Abstract
Dry eye disease (DED) is a highly prevalent and debilitating condition affecting several hundred million people worldwide. Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan commonly used in the treatment of DED. This review aims to critically evaluate the literature on the safety and efficacy of artificial tears containing HA used in DED treatment. Literature searches were conducted in PubMed, including MEDLINE, and in Embase via Ovid with the search term: “(hyaluronic acid OR hyaluronan OR hyaluronate) AND (dry eye OR sicca)”. A total of 53 clinical trials are included in this review, including eight placebo‐controlled trials. Hyaluronic acid concentrations ranged from 0.1% to 0.4%. Studies lasted up to 3 months. A broad spectrum of DED types and severities was represented in the reviewed literature. No major complications or adverse events were reported. Artificial tears containing 0.1% to 0.4% HA were effective at improving both signs and symptoms of DED. Two major gaps in the literature have been identified: 1. no study investigated the ideal drop frequency for HA‐containing eyedrops, and 2. insufficient evidence was presented to recommend any specific HA formulation over another. Future investigations assessing the optimal drop frequency for different concentrations and molecular weights of HA, different drop formulations, including tonicity, and accounting for DED severity and aetiology are essential for an evidence‐based, individualized approach to DED treatment.
Keywords: artificial tears, dry eye disease, dry eye treatment, hyaluronate, hyaluronic acid
Introduction
Dry eye disease (DED) is a complex and debilitating inflammatory condition of the ocular surface. World‐wide epidemiological studies find that DED prevalence ranges from 5–50% (Stapleton et al. 2017). When extrapolated, this would equate to between 400 million and 3.7 billion DED patients globally. Dry eye disease increases with age, with the prevalence of the condition rising steeply from around 50 years of age (Vehof et al. 2021). Through limiting participation in working life and daily activities and increasing healthcare costs, DED introduces a substantial financial burden for both the individual patient and society as a whole (Stapleton et al. 2017). The estimated yearly direct and indirect costs of DED to the US society alone is 56 billion USD (Yu et al. 2011). For patients, DED causes a considerable reduction in quality of life and quality of vision (Morthen et al. 2021, 2022).
Dry eye disease results in deterioration of the ocular surface including dysfunction of the tear film, lacrimal system, eyelids, conjunctiva, and cornea (Bron et al. 2017). The healthy tear film serves to protect and lubricate the ocular surface by providing a physical, chemical, and immunological barrier to the environment. The tear film consists of an inner muco‐aqueous layer and an outer lipid layer that combined contribute to a stable ocular surface in a normal eye (Fig. 1; Craig et al. 2017). Aetiologically, DED is divided into aqueous deficient, evaporative, and mixed types. In DED, regardless of aetiology, ocular surface instability promotes a vicious circle of inflammation, exacerbating signs and symptoms of disease, and damage of the ocular surface (Bron et al. 2017). Breaking this vicious circle, plays an essential role in the treatment of DED.
Fig. 1.
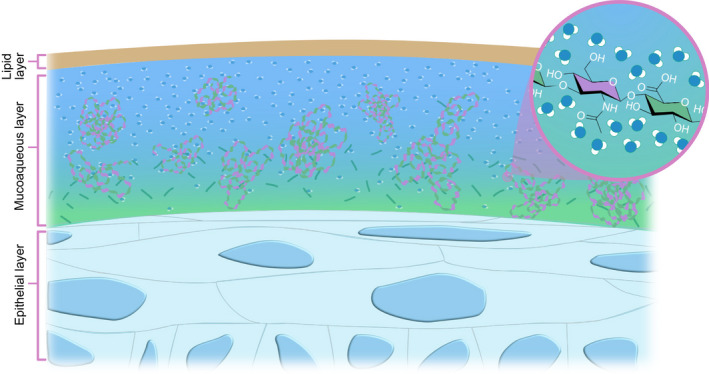
Illustration of the pre‐corneal tear film containing hyaluronic acid. Hyaluronic acid attracts surrounding water molecules with its numerous hydroxyl groups, thickening and stabilizing the mucoaqueous layer of the tear film. Illustration by Emily Moschowits.
Artificial tears are the first‐line treatment for DED. They help restore and stabilize the tear film and protect the ocular surface (Jones et al. 2017). This aids in slowing or stopping DED progression which decreases signs and symptoms and prevents further damage (Nebbioso et al. 2016). There is a wide array of artificial tears on the market with various active ingredients. One clinically proven and commonly used component is hyaluronic acid (HA; Ang et al. 2017).
Hyaluronic acid is a naturally occurring, non‐toxic (Debbasch et al. 2002) glycosaminoglycan disaccharide bi‐polymer (Silvani et al. 2020; Fig. 2). Hyaluronic acid serves several crucial purposes in the human body, including joint and tendon lubrication (Lin et al. 2019) and cell‐to‐cell communication (Bayer 2020). Hyaluronic acid was first discovered in bovine vitreous humour in the 1930s and has since become an important addition in many fields of medicine (Kotla et al. 2021). It is a frequently used component of slow‐release drug formulas (Bayer 2020), skin‐care products, and fillers for cosmetic and reconstructive purposes (Vorvolakos et al. 2011). Due to HA's safety profile and physiological effects, it has become an important substance in ophthalmology (Higashide & Sugiyama 2008; Salwowska et al. 2016; Fig. 3).
Fig. 2.
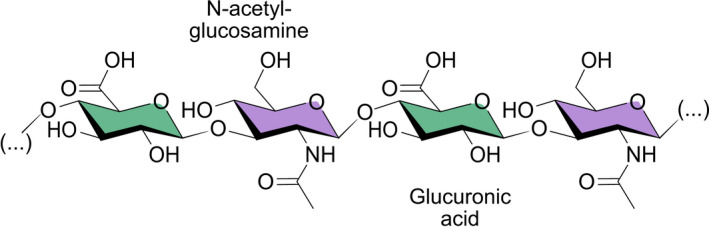
The hyaluronic acid molecule made up of repeating N‐acetyl‐glucosamine and glucuronic acid linked together via alternating β‐1,3 and β‐1,4 glycosidic bonds. Illustration by Emily Moschowits.
Fig. 3.
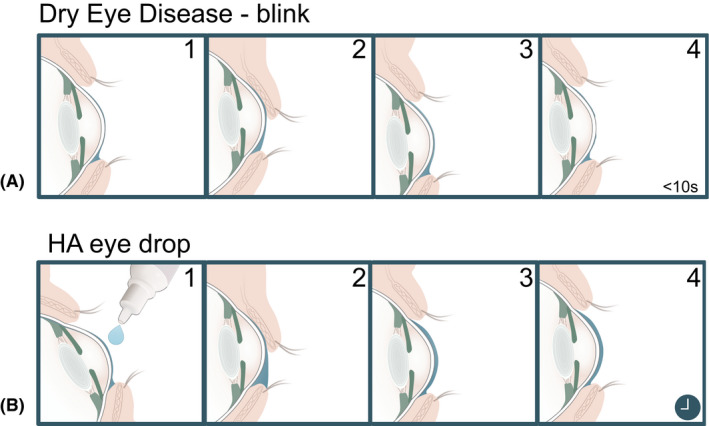
Hyaluronic acid increases tear film stability. (A, 1–4) Cross section of a thin tear film in dry eye disease with short tear film break up time (TBUT). (B, 1–4) After application of hyaluronic acid, the tear film increases in viscosity and thickness and allows for even distribution across the ocular surface. Proportions are exaggerated for illustrative purposes. Illustration by Emily Moschowits.
Hyaluronic acid is found naturally in the tear film, outer cornea, and vitreous humour (Posarelli et al. 2019). However, the highest concentration of HA is found as a chief component of the extracellular matrix in soft connective tissues (Gudowska‐Sawczuk et al. 2017; Mateo Orobia et al. 2018). Under physiological conditions, HA takes the form of a highly hydrophilic, negatively charged bipolymer. It is made of repeating units of N‐acetyl‐d‐glucosamine and d‐glucuronic acid. These units are linked together via alternating β‐1,3 and β‐1,4 glycosidic bonds (Fig. 2). The in‐vivo structure of HA is largely homogeneous, with variable chain lengths and occasional deacetylated glucosamine residues (Mateo Orobia et al. 2018; Bayer 2020). Hyaluronic acid exhibits high pseudo‐plasticity and introduces non‐Newtonian mechanics in fluids (Chernos et al. 2017). This means that the viscosity of the liquid changes depending on the applied shear forces. At the ocular surface, the viscosity of an HA‐containing tear film will decrease during a blink, allowing even distribution of the tear film. Once at rest, higher viscosity is restored which prolongs its residence time on the ocular surface (López‐García et al. 2014; Fig. 3).
Hyaluronic acid is rich in hydroxyl‐groups that attract water molecules, thus thickening and stabilizing the tear film (Kaya et al. 2015; Szegedi et al. 2018), and reducing the effects of mechanical trauma to the ocular surface by lubrication (van Setten 2020), and contributing to re‐epithelialization (Carlson et al. 2018; Fig. 1). Hyaluronic acid also reduces evaporation from the ocular surface (Tsubota & Yamada 1992), the driving force behind hyperosmolarity, which in turn is one of the main causes of inflammation and ocular surface damage in DED (Bron et al. 2017). Additionally, HA binds to hyaladherins, also known as hyaluronan‐binding protein family receptors, which are expressed throughout the body including by the epithelial cells of the ocular surface (Lardner & van Setten 2020). Binding to these receptors activate various intracellular signalling pathways dependent on concentration, molecular weight and modifications of the HA molecule (Abatangelo et al. 2020), which can modulate inflammation, cellular migration and angiogenesis, which are the main phases of wound healing (Litwiniuk et al. 2016). Hyaluronic acid also protects damaged surfaces during wound healing (Debbasch et al. 2002; Pauloin et al. 2009b; Rah 2011; Carracedo et al. 2019; Lardner & van Setten 2020; Kotla et al. 2021). The specific biological effects and physical properties of the HA molecule vary with changing molecular weight, which ranges several orders of magnitude, from a few‐ to several thousand kilodaltons (Snetkov et al. 2020; Kotla et al. 2021). Generally, low molecular weight HA tends to have pro‐inflammatory properties and lower viscosity while high molecular weight HA is anti‐inflammatory and more viscous (Snetkov et al. 2020; Kotla et al. 2021).
Hyaluronic acid has been used as a viscoelastic for intraocular surgery since the 1970s (Higashide & Sugiyama 2008). The first study exploring the effects of HA on DED was published in 1982 (Polack & McNiece 1982). Four years later Mengher et al. (1986) showed that eye drops containing 0.1% HA could improve tear film break‐up time (TBUT) in patients with DED. Since then, HA has become a key component in many artificial tear fluids, improving lubrication and tear film properties. The number of commercial options available on the market that use HA are ever growing (Salwowska et al. 2016). Figure 4 shows the increasingly important role of HA in ophthalmology over time.
Fig. 4.
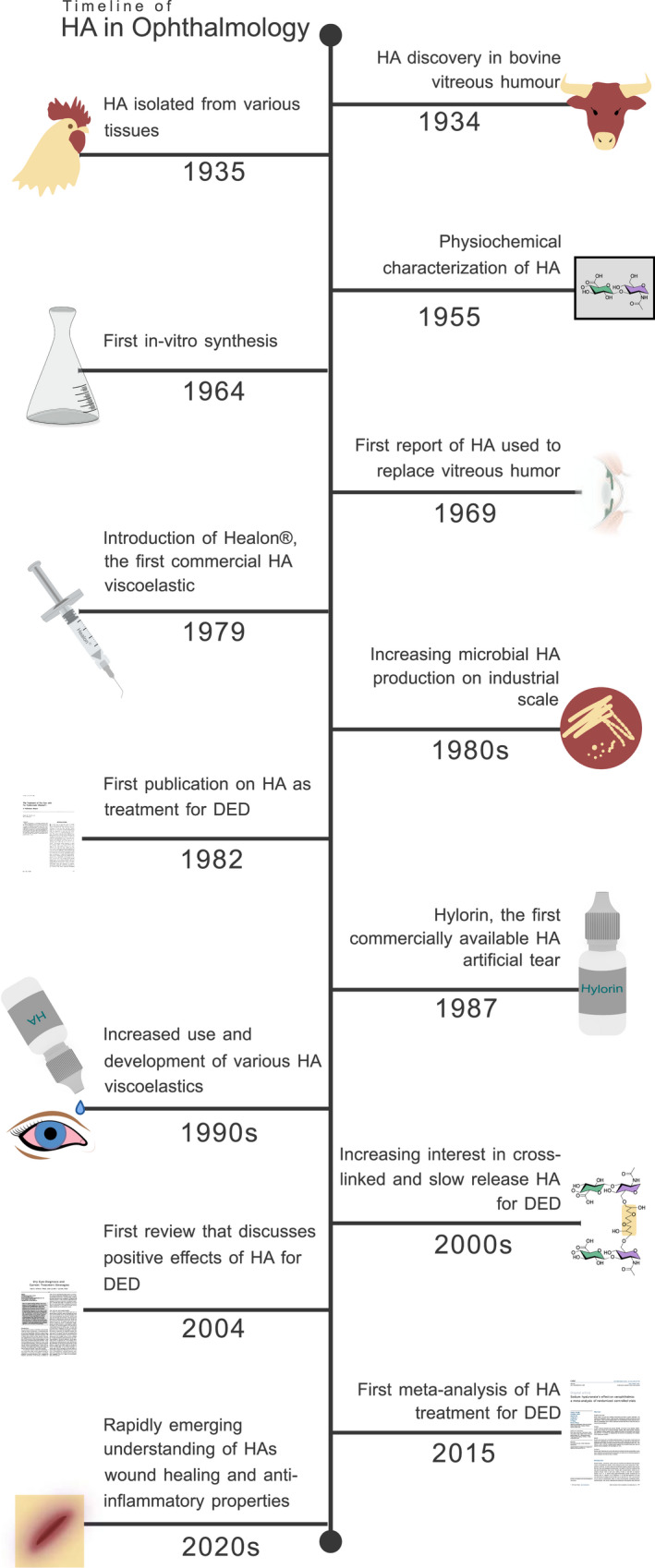
Timeline of the discovery and development of HA in ophthalmology. DED = dry eye disease, HA = Hyaluronic acid. Illustration by Emily Moschowits. [Correction added on 14‐May‐2022, after first online publication: Figure 4 was corrected in this version.]
Several meta‐analyses and reviews have shown that HA is safe and effective in the treatment of DED (Doughty & Glavin 2009; Salwowska et al. 2016; Ang et al. 2017; Yang et al. 2021). This review will summarize and discuss the current literature on the treatment of DED with artificial tears containing HA, the safety and efficacy of HA in treating DED, explore how physiochemical properties of various HA formulations may influence treatment, and shed light on gaps in the literature.
Methods
A literature review was conducted in Embase using Ovid on the 24th of August 2021 and PubMed, including MEDLINE, on the 20th of September 2021 (Fig. 5). The search terms “(hyaluronic acid OR hyaluronan OR hyaluronate) AND (dry eye OR sicca)” were used in both searches. All original English language published articles available in full text were considered. Titles and abstracts were screened to ensure relevance to the topic. Reviews, meta‐analyses, case studies, and papers on unrelated subjects were not considered. When duplicates were identified, the latest version was included. Studies were narrowed down by checking against the exclusion criteria: 1) effect of HA treatment not isolated, 2) no baseline measurements before initiating HA treatment, 3) no appropriate statistical tests reported. Only studies investigating treatment of DED with artificial tears containing HA with reported statistical tests for subjective or objective measurements against baseline or placebo were included.
Fig. 5.
Methodology for determining studies of relevance for the present review.
Results
Review of existing literature
The search term “(hyaluronic acid OR hyaluronan OR hyaluronate) AND (dry eye OR sicca)” in Embase the 24th of August 2021 through Ovid produced 661 results. The same search term in PubMed on the 20th of September 2021 produced 351 results. Studies that investigated eye drops containing HA along with other active ingredients like steroids, cyclosporine, trehalose, or polyethylene glycol were excluded if the effect of HA could not be isolated (Versura et al. 2010; Montani 2013; Macri et al. 2015; Pinto‐Bonilla et al. 2015; Kim et al. 2017; Rolando & Vagge 2017; Fariselli et al. 2018; Fondi et al. 2018; Wu et al. 2021). Studies where the effects of HA treatment could not be isolated for other reasons were excluded (Laflamme & Swieca 1988; Ibrahim et al. 2012; Kamiya et al. 2012; Çakır et al. 2018). Studies without statistical tests comparing the results after HA treatment to either baseline values or to a control group receiving placebo were excluded (Limberg et al. 1987; Nepp et al. 2001; Matsuo 2004; Brignole et al. 2005; Baudouin et al. 2012; Robert et al. 2016; Labetoulle et al. 2018; van Setten et al. 2020).
Finally, 53 clinical trials remained. A flow chart of the process is shown in Fig. 5. The final list of clinical treatment studies (with some overlap due to variations in combinations of study design) includes 8 randomized placebo‐controlled trials (RCTs), 44 baseline controlled RCTs, 6 baseline controlled non‐randomized prospective‐longitudinal studies, and 10 cross‐over studies (Tables 1 and 2). The studies recruited patients in 17 different countries across Europe, Asia, North America, and Africa, including 5 multicenter studies that recruited patients in more than one country (Condon et al. 1999; Baeyens et al. 2012; Gong et al. 2015; Chiambaretta et al. 2017; Labetoulle et al. 2017). The geographical spread of the studies is shown in Fig. 6. Tables 1 and 2 summarize the results of each article. Table 1 summarizes the 50 studies that provided HA treatment results compared to baseline, focusing on reported efficacy in subjective and objective measures. Table 2 summarizes results of HA against placebo in the 8 RCTs that had a double‐blinded study design with a placebo group. Five studies are represented in both tables as they provided statistical results both against baseline and against placebo (Condon et al. 1999; Vogel et al. 2010; Baeyens et al. 2012; López‐de la Rosa et al. 2017; Pinto‐Fraga et al. 2017). Seven studies comparing HA treatment against baseline (Papa et al. 2001; Aragona et al. 2002a; Milafzzo et al. 2002; Troiano & Monaco 2008; Lee et al. 2014a; Groß et al. 2017; Park et al. 2017) and one study comparing against placebo (Sand et al. 1989) had more than one HA treatment arm, making a total of 58 treatment arms in Table 1 and 9 treatment arms in Table 2. Important factors examined in these tables includes the type of study, sample size, disease severity of the sampled population, HA concentration, drop frequency, patient outcomes at last follow up, and other key findings. The results are broken down into subjective and objective measures and compared across articles. Safety features are not represented in the tables as there were no serious adverse effects associated with HA use in any of the studies.
Table 1.
Changes in commonly measured clinical signs and symptoms with HA treatment compared to baseline, sorted by publication date.
| First Author (year) | Design | Population | Setup | Duration | Symp. | TBUT | OSS | Schi. | Other outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Brar S. (Brar et al. 2021) | RCT | 60 DED | 0.1% HA x4/d | 3 mo | ↑ | ↑ |
↑ tear film osmolarity, ↑ TMH, ↑ MG loss, ↑ objective scatter index, ⟷ VA |
||
| Morya A. K. (Morya et al. 2021) | SB RCT | 384 mild‐to‐severe DED | 0.1% HA x4/d | 2 mo | ↑ | ⟷ | ↑ | ⟷ | ⟷ TMH |
| Balestrazzi A. (Balestrazzi et al. 2020) | OL RCT | 19 DED with gastric reflux | 0.2% HA x3/d | 3 mo | ↑ | ⟷ | ⟷ | ||
| Cai M. (Cai & Zhang 2020) | SB RCT | 45 moderate‐to‐severe DED | HA x4/d | 1mo | ↑ | ⟷ | ↑ | ⟷ | |
| Laihia J. (Laihia et al. 2020) | DB RCT | 52 moderate‐to‐severe DED | 0.2% x3/d | 1 mo | ↑ | ⟷ | ⟷ | ||
| Garcia‐Conca V. (García‐Conca et al. 2019) | SB RCT | 84 mild‐to‐severe ADDE | 0.18% HA x6/d | 1 mo | ↑ | ⟷ | ⟷ | ⟷ tear osmolarity, ⟷ hyperemia, ⟷ VA, ⟷ CIC measures | |
| Kim Y. (Kim et al. 2019) | OL RCT | 54 mild‐to‐moderate DED | 0.15% HA x5‐6/d | 3 mo | ↑ | ⟷ | ↑ | ||
| Essa L. (Essa et al. 2018) | SB RCT XO | 50 DED | 0.15% and 0.4% HA x3/d | 1 mo | ↑ | ⟷ | ↑/⟷ | ⟷r | ↑ LIPCOF, ⟷ tear meniscus height, |
| Groß D. (Groß et al. 2018) | SB RCT | 80 moderate DED | 0.1% HA x3/d | 3 mo | ↑ | ⟷ | ↑ | ||
| Miháltz K. (Miháltz et al. 2018) | SB RCT | 25 mild‐to‐moderate DED | 0.2% HA x4/d | 3 mo | ↑ | ↑ | ↑ | ↑ | |
| Postorino E. (Postorino et al. 2018) | SB RCT | 40 mild‐to‐moderate DED | 0.15% HA x4/d | 3 mo | ↑ | ⟷ | ↑ | ⟷ MG assessment, ⟷ corneal sensitivity, ⟷ VA | |
| Roberti G. (Roberti et al. 2018) | SB RCT | 39 DED using long term preserved glaucoma medication | 0.2% HA x4/d | 3 mo | ⟷ | ⟷ | ⟷ | ⟷ | ⟷ goblet cell density by IVCM |
| Groß D. (Groß et al. 2017) | SB RCT | 60 moderate‐to‐severe DED | 0.2% HA x3/d | 3 mo | ↑ | ⟷ | ↑ | ||
| 0.18% HA x3/d | ↑ | ⟷ | ↑ | ||||||
| Labetoulle M. (Labetoulle et al. 2017) | SB RCT | 80 moderate‐to‐severe DED | 0.18% HA hypotonic x2‐6/d | 3 mo | ↑ | ⟷ | ↑ | ⟷ | |
| Lambiase A. (Lambiase et al. 2017) | DB RCT | 35 moderate DED | 0.18% HA 2‐6/d | 2 w | ↑ | ↑ | |||
| López‐de la Rosa A. (López‐de la Rosa et al. 2017) | DB RCT XO | 16 moderate‐to‐severe DED | 0.3% HA hypotonic x3‐8/d | 1 mo x 2 | ↑ | ↑ | ↑/⟷ | ⟷ | ↑ tarsal hyperemia, ⟷ bulbar hyperemia |
| Park Y. (Park et al. 2017) | SB RCT | 176 moderate‐to‐severe DED | 0.1% HA x5‐6/d | 3 mo | ↑ | ↑ | ↑ | ⟷ | ⟷ MG parameters |
| 0.15% HA x5‐6/d | ↑ | ↑ | ↑ | ↑ | |||||
| 0.3% HA x5‐6/d | ↑ | ↑ | ↑ | ⟷ | |||||
| Pinto‐Fraga J. (Pinto‐Fraga et al. 2017) | DB RCT XO | 16 mild DED | 0.2% HA x3‐8/d | 1 mo x 2 | ↑ | ⟷ | ↑ | ⟷ | ↑ bulbar hyperemia, ⟷ tarsal hyperemia |
| Chiambaretta F. (Chiambaretta et al. 2017) | SB RCT | 105 moderate‐to‐severe DED | 0.18% HA x3‐6/d | 3 mo | ↑ | ↑ | ↑ | ↑ | ↑ conjunctival hyperemia |
| Gong L. (Gong et al. 2015) | SB RCT | 489 moderate DED | 0.1% HA x6/d | 1 mo | ↑ | ↑ | ↑ | ||
| Lanzini M. (Lanzini et al. 2015) | PL | 24 DED | 0.2% HA x4/d | 3 mo | ⟷ | ↑ | ⟷ | ↑ IVCM, ⟷ CIC measures | |
| Liu X. (Liu et al. 2015) | SB RCT | 58 severe DED + glaucoma | 0.3% HA x3/d | 3 mo | ↑ | ↑ | ↑ | ↑ | ↑ goblet cell density by CIC |
| Hwang H. S. (Hwang et al. 2014) | OL RCT | 128 moderate ADDE | 0.1% HA x4/d | 3 mo | ↑ | ↑ | ↑ | ↑ | ↑ goblet cell density and CIC grade |
| Lee H. S. (Lee et al. 2014a) | PL | 30 mild DED | 0.1% HA isotonic x4/d | 3 mo | ⟷ | ⟷ | ⟷ | ⟷ | |
| 0.18% HA hypotonic x4/d | ⟷ | ⟷ | ↑ | ↑ | |||||
| Lee J. E. (Lee et al. 2014b) | OL RCT | 86 moderate DED | 0.1% HA x5/d | 1 mo | ↑ | ↑ | ⟷ | ⟷ | ↑/⟷ tear osmolarity |
| Aragona P. (Aragona et al. 2013) | DB RCT | 40 moderate‐to‐severe DED, SS | 0.15% HA x5/d | 3 mo | ↑ | ↑ | ⟷ | ⟷ | ↑ morphometric analysis by IVCM |
| Kinoshita S. (Kinoshita et al. 2013) | SB RCT | 182 moderate DED | 0.1% HA x6/d | 1 mo | ↑ | ↑ | ↑ | ↑ | 70% of patients reported symptomatic improvement with HA |
| Saeed N. (Saeed et al. 2013) | OL PL | 240 DED | HA x2‐4/d | 2 mo | ↑ | ↑ | ↑ | ||
| Baeyens V. (Baeyens et al. 2012) | DB RCT | 303 mild‐to‐moderate DED | 0.18% HA x2‐4/d | 3 mo | ↑ | ↑ | ↑ | ↑ | Evaluated as moderately‐very effective by >60% of evaluators and participants |
| Liu X. (Liu et al. 2012) | DB RCT | 60 moderate‐to‐severe DED | 0.1% HA x4/d | 1 mo | ⟷ | ⟷ | ⟷ | ⟷ | ⟷ CIC measures |
| McCann L. C. (McCann et al. 2012) | SB RCT | 73 mild‐to‐moderate DED | 0.15% HA x4/d | 3 mo | ↑ | ↑ | ⟷ | ↑ evaporation, ↑ tear film stability by interferometry, ⟷ osmolarity | |
| Takamura E. (Takamura et al. 2012) | DB RCT | 286 moderate DED | 0.1% HA x6/d | 1 mo | ↑ | ↑ | |||
| Lee J. H. (Lee et al. 2011) | SB RCT | 65 mild‐to‐moderate DED | 0.1% HA x6/d | 2 mo | ↑ | ↑ | ↑ | ||
| Monaco G. (Monaco et al. 2011) | DB RCT XO | 20 glaucoma patients with dry eye symptoms | 0.2% HA x4/d | 2 w x2 | ⟷ | ⟷ | |||
| Sanchez M. (Sanchez et al. 2010) | SB RCT | 15 mild‐to‐moderate DED | 0.15% HA x4/d | 1 mo | ⟷ | ⟷ | ⟷ | ↑ HLA‐DR by CIC flow cytometry | |
| Vogel R. (Vogel et al. 2010) | DB RCT | 436 moderate DED | 0.18% HA hypotonic x3‐6/d | 2 w | ↑ | ↑ | |||
| Johnson M. E. (Johnson et al. 2008) | DB RCT | 65 moderate DED | 0.18% HA x2/d | 1 mo | ↑ | ⟷ | ↑ | ||
| Troiano P. (Troiano & Monaco 2008) | SB RCT XO | 28 moderate DED | 0.4% HA hypotonic x4/d | 1 w x 2 | ↑↑ | ↑↑ | ↑ conjunctival hyperemia, 61% preferred hypotonic over isotonic drops | ||
| 0.4% HA isotonic x4/d | ↑ | ↑ | ↑ conjunctival hyperemia | ||||||
| Rolando M. (Rolando & Valente 2007) | OL RCT | 30 mild‐to‐moderate DED | 0.2% HA x3‐4/d | 3 mo | ↑ | ↑ | ↑ | ||
| Lee H. K. (Lee et al. 2006) | DB RCT | 41 DED (severity NG) | 0.1% HA x3/d | 1 mo | ↑ | ↑ | ⟷ | ⟷ NFG/TP, ⟷ CIC measures | |
| Benitez‐del‐Castillo J. M. (Benitez‐del‐Castillo et al. 2002) | PL | 6 moderate‐to‐severe DED | 0.18% HA x4/d | 2 w | ↑s | ||||
| Aragona P. Aug. 2002 (Aragona et al. 2002a) | SB RCT | 40 severe DED with SS | 0.4% HA hypotonic x6/d | 3 mo | ↑ | ↑↑ | ↑↑ | ⟷ | ↑↑ CIC measures |
| 0.4% HA isotonic x6/d | ↑ | ↑ | ↑ | ⟷ | ↑ CIC measures | ||||
| McDonald C. C. (McDonald et al. 2002) | DB RCT XO | 32 severe DED with SS | 0.1% HA x3‐4/d | 2 mo | ↑/⟷ | ||||
| Milafzzo G. (Milafzzo et al. 2002) | DB RCT XO | 139 moderate KCS | HA hypotonic | 1 mo x 2 | ↑ | ↑↑ | ↑ | ↑ | |
| HA isotonic | ↑ | ↑ | ↑ | ↑ | |||||
| Papa V. (Papa et al. 2001) | DB RCT XO | 139 moderate DED | HA hypotonic up to x 6/d | 1 mo x 2 | ↑ | ↑↑ | ↑ | ↑ | ↑ conjunctival hyperemia |
| HA isotonic up to x 6/d | ↑ | ↑ | ↑ | ↑ | ↑ conjunctival hyperemia | ||||
| Iester M. (Iester et al. 2000) | OL RCT | 113 moderate‐to‐severe DED | 0.4% HA hypotonic x6/d | 3 mo | ↑ | ↑ | ↑ | ↑ | ↑ tear osmolarity, ↑ CIC score |
| Condon P. I. (Condon et al. 1999) | DB RCT XO | 70 KCS, SS | 0.1% HA x3‐4/d | 1 mo x2 | ↑ | ↑ | ↑ | ||
| Yokoi N. (Yokoi et al. 1997) | PL | 7 SS + 4 moderate KCS | 0.1% HA x5/d | 1 mo | ↑ | ||||
| Nelson J. D. (Nelson & Farris 1988) | DB RCT | 35 moderate KCS | 0.1% HA x8/d | 2 mo | ↑ | ↑ | ⟷ | ⟷ | ↑ tear film osmolality, ↑ conjunctival hyperemia, ↑/⟷ CIC measures |
| DeLuise V. P. (DeLuise & Peterson 1984) | PL | 28 severe DED, SS | 0.1% HA x4/d | 2 mo | ↑ | ↑ | ↑ | ⟷ | ↑ mucus strand formation, ⟷ TMH |
⟷ = no statistically significant difference at p > 0.05 compared to baseline, ↑ = statistically significant improvement at p < 0.05 compared to baseline, ↑/⟷ = statistically significant improvement at p < 0.05 in some parameters and no difference in others compared to baseline, ↑↑ = statistically significant improvement at p > 0.05 compared to baseline and compared to other hyaluronic acid treatment arm (empty cell) = not described, ADDE = Aqueous‐deficient dry eye, CIC = conjunctival impression cytology, DB = double‐blinded, DED = dry eye disease/syndrome, IVCM = in vivo confocal microscopy, KCS = keratoconjunctivitis sicca, LIPCOF = lid parallel conjunctival folds, MG = meibomian gland, mo = month(s), NFG/TP = Nerve Growth Factor/total protein ratio, OL = open label, OSS = ocular surface staining, PL = prospective longitudinal study, r = phenol red test, RCT = randomized controlled trial, s = stromal fluorescein uptake by fluorophotometer, SB = single‐blinded, Schi. = Schirmer's test, SS = Sjögren's syndrome, Symp. = subjective symptoms, TBUT = tear film break‐up time, TMH = tear meniscus height, VA = visual acuity, w = weeks, XO = cross‐over design.
Table 2.
Changes in HA treatment versus placebo in randomized double‐blinded placebo‐controlled trials, sorted by publication date.
| First Author (year) | Design | Setup | Placebo arm | Duration | Symp. | TBUT | OSS | Schi. | Other outcomes |
|---|---|---|---|---|---|---|---|---|---|
| López‐de la Rosa A.xo (López‐de la Rosa et al. 2017) | 16 moderate‐to‐severe DED | 0.3% HA x3‐8/d | saline | 1 mo x2 | ⟷ | ↑ | ⟷ | ⟷ | |
| Pinto‐Fraga J.xo (Pinto‐Fraga et al. 2017) | 16 mild DED | 0.2% HA x3‐8/d | saline | 1 mo x2 | ↑ | ⟷ | ↑ | ⟷ | ↑ conjunctival hyperemia, ↑ subjective satisfaction |
| Baeyens V. (Baeyens et al. 2012) | 303 mild‐to‐moderate DED | 0.18% HA x2‐4/d | saline | 3 mo | ↑/⟷ | ↑ | ↑ | ↑ | ↑ patient and investigator efficacy evaluation, ⟷ VA, ↑ blurry vision, less average drop instillations |
| Vogel R. (Vogel et al. 2010) | 436 moderate DED | 0.18% HA x6/d | vehicle | 2 w | ↑ | ↑ | |||
| Aragona P. Feb. 2002 (Aragona et al. 2002b) | 44 moderate‐to‐severe DED | 0.15% HA x4‐8/d | saline | 3 mo | ⟷ | ⟷ | ⟷ | ⟷ | ↑ CIC |
| Condon P. I.xo (Condon et al. 1999) | 70 KCS or SS | 0.1% HA x3‐4/d | saline | 1 mo x2 | ↑ | ↑ | ↑ | 3:1 patient preference for HA over saline | |
| Shimmura S. (Shimmura et al. 1995) | 91 DED, SS | 0.1% HA x6/d | vehicle | 1 mo | ⟷ | ⟷ | ↑/⟷ | ⟷t | ⟷ patient preference |
| Sand B. B.xo (Sand et al. 1989) | 18 severe KCS | 0.1% HA x6/d | vehicle | 2 w x4 | ⟷ | ⟷ | ⟷ | ⟷ |
4/14 preferred 0.1% 8/14 preferred 0.2% 2/14 preferred placebo |
| 0.2% HA x6/d | ⟷ | ↑ | ↑ | ⟷ |
⟷ = no statistically significant difference compared to placebo with p > 0.05, ↑ = statistically significant improvement compared to placebo with p < 0.05, ↑/⟷ = statistically significant improvement with p < 0.05 compared to placebo in some but not all measures of this category, CIC = conjunctival impression cytology, d = day, DED = dry eye disease, HA = hyaluronic acid, KCS = keratoconjunctivitis sicca, mo = months, SS = Sjögren's syndrome, t = Schirmer's and tear clearance test, VA = visual acuity, w = weeks, xo = cross‐over study.
Fig. 6.
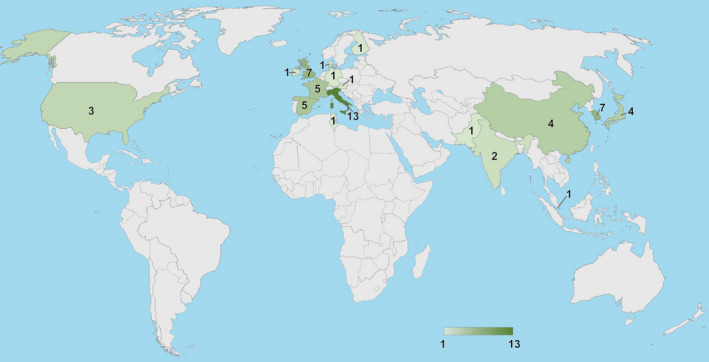
Number of included studies conducted in each country. Generated by Bing in Excel by Emily Moschowits.
Changes in subjective scores against baseline in treatment studies
As seen in Table 1, 45 treatment studies provided subjective symptom data in 53 treatment arms on HA treatment compared to baseline (DeLuise & Peterson 1984; Nelson & Farris 1988; Condon et al. 1999; Iester et al. 2000; Papa et al. 2001; Aragona et al. 2002a; McDonald et al. 2002; Milafzzo et al. 2002; Lee et al. 2006; Rolando & Valente 2007; Johnson et al. 2008; Troiano & Monaco 2008; Vogel et al.2010; Lee et al. 2011; Monaco et al. 2011; Baeyens et al. 2012; Liu et al. 2012; McCann et al. 2012; Aragona et al. 2013; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Lee et al. 2014a, b; Gong et al. 2015; Liu et al. 2015; Chiambaretta et al. 2017; Groß et al. 2017; Labetoulle et al. 2017; Lambiase et al. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Essa et al. 2018; Groß et al. 2018; Miháltz et al. 2018; Postorino et al. 2018; Roberti et al. 2018; García‐Conca et al. 2019; Kim et al. 2019; Balestrazzi et al. 2020; Cai & Zhang 2020; Laihia et al. 2020; Brar et al. 2021; Morya et al. 2021). The most frequently used validated questionnaire was the Ocular Surface Disease Index (OSDI), used in 20 treatment studies (Monaco et al. 2011; Liu et al. 2012, 2015; Hwang et al. 2014; Lee et al. 2014a,b; Chiambaretta et al. 2017; Labetoulle et al. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Essa et al. 2018; Postorino et al. 2018; Roberti et al. 2018; García‐Conca et al. 2019; Kim et al. 2019; Balestrazzi et al. 2020; Cai & Zhang 2020; Laihia et al. 2020; Morya et al. 2021). The second most common was the Visual Analogue Scale (VAS), used in eight treatment studies (Nelson & Farris 1988; Papa et al. 2001; McDonald et al. 2002; Rolando & Valente 2007; Vogel et al. 2010; Baeyens et al. 2012; Aragona et al. 2013; Lambiase et al. 2017). Other symptom assessments included the ocular comfort index (Johnson et al. 2008; Groß et al. 2017, 2018), Symptom Assessment in Dry Eye (SANDE) questionnaire (McCann et al. 2012; Lambiase et al. 2017), global discomfort index for ocular symptoms (Aragona et al. 2002a), Standardized Patient Evaluation of Eye Dryness (SPEED) questionnaire (Brar et al. 2021), and patient or researcher assessment or preference (Troiano & Monaco 2008; Baeyens et al. 2012; Kinoshita et al. 2013). Eleven studies reported results of dry eye symptom assessments without the use of validated questionnaires (DeLuise & Peterson 1984; Condon et al. 1999; Iester et al. 2000; Milafzzo et al. 2002; Lee et al. 2006, 2011; Troiano & Monaco 2008; Kinoshita et al. 2013; Saeed et al. 2013; Gong et al. 2015; Miháltz et al. 2018). Symptoms are presented in the “Symp.” column in Table 1 as a single group of results, while additional investigator and patient assessments and preferences are presented in the “Other outcomes” column of Table 1.
Generally, there was a clear improvement in subjective scores against baseline in studies of both shorter and longer follow‐up (Table 1). Forty‐seven of the 53 treatment arms showed statistically significant improvement in subjective symptoms with HA treatment against baseline (DeLuise & Peterson 1984; Nelson & Farris 1988; Condon et al. 1999; Iester et al. 2000; Papa et al. 2001; Aragona et al. 2002a; Milafzzo et al. 2002; Lee et al. 2006; Rolando & Valente 2007; Johnson et al. 2008; Troiano & Monaco 2008; Vogel et al. 2010; Lee et al. 2011; Baeyens et al. 2012; McCann et al. 2012; Aragona et al. 2013; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Lee et al. 2014b; Gong et al. 2015; Liu et al. 2015; Chiambaretta et al. 2017; Groß et al. 2017; Labetoulle et al. 2017; Lambiase et al. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Essa et al. 2018; Groß et al. 2018; Miháltz et al. 2018; Postorino et al. 2018; García‐Conca et al. 2019; Kim et al. 2019; Balestrazzi et al. 2020; Cai & Zhang 2020; Laihia et al. 2020; Brar et al. 2021; Morya et al. 2021). One study arm found improvement in some but not all subjective parameters (McDonald et al. 2002). Only five treatment arms found no statistically significant change in symptoms compared to baseline (Monaco et al. 2011; Liu et al. 2012; Lee et al. 2014a; Roberti et al. 2018). No studies reported worsening of subjective scores with HA treatment.
Changes in objective measures against baseline in treatment studies
As seen in Table 1, the most commonly reported objective measure compared to baseline was ocular surface staining (OSS) reported in 53 treatment arms in 45 studies (DeLuise & Peterson 1984; Nelson & Farris 1988; Yokoi et al. 1997; Condon et al. 1999; Iester et al. 2000; Papa et al. 2001; Aragona et al. 2002a; Benitez‐del‐Castillo et al. 2002; Milafzzo et al. 2002; Rolando & Valente 2007; Johnson et al. 2008; Troiano & Monaco 2008; Sanchez et al. 2010; Vogel et al. 2010; Lee et al. 2011; Monaco et al. 2011; Baeyens et al. 2012; Liu et al. 2012; McCann et al. 2012; Takamura et al. 2012; Aragona et al. 2013; Kinoshita et al. 2013; Hwang et al. 2014; Lee et al. 2014a, b; Gong et al. 2015; Lanzini et al. 2015; Liu et al. 2015; Chiambaretta et al. 2017; Groß et al. 2017; Labetoulle et al. 2017; Lambiase et al. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Essa et al. 2018; Groß et al. 2018; Miháltz et al. 2018; Postorino et al. 2018; Roberti et al. 2018; García‐Conca et al. 2019; Kim et al. 2019; Cai & Zhang 2020; Laihia et al. 2020; Morya et al. 2021), followed by TBUT reported in 48 treatment arms from 41 studies (DeLuise & Peterson 1984; Nelson & Farris 1988; Iester et al. 2000; Papa et al. 2001; Aragona et al. 2002a; Milafzzo et al. 2002; Lee et al. 2006; Rolando & Valente 2007; Johnson et al. 2008; Sanchez et al. 2010; Lee et al. 2011; Baeyens et al. 2012; Liu et al. 2012; McCann et al. 2012; Takamura et al. 2012; Aragona et al. 2013; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Lee et al. 2014a, b; Gong et al. 2015; Lanzini et al. 2015; Liu et al. 2015; Chiambaretta et al. 2017; Groß et al. 2017; Labetoulle et al. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Essa et al. 2018; Groß et al. 2018; Miháltz et al. 2018; Postorino et al. 2018; Roberti et al. 2018; Kim et al. 2019; Balestrazzi et al. 2020; Cai & Zhang 2020; Laihia et al. 2020; Brar et al. 2021; Morya et al. 2021), and Schirmer's test in 37 treatment arms from 31 studies (DeLuise & Peterson 1984; Nelson & Farris 1988; Condon et al. 1999; Iester et al. 2000; Papa et al. 2001; Aragona et al. 2002a; Milafzzo et al. 2002; Lee et al. 2006; Sanchez et al. 2010; Baeyens et al. 2012; Liu et al. 2012; Aragona et al. 2013; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Lee et al. 2014a, b; Lanzini et al. 2015; Liu et al. 2015; Chiambaretta et al. 2017; Labetoulle et al. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Essa et al. 2018; Miháltz et al. 2018; Roberti et al. 2018; García‐Conca et al. 2019; Balestrazzi et al. 2020; Cai & Zhang 2020; Morya et al. 2021). The most commonly used method of measuring OSS was the Oxford system (Bron et al. 2003) and variations of the Oxford system, or comparable standardized OSS grading methods. One study measured stromal fluorescein uptake by fluorophotometry (Benitez‐del‐Castillo et al. 2002) as a measure of corneal epithelial barrier integrity which is listed as an OSS measure in Table 1. Twenty‐three studies performed Schirmer's test without topical anaesthetics (DeLuise & Peterson 1984; Nelson & Farris 1988; Condon et al. 1999; Iester et al. 2000; Aragona et al. 2002a; Lee et al. 2006; Baeyens et al. 2012; Liu et al. 2012; Aragona et al. 2013; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Lanzini et al. 2015; Labetoulle etal. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Miháltz et al. 2018; Roberti et al. 2018; García‐Conca et al. 2019; Balestrazzi et al. 2020; Cai & Zhang 2020; Morya et al. 2021) while six studies performed Schirmer's test with topical anaesthetics (Papa et al. 2001; Milafzzo et al. 2002; Sanchez et al. 2010; Lee et al. 2014a,b; Liu et al. 2015). One study did not clearly state whether anaesthetics were used (Chiambaretta et al. 2017), and one study used the Phenol Red test, which is comparable to Schirmer's test and is therefore listed with Schirmer in Table 1 (Essa et al. 2018). Only one of the 53 studies listed in Table 1 did not report any objective measures with HA treatment against baseline (McDonald et al. 2002).
Total OSS scores showed statistically significant improvement compared to baseline in 40 out of 53 treatment arms (DeLuise & Peterson 1984; Yokoi et al. 1997; Condon et al. 1999; Iester et al. 2000; Papa et al. 2001; Aragona et al. 2002a; Benitez‐del‐Castillo et al. 2002; Milafzzo et al. 2002; Rolando & Valente 2007; Johnson et al. 2008; Troiano & Monaco 2008; Vogel et al. 2010; Lee et al. 2011; Baeyens et al. 2012; Takamura et al. 2012; Kinoshita et al. 2013; Hwang et al. 2014; Lee et al. 2014a; Gong et al. 2015; Lanzini et al. 2015; Liu et al. 2015; Chiambaretta et al. 2017; Groß et al. 2017; Labetoulle et al. 2017; Lambiase et al. 2017; Park et al. 2017; Pinto‐Fraga et al. 2017; Groß et al. 2018; Miháltz et al. 2018; Postorino et al. 2018; Kim et al. 2019; Cai & Zhang 2020; Morya et al. 2021). Two treatment arms showed improvement in some but not all OSS parameters (López‐de la Rosa et al. 2017; Essa et al. 2018). Eleven treatment arms showed no statistically significant change in OSS (Nelson & Farris 1988; Sanchez et al. 2010; Monaco et al. 2011; Liu et al. 2012; McCann et al. 2012; Aragona et al. 2013; Lee et al. 2014a,b; Roberti et al. 2018; García‐Conca et al. 2019; Laihia et al. 2020). Tear film break‐up time improved in 29 of 48 treatment arms compared to baseline (DeLuise & Peterson 1984; Nelson & Farris 1988; Iester et al. 2000; Papa et al. 2001; Aragona et al. 2002a; Milafzzo et al. 2002; Lee et al. 2006; Rolando & Valente 2007; Lee et al. 2011; Baeyens et al. 2012; McCann et al. 2012; Takamura et al. 2012; Aragona et al. 2013; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Lee et al. 2014b; Gong et al. 2015; Liu et al. 2015; Chiambaretta et al. 2017; López‐de la Rosa et al. 2017; Park et al. 2017; Miháltz et al. 2018; Brar et al. 2021). Schirmer's improved in 15 of 37 treatment arms (Condon et al. 1999; Iester et al. 2000; Papa et al. 2001; Milafzzo et al. 2002; Baeyens et al. 2012; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Liu et al. 2015; Chiambaretta et al. 2017; Miháltz et al. 2018). No studies reported worsening of objective measures compared to baseline.
Additional objective parameters were measured in some studies and compared to baseline (as seen in Table 1’s “Other outcomes” column). Six treatment arms found improvement in conjunctival impression cytology (CIC) measures (Iester et al. 2000; Aragona et al. 2002a; Sanchez et al. 2010; Hwang et al. 2014; Liu et al. 2015), while one found some CIC measures to be improved and others not (Nelson & Farris 1988), and five treatment arms found no change (Nelson & Farris 1988; Lee et al. 2006; Liu et al. 2012; Lanzini et al. 2015; García‐Conca et al. 2019). Three treatment arms showed improved tear osmolality or osmolarity (Nelson & Farris 1988; Iester et al. 2000; Brar et al. 2021), one showed improved tear osmolarity in one eye and no change in the other (Lee et al. 2014b), while two did not find any change in tear osmolarity from baseline (McCann et al. 2012; García‐Conca et al. 2019). Six treatment arms found improvement in conjunctival hyperemia (Nelson & Farris 1988; Papa et al. 2001; Troiano & Monaco 2008; Chiambaretta et al. 2017), while two found improvement in either tarsal or bulbar hyperemia but not in both (López‐de la Rosa et al. 2017; Pinto‐Fraga et al. 2017), and one found no change in hyperemia (García‐Conca et al. 2019). Tear meniscus height was improved in one (Brar et al. 2021) and unchanged in two (DeLuise & Peterson 1984; Morya et al. 2021) treatment arms. Meibomian gland measurements improved in one (Brar et al. 2021) and were unchanged in two (Park et al. 2017; Postorino et al. 2018) treatment arms.
Changes against control in placebo controlled clinical trials
The nine HA treatment arms in the eight placebo‐controlled trials comparing HA treatment with either saline or vehicle are presented in Table 2. All but one treatment arm found improvement in at least one subjective or objective measure (Sand et al. 1989; Shimmura et al. 1995; Condon et al. 1999; Aragona et al. 2002b; Vogel et al. 2010; Baeyens et al. 2012; López‐de la Rosa et al. 2017; Pinto‐Fraga et al. 2017). Three of nine treatment arms showed statistically significant subjective improvement (Condon et al. 1999; Vogel et al. 2010; Pinto‐Fraga et al. 2017), one of nine found improvement in some and no change in other subjective measures (Baeyens et al. 2012), and five of nine arms found no change in subjective measures (Sand et al. 1989; Shimmura et al. 1995; Aragona et al. 2002b; López‐de la Rosa et al. 2017). Three of seven treatment arms found improvement in TBUT (Sand et al. 1989; Baeyens et al. 2012; López‐de la Rosa et al. 2017). Five of nine treatment arms found improvement in OSS (Sand et al. 1989; Condon et al. 1999; Vogel et al. 2010; Baeyens et al. 2012; Pinto‐Fraga et al. 2017). One of nine treatment arm found improvement in some but not all OSS measures (Shimmura et al. 1995). Only two of eight treatment arms found improvement in Schirmer's test (Condon et al. 1999; Baeyens et al. 2012). No studies showed worsening in HA treatment compared to placebo.
Preservatives
Four studies used HA formulations with benzalkonium chloride as a preservative (Lee et al. 2006; Liu et al. 2012; Takamura et al. 2012; Hwang et al. 2014). Two of these studies found improvement in all measures compared to baseline (Takamura et al. 2012; Hwang et al. 2014), one found improvement in some measures compared to baseline (Lee et al. 2006), and one found no improvement compared to baseline (Liu et al. 2012). 34 studies used preservative‐free HA formulations (DeLuise & Peterson 1984; Nelson & Farris 1988; Sand et al. 1989; Shimmura et al. 1995; Yokoi et al. 1997; Condon et al. 1999; Iester et al. 2000; Benitez‐del‐Castillo et al. 2002; McDonald et al. 2002; Aragona et al. 2002a,b; Rolando & Valente 2007; Johnson et al. 2008; Troiano & Monaco 2008; Sanchez et al. 2010; Vogel et al. 2010; Lee et al. 2011, 2014a,b; Monaco et al. 2011; McCann et al. 2012; Kinoshita et al. 2013; Liu et al. 2015; Chiambaretta et al. 2017; Groß et al. 2017, 2018; Labetoulle et al. 2017; Lambiase et al. 2017; López‐de la Rosa et al. 2017; Essa et al. 2018; Miháltz et al. 2018; Postorino et al. 2018; Roberti et al. 2018; Laihia et al. 2020). No studies compared clinical effects of HA treatment with preservatives compared to without preservatives.
Safety and complications
Hyaluronic acid was found safe in all reviewed literature. There were no serious adverse events associated with the use of HA in any of the included studies. However, some studies mentioned cases of conjunctival hyperemia, conjunctivitis, burning sensation, and/or discomfort with HA use (Sand et al. 1989; Vogel et al. 2010; Chen et al. 2014; Lee et al. 2014b; Labetoulle et al. 2017). One study discussed in general the very low adverse effects reporting from consumers of artificial tears with HA (Vogel et al. 2010). None of the double‐blinded controlled treatment studies found clinically relevant differences in adverse events or tolerability between groups (Sand et al. 1989; Condon et al. 1999; Aragona et al. 2002b; Vogel et al. 2010; López‐de la Rosa et al. 2017; Pinto‐Fraga et al. 2017), with the exception of more blurry vision after instillation of 0.18% HA than saline in one study (Baeyens et al. 2012). Two studies using 0.15% and 0.18% HA respectively reported minor and tolerable temporary visual changes after instillation (Johnson et al. 2008; Sanchez et al. 2010).
Discussion
Summary
With over 30 years of clinical use, HA has proven to be an enduring element of DED treatment. This review summarizes the current knowledge on the safety and efficacy of HA in the treatment of DED against baseline measures and against placebo. Hyaluronic acid is shown to be effective at improving symptoms and objective measures of dry eye, such as TBUT and OSS compared to baseline and to placebo.
Change in Schirmer's test
Interestingly, despite improvements in symptoms, TBUT, and OSS in most studies that included these measures, Schirmer's scores improved in less than half. Schirmer's test mainly measures the lacrimal gland's aqueous production (Willcox et al. 2017). Only some DED patients have decreased Schirmer's values (Bron et al. 2017). Reduction in aqueous production in DED can be due to decreased function, destruction, fibrosis or atrophy of the lacrimal gland or adjacent conjunctiva (Conrady et al. 2016; Bron et al. 2017). Hyaluronic acid's mechanism for improving Schirmer's score may be through resolving the ocular inflammation of DED (Pauloin et al. 2009a), which may improve lacrimal gland function (McMonnies 2020), though restoration of an auto‐immunologically damaged lacrimal gland as in the course of Sjögren's syndrome (Bjordal et al. 2020) is less likely.
Study populations
A full range of DED severities and types was covered in the reviewed literature (Tables 1 and 2). Only one study looked at mild and moderate DED separately, however the participants with moderate DED also received topical cyclosporine and steroid treatment, so the HA effect could not be isolated in the moderate DED groups (Lee et al. 2014a). The authors of that study suggested the small sample size and low drop frequency to contribute to the absence of significant improvements in the mild DED groups. Studies with across‐the‐board improvement were found among both smaller (DeLuise & Peterson 1984; Nelson & Farris 1988; Rolando & Valente 2007; Troiano & Monaco 2008; Miháltz et al. 2018; Brar et al. 2021), and larger (Iester et al. 2000; Papa et al. 2001; Milafzzo et al. 2002; Baeyens et al. 2012; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Gong et al. 2015; Chiambaretta et al. 2017; Park et al. 2017) study sizes. Non‐improvement or improvement in only one measure was only found in studies with less than 100 participants (Shimmura et al. 1995; Aragona et al. 2002b; Sanchez et al. 2010; Monaco et al. 2011; Liu et al. 2012; Lee et al. 2014a; Roberti et al. 2018; Balestrazzi et al. 2020). All studies with more than 100 participants found improvements in two or more measures (Iester et al. 2000; Papa et al. 2001; Milafzzo et al. 2002; Vogel et al. 2010; Baeyens et al. 2012; Takamura et al. 2012; Kinoshita et al. 2013; Saeed et al. 2013; Hwang et al. 2014; Gong et al. 2015; Chiambaretta et al. 2017; Park et al. 2017; Morya et al. 2021). This could indicate that some studies with little to no improvement were underpowered. The 10 studies that recruited patients with aqueous deficient dry eye, Sjögren's syndrome and keratoconjunctivitis sicca found improvement in most or all reported subjective and objective measures (DeLuise & Peterson 1984; Nelson & Farris 1988; Yokoi et al. 1997; Condon et al. 1999; Aragona et al. 2002a; McDonald et al. 2002; Milafzzo et al. 2002; Aragona et al. 2013; Hwang et al. 2014; García‐Conca et al. 2019).
HA concentration
Concentrations of HA used in HA treatment studies ranged from 0.1% to 0.4% (Tables 1 and 2). Comparisons of treatment with different HA concentrations was limited. Only two baseline‐controlled studies isolated the effects of different HA concentrations in parallel treatment arms (Groß et al. 2017; Park et al. 2017), both with a non‐inferiority study design, thus not powered to find superiority of one treatment over the other. One placebo controlled study found no difference in any measures in the 0.1% HA treatment arm against placebo but found improvement in TBUT and OSS against placebo with 0.2% HA (Sand et al. 1989). In the same study, 0.2% concentration also won patient preference compared to both placebo and 0.1% HA (Sand et al. 1989). Increasing HA concentration from 0.1% to 0.3% has been found to cause further improvements in experimental DED in mice, including decreased goblet cell and corneal epithelial cell damage and increased tear film stability (You et al. 2018). Generally, HA in concentrations between 0.1% and 0.2% appear to provide objective improvement, symptom‐relief, and patient comfort without substantial blurring of vision (Johnson et al. 2008; Sanchez et al. 2010; Carracedo et al. 2019). 94% of patients in the 2008 Johnson et al. study reported less than one minute of visual disturbance after drop installation with 0.18% HA (Johnson et al. 2008). Higher concentrations of HA (>0.2%) provide longer tear film stability, but also show increased complaints of blurry vision (Aragona et al. 2019; Carracedo et al. 2019). Ishioka et al. showed that 0.3% HA caused significantly more visual acuity loss compared to 0.1% HA immediately after drop instillation, but that this difference disappeared within 5 minutes of administering drops (Ishioka et al. 2009). This is supported by a study that found increased optical higher order aberrations and forward light scatter of the cornea for five minutes after instilling 0.3% HA drops (Koh et al. 2013). Future studies should investigate differences in treatment effects and tolerance with varying HA concentrations.
Drop frequency
Per‐protocol drop frequency in treatment studies, as seen in Tables 1 and 2, ranged from 2 to 8 drops per day across studies. There was no clear pathophysiological or evidence‐based reasoning behind the choice of drop frequency in any of the studies. No study aimed to find the ideal drop frequency for HA treatment. The three studies that compared different concentrations of HA used the same drop frequency for all HA treatment arms (Lee et al. 2014a; Groß et al. 2017; Park et al. 2017). One study found patients to use a significantly lower average drop frequency in the HA group compared to the placebo group (Baeyens et al. 2012). Six studies reported on compliance with protocol instructions (Aragona et al. 2002b; Rolando & Valente 2007; Johnson et al. 2008; Baeyens et al. 2012; Essa et al. 2018; Laihia et al. 2020). Five of those studies reported good compliance. One of those studies found the mean daily drop frequency of participants to be 9 drops per day (Aragona et al. 2002b). Another study, instead of specific instructions, asked severe DED patients to administer drops whenever they experienced DED symptoms (van Setten et al. 2020). They reported an average drop frequency among patients ranging from 2 to 23.8 drops per day with an average of 7.1 drops. Dry eye disease symptoms often do not correspond with objective findings in the office (Craig et al. 2017), and symptom relief does not necessarily correspond to objective improvement. It would be useful for clinicians and patients to have evidence‐based drop frequency recommendations, ideally for any given formulation and DED severity or type. This would require a study design with several treatment‐arms of varying strict drop frequency protocols with robust methods of measuring compliance. Such studies are currently missing from the literature. There is also the potential risk of patients over‐ or under‐treating when self‐administering, either not achieving the full clinical effect, or washing away the many trophic, anti‐inflammatory, and antioxidating proteins, lipids, and mucins that are naturally present in the tear film (Willcox et al. 2017). Few prescribed medical interventions have such a wide range of treatment regimens across studies or allow patients to self‐determine their own treatment based on symptoms. Future clinical studies need to investigate optimal drop frequency of HA in dry eye treatment and explore the possibility that different patient groups may need different drop frequencies.
Hypotonic versus isotonic HA drops
Five treatment studies compared hypotonic and isotonic HA treatment for DED (Papa et al. 2001; Aragona et al. 2002a; Milafzzo et al. 2002; Troiano & Monaco 2008; Lee et al. 2014a; Table 1). One treatment study using 0.4% HA found significantly more subjective and objective improvements in the hypotonic group compared to the isotonic group (Troiano & Monaco 2008). In the same study, the hypotonic group also won patient preference (Troiano & Monaco 2008). Three of the studies found significantly greater objective but not subjective improvement in the hypotonic group compared to the isotonic group (Papa et al. 2001; Aragona et al. 2002a; Milafzzo et al. 2002). One of the studies looking at hypotonic and isotonic HA drops also used different HA concentrations and thus did not separate the effect of different osmolarity or concentration (Lee et al. 2014a). More studies are needed to determine if hypotonic HA formulations are better than isotonic formulations in DED treatment.
Molecular weight of HA
The molecular weights of the HA used was provided in only 10 of the included studies (Shimmura et al. 1995; Yokoi et al. 1997; Aragona et al. 2002a,b; Johnson et al. 2008; Groß et al. 2017, 2018; Roberti et al. 2018; García‐Conca et al. 2019; Laihia et al. 2020) of which one study only provided molecular weights for one of two treatment arms (Groß et al. 2017). The molecular weight of HA ranged from 60 kilo‐Daltons (Yokoi et al. 1997) to 3000 kilo‐Daltons (Aragona et al. 2002b), which is in the low to high molecular weight range (Aragona et al. 2019; Lee et al. 2021). No studies provided comparative results between HA of different molecular weights. High molecular weight HA, especially above 1000 kiloDaltons, has known anti‐inflammatory, immunosuppressive and antiangiogenic activity, including pro‐inflammatory cytokine and chemokine suppression through cell‐surface receptor‐binding (Altman et al. 2019), while low molecular weight, and even medium molecular weight HA, may produce pro‐inflammatory responses (Altman et al. 2019; Lee et al. 2021). Given the changing and even opposing physical characteristics and immunological effects of HA depending on its molecular weight (Litwiniuk et al. 2016; Snetkov et al. 2020), investigation into the effects of varying molecular weights in the treatment of DED is warranted.
Pathophysiology of DED and HA drop formulation
Hyaluronic acid works through breaking the vicious circle of dry eye disease that self‐perpetuates the DED pathophysiology (Jones et al. 2017) as illustrated in our modified version of The Vicious Circle of Dry Eye Disease (Bron et al. 2017; Fig. 7). Across the reviewed literature it is shown that DED treatment with HA improves tear film stability, evaporation, osmolarity, inflammation, ocular surface damage, as well as goblet‐cell, epithelial, and meibomian gland health (Tables 1 and 2). Some of these improvements can be explained through the direct or indirect effect of HA on the osmolarity of the tear film, which in turn will reduce the osmotic stress at the ocular surface (Yu et al. 2021). Osmotic stress results from hyperosmolarity of the tear film and is one of the main mechanisms behind the vicious circle of DED, leading to inflammation and ocular surface damage (Bron et al. 2017). The frictional damage to the ocular surface, conjunctival thinning, pain, and inflammation of DED (Bron et al. 2017; van Setten 2020; Aragona et al. 2021) is improved by HA through lubrication of the ocular surface by its viscous, mucoadhesive and non‐Newtonian properties, which reduces shear‐forces (van Setten 2020) as well as through HA's established anti‐inflammatory effects (Kotla et al. 2021). Hyaluronic acid treatment appears to address the pathophysiology of both evaporative and aqueous deficient DED at multiple action points.
Fig. 7.
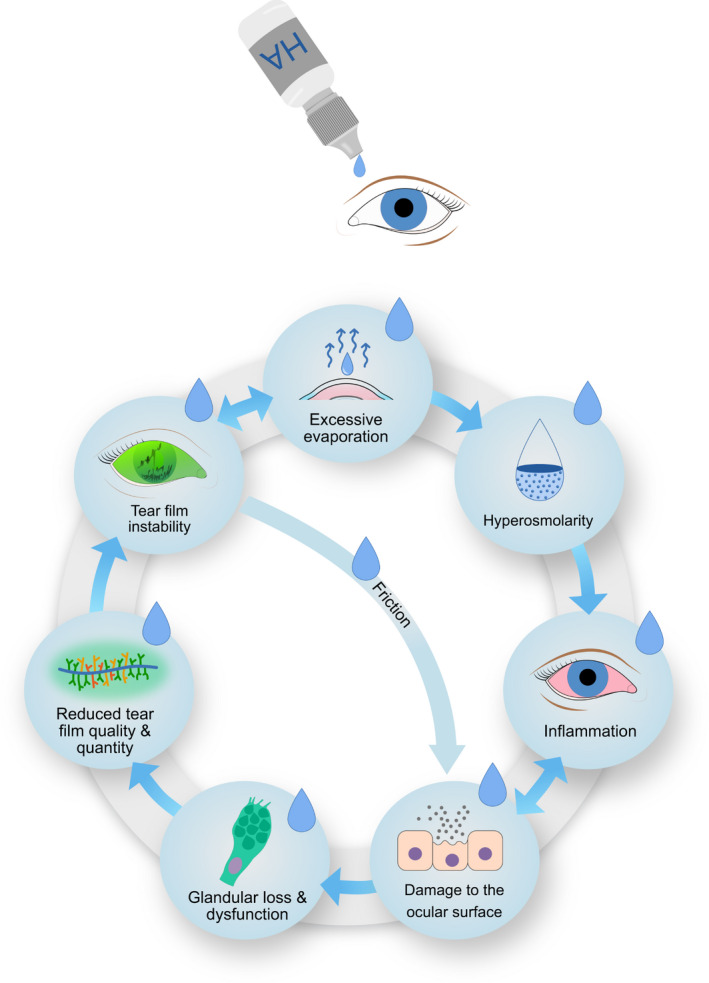
Modified Vicious Circle of Dry Eye Disease inspired by Bron et al. Hyaluronic acid treatment attempts to break the circle. Blue drops illustrate action‐points where hyaluronic acid contributes. Illustration by Emily Moschowits, using elements from Sara Nøland with permission.
The variation in the physiochemical properties of HA and its effects in DED treatment is dependent on its concentration, molecular weight, chemical modifications, and the drop formulation (Abatangelo et al. 2020; van Setten 2020). Guillaumie et al. found that higher molecular weights of HA at equal concentrations provide prolonged residence time in the tear film of rabbits (Guillaumie et al. 2010). These actions were also found in human cells and tissues, including the cornea (Jiang et al. 2007; Pauloin et al. 2009a,b; Wu et al. 2013). One study found improved tear film evaporation rates after 90 days of HA treatment which suggests that the lipid layer of the tear film may also be improved with long term HA treatment (McCann et al. 2012). In a study published in 2019, Aragona et al. assessed the physiochemical properties of 18 commercially available HA‐based artificial tears and concluded that the ideal HA‐based artificial tear should include high molecular weight HA, and discussed the possibility of unique formulations that could target specific ocular surface conditions (Aragona et al. 2019).
During the data collection process, details on product used, exact ingredients, concentrations, molecular weights, osmolarity, and pH of HA containing artificial tear formulations were often unavailable. Only eight of the included studies provided complete, or nearly complete information on the HA formulation used (Yokoi et al. 1997; Aragona et al. 2002a,b; Johnson et al. 2008; Troiano & Monaco 2008; Vogel et al. 2010; Chiambaretta et al. 2017; Laihia et al. 2020). Commercially available artificial tear formulations containing HA have limited details listed online. A greater availability of information regarding drop formulation, osmolarity, HA concentration, molecular weight, chemical modifications, and preservatives, could help strengthen the available evidence for DED treatment, aid in the planning of future clinical trials, and enable the development of better treatments for DED.
Future directions for HA in DED
There are promising future directions for HA in the treatment of DED. Other modes of HA delivery have been tested, including oral administration (Kim et al. 2019), slow‐release HA in contact lenses (Ali & Byrne 2009; Maulvi et al. 2015, 2017; Scheuer et al. 2016; Desai et al. 2018, 2020; Wei et al. 2020; Huang et al. 2021), and canaliculus HA gel‐occlusion (Fezza 2018). Hyaluronic acid shows promise as part of an ocular surface delivery device for other drugs, such as dexamethasone and cyclosporine (Soiberman et al. 2017; Liu et al. 2019). The HA molecule allows for chemical manipulation, including elongation, cross‐linking (Williams & Mann 2014), and for use as a derivative of new muco‐adhesives (Laffleur & Dachs 2015; Posarelli et al. 2019). A 2019 review concluded that HA is superior to other artificial tears and that the HA molecule still has potential for further improvement including cross‐linking to increase bioavailability and resistance to degradation (Posarelli et al. 2019). Hyaluronic acid lends itself to combination treatments and has been combined successfully with multiple other dry eye treatments, both experimentally and clinically, with promising results. A few examples of combination treatments include taurine (Roberti et al. 2018), which has osmoprotective and antioxidant properties, trehalose with similar properties as HA (Matsuo 2001; Schmidl et al. 2015; Bucolo et al. 2018; Fariselli et al. 2018; Fondi et al. 2018; Laihia & Kaarniranta 2020), omega‐3 fatty acids (Li et al. 2014), and glycerol (Kiss & Németh 2015).
Limitations
A limitation of this review was that only articles available with English full text were included. Exclusion of non‐English and non‐full‐text articles was a necessary step to ensure that thorough and correct information was available for review. None of the reviewed studies lasted more than three months. Longer studies could potentially reveal long‐term beneficial aspects of HA treatment, considering HA's known anti‐inflammatory and antioxidant properties. There were only eight placebo‐controlled trials among the reviewed literature (Sand et al. 1989; Shimmura et al. 1995; Condon et al. 1999; Aragona et al. 2002b; Vogel et al. 2010; Baeyens et al. 2012; López‐de la Rosa et al. 2017; Pinto‐Fraga et al. 2017). Placebo brings a unique challenge in DED trials as both saline and vehicle control groups tend to improve signs and symptoms of DED. This is a recurring problem in artificial tear research (Vogel et al. 2010). Lubrication effects of placebo drops as well as regression to the mean are likely explanations for this phenomenon.
Conclusion
Hyaluronic acid as an active ingredient in artificial tears in concentrations between 0.1% and 0.4% is a safe and effective treatment for DED. Hyaluronic acid has lubricating, anti‐inflammatory, antioxidant, and anti‐toxic effects at the ocular surface. Hyaluronic acid treatment improved both signs and symptoms of DED in most of the reviewed literature. Hypotonic HA drops appear to have some clinical benefit over isotonic drops but more studies isolating the effects of tonicity are needed. There is a literature gap in determining optimal HA concentration, drop frequency, molecular weight of HA, and potential differences in optimal treatment for different DED severities and sub‐types. Researchers should aim to isolate and investigate these variables in future studies of HA treatment for DED.
Darlene Dartt is supported by US NIH grants R01EY019470 and R01EY029789.
For the sake of transparency, Tor Paaske Utheim is co‐founder and co‐owner of The Norwegian dry eye clinic and the Clinic of eye health, Oslo, Norway, which delivers talks for and/or receives financial support from: ABIGO, Alcon, Allergan, AMWO, Bausch&Lomb, Bayer, European school for advanced studies in ophthalmology, InnZ Medical, Medilens Nordic, Medistim, Novartis, Santen, Specsavers, Shire Pharmaceuticals and Thea Laboratories. He has served on the global scientific advisory board for Novartis and Alcon, and on the European advisory board for Shire Pharmaceuticals. Utheim is the Norwegian Global Ambassador for the Tear Film and Ocular Surface Society (TFOS), a Board Member of the International Ocular Surface Society, an international member of the Japanese Lid and Meibomian gland working group (LIME), a Consultant at the Norwegian Association for the Blind and Partially Sighted, the President of the Oslo Society of ophthalmology, and the Editor‐in‐Chief of the eye journal Oftalmolog which contains advertisements from pharmaceutical companies, companies selling ophthalmological equipment, and associations organizing conferences and events in ophthalmology.
References
- Abatangelo G, Vindigni V, Avruscio G, Pandis L & Brun P (2020): Hyaluronic acid: redefining its role. Cell 9: 1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M & Byrne ME (2009): Controlled release of high molecular weight hyaluronic acid from molecularly imprinted hydrogel contact lenses. Pharm Res 26: 714–726. [DOI] [PubMed] [Google Scholar]
- Altman R, Bedi A, Manjoo A, Niazi F, Shaw P & Mease P (2019): Anti‐inflammatory effects of intra‐articular hyaluronic acid: a systematic review. Cartilage 10: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang BCH, Sng JJ, Wang PXH, Htoon HM & Tong LHT (2017): Sodium hyaluronate in the treatment of dry eye syndrome: a systematic review and meta‐analysis. Sci Rep 7: 9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona P, Di Stefano G, Ferreri F, Spinella R & Stilo A (2002a): Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjögren's syndrome patients. Br J Ophthalmol 86: 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E & Rolando M (2021): Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.a.S.S.O. board review. Br J Ophthalmol 105: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona P, Papa V, Micali A, Santocono M & Milazzo G (2002b): Long term treatment with sodium hyaluronate‐containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol 86: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona P, Rania L, Roszkowska AM, Spinella R, Postorino E, Puzzolo D & Micali A (2013): Effects of amino acids enriched tears substitutes on the cornea of patients with dysfunctional tear syndrome. Acta Ophthalmol 91: e437–e444. [DOI] [PubMed] [Google Scholar]
- Aragona P, Simmons PA, Wang H & Wang T (2019): Physicochemical properties of hyaluronic acid‐based lubricant eye drops. Transl Vis Sci Technol 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens V, Bron A, Baudouin C & Vismed/Hylovis Study Group (2012): Efficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J Fr Ophtalmol 35: 412–419. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Passali GC, Passali D, Damiani V, Ciprandi G & Balestrazzi E (2020): A new therapeutic approach for the dry eye syndrome in patients with laryngopharyngeal reflux: first data. Acta Biomed 91: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C, Cochener B, Pisella PJ, Girard B, Pouliquen P, Cooper H & Creuzot‐Garcher C (2012): Randomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Bayer IS (2020): Hyaluronic acid and controlled release: a review. Molecules 25: 2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez‐del‐Castillo JM, Aranguez C & Garcia‐Sanchez J (2002): Corneal epithelial permeability and dry eye treatment. Adv Exp Med Biol 506: 703–706. [DOI] [PubMed] [Google Scholar]
- Bjordal O, Norheim KB, Rødahl E, Jonsson R & Omdal R (2020): Primary Sjögren's syndrome and the eye. Surv Ophthalmol 65: 119–132. [DOI] [PubMed] [Google Scholar]
- Brar S, Vanga HR & Ganesh S (2021): Comparison of efficacy of trehalose‐based eye drops versus topical 0.1% hyaluronic acid for management of clinically significant dry eye using non‐invasive investigational modalities. Int Ophthalmol 41: 3349–3359. [DOI] [PubMed] [Google Scholar]
- Brignole F, Pisella PJ, Dupas B, Baeyens V & Baudouin C (2005): Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol 243: 531–538. [DOI] [PubMed] [Google Scholar]
- Bron AJ, de Paiva CS, Chauhan SK et al. (2017): TFOS DEWS II pathophysiology report. Ocul Surf 15: 438–510. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Evans VE & Smith JA (2003): Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 22: 640–650. [DOI] [PubMed] [Google Scholar]
- Bucolo C, Fidilio A, Platania CBM, Geraci F, Lazzara F & Drago F (2018): Antioxidant and Osmoprotecting activity of taurine in dry eye models. J Ocul Pharmacol Ther 34: 188–194. [DOI] [PubMed] [Google Scholar]
- Cai MM & Zhang J (2020): Effectiveness of transcutaneous electrical stimulation combined with artificial tears for the treatment of dry eye: a randomized controlled trial. Exp Ther Med 20: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakır B, Doğan E, Çelik E, Babashli T, Uçak T & Alagöz G (2018): Effects of artificial tear treatment on corneal epithelial thickness and corneal topography findings in dry eye patients. J Fr Ophtalmol 41: 407–411. [DOI] [PubMed] [Google Scholar]
- Carlson E, Kao WWY & Ogundele A (2018): Impact of hyaluronic acid‐containing artificial tear products on Reepithelialization in an in vivo corneal wound model. J Ocul Pharmacol Ther 34: 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo G, Pastrana C, Serramito M & Rodriguez‐Pomar C (2019): Evaluation of tear meniscus by optical coherence tomography after different sodium hyaluronate eyedrops instillation. Acta Ophthalmol 97: e162–e169. [DOI] [PubMed] [Google Scholar]
- Chen J, Dong F, Chen W et al. (2014): Clinical efficacy of 0.1% pranoprofen in treatment of dry eye patients: a multicenter, randomized, controlled clinical trial. Chin Med J 127: 2407–2412. [PubMed] [Google Scholar]
- Chernos M, Grecov D, Kwok E, Bebe S, Babsola O & Anastassiades T (2017): Rheological study of hyaluronic acid derivatives. Biomed Eng Lett 7: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiambaretta F, Doan S, Labetoulle M et al. (2017): A randomized, controlled study of the efficacy and safety of a new eyedrop formulation for moderate to severe dry eye syndrome. Eur J Ophthalmol 27: 1–9. [DOI] [PubMed] [Google Scholar]
- Condon PI, McEwen CG, Wright M, Mackintosh G, Prescott RJ & McDonald C (1999): Double blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br J Ophthalmol 83: 1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrady CD, Joos ZP & Patel BCK (2016): Review: The lacrimal gland and its role in dry eye. J Ophthalmol 2016: 7542929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JP, Nichols KK, Akpek EK et al. (2017): TFOS DEWS II definition and classification report. Ocul Surf 15: 276–283. [DOI] [PubMed] [Google Scholar]
- Debbasch C, De La Salle SB, Brignole F, Rat P, Warnet JM & Baudouin C (2002): Cytoprotective effects of hyaluronic acid and Carbomer 934P in ocular surface epithelial cells. Invest Ophthalmol Vis Sci 43: 3409–3415. [PubMed] [Google Scholar]
- DeLuise VP & Peterson WS (1984): The use of topical Healon tears in the management of refractory dry‐eye syndrome. Ann Ophthalmol 16: 823–824. [PubMed] [Google Scholar]
- Desai AR, Maulvi FA, Desai DM et al. (2020): Multiple drug delivery from the drug‐implants‐laden silicone contact lens: addressing the issue of burst drug release. Mater Sci Eng C Mater Biol Appl 112: 110885. [DOI] [PubMed] [Google Scholar]
- Desai AR, Maulvi FA, Pandya MM, Ranch KM, Vyas BA, Shah SA & Shah DO (2018): Co‐delivery of timolol and hyaluronic acid from semi‐circular ring‐implanted contact lenses for the treatment of glaucoma: in vitro and in vivo evaluation. Biomater Sci 6: 1580–1591. [DOI] [PubMed] [Google Scholar]
- `Doughty MJ & Glavin S (2009): Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt 29: 573–583. [DOI] [PubMed] [Google Scholar]
- Essa L, Laughton D & Wolffsohn JS (2018): Can the optimum artificial tear treatment for dry eye disease be predicted from presenting signs and symptoms? Cont Lens Anterior Eye 41: 60–68. [DOI] [PubMed] [Google Scholar]
- Fariselli C, Giannaccare G, Fresina M & Versura P (2018): Trehalose/hyaluronate eyedrop effects on ocular surface inflammatory markers and mucin expression in dry eye patients. Clin Ophthalmol 12: 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezza JP (2018): Cross‐linked hyaluronic acid gel occlusive device for the treatment of dry eye syndrome. Clin Ophthalmol 12: 2277–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondi K, Wozniak PA, Schmidl D, Bata AM, Witkowska KJ, Popa‐Cherecheanu A, Schmetterer L & Garhöfer G (2018): Effect of hyaluronic acid/Trehalose in two different formulations on signs and symptoms in patients with moderate to severe dry eye disease. J Ophthalmol 2018: 4691417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Conca V, Abad‐Collado M, Hueso‐Abancens JR, Mengual‐Verdú E, Piñero DP, Aguirre‐Balsalobre F & Molina JC (2019): Efficacy and safety of treatment of hyposecretory dry eye with platelet‐rich plasma. Acta Ophthalmol 97: e170–e178. [DOI] [PubMed] [Google Scholar]
- Gong L, Sun X, Ma Z et al. (2015): A randomised, parallel‐group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br J Ophthalmol 99: 903–908. [DOI] [PubMed] [Google Scholar]
- Groß D, Childs M & Piaton JM (2017): Comparison of 0.2% and 0.18% hyaluronate eye drops in patients with moderate to severe dry eye with keratitis or keratoconjunctivitis. Clin Ophthalmol 11: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groß D, Childs M & Piaton JM (2018): Comparative study of 0.1% hyaluronic acid versus 0.5% carboxymethylcellulose in patients with dry eye associated with moderate keratitis or keratoconjunctivitis. Clin Ophthalmol 12: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudowska‐Sawczuk M, Cylwik B & Chrostek L (2017): The role of serum hyaluronic acid determination in the diagnosis of liver fibrosis. Acta Biochim Pol 64: 451–457. [DOI] [PubMed] [Google Scholar]
- Guillaumie F, Furrer P, Felt‐Baeyens O, Fuhlendorff BL, Nymand S, Westh P, Gurny R & Schwach‐Abdellaoui K (2010): Comparative studies of various hyaluronic acids produced by microbial fermentation for potential topical ophthalmic applications. J Biomed Mater Res A 92: 1421–1430. [DOI] [PubMed] [Google Scholar]
- Higashide T & Sugiyama K (2008): Use of viscoelastic substance in ophthalmic surgery ‐ focus on sodium hyaluronate. Clin Ophthalmol 2: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zhang X, Li Y & Yang X (2021): Hyaluronic acid and graphene oxide loaded silicon contact lens for corneal epithelial healing. J Biomater Sci Polym Ed 32: 372–384. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Sung YM, Lee WS & Kim EC (2014): Additive effect of preservative‐free sodium hyaluronate 0.1% in treatment of dry eye syndrome with diquafosol 3% eye drops. Cornea 33: 935–941. [DOI] [PubMed] [Google Scholar]
- Ibrahim OM, Dogru M, Kojima T, Matsumoto Y, Wakamatsu TH, Tsubota K & Fujishima H (2012): OCT assessment of tear meniscus after punctal occlusion in dry eye disease. Optom Vis Sci 89: E770–E776. [DOI] [PubMed] [Google Scholar]
- Iester M, Orsoni GJ, Gamba G, Taffara M, Mangiafico P, Giuffrida S & Rolando M (2000): Improvement of the ocular surface using hypotonic 0.4% hyaluronic acid drops in keratoconjunctivitis sicca. Eye (Lond) 14: 892–898. [DOI] [PubMed] [Google Scholar]
- Ishioka M, Kato N, Takano Y, Shimazaki J & Tsubota K (2009): The quantitative detection of blurring of vision after eyedrop instillation using a functional visual acuity system. Acta Ophthalmol 87: 574–575. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J & Noble PW (2007): Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23: 435–461. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Murphy PJ & Boulton M (2008): Carbomer and sodium hyaluronate eyedrops for moderate dry eye treatment. Optom Vis Sci 85: 750–757. [DOI] [PubMed] [Google Scholar]
- Jones L, Downie LE, Korb D et al. (2017): TFOS DEWS II management and therapy report. Ocul Surf 15: 575–628. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Nakanishi M, Ishii R, Kobashi H, Igarashi A, Sato N & Shimizu K (2012): Clinical evaluation of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: a prospective, randomized, multicenter study. Eye (Lond) 26: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya S, Schmidl D, Schmetterer L et al. (2015): Effect of hyaluronic acid on tear film thickness as assessed with ultra‐high resolution optical coherence tomography. Acta Ophthalmol 93: 439–443. [DOI] [PubMed] [Google Scholar]
- Kim Y, Moon CH, Kim BY & Jang SY (2019): Oral hyaluronic acid supplementation for the treatment of dry eye disease: a pilot study. J Ophthalmol 2019: 5491626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Kang YS, Lee HS, Choi W, You IC & Yoon KC (2017): Effectiveness of combined tear film therapy in patients with evaporative dry eye with short tear film breakup time. J Ocul Pharmacol Ther 33: 635–643. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Oshiden K, Awamura S, Suzuki H, Nakamichi N, Yokoi N & Rebamipide Ophthalmic Suspension Phase 3 Study Group (2013): A randomized, multicenter phase 3 study comparing 2% rebamipide (OPC‐12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology 120: 1158–1165. [DOI] [PubMed] [Google Scholar]
- Kiss HJ & Németh J (2015): Isotonic glycerol and sodium hyaluronate containing artificial tear decreases Conjunctivochalasis after one and three months: a self‐controlled, unmasked study. PLoS ONE 10: e0132656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Maeda N, Ikeda C et al. (2013): Effect of instillation of eyedrops for dry eye on optical quality. Invest Ophthalmol Vis Sci 54: 4927–4933. [DOI] [PubMed] [Google Scholar]
- Kotla NG, Bonam SR, Rasala S, Wankar J, Bohara RA, Bayry J, Rochev Y & Pandit A (2021): Recent advances and prospects of hyaluronan as a multifunctional therapeutic system. J Control Release 336: 598–620. [DOI] [PubMed] [Google Scholar]
- Labetoulle M, Chiambaretta F, Shirlaw A, Leaback R & Baudouin C (2017): Osmoprotectants, carboxymethylcellulose and hyaluronic acid multi‐ingredient eye drop: a randomised controlled trial in moderate to severe dry eye. Eye (Lond) 31: 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labetoulle M, Schmickler S, Galarreta D, Böhringer D, Ogundele A, Guillon M & Baudouin C (2018): Efficacy and safety of dual‐polymer hydroxypropyl guar‐ and hyaluronic acid‐containing lubricant eyedrops for the management of dry‐eye disease: a randomized double‐masked clinical study. Clin Ophthalmol 12: 2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffleur F & Dachs S (2015): Development of novel mucoadhesive hyaluronic acid derivate as lubricant for the treatment of dry eye syndrome. Ther Deliv 6: 1211–1219. [DOI] [PubMed] [Google Scholar]
- Laflamme MY & Swieca R (1988): A comparative study of two preservative‐free tear substitutes in the management of severe dry eye. Can J Ophthalmol 23: 174–176. [PubMed] [Google Scholar]
- Laihia J, Järvinen R, Wylęgała E & Kaarniranta K (2020): Disease aetiology‐based design of multifunctional microemulsion eye drops for moderate or severe dry eye: a randomized, quadruple‐masked and active‐controlled clinical trial. Acta Ophthalmol 98: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laihia J & Kaarniranta K (2020): Trehalose for ocular surface health. Biomolecules 10: 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Sullivan BD, Schmidt TA et al. (2017): A two‐week, randomized, double‐masked study to evaluate safety and efficacy of Lubricin (150 μg/mL) eye drops versus sodium hyaluronate (HA) 0.18% eye drops (Vismed®) in patients with moderate dry eye disease. Ocul Surf 15: 77–87. [DOI] [PubMed] [Google Scholar]
- Lanzini M, Curcio C, Colabelli‐Gisoldi RA, Mastropasqua A, Calienno R, Agnifili L, Nubile M & Mastropasqua L (2015): In vivo and impression cytology study on the effect of compatible solutes eye drops on the ocular surface epithelial cell quality in dry eye patients. Mediat Inflamm 2015: 351424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardner E & van Setten GB (2020): Detection of TSG‐6‐like protein in human corneal epithelium. Simultaneous presence with CD44 and hyaluronic acid. J Fr Ophtalmol 43: 879–883. [DOI] [PubMed] [Google Scholar]
- Lee BM, Park SJ, Noh I & Kim C‐H (2021): The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater Res 25: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Ryu IH, Seo KY, Hong S, Kim HC & Kim EK (2006): Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology 113: 198–205. [DOI] [PubMed] [Google Scholar]
- Lee HS, Ji YS & Yoon KC (2014a): Efficacy of hypotonic 0.18% sodium hyaluronate eye drops in patients with dry eye disease. Cornea 33: 946–951. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kim NM, Yang JW, Kim SJ, Lee JS & Lee JE (2014b): A randomised controlled trial comparing a thermal massager with artificial teardrops for the treatment of dry eye. Br J Ophthalmol 98: 46–51. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ahn HS, Kim EK & Kim TI (2011): Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea 30: 175–179. [DOI] [PubMed] [Google Scholar]
- Li Z, Choi JH, Oh HJ, Park SH, Lee JB & Yoon KC (2014): Effects of eye drops containing a mixture of omega‐3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress‐induced murine dry eye. Curr Eye Res 39: 871–878. [DOI] [PubMed] [Google Scholar]
- Limberg MB, McCaa C, Kissling GE & Kaufman HE (1987): Topical application of hyaluronic acid and chondroitin sulfate in the treatment of dry eyes. Am J Ophthalmol 103: 194–197. [DOI] [PubMed] [Google Scholar]
- Lin W, Mashiah R, Seror J, Kadar A, Dolkart O, Pritsch T, Goldberg R & Klein J (2019): Lipid‐hyaluronan synergy strongly reduces intrasynovial tissue boundary friction. Acta Biomater 83: 314–321. [DOI] [PubMed] [Google Scholar]
- Litwiniuk M, Krejner A, Speyrer MS, Gauto AR & Grzela T (2016): Hyaluronic acid in inflammation and tissue regeneration. Wounds 28: 78–88. [PubMed] [Google Scholar]
- Liu X, Wang S, Kao AA & Long Q (2012): The effect of topical pranoprofen 0.1% on the clinical evaluation and conjunctival HLA‐DR expression in dry eyes. Cornea 31: 1235–1239. [DOI] [PubMed] [Google Scholar]
- Liu X, Yu FF, Zhong YM, Guo XX & Mao Z (2015): Therapeutic effects of sodium hyaluronate on ocular surface damage induced by Benzalkonium chloride preserved anti‐glaucoma medications. Chin Med J 128: 2444–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Yang J, Zhang H & Gan L (2019): Cationized hyaluronic acid coated spanlastics for cyclosporine a ocular delivery: prolonged ocular retention, enhanced corneal permeation and improved tear production. Int J Pharm 565: 133–142. [DOI] [PubMed] [Google Scholar]
- López‐de la Rosa A, Pinto‐Fraga J, Blázquez Arauzo F, Urbano Rodríguez R & González‐García MJ (2017): Safety and efficacy of an artificial tear containing 0.3% hyaluronic acid in the Management of Moderate‐to‐Severe dry eye Disease. Eye Contact Lens 43: 383–388. [DOI] [PubMed] [Google Scholar]
- López‐García JS, García‐Lozano I, Rivas L, Ramírez N, Raposo R & Méndez MT (2014): Autologous serum eye drops diluted with sodium hyaluronate: clinical and experimental comparative study. Acta Ophthalmol 92: e22–e29. [DOI] [PubMed] [Google Scholar]
- Macri A, Scanarotti C, Bassi AM, Giuffrida S, Sangalli G, Traverso CE & Iester M (2015): Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative‐free hyaluronic acid 0.15% and vitamin B12 eye drops. Graefes Arch Clin Exp Ophthalmol 253: 425–430. [DOI] [PubMed] [Google Scholar]
- Mateo Orobia AJ, Saa J, Ollero Lorenzo A & Herreras JM (2018): Combination of hyaluronic acid, carmellose, and osmoprotectants for the treatment of dry eye disease. Clin Ophthalmol 12: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T (2001): Trehalose protects corneal epithelial cells from death by drying. Br J Ophthalmol 85: 610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T (2004): Trehalose versus hyaluronan or cellulose in eyedrops for the treatment of dry eye. Jpn J Ophthalmol 48: 321–327. [DOI] [PubMed] [Google Scholar]
- Maulvi FA, Shaikh AA, Lakdawala DH et al. (2017): Design and optimization of a novel implantation technology in contact lenses for the treatment of dry eye syndrome: in vitro and in vivo evaluation. Acta Biomater 53: 211–221. [DOI] [PubMed] [Google Scholar]
- Maulvi FA, Soni TG & Shah DO (2015): Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome. J Biomater Sci Polym Ed 26: 1035–1050. [DOI] [PubMed] [Google Scholar]
- McCann LC, Tomlinson A, Pearce EI & Papa V (2012): Effectiveness of artificial tears in the management of evaporative dry eye. Cornea 31: 1–5. [DOI] [PubMed] [Google Scholar]
- McDonald CC, Kaye SB, Figueiredo FC, Macintosh G & Lockett C (2002): A randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye (Lond) 16: 601–607. [DOI] [PubMed] [Google Scholar]
- McMonnies CW (2020): Aqueous deficiency is a contributor to evaporation‐related dry eye disease. Eye Vision 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengher LS, Pandher KS, Bron AJ & Davey CC (1986): Effect of sodium hyaluronate (0.1%) on break‐up time (NIBUT) in patients with dry eyes. Br J Ophthalmol 70: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miháltz K, Faschinger EM & Vécsei‐Marlovits PV (2018): Effects of lipid‐ versus sodium hyaluronate‐containing eye drops on optical quality and ocular surface parameters as a function of the Meibomian gland dropout rate. Cornea 37: 886–892. [DOI] [PubMed] [Google Scholar]
- Milafzzo G, Papa V, Aragona P, Russo S, Russo P & Di Bella A (2002): Efficacy of sodium hyaluronate eye drops of different osmolarities in the symptomatic treatment of dry eye patients. Adv Exp Med Biol 506: 1233–1235. [DOI] [PubMed] [Google Scholar]
- Monaco G, Cacioppo V, Consonni D & Troiano P (2011): Effects of osmoprotection on symptoms, ocular surface damage, and tear film modifications caused by glaucoma therapy. Eur J Ophthalmol 21: 243–250. [DOI] [PubMed] [Google Scholar]
- Montani G (2013): Intrasubject tear osmolarity changes with two different types of eyedrops. Optom Vis Sci 90: 372–377. [DOI] [PubMed] [Google Scholar]
- Morthen MK, Magno MS, Utheim TP, Snieder H, Hammond CJ & Vehof J (2021): The physical and mental burden of dry eye disease: a large population‐based study investigating the relationship with health‐related quality of life and its determinants. Ocul Surf 21: 107–117. [DOI] [PubMed] [Google Scholar]
- Morthen MK, Magno MS, Utheim TP, Snieder H, Jansonius N, Hammond CJ & Vehof J (2022): The vision‐related burden of dry eye. Ocul Surf 23: 207–215. [DOI] [PubMed] [Google Scholar]
- Morya AK, Solanki K, Prakash S, Samota M & Gupta A (2021): Randomized controlled trial of trehalose: an efficient autophagic bioprotectant in the management of dry eye disease. Taiwan J Ophthalmol 11: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbioso M, Fameli V, Gharbiya M, Sacchetti M, Zicari AM & Lambiase A (2016): Investigational drugs in dry eye disease. Expert Opin Investig Drugs 25: 1437–1446. [DOI] [PubMed] [Google Scholar]
- Nelson JD & Farris RL (1988): Sodium hyaluronate and polyvinyl alcohol artificial tear preparations. a comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol 106: 484–487. [DOI] [PubMed] [Google Scholar]
- Nepp J, Schauersberger J, Schild G, Jandrasits K, Haslinger‐Akramian J, Derbolav A & Wedrich A (2001): The clinical use of viscoelastic artificial tears and sodium chloride in dry‐eye syndrome. Biomaterials 22: 3305–3310. [DOI] [PubMed] [Google Scholar]
- Papa V, Aragona P, Russo S, Di Bella A, Russo P & Milazzo G (2001): Comparison of hypotonic and isotonic solutions containing sodium hyaluronate on the symptomatic treatment of dry eye patients. Ophthalmologica 215: 124–127. [DOI] [PubMed] [Google Scholar]
- Park Y, Song JS, Choi CY, Yoon KC, Lee HK & Kim HS (2017): A randomized multicenter study comparing 0.1%, 0.15%, and 0.3% sodium hyaluronate with 0.05% cyclosporine in the treatment of dry eye. J Ocul Pharmacol Ther 33: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauloin T, Dutot M, Joly F, Warnet J‐M & Rat P (2009a): High molecular weight hyaluronan decreases UVB‐induced apoptosis and inflammation in human epithelial corneal cells. Mol Vis 15: 577–583. [PMC free article] [PubMed] [Google Scholar]
- Pauloin T, Dutot M, Liang H, Chavinier E, Warnet JM & Rat P (2009b): Corneal protection with high‐molecular‐weight hyaluronan against in vitro and in vivo sodium lauryl sulfate‐induced toxic effects. Cornea 28: 1032–1041. [DOI] [PubMed] [Google Scholar]
- Pinto‐Bonilla JC, Del Olmo‐Jimeno A, Llovet‐Osuna F & Hernández‐Galilea E (2015): A randomized crossover study comparing trehalose/hyaluronate eyedrops and standard treatment: patient satisfaction in the treatment of dry eye syndrome. Ther Clin Risk Manag 11: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto‐Fraga J, Lopez‐de A, la Rosa F, Blazquez Arauzo R, Rodriguez U & Gonzalez‐Garcia MJ (2017): Efficacy and safety of 0.2% hyaluronic acid in the Management of dry eye Disease. Eye Contact Lens 43: 57–63. [DOI] [PubMed] [Google Scholar]
- Polack FM & McNiece MT (1982): The treatment of dry eyes with NA hyaluronate (Healon)‐apreliminiary report. Cornea 1: 133–136. [Google Scholar]
- Posarelli C, Passani A, Del Re M, Fogli S, Toro MD, Ferreras A & Figus M (2019): Cross‐linked hyaluronic acid as tear film substitute. J Ocul Pharmacol Ther 35: 381–387. [DOI] [PubMed] [Google Scholar]
- Postorino EI, Rania L, Aragona E, Mannucci C, Alibrandi A, Calapai G, Puzzolo D & Aragona P (2018): Efficacy of eyedrops containing cross‐linked hyaluronic acid and coenzyme Q10 in treating patients with mild to moderate dry eye. Eur J Ophthalmol 28: 25–31. [DOI] [PubMed] [Google Scholar]
- Rah MJ (2011): A review of hyaluronan and its ophthalmic applications. Optometry 82: 38–43. [DOI] [PubMed] [Google Scholar]
- Robert PY, Cochener B, Amrane M, Ismail D, Garrigue JS, Pisella PJ & Baudouin C (2016): Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol 26: 546–555. [DOI] [PubMed] [Google Scholar]
- Roberti G, Agnifili L, Berardo F, Riva I, Figus M, Manni G, Quaranta L & Oddone F (2018): Prospective, randomized, single masked, parallel study exploring the effects of a preservative‐free ophthalmic solution containing hyaluronic acid 0.4% and taurine 0.5% on the ocular surface of glaucoma patients under multiple Long‐term topical hypotensive therapy. Adv Ther 35: 686–696. [DOI] [PubMed] [Google Scholar]
- Rolando M & Vagge A (2017): Safety and efficacy of cortisol phosphate in hyaluronic acid vehicle in the treatment of dry eye in Sjogren syndrome. J Ocul Pharmacol Ther 33: 383–390. [DOI] [PubMed] [Google Scholar]
- Rolando M & Valente C (2007): Establishing the tolerability and performance of tamarind seed polysaccharide (TSP) in treating dry eye syndrome: Results of a clinical study. BMC Ophthalmol 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed N, Qazi Z, Butt NH, Siddiqi A, Maheshwary N & Athar Khan M (2013): Effectiveness of sodium hyaluronate eye gel in patients with dry eye disease: a multi‐centre, open label, uncontrolled study. Pak J Med Sci 29: 1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salwowska NM, Bebenek KA, Żądło DA & Wcisło‐Dziadecka DL (2016): Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol 15: 520–526. [DOI] [PubMed] [Google Scholar]
- Sanchez MA, Torralbo‐Jimenez P, Giron N et al. (2010): Comparative analysis of carmellose 0.5% versus hyaluronate 0.15% in dry eye: a flow cytometric study. Cornea 29: 167–171. [DOI] [PubMed] [Google Scholar]
- Sand BB, Marner K & Norn MS (1989): Sodium hyaluronate in the treatment of keratoconjunctivitis sicca. A double masked clinical trial. Acta Ophthalmol 67: 181–183. [DOI] [PubMed] [Google Scholar]
- Scheuer CA, Rah MJ & Reindel WT (2016): Increased concentration of hyaluronan in tears after soaking contact lenses in biotrue multipurpose solution. Clin Ophthalmol 10: 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidl D, Schmetterer L, Witkowska KJ et al. (2015): Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease. Cornea 34: 421–426. [DOI] [PubMed] [Google Scholar]
- Shimmura S, Ono M, Shinozaki K, Toda I, Takamura E, Mashima Y & Tsubota K (1995): Sodium hyaluronate eyedrops in the treatment of dry eyes. Br J Ophthalmol 79: 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvani L, Bedei A, De Grazia G & Remiddi S (2020): Arabinogalactan and hyaluronic acid in ophthalmic solution: experimental effect on xanthine oxidoreductase complex as key player in ocular inflammation (in vitro study). Exp Eye Res 196: 108058. [DOI] [PubMed] [Google Scholar]
- Snetkov P, Zakharova K, Morozkina S, Olekhnovich R & Uspenskaya M (2020): Hyaluronic acid: The influence of molecular weight on structural, physical, Physico‐chemical, and degradable properties of biopolymer. Polymers (Basel) 12: 1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiberman U, Kambhampati SP, Wu T et al. (2017): Subconjunctival injectable dendrimer‐dexamethasone gel for the treatment of corneal inflammation. Biomaterials 125: 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Alves M, Bunya VY et al. (2017): TFOS DEWS II epidemiology report. Ocul Surf 15: 334–365. [DOI] [PubMed] [Google Scholar]
- Szegedi S, Scheschy U, Schmidl D et al. (2018): Effect of single instillation of two hyaluronic acid‐based topical lubricants on tear film thickness in patients with dry eye syndrome. J Ocul Pharmacol Ther 34: 605–611. [DOI] [PubMed] [Google Scholar]
- Takamura E, Tsubota K, Watanabe H & Ohashi Y (2012): A randomised, double‐masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol 96: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano P & Monaco G (2008): Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: a cross‐over study. Cornea 27: 1126–1130. [DOI] [PubMed] [Google Scholar]
- Tsubota K & Yamada M (1992): Tear evaporation from the ocular surface. Invest Ophthalmol Vis Sci 33: 2942–2950. [PubMed] [Google Scholar]
- van Setten G‐B, Baudouin C, Horwath‐Winter J et al. (2020): The HYLAN M study: efficacy of 0.15% high molecular weight Hyaluronan fluid in the treatment of severe dry eye disease in a multicenter randomized trial. J Clin Med 9: 3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Setten GB (2020): Impact of attrition, intercellular shear in dry eye disease: when cells are challenged and neurons are triggered. Int J Mol Sci 21: 4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehof J, Snieder H, Jansonius N & Hammond CJ (2021): Prevalence and risk factors of dry eye in 79,866 participants of the population‐based lifelines cohort study in The Netherlands. Ocul Surf 19: 83–93. [DOI] [PubMed] [Google Scholar]
- Versura P, Profazio V, Balducci N & Campos EC (2010): Efficacy of two‐month treatment with Xiloial eyedrops for discomfort from disposable soft contact lenses. Clin Ophthalmol 4: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R, Crockett RS, Oden N, Laliberte TW & Molina L (2010): Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (vismed, rejena). Am J Ophthalmol 149: 594–601. [DOI] [PubMed] [Google Scholar]
- Vorvolakos K, Isayeva IS, Luu H‐MD, Patwardhan DV & Pollack SK (2011): Ionically cross‐linked hyaluronic acid: wetting, lubrication, and viscoelasticity of a modified adhesion barrier gel. Med Devices (Auckl) 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N, Xu X, Huang C & Cao L (2020): Hyaluronic acid‐Pluronic®F127‐laden soft contact lenses for corneal epithelial healing: in vitro and in vivo studies. AAPS PharmSciTech 21: 162. [DOI] [PubMed] [Google Scholar]
- Willcox MDP, Argüeso P, Georgiev GA et al. (2017): TFOS DEWS II tear film report. Ocul Surf 15: 366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL & Mann BK (2014): Efficacy of a crosslinked hyaluronic acid‐based hydrogel as a tear film supplement: a masked controlled study. PLoS ONE 9: e99766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Chou HC, Li JM, Chen YW, Chen JH, Chen YH & Chan HL (2013): Hyaluronic acid‐dependent protection against alkali‐burned human corneal cells. Electrophoresis 34: 388–396. [DOI] [PubMed] [Google Scholar]
- Wu Y, Jin X, Mou Y, Yuan K, Min J & Huang X (2021): A 4‐week, randomized, double‐masked study to evaluate efficacy of deproteinized calf blood extract eye drops versus sodium hyaluronate 0.3% eye drops in dry eye patients with ocular pain. Ann Palliat Med 10: 3617–3625. [DOI] [PubMed] [Google Scholar]
- Yang Y‐J, Lee W‐Y, Kim Y‐j & Hong Y‐p (2021): A meta‐analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int J Environ Res Public Health 18: 2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi N, Komuro A, Nishida K & Kinoshita S (1997): Effectiveness of hyaluronan on corneal epithelial barrier function in dry eye. Br J Ophthalmol 81: 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You IC, Li Y, Jin R, Ahn M, Choi W & Yoon KC (2018): Comparison of 0.1%, 0.18%, and 0.3% hyaluronic acid eye drops in the treatment of experimental dry eye. J Ocul Pharmacol Ther 34: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Asche CV & Fairchild CJ (2011): The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 30: 379–387. [DOI] [PubMed] [Google Scholar]
- Yu L, Yu C, Dong H et al. (2021): Recent developments about the pathogenesis of dry eye disease: Based on immune inflammatory mechanisms. Front Pharmacol 12: 732887. [DOI] [PMC free article] [PubMed] [Google Scholar]


