Abstract
Background
Although long‐term management of psoriasis is paramount, this approach is challenging in clinical practice. In the recent PSO‐LONG trial, a fixed‐dose combination of betamethasone dipropionate (BD) and calcipotriol (Cal) foam applied twice a week on non‐consecutive days for 52 weeks (proactive treatment) reduced the risk of relapse. However, the role of Cal/BD foam in the long‐term management of psoriasis needs further clarifications. The ProActive Management (PAM) program, a nationwide Italian project, aims at reaching a consensus on the role of proactive management of psoriasis.
Methods
A steering committee generated some statements through the nominal group technique (NGT). The statements were voted by an expert panel in an adapted Delphi voting process.
Results
Eighteen statements were proposed, and the majority of them (14/18) reached a consensus during the Delphi voting. The need to provide long‐term proactive topical treatment to reduce the risk of relapse for the treatment of challenging diseases sites or in patients where phototherapy or systemic therapies are contraindicated/ineffective was widely recognized. A consensus was reached about the possibility to associate the proactive treatment with systemic and biological therapies, without the need for dose intensification, thus favoring a prolonged remission. Moreover, the proactive treatment was recognized as more effective than weekend therapy in increasing time free from relapses. Approaches to improve adherence, on the other hand, need further investigation.
Conclusions
The inclusion in guidelines of a proactive strategy among the effective treatment options will be a fundamental step in the evolution of a mild‐moderate psoriasis therapeutic approach.
Keywords: adherence, Cal/BD , consensus, long‐term, proactive management, psoriasis
1. Introduction
Psoriasis requires long‐term management in clinical practice. Indeed, even once the skin lesions are fully resolved, subclinical inflammation may persist, eventually leading to flares of disease in the same site. 1 , 2 However, although long‐term management of psoriasis is paramount, this approach is still challenging in clinical practice (especially for mild‐to‐moderate forms of the disease) partly due to the lack of suitable treatments or the difficulties in maintaining adherence. Hence, many patients are not treated or undertreated. 3
Topical treatments are recommended by international guidelines for the first‐line treatment of mild‐to‐moderate psoriasis. 4 , 5 Among these treatments, approved therapies include corticosteroids, vitamin D analogs, combined corticosteroid/vitamin D analog (calcipotriol [Cal]) formulations, vitamin A derivatives, anthralin, and newer formulations of tar. 2 However, to date, the use of topical treatments has mainly been explored in short‐term therapy, and clinical data on the long‐term use of this therapeutic strategy remain scant. 3 Furthermore, long‐term management by topical therapy is usually based upon a reactive approach – i.e., the administration of treatment when psoriasis relapses.
Noteworthy, a proactive approach—i.e., the regular application of maintenance therapy to previously affected areas, with calcineurin inhibitors prevents and delays relapses in another chronic dermatological condition, namely atopic dermatitis. Therefore, in line with the principle, proactive management can also be effective in psoriasis. 1 , 3
This speculation found confirmation in the recently published PSO‐LONG trial, in which the fixed‐dose combination of a corticosteroid (betamethasone dipropionate [BD]) and vitamin D analog (Cal) foam applied twice weekly on non‐consecutive days for 52 weeks, in patients who had achieved treatment success after a 4‐week open‐label phase, prolonged the time to first relapse, increased time with disease remission, and reduced the number of relapses compared with reactive management. 6 The PSO‐LONG trial represents an unprecedented advance in the treatment of psoriasis, and according to its results, Cal/BD foam is now indicated also in the maintenance treatment of this disease. 3 , 7 However, the role of Cal/BD foam and the potential therapeutic schemes in the long‐term management of psoriasis need further clarifications, and suggestions should be provided to improve adherence and optimize the patient journey in clinical practice. 3
Consensus approaches play a major role in current research in dermatology. This methodology is used to obtain consensus definitions that would be useful for daily practice, especially for areas in which evidence from well‐grounded studies is scant. 3 , 5 , 8 , 9 , 10 We have, therefore, conducted the ProActive Management (PAM) program, a nationwide Italian project, to reach a consensus on the role of proactive management of psoriasis, with a focus on Cal/BD, in terms of its rationale, therapeutic approaches, and optimization of a patient's journey. In particular, we used a combined approach of two different consensus methods, the nominal group technique (NGT) and the Delphi method. The combination of NGT and Delphi is increasingly used in clinical qualitative methdology, to provide a robust collection of expert opinions. 11 , 12 , 13
2. Methods
2.1. Overview of the PAM project
The structure of this nationwide project is shown in the flowchart (Fig. 1), and more details are provided in the following paragraphs. In short, a steering committee reviewed the available literature and generated some statements through the NGT. 14 The steering committee consisted of six Italian dermatologists who designed and developed the project, identified the expert panel, generated the statements, and reviewed and discussed the voting results. The statements were voted by an expert panel of Italian dermatologists in a round of an adapted Delphi voting process during eight macroregional meetings. Candidate experts were proposed and approved by the steering committee.
Figure 1.
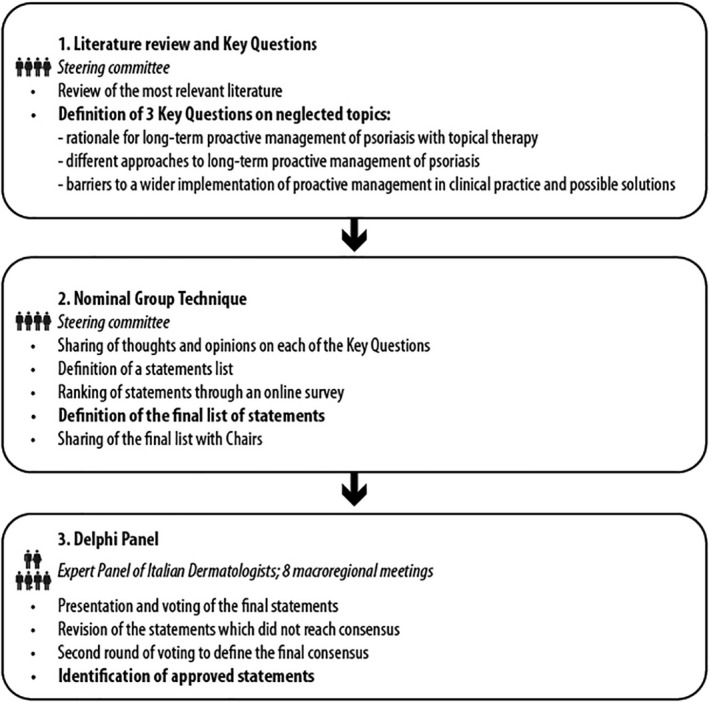
The overall structure of the ProActive Management project Source: Original—no permissions required
This methodology has already been applied elsewhere. 13 The project was supported by an unconditioned grant from Leo Pharma. The steering committee sought the assistance of an independent scientific consultancy agency (Polistudium srl, Milan, Italy) to facilitate meetings and provide assistance in the preparation of materials.
2.2. Literature review and key questions
The steering committee reviewed the most recent literature on long‐term and proactive management of psoriasis and drafted three key questions to be used to generate statements through an NGT round.
Domains of the questions were as follows: (i) rationale for long‐term proactive management of psoriasis with topical therapy; (ii) different approaches to long‐term proactive management of psoriasis; and (iii) barriers to a wider implementation of proactive management in clinical practice and possible solutions.
2.3. Definition of statements
The NGT is a direct and structured technique, based on experts' opinions, aimed at managing meetings organized to make decisions and provide guidance for a specific topic that is not supported by robust evidence. 14 In the PAM project, an NGT was used to generate the statements for the Delphi Panel. First, each member of the steering committee independently developed his/her thoughts and opinions on each of the identified three questions. All opinions were presented during an online meeting in November 2020, chaired by a professional facilitator. All the opinions were refined and converted into statements and shared with the members of the steering committee, who ranked them through an online voting process in terms of priority and relevance using a 1–5 scale. The participants then had the opportunity to review and/or comment on all statements during a second, remotely performed meeting. Eventually, the final list of statements was drafted.
2.4. Adapted Delphi process
The Delphi method is a standard method of consensus which is evaluated interactively and anonymously through online voting, the level of agreement (consensus quantification) using a Likert scale (1–5; 1 = total disagreement; 5 = total agreement). According to the indications of the Ministry of Health, 15 consensus on the agreement is reached when ≥75% of voters express a vote equal to 4 or 5. Further rounds of voting are performed after collecting the feedback of participants on each statement.
During an online meeting held in February 2021, the steering committee presented the overall architecture of the project and the statements to a group of nine other Italian dermatologists (chairs), who guided and chaired a series of other eight online meetings (March–May 2021) in which the statements were presented to and voted on by the Delphi Panel (one meeting was chaired by two chairs).
Eight to 10 other Italian dermatologists participated in each of those online meetings, for a total of 77 members of the Delphi Panel. During each meeting, the members of the Delphi Panel, including the chairs, voted the statements according to the Delphi methodology, and results from each meeting were pooled. Advice from participants was collected, and suggestions were integrated with the statements which did not reach consensus during the online meetings. Those statements were then voted online again by the Delphi Panel.
2.5. Statistical analysis
All data were analyzed with descriptive statistics.
3. Results
3.1. NGT outcome
During the NGT round, the steering committee provided an opinion on the three key questions and defined 18 statements to be administered during the Delphi voting (Table 1). One statement, which concerned the outcomes for monitoring the efficacy of proactive management, was composed of seven substatements, one for each outcome.
Table 1.
Results of Delphi panel
| Statements | Response | Consensus score a | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Agree | Disagree | |
| 1. Rationale for long‐term proactive management of psoriasis with topical therapy | |||||||
| 1.1 The skin of patients with psoriasis, even when treated and not showing overt lesions, still presents a residual risk of relapse. Long‐term proactive treatment can deactivate the inflammatory pathway and prevent relapses | 2 (2.7%) | 14 (18.7%) | 59 (78.7%) | 97.3% | |||
| 1.2 Based on available evidence on long‐term proactive treatment with calcineurin inhibitors in atopic dermatitis, a revision of the guidelines is suggested to also recommend proactive treatment in psoriasis | 5 (6.7%) | 13 (17.3%) | 57 (76.0%) | 93.3% | |||
| 1.3 Long‐term proactive topical treatment avoids worsening of a mild‐to‐moderate disease and can extend the disease‐free period, improving quality of life with minimum risks | 4 (5.3%) | 23 (30.7%) | 48 (64.0%) | 90.7% | |||
| 1.4 Long‐term topical treatment allows targeted intervention on sites where systemic drugs or phototherapy are less effective in the individual patient | 2 (2.7%) | 40 (53.3%) | 7 (9.3%) | 26 (34.7%) | 88.0% | ||
| 1.5 When phototherapy, systemic or biological therapy are contraindicated, ineffective or only partially effective, chronic topical treatment is useful, both in reactive and in proactive maintenance | 1 (1.3%) | 3 (3.9%) | 15 (19.7%) | 57 (75.0%) | 94.7% | ||
| 1.6 To evaluate the effectiveness of proactive treatment, the following outcome(s) should be considered: | |||||||
| 1.6.1 duration of the remission period | 1 (1.3%) | 2 (2.7%) | 20 (26.7%) | 52 (69.3%) | 96.0% | ||
| 1.6.2 number of relapses | 2 (2.7%) | 1 (1.3%) | 8 (10.7%) | 28 (37.3%) | 36 (48.0%) | 85.3% | |
| 1.6.3 time free from subjective symptoms important for the patient's QoL (heat, burning, itching) | 1 (1.3%) | 11 (14.7%) | 27 (36.0%) | 36 (48.0%) | 84.0% | ||
| 1.6.4 tolerability by monitoring any local adverse reaction | 1 (1.3%) | 14 (18.7%) | 28 (37.3%) | 32 (42.7%) | 80.0% | ||
| 1.6.5 patient's ability to maintain a proper adherence | 1 (1.3%) | 5 (6.7%) | 25 (33.3%) | 44 (58.7%) | 92.0% | ||
| 1.6.6 modified PASI | 3 (4.0%) | 10 (13.3%) | 33 (44.0%) | 29 (38.7%) | 82.7% | ||
| 1.6.7 PGA | 2 (2.7%) | 5 (6.7%) | 14 (18.7%) | 32 (42.7%) | 22 (29.3%) | 72.8% | |
| 2. Different approaches to long‐term proactive management of psoriasis | |||||||
| 2.1 The mechanistic rationale of the Cal/BD combination and available evidence allow its use as a long‐term topical therapy in patients with psoriasis | 3 (4.0%) | 23 (30.7%) | 49 (65.3%) | 96.0% | |||
| 2.2 The good tolerability and lack of clinically relevant systemic effects (e.g., on the hypothalamic–pituitary–adrenal axis and calcium metabolism) support the twice‐weekly use of Cal/BD foam in the long‐term proactive treatment of psoriasis | 1 (1.3%) | 1 (1.3%) | 16 (21.3%) | 57 (76.0%) | 97.3% | ||
| 2.3 The proactive treatment with Cal/BD foam can be associated with systemic conventional and biological therapy, without the need for an intensification of the dose or even with the possibility of a dose reduction of the systemic therapy, thus favoring a prolonged remission | 1 (1.3%) | 8 (10.7%) | 25 (33.3%) | 41 (54.7%) | 88.0% | ||
| 2.4 Proactive treatment with Cal/BD foam may also be useful in combination with biologics, for example in unresponsive sites or when a complete response is not achieved | 1 (1.3%) | 2 (2.7%) | 7 (9.3%) | 22 (29.3%) | 43 (57.3%) | 86.6% | |
| 2.5 The twice‐weekly non‐consecutive dosing regimen recommended for long‐term therapy with Cal/BD foam (proactive treatment) may allow for a reduction in the number of relapses | 3 (4.0%) | 29 (38.7%) | 43 (57.3%) | 96.0% | |||
| 2.6 Proactive treatment allows for greater efficacy than weekend therapy, increasing the time free from relapses | 2 (2.7%) | 1 (1.3%) | 6 (8.0%) | 22 (29.3%) | 44 (58.7%) | 88.0% | |
| 3. Barriers to a wider implementation of proactive management in clinical practice and possible solutions | |||||||
| 3.1 Talking with the patient, it is necessary to clarify the importance of the correct adherence to long‐term topical treatment, explaining how this therapeutic regimen will help in delaying relapses | 1 (1.3%) | 1 (1.3%) | 9 (12.0%) | 64 (85.3%) | 97.3% | ||
| 3.2 Continued dermatologist‐patient relationship is central to maintain correct adherence, optimizing the therapeutic outcomes of long‐term treatment, and educating patients to avoid the use of self‐prescribed remedies | 3 (4.0%) | 16 (21.3%) | 55 (73.3%) | 94.6% | |||
| 3.3 The general practitioner can help in the monitoring of long‐term therapy, forming a network with the dermatologist | 3 (4.0%) | 10 (13.3%) | 21 (28.0%) | 23 (30.7%) | 18 (24.0%) | 54.7% | |
| 3.4 For the active involvement of the patient, mixed approaches are preferred, based on visits, teleconsultations, visuals, apps | 5 (6.7%) | 17 (22.7%) | 31 (41.3%) | 15 (20.0%) | 7 (9.3%) | 29.3% | |
| 3.5 Apps for long‐term therapy monitoring should be easy to use, not time‐consuming for the dermatologist, and developed with the support of scientific societies | 2 (2.7%) | 7 (9.3%) | 20 (26.7%) | 24 (32.0%) | 22 (29.3%) | 61.3% | |
| 3.6 Monitoring of moderate psoriasis cases has worsened considerably since the onset of the COVID‐19 pandemic; this reinforces the importance of remote monitoring and teleconsultation | 5 (6.7%) | 1 (1.3%) | 30 (40.0%) | 19 (25.3%) | 6 (8.0%) | 33.3% | |
Abbreviations: PGA, Physician Global Assessment; PASI, Psoriasis Area Severity Index.
Consensus on the agreement is reached when ≥75% of voters express a vote equal to 4 or 5. 50
3.2. Delphi round
During the first round of the Delphi process, held during the online meetings, consensus on the agreement was reached for 14 out of 18 statements (Table 1). The consensus was not reached for four statements, all derived from the ‘barriers to a wider implementation of proactive management in clinical practice and possible solutions’ key questions, and one substatement.
Statements in which consensus was not reached were modified by the steering committee according to the suggestions collected during the meetings and re‐proposed to the Delphi Panel. However, a consensus was not reached again (data not shown).
4. Discussion
The PSO‐LONG can be considered a landmark trial for the treatment of psoriasis since it is the first study to show the efficacy of proactive long‐term therapy of mild‐to‐moderate disease. 3 , 6 In the PSO‐LONG trial, twice‐weekly treatment with Cal/BD foam resulted in delayed time to first relapse, reduced number of relapses, and prolonged time free in remission compared with on‐demand reactive treatment. These outcomes were paralleled by a favorable safety profile and a positive effect on the quality of life. 6 Of note, the good cosmetic appearance of Cal/BD foam may further contribute to patients' acceptance of this long‐term proactive therapy, 6 , 16 as also shown in the very recent field‐practice, Italian multicenter LION study. 17 In addition, the reported action of Cal/BD foam on skin pain associated with psoriasis lesions represents another adjunctive factor, which further supports its use in the long term. 18
However, to date, the PSO‐LONG trial represents the only evidence on the proactive management of mild‐to‐moderate psoriasis. Therefore, while new evidence appears eagerly awaited, consensus approaches have a major role in forming expert opinions on the use of proactive regimens in psoriasis and on educational and monitoring interventions.
We have, therefore, conducted the PAM, a nationwide Italian project, which aims to reach a consensus on the role of proactive management of psoriasis, with a focus on Cal/BD. In this project, we sequentially used two different consensus methods, the NGT and the Delphi method, with the aim to collect and form experts' opinions through a robust approach. A high number of dermatologists from all the Italian territory participated in the project.
In total, three main areas of discussion were identified, and 18 statements were proposed during the NGT round. The majority of the statements (14/18) reached a consensus during the Delphi round. We comment here on the outcomes of the NGT and Delphi rounds.
4.1. The rationale for long‐term proactive management of psoriasis with topical therapy
Since psoriasis presents subtle inflammation even during remission phases, 19 there was a high level of consensus on the need to provide long‐term proactive topical treatment to reduce the risk of relapse in patients with mild‐to‐moderate psoriasis and prolong time free from disease, in line with current practice in atopic dermatitis. 20 , 21 Furthermore, according to the Panel, long‐term topical treatment can have a role in the treatment of challenging disease sites on which systemic treatment is often not fully effective, such as the hands, elbows, and scalp. 22 , 23 In addition, according to the Panel, topical long‐term proactive treatment of psoriasis can be useful when phototherapy or systemic treatment are contraindicated or ineffective, two conditions that may be frequently encountered in clinical practice. 24 , 25 On these bases, the Panel suggested that a revision of current guidelines for psoriasis may be considered to include the possibility of proactive long‐term treatment.
However, the efficacy of topical long‐term treatment in clinical practice should be properly monitored. According to the Panel, several outcomes can be considered as relevant to this end, namely duration of the remission, the number of relapses in the already‐treated areas, time free from symptoms effect on quality of life (e.g., itching, pain, burning), ability to maintain adherence, and modified Psoriasis Area and Severity Index (PASI). Remarkably, the efficacy on the symptoms constitutes a not negligible advantage given the high impact of itching, pain, and burning on the quality of life of patients. In addition, the patient's opinion and expectations together with the impact on quality of life (DLQI) represent factors that have to be considered when evaluating treatment efficacy. Among the outcomes, the patient's ability to maintain proper adherence achieved the highest percentage of agreement (92%): adherence to treatment, which is of paramount relevance in topical therapy, is indeed influenced not only by the efficacy and safety of the treatment but also by its cosmetic acceptability and the patient's preferences. Interestingly, a consensus was not reached on the use of PGA (Physician Global Assessment) to monitor the efficacy of topical long‐term treatment in clinical practice. A possible explanation for this finding could be that, although PGA is a practical tool for measuring the severity of psoriasis, its value is limited as a stand‐alone instrument because it does not assess the affected body surface area. However, in the sole trial available to date, the PSO‐LONG study, PGA was assessed. 6 Although the European Medicines Agency recommends using PASI together with PGA to evaluate the efficacy of new treatments in clinical trials, PGA can be used in field practice since it is reliable and time‐saving. 26 Indeed, a close correlation between PGA and PASI, irrespective of treatment modality, has been recently demonstrated. 27 We, therefore, believe that a wider application of this method of assessment of treatment efficacy would be welcome, in line with recent suggestions. Other approaches (e.g., dermoscopy, which allows an objective measure of the efficacy of therapy over time) can be also worth further evaluation.
4.2. Different approaches to long‐term proactive management of psoriasis
When dealing with this key question, the focus was on treatment with Cal/BD topical foam, since the PSO‐LONG trial is the only phase III trial in this setting available to date, and Cal/BD foam is currently the only treatment for which the approved label allows either reactive treatment of relapse or proactive use. 6 , 28 Furthermore, Cal/BD foam showed to be more effective and convenient than other formulations, although in the short‐term setting so far. 3 , 29 , 30 , 31 All statements in this area reached a consensus.
First, the Delphi Panel pointed out that the use of Cal/BD foam in the long‐term proactive treatment of psoriasis is based upon a strong mechanistic rationale, given the well‐documented synergistic effect of the two components and the immunomodulatory properties shown by this compound. 32 , 33 , 34 , 35 In short, Cal, a vitamin D analog, acts on epidermal dysregulation and promotes the differentiation of keratinocytes, while BD targets pro‐inflammatory cytokines and chemokines and also enhances keratinocyte differentiation. Furthermore, Cal/BD decreases the expansion of Th1 and Th17 cells into the psoriatic lesions. Last, local skin reactions potentially associated with Cal are decreased in the presence of BD, and, in turn, Cal contrasts the skin atrophy induced by BD.
This mechanistic rationale poses the basis for the marked and sustained efficacy shown by Cal/BD foam in the PSO‐LONG trial and to its favorable safety profile, with no relevant effects on calcium metabolism and hypothalamic–pituitary–adrenal axis. 6
Given the favorable results shown in the PSO‐LONG trial and other experiences, 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 the possibility to associate the proactive treatment with Cal/BD foam with systemic conventional and biological therapy was discussed for the first time. The Panel widely considered this association as a viable and promising therapeutic possibility, especially on challenging areas like elbows, feet, and hands. Another possible setting of use is when a response is poor/systemic therapy is contraindicated. Of note, the Panel believes that the association of the proactive treatment to systemic and biological therapy does not need for a dose intensification, instead presents the possibility of a dose reduction of the systemic therapy, thus favoring a prolonged remission.
Importantly, the Panel reached a consensus on the preferential use of the schedule applied in the PSO‐LONG trial over the so‐called weekend therapy—i.e., the administration of Cal/BD foam on 2 consecutive days (“weekend therapy”), which is sometimes suggested and applied in clinical practice. 44 , 45
Barriers to a wider implementation of proactive management in clinical practice and possible solutions.
Adherence remains the most immediate issue when prescribing any long‐term therapy in clinical practice, and this is also true in psoriasis. 1 , 3 With specific reference to proactive management of psoriasis, the Panel pointed out the importance of proper communication between the dermatologist and the patient, with the aim to clearly explain the benefits of long‐term proactive management of psoriasis and optimize adherence, minimizing at the same time the use of self‐prescribed therapies by the patient. This is in line with current suggestions in the field of psoriasis 5 , 46 and was somehow expected. However, while there was a consensus on the importance of adherence and patient/physician communication, no consensus was reached on which measures should be implemented in clinical practice to enhance adherence. Interventions for improving adherence should be reliable, contemporary, acceptable, and readily available to the person. With reference to proactive management of psoriasis, the establishment of a GP/Dermatologist network, the use of apps, and the wider implementation of telemedicine were proposed, and all of them were not considered as suitable tools at the current time. In some previous experiences, the use of telemedicine proved to increase patients' adherence in clinical practice. 5 , 47 , 48 However, those experiences refer to a period before the boost of telemedicine because of the COVID‐19 pandemic, which posed new and unexpected challenges also to the proper dissemination of telemedicine. 49 Further investigation in this field and, most importantly, a proper definition of the legal limits of telemedicine are required before these approaches enter routine daily practice.
5. Conclusion
Although the treatment of severe psoriasis has more and more new and effective weapons with the advent of biological drugs, mild and moderate forms are treated with consolidated strategies, without significant additional novelties. The proactive approach constitutes an effective novelty useful not only in the treatment of the disease itself in mild‐to‐moderate forms but also in the prevention of relapses, with an improvement of QoL for the patient.
This nationwide Italian project reported a consensus on the role of proactive management of psoriasis with Cal/BD foam. Indeed, a consensus was reached for the need of providing long‐term proactive topical treatment to reduce the risk of relapse and to treat challenging diseases sites or difficult patients where phototherapy or systemic therapies are contraindicated or ineffective. The Panel also reached a consensus on the possibility that the proactive topical treatment can be used as an adjunct treatment to systemic therapy, either conventional or biological, and the preferential use of the schedule applied in the PSO‐LONG trial over the so‐called weekend therapy. On the contrary, no consensus was reached on which measures should be implemented in clinical practice to enhance adherence, and this should represent a field of future research efforts.
The inclusion in guidelines of a proactive strategy among the effective treatment options will be a fundamental step in the evolution of a mild‐moderate psoriasis therapeutic approach.
Data availability statement
All data are available from the Corresponding Author upon reasonable request.
Acknowledgment
Editorial, graphical, and statistical assistance were provided by Simonetta Papa, PhD, Valentina Mirisola, PhD, Massimiliano Pianta, and Aashni Shah (Polistudium SRL, Milan, Italy). Open Access Funding provided by Universita Cattolica del Sacro Cuore within the CRUI‐CARE Agreement.
Appendix A.
The PAM Italian Working Group.
Giuseppe Fabrizio Amoruso, Francesco Baglieri, Anna Silvia Biamonte, Tommaso Bianchelli, Laura Bigi, Jarno Bortoli, Bruno Brunetti, Cinzia Buligan, Elisabetta Cagni, Ombretta Calderoni, Piergiacomo Calzavara‐Pinton, Anna Campanati, Alighiero Caputo, Carlo Giovanni Carrera, Andrea Carugno, Karin Chersi, Stefano Cicchelli, Flora De Natale, Clara De Simone, Paolo Dapavo, Domenico Di Maria, Enzo Errichetti, Gabriella Fabbrocini, Angelo Salvatore Ferrari, Emanuela Fogli, Riccardo Forconi, Chiara Franchi, Augusto Galeazzi, Alessio Gambardella, Andrea Giovannini, Maria Teresa Giura, Massimo Iuculano, Giuseppe Lazzaretti, Claudia Leporati, Massimiliano Magnanini, Piergiorgio Malagoli, Barbara Marconi, Alessandro Martella, Adriana Maruccia, Matteo Megna, Roberta Miglietta, Anna Minuti, Luigi Mocci, Sonia Modica, Alessandra Narcisi, Giulia Odorici, Federica Osti, Massimiliano Pazzaglia, Rossana Peila, Ginevra Pertusi, Michele Pezza, Elio Pezzullo, Nunzio Puccia, Umberto Raulo, Simone Ribero, Mariateresa Rossi, Sergio Rusignuolo, Giada Sapienza, Catello Savarese, Mariaelena Scalisi, Davide Strippoli, Elena Stroppiana, Rossana Tiberio, Antonino Trischitta, Maria Giovanna Tucci, Fabrizio Vaira, Anna Verrone, Lucia Villa, Fabio Zagni, Andrea Zoccali.
Piergiacomo Calzavara‐Pinton and Gabriella Fabbrocini are co‐senior authors.
Conflicts of interest: EE was a consultant for Jannsen. MM acted as speaker or consultant for Abbvie, Amgen, Eli Lilly, Janssen, Leo Pharma, Novartis, and UCB. The other authors report no conflict of interest.
Funding source: The PAM project and editorial assistance were supported by an unrestricted grant from LEO Pharma.
Members of the PAM Italian Working Group are listed in the Appendix A.
References
- 1. Megna M, Cinelli E, Camela E, et al. Calcipotriol/betamethasone dipropionate formulations for psoriasis: an overview of the options and efficacy data. Expert Rev Clin Immunol 2020; 16: 599–620. [DOI] [PubMed] [Google Scholar]
- 2. Segaert S, Calzavara‐Pinton P, de la Cueva P, et al. Long‐term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2022; 33: 111–120. [DOI] [PubMed] [Google Scholar]
- 3. Fabbrocini G, De Simone C, Dapavo P, et al. Long‐term maintenance treatment of psoriasis: the role of calcipotriol/betamethasone dipropionate aerosol foam in clinical practice. J Dermatol Treat 2021; 10.1080/09546634.2021.1998310. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4. NICE. Psoriasis: assessment and management 2012 [cited 2019. Nov 1]. Available from: https://www.nice.org.uk/Guidance/CG153
- 5. Thaçi D, de la Cueva P, Pink AE, et al. General practice recommendations for the topical treatment of psoriasis: a modified‐Delphi approach. BJGP Open 2020; 4: bjgpopen20X101108 . 10.3399/bjgpopen20X101108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lebwohl M, Kircik L, Lacour JP, et al. Twice‐weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO‐LONG trial). J Am Acad Dermatol 2021; 84: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 7. Enstilar SmPC. Available at: https://www.medicines.org.uk/emc/product/2139/pil#gref. .
- 8. Grandi V, Baldo A, Berti E, et al. Italian expert‐based recommendations on the use of photo(chemo)therapy in the management of mycosis fungoides: results of an e‐Delphi consensus. Photodermatol Photoimmunol Photomed 2021; 37: 334–342. [DOI] [PubMed] [Google Scholar]
- 9. Seoane J, Warnakulasuriya S, Bagán JV, et al. Assembling a consensus on actinic cheilitis: a Delphi study. J Oral Pathol Med 2021; 50: 962–970. [DOI] [PubMed] [Google Scholar]
- 10. Abo‐Tabik M, Parisi R, Willis SC, et al. Development of clinical diagnostic criteria for chronic plaque psoriasis: an international e‐Delphi study. Br J Dermatol 2021; 185: 455–456. [DOI] [PubMed] [Google Scholar]
- 11. Petosa JJ Jr, Martini A, Klein M, et al. Resident sensitive quality measures for general pediatrics: alignment with existing care recommendations. Acad Pediatr 2021; 21: 943–947. [DOI] [PubMed] [Google Scholar]
- 12. Hellberg C, Österberg M, Jonsson AK, et al. Important research outcomes for treatment studies of perinatal depression: systematic overview and development of a core outcome set. BJOG 2021; 128: 2141–2149. [DOI] [PubMed] [Google Scholar]
- 13. Bosello SL, Beretta L, Del Papa N, et al. Interstitial lung disease associated with autoimmune rheumatic diseases: checklists for clinical practice. Front Med (Lausanne). 2021; 8: 732761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Come organizzare una conferenza di consenso. Manuale metodologico Sistema nazionale per le Linee Guida. https://www.psy.it/wp‐content/uploads/2018/02/Manuale‐Metodologico‐Consensus.pdf
- 16. Giovene GL, Giacomelli L, AIDA (Italian Association of Outpatient Dermatologists) Working Group . Calcipotriene plus betamethasone dipropionate in aerosol foam formulation: will this effective treatment for mild‐to‐moderate psoriasis change clinical practice? G Ital Dermatol Venereol 2018; 153: 872–876. [DOI] [PubMed] [Google Scholar]
- 17. Campanati A, Atzori L, Potenza C, et al. Patient satisfaction with calcipotriol/betamethasone dipropionate cutaneous foam for the treatment of plaque psoriasis: the LION real‐life multicenter prospective observational cohort study. Dermatol Ther 2021; 34: e15077 . 10.1111/dth.15077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallo L, Megna M, Cirillo T, et al. Psoriasis and skin pain: real‐life effectiveness of calcipotriol plus betamethasone dipropionate in aerosol foam formulation. J Eur Acad Dermatol Venereol 2019; 33: 1312–1315. [DOI] [PubMed] [Google Scholar]
- 19. Benezeder T, Wolf P. Resolution of plaque‐type psoriasis: what is left behind (and reinitiates the disease). Semin Immunopathol 2019; 41: 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siegfried EC, Jaworski JC, Kaiser JD, et al. Systematic review of published trials: long‐term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr 2016; 16: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ibrahim S, Amer A, Nofal H, et al. Practical compendium for psoriasis management. Dermatol Ther 2020; 33: e13243 . 10.1111/dth.13243 [DOI] [PubMed] [Google Scholar]
- 23. Foley P, Gordon K, Griffiths CEM, et al. Efficacy of Guselkumab compared with Adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol 2018; 154: 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lockwood SJ, Prens LM, Kimball AB. Adverse reactions to biologics in psoriasis. Curr Probl Dermatol 2018; 53: 1–14. [DOI] [PubMed] [Google Scholar]
- 25. Balak DMW, Gerdes S, Parodi A, et al. Long‐term safety of oral systemic therapies for psoriasis: a comprehensive review of the literature. Dermatol Ther (Heidelb). 2020; 10: 589–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. European Medicines Agency . Clinical investigation of medicinal products indicated for the treatment of psoriasis. https://www.ema.europa.eu/en/clinical‐investigation‐medicinalproducts‐indicated‐treatment‐psoriasis
- 27. Mahil SK, Wilson N, Dand N, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real‐world, population‐based cohort study (the British Association of Dermatologists biologics and Immunomodulators register, BADBIR). Br J Dermatol 2020; 182: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bark C, Brown C, Svangren P. Systematic literature review of long‐term efficacy data for topical psoriasis treatments. J Dermatolog Treat 2021. 10.1080/09546634.2021.1925211 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29. Fabbrocini G, Dauden E, Jalili A, et al. Calcipotriol/betamethasone dipropionate aerosol foam in the treatment of psoriasis: new perspectives for the use of an innovative topical treatment from real‐life experience. G Ital Dermatol Venereol 2020; 155: 212–219. [DOI] [PubMed] [Google Scholar]
- 30. Girolomoni G, Calzavara Pinton P, Cristaudo A, et al. Back to the future: a new topical approach for mild‐to‐moderate psoriasis. G Ital Dermatol Venereol 2018; 153: 375–382. [DOI] [PubMed] [Google Scholar]
- 31. Balak DMW, Carrascosa JM, Gregoriou S, et al. Cost per PASI‐75 responder of calcipotriol plus betamethasone dipropionate cutaneous foam versus nonbiologic systemic therapies for the treatment of plaque psoriasis in seven European countries. J Dermatolog Treat 2020; 6: 1–8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32. Fleming C, Ganslandt C, Guenther L, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double‐blind, exploratory study. Eur J Dermatol 2010; 20: 465–471. [DOI] [PubMed] [Google Scholar]
- 33. Segaert S, Shear NH, Chiricozzi A, et al. Optimizing anti‐inflammatory and immunomodulatory effects of corticosteroid and vitamin D analogue fixed‐dose combination therapy. Dermatol Ther (Heidelb) 2017; 7: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fujiyama T, Ito T, Umayahara T, et al. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion. J Allergy Clin Immunol 2016; 138: 517–528.e5. [DOI] [PubMed] [Google Scholar]
- 35. Lovato P, Norsgaard H, Tokura Y, et al. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell‐Th17 cell axis in psoriasis. J Dermatol Sci 2016; 81: 153–164. [DOI] [PubMed] [Google Scholar]
- 36. Del Rosso JQ, Kircik LH. The effect of Calcipotriene‐betamethasone Dipropionate aerosol foam versus vehicle on target lesions in moderate severity plaque psoriasis: focus on elbows and knees. J Drugs Dermatol 2019; 18: 358–361. [PubMed] [Google Scholar]
- 37. Petersen B, Lebwohl M. Treating scalp psoriasis with Calcipotriene/betamethasone Dipropionate fixed‐dose combination cutaneous foam: review of phase 2 data. J Drugs Dermatol. 2020; 19: 784–786. [DOI] [PubMed] [Google Scholar]
- 38. Patel DS, Veverka KA, Hansen JB, et al. Efficacy of fixed‐combination Calcipotriene 0.005% and betamethasone Dipropionate 0.064% foam for scalp plaque psoriasis: additional analysis of a phase II, randomized clinical study. J Clin Aesthet Dermatol 2020; 13: 12–18. [PMC free article] [PubMed] [Google Scholar]
- 39. Bagel J, Zapata J, Nelson E. A prospective, open‐label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018; 17: 845–850. [PubMed] [Google Scholar]
- 40. Kircik LH, Schlesinger TE, Tanghetti E. Efficacy and safety of calcipotriene 0.005%/betamethasone dipropionate 0.064% foam with apremilast for moderate plaque psoriasis. J Drugs Dermatol. 2020; 19: 874–880. [DOI] [PubMed] [Google Scholar]
- 41. Pink AE, Jalili A, Berg P, et al. Rapid onset of action of calcipotriol/betamethasone dipropionate cutaneous foam in psoriasis, even in patients with more severe disease. J Eur Acad Dermatol Venereol 2019; 33: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 42. Jalili A, Lebwohl M, Stein Gold L, et al. Itch relief in patients with psoriasis: effectiveness of calcipotriol plus betamethasone dipropionate foam. J Eur Acad Dermatol Venereol 2019; 33: 709–717. [DOI] [PubMed] [Google Scholar]
- 43. Bewley AP, Shear NH, Calzavara‐Pinton PG, et al. Calcipotriol plus betamethasone dipropionate aerosol foam vs. apremilast, methotrexate, acitretin or fumaric acid esters for the treatment of plaque psoriasis: a matching‐adjusted indirect comparison. J Eur Acad Dermatol Venereol 2019; 33: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imafuku S, Zheng M, Tada Y, et al. Asian consensus on assessment and management of mild to moderate plaque psoriasis with topical therapy. J Dermatol 2018; 45: 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fernandes IC, Torres T, Selores M. Maintenance treatment of psoriasis with cyclosporine a: comparison between continuous and weekend therapy. J Am Acad Dermatol 2013; 68: 341–342. [DOI] [PubMed] [Google Scholar]
- 46. Kircik LH, Stein Gold LF, Pariser DM. Improving adherence to topical therapies through improved clinician‐patient communication and shared decision making. Cutis 2019; 103: S13–S15. [PubMed] [Google Scholar]
- 47. Svendsen MT, Andersen F, Andersen KH, et al. A smartphone application supporting patients with psoriasis improves adherence to topical treatment: a randomized controlled trial. Br J Dermatol 2018; 179: 1062–1071. [DOI] [PubMed] [Google Scholar]
- 48. Armitage LC, Kassavou A, Sutton S. Do mobile device apps designed to support medication adherence demonstrate efficacy? A systematic review of randomised controlled trials, with meta‐analysis. BMJ Open 2020; 10: e032045 . 10.1136/bmjopen-2019-032045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Palabindala V, Bharathidasan K. Telemedicine in the COVID‐19 era: a tricky transition. J Community Hosp Intern Med Perspect 2021; 11: 302–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Candiani 2019 . Sistema nazionale per le Linee Guida: Zadig Editore, Milano: 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the Corresponding Author upon reasonable request.


