the first low TSH was 203 days.
Spontaneous hypothyroidism occurred in 5% of autoimmune cases during remission monitoring and in a similar proportion of other aetiologies—with these patients remaining hypothyroid a mean of 10.5 years later.
The probability of relapse over time was related to the aetiology of thyrotoxicosis, resulting in around 40% within 1 year in subjects with mixed or nodular thyrotoxicosis and 25% for autoimmune aetiology (Figure 2).
Figure 2.
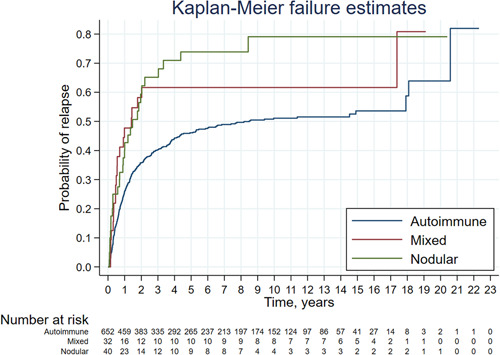
Kaplan–Meier curve showing the probability of thyrotoxicosis relapse based on aetiology of the 831 post‐ATD remission phases following the first course of ATD; 107 (102 with relapse and 5 with remission/hypothyroidism at starting of observation) were excluded. ATD, antithyroid drug [Color figure can be viewed at wileyonlinelibrary.com]
3.7. Other factors influencing relapse
Patients with definite evidence of ophthalmopathy were more likely to relapse after the first course of ATD (62% vs. 49%; p < .05) but not after subsequent courses (p > .4). We found no difference in relapse rate in women versus men after the first ATD course (53% vs. 52%; p > .5) or subsequent courses (60% vs. 57%; p > .5). There was no significant difference in relapse rate between CBZ and PTU (p > .3).
There was a trend to increasing remission rate with increasing ATD course length in the first remission in autoimmune aetiology, but this was not seen in other aetiologies or in subsequent courses (data not shown). However, since our standard clinical strategy was an 18‐month course of ATD, courses of shorter and longer length may well represent a significant selection bias rather than the effect of treatment duration per se, and we therefore did not analyse further.
3.8. Outcomes after RAI
We identified RAI treatment in 393 cases. Outcomes and times to relevant diagnostic markers are shown in Table 5. At the latest follow‐up, the development of hypothyroidism was most common and occurred earlier in patients with autoimmune aetiology (80% at median 168 days) and was least common and occurred later in patients with nodular thyrotoxicosis (38% at median 748 days) with intermediate values in mixed cases. Overall, 11% of autoimmune and mixed cases and 18% of nodular cases remained on ATD at the latest follow‐up (at a mean of 6.5 years after RAI), but only 6% autoimmune and 13% nodular cases had received more than one dose of RAI (at a median of 679 days after the first dose).
Table 5.
Outcomes of RAI treatment in POTC analysis, stratified by aetiology
| Parameter | Autoimmune | Nodular | Mixed |
|---|---|---|---|
| Number of cases | 303 | 62 | 28 |
| Hypothyroidism diagnosed | 80% | 38% | 71% |
| On ATD at the latest F/U | 11% | 18% | 11% |
| Off Rx at the latest F/U | 9% | 44% | 18% |
| Days F/U [median (range)] | 2772 (23–8560) | 2798 (405–7908) | 2929 (400–7455) |
| Median days to TSH normalizationa | 92 | 202 | 112 |
| Median days to hypothyroidism (range) | 168 (47–5978) | 745 (123–2751) | 196 (124–2791) |
| Still on ATD > 360 days after RAI | 11% | 18% | 11% |
| RAI dose repeated | 6% | 13% | 21% |
Abbreviations: ATD, antithyroid drug; F/U, follow‐up; POTC, phase of thyroid care; RAI, radioiodine; Rx, treatment; TSC, thyrotoxicosis shared‐care scheme; TSH, thyroid‐stimulating hormone.
Days to first TSH, which was normal or high off all treatment after RAI (excluding patients in whom TSC started >60 days after RAI).
3.9. Adverse events on ATD
Patients who were continuing long‐term ATD at the latest follow‐up were by definition tolerating treatment without clinically significant adverse effects, but we sought any mention of a definite or possible ATD adverse reaction or allergy in LCW for every patient, including diagnosis of neutropenia or agranulocytosis; these adverse reactions were found by searching for the SNOMED CT clinical terms entered by the supervising senior clinician at the time of diagnosis, rather than predefined haematological parameters. Definite or possible neutropenia was recorded as a diagnosis in 1.3% of patients, but this was often mild and usually not considered drug‐related. None of the patients had a formal diagnosis of agranulocytosis. Overall, a definite or possible adverse reaction to one or more ATD and/or a diagnosis of neutropenia was present in 6% cases, but this was no more common in long‐term ATD patients and was broadly similar to rates reported in other studies. 1
4. DISCUSSION
There are few studies observing the natural history of thyrotoxicosis in a large number of patients in a single centre over an extended period of time. Despite the unavoidable inaccuracies inherent in retrospective analyses, this was a real‐life observation of the long‐term follow‐up of thyrotoxicosis in a single specialist centre. The most striking observation is that it is possible to achieve excellent control of thyroid function with a virtual clinic without the need to physically bring patients to the clinic more than annually. We developed our own software to achieve this over 20 years ago, but most NHS departments still do not have access to clinical systems that are capable of supporting these functions. We suggest that attempting to manage thyrotoxicosis in the community without access to specialist advice in this ‘shared‐care’ way is unlikely to achieve the good control of TFTs, which we report here.
Our department has long advocated patient choice in the long‐term treatment of thyrotoxicosis and has been happy to use repeated courses of antithyroid medication. We found that many patients opt for long‐term ATDs rather than definitive treatment, and this group of patients had good control with no apparent serious side effects of long‐term drug therapy. As a result, we have managed a large number of patients with ‘long‐term’ medical treatment. The results of our real‐life observational study confirm that such long‐term therapy is frequently chosen, safe and well‐tolerated in clinical practice. In our cohort, patients achieved control at least as good as those who had RAI or thyroidectomy. We do not argue that long‐term ATD is better than surgery or RAI, but this study confirms that it may be a valid patient treatment choice, particularly in the context of a virtual clinic, which can cope with the long‐term follow‐up of such patients. The pros and cons of RAI and surgery continue to apply and are often the treatment of choice for both patients and physicians. Our findings confirm a previous study comparing outcomes of RAI and long‐term methimazole. 14 We found no evidence in our data that longer courses of antithyroid treatment improve remission rates after ATD is withdrawn, but confirm a widely held clinical impression that they can effectively maintain control long‐term while treatment continues.
After ATD, we observed relapse rates of over 50% in all aetiologies. Remission rates were highest in autoimmune disease and lowest in patients with the nodular disease, although remission was still observed in this group. We observed that relapse was more likely to occur in the presence of ophthalmopathy, but did not show any difference between the sexes in our cohort. We used a standard 18‐month ATD course as our routine strategy, which is suggested to be sufficient to optimize the posttreatment remission rate. 1 Our study followed up patients for longer than many previous clinical trials, so our long‐term remission and relapse rates are likely to represent the true final outcome of this cohort of patients (Figure 2 and Table 5). The pattern of relapse we observed was very similar to other large long‐term studies of Graves' disease. 15 As expected, relapse was more common when there was evidence of nodular disease, but we were surprised that a sizeable minority of cases, at least 20%, remained in remission at the latest follow‐up, meaning that a course of ATD may well be a valid patient choice in these circumstances. It is quite possible that a proportion of patients labelled as having a nodular disease based on negative antibodies and ultrasound findings may have had autoimmune disease within a nodular goitre. The current use of TSHrAbs and increased use of thyroid uptake scans would give a better characterization of the precise aetiology of thyrotoxicosis, thus we are reluctant to make definitive conclusions regarding the natural history of nodular thyroid disease. We found that relapse of thyrotoxicosis occurred most commonly in the first year after completion of the ATD course, and was uncommon after 5 years, particularly in patients with a nodular disease. Relapse was very rare after 10 years of remission, but did occur in a small number of patients, suggesting that there needs to be some form of long‐term follow‐up in patients with a history of thyrotoxicosis even if from primary care after 10 years of remission. Our findings might allow more individualized estimations of appropriate follow‐up intervals and risk of relapse in patients with thyrotoxicosis. A more recent study looking at Grave's disease in 659 patients showed a higher remission (73.6%) and lower relapse (36.7%) rate. In this study, the diagnosis of Grave's was improved in our study by the use of TSRAbs in 56% of patients and isotope scanning in 26.7%, although it was still not possible to be unequivocal about the aetiology. 16
UK RAI Guidelines 1 , 17 currently allow a wide range of different treatment approaches and RAI doses and these differences have led to conflicting outcomes in the literature. 18 We used a fixed ablative dose of 555 MBq in line with the current practice that calculated doses are not superior to calculated doses in patients with thyrotoxicosis. 1 The best control of thyroid status after RAI in our study was seen in patients who had become hypothyroid on levothyroxine for autoimmune thyrotoxicosis, which was achieved in most patients with 555 MBq. In our study, only a minority of patients with the nodular disease became hypothyroid, but only 2% had persistent thyrotoxicosis, supporting our longstanding recommendation for RAI when such patients relapse after ATD. This majority of patients with toxic multinodular goitre were euthyroid in the long term after RAI in line with some previous reports, 19 but not others. 20 Overall, our results confirm the effectiveness of RAI in control of thyrotoxicosis in a large majority of patients.
The main weakness of this study is that the aetiology of hyperthyroidism was defined by the supervising clinican as the most likely clinical diagnosis based on the contemporaneous clinical and laboratory data at presentation. In many patients, this was before TRAbs had been introduced into routine practice to confirm autoimmune aetiology. Another weakness is that reference ranges and assay platforms have changed during the course of the study, giving inherent difficulty with uniformity of analysis and categorization of results as in other similar large studies. Nevertheless, we think these weaknesses are offset by the very large number of patients, the length of time of follow‐up, and the fact that the likely aetiology was a diagnosis made by a senior endocrinologist, which reflects clinical real‐life practice. In fact, the observation that nodular hyperthyroidism may go into remission strongly suggests that patients may have nodularity within an autoimmune presentation, indicating that dual pathology can occur and that predominant pathology may only be determined after isotope scanning in addition to other clinical and laboratory information, which is often not done even today.
Whilst a virtual clinic is a convenient way to manage a hyperthyroid service for both patient and clinician, it is important to emphasize that there is no substitute for a clinical consultation at the appropriate time. All patients are seen as new patients, which allows the clinician to detect subtle clinical features such as mild orbital disease, but previous cases may have been missed if they developed symptoms during follow‐up in‐between face‐to‐face appointments. Given the potential to miss orbitopathy, our practice is now to advise patients to inform the endocrinology specialist nurses if symptoms arise. We have also started to regularly check TSHrAbs in all new patients and after stopping CBZ to see if this might predict future recurrence in future analyses of our TSC cohort.
In this real‐life observation study of thyrotoxicosis in a single centre using a virtual clinic, we found that good control of thyrotoxicosis can be readily achieved with appropriate endocrine expertise and good software. We have found this to be an efficient way of controlling large numbers of patients with thyrotoxicosis as well as a very useful way of observing the outcomes of this disease over a long period of time. The findings of this study have confirmed some of the long‐held beliefs about hyperthyroidism and questioned others. We found that patients who request long‐term antithyroid treatment had good control of thyroid function and this appears to be a safe and effective treatment strategy in patients who do not want definitive treatment. We would advocate that as long as patients are seen intermittently to discuss overarching management options and where problems arise, the use of a virtual clinic is a safe and efficient way of managing thyrotoxicosis in a large number of patients.
ACKNOWLEDGEMENT
This manuscript is dedicated to Nikki Kieffer, endocrine specialist nurse, who ran the majority of the virtual shared‐care clinic in this time period.
Levy M, Reddy N, Price D, et al. Audit of long‐term treatment outcomes of thyrotoxicosis in a single‐centre virtual clinic: the utility of long‐term antithyroid drugs. Clin Endocrinol (Oxf). 2022;97:643‐653. 10.1111/cen.14721
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. NICE National Institute for Health and Care Excellence .Thyroid Disease: Assessment and Management. NICE Guideline (NG145). 2019. [PubMed] [Google Scholar]
- 2. Ross DS, Burch HB, Cooper DS, et al. American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343‐1421. [DOI] [PubMed] [Google Scholar]
- 3. Codaccioni JL, Orgiazzi J, Blanc P, Pugeat M, Roulier R, Carayon P. Lasting remissions in patients treated for Graves' hyperthyroidism with propranolol alone: a pattern of spontaneous evolution of the disease. J Clin Endocrinol Metab. 1988;67(4):656‐662. [DOI] [PubMed] [Google Scholar]
- 4. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet. 2016;388(10047):906‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the diagnosis and treatment of Graves' disease in Europe, Japan, and the United States. Thyroid. 1991;1:129‐135. [DOI] [PubMed] [Google Scholar]
- 6.Carbimazole: risk of acute pancreatitis. Medicines and Healthcare products Regulatory Agency. 2019. http://www.government/organisations/medicines-and-healthcare-products-regulatory-agency
- 7. Page SR, Sheard CE, Herbert M, Hopton M, Jeffcoate WJ. A comparison of 20 or 40 mg per day of carbimazole in the initial treatment of hyperthyroidism. Clin Endocrinol. 1996;45:511‐516. [DOI] [PubMed] [Google Scholar]
- 8. Romaldini JH, Bromberg N, Werner RS, et al. Comparison of effects of high and low dosage regimens of antithyroid drugs in the management of Graves' hyperthyroidism. J Clin Endocrinol Metab. 1983;57:563‐570. [DOI] [PubMed] [Google Scholar]
- 9. Hashizume K, Ichikawa K, Sakurai A, et al. Administration of thyroxine in treated Graves' disease. Effects on the level of antibodies to thyroid‐stimulating hormone receptors and on the risk of recurrence of hyperthyroidism. N Engl J Med. 1991;324:947‐953. [DOI] [PubMed] [Google Scholar]
- 10. Chatterjee S, Kieffer V, Howlett T. Analysis of a clinical workstation thyrotoxicosis share‐care scheme. Endocr Abstr. 2002;3:P279. [Google Scholar]
- 11. McIver B, Rae P, Beckett G, Wilkinson E, Gold A, Toft A. Lack of effect of thyroxine in patients with Graves' hyperthyroidism who are treated with an antithyroid drug. N Engl J Med. 1996;334:220‐224. [DOI] [PubMed] [Google Scholar]
- 12. Abraham P, Avenell A, Park CM, Watson WA, Bevan JS. A systematic review of drug therapy for Graves' hyperthyroidism. Eur J Endocrinol. 2005;153:489‐498. [DOI] [PubMed] [Google Scholar]
- 13. De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2012;97:2543‐2565. [DOI] [PubMed] [Google Scholar]
- 14. Azizi F, Ataie L, Hedayati M, Mehrabi Y, Sheikholeslami F. Effect of long‐term continuous methimazole treatment of hyperthyroidism: comparison with radioiodine. Eur J Endocrinol. 2005;152:695‐701. [DOI] [PubMed] [Google Scholar]
- 15. Benker G, Reinwein D, Kahaly G, et al. Is there a methimazole dose effect on remission rate in Graves' disease? Results from a long‐term prospective study. The European Multicentre Trial Group of the Treatment of Hyperthyroidism with Antithyroid Drugs. Clin Endocrinol. 1998;49:451‐457. [DOI] [PubMed] [Google Scholar]
- 16. Hussein YS, Hookham JC, Allahabadia A, Balasubramanian SP. Epidemiology, management and outcome of Grave's disease—real life data. Endocrine. 2017;56:568‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Royal College of Physicians Working Party . Radioiodine in the Management of Benign Thyroid Disease: Clinical Guidelines. Royal College of Physicians; 2007. [Google Scholar]
- 18. Bonnema SJ, Hegedus L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33:920‐980. [DOI] [PubMed] [Google Scholar]
- 19. Leary AC, Grealy G, Higgins TM, et al. Long‐term outcomes of treatment of hyperthyroidism in Ireland. Ir J Med Sci. 1999;168:47‐52. [DOI] [PubMed] [Google Scholar]
- 20. Kahraman D, Keller C, Schneider C, et al. Development of hypothyroidism during long‐term follow‐up of patients with toxic nodular goitre after radioiodine therapy. Clin Endocrinol. 2012;76:297‐303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


