Abstract
Objective
To compare recurrent urinary tract infection (rUTI) guidelines from major urological and non‐urological organisations internationally and identify areas of consensus and discrepancy.
Methods
PubMed, Google Scholar and the official webpages of major urological, gynaecological, infectious diseases and general practice organisations were searched for rUTI guidelines in March 2022. Nine guidelines were included for review: European Association of Urology, National Institute for Health and Care Excellence (NICE), Society of Obstetricians and Gynaecologists of Canada, American Academy of Family Physicians, Mexican College of Gynaecology and Obstetrics Specialists, Swiss Society of Gynaecology and Obstetrics, Spanish Society of Infectious Diseases and Clinical Microbiology, German Association of Scientific Medical Societies, and the combined American Urological Association/Canadian Urological Association/Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction.
Results
The definition and evaluation of rUTIs, and antibiotic prophylaxis strategies, were mostly consistent across guidelines, and emphasised the importance of obtaining urine cultures and limiting cystoscopy and upper tract imaging in women without risk factors. Variable recommendations were noted for symptomatic treatment, self‐initiated antibiotics, and antibiotic‐sparing preventative strategies such as cranberry, vaginal oestrogen, immunoactive prophylaxis with OM‐89, intravesical glycosaminoglycan instillation, and phytotherapeutics. Recent randomised evidence supports the use of methenamine hippurate. Either continuous or post‐coital prophylactic antibiotics were supported by all guidelines. None of the guidelines were tailored to the management recurrent complicated UTI.
Conclusion
Multiple rUTI guidelines were identified and mostly limited their recommendations to otherwise healthy non‐pregnant women with uncomplicated cystitis. Variation was noted, particularly in antibiotic‐sparing preventative strategies. Some conflicting recommendations are due to more recent guidelines including updated evidence. Future guidelines should consider recommendations to assist management of complex patient groups, such as recurrent complicated UTI.
Keywords: recurrent urinary tract infections, treatment, antibiotics, prophylaxis, prevention, #Urology, #UroUTI
Introduction
A UTI is a common reason to seek health care and for antimicrobial prescribing. About 60% of women will experience at least one UTI in their life and 30%–40% will experience recurrent UTIs (rUTIs). rUTIs are associated with significant morbidity and decrease in quality of life [1] with a subsequent economic impact on healthcare. Specifically, rUTIs account for >76 000 Australian hospitalisations/year at an annual cost of AUD$909 million that is increasing due to antimicrobial resistance [2, 3]. In the USA, costs are estimated in excess of $2 billion/year [4].
Accurate diagnosis and optimal management of UTIs is essential given the significant burden of disease of UTIs both for individuals as well as society. Urine microscopy and culture have been considered the diagnostic ‘gold standard’ for decades, despite known suboptimal diagnostic accuracy [5, 6]. Promising molecular research, including whole genome and metagenomic sequencing technologies, coupled with an increased understanding of the urinary tract microbiome may improve diagnosis, but are not validated for use in clinical practice [7, 8]. While the use of antibiotics in asymptomatic bacteriuria in people without specific risk factors is now universally discouraged, there remains widespread and heterogeneous use in both asymptomatic and symptomatic populations, raising concerns about fuelling the global crisis of emerging multidrug resistance [9]. Antibiotic‐sparing therapies including vaginal oestrogen in postmenopausal women, cranberry, acupuncture and immunoactive prophylaxis are variably recommended.
Urinary tract infections, in particular rUTIs, are managed by various specialities including general practice, internal and geriatric medicine, infectious diseases, gynaecology and urology. Various guidelines from these groups have been developed and published to help standardise evidence‐based management. However, there remain significant areas of uncertainty, as well as variability in the strength and content of recommendations. Therefore, to address confusion for clinicians treating patients with rUTIs, the aim of this study was to compare the published guidelines based on the content and strength of evidence for their recommendations and to determine areas of consensus and discrepancy.
Methods
PubMed (including MEDLINE) and Google Scholar were searched for rUTI guidelines published between January 2010 to March 2021 using terms ‘recurrent urinary tract infections’, ‘prevention’ or ‘prophylaxis’ and filters for ‘review’, ‘systematic review’ and ‘meta‐analysis’ were applied in PubMed. The official webpages of major urological, gynaecological, infectious diseases and general practice organisations, as well as reference lists of relevant papers and guidelines were searched. Articles published as clinical guidelines for the management of rUTI were considered for inclusion. We excluded articles that were not constructed with a clinical guidelines framework and that did not outline overall patient management (e.g., only imaging). Overall, nine rUTI guidelines from major urological and non‐urological organisations internationally were obtained and compared for the evidence base and strength of included recommendations. The methodology used by each guideline to assess the evidence base and to provide a strength of recommendations is outlined in Table S1. Quality assessment of included guidelines was performed using the Appraisal of Guidelines Research and Evaluation (AGREE II) International tool (Table S2) [10].
The combined AUA, Canadian Urological Association (CUA) and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) guideline titled ‘Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline’ was published in 2019 and provides guidance on the evaluation and management of otherwise healthy women with uncomplicated rUTI, excluding pregnant women, immunocompromised patients, those with anatomical or functional urinary tract abnormalities, those with an in‐dwelling catheter or self‐catheterise, systemically unwell patients, and those with comorbidities affecting the lower urinary tract, such as peripheral neuropathy, diabetes, and spinal cord injury [11]. The AUA/CUA/SUFU Guideline supersedes the 2011 CUA ‘Guidelines for the diagnosis and management of recurrent urinary tract infection in women’ [12].
The European Association of Urology (EAU) Guidelines on Urological Infections updated in 2022, under section 3.4 and 3.5 have recommendations regarding uncomplicated cystitis and recurrent uncomplicated UTIs and/or complicated UTIs (cUTIs), including both lower and upper tract infection [13].
The UK National Institute for Health and Care Excellence (NICE) have published guidelines in 2018 titled ‘Urinary tract infection (recurrent): antimicrobial prescribing’ and ‘Urinary tract infection (lower): antimicrobial prescribing’, providing guidance on the antibiotic prescribing strategy for UTI treatment and prevention in children, young people, and adults, including men, who do not have a catheter [14].
The Society of Obstetricians and Gynaecologists of Canada (SOGC) have published a ‘Recurrent Urinary Tract Infection’ clinical practice guideline in 2010, covering rUTI in women, including pregnant women [15].
The American Academy of Family Physicians (AAFP) have two published papers providing guidelines on rUTI in low‐risk non‐pregnant women [16, 17]. The more recent paper published in 2016 is titled ‘Common Questions About Recurrent Urinary Tract Infections in Women’.
The Mexican College of Gynaecology and Obstetrics Specialists (COMEGO) published in 2010 clinical practice guidelines on recurrent uncomplicated UTI in non‐pregnant women [18].
The Swiss Society of Gynaecology and Obstetrics (SSGO) published their guidelines in 2020 that contain acute and rUTI in women, including in the context of pregnancy, and urogynaecological diagnoses and surgery [19]. rUTI management in men was not included.
The Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) published in 2017 their consensus statement paper ‘Executive summary of the diagnosis and treatment of urinary tract infection: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC)’ [20].
The Association of Scientific Medical Societies in Germany (AWMF [Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften]) S3 Guideline is written in German. The main content is summarised in two English papers that were reviewed: ‘The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients: Part 1’ and ‘The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients. Part II: Therapy and Prevention’ [21, 22].
We identified several other clinical practice guidelines (Infectious Diseases Society of America, American College of Radiology) that were partially relevant but not included as rUTI was not a specified patient group or management was not considered [23, 24, 25, 26]. Additionally, we identified a number of notable review papers [27, 28, 29, 30], which did not meet inclusion criteria but were considered during the synthesis of findings.
Results
A comparison of key recommendations from the various guidelines on the diagnosis and initial management of rUTI are detailed in Table 1, and the long‐term preventative management options are detailed in Table 2.
Table 1.
Comparison of guidelines’ key recommendations on the diagnosis and initial management of recurrent UTI.
| Issue/recommendation | EAU 2022 | AUA/CUA/SUFU 2019 | NICE 2018 | SOGC 2010 | AAFP 2016 | COMEGO 2010 | SSGO 2020 | SEIMC 2017 | AWMF 2017 |
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of rUTI using urine dipstick/culture | Usually not required if typical symptoms are present, but indicated if failing to respond to antibiotics | Urine analysis and urine culture with each episode of symptomatic acute cystitis prior to initiating treatment (moderate recommendation, evidence level C) | – | Initiating empirical therapy based on symptoms is accurate and cost‐effective | Usually not required if typical symptoms are present, but indicated if failing to respond to antibiotics | Urine culture prior to treatment, for sensitivities (Grade B recommendation) | Urine culture is indicated | Urine culture is indicated for early symptomatic recurrences | Urine culture is indicated |
| Evaluation of rUTI: cystoscopy and imaging should not be routinely obtained in the absence of risk factors | Weak recommendation, LE 3 (avoid extensive evaluation in women aged <40 years with no risk factors) | Expert opinion | – | Expert opinion (unless haematuria or non‐E. coli culture) | Level B evidence | Grade B recommendation | Cystoscopy if ≥3 UTIs/year; kidney contrast CT if ≥2 pyelonephritis episodes/year | Strong recommendation (pre‐menopausal sexually active women where there is no suspicion for underlying urological disease) | Recommend routine ultrasound (unless haematuria or persistent non‐E. coli culture, then for further cystoscopy and imaging) |
| Expectant management of rUTI with analgesia | Antibiotics are recommended but can consider symptomatic therapy in consultation with patient | Likely underutilised, can attempt whilst awaiting cultures | Non‐pregnant women: either 48 h of symptomatic management with back‐up antibiotics, or immediate antibiotics | – | Immediate antibiotics leads to better clinical outcomes and delaying antibiotics whilst awaiting cultures is not recommended | – | Recommend delay antibiotics for 48 h (non‐pregnant, uncomplicated UTI, age <65 years) but observe closely for pyelonephritis | – | Can be considered in uncomplicated cystitis with mild–moderate symptoms (after counselling regarding risks and depending on patient preferences) |
| Recommend short course of antibiotic treatments (no longer than 7 days) | Recommend short courses in uncomplicated cystitis | Moderate recommendation, evidence level B | – | Recommended antibiotics for duration up to 7 days | Recommend short course of antibiotics | At least 3 days (Grade B) | Short course unless pyelonephritis | – | Recommend short course in uncomplicated cystitis in premenopausal women |
| Recommend self‐diagnosis and self‐initiated acute treatment for compliant patients | Strong recommendation | Moderate recommendation | – | Moderate recommendation | Offer for those who decline prophylactic antibiotics | Not enough evidence to support this practice | Can be an option if no predisposing factors | Recommend if <3 UTIs/year | – |
Table 2.
Comparison of guidelines’ key recommendations on the long‐term preventative management options for recurrent UTI.
| Issue/recommendation | EAU 2022 | AUA/CUA/SUFU 2019 | NICE 2018 | SOGC 2010 | AAFP 2016 | COMEGO 2010 | SSGO 2020 | SEIMC 2017 | AWMF 2017 |
|---|---|---|---|---|---|---|---|---|---|
| Antibiotic prophylaxis (continuous and post‐coital) | Strong recommendation | Moderate recommendation | Recommend | Strong recommendation | Strong recommendation | Grade B recommendation | Recommend (as a last resort) | Strong recommendation | Weak recommendation |
| Topical vaginal oestrogen replacement | Strong recommendation (postmenopausal women) | Moderate recommendation | Consider | Offer | Weak recommendation | Grade D recommendation | Strongly recommend | Strong recommendation, (especially postmenopause, vaginal atrophy) | – |
| Cranberry | Conflicting evidence, no recommendation | Weak recommendation | Weak recommendation | Strong recommendation | Consider; however, data conflicting | Grade C recommendation | Insufficient evidence to support use | Moderate recommendation | – |
| D‐mannose | Weak recommendation | – | Consider as self‐care treatment | – | – | – | Recommend | Strong recommendation | Consider recommending |
| Methenamine | Contradictory evidence, no recommendation | Insufficient evidence | – | – | – | – | Consider (short‐term therapy) | Do not recommend use | – |
| Behavioural modifications | Weak recommendation | Weak recommendation | Weak recommendation | No evidence | Recommend | Recommend | Recommend | Weak | Strong recommendation (prior to long‐term prophylactic drugs) |
| Increasing fluid intake | Weak recommendation (pre‐menopausal women) | Recommend | Recommend | No evidence | – | Recommend | Recommend | – | Strong recommendation |
| Immunoprophylaxis | Strong recommendation (OM‐89) | Insufficient evidence | – | Do not recommend vaccines | Evidence is sparse and conflicting | Grade B recommendation for Uro‐Vaxom (OM‐89) | Recommend OM‐89 | Moderate recommendation (OM‐89) | Weak recommendation for OM‐89 prior to other long‐term prophylaxis |
| Intravesical GAG therapy (hyaluronic acid and chondroitin sulphate) | Weak recommendation (where less invasive measures failed) | – | – | – | – | – | Strong recommendation | – | – |
| Phytotherapeutics | – | – | – | – | – | – | Recommend Angocin: active ingredients are mustard oils from nasturtium and horseradish root | – | Consider e.g. bearberry leaves with dandelion root, and horseradish root with nasturtium herb |
Diagnosis
The definition of rUTI was consistent across guidelines, defined as two or more episodes of symptomatic UTI within 6 months, or three or more UTIs within 12 months. The AUA/CUA/SUFU guidelines additionally included ‘culture proven episodes of acute bacterial cystitis and associated symptoms’. Culture positivity was defined by the SOGC guideline to be >100 000 colony‐forming units (CFU)/high‐power field (HPF), or >1000 CFU/HPF for symptomatic patients, while the AAFP and AWMF guidelines similarly defined culture positivity in symptomatic patients as ≥1000 bacterial colonies/mL of a known urinary pathogen. The SEIMC definition was cystitis in symptomatic women with a slightly lower cut‐off of ≥100 CFU/mL. All guidelines except the NICE emphasised the importance of urine cultures for the initial diagnosis of rUTI. The definition of uncomplicated cystitis was defined by EAU as acute, sporadic, or recurrent cystitis limited to non‐pregnant women with no relevant anatomical and functional abnormalities within the urinary tract or comorbidities [13].
For diagnosis of each acute rUTI episode, guidelines were variable. The AUA/CUA/SUFU and COMEGO guidelines recommend dipstick and culture with each acute episode. In contrast, the EAU and AAFP indicated that repeat urine culture is usually not required if typical symptoms are present and patients are appropriately responding to antibiotics. This approach is supported by the SOGC guidelines due to diagnostic accuracy and cost‐effectiveness. However, in the presence of atypical symptoms or cases who fail to respond to ‘appropriate’ antibiotics then the EAU and AAFP specify that urine cultures are indicated. The SOGC suggest urine cultures in women who are developing a rUTI problem.
Evaluation
The AUA/CUA/SUFU, SOGC and AAFP guidelines all recommend pelvic examination in women with rUTI, specifically for vaginal atrophy or pelvic organ prolapse. Most guidelines agreed that extensive investigations including cystoscopy and imaging generally have a low diagnostic yield and therefore should not be routinely obtained in women without other risk factors, in agreement with the American College of Radiology Appropriateness Criteria [26]. However, strength of recommendations were variable (Table 1).
Further investigation is indicated when urothelial cancer is suspected. Consequently, the SOGC and AWMF guidelines recommend cystoscopy and upper tract imaging in patients with haematuria and persistent urine culture of bacteria other than Escherichia coli (expert opinion; insufficient evidence). The SSGO recommended cystoscopy in those with three or more UTIs/year, and contrast‐enhanced renal CT in those with two or more episodes of pyelonephritis/year.
Antibiotic Use
All guidelines recommended short (<7 days) courses of antibiotics for treatment of acute episodes in those with rUTI, rather than prolonged courses. For prophylactic antibiotics, both continuous and post‐coital regimens are supported by all the guidelines. The SSGO and AWMF guidelines emphasised that prophylactic antibiotics should be a last resort after other preventative measures have been exhausted. Additionally, the EAU, AUA/CUA/SUFU and AAFP guidelines all discourage treatment of asymptomatic bacteriuria.
Self‐diagnosis of UTI and self‐initiated antibiotics for compliant patients is a moderate‐strong recommendation in the EAU, AUA/CUA/SUFU, SOGC and SSGO guidelines. The AAFP suggest offering this management strategy in those who decline prophylactic antibiotics and the SEIMC only recommend this in those with <3 UTIs/year. The COMEGO reported that there was not enough evidence to support this practice.
Symptomatic management with analgesia in patients with suspected UTI is supported by the AUA/CUA/SUFU (while awaiting results of urine culture), SSGO (delay antibiotics for 48 h and administer analgesia in non‐pregnant women aged <65 years with an uncomplicated UTI) and AWMF (for uncomplicated cystitis with mild–moderate symptoms and depending on patient preferences after counselling regarding risks) guidelines. Similarly, the NICE guidelines recommend giving advice on symptomatic management for all patients, but recommended immediate antibiotics for pregnant women, men or children and non‐pregnant women, either immediately or deferred if stable or worsening symptoms after 48 h. The AAFP guidelines, while acknowledging that this strategy may limit antibiotic use, did not recommend delay in giving antibiotics. Similarly, the EAU guidelines acknowledged that symptomatic treatment can be discussed as an alternative in select patients, but antibiotics were still recommended on the basis of randomised clinical trials findings improved clinical success although this was not further specified.
Preventative Measures
Following successful treatment of the acute episode, preventative measures are important to reduce rUTI frequency and morbidity. Vaginal oestrogen replacement is strongly recommended for rUTI prevention by the EAU (in postmenopausal women), SSGO and SEIMC (especially in postmenopausal women or those with vaginal atrophy), whereas other guidelines made a weak to moderate recommendation. Cranberry supplementation was strongly recommended by the SOGC guidelines, whereas others only provided a weak–moderate recommendation. The majority of guidelines made a weak recommendation for behavioural modifications for UTI prevention (except AWMF: strong recommendation). Specifically, the AUA/CUA/SUFU guidelines recommended increasing oral fluid intake and changing mode of contraception. The NICE guidelines also suggest wiping front to back following defecation, not delaying urination including post‐coital voiding, not douching, or wearing occlusive underwear; however, both the EAU and AUA/CUA/SUFU guidelines highlight the lack of evidence for these behavioural interventions. All guidelines agreed there is insufficient evidence to make a recommendation for probiotics and lactobacillus products. D‐mannose was strongly recommended by the SEIMC due to its similar effectiveness to nitrofurantoin for this indication [31]. The EAU, SSGO and AWMF also support D‐mannose use (weak–moderate recommendations), while the NICE guidelines recommended for some non‐pregnant women as a self‐care treatment based on a low number needed to treat of three based on one small RCT [31]. The SOGC, COMEGO, SSGO and AAFP recommended avoiding spermicide use. The EAU, COMEGO, SEMIC, SSGO and AWMF guidelines recommended immunoactive prophylaxis with OM‐89 (Table 2). The SOGC guidelines suggested consideration of acupuncture to prevent rUTI in women who are unresponsive or intolerant of antibiotic prophylaxis [32, 33], although acupuncture was not included in other guidelines. Methenamine hippurate was recommended by the SSGO for short‐term therapy, whereas the SEIMC recommended against its use and the other guidelines (EAU, AUA/CUA/SUFU) reported contradictory/insufficient evidence to make a recommendation. Phytotherapeutics were briefly included in the SSGO and AWMF guidelines.
A summarised algorithm for the assessment and initial management of rUTI in women is presented in Fig. 1, and long‐term preventative management options in Fig. 2.
Fig. 1.
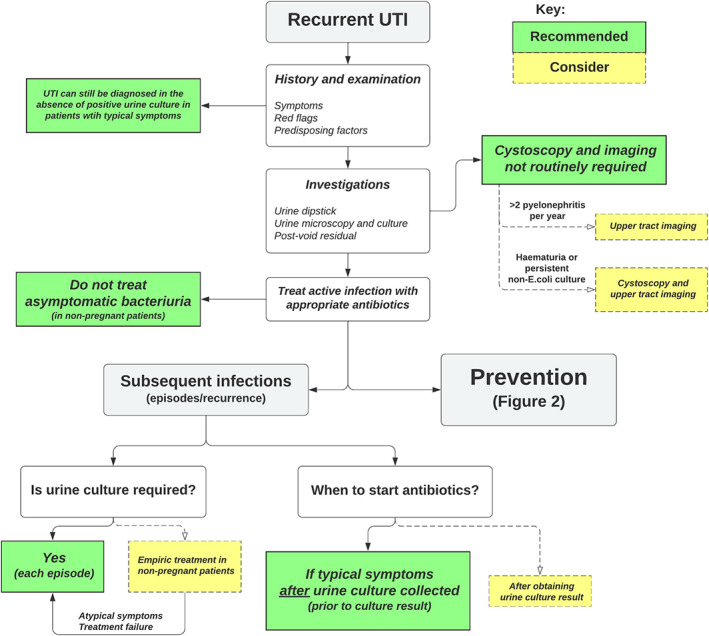
Recurrent UTI in women – assessment and initial management algorithm [30] with guideline recommendations (if general consensus) or considerations (if variation).
Fig. 2.
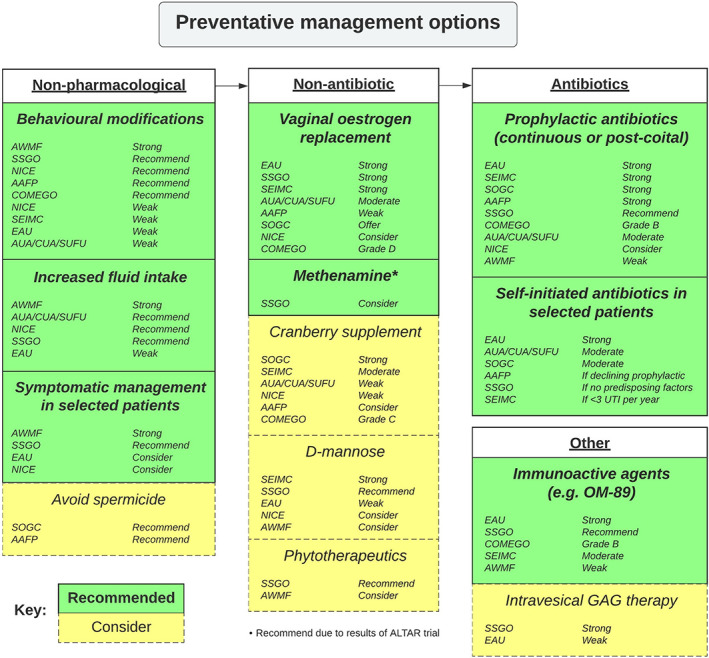
Recurrent UTI in women – long‐term preventative management options.
When to Refer
The NICE guidelines suggest referral or consultation with specialists for men aged ≥16 years, patients with upper tract rUTI, lower tract rUTI when the cause is unknown, pregnant women, children aged <16 years, and those with suspected cancer. The AAFP guidelines highlight the lack of clear guidelines to assist primary care physicians in appropriately referring to specialists.
Discussion
We reviewed rUTI guidelines published by urological, gynaecological, and general practice organisations, which represent the most common health professionals involved in the evaluation and management of rUTI. The guidelines predominantly reported on otherwise healthy non‐pregnant women with uncomplicated rUTI; the NICE reported on antimicrobial prescribing in men with rUTI; the SOGC discussed rUTI antibiotic prophylaxis in pregnant women. None of the guidelines were specifically tailored to the management of recurrent cUTI in those with an anatomical or functional abnormality of the urinary tract, in‐dwelling catheters, or immunocompromising comorbidities and risk factors (age, pregnancy, spinal cord injury, diabetes mellitus) [4].
Variation in recommendations between guidelines can be a source of confusion for clinicians. We noted there was consensus in the definition of rUTI and recommendations for antibiotic courses of <7 days for acute uncomplicated UTI episodes, continuous or post‐coital prophylactic antibiotics and behavioural modifications (weak recommendation).
There was variation in the recommendations regarding symptomatic or deferred antibiotic management in subsequent episodes of rUTI. There may be concern that delayed treatment of uncomplicated cystitis can lead to pyelonephritis. A meta‐analysis comparing antibiotic treatment with placebo for uncomplicated culture‐confirmed cystitis found that antibiotics led to increased treatment success clinically and microbiologically within 3–17 days [34]. No difference in the incidence of pyelonephritis (range 0.4%–2.6%) or emergence of antibiotic resistance was noted, while one in five patients developed adverse effects from antibiotics. Additionally, a meta‐analysis of the natural history of untreated uncomplicated cystitis reported one‐third of women have spontaneous resolution of symptoms within 7–10 days and only 5% developed pyelonephritis within 6 weeks [35]. On this basis, guidelines for deferred antibiotics may apply to a short timeframe for trial of symptomatic management (AUA/CUA/SUFU: until urine culture results are available, NICE: if not improved by 48 h). Furthermore, the duration of symptoms prior to presentation was not considered for these recommendations.
Major variability in recommendation of non‐antibiotic preventative therapies was observed between guidelines. Cranberry products contain proanthocyanidins, which are thought to prevent adhesion of bacteria to urothelium and consequently utilised for UTI prophylaxis [11]. In contrast with the other guidelines, the SOGC 2010 guideline strongly recommended cranberry for UTI prophylaxis on the basis of a Cochrane review published in 2008 where cranberry decreased symptomatic UTIs over 12 months, particularly for women with rUTI [15]. However, the Cochrane review was updated in 2012 to report that cranberry products did not confer significant benefit and dropouts/withdrawals were high in the cranberry group so treatment adherence may further influence results [28]. Furthermore, cranberry juice should be used in caution in patients with diabetes mellitus due to the high sugar content, while the amount of active ingredient within cranberry tablets and capsules was inconsistent or not reported [28].
The SOGC strongly recommended offering postmenopausal women vaginal oestrogen, whereas the other guidelines made a weak–moderate recommendation. Vaginal oestrogen is used to treat atrophic vaginitis in postmenopausal women, as its effect on vaginal flora and pH is thought to assist in reducing the incidence of UTI [15]. A Cochrane review found that vaginal oestrogen reduced UTI recurrence; however, this was based on only two small studies; topical vaginal oestrogen cream may be more effective with relative risk (RR) of 0.25 (95% CI 0.13–0.50), compared with vaginal rings with a RR of 0.64 (95% CI 0.47–0.86) [29]. Topical cream may be difficult to apply and can lead to adverse effects such as vaginal itching, burning, or bleeding in 20% of women [36], whereas vaginal oestrogen pessaries may be better tolerated but more expensive. Oestrogen therapy, in particular topical vaginal application, does not appear to increase the risks of developing breast or endometrial cancer [37, 38]. However, patient or clinician anxiety from dogma may limit its utility. In patients with an existing diagnosis of breast cancer, the suitability of vaginal oestrogen therapy should be discussed with the treating oncologist [11].
OM‐89 is a bacterial extract from E. coli that is administered orally and thought to stimulate the host immune system to produce antibodies and cytokines as immunoactive prophylaxis for rUTI [36, 39]. The effect of OM‐89 does not appear to be limited to E. coli, likely due to sharing of similar antigenic structures and toxin secretion mechanisms between various common uropathogens [39]. While the majority of guidelines have only briefly discussed immunoactive prophylaxis, noting insufficient or conflicting evidence to recommend it, the EAU and COMEGO guidelines do make a recommendation of immunoprophylaxis for rUTI. This is supported by several meta‐analyses [36, 39, 40], where OM‐89 led to a mean reduction in UTI of 39%, with minimal side‐effects compared with placebo [36, 39]. However, limitations in availability and awareness may result in OM‐89 being a generally underutilised strategy for UTI prophylaxis.
Methenamine hippurate was recommended by the SSGO as a short‐term therapy, whereas the SEIMC did not. A Cochrane meta‐analysis of four studies, including 456 patients, reported a possible benefit (RR 0.24, 95% CI 0.07–0.89) with limited reports of adverse events [41]. The ALTAR trial (alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women) was a randomised, non‐inferiority trial that allocated 240 women with rUTI to daily low‐dose antibiotics or methenamine hippurate [42, 43]. Overall, methenamine hippurate was not inferior to daily low‐dose antibiotics based on an absolute difference of 0.49 episodes/person year (90% CI 0.15–0.84) and similar adverse reaction and antibiotic resistance profile [43]. Health economics evaluation favoured methenamine hippurate when the study was presented at the BAUS 2021 Annual Meeting. It is expected that this non‐antibiotic strategy will be included in future guidelines.
Increasing fluid intake was strongly recommended by the AWMF but was a weak recommendation by the EAU (pre‐menopausal women) and only briefly discussed in other guidelines. A meta‐analysis of seven studies (875 patients) reported that increased fluid intake resulted in a significant 64% reduction in the overall risk of rUTI (RR 0.46, 95% CI 0.40–0.54, P < 0.001) [44], so it remains a potentially effective, low risk non‐antibiotic prevention strategy.
Replenishment of the glycosaminoglycan (GAG) layer within the bladder urothelium, often deficient in patients with rUTI, is thought to reduce bacterial adherence and consequent infection [45]. Intravesical GAG therapy is already being used in the context of interstitial cystitis, radiation cystitis, and overactive bladder. The combination of hyaluronic acid and chondroitin sulphate is supported by the SSGO and EAU guidelines, and there is meta‐analytical evidence (two randomised and six non‐randomised studies, n = 800) that it reduces the UTI rate/patient‐year (pooled mean difference −2.56, 95% CI −3.86 to −1.26; P < 0.001) and increased time to first UTI recurrence (pooled mean difference 130.05 days, 95% CI 5.84–254.26; P = 0.04) [46].
We have included some of the more commonly cited guidelines from multiple specialties, as rUTI can be managed by urologists, gynaecologists, GPs and infectious disease physicians. This is not a systematic review of the evidence basis for these guidelines. The SOGC guideline was published over a decade ago and so recommendations differed due to updated evidence used in more recent publications by other organisations, thus highlighting the importance of regular guideline updates, such as that performed by the EAU Guidelines Office. Comparison of recommendations was also limited by the variations in the reporting of the evidence basis. Not all guidelines specified their strength of recommendation (Table S1), which had to be inferred. The COMEGO guideline was published in Spanish and therefore its interpretation may be limited by the accuracy of the translation. In contrast with other urological problems where a defined clinical pathway for management can be established [47], heterogeneity in the rUTI phenotype and effectiveness of management strategies complicates the simple construction of a clinical pathway for rUTI. However, following review of multiple guidelines in this manuscript, we have provided a proposed clinical pathway for rUTI with indication of guideline concordance and variation (Figs 1, 2).
Priority Areas for Future Guidelines
Future guidelines could consider particular patient groups and recommend an assessment and treatment approach to help a wide clinical audience. To aid assessment and initial treatment for primary care and other non‐urological clinicians, key features in the history and examination should be outlined (e.g., infection chronology and complications, LUTS and incontinence, paediatric and obstetric history, red flags for malignancy and relevant comorbidities). In complex clinical scenarios, use of case‐based discussions of ‘Index patients’ have been adopted in cancer guidelines [48], which could be applied for rUTI (e.g., pre‐ vs postmenopausal women, potential urinary tract dysfunction, use of self or indwelling catheterisation). Furthermore, emerging therapies and their evidence base would be helpful to include. Emerging therapies include GAG layer supplementation, other immunotherapies (including vaccines), phytotherapeutics, faecal microbiota transplantation, and minimally invasive surgical options [30]. Most guidelines only consider simple rUTI in women, whereas more guidance is needed for rUTI in men and recurrent cUTIs. cUTIs are associated with increased antimicrobial resistance, rUTI and severe consequences such as urosepsis, renal scarring or even end‐stage renal failure [49]. European guidelines exist for cUTI management [13]; however, further guidance on assessment and preventative strategies for this heterogenous patient group would be helpful. Furthermore, regular guideline updates are required to provide up‐to‐date evidence‐based recommendations for clinicians. Finally, involvement of primary care and internal medicine colleagues in guideline development may help in outlining appropriate referral criteria and expectations of management prior to referral so that specialist management efficiency is optimal. Additionally, other specialty involvement may provide guidance on how to optimise non‐urological contributors to rUTI, such as substitution of sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors for patients with diabetes mellitus or rationalisation of immunosuppressants. It is our clinical experience that this multidisciplinary approach is important for improving rUTI outcomes in patients with multiple medical comorbidities.
Conclusions
We found that various international guidelines on rUTI management exist but have differing recommendations that potentially limit clinical use and value. The definition and evaluation of rUTI was mostly consistent amongst the various guidelines. There were differing recommendations on symptomatic management and deferred antibiotic treatment for acute episodes. Non‐antibiotic prophylaxis recommendations were variable, particularly relating to cranberry products, vaginal oestrogen, and lifestyle measures. Methenamine hippurate was not widely recommended but expected to be included in future updates following recent publication of the ALTAR trial. Immunoactive prophylaxis may be underutilised due to limitations in access and awareness despite supportive data. The majority of guidelines limited their recommendations to otherwise healthy non‐pregnant women with uncomplicated cystitis. Future guideline versions could consider widening their audience appeal and varying content appropriately to optimise assessment, management, and specialist referral for timely treatment.
Disclosures of Interest
The authors declare that they have no disclosures of interest.
Funding
This study did not receive any funding.
Abbreviations
- AAFP
American Academy of Family Physicians
- ALTAR
alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women (trial)
- AWMF
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften
- CFU
colony‐forming units
- COMEGO
Mexican College of Gynaecology and Obstetrics Specialists
- CUA
Canadian Urological Association
- EAU
European Association of Urology
- HPF
high‐power field
- NICE
UK National Institute for Health and Care Excellence
- rUTI
recurrent UTI
- SEIMC
Spanish Society of Infectious Diseases and Clinical Microbiology
- SOGC
Society of Obstetricians and Gynaecologists of Canada
- SSGO
Swiss Society of Gynaecology and Obstetrics
- SUFU
Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction
Supporting information
Table S1 . Quality of evidence and strength of recommendations.
Table S2. Appraisal of Guidelines Research and Evaluation (AGREE II) quality assessment of included guidelines.
Acknowledgements
Matthew J. Roberts is supported by a Clinician Research Fellowship from the Metro North Office of Research, Queensland Health. Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
References
- 1. Renard J, Ballarini S, Mascarenhas T et al. Recurrent lower urinary tract infections have a detrimental effect on patient quality of life: a prospective, observational study. Infect Dis Ther 2014; 4: 125–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Australian Commission on Safety and Quality in Health Care and Australian Institute of Health and Welfare . The Fourth Australian Atlas of Healthcare Variation. Sydney, NSW: ACSQHC, 2021. [Google Scholar]
- 3. OUTBREAK Consortium . A One Health Antimicrobial Resistance Economic Perspective. Sydney, NSW: UTS, 2020. [Google Scholar]
- 4. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002; 113(Suppl. 1): 5–13 [DOI] [PubMed] [Google Scholar]
- 5. Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med 2013; 369: 1883–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heytens S, De Sutter A, Coorevits L et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR‐based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017; 23: 647–52 [DOI] [PubMed] [Google Scholar]
- 7. Horváth J, Wullt B, Naber KG, Köves B. Biomarkers in urinary tract infections–which ones are suitable for diagnostics and follow‐up? GMS Infect Dis 2020; 8: Doc24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fritzenwanker M, Imirzalioglu C, Chakraborty T, Wagenlehner FM. Modern diagnostic methods for urinary tract infections. Expert Rev Anti Infect Ther 2016; 14: 1047–63 [DOI] [PubMed] [Google Scholar]
- 9. Cai T, Nesi G, Mazzoli S et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015; 61: 1655–61 [DOI] [PubMed] [Google Scholar]
- 10. Brouwers MC, Kho ME, Browman GP et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010; 182: E839–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anger J, Lee U, Ackerman AL et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol 2019; 202: 282–9 [DOI] [PubMed] [Google Scholar]
- 12. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J 2011; 5: 316–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonkat G, Bartoletti R, Bruyére F et al. EAU Guidelines on Urological Infections 2022. European Association of Urology Guidelines 2022 Edition. Presented at the EAU Annual Congress Amsterdam 2022. Arnhem: European Association of Urology Guidelines Office, 2022. [Google Scholar]
- 14. National Institute for Health and Care Excellence [Internet] . Urinary Tract Infection (Recurrent): Antimicrobial Prescribing. London: NICE, 2018. Available at: https://www.nice.org.uk/guidance/ng112. Accessed April 2021 [Google Scholar]
- 15. Epp A, Larochelle A, Lovatsis D et al. Recurrent urinary tract infection. J Obstet Gynaecol Can 2010; 32: 1082–90 [DOI] [PubMed] [Google Scholar]
- 16. Arnold JJ, Hehn LE, Klein DA. Common questions about recurrent urinary tract infections in women. Am Fam Physician 2016; 93: 560–9 [PubMed] [Google Scholar]
- 17. Kodner CM, Thomas Gupton EK. Recurrent urinary tract infections in women: diagnosis and management. Am Fam Physician 2010; 82: 638–43 [PubMed] [Google Scholar]
- 18. Del Pilar Velázquez M, Romero Nava LE, López de Avalos DR et al. Clinical practice guidelines. Recurrent infection of the urinary tract in women. Colegio Mexicano de Especialistas en Ginecología y Obstetricia. Ginecol Obstet Mex 2010; 78: S437–59 [PubMed] [Google Scholar]
- 19. Betschart C, Albrich WC, Brandner S et al. Guideline of the swiss Society of Gynaecology and Obstetrics (SSGO) on acute and recurrent urinary tract infections in women, including pregnancy. Swiss Med Wkly 2020; 150: w20236 [DOI] [PubMed] [Google Scholar]
- 20. de Cueto M, Aliaga L, Alós J‐I et al. Executive summary of the diagnosis and treatment of urinary tract infection: guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm Infecc Microbiol Clin 2017; 35: 314–20 [DOI] [PubMed] [Google Scholar]
- 21. Kranz J, Schmidt S, Lebert C et al. The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients: Part 1. Urol Int 2018; 100: 263–70 [DOI] [PubMed] [Google Scholar]
- 22. Kranz J, Schmidt S, Lebert C et al. The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients. Part II: therapy and prevention. Urol Int 2018; 100: 271–8 [DOI] [PubMed] [Google Scholar]
- 23. Gupta K, Hooton TM, Naber KG et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20 [DOI] [PubMed] [Google Scholar]
- 24. Nicolle LE, Gupta K, Bradley SF et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis 2019; 68: e83–110 [DOI] [PubMed] [Google Scholar]
- 25. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 1999; 29: 745–58 [DOI] [PubMed] [Google Scholar]
- 26. Venkatesan AM, Oto A, Allen BC et al. ACR appropriateness criteria® recurrent lower urinary tract infections in females. J Am Coll Radiol 2020; 17: S487–96 [DOI] [PubMed] [Google Scholar]
- 27. Gupta K, Trautner BW. Diagnosis and management of recurrent urinary tract infections in non‐pregnant women. BMJ 2013; 346: f3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2012; (10): Cd001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev 2008;(2); Cd005131 [DOI] [PubMed] [Google Scholar]
- 30. Sihra N, Malde S, Greenwell T, Pakzad M, Kujawa M, Sinclair A. Management of recurrent urinary tract infections in women. J Clin Urol 2022; 15: 152–64 [Google Scholar]
- 31. Kranjčec B, Papeš D, Altarac S. D‐mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol 2014; 32: 79–84 [DOI] [PubMed] [Google Scholar]
- 32. Aune A, Alraek T, Lihua H, Baerheim A. Acupuncture in the prophylaxis of recurrent lower urinary tract infection in adult women. Scand J Prim Health Care 1998; 16: 37–9 [DOI] [PubMed] [Google Scholar]
- 33. Alraek T, Soedal LIF, Fagerheim SU, Digranes A, Baerheim A. Acupuncture treatment in the prevention of uncomplicated recurrent lower urinary tract infections in adult women. Am J Public Health 2002; 92: 1609–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falagas ME, Kotsantis IK, Vouloumanou EK, Rafailidis PI. Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: a meta‐analysis of randomized controlled trials. J Infect 2009; 58: 91–102 [DOI] [PubMed] [Google Scholar]
- 35. Hoffmann T, Peiris R, Mar CD, Cleo G, Glasziou P. Natural history of uncomplicated urinary tract infection without antibiotics: a systematic review. Br J Gen Pract 2020; 70: e714–e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beerepoot MA, Geerlings SE, van Haarst EP, van Charante NM, ter Riet G. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta‐analysis of randomized controlled trials. J Urol 2013; 190: 1981–9 [DOI] [PubMed] [Google Scholar]
- 37. Crandall CJ, Hovey KM, Andrews CA et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause 2018; 25: 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chlebowski RT, Anderson GL, Aragaki AK et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long‐term follow‐up of the women's health initiative randomized clinical trials. JAMA 2020; 324: 369–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naber KG, Cho YH, Matsumoto T, Schaeffer AJ. Immunoactive prophylaxis of recurrent urinary tract infections: a meta‐analysis. Int J Antimicrob Agents 2009; 33: 111–9 [DOI] [PubMed] [Google Scholar]
- 40. Bauer HW, Rahlfs VW, Lauener PA, Blessmann GS. Prevention of recurrent urinary tract infections with immuno‐active E. coli fractions: a meta‐analysis of five placebo‐controlled double‐blind studies. Int J Antimicrob Agents 2002; 19: 451–6 [DOI] [PubMed] [Google Scholar]
- 41. Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev 2012;(10); CD003265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forbes R, Ali A, Abouhajar A et al. ALternatives to prophylactic antibiotics for the treatment of recurrent urinary tract infection in women (ALTAR): study protocol for a multicentre, pragmatic, patient‐randomised, non‐inferiority trial. Trials 2018; 19: 616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harding C, Mossop H, Homer T et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, non‐inferiority trial. BMJ 2022; 376: e068229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scott AM, Clark J, Del Mar C, Glasziou P. Increased fluid intake to prevent urinary tract infections: systematic review and meta‐analysis. Br J Gen Pract 2020; 70: e200–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Vita D, Antell H, Giordano S. Effectiveness of intravesical hyaluronic acid with or without chondroitin sulfate for recurrent bacterial cystitis in adult women: a meta‐analysis. Int Urogynecol J 2013; 24: 545–52 [DOI] [PubMed] [Google Scholar]
- 46. Goddard JC, Janssen DAW. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: systematic review and meta‐analysis. Int Urogynecol J 2018; 29: 933–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung E, Gillman M, Tuckey J, La Bianca S, Love C. A clinical pathway for the management of Peyronie's disease: integrating clinical guidelines from the International Society of Sexual Medicine, American Urological Association and European Urological Association. BJU Int 2020; 126: 12–7 [DOI] [PubMed] [Google Scholar]
- 48. Lowrance WT, Murad MH, Oh WK, Jarrard DF, Resnick MJ, Cookson MS. Castration‐resistant prostate cancer: AUA guideline amendment 2018. J Urol 2018; 200: 1264–72 [DOI] [PubMed] [Google Scholar]
- 49. Melekos MD, Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents 2000; 15: 247–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 . Quality of evidence and strength of recommendations.
Table S2. Appraisal of Guidelines Research and Evaluation (AGREE II) quality assessment of included guidelines.


