Abstract
A biomarker associated with cognition in neurodegenerative dementias would aid in the early detection of disease progression, complement clinical staging and act as a surrogate endpoint in clinical trials. The current systematic review evaluates the association between cerebrospinal fluid protein markers of synapse loss and neuronal injury and cognition. We performed a systematic search which revealed 67 studies reporting an association between cerebrospinal fluid markers of interest and neuropsychological performance. Despite the substantial heterogeneity between studies, we found some evidence for an association between neurofilament‐light and worse cognition in Alzheimer's diseases, frontotemporal dementia and typical cognitive ageing. Moreover, there was an association between cerebrospinal fluid neurogranin and cognition in those with an Alzheimer's‐like cerebrospinal fluid biomarker profile. Some evidence was found for cerebrospinal fluid neuronal pentraxin‐2 as a correlate of cognition across dementia syndromes. Due to the substantial heterogeneity of the field, no firm conclusions can be drawn from this review. Future research should focus on improving standardization and reporting as well as establishing the importance of novel markers such as neuronal pentraxin‐2 and whether such markers can predict longitudinal cognitive decline.
Keywords: Alzheimer disease, biomarkers, cerebrospinal fluid, cognition, cognitive aging, dementia
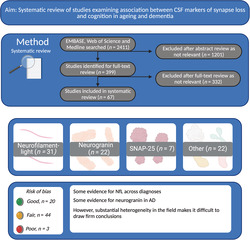
We conducted a systematic review of studies examining the association between cerebrospinal fluid markers and neuropsychological performance. Despite substantial heterogeneity in the field, there was some evidence of an association between cognition and neurofilament‐light and neurogranin. Risk of bias in the field requires improvement (Created with Biorender.com).
1. INTRODUCTION
Dementia is a syndrome characterised by progressive cognitive decline. An estimated 50 million people are living with a form of dementia worldwide, which is expected to reach 82 million by 2030 (World Health Organisation, 2020). The identification of a biomarker which correlates with cognition would have numerous benefits. An earlier indication of the pathophysiological processes underlying cognitive impairment is needed, as neuronal loss precedes detectable cognitive symptoms and so may be used to predict prognosis (Counts et al., 2017; DeKosky & Marek, 2003). Moreover, such markers could benefit our aetiological understanding of dementias as different synaptic markers could reflect different pathophysiological mechanisms. Next, in clinical trials, they could be used as surrogate endpoints for synapse‐targeting pharmacological interventions and could aid in the selection of participants who are in the earliest stages of dementia (Atri, 2011; Yiannopoulou & Papageorgiou, 2013). However, at present, there are no widely used biomarkers that predict cognitive status or cognitive decline in dementias.
Alzheimer's disease, the leading cause of dementia (World Health Organisation, 2020), is characterised by the pathological hallmarks of extracellular deposition of amyloid‐β (Aβ), intracellular accumulation of abnormally hyperphosphorylated tau into neurofibrillary tangles and brain atrophy due to neuronal and synapse loss (Blennow et al., 2006). These hallmarks of AD are present in mild cognitive impairment (MCI) and even before detectable symptoms begin to emerge—with Aβ accumulation possibly beginning up to two decades before symptom manifestation (Counts et al., 2017; Jack et al., 2010). Changes in the levels of these pathological proteins in the cerebrospinal fluid (CSF) have been observed as they aggregate in the brain and so the CSF may be a viable source of potential biomarkers.
The cerebrospinal fluid is a clear liquid which surrounds the brain and provides mechanical support, transfers micronutrients and signalling molecules to neurons and is involved in the removal of unnecessary metabolites (Spector et al., 2015). The CSF is an ideal source for biomarkers associated with cognition as it directly interacts with the extracellular space of the brain and so it can reflect the occurrence of pathophysiological changes (Hampel et al., 2012). In AD, the deposition of extracellular Aβ is reflected by reduced CSF levels of the 42‐amino acid form of Aβ (Aβ42) or the Aβ42/Aβ40 ratio, likely reflecting the reduced clearance of the protein (Potter et al., 2013; Tarasoff‐Conway et al., 2015). In contrast, levels of both total tau (t‐tau) and phosphorylated tau (p‐tau) are increased in the brain and in the CSF in AD (Counts et al., 2017; Ortega et al., 2019; Savage et al., 2014). These core CSF biomarkers of AD have high diagnostic accuracy (Counts et al., 2017; Ortega et al., 2019; Savage et al., 2014) and can predict conversion from MCI to AD (Caminiti et al., 2018; Li et al., 2016; Ortega et al., 2019). Indeed, they are currently accepted in international diagnostic criteria for use in the research diagnosis of AD and pre‐clinical AD (Dubois et al., 2014; Jack et al., 2018). However, despite the utility of these core CSF biomarkers as diagnostic tools, they correlate weakly with cognitive impairment. Studies report weak or no significant associations between cognitive performance and CSF Aβ (Kester et al., 2009; Ottoy et al., 2019; Zhou et al., 2009) and moderate‐to‐poor relationships with CSF t‐tau and p‐tau (Buchhave et al., 2009; Ecay‐Torres et al., 2018; Mattsson, Schöll, et al., 2017; Wattmo et al., 2020; Zhou et al., 2009). Meanwhile, other neurodegenerative dementias such as frontotemporal dementia (FTD), vascular dementia (VaD) and dementia with Lewy bodies (DLB) also lack a validated biomarker that associated with cognition. For example, CSF t‐tau and p‐tau can accurately discriminate FTD from controls (Meeter, Vijverberg, et al., 2018) but only have a moderate‐to‐weak correlation with neuropsychological performance (Bian et al., 2008; Borroni et al., 2011; Goossens et al., 2018). Accordingly, there is a need for additional validated CSF biomarkers which correlate with cognition and biomarkers of synapse loss that have been proposed as potential candidates.
Healthy synapse function enables neuronal signal transmission to occur, which is facilitated by pre‐synaptic and post‐synaptic compartments. Synaptic plasticity, formation, maturation and elimination involve processes essential for learning and memory, namely, long‐term potentiation (LTP) and long‐term depression (LTD) (Bear & Malenka, 1994). LTP refers to the strengthening of synaptic transmission by the addition of new receptors at the post‐synaptic density and the enlargement of dendritic spine heads. Conversely, LTD refers to the weakening of synaptic strength and spine shrinkage/loss (Citri & Malenka, 2008). The total number of synapses in the brain decreases with typical ageing, which is exacerbated in AD and other dementias (Bertoni‐Freddari et al., 1990; DeKosky & Scheff, 1990; Masliah et al., 1994, 2006). What is more, synapse loss is the strongest pathological correlate of cognitive decline in AD (De Wilde et al., 2016; DeKosky & Scheff, 1990; Masliah et al., 1994; Terry et al., 1991). Accordingly, CSF markers of synapse loss would be expected to correlate with cognitive impairment. Indeed, a number of CSF synapse and neuronal marker levels are altered in dementia syndromes and age‐related cognitive decline, some of which will be discussed. Before continuing, it is important to note that any CSF biomarker associated with cognition is primarily a marker of changes in the brain. Such pathophysiological changes may lead to neuronal network breakdown/damage, which may translate into cognitive symptoms at a point in the future. Therefore, the term ‘biomarker for cognition’ is erroneous and should be avoided.
1.1. Neurofilament‐light
Neurofilaments are classed as type IV intermediate filaments and are primarily located in axons. They play essential roles in radial growth, cytoskeletal support and transmission of electrical impulses along axons (Fuchs & Cleveland, 1998; Petzold, 2005). Neurofilaments are heteropolymers and are composed of four subunits in the CNS: neurofilament‐light (NfL), neurofilament‐medium (NfM), neurofilament‐heavy (NfH) and α‐internexin, of which NfL is the essential component. CSF NfL has been established as a general marker of axonal damage across neurodegenerative diseases as NfL is released into the extracellular fluid following axonal injury (Petzold, 2005). Indeed, CSF NfL levels correlate with brain atrophy (Dhiman et al., 2020; Pereira et al., 2017) and are elevated across dementias, MCI (Olsson et al., 2016; Petzold et al., 2007; Rosengren et al., 1999; Zetterberg et al., 2016) and neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Parkinson's disease (PD) (Gaetani et al., 2019).
1.2. Neurogranin (Ng)
Ng is a post‐synaptic peripheral membrane protein involved in LTP and memory formation. Ng binds calmodulin (CaM) in the absence of calcium (Ca2+) and thus regulates CaM availability (Petersen & Gerges, 2015). In the AD brain, full‐length Ng levels are reduced (Kvartsberg et al., 2019; Reddy et al., 2005), whereas CSF levels are increased in AD and MCI (Dulewicz et al., 2020). Elevated CSF Ng levels appear to be specific to AD, rather than reflecting general synapse damage in other neurodegenerative diseases or dementias (Portelius et al., 2018; Wellington et al., 2016).
1.3. Pre‐synaptic and neuronal markers
Cerebrospinal fluid levels of proteins localised at the pre‐synapse and post‐synapse are an obvious choice for a CSF marker of synapse loss/damage. The localization and normal function of such proteins suggest that they could be adequate surrogate markers for synapse loss, as they may be released into the extracellular fluid following synapse damage (Vergallo et al., 2018). Both NfL and Ng are some of the most researched markers. Next, we briefly discuss other pre‐synaptic and neuronal markers with a short description of their function, localization and potential roles in dementia syndromes.
Alpha‐synuclein (α‐syn) is a pre‐synaptic protein, expressed predominately in the neocortex and subcortical areas, including the hippocampus (Emamzadeh, 2016; Kim et al., 2014). Aggregates of hyperphosphorylated, misfolded α‐syn are the main component of Lewy bodies (LBs), the characteristic pathological accumulates of α‐synucleinopathies such as PD, Parkinson's disease dementia (PDD) and DLB (Kim et al., 2014). The normal function of α‐syn is not fully understood; however, it is thought to be involved in vesicle fusion and neurotransmitter release (Kim et al., 2014). The localization and normal function of α‐syn suggests that it could be used as a surrogate marker for synapse loss as it may be released into the extracellular fluid following synapse damage (Vergallo et al., 2018). Studies measuring full‐length α‐syn (rather than LB‐specific fragments) report significant elevations in AD and MCI and those with α‐synucleinopathies (Hansson et al., 2014; Korff et al., 2013; Slaets et al., 2014).
Beta‐synuclein (β‐syn) is a pre‐synaptic protein which is highly enriched in the hippocampus (Uhlén et al., 2015). It is homologous to and co‐localises with α‐syn (Williams et al., 2018). The normal function of β‐syn is unknown, although there is evidence to suggest that it has a role in the inhibition of α‐syn aggregation (Williams et al., 2018). Independent of its pathological form, β‐syn may be a good marker of synapse loss due to its localization at the pre‐synapse.
Contactin‐2 is a pre‐synaptic and axonal protein (Furley et al., 1990), expressed in frontal and temporal lobes—including hippocampal pyramidal cells (Gautam et al., 2014; Murai et al., 2002). Contactin‐2 is involved in axonal guidance during development, neuronal fasciculation and axonal domain organisation (Masuda, 2017; Wolman et al., 2008). In AD, contactin‐2 levels are reduced in the brain (Chatterjee, Del Campo, et al., 2018; Gautam et al., 2014) and altered in the CSF, although findings are somewhat discrepant with regard to whether CSF levels are elevated or decreased (Chatterjee, Del Campo, et al., 2018; Yin et al., 2009). Contactin‐2 may be a potential marker of general synapse and axonal damage for neurodegenerative diseases as CSF levels are also increased in multiple sclerosis (MS) (Chatterjee, Koel‐Simmelink, et al., 2018).
GAP‐43 is a pre‐synaptic protein widely expressed in the CNS during the development, which reduces with maturation (Holahan, 2017). In adulthood, GAP‐43 is expressed in hippocampal pyramidal cells and association cortices (Chung et al., 2020; Neve et al., 1988; Riascos et al., 2014) and is involved in axonal outgrowth, synaptic plasticity and functions associated with learning and memory (Chung et al., 2020; Holahan, 2017). Levels of GAP‐43 in the frontal cortex are reduced in a number of dementia syndromes (Bogdanovic et al., 2000; Davidsson & Blennow, 1998; Rekart et al., 2004). Moreover, CSF GAP‐43 levels are increased in AD, FTD‐syndromes (Remnestål et al., 2016) and other neurodegenerative diseases such as PD and ALS (Sandelius et al., 2019).
The neuronal pentraxin family includes neuronal pentraxin I (NPTX1), neuronal pentraxin 2 (NPTX2) and neuronal pentraxin receptor (NPTXR) which are highly enriched in excitatory pyramidal neurons of the hippocampus and cerebellum (Chang et al., 2010; Dodds et al., 1997). All three neuronal pentraxins are involved in developmental and adult synaptic plasticity, formation and remodelling, as well as the maintenance of parvalbumin interneuron activity (Chang et al., 2010; Osera et al., 2012). NPTX1/2 are secreted pre‐synaptic proteins, whereas NPTXR is a membrane‐anchored protein (Lee et al., 2017). In the brain and the CSF, NPTX2 levels are reduced in AD, MCI, FTD and aged controls (Soldan et al., 2019; van der Ende et al., 2020, 2019; Xiao et al., 2017).
Neuregulin 1 (nrg1), a substrate of BACE1, is a pre‐synaptic protein thought to be implicated in a number of neurodegenerative diseases and psychiatric/neurodevelopmental disorders such as AD, attention deficit hyperactive disorder (ADHD) and schizophrenia (Shi & Bergson, 2020). Nrg1 is thought to be involved in synaptic transmission and plasticity (Fischbach, 2007); however, at least 31 isoforms have been described which all perform a broad range of functions throughout the body. It is unclear whether Nrg1 in the brain exerts protective or detrimental effects on cognition as both high and low levels of Nrg1 at synapses lead to cognitive impairment in animal models (Agarwal et al., 2014). There are no known human post‐mortem brain studies examining Nrg1 levels in dementias; however, elevations of CSF Nrg1 have been reported in AD and MCI (Mouton‐Liger et al., 2020; Pankonin et al., 2009).
Synaptosomal‐associated protein 25 (SNAP‐25) is a pre‐synaptic protein involved in vesicular exocytosis, LTP and the formation of SNARE complexes (Noor & Zahid, 2017). In post‐mortem brain studies, levels of SNAP‐25 are reduced across dementia syndromes (Connelly et al., 2011; Minger et al., 2001; Mukaetova‐Ladinska et al., 2009; Sinclair et al., 2015). Levels of CSF SNAP‐25 are increased in AD and MCI (Brinkmalm et al., 2014; Galasko et al., 2019; Wang, Zhou, & Zhang, 2018; Zhang, Therriault, et al., 2018), potentially reflecting the release of SNAP‐25 from synapses into the extracellular space. Elevations have also been reported in PD, Creutzfeldt‐Jakob Disease (CJD) (Noor & Zahid, 2017) and a number of psychiatric disorders; hence, CSF SNAP‐25 could be a general marker of synapse damage (Najera et al., 2019).
Synaptotagmin‐1 is a pre‐synaptic protein involved in synaptic vesicle exocytosis and synaptic transmission (Baker et al., 2015; Jahn & Fasshauer, 2012). Across dementia syndromes, synaptotagmin‐1 levels are reduced in the brain (Bereczki et al., 2018; Davidsson & Blennow, 1998; Yoo et al., 2001) and elevated in the CSF (Öhrfelt et al., 2016, 2019; Tible et al., 2020).
Visinin‐like protein‐1 (VILIP‐1) is a neuronal calcium sensor protein which is widely expressed in neurons and involved in signalling pathways related to synaptic plasticity (Braunewell, 2012). In AD and FTD, VILIP‐1 expression is reduced in the temporal/entorhinal cortices (Braunewell et al., 2001; Kirkwood et al., 2016) and the superior frontal gyrus, respectively (Kirkwood et al., 2016). Additionally, in the CSF, a recent meta‐analysis reported elevated CSF VILIP‐1 levels in AD and MCI due to AD (Dulewicz et al., 2020).
To date, there is no summary of the evidence examining the relationship between CSF markers of synapse loss and neuronal damage and cognition in ageing and disease. Hence, we conducted a systematic review examining the scientific literature for associations between these markers and cognition in healthy ageing and dementia syndromes. We searched for papers examining any type of dementia or cognition in typical ageing to characterise the cross‐diagnostic specificity of markers. Levels of CSF Aβ or tau were not considered as this was beyond the scope of the current review. We searched for correlates of both cross‐sectional cognition only.
2. MATERIALS AND METHODS
The protocol for this review was prospectively registered on PROPSERO (CRD42020164456).
2.1. Search strategy
The initial search was conducted in December 2019 within MEDLINE, EMBASE and Web of Science. The most recent update search was conducted on 4 January 2021. Search terms can be found in the supporting information Table S1. Reference lists of studies and reviews were manually searched to identify additional studies. No restrictions were applied for language or date of publication. Only published studies in peer reviewed journals were included; conference abstracts were excluded.
2.2. Eligibility criteria
The inclusion criteria were that the study: (i) included a population with a diagnosis of Alzheimer's disease, MCI, FTD, any other type of dementia or a cognitively unimpaired (CU) sample; (ii) measured a cerebrospinal fluid marker of synapse loss and/or neuronal damage, excluding Aβ or tau; (iii) assessed cognition using a validated tool; and (iv) directly examined the relationship between the CSF marker and cognition.
Exclusion criteria included studies (i) where participants were diagnosed with a psychiatric disorder, (ii) review articles, (iii) conference abstracts, (iv) animal studies and (v) studies which only examined CSF Aβ or tau.
Two researchers (T.S.S. and D.A.G.) independently screened studies for inclusion/exclusion and resolved any discrepancies through discussion.
2.3. Data extraction
T.S.S. and D.A.G. independently extracted data from eligible studies using Covidence software. This included the following: year of publication, demographics, sample size, medication status, apolipoprotein E (ApoE) status, mean/median CSF marker levels with the appropriate measure of variation and other related information. Researchers were not blinded to authors, journals or institutions. Any discrepancies were resolved by discussion and joint data extraction. Authors were contacted for additional clarification and to request missing data wherever possible.
2.4. Risk of bias assessment
The Cochrane network advise against quality scales which generate a summary score and instead suggest placing importance on how each study performed on individual criterion (Boutron et al., 2020). Therefore, we assessed the risk of bias in study design and reporting using the National Institute of Health Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies (National Institutes of Health, 2014). T.S.S. and D.A.G. independently assessed risk of bias, and any discrepancies were resolved by discussion.
2.5. Synthesis of results
Correlation coefficients were selected as the standardised metric of the review. After extraction of results, a meta‐analysis was not conducted due to substantial differences in study methodologies and a lack of reporting of correlation coefficients in published reports. Therefore, we grouped studies according to the CSF marker being measured due to a number of studies pooling participants across diagnostic groups in statistical analysis.
3. RESULTS
3.1. Search results
Two thousand, four hundred and eleven studies were identified. After screening studies for eligibility, 67 studies met criteria for inclusion in the systematic review (see Figure 1).
FIGURE 1.
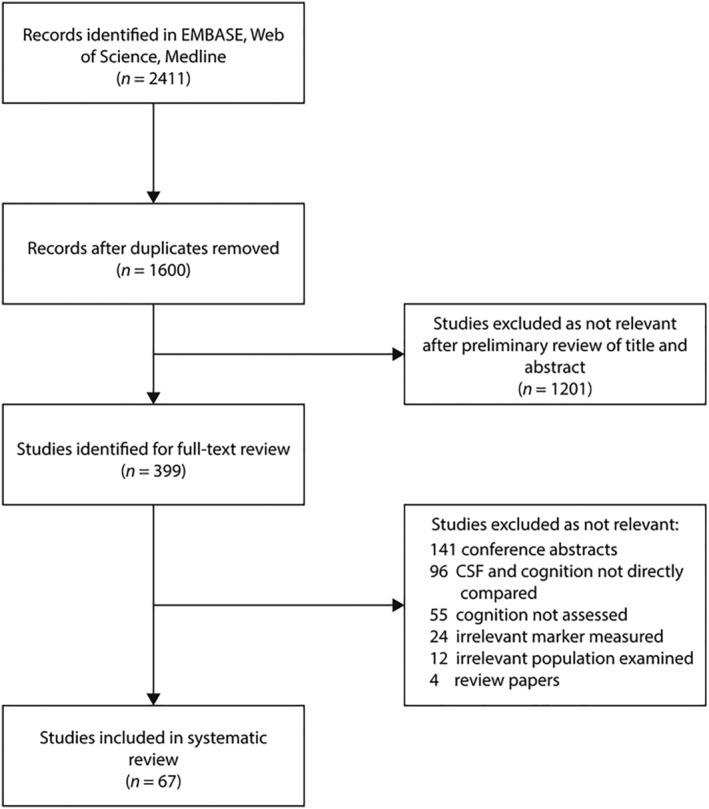
Search process
3.2. Study characteristics
3.2.1. Sample size
Characteristics of included studies can be found in Table 1. Some cohorts were used in multiple studies. Ten studies used the Alzheimer's Disease Neuroimaging Initiative (ADNI) (Galasko et al., 2019; Headley et al., 2018; Mattsson et al., 2016; Petersen et al., 2010; Portelius et al., 2015; Sun et al., 2016; Swanson et al., 2016; Wang, 2019; Wang, Zhou, & Zhang, 2018; Zetterberg et al., 2016; Zhang, Ng, et al., 2018; Zhang, Therriault, et al., 2018), five used the Amsterdam Dementia Cohort (Boiten et al., 2021; Chatterjee, Del Campo, et al., 2018; Kvartsberg, Duits, et al., 2015; Meeter, Vijverberg, et al., 2018; van Der Flier & Scheltens, 2018; van Steenoven et al., 2020), three used the Wisconsin Registry for Alzheimer's Prevention (Bendlin et al., 2012; Casaletto et al., 2017; Racine et al., 2016; Sager et al., 2005) and three used the Genetic Frontotemporal Dementia Initiative (GENFI—The Genetic Frontotemporal Initiative) (GENFI – The Genetic Frontotemporal Initiative, n.d.; Meeter et al., 2016; Meeter, Vijverberg, et al., 2018; van der Ende et al., 2020). The Gothenburg Mild Cognitive Impairment Study (Bjerke et al., 2009; Brinkmalm et al., 2014; Rolstad, Berg, et al., 2015; Wallin et al., 2016) was used in three studies, the Mayo Clinic Study of Ageing (Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al., 2019; Mielke, Syrjanen, Blennow, Zetterberg, Vemuri, et al., 2019; Roberts et al., 2008) in two studies, the Vanderbilt Memory and Ageing Project (Gifford et al., 2018; Jefferson et al., 2016; Osborn et al., 2019) in two studies and finally, the University of California San Diego (UCSD) Shiley‐Marcos Alzheimer's Disease Research Center (Galasko et al., 2019; Xiao et al., 2017) in two studies.
TABLE 1.
Characteristics of included studies
| Study | CSF Marker | CSF analysis assay and brand | Population (N) | Age (years) * | Sex (N, % female) | CSF marker level (pg/mL) * : | Cognitive assessment | Adjustment factors | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
Abu‐Rumeileh et al., 2018 |
NfL |
ELISA (IBL, Germany) |
AD (60) FTD (141) |
67.1 (8.7) 64.9 (9.8) |
27 (45%) 66 (46.8%) |
Median [IQR] 2160 [1614‐2878] 3293 [2120‐7596] |
BMDB, FAB |
None |
||
| Agnello et al., 2020 |
Ng Alpha‐Synuclein |
ELISA (ADx Neurosciences, Belgium) |
AD (29) |
67.8 (6.4) |
15 (51.7%) |
Median [IQR] |
MMSE |
None |
||
|
Ng: 460 [410‐647] |
α‐syn: 2844[2326.9‐3524.5] |
|||||||||
| Alcolea et al., 2017 | NfL | ELISA (UmanDiagnostics, Sweden) | FTD (249) | 67.12 (8.87) | 121 (48.4%) | 2069.39 (1833.92) | MMSE | None | ||
| Aschenbrenner et al., 2020 | NfL | ELISA (UmanDiagnostics, Sweden) |
CU Aβ + (94) CU Aβ ‐ (161) |
67.31 (8.99) 65.60 (8.47) |
40 (43%) 100 (62%) |
1356.29 (574.42) 1505.72 (703.91) |
Global, episodic memory, attention composites | Age, amyloid status | ||
| Bartos et al., 2012 | NfL | ELISA (Progen, Germany) |
AD (25) PSP, FTD, CJD, CBS, WE (13) |
73 (8) 64 (8) |
21 (84%) 4 (23%) |
N.R | MMSE (derived from ACE‐CZ), ACE‐CZ | None | ||
| Begcevic et al., 2020 |
NPTX1 NPTXR |
Mass spectrometry |
Cohort 1 (58): MCI (8) Mild AD (11) Moderate AD (24) Severe AD (15) Cohort 2 (43): MCI (6) Mild AD (8) Moderate AD (16) Severe AD (15) |
74.5 (7.8) 71.4 (8.4) 75.7 (6.4) 74.4 (9.3) 67.6 (9.2) 76.2 (8.8) 78.1 (6.9) 71.1 (9.0) |
3 (38%) 3 (27%) 13 (54%) 6 (40%) 5 (83%) 3 (38%) 6 (38%) 2 (15%) |
N.R | MMSE | None | ||
| Bendlin et al., 2012 | NfL | ELISA (in‐house) | CU with family history of AD (43) | 53.67 (7.77) | 31 (72.1%) | N.R | BVMT, COWAT, TMT‐A, TMT‐B, WAIS‐working memory index, AVLT | Age, education | ||
| Bjerke et al., 2009 | NfL | ELISA (in‐house) |
MCI‐SVD (9) MCI‐MD (15) MCI‐MCI (118) MCI‐AD (20) CU (52) |
Median {25th, 75th percentile} 68 {66, 74} 69 {65, 74} 62 {57, 68} 68 {58, 72} 66 {63, 70} |
4 (44.4%) 13 (86.7%) 65 (55.1%) 12 (60%) 30 (57.7%) |
Median {25th, 75th percentile} 424 {255, 1414} 250 {250, 406} 250 {250, 250} 250 {250, 341} 250 {250, 250} |
MMSE | None | ||
|
Boiten et al., 2021 |
NPTX2 |
ELISA (in‐house) |
AD (20) DLB (48) |
65.3 (6.0) 67.7 (6.4) |
2 (10%) 6 (13%) |
Median [95% interval] 453 [317‐696] 474 [279 – 659] |
Global, memory, attention, executive function, language, visual composites, MMSE |
Age, education |
||
| Bos et al., 2019 |
NfL Ng |
ELISA (UmanDiagnostics) Electrochemiluminescence (in‐house) |
AD (180) Aβ + (157) Aβ ‐ (23) MCI (450) Aβ + (263) Aβ ‐ (187) CU (140) Aβ + (45) Aβ ‐ (95) |
69.8 (8.8) 74.2 (7.9) 71.4 (7.1) 68.6 (8.2) 69.5 (8.1) 62.7 (7.3) |
85 (54%) 8 (34%) 145 (55%) 89 (48%) 23 (51%) 49 (52%) |
NfL 1742.2 (2893.2) 1931.9 (1934.8) 1242.3 (2556.1) 1031.2 (919.1) 983.13 (678.4) 627.4 (293.3 |
Ng 155.2 (121.4) 118.3 (136) 175.5 (217.8) 99.2 (102.9) 152.6 (149.6) 110.8 (224) |
MMSE | Age, sex, years of education, baseline diagnosis | |
| Brinkmalm et al., 2014 | SNAP‐25 | Mass spectrometry |
AD (36) CU (33) |
Median [IQR] Cohort 1: 68 [68‐79] Cohort 2: 77 [73‐82] Cohort 3: 68 [66‐70] Cohort 1: 70 [68‐74] Cohort 2: 54 [48‐63] Cohort 3: 66 [64‐68] |
Cohort 1: 6 (66.7%) Cohort 2: 7 (70%) Cohort 3: 12 (70.6%) Cohort 1: 7 (77.8%) Cohort 2: 5 (83.3%) Cohort 3: 8 (47.1%) |
N.R. | MMSE | None | ||
| Bruno et al., 2020 |
Ng Alpha‐Synuclein |
ELISA (in‐house) ELISA (Tecan Sunrise, Austria) |
CU (19) | 68.1 (7.3) | 12 (63%) |
Ng: 100.8 (91.4) |
α‐syn: 14.1 (16.1) |
BSRT | None | |
|
Casaletto et al., 2017 |
Ng |
ELISA (in‐house) |
CU with family history of dementia (132) |
64.5 (7.4) |
86 (65.2%) |
Median [IQR] 335.9 [250.6‐482.8] |
AVLT, WAIS‐III symbol digit coding, BNT, WAIS‐III digit span forwards, WAIS‐III digit span backwards. |
Sex CSF Aβ42 CSF t‐tau CSF p‐tau Hippocampal volume ApoE status Family history of AD |
||
|
Chatterjee et al., 2018 |
Contactin‐2 |
ELISA (R&D, USA) |
AD (106) CU (48) |
Cohort 1: 62 (6) Cohort 2: 62 (5) Cohort 1: 60 (7) Cohort 2: 62 (3) |
21 (58.3%) 41 (58 %) 15 (53.6%) 6 (30.6%) |
Median [IQR] 59 [42‐74] 61 [39‐78] 78 [69‐110] 65 [54‐99] |
MMSE |
None |
||
| De Vos et al., 2016 | Ng | ELISA (in‐house) |
AD (50) MCI (38) |
Median {25th, 75th percentile} 75 {68, 78} 73 {69, 79} |
27 (54%) 23 (60.5%) |
Median [IQR] 172 [141‐230] 214 [161‐256] |
MMSE |
Age Sex |
||
| De Jong et al., 2007 | NfL | ELISA (in‐house) |
EAD (37) LAD (33) DLB (18) FTD (28) |
Median [IQR] 61 [52‐69] 76 [69‐90] 72 {58‐90] 63 [43‐79] |
22 (59.4%) 20 (60.6%) 5 (27.8%) 8 (28.6%) |
Median [range] 6.1 [0.0‐40.3] 15.2 [0.0‐70.1] 10.4 [0.0‐60.4] 16.9 [0.0‐76.4] |
MMSE | None | ||
|
Delaby et al., 2020 |
NfL |
ELISA (UmanDiagnostics, Sweden) |
CU (118) AD (116) FTD (56) DLB (37) Prodromal DLB (26) PSP (12) CBS (26) |
59.4 (9.7) 70.4 (8.0) 65.8 (5.2) 76.7 (4.9) 82.2 (6.1) 70.5 (7.8) 72 (7.3) |
68 (57.6%) 71 (61.2%) 15 (26.8%) 19 (51.4%) 13 (50%) 7 (58.3%) 13 (50%) |
Median [IQR] 411 [343‐567] 940 [765‐1229] 1240 [859‐2378] 1135 [803‐1321] 934 [643‐1094] 1422 [1034‐1727] 1637 [923‐2797] |
MMSE |
None |
||
| Dhiman et al., 2020 | NfL | ELISA (UmanDiagnostics, Sweden) |
AD (28) MCI (34) CU (159) |
74.6 (7.5) 74.1 (7.6) 72.8 (5.5) |
12 (43%) 13 (38%) 84 (53%) |
2201 (626.96) 1977 (908.44) 1506 (510.59) |
MMSE |
Age Sex ApoE status |
||
| Galasko et al., 2019 |
Ng (Cohort 1) SNAP‐25 (Cohort 1) NPTX2 (Cohort 1, Cohort 2) |
ELISA (EUROIMMUN, Germany) SIMOA (home‐brew) ELISA (in‐house) |
Cohort 1 (193): AD MCI CU Cohort 2 (292): AD MCI CU |
70.7 (9.4) 74.3 (6.5) 73 (5.2) 75.1 (7.6 74.7 (7.2) 75.7 (5.5) |
19 (41%) 20 (35%) 52 (35%) 28 (42%) 44 (31%) 43 (50%) |
Ng 347.6 (235.6) 332.2 (199.9) 324.5(163.4) |
SNAP‐25 36 (15.6) 34.9(15.5) 32.1( 9.8) |
NPTX2 715.1 (426.6) 826.5 (474.4) 1075 (504.8) 10.3 (0.9) 10.6 (0.7) 10.7 (0.5). |
CVLT |
Age Sex Education ApoE status |
| Gifford et al., 2018 | NfL | ELISA (UmanDiagnostics, Sweden) |
Early MCI (9) MCI (37) CU (65) |
72 (7) 74(7) 73 (7) |
2 (22%) 13 (35%) 20 (31%) |
1145 (477) 1395 (795) 959 (466) |
PVLT | Age, sex, ethnicity, ApoE status, cognitive diagnosis | ||
| Headley et al., 2018 | Ng | Electrochemiluminescence (Meso Scale Discovery, USA) |
MCI (193) CU (111) |
75 (7) 75 (6) |
64 (33%) 55 (50%) |
494 (353) 352 (294) |
MMSE, ADAS‐Cog, ADAS‐Cog13, memory composite, executive function composite | Age, sex, years of education, ApoE status, CSF t‐tau, CSF Aβ42 | ||
| Hellwig et al., 2015 | Ng | Electrochemiluminescenc (Meso Scale Discovery, USA) |
AD (39) MCI‐AD (13) Non‐AD dementia (14) MCI‐O (29) |
Median (range) 72.5 (68‐76) 73.3 (69‐77) 65.1 (59‐71) 69.4 (61‐75) |
21 (53.9%) 8 (61.5%) 8 (57.1%) 14 (48.3%) |
N.R | MMSE | None | ||
| Hoglund et al., 2015 |
NfL Ng VILIP‐1 |
ELISA (UmanDiagnostics, Sweden) Electrochemiluminescence (Meso Scale Discovery, USA) ELISA (BioVendor R&D, Germany) |
CU Aβ‐ (43) CU Aβ+ (86) |
Total: 81.9 (3.4) | Total: 73 (56.6%) |
NfL 1847 (987.2) 1940 (1353) |
Ng 889.3 (414.5) 686.1 (322.8) |
VILIP‐1 0.13 (0.06) 0.12 (0.05) |
MMSE | N.R |
| Jia et al., 2020 |
Ng GAP‐43 SNAP‐25 Synaptotagmin‐1 |
ELISA (American Research Products, USA) ELISA (MyBiosource, USA) ELISA (Proteintech, USA) ELISA (Abbkine, China) |
Cohort 1: AD (28) Cohort 2: AD (73) |
66 (6) 65 (6) |
16 (57.1%) 42 (57.5%) |
N.R | MMSE | Age, sex, ApoE status | ||
| Kirsebom et al., 2018 | Ng | ELISA (EUROIMMUN, Germany) |
Aβ+ MCI (20) Aβ+ SCI (18) CU (36) |
66.8 (7.4) 66.7 (6.8) 61.2 (9.2) |
12 (57%) 8 (44%) 19 (52.8%) |
428 (179) 468 (217) 374 (128) |
MMSE CERAD word list test, TMT‐A, TMT‐B |
Age | ||
| Kvartsberg et al., 2015 | Ng | ELISA (in‐house) |
MCI (40) |
Median [IQR] 64 [58‐71] |
19 (48%) |
Median [IQR] 210 [83‐433] |
MMSE | Age, sex | ||
| Lee et al., 2008 | VILIP‐1 | ELISA (in‐house) |
AD (33) |
Mean ± SE 67.0 ± 1.8 |
18 (55%) |
N.R | MMSE | None | ||
| Lim et al., 2019 | NPTXR | ELISA (RayBiotech, USA) |
MCI (14) Mild AD (21) Moderate AD (43) Severe AD (30) |
72.1 (9.3) 73.7 (8.5) 77.0 (9.0) 72.8 (9.6) |
8 (57%) 6 (29%) 19 (44%) 9 (30%) |
N.R | MMSE | None | ||
| Mattsson et al., 2016 |
NfL Ng |
ELISA (UmanDiagnostics, Sweden) ELISA (in‐house) |
AD MCI CU |
74.7 (8) 74.5 (7.5) 75.7 (5.2) |
41 (44%) 62 (33%) 54 (50%) |
N.R | MMSE, ADAS‐Cog11 | Age, sex, years of education | ||
| McGuire et al., 2015 |
NfL pNfH |
ELISA (UmanDiagnostics, Sweden ELISA (BioVendor,Czech Republic) |
HAD (3) ANI (15) MNCD (15) CU (15) |
Median [IQR] 47 [38‐50] 38 [31‐40] 40 [35‐48] 44 [36‐49] |
0 (0%) 6 (40%) 3 (20%) 3 (20%) |
N.R |
WAIS‐III Digit symbol WAIS‐III Symbol search TMT‐A Story memory test Figure memory test WCST TMT‐B COWAT ANT WAIS‐III letter‐number sequencing PASAT |
None |
||
| Meeter et al., 2016 |
NfL |
ELISA (UmanDiagnostics, Sweden) |
FTD with GRN, MAPT, C9orf72 mutation (101) |
Median [IQR] 59 [56‐65] |
52 (51%) |
6762 (N.R) |
MMSE |
None |
||
|
Meeter et al., 2018 |
NfL |
ELISA (UmanDiagnostics) |
FTD with C9orf72 mutation (64) Presymptomatic carriers of C9orf72 mutation (25) |
Median [IQR] 60 [55‐66] 47 [41‐57] |
29 (45.3%) 17 (68%) |
Median [IQR] 1885 [848‐2841] 429 [336‐830] |
MMSE |
None |
||
|
Meeter et al., 2019 |
NfL |
ELISA (UmanDiagnostics, Sweden) |
svPPA (147) |
Median [IQR] 64 [58‐68] |
87 (54%) |
Median [IQR] 2326 [1628‐3593] |
BNT, ANT, letter fluency, WAIS‐III digit span forward and backwards, TMT‐A, TMT‐B, SCWT, CDT, AVLT, CVLT, CERAD word list test, Rey complex figure test |
Age, sex, laboratory |
||
|
Meeter et al., 2017 |
NfL |
ELISA (UmanDiagnostics) |
bvFTD (164) svPPA (36) nfvPPA (19) lvPPA (4) CBS (40) PSP (58) |
Median [IQR] 61 [55‐67] 62 [58‐65] 62 [52‐66] 64 [51‐69] 65 [60‐73] 66 [62‐70] |
78 (44%) 10 (53%) 10 (53%) 3 (75%) 14 (33%) 36 (56%) |
Median [IQR] 3168 [1752‐4818] 3151[1906=4802] 2345 [1956‐2957] 1731 [1181‐2472] 2664 [1715‐4158] 1907 [1474‐2755] |
MMSE, FAB |
None |
||
|
Mielke et al., 2019a |
NfL Ng |
ELISA (in‐house) ELISA (in‐house) |
Dementia MCI CU Total (777) |
Median [IQR] Total = 72.9 [64‐79.3] |
Total = 334 (43%) |
NfL (total) 520.2 [374.3‐745.4] |
Ng (total) 166.6 [132.9‐220.8] |
Global, Memory, language, attention, visuospatial composites |
Age, sex |
|
|
Mielke et al., 2019b |
NfL |
ELISA (in‐house) |
MCI CU Total (79) |
Median [IQR] Total = 76.4 [71.7‐80.7] |
Total = 27 (34%) |
Median [IQR] Total = 608.3 [429.1‐817.7] * |
Memory, language, executive function, visuospatial composites |
Age, sex, years of education |
||
| Mouton‐Liger et al., 2020 | Nrg1 | ELISA (R&D Systems, USA) |
AD (54) MCI‐AD (20) Non‐AD dementia (30) Non‐AD MCI (31) CU (27) |
69.4 (7.9) 70.2 (8.0) 68.7 (7.6) 61.5 (9.6) 62 (11.3) |
33 (61.1%) 12 (60%) 11 (36.7%) 11 (35.5%) 23 (85.2%) |
364.7 (149.2) 342.6 (161.5) 287.5 (106.5) 304.9 (113.0) 267.7 (104.2) |
MMSE | None | ||
|
Oeckl et al., 2020 |
Beta‐synuclein |
Mass spectrometry |
Cohort 1: AD (64) Cohort 2: AD (40) Cohort 3: AD (49) |
Median [IQR] 73 [68‐78] 70 [63‐74] 72 [64‐77] |
42 (65.6%) 20 (50%) 25 (51.0%) |
Median [IQR] 979 [738‐1223] 694 [532‐990] 917 [746‐1185] |
MMSE |
None |
||
| Öhrfelt et al., 2016 | Synaptotagmin | Mass spectrometry |
Cohort 1: AD (17) Cohort 2: AD (24) Cohort 1: MCI‐AD (5) Cohort 2: MCI‐AD (18) Cohort 1: CU (17) Cohort 2: CU (36) |
Median [IQR] 65 [58‐81] 68 [64‐72] 78 [73‐81] 70 [69‐78] 60 [53‐67] 62 [55‐69] |
12 (70.6%) 17 (70.8%) 4 (80%) 13 (72.2%) 10 (58.8%) 23 (63.9%) |
N.R | MMSE | None | ||
| Öhrfelt et al., 2019 | SNAP‐25 | ELISA (in‐house) |
Cohort 1: AD (17) Cohort 2: AD (24) Cohort 1: MCI‐AD (5) Cohort 2: MCI‐AD (18) Cohort 1: CU (17) Cohort 2: CU (36) |
Median [IQR] 65 [58‐81] 68 [64‐72] 78 [73‐81] 70 [69‐78] 60 [53‐67] 62 [55‐69] |
12 (70.6%) 17 (70.8%) 4 (80%) 13 (72.2%) 10 (58.8%) 23 (63.9%) |
N.R | MMSE | None | ||
| Osborn et al., 2019 | NfL | ELISA (UmanDiagnostics, Sweden) |
Early MCI (27) MCI (132) CU (174) |
73 (6) 73 (8) 72 (7) |
7 (26%) 58 (44%) 71 (41%) |
1088 (465) 1250 (712) 930 (448) |
Episodic memory composite, executive function composite, BNT, ANT, WAIS‐IV coding, DKEFS number sequencing, Hooper visual organisation test | Age, sex, ethnicity, ApoE status | ||
|
Portelius et al., 2015 |
Ng |
Electrochemiluminescence (in‐house) |
AD (95) pMCI (105) sMCI (68) CU (110) |
Median [IQR] 76 [70‐80] 75 [70‐80] 74 [70‐80] 76 [72‐78] |
42 (44%) 37 (35%) 22 (32%) 55 (50%) |
Median [IQR] 485 [349‐744] * 492 [330‐672] * 386 [190‐582] * 304 [161‐453] * |
MMSE, ADAS‐Cog |
Age, sex, education |
||
| Racine et al., 2016 | NfL | ELISA (UmanDiagnostics, Sweden) |
MCI + CU (70) |
66.26 (6.1) |
40 (57.1%) |
N.R |
CAB CPAL errors GMCT moves/sec GML errors GMR errors OCL accuracy ONB accuracy TWOB accuracy RAVLT delayed Logical memory delayed BVMT‐R delayed |
None | ||
| Rojas et al., 2018 | NfL | ELISA (UmanDiagnostics, Sweden) | PSP (50) | 67.7 (5.7) | 30 (60%) | 5929 (6196) |
RBANS Color trails 1 & 2 Letter‐number sequencing, Phonemic fluency |
Age, sex | ||
| Rolstad et al., 2015a | NfL | ELISA (in‐house) |
Dementia‐ vascular (65) Dementia‐ non‐vascular (128) MCI‐ vascular (86) MCI‐ non‐vascular (175) SCI‐ vascular (48) SCI‐ non‐vascular (120) |
68.9 (6.5) 66.4 (7.8) 67.4 (7.2) 63.9 (7.7) 65.6 (7.4) 60.6 (7.1) |
32 (49.2%) 78 (60.9%) 50 (58.1%) 60 (34.3%) 28 (58.3%) 72 (60%) |
567.5 (635.0) 569.4 (720.3) 611.2 (1110.9) 360.7 (299.6) 308.5 (158.2) 328.3 (295.8) |
Attention, learning/memory, visuospatial, language, executive function composites, | Age, sex | ||
| Rolstad et al., 2015b | NfL | ELISA (UmanDiagnostics) | CU (71) | 37.8 (14.6) | 44 (61.9%) | 254.38 (55.42) | Memory , executive function, visuospatial, speed/attention, verbal composites | Age, sex | ||
| Sancesario et al., 2020 | Ng | ELISA (EUROIMMUN, Germany) | CU (30) | 64.04 (11.83) | 18 (61%) | 336.53 (193.40) | MMSE | None | ||
| Sandelius et al., 2019 | GAP‐43 | ELISA (in‐house) |
AD (275) MCI (84) CU (43) FTD (39) DLB (27) lvPPA (10) svPPA (15) PSP (18) CBS (19) |
71.2 (9.2) 72 (8.9) 69 (9.1) |
58.2% 46.4% 69.8% |
N.R | MMSE | None | ||
|
Sanfilippo et al., 2016 |
Ng |
ELISA (in‐house) |
AD (25) MCI (50) MCI‐AD (36) CU (44) |
Median [IQR] 76 [67‐85] 71 [68‐76] 73 [71‐76] 71 [67.5‐75] |
19 (76%) 30 (60%) 22 (61%) 31 (70.5%) |
Median [IQR] 687 [474‐956] * 182 [83‐310] * 481 [326‐841] * 235.5 [171‐358] * |
MMSE, CAMCOG |
None |
||
| Santillo et al., 2019 | Ng | Electrochemiluminescence (Meso Scale Discovery, USA) | CU (20) | 25 (4) | 9 (45%) | 427 (189) | MCCB | None | ||
| Scherling et al., 2014 | NfL | ELISA (UmanDiagnostics, Sweden) |
Asymptomatic FTD mutation carriers (8) bvFTD (45) nfvPPA (18) svPPA (16) CBS (17) AD (50) PSP (22) CU (47) |
54 (10) 61 (8) 70 (7) 63 (7) 68 (8) 66 (9) 68 (7) 66 (11) |
4 (100%) 13 (28.9%) 7 (38.9%) 10 (62.5%) 11 (64.7%) 22 (44%) 11 (50%) 21 (44.7%) |
MMSE, Rey‐Osterrieth figure, FDS, BDS, TMT, Stroop task, BNT, ANT, CVLT, phonemic fluency | None | |||
| Schindler et al., 2019 |
Ng SNAP‐25 VILIP‐1 |
SIMOA (Millipore, USA) SIMOA (Millipore, USA) SIMOA (Millipore, USA) |
Carriers of mutations in PSEN1, PSEN2, or APP (235) Mutation non‐carriers (145) |
38.4 (10.4) 38.8 (12.1) |
127 (54%) 89 (61%) |
Ng 2269 (1189) 1572 (741) |
SNAP‐25 4.6 (1.9) 3.7 (1.3) |
VILIP‐1 173.4 (77.9) 132.9 (50.2) |
DIAN cognitive composite | Age, sex, education, ApoE status |
| Sjögren et al., 2001 | NfL | ELISA (in‐house) |
AD (22) SVD (9) CU (20) |
64.4 (7.7) 70.1 (6.3) 66.4 (9.9) |
7 (31.8%) 9 (100%) 15 (75%) |
569 (308) 1977 (1436) 156 (66) |
MMSE | None | ||
| Sjögren et al., 2000 | NfL | ELISA (in‐house) |
FTD (18) AD (21) |
62.4 (10) 73.4 (3.2) |
7 (38.9%) 14 (66.7%) |
1442 (1183) 1006 (727) |
MMSE | None | ||
| Skillback et al., 2014 | NfL | ELISA (UmanDiagnostics, Sweden) |
EAD (223) AD (1194) FTD (146) DLB (114) VaD (465) MIX (517) PDD (45) Dementia NOS (437) |
59 (4) 76 (6) 68 (9) 73 (7) 76 (8) 78 (7) 70 (8) 74 (9) |
Total = 54.4% |
448 (415) 667 (664) 1220 (1026) 622 (1217) 1059 (1207) 928 (1056) 503 (374) 807 (1237) |
MMSE | Age, sex | ||
| Sun et al., 2016 | Ng | Electrochemiluminescence (Meso Scale Discovery, USA) |
ApoE ε4 carriers: AD (67) MCI (102) CU (27) |
75 (8) 74 (8) 76 (5) |
42 (44%) 64 (33%) 55 (50%) |
N.R | MMSE | None | ||
| Swanson et al., 2016 |
NPTX2 |
Mass spectrometry |
AD (64) MCI (135) CU (86) |
74.98 (7.57) 74.69 (7.35) 75.70 (5.54) |
29 (45.3%) 44 (32.6%) 42 (48.8%) |
Mean ± SE 10.31 ± 0.09 10.62 ± 0.06 10.70 ± 0.08 |
MMSE, ADAS‐Cog, memory composite |
Age, sex, education, ApoE status |
||
| Teitsdottir et al., 2020 | NfL | ELISA (UmanDiagnostics, Sweden) |
AD CSF profile (28) SCI (2) MCI (9) AD (16) DLB (1) Non‐AD CSF profile SCI (10) MCI (13) DLB (1) |
Median (range) 70 (51‐84) 67 (46‐80) |
11 (39.3%) 8 (80%) |
Median (range) 2500 (1200 – 4500) 1900 (900 – 6500) |
Verbal episodic memory composite | Age, education | ||
| Van Der Ende et al., 2020 |
NPTX2 |
ELISA (in‐house) |
Symptomatic genetic FTD (54) Presymptomatic genetic FTD (106) |
Median [IQR] 63 [56‐69] 45 [34‐56] |
22 (40.7%) 59 (55.7%) |
Median [IQR] 643 [301‐872] 1003 [624‐1358] |
MMSE, TMT‐B, phonemic verbal fluency |
Age, sex, years of education, study site |
||
| Van Steenoven et al., 2020 |
NPTX2 NPTXR |
Mass spectrometry Mass spectrometry |
Cohort 1: DLB (20) Cohort 2: DLB (17) Cohort 3: DLB (48) |
65.3 (5.8) 66.9 (7.5) 67.8 (6.3) |
3 (15%) 4 (24%) 6 (12%) |
N.R | MMSE | Cohort | ||
|
Wang et al., 2019 |
Ng |
Electrochemiluminescence (Meso Scale Discovery, USA) |
AD (81) MCI (171) CU (99) |
74.6 (7.8) 74.2 (7.6) 75.5 (5.3) |
37 (45.7%) 58 (33.9%) 49 (49.5%) |
Median [IQR] 471 [347‐675] 455 [267‐657] 324 [191‐468] |
MMSE |
None |
||
| Wang et al., 2018 | SNAP‐25 | ELISA (Erenna, USA) |
AD (16) MCI (75) CU (55) |
73.4 (6.8) 74.3 (6.5) 76 (5) |
10 (62.5%) 21 (28%) 24 (43.6%) |
N.R | MMSE | None | ||
|
Wellington et al., 2016 |
Ng |
Electrochemiluminescence (in‐house) |
AD (100) Genetic AD (2) bvFTD (20) svFTD (21) LBD (13) PSP (46) CU (19) |
Median [IQR] 63 [57‐68] 43, 47 61 [57‐69] 69 [61‐73] 68 [66‐76] 70 [66‐72] 61 [50‐64] |
59 (59%) 2 (100%) 8 (40%) 10 (48%) 2 (15%) 19 (41%) 11 (58%) |
Median [IQR] 463 [275‐669] 252, 1162 150 [120‐317] 244 [138‐426] 120 [120‐304] 188 [120‐302] 196 [120‐297] |
MMSE |
None |
||
|
Xiao et al., 2017 |
NPTX2 |
ELISA (in‐house) |
AD (30) |
Mean ± SE 72.24 ± 10.15 |
16 (53.3%) |
Mean ± SE 716.12 ± 388.22 |
MMSE, DSS, BNT, phonemic verbal fluency, semantic verbal fluency, Wisconsin card sorting task, visual reproduction test, block design, CDT, CVLT |
None |
||
|
Zetterberg et al., 2016 |
NfL |
ELISA (UmanDiagnostics, Sweden) |
AD (95) pMCI (101) sMCI (91) CU (110) |
Median [IQR] 76 [69‐80] 74 [69‐80] 74 [71‐80] |
42 (44.2%) 37 (36.6%) 26 (28.6%) |
Median [IQR] 1479 [1134‐1842] 1336 [1061‐1693] 1182 [923‐1687] |
MMSE, ADAS‐Cog |
Age, sex, education |
||
| Zhang et al., 2018a | VILIP‐1 | ELISA (Erenna, USA) |
AD (18) sMCI (24 pMCI (47) CU (32) |
74.3 (6.79) 76.7 (5.34) 73.1 (6.86) 76 (5.66) |
11 (61.1%) 7 (29.2%) 14 (29.8%) 13 (40.6%) |
189.7 (70.43) 146 (51.93) 184.3 (64.44) 133.0 (37.9) |
MMSE | Age, sex, education | ||
| Zhang et al., 2018b | SNAP‐25 | ELISA (Erenna USA) |
AD (18) sMCI (22) pMCI (47) CU (52) |
74.3 (7) 76 (5.1) 73.1 (6.6) 76.2 (5.1) |
11 (61.1%) 7 (31.8%) 14 (29.8%) 22 (42.3%) |
N.R | MMSE1 | Age, sex, education | ||
Age and CSF levels presented as mean (SD) unless otherwise specified
ACE‐CZ, Addenbrooke’s Cognitive Examination‐ Czech Version; AD, Alzheimer’s Disease; ADAS‐Cog, Alzheimer Disease Assessment Scale cognitive subscale; ALS, Amyotrophic Lateral Sclerosis; ANI, Asymptomatic Neurocognitive Impairment; ANT, Animal Naming Test; ApoE, Apolipoprotein E; AVLT, Rey Auditory Verbal Learning Test; Aβ‐, Amyloid beta negative; Aβ+, Amyloid beta positive; BMDB, Brief Mental Deterioration Battery; BNT, Boston Naming Test; BSRT, Buschke Selective Reminding Test; bvFTD, Behaviour Variant FTD; BVMT‐R, Brief Visuospatial Memory Test‐ Revised; BVMT, Brief Visuospatial Memory Test; CAMCOG, Cambridge Cognitive Examination; CBS, Corticobasal Syndrome; CDT, Clock Drawing Test; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CJD, Creutzfeldt‐Jacob Disease; COWAT, Controlled Oral Word Association Test; CPAL, Continuous Paired Associate Learning; CU, Cognitive unimpaired; CVLT, California Verbal Learning Test; DIAN, Dominantly Inherited Alzheimer Network; DKEFS, Delis‐Kaplan Executive Function System; DLB, Dementia with Lewy Bodies; DSB, Digit Span Backwards; DSF, Digit Span Forwards; DSS, Digit Symbol Substitution; EAD, Early onset Alzheimer’s Disease; FAB, Frontal Assessment Battery; FTD, Frontotemporal Dementia; GMCT, Groton Maze Times Chase Test; GML, Groton Maze Learning Test; GMR, Groton Maze Learning Test delayed recall; HAD, HIV‐Associated Dementia; lvPPA, logopenic variant Primary Progressive Aphasia; MCCB, MATRICS Consensus Cognitive Battery; MCI‐AD, Mild Cognitive Impairment due to Alzheimer’s Disease; MCI‐o, Mild Cognitive Impairment not due to Alzheimer’s Disease; MCI, Mild Cognitive Impairment; MIX, Mixed Dementia; MMSE, Mini Mental State Examination; MNCD, Mild Neurocognitive Disorder; MND, Motor Neuron Disease; MSA, Multiple System Atrophy; NfL, Neurofilament‐Light; nfvPPA, non‐fluent variant Primary Progressive Aphasia; Ng, Neurogranin; NOS, Not Otherwise Specified; OCL, One‐Card Learning; ONB, One‐Back Memory; PASAT, Paced Auditory Serial Addition Test; PCA, Posterior Cortical Atrophy; PD, Parkinson’s Disease; PDD, Parkinson’s Disease Dementia; pDLB, Prodromal Dementia with Lewy Bodies; pMCI, progressive MCI; pNfH, Phosphorylated Neurofilament Heavy; PSP, Progressive Supranuclear Palsy; PVLT, Philadelphia Verbal Learning Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SCWT, Stroop Color Word Test; sMCI, stable MCI; SVD, Small Vessel Disease; svPPA, Semantic Variant Primary Progressive Aphasia; TMT‐ B, Trail Making Test B; TMT‐A, Trail Making Test A; TWOB, Two‐Back Memory; VaD, Vascular Dementia; WAIS, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Test; WE, Wernicke’s Encephalopathy
Sample sizes of included studies ranged from 19 to 770. Only one of the included studies conducted a power analysis (Xiao et al., 2017), although others acknowledged a possible lack of power.
3.2.2. Sociodemographic factors
Participants with AD were aged between 62 and 77 years, those with FTD were aged between 59 and 72 years and MCI participants' age ranged from 62 to 76 years. The age ranges of participants are within the typical range for the detection of dementia/MCI‐related cognitive decline. Those with an ‘other’ form of dementia were aged between 39.5 and 76.7 years. CU participants' age varied widely (between 37.8 and 81.9) due to the nature of the healthy ageing groups; some findings were taken from studies investigating neurodegenerative diseases with age‐matched controls, while few focused solely on CU younger participants. Most studies included a mix of both males and females.
3.2.3. Group status and dementia definitions
As reported in Table 1, 46 studies included participants with AD or MCI, 15 examined those with an FTD‐related syndrome, 39 examined controls or CU samples and 9 studies included those with an ‘other’ dementia. All studies used validated criteria for diagnosing dementia, MCI or identifying the absence of dementia. In AD, most studies used the National Institute of Neurological and Communicative Disorders and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) (McKhann et al., 1984) criteria to diagnose probable AD and others used the updated National Institute on Ageing/Alzheimer's Association (NIA‐AA) criteria (Jack et al., 2018). One study used The International Working Group 2 (IWG‐2) (Dubois et al., 2014) criteria, and in five, diagnoses were made by clinicians (which were supplemented with CSF marker information in two). To confirm familial AD, one study used autopsy and medical records matched with NINCDS‐ADRDA criteria, while another used autopsy records and the Kawas Dementia Questionnaire (Kawas et al., 1994). Studies with MCI patients used established criteria proposed by the IWG‐2 (Winblad et al., 2004), NIA‐AA criteria (Albert et al., 2011) or criteria proposed by Petersen and colleagues (Petersen, 2004). Two studies used criteria for early MCI proposed by Aisen and colleagues (Aisen et al., 2010) which defines early MCI as a milder episodic memory impairment relative to ‘late MCI’. All FTD studies used established criteria for the relevant subsyndrome, which were appropriate for the time of publication (Armstrong et al., 2013; Gorno‐Tempini et al., 2011; Litvan et al., 1996; Neary et al., 1998; Rascovsky et al., 2011). Most CU studies ruled out dementia or cognitive impairment if participants had a Clinical Dementia Rating (CDR) of 0 or did not meet DSM‐III‐R criteria.
3.2.4. Adjustment factors
As seen in Table 1, adjustment factors varied between studies. Thirty‐four studies did not adjust for any covariates. One study conducted a partial correlation and adjusted for multiple cohorts (van Steenoven et al., 2020). Studies using regression techniques most often controlled for age, sex and years of education. Nine studies controlled for ApoE e4 status, and two controlled for ethnicity.
3.2.5. Cognitive assessments
A number of tools were used to assess neuropsychological performance (Table 1). Including composite measures as single tests, there were 37 different cognitive tests analysed across all 67 studies. The most commonly used test was the Mini Mental State Examination (MMSE) (Folstein et al., 1975), which was employed in 48 studies. The main domains assessed were global cognition, visuospatial abilities, language, attention, general executive functions and memory (working, episodic and semantic).
3.2.6. Risk of bias
Risk of bias ratings is provided in the supporting information Table S2. Twenty studies were rated as ‘Good’, 45 rated as ‘Fair’ and 3 as ‘Poor’.
3.2.7. CSF markers
As reported in Table 1, most studies assayed multiple markers. Thirty‐one studies examined NfL, 22 examined Ng and 24 studies examined a different marker of interest. A description of each marker can be found in Table 2.
TABLE 2.
Summary of CSF markers from included studies
| CSF marker | Function | Localization |
|---|---|---|
| Alpha‐Synuclein | Regulation of synaptic vesicle trafficking | Pre‐synaptic |
| Beta‐Synuclein | Unknown | Pre‐synaptic |
| Contactin‐2 |
Axonal guidance Axonal fasciculation |
Pre‐synaptic Axonal |
| GAP‐43 |
Axonal outgrowth Synaptic plasticity |
Pre‐synaptic |
| NfH | Neuronal structure | Axonal |
| NfL | Neuronal structure | Axonal |
| Ng |
Calmodulin‐binding LTP signalling |
Post‐synaptic |
| NPTX1 |
Synaptic plasticity Facilitates excitatory synapse formation |
Pre‐synaptic |
| NPTX2 |
Synaptic plasticity Facilitates excitatory synapse formation |
Pre‐synaptic |
| NPTXR |
Synaptic plasticity Facilitates excitatory synapse formation |
Trans‐synaptic |
| Nrg1 | Synaptic plasticity | Pre‐synaptic |
| SNAP‐25 | SNARE | Pre‐synaptic |
| Synaptotagmin | Calcium sensor | Pre‐synaptic |
| VILIP‐1 | Calcium sensor | Neuronal |
Abbreviations: CSF, cerebrospinal fluid; GAP‐43, growth‐associated protein 43; NfH, neurofilament‐heavy; NfL, neurofilament‐light; Ng, neurogranin; NPTX1, neuronal pentraxin I; NPTX2, neuronal pentraxin 2; NPTXR, neuronal pentraxin receptor; Nrg1, neuregulin‐1; SNAP‐25, synaptosomal‐associated protein 25; VILIP‐1, visinin‐like protein‐1.
A number of immunoassay methods were used to measure CSF analytes. Enzyme‐linked immunosorbent assays (ELISAs) were the most common immunoassay method, followed by electrochemiluminescence and mass‐spectrometry based methods. Two studies used SIMOA assays. Of the included 67 studies, only 29 reported the intra‐assay coefficient of variability (CV) (Abu‐Rumeileh et al., 2018; Bartos et al., 2011; Bendlin et al., 2012; Bjerke et al., 2009; Brinkmalm et al., 2014; Casaletto et al., 2017; Chatterjee, Del Campo, et al., 2018; Dhiman et al., 2020; Gifford et al., 2018; Hellwig et al., 2015; Hoglund et al., 2017; Kirsebom et al., 2018; Kvartsberg, Duits, et al., 2015; Lim et al., 2019; Meeter et al., 2016; Meeter, Gendron, et al., 2018; Meeter et al., 2019; Meeter, Vijverberg, et al., 2018; Mielke, Syrjanen, Blennow, Zetterberg, Vemuri, et al., 2019; Öhrfelt et al., 2019; Osborn et al., 2019; Rolstad, Jakobsson, et al., 2015; Sandelius et al., 2019; Skillback et al., 2014; Teitsdottir et al., 2020; van der Ende et al., 2020; Wellington et al., 2016; Zetterberg et al., 2016) and only 22 reported inter‐assay CVs (Abu‐Rumeileh et al., 2018; Bartos et al., 2011; Bjerke et al., 2009; Brinkmalm et al., 2014; Chatterjee, Del Campo, et al., 2018; Dhiman et al., 2020; Hellwig et al., 2015; Hoglund et al., 2017; Kvartsberg et al., 2015; Meeter et al., 2016; Meeter, Gendron, et al., 2018; Meeter et al., 2019; Meeter, Vijverberg, et al., 2018; Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al., 2019; Mielke, Syrjanen, Blennow, Zetterberg, Vemuri, et al., 2019; Mouton‐Liger et al., 2020; Rolstad, Jakobsson, et al., 2015; Sandelius et al., 2019; Singh et al., 2016; Teitsdottir et al., 2020; van der Ende et al., 2020); therefore, the repeatability and technical heterogeneity of results was not reported in the majority of studies.
3.3. Main outcome: Associations between CSF markers and neuropsychological performance
3.3.1. Papers on CSF NfL
In total, 31 studies examined the relationship between CSF NfL levels and neuropsychological performance. All studies analysed CSF NfL using ELISAs.
As reported in Table 3, a significant association between CSF NfL and neuropsychological performance was consistently reported in AD samples. Most studies found significant moderate‐to‐weak relationships with MMSE scores (Abu‐Rumeileh et al., 2018; Bos et al., 2019; Delaby et al., 2020; Sjogren et al., 2000; Skillback et al., 2014; Zetterberg et al., 2016), while others showed no relationship (Bartos et al., 2011; de Jong et al., 2007; Rolstad, Berg, et al., 2015). However, sample sizes were relatively small in two of these studies. Only two studies included early‐onset Alzheimer's (EAD) samples, and both reported no significant associations with MMSE scores (de Jong et al., 2007; Skillback et al., 2014).
TABLE 3.
Summary of results
| Study | CSF marker | Population (N) | Cognitive assessment and direction of relationship ( ‐positive relationship, ‐positive relationship,  ‐ negative, * non‐significant; non‐adjusted results reported where available) ‐ negative, * non‐significant; non‐adjusted results reported where available) |
|
|---|---|---|---|---|
| Abu‐Rumeileh et al. (2018) | NfL | AD (60) | MMSE |

|
|
FTD (141) |
BMDB |

|
||
| FAB |

|
|||
| MMSE | * | |||
| Agnello et al. (2020) | Ng | AD (29) | MMSE |

|
| Alpha‐synuclein | AD (29) | MMSE | * | |
| Alcolea et al. (2017) | NfL | FTD (249) | MMSE |

|
| Aschenbrenner et al. (2020) | NfL |
CU Aβ + (94) CU Aβ‐ (161) |
Global cognition composite | * |
| Episodic memory composite |

|
|||
| Attention composite | * | |||
| Bartos et al. (2011) | NfL | AD (25) | MMSE (derived from ACE‐CZ) | * |
| ACE‐CZ | * | |||
| PSP, FTD, CJD, CBS, WE (13) | MMSE (derived from ACE‐CZ) | * | ||
| ACE‐CZ | * | |||
| Begcevic et al. (2018) | NPTX1 |
Cohort 1 (58): MCI (8) Mild AD (11) Moderate AD (24) Severe AD (15) |
MMSE | * |
|
Cohort 2 (43): MCI (6) Mild AD (8) Moderate AD (16) Severe AD (15) |
MMSE | * | ||
| NPTXR |
Cohort 1 (58): MCI (8) Mild AD (11) Moderate AD (24) Severe AD (15) |
MMSE | * | |
|
Cohort 2 (43): MCI (6) Mild AD (8) Moderate AD (16) Severe AD (15) |
MMSE | * | ||
| Bendlin et al. (2012) | NfL | CU with family history of AD (43) | BVMT | * |
| COWAT | * | |||
| TMT‐A | * | |||
| TMT‐B | * | |||
| WAIS‐working memory index | * | |||
| AVLT | * | |||
| Bjerke et al. (2009) | NfL |
MCI‐SVD (9) MCI‐MD (15) MCI‐MCI (118) MCI‐AD (20) CU (52) |
MMSE | * |
| Boiten et al. (2021) | NPTX2 | AD (20) | Global composite | * |
| Memory composite | * | |||
| Attention composite | * | |||
| Executive function composite | * | |||
| Language composite | * | |||
| Visuospatial composite | * | |||
| MMSE | * | |||
| DLB (48) | Global composite |

|
||
| Memory composite | * | |||
| Attention composite |

|
|||
| Executive function composite | * | |||
| Language composite | * | |||
| Visuospatial composite | * | |||
| MMSE | * | |||
| Bos et al. (2019) | NfL | Total Aβ + (465) | MMSE |

|
| Total Aβ‐ (305) | * | |||
| AD (180) |

|
|||
| MCI (450) | * | |||
| CU (140) | * | |||
| Ng |
Total Aβ + (465) Total Aβ‐ (305) |
MMSE |
* |
|
| AD (180) |

|
|||
| MCI (450) | * | |||
| CU (140) | * | |||
| Brinkmalm et al. (2014) | SNAP‐25 | AD (36) | MMSE |

|
| CU (33) | MMSE | * | ||
| Bruno et al. (2020) | Alpha‐Synuclein | CU (19) | BSRT | * |
| Ng | CU (19) | BSRT | * | |
| Casaletto et al. (2017) | Ng | CU with family history of dementia (132) | AVLT |

|
| WAIS‐III symbol digit coding |

|
|||
| BNT | * | |||
| WAIS‐III DSF | * | |||
| WAIS‐III DSB | * | |||
| Chatterjee, Del Campo, et al. (2018) | Contactin‐2 | Total sample (154) | MMSE |

|
| AD (106) | MMSE | * | ||
| CU (48) | MMSE | * | ||
| De Vos et al. (2016) | Ng | AD (50) | MMSE | * |
| MCI (38) | MMSE | * | ||
| De de Jong et al. (2007) | NfL | EAD (37) | MMSE | * |
| AD (33) | MMSE | * | ||
| DLB (18) | MMSE | * | ||
| FTD (28) | MMSE | * | ||
| Delaby et al. (2020) | NfL | CU (118) | MMSE |

|
| AD (116) | MMSE |

|
||
| FTD (56) | MMSE |

|
||
| DLB (37) | MMSE | * | ||
| pDLB (26) | MMSE |

|
||
| PSP (12) | MMSE | * | ||
| CBS (26) | MMSE | * | ||
| Dhiman et al. (2020) | NfL |
Total sample (221) AD (28) MCI (34) CU (159) |
MMSE |

|
| Galasko et al. (2019) | Ng | Total AD, MCI, CU (193) |
CVLT immediate recall CVLT delayed recall |
|
| Aβ/tau+ |
CVLT immediate recall CVLT delayed recall |
|
||
| Aβ/tau‐ |
CVLT immediate recall CVLT delayed recall |
|
||
| NPTX2 | Total AD, MCI, CU (193) |
CVLT immediate recall CVLT delayed recall |
|
|
| Aβ/tau+ |
CVLT immediate recall CVLT delayed recall |
|
||
| Aβ/tau‐ |
CVLT immediate recall CVLT delayed recall |
* |
||
| SNAP‐25 | Total AD, MCI, CU (193) |
CVLT immediate recall CVLT delayed recall |
*
|
|
| Aβ/tau+ |
CVLT immediate recall CVLT delayed recall |
*
|
||
| Aβ/tau‐ |
CVLT immediate recall CVLT delayed recall |
* * |
||
| Gifford et al. (2018) | NfL |
Early MCI (9) MCI (37) CU (65) |
PVLT List Total learning |
* |
| Short delay free recall | * | |||
| Short delay cued recall | * | |||
| Long delay free recall | * | |||
| Long delay cued recall |

|
|||
| Discrimination | * | |||
| CU (65) |
PVLT List Total learning |
|
||
| Short delay free recall |

|
|||
| Short delay cued recall |

|
|||
| Long delay free recall |

|
|||
| Long delay cued recall |

|
|||
| Discrimination |

|
|||
| Headley et al. (2018) | Ng |
MCI (193) |
Memory composite |

|
| Executive function composite |

|
|||
| CU (111) | Memory composite | * | ||
| Executive function composite | * | |||
| Total (304) | MMSE |

|
||
| ADAS‐cog |
|
|||
| ADAS‐Cog13 |
|
|||
| Memory composite |

|
|||
| Executive function composite | * | |||
| Hellwig et al. (2015) | Ng | AD + MCI‐AD (53) | MMSE | * |
| Non‐AD dementia + MCI‐o (43) | MMSE | * | ||
| Hoglund et al. (2017) |
NfL |
CU Aβ‐ (43) | MMSE | * |
| CU Aβ + (86) | MMSE | * | ||
| Ng | CU Aβ‐ (43) | MMSE | * | |
| CU Aβ + (86) | MMSE | * | ||
| VILIP‐1 | CU Aβ‐ (43) | MMSE | * | |
| CU Aβ + (86) | MMSE | * | ||
| Jia et al. (2020) | Ng |
Discovery cohort AD (28) |
MMSE |

|
| Validation cohort (73) |

|
|||
| GAP‐43 |
Discovery cohort AD (28) |
MMSE |

|
|
| Validation cohort (73) |

|
|||
| SNAP‐25 |
Discovery cohort AD (28) |
MMSE |

|
|
| Validation cohort (73) |

|
|||
| Synaptotagmin‐1 |
Discovery cohort AD (28) |
MMSE |

|
|
| Validation cohort (73) |

|
|||
| Kirsebom et al. (2018) | Ng |
Aβ + MCI (20) Aβ + SCI (18) CU (36) |
MMSE | * |
| CERAD word list test | * | |||
| TMT‐A | * | |||
| TMT‐B | * | |||
| Kvartsberg, Duits, et al. (2015) | Ng | MCI (40) | MMSE | * |
| Lee et al. (2008) | VILIP‐1 | AD (33) | MMSE |

|
| Lim et al. (2019) | NPTXR |
MCI (14) Mild AD (21) Moderate AD (43) Severe AD (30) |
MMSE |
|
| Mattsson et al. (2016) |
NfL |
Aβ + AD, MCI, CU (262) | MMSE | * |
| ADAS‐Cog11 |

|
|||
| Aβ‐ AD, MCI, CU (127) | MMSE |

|
||
| ADAS‐Cog11 |

|
|||
| Ng | Aβ + AD, MCI, CU (262) | MMSE | * | |
| ADAS‐Cog11 | * | |||
| Aβ‐ AD, MCI, CU (127) | MMSE | * | ||
| ADAS‐Cog11 | * | |||
| McGuire et al. (2015) | NfL |
HAD (3) ANI (15) MNCD (15) CU (15) |
WAIS‐III digit symbol | * |
| WAIS‐III symbol search | * | |||
| TMT‐A | * | |||
| Story memory test | * | |||
| Figure memory test | * | |||
| WCST | * | |||
| TMT‐B | * | |||
| COWAT | * | |||
| ANT | * | |||
| WAIS‐III letter‐number sequencing | * | |||
| PASAT | * | |||
| pNfH |
HAD (3) ANI (15) MNCD (15) CU (15) |
WAIS‐III digit symbol |

|
|
| WAIS‐III symbol search |

|
|||
| TMT‐A |

|
|||
| Story memory test |

|
|||
| Figure memory test |

|
|||
| WCST | * | |||
| TMT‐B | * | |||
| COWAT | * | |||
| ANT | * | |||
| WAIS‐III letter‐number sequencing | * | |||
| PASAT | * | |||
| Meeter et al. (2016) | NfL | FTD with GRN, MAPT, C9orf72 mutation (101) | MMSE | * |
| Meeter, Gendron, et al. (2018) | NfL | FTD with C9orf72 mutation (64) | MMSE |

|
| Presymptomatic carriers of C9orf72 mutation (25) | MMSE | * | ||
| Total (89) | MMSE |

|
||
| Meeter et al. (2019) | NfL | svPPA (147) | BNT |

|
| ANT | * | |||
| Letter fluency | * | |||
| WAIS‐III DSF | * | |||
| WAIS‐III DSB | * | |||
| TMT‐A |

|
|||
| TMT‐B |

|
|||
| SCWT | * | |||
| CDT | * | |||
| AVLT | * | |||
| CVLT | * | |||
| CERAD word list test | * | |||
| Rey complex figure test | * | |||
| Meeter, Vijverberg, et al. (2018) | NfL | bvFTD (164) | MMSE | * |
| FAB |

|
|||
| svPPA (36) | MMSE | * | ||
| FAB | * | |||
| nfvPPA (19) | MMSE | * | ||
| FAB | * | |||
| lvPPA (4) | MMSE | * | ||
| FAB | * | |||
| CBS (40) | MMSE |

|
||
| FAB | * | |||
| PSP (58) | MMSE | * | ||
| FAB | * | |||
| Total sample (including FTD‐MND; | MMSE |

|
||
| FAB |

|
|||
| Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al. (2019) | NfL |
Dementia (7) MCI (83) |
Global composite | * |
| Memory composite |

|
|||
| Language composite | * | |||
| Attention composite | * | |||
| Visuospatial composite | * | |||
|
CU (687) |
Global composite |

|
||
| Memory composite |

|
|||
| Language composite |

|
|||
| Attention composite |

|
|||
| Visuospatial composite |

|
|||
| Total (777) | Global composite |

|
||
| Memory composite |

|
|||
| Language composite |

|
|||
| Attention composite |

|
|||
| Visuospatial composite |

|
|||
| Ng |
Dementia (7) MCI (83) |
Global composite | * | |
| Memory composite |

|
|||
| Language composite | * | |||
| Attention composite |

|
|||
| Visuospatial composite | * | |||
| CU (687) | Global composite | * | ||
| Memory composite | * | |||
| Language composite | * | |||
| Attention function composite | * | |||
| Visuospatial composite | * | |||
| Total (777) | Global composite |

|
||
| Memory composite |

|
|||
| Language composite |

|
|||
| Attention composite | * | |||
| Visuospatial composite |

|
|||
| Mielke, Syrjanen, Blennow, Zetterberg, Vemuri, et al. (2019) | NfL |
MCI (15) CU (64) Total (79) |
Global composite | * |
| Memory composite | * | |||
| Language composite | * | |||
| Attention composite | * | |||
| Visuospatial composite | * | |||
| Mouton‐Liger et al. (2020) | Nrg1 | AD (54) | MMSE |

|
| MCI‐AD (20) | MMSE |

|
||
|
Total: AD (54) MCI‐AD (20) Non‐AD dementia (30) Non‐AD MCI (31) CU (27) |
MMSE |

|
||
| Oeckl et al. (2020) | Beta‐synuclein | Cohort 1: AD (64) | MMSE |

|
| Cohort 2: AD (40) | MMSE | * | ||
| Cohort 3: AD (49) | MMSE | * | ||
| Öhrfelt et al. (2016) | Synaptotagmin | Cohort 1: AD (17) | MMSE | * |
| Cohort 2: AD (24) | MMSE | * | ||
| Cohort 1: MCI‐AD (5) | MMSE | * | ||
| Cohort 2: MCI‐AD (18) | MMSE | * | ||
| Cohort 1: CU (17) | MMSE | * | ||
| Cohort 2: CU (36) | MMSE | * | ||
| Öhrfelt et al. (2019) | SNAP‐25 | Cohort 1: AD (17) | MMSE | * |
| Cohort 2: AD (24) | MMSE | * | ||
| Cohort 1: MCI‐AD (5) | MMSE | * | ||
| Cohort 2: MCI‐AD (18) | MMSE | * | ||
| Cohort 1: CU (17) | MMSE | * | ||
| Cohort 2: CU (36) | MMSE | * | ||
| Osborn et al. (2019) | NfL |
Early MCI (27) MCI (132) |
Episodic memory composite | * |
| Executive function composite | * | |||
| BNT | * | |||
| ANT |

|
|||
| WAIS‐IV coding | * | |||
| DKEFS number sequencing | * | |||
| Hooper visual organisation test | * | |||
| CU (174) | Episodic memory composite |

|
||
| Executive function composite | * | |||
| BNT | * | |||
| ANT | * | |||
| WAIS‐IV coding | * | |||
| DKEFS number sequencing | * | |||
| Hooper visual organisation test | * | |||
| Total (333) | Episodic memory composite |

|
||
| Executive function composite | * | |||
| BNT | * | |||
| ANT |

|
|||
| WAIS‐IV coding | * | |||
| DKEFS number sequencing | * | |||
| Hooper visual organisation test | * | |||
| Portelius et al. (2015) | Ng | AD (95) | MMSE | * |
| ADAS‐cog | * | |||
|
pMCI (105) |
MMSE | * | ||
| ADAS‐cog | * | |||
|
sMCI (68) |
MMSE | * | ||
| ADAS‐cog | * | |||
| CU (110) | MMSE | * | ||
| ADAS‐cog | * | |||
| Racine et al. (2016) | NfL |
MCI + CU (70) |
CPAL errors‐ visual memory |

|
| GMCT moves/sec ‐speed of visual processing |

|
|||
| GML errors | * | |||
| GMR errors | * | |||
| OCL accuracy | * | |||
| ONB accuracy | * | |||
| TWOB accuracy | * | |||
| AVLT delayed |

|
|||
| Logical memory delayed |

|
|||
| BVMT‐R delayed |

|
|||
| Rojas et al. (2018) | NfL | PSP (50) | RBANS | * |
| Colour trails 1 | * | |||
| Colour trails 2 |

|
|||
| Letter‐number sequencing |

|
|||
| Phonemic fluency | * | |||
| Rolstad, Berg, et al. (2015) | NfL | Dementia‐ vascular (65) | Attention composite | * |
| Learning/memory composite | * | |||
| Visuospatial composite | * | |||
| Language composite | * | |||
| Executive function composite | * | |||
| Dementia‐ non‐vascular (128) | Attention composite | * | ||
| Learning/memory composite | * | |||
| Visuospatial composite | * | |||
| Language composite |

|
|||
| Executive function composite | * | |||
| MCI‐ vascular (86) | Attention composite |

|
||
| Learning/memory composite | * | |||
| Visuospatial composite | * | |||
| Language composite | * | |||
| Executive function composite |

|
|||
| MCI‐ non‐vascular (175) | Attention composite | * | ||
| Learning/memory composite |

|
|||
| Visuospatial composite | * | |||
| Language composite | * | |||
| Executive function composite | * | |||
| SCI‐ vascular (48) | Attention composite |

|
||
| Learning/memory composite | * | |||
| Visuospatial composite |

|
|||
| Language composite | * | |||
| Executive function composite |

|
|||
| SCI‐ non‐vascular (120) | Attention composite | * | ||
| Learning/memory composite | * | |||
| Visuospatial composite | * | |||
| Language composite | * | |||
| Executive function composite | * | |||
| Rolstad, Jakobsson, et al. (2015) | NfL | CU (71) | Memory composite | * |
| Executive functions composite | * | |||
| Visuospatial composite | * | |||
| Attention composite | * | |||
| Verbal functions composite | * | |||
| Sancesario et al. (2020) | Ng | CU (30) | MMSE | * |
| Sandelius et al. (2019) | GAP‐43 | AD (275) | MMSE | * |
| MCI (84) | MMSE | * | ||
| CU (43) | MMSE | * | ||
| FTD (39) | MMSE | * | ||
| DLB (27) | MMSE | * | ||
| lvPPA (10) | MMSE | * | ||
| svPPA (15) | MMSE | * | ||
| PSP (18) | MMSE | * | ||
| CBS (19) | MMSE | * | ||
| Total sample (662; CU, MCI, AD, ALS, FTD, PD, PD‐MCI, PD‐dementia, DLB, lvPPA, svPPA, PSP, CBS, PCA) | MMSE |

|
||
| Sanfilippo et al. (2016) | Ng | AD (25) | MMSE |

|
| CAMCOG | * | |||
| MCI (50) | MMSE | * | ||
| CAMCOG | * | |||
| MCI‐AD (36) | MMSE | * | ||
| CAMCOG | * | |||
| CU (44) | MMSE | * | ||
| CAMCOG | * | |||
| Santillo et al. (2019) | Ng | CU (20) | MCCB | * |
| Scherling et al. (2014) | NfL |
Total: Asymptomatic FTD mutation carriers (8) bvFTD (45) nfvPPA (18) svPPA (16) CBS (17) AD (50) PSP (22) CU (47) |
MMSE | * |
| Rey‐Osterrieth figure | * | |||
| DSF | * | |||
| DSB |

|
|||
| TMT | * | |||
| Stroop colour naming task |

|
|||
| BNT |

|
|||
| ANT |

|
|||
| CVLT |

|
|||
| Phonemic fluency |

|
|||
| bvFTD (45) | MMSE |

|
||
| Rey‐Osterrieth figure | * | |||
| DSF | * | |||
| DSB |

|
|||
| TMT | * | |||
| Stroop colour naming task |

|
|||
| BNT | * | |||
| ANT |

|
|||
| CVLT |

|
|||
| Phonemic fluency |

|
|||
| nfvPPA (18) | MMSE | * | ||
| Rey‐Osterrieth figure | * | |||
| DSF | * | |||
| DSB | * | |||
| TMT | * | |||
| Stroop colour naming task | * | |||
| BNT |

|
|||
| ANT | * | |||
| CVLT | * | |||
| Phonemic fluency | * | |||
| Schindler et al. (2019) | Ng | Carriers of mutations in PSEN1, PSEN2, or APP (235) | DIAN cognitive composite |

|
| Mutation non‐carriers (145) | DIAN cognitive composite | * | ||
| SNAP‐25 | Carriers of mutations in PSEN1, PSEN2, or APP (235) | DIAN cognitive composite |

|
|
| Mutation non‐carriers (145) | DIAN cognitive composite | * | ||
| VILIP‐1 | Carriers of mutations in PSEN1, PSEN2, or APP (235) | DIAN cognitive composite |

|
|
| Mutation non‐carriers (145) | DIAN cognitive composite | * | ||
| Sjogren et al. (2001) | NfL | Insignificant white matter changes (61; AD, SVD, CU) | MMSE |

|
| Extensive white matter changes (14; AD, SVD, CU) | MMSE | * | ||
| Sjogren et al. (2000) | NfL | FTD (18) | MMSE |

|
| AD (21) | MMSE |

|
||
| Skillback et al. (2014) | NfL | EAD (223) | MMSE | * |
| AD (1194) | MMSE |

|
||
| FTD (146) | MMSE | * | ||
| DLB (114) | MMSE | * | ||
| VaD (465) | MMSE | * | ||
| MIX (517) | MMSE |

|
||
| PDD (45) | MMSE | * | ||
| Dementia NOS (437) | MMSE | * | ||
| Total (3103) | MMSE |

|
||
| Sun et al. (2016) | Ng |
ApoE ε4 carriers: AD (67) MCI (102) CU (27) |
MMSE |

|
| Swanson et al. (2016) | NPTX2 |
Total: AD (64) MCI (135) CU (86) |
MMSE |

|
| ADAS‐Cog11 |

|
|||
| Memory composite |

|
|||
| Teitsdottir et al. (2020) | NfL |
AD CSF profile (28): SCI (2) MCI (9) AD (16) DLB (1) |
Verbal episodic memory composite |

|
|
Non‐AD CSF profile (14): SCI (10) MCI (13) DLB (1) |
Verbal episodic memory composite | * | ||
| van der Ende et al., (2020) | NPTX2 |
Symptomatic genetic FTD (54) |
MMSE |

|
| TMT‐B |

|
|||
| Phonemic verbal fluency | * | |||
| Presymptomatic genetic FTD (106) | MMSE | * | ||
| TMT‐B | * | |||
| Phonemic verbal fluency | * | |||
| van Steenoven et al. (2020) | NPTX2 | DLB (85) | MMSE |

|
| NPTXR | DLB (85) | MMSE |

|
|
| Wang (2019) | Ng | AD (81) | MMSE | * |
| MCI (171) | MMSE | * | ||
| CU (99) | MMSE | * | ||
| Total (351) | MMSE |

|
||
| Wang, Zhou, and Zhang (2018) | SNAP‐25 |
AD (16) MCI (75) CU (55) |
MMSE |

|
| Wellington et al. (2016) | Ng | AD (100) | MMSE | * |
| bvFTD (20) | MMSE | * | ||
| svFTD (21) | MMSE | * | ||
| DLB (13) | MMSE | * | ||
| PSP (46) | MMSE | * | ||
| CU (19) | MMSE | * | ||
| Total (including PD, MSA, mood disorders) | MMSE |

|
||
| AD‐like biomarker profile (151) | MMSE | * | ||
| Non‐AD‐like biomarker (109) | MMSE | * | ||
| Xiao et al. (2017) | NPTX2 | AD (30) | MMSE | * |
| DSS |

|
|||
| BNT | * | |||
| Phonemic verbal fluency |

|
|||
| Semantic verbal fluency |

|
|||
| WCST |

|
|||
| Visual reproduction test |

|
|||
| Block design |

|
|||
| CDT | * | |||
| CVLT |

|
|||
| Zetterberg et al. (2016) | NfL | AD (95) | MMSE |

|
| ADAS‐cog |

|
|||
| pMCI (101) | MMSE | * | ||
| ADAS‐cog | * | |||
| sMCI (91) | MMSE | * | ||
| ADAS‐cog |

|
|||
| CU (110) | MMSE | * | ||
| ADAS‐cog | * | |||
| Zhang, Ng, et al. (2018) | VILIP‐1 | Aβ + AD, MCI, CU (83) | MMSE |

|
| Aβ‐ MCI, CU (38) | MMSE | * | ||
| Zhang, Therriault, et al. (2018) | SNAP‐25 | AD (18) | MMSE | * |
| ADAS‐cog | * | |||
| sMCI (22) | MMSE | * | ||
| ADAS‐cog | * | |||
| pMCI (47) | MMSE | * | ||
| ADAS‐cog | * | |||
| CU (52) | MMSE | * | ||
| ADAS‐cog | * | |||
Abbreviations: ACE‐CZ, Addenbrookes Cognitive Examination‐Czech Version; AD, Alzheimers disease; ADAS‐Cog, Alzheimer Disease Assessment Scale‐Cognitive Subscale; ALS, amyotrophic lateral sclerosis; ANI, asymptomatic neurocognitive impairment; ANT, animal naming test; ApoE, apolipoprotein E; AVLT, Rey auditory verbal learning Test; Aβ‐, amyloid beta negative; Aβ+, amyloid beta positive; BMDB, brief mental deterioration battery; BNT, Boston naming test; BSRT, Buschke selective reminding test; bvFTD, behaviour variant FTD; BVMT‐R, Brief visuospatial memory test‐revised; BVMT, brief visuospatial memory test; CAMCOG, Cambridge cognitive examination; CBS, corticobasal syndrome; CDT, clock drawing test; CERAD, consortium to establish a registry for Alzheimers disease; CJD, Creutzfeldt‐Jacob disease; COWAT, controlled oral word association test; CPAL, continuous paired associate learning; CU, cognitive unimpaired; CVLT, California verbal learning test; DIAN, dominantly inherited Alzheimer network; DKEFS, Delis‐Kaplan executive function system; DLB, dementia with Lewy bodies; DSB, digit span backwards; DSF, digit span forwards; DSS, digit symbol substitution; EAD, early‐onset Alzheimers disease; FAB, frontal assessment battery; FTD, frontotemporal dementia; GMCT, groton maze times chase test; GML, groton maze learning test; GMR, groton maze learning test delayed recall; HAD, HIV‐associated dementia; lvPPA, logopenic variant primary progressive aphasia; MCCB, MATRICS consensus cognitive battery; MCI‐AD, mild cognitive impairment due to Alzheimers disease; MCI‐o, mild cognitive impairment not due to Alzheimers disease; MCI, mild cognitive impairment; MIX, mixed dementia; MMSE, mini mental state examination; MNCD, mild neurocognitive disorder; MND, motor neuron disease; MSA, multiple system atrophy; NfL, neurofilament‐light; nfvPPA, non‐fluent variant primary progressive aphasia; Ng, neurogranin; NOS, not otherwise specified; OCL, one‐card learning; ONB, one‐back memory; PASAT, paced auditory serial addition test; PCA, posterior cortical atrophy; PD, Parkinsons disease; PDD, Parkinsons disease dementia; pDLB, prodromal dementia with Lewy bodies; pMCI, progressive MCI; pNfH, phosphorylated neurofilament heavy; PSP, progressive supranuclear palsy; PVLT, Philadelphia verbal learning test; RBANS, repeatable battery for the assessment of neuropsychological status; SCWT, stroop colour word test; sMCI, stable MCI; SVD, small vessel disease; svPPA, semantic variant primary progressive aphasia; TMT‐B, trail making test B; TMT‐A, trail making test A; TWOB, two‐back memory; VaD, vascular dementia; WAIS, Wechsler adult intelligence scale; WCST, Wisconsin card sorting test; WE, Wernicke's encephalopathy.
A relationship between CSF NfL and neuropsychological performance was not consistently reported in MCI samples, although cognitive assessments used may have influenced findings. Three studies, with relatively large sample sizes, reported no significant association with MMSE scores (Bjerke et al., 2009; Bos et al., 2019; Zetterberg et al., 2016). However, several studies using other cognitive tests such as the ADAS‐Cog and cognitive composite scores reported associations with CSF NfL levels (Osborn et al., 2019; Rolstad, Berg, et al., 2015; Zetterberg et al., 2016). One study included participants with subjective cognitive impairment (SCI) and showed a significant association with a number of cognitive composite scores in those with a vascular burden (Rolstad, Berg, et al., 2015). Four studies pooled MCI and age‐matched CU samples and most reported a significant association with neuropsychological performance (Gifford et al., 2018; Osborn et al., 2019; Racine et al., 2016), while one reported no associations after controlling for demographics (Mielke, Syrjanen, Blennow, Zetterberg, Vemuri, et al., 2019). Five studies pooled AD, MCI and CU participants, and all reported a significant association with a number of neuropsychological assessments including the MMSE and ADAS‐Cog11(Bos et al., 2019; Dhiman et al., 2020; Mattsson et al., 2016; Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al., 2019; Teitsdottir et al., 2020). Interestingly, one study reported a stronger association in Aβ‐participants (Mattsson et al., 2016), while another reported a stronger association in Aβ + participants (Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al., 2019).
A significant association between CSF NfL and neuropsychological performance was consistently reported in 15 FTD studies; however, the cognitive assessment used may have influenced results. In FTD, three studies report significant moderate‐to‐weak relationships with MMSE scores (Alcolea et al., 2017; Delaby et al., 2020; Sjogren et al., 2000), although three studies showed no significant correlation (Abu‐Rumeileh et al., 2018; de Jong et al., 2007; Skillback et al., 2014). Despite a lack of association with MMSE scores, one study showed a weak but significant correlation with the frontal assessment battery (FAB)—a tool which is more sensitive to FTD (Dubois et al., 2000). Similarly, findings in studies of familial FTD were also mixed. One study found a significant correlation with MMSE scores in patients with a C9orf72 mutation (Meeter, Gendron, et al., 2018), while another reported no association in those with mutations in the MAPT, GRN, or C9orf72 genes (Meeter et al., 2016). Nevertheless, four studies examining subvariants of FTD consistently reported significant associations between CSF NfL and neuropsychological performance across subvariants (Meeter, Vijverberg, et al., 2018; Meeter et al., 2019; Rojas et al., 2018; Scherling et al., 2014).
Other types of dementia, including DLB and VaD, were investigated in five studies. Most studies reported no association between CSF NfL and MMSE in DLB (de Jong et al., 2007; Delaby et al., 2020; Skillback et al., 2014), although interestingly one showed a significant correlation in prodromal DLB. In two studies, NfL was correlated with neuropsychological performance in VaD and mixed dementia (Sjogren et al., 2001; Skillback et al., 2014). Finally, one study investigated HIV‐associated neurocognitive disorders (HAND) and while there were no associations between NfL and neuropsychological performance, there was a significant correlation with CSF levels of phosphorylated neurofilament heavy (pNfH) domains (McGuire et al., 2015).
Most studies including CU participants reported a significant association between CSF NfL and neuropsychological performance (Aschenbrenner et al., 2020; Gifford et al., 2018; Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al., 2019; Osborn et al., 2019); however, three reported no significant correlation (Bendlin et al., 2012; Bos et al., 2019; Hoglund et al., 2017). Moreover, studies using the MMSE consistently reported no significant correlation with CSF NfL levels, while most studies using other validated cognitive assessments reported significant results. One study included a younger sample (mean age = 37.8 years) and reported no significant association with cognitive composite test scores (Rolstad, Jakobsson, et al., 2015).
As reported in Table 4, CSF NfL appears to be related to neuropsychological performance in AD, MCI, CU and some forms of FTD. Conflicting results could be attributed to the cognitive assessment used; many studies employing the MMSE tended to report no associations, whereas more sensitive test scores appear to correlate with CSF NfL levels.
TABLE 4.
Summary of results grouped by CSF marker and diagnosis
| CSF marker | AD | MCI | CU | AD, MCI, CU Aβ+ | AD, MCI, CU Aβ‐ | AD, MCI, CU | MCI, CU | CUf | FTD‐related syndromes | DLB | VaD | EAD | PSP | CBS | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NfL |

|

|

|

|
|

|

|

|

|

|

|

|

|
||

|

|

|

|

|

|

|

|

|

|

|

|
||||
| Ng |

|

|

|

|

|

|

|

|
|||||||

|

|

|

|

|

|

|

|

|

|
||||||
| α‐Syn |

|

|
|||||||||||||
| β‐Syn |

|
||||||||||||||
| Contactin‐2 |

|
||||||||||||||
| GAP‐43 |
|

|

|

|

|

|

|
||||||||
| NPTX1/2/R |
|

|

|

|

|

|
|||||||||
| Nrg1 |

|

|

|
||||||||||||
| SNAP‐25 |

|

|

|

|
|||||||||||

|

|

|

|
||||||||||||
| Synaptotagmin‐1 |
|
|
|
||||||||||||
| VILIP‐1 |

|

|

|
||||||||||||

|

|
Note: Blue inverted triangles ( ) indicate a significant negative association, and green triangles (
) indicate a significant negative association, and green triangles ( ), respectively, indicate a significant positive association between CSF marker levels and neuropsychological performance. Black circles indicate no significant associations. Numeric value within shape corresponds to number of studies with this finding.
), respectively, indicate a significant positive association between CSF marker levels and neuropsychological performance. Black circles indicate no significant associations. Numeric value within shape corresponds to number of studies with this finding.
Abbreviations: AD, Alzheimers disease; Aβ‐, amyloid beta negative; Aβ+, amyloid beta positive; CBS, corticobasal syndrome; CU, cognitively unimpaired; CUf, cognitively unimpaired with familial history of AD; DLB, dementia with Lewy bodies; EAD, early‐onset Alzheimers disease; FTD, frontotemporal dementia; GAP‐43, growth‐associated protein 43; MCI, mild cognitive impairment; NfL, neurofilament‐light; Ng, neurogranin; NPTX1/R, neuronal pentraxin 1/receptor; Nrg1, neuregulin‐1; PSP, progressive supranuclear palsy; SNAP‐25, synaptosomal‐associated protein 25; VaD, vascular dementia; VILIP‐1, visinin‐like protein 1; α‐syn, alpha‐synuclein; β‐syn, beta‐synuclein.
3.3.2. Papers on CSF Ng
In total, 22 studies examined the association between CSF Ng and neuropsychological performance. Overall, CSF Ng was associated with neuropsychological performance in larger AD and MCI samples but not in CU or non‐AD dementias.
Nine studies examined the relationship with neuropsychological performance in AD samples. Some studies reported significant correlations with global cognition (Agnello et al., 2020; Bos et al., 2019; Jia et al., 2020; Sanfilippo et al., 2016), while a number of others reported no significant associations (De Vos et al., 2016; Hellwig et al., 2015; Portelius et al., 2015; Wang, 2019; Wellington et al., 2018). However, all studies reporting no association had sample sizes of fewer than 100 participants. In studies pooling AD, MCI and CU participants, sample sizes ranged from 193 to 770 and all three studies reported significant associations with neuropsychological performance (Bos et al., 2019; Galasko et al., 2019; Wang, 2019). In one study, this relationship was limited to Aβ+ participants (Bos et al., 2019), while in another it was independent of CSF Aβ and tau (Galasko et al., 2019). In carriers of autosomal dominant AD mutations in PSEN1, PSEN2 or APP genes, one study reported a significant association between Ng and neuropsychological performance (Schindler et al., 2019). Finally, one study with a CU sample enriched for a familial history of AD and ApoE e4 carriers reported a weak correlation with neuropsychological performance (Casaletto et al., 2017).
Most studies examining MCI samples found no significant association between CSF Ng and MMSE or ADAS‐Cog scores (Bos et al., 2019; De Vos et al., 2016; Hellwig et al., 2015; Kvartsberg, Duits, et al., 2015; Portelius et al., 2015; Wang, 2019). Moreover, two studies using domain‐specific tests reported significant correlations (Headley et al., 2018; Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al., 2019). Interestingly, one study that reported no associations in MCI or SCI, however, did show a significant correlation between CSF Ng/BACE1 ratio and neuropsychological performance (Kirsebom et al., 2018).
Only two studies examined CSF Ng and neuropsychological performance in non‐AD dementias. Both showed no significant relationships in bvFTD, nfvPPA, PSP, DLB and non‐AD related MCI (Hellwig et al., 2015; Wellington et al., 2016).
Eleven studies reported no associations between CSF Ng and neuropsychological performance in CU samples (Bruno et al., 2020; Headley et al., 2018; Hoglund et al., 2017; Mielke, Syrjanen, Blennow, Zetterberg, Skoog, et al., 2019; Sancesario et al., 2020; Santillo et al., 2019; Schindler et al., 2019; Wang, 2019; Wellington et al., 2018). Cognitive domains assessed, immunoassay methods used or mean sample ages did not appear to influence results.
Overall, CSF Ng is associated with neuropsychological performance in AD studies (see Table 4) with large samples. Most studies reporting significant correlations had sample sizes of ~200 or above, while those reporting no relationship tended to have smaller samples. Findings for MCI were less convincing, as the majority of studies found no associations. No significant results were found for CU or non‐AD dementia samples.
3.3.3. Papers on other CSF markers
Twenty‐two papers examined another CSF marker of interest. Overall, CSF NPTX2, and to a lesser extent CSF SNAP‐25, had the most promising evidence as markers associated with neuropsychological performance across diagnoses. Studies examining other CSF markers largely reported negative results.
A significant association between CSF NPTX2 and neuropsychological performance was consistently reported across studies. Three studies found a significant positive relationship with MMSE and domain‐specific assessments across the AD spectrum (Galasko et al., 2019; Swanson et al., 2016; Xiao et al., 2017), while one study reported no significant association (Boiten et al., 2021). Additionally, three studies reported associations in non‐AD dementias, namely, DLB (Boiten et al., 2021; van Steenoven et al., 2020) and FTD patients with GRN, C9orf72 and MAPT mutations (van der Ende et al., 2020). Moreover, two studies found significant associations between MMSE scores and CSF NPTXR levels (Lim et al., 2019; van Steenoven et al., 2020). One study investigated CSF NPTX1 levels but reported no associations with MMSE scores (Begcevic et al., 2018).
Seven studies examined CSF SNAP‐25 across the AD‐spectrum, although findings were slightly more mixed. Two studies using the ultrasensitive SIMOA assay reported significant associations with neuropsychological performance in carriers of autosomal AD mutations and in a pooled sample of AD, MCI and CU, respectively (Galasko et al., 2019; Schindler et al., 2019). The use of other immunoassay methods did not appear to impact findings as studies using ELISAs and MS methods both reported significant (Brinkmalm et al., 2014; Jia et al., 2020; Wang, Zhou, & Zhang, 2018) and non‐significant (Öhrfelt et al., 2019; Zhang, Therriault, et al., 2018) associations. One papers did show an association between neuropsychological performance and a CSF SNAP‐25/Aβ42 ratio but not CSF SNAP‐25 alone.
Three studies reported significant correlations between MMSE scores and CSF VILIP‐1 in AD (Lee et al., 2008), a pooled sample of Aβ+ AD, MCI and CU participants (Zhang, Ng, et al., 2018) and in carriers of autosomal dominant AD mutations (Schindler et al., 2019). This relationship may be specific to those with Aβ pathology as one study reported no associations in a CU sample (Hoglund et al., 2017).
Few studies investigated the remaining CSF markers. Firstly, one small study showed a significant association between CSF nrg1 levels and MMSE scores in AD and MCI but not in non‐AD dementias (Mouton‐Liger et al., 2020). Secondly, CSF contactin‐2 levels were correlated with MMSE scores across the AD‐spectrum (Chatterjee, Del Campo, et al., 2018), but this failed to replicate in a validation cohort. Thirdly, CSF beta‐synuclein was correlated with MMSE scores but also failed to replicate in a validation cohort (Oeckl et al., 2020). No relationship was found between neuropsychological performance and alpha‐synuclein (Agnello et al., 2020; Bruno et al., 2020). Finally, findings concerning CSF GAP‐43 (Sandelius et al., 2019) and synaptotagmin‐1 were mixed; one small study reported significant associations with neuropsychological performance (Jia et al., 2020) while others failed to find such relationships (Öhrfelt et al., 2016; Sandelius et al., 2019).
Overall, CSF NPTX2 appears to be associated with neuropsychological performance across diagnoses (see Table 4). There was some evidence for an association with CSF SNAP‐25 across the AD‐spectrum; however, findings were somewhat mixed. Additionally, the few studies examining CSF VILIP‐1 levels reported significant relationships across the AD‐spectrum. Conversely, evidence for the remaining CSF markers is limited, owing to small samples and few studies examining such markers.
3.4. Heterogeneity
There was significant heterogeneity documented between the studies included in this review. Sources of variability were most evident in the number of difference cognitive assessments used. Although the MMSE was the most commonly employed test, many studies used cognitive composite scores, which hampered our ability to conduct a comparison between the studies. Moreover, across studies using the MMSE only, many non‐significant correlation coefficients were not reported.
Differences in statistical analyses also contributed to heterogeneity, while some studies used Spearman or Pearson correlations to analyse data and others used various regression models with different adjustment factors. For these reasons, a quantitative meta‐analysis of results was not possible.
4. DISCUSSION
We conducted a systematic review to investigate the relationship between CSF markers of synapse and neuronal loss and neuropsychological performance in dementia and typical ageing. Overall, the substantial heterogeneity between studies makes it difficult to draw firm conclusions on any markers associated with cognition. However, there may be evidence for an association between cognition and CSF NfL across dementia syndromes/cognitive ageing and CSF Ng in those with an AD‐like biomarker profile. There was some evidence CSF NPTX2 and SNAP‐25 are associated with cognition.
We found evidence for an association between CSF NfL and neuropsychological performance in AD, FTD and aged CU samples. There was some evidence for an association in MCI participants, but these findings were conflicting. Elevations of CSF NfL have been reported across neurodegenerative diseases and is thought to reflect global degeneration as neurofilaments ‘leak’ out of damaged axons into the CSF (see Figure 2). However, the lack of consistent findings for MCI samples was surprising. Most studies reporting non‐significant associations across diagnoses used the MMSE to assess cognitive impairment, while those using the ADAS‐Cog or domain‐specific tests tended to report significant correlations with CSF NfL levels. The MMSE is known to lack sensitivity, particularly in detecting MCI (Mitchell, 2009) and so it could be speculated that this test is not the most adequate to capture subtle cognitive impairments and therefore not a suitable tool for studies investigating potential biomarkers associated with cognition.
FIGURE 2.
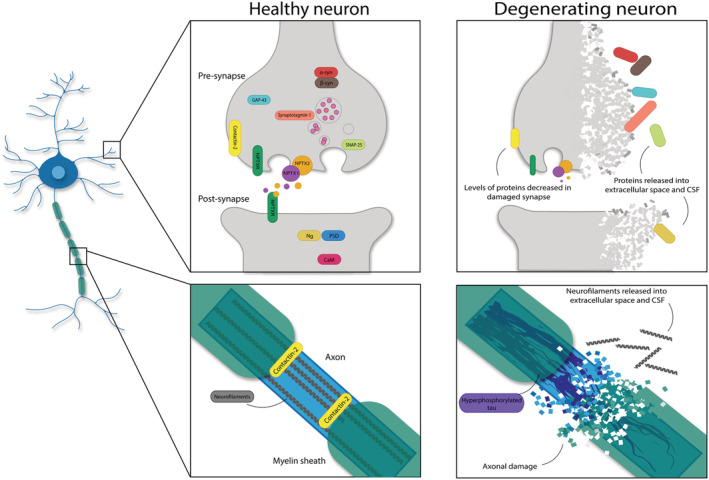
Schematic of localisation of synaptic and axonal markers included in the current review. Left: localisation in healthy synapses and axons. Right: possible mechanism of release into the cerebrospinal fluid (CSF) in degrading and damaged neurons
We also found some evidence that CSF Ng is associated with cognition in studies with large samples, possibly in Aβ+ participants (Bos et al., 2019) and Aβ+/Tau+ participants (Galasko et al., 2019). However, several studies focusing solely on participants with a clinical AD diagnosis reported no significant results. The use of the MMSE and small samples was a common feature of such studies indeed; those using larger samples tended to report significant associations. Meanwhile, CSF Ng was not associated with neuropsychological performance in non‐AD dementias. It is possible that Ng is specifically lost from synapses damaged by Aβ or tau, which are both associated with synaptotoxicity (Jackson et al., 2019; Koffie et al., 2009, 2012; Pickett et al., 2019) (see Figure 2). Indeed, CSF Ng was only associated with neuropsychological performance in a CU sample when enriched for a familial history of AD. However, the substantial heterogeneity between studies makes it difficult to draw firm conclusions on the use of CSF Ng as a biomarker associated with cognition.
The current review also highlighted other potential emerging biomarkers associated with cognition, namely, NPTX2 and SNAP‐25. CSF NPTX2 was consistently associated with neuropsychological performance in FTD, DLB and across the AD‐spectrum. In addition to its essential role at the synapse, low CSF NPTX2 levels are associated with hippocampal atrophy (Swanson et al., 2016), supporting its role as a biomarker of synapse dysfunction. Our findings suggest that CSF NPTX2 is not a disease‐specific marker of synapse loss but may instead reflect general synaptic dysfunction, although further research will be needed. CSF NPTX2, along with contactin‐2, was positively correlated with neuropsychological performance, unlike all other markers which had negative correlations. A potential explanation for these findings is that some synaptic and axonal proteins may leak out into the CSF after neuronal damage (those which show negative correlations with cognition); however, NPTXs and contactin‐2 levels may be reduced in surviving synapses, causing less to be secreted into the CSF as part of healthy synaptic turnover (see Figure 2).
SNAP‐25 was also a promising marker associated with cognition, although the evidence was less convincing and findings may have been influenced by small sample sizes. Prior to 2019, an ELISA assay available for CSF SNAP‐25 analysis was not available (Öhrfelt et al., 2019). With the growing accessibility of ELISA sampling technologies, we expect that further research will be able to employ larger sample sizes than those which are practical with mass‐spectrometry methods. Both studies using the ultrasensitive SIMOA immunoassay reported an association between SNAP‐25 levels and neuropsychological performance. Given the relatively low detected concentrations of CSF SNAP‐25 in the included studies, the improved sensitivity provided by SIMOA immunoassays may be more suited for future research.
While out of the scope of the current review, longitudinal studies of cognitive decline are also needed and useful. Cross‐sectional cognition can be dependent on several factors, such as age. While cross‐sectional age trends in cognitive measures have been reported to have a linear pattern, different samples with different ages may not be directly comparable (Salthouse, 2019). Longitudinal studies are needed to provide a direct measure of change with the same individuals assessed at each age. Longitudinal cohort studies such as the EPAD‐LCS (Ritchie et al., 2020; Solomon et al., 2018) may provide useful insights into how CSF markers relate to cognitive decline.
4.1. Beyond CSF markers
It is unlikely that a single CSF marker will act as a reliable biomarker for neuronal and synaptic changes affecting cognition. As assays become more sensitive and specific, a combination of CSF markers capturing different aspects of neurodegeneration may be a better correlate of cognition than single markers alone. However, CSF biomarkers are a relatively crude measure of brain function as regional differences cannot be examined. Incorporating both structural imaging (e.g. MRI) and functioning imaging (e.g. FDG‐PET and qEEG) along with cognitive testing is likely to provide a strong indication of neurodegeneration and cognitive status (Colom‐Cadena et al., 2020). Magnetic resonance imaging (MRI) can provide further information on neurodegeneration occurring in the brain. As one of the most widely used and accessible imaging methods, it is currently recommended in diagnostic criteria for AD (Jack et al., 2018). T1‐ and T2‐weighted images show different atrophy patterns and white matter alterations across different dementia syndromes (Harper et al., 2017), which all correlate with degree of the cognitive impairment (Bayram et al., 2018; Sudo et al., 2019; Wolk & Dickerson, 2011). The 7T MRI can provide further information about cognitive decline at an ultrahigh resolution, such as hippocampal subfield changes across dementias and MCI (McKiernan & OBrien, 2017).
Functional imaging can also provide information about brain functioning. Position emission tomography (PET) with 2 [(18)F]fluoro‐2‐deoxy‐D‐glucose (FDG‐PET) provides visualisation of the metabolic rate of glucose in the brain (Hoffman et al., 2000; Phelps et al., 1979) which is a direct index of synaptic functioning and an indirect index of synaptic density (Attwell & Iadecola, 2002; Rocher et al., 2003; Sokoloff, 1977). Reduced (18F) FDG uptake correlates with cognition in AD and MCI (Chiaravalloti et al., 2020; Landau et al., 2011). Recently, a direct measure of synapse density has been developed by targeting proteins critical for synaptic functioning (Finnema et al., 2016, 2018). PET ligands such as [11C]UCB‐J target synaptic vesicle glycoprotein 2A (SV2A), a ubiquitous protein expressed in pre‐synaptic terminals which is critical to synaptic function (Vogl et al., 2015). SV2A PET provides the opportunity to visualise synapses in vivo which is vital when investigating synapse loss. Decreased [11C]UCB‐J binding has been reported in early AD (Chen et al., 2018; Mecca et al., 2020) and correlates with episodic memory (Chen et al., 2018).
Additional functional imaging techniques, such as electroencephalography (EEG), provide a direct measure of neuronal field potentials. Reflecting the summed post‐synaptic potentials of excitatory and inhibitory neurons (Lopes da Silva, 2013), EEG is able to detect synapse dysfunction in vivo. Quantitative EEG analysis provides data reflecting neuronal circuit changes as a result of synapse dysfunction. Increases in delta (0.5–4 Hz) and theta (4–8 Hz) power bands, with a parallel decrease in alpha (8–13 Hz) and beta (13–30 Hz) power, have been reported in AD (Jelic et al., 2000). Furthermore, an increase in theta power is associated with clinical progression from SCI to MCI in those with Aβ pathology (Gouw et al., 2017), suggesting that changes in theta power may be associated with synapse dysfunction or loss. Magnetoencephalography (MEG) also records a signal based on post‐synaptic potentials; however, where EEG records electric potentials, MEG records the magnetic fields that are induced by electrical fields in the cortex (Lopes da Silva, 2013). Alterations have been reported in AD, MCI and SCI (López‐Sanz et al., 2018; Serrano et al., 2020; Xie et al., 2019), and increases in theta and beta2 power (20–30 Hz) have been reported in progressive MCI versus stable MCI (López et al., 2016). An increase in parietal delta power was found to increase the probability of conversion from MCI to AD by 350% (Fernández et al., 2006). Advantages of EEG and MEG include accessibility and non‐intrusive nature, as well as the excellent temporal resolution provided. Both of these functional techniques could contribute to an accurate readout of brain function at the network level.
With the exception of EEG and MRI in certain cases, the above methods are not part of routine practice. The costs associated with these methods, along with the invasive nature of CSF sampling and PET scans, could be a barrier to implementation in general practice. A biomarker detectable in the blood via a blood test would be more accessible, relatively invasive and most patients would be familiar with the procedure. A robust blood‐based biomarker of synapse loss or neuronal injury is not yet available; however, there is promising evidence for several markers.
Aβ and tau show promise as blood biomarkers for AD. Plasma Aβ is reduced in AD (Janelidze et al., 2016; Ovod et al., 2017; Zetterberg et al., 2011), correlates with CSF Aβ42 and can predict amyloid PET positivity (Nakamura et al., 2018). Plasma t‐tau and p‐tau levels are significantly increased in AD (Olsson et al., 2016; Randall et al., 2013; Zetterberg et al., 2013) and MCI (Yang et al., 2018). Plasma t‐tau correlates with cognitive decline in MCI ( Mielke et al., 2017), and plasma p‐tau181 is associated with both Aβ and tau PET (Mielke et al., 2018) and is more closely associated with AD neuropathology than a clinical diagnosis (Lantero Rodriguez et al., 2020). Blood levels of p‐tau217 are also elevated in AD and MCI and correlate with cognitive decline (Janelidze et al., 2020; Mattsson‐Carlgren et al., 2020). Blood levels of NfL show promise as a marker of general neurodegeneration; plasma or serum NfL levels are altered and correlate with MMSE scores in dementia syndromes and other neurodegenerative diseases (Al Shweiki et al., 2019; Khalil et al., 2020; Mattsson, Andreasson, et al., 2017; Sugarman et al., 2020; Zetterberg, 2016). However, not all CSF markers may be useful as blood biomarkers. In the CSF, Ng is a promising marker associated with cognition whereas in the blood, evidence suggests its use may be limited. While detectable in the blood, levels do not correlate with CSF Ng nor do they differ between AD and controls (De Vos et al., 2015; Kvartsberg, Portelius, et al., 2015). However, advancing technologies have made it possible to analyse neuron‐derived exosomes (NDEs) in blood which may offer increased sensitivity (Zetterberg, 2019). Indeed, a meta‐analysis reported a significant reduction of Ng plasma NDEs in AD and MCI (Liu et al., 2020). One study found an inverse correlation between GAP‐43, SNAP‐25, Ng and synaptotagmin‐1 NDEs and CSF levels of the protein, as well as a significant reduction in AD and MCI, and a significant correlation with MMSE scores (Jia et al., 2020). While this is promising evidence, the validation of blood biomarkers faces additional challenges. The CSF contains more neuronally derived molecules than blood (Zetterberg, 2019) which is particularly important to consider if the analyte of interest is expressed elsewhere in the body other than the brain, such as Ng expression in the lungs and kidneys which could explain the lack of correlation between blood and CSF levels (Díez‐Guerra, 2010). Blood biomarkers require sensitive and specific assays with meticulous validation studies (Zetterberg & Burnham, 2019), and the issues surrounding low reproducibility for CSF markers is also relevant for the validation of blood biomarkers.
4.2. Limitations
While this is the first known systematic review to examine CSF biomarkers associated with cognition in ageing and disease, it was not possible to conduct a meta‐analysis. An independent academic librarian was consulted with regard to the overall search strategy; however, they did not validate search terms. Furthermore, T.S.S. and D.A.G. were not blinded to studies when extracting data or rating the quality of studies which could introduce bias. Publication bias could also have affected the results of this review.
4.3. Recommendations
The current review reported conflicting findings between similar populations. While biologically important differences could explain these apparent discrepant findings, methodological heterogeneity could also be a contributing factor. We were unable to assess heterogeneity statistically; however, our review indicated substantial variability in methodology between studies. For example, differences in adjustment factors, cognitive tests and statistical analyses performed were some of the most common variations noted. A recent review has discussed low reproducibility as a common issue for biomarker findings (Mattsson‐Carlgren, Palmqvist, et al., 2020). The authors highlighted a number of sources of variability including cohort factors, assay factors, pre‐analytical factors and lack of validation methods. The field could improve on standardization with selecting a gold‐standard cognitive assessment, common adjustment factors, and the complete reporting of results. For novel biomarkers, validation cohorts are the most robust validation method (Mattsson‐Carlgren, Palmqvist, et al., 2020) and may improve the low reproducibility in the field. The overall quality of studies was good/fair. All studies clearly stated research objectives and most defined the study population clearly. However, only one of the included studies conducted a power analysis which limits confidence in findings, particularly in studies with smaller sample sizes.
To improve study quality and reporting, we recommend that future studies should address standardising cognitive assessments. The MMSE may not be the most appropriate tool due to floor and ceiling effects and a lack of sensitivity in detecting MCI (Mitchell, 2009). Other tests of global cognition such as The Repeatable Battery for the Assessment of Neuropsychological Status (Randolph, 1998) and the Addenbrooke's Cognitive Examination (Mathuranath et al., 2000) could be potential gold‐standard assessments for future studies, although further research is required. In addition to the assessment of global cognition, domain‐specific tests should also be used in future research. The International Working Group note a specific episodic memory disorder in AD which can be identified by tests that include list learning, such as the free and cued selective reminding test, paired associate learning and the Rey auditory verbal learning tasks (Dubois et al., 2014). Such tests are likely to be important in exploring potential biomarkers associated with disease‐specific cognitive impairments. A number of studies in the review used cognitive composite scores composed of various cognitive tools. These unstandardised composites contribute to variability in the field as they cannot be directly compared. Studies could improve on this by reporting the individual test scores in addition to composite scores or electing gold‐standard cognitive composites.
Future studies should also improve on the balanced reporting of data, as many studies did not report non‐significant correlation coefficients. Finally due to the nature of cohort studies, power analyses are unlikely to affect the final available sample but would still provide insight into whether individual studies are sufficiently powered to detect true relationships.
The reporting of sex and ethnicity differences was sparse. Concentrations of CSF biomarkers can vary with sex and ethnicity; CSF NfL is elevated in males, and elevations in CSF Ng have been reported for females (Mielke, 2020). Few studies have examined CSF marker changes across ethnicities; however, two studies report significant differences in CSF tau between African American and Caucasian groups (Garrett et al., 2019; Howell et al., 2017). Some studies in the current review controlled for sex (and less often for ethnicity), however, to work towards precision medicine, sex and ethnicity should be considered in the progression of cognitive decline, rather than treated as sources of random variability.
5. CONCLUSION
The current systematic review aimed to examine the relationship between CSF levels of markers for synaptic and neuronal damage with cognition in ageing and disease. Overall, heterogeneity between studies means no firm conclusions can be drawn from our results. We found some evidence for an association between neuropsychological performance and CSF NfL across diagnoses and CSF Ng in those with AD‐like pathology. Some studies found relationships with CSF NPTX2 across diagnoses. Recommendations for the field include the improvement of consistent analyses, measurements and reporting, as well as the exploration of important demographic differences in samples. In future research, a combination of CSF biomarkers of synaptic and neuronal loss and structural and functional imaging is likely to be a powerful tool for tracking changes affecting cognition and as a readout for interventions aiming to preserve cognitive function.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
AUTHOR CONTRIBUTIONS
TS, DK, TSJ, GM and CR conceived and designed the review. TS and DG performed the search, screened papers and extracted data. TSJ, DK, CR and GM provided supervision and guidance. TS wrote the original manuscript, and DG, TSJ, DK, GM and CR provided feedback and corrections.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15656.
ABBREVIATIONS
- α‐syn
Alpha‐synuclein
- β‐syn
Beta‐synuclein
- Aβ
Amyloid beta
- ACE‐CZ
Addenbrooke's Cognitive Examination‐Czech Version
- AD
Alzheimer's disease
- ADAS‐Cog
Alzheimer's Disease Assessment Scale‐Cognitive Subscale
- ADHD
Attention deficit hyperactivity disorder
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ALS
Amyotrophic lateral sclerosis
- ANI
Asymptomatic neurocognitive impairment
- ANT
Animal naming test
- APOE
Apolipoprotein E
- APP
Amyloid beta precursor protein
- AVLT
Rey auditory verbal learning test
- BACE
Beta‐secretase 1
- BMDB
Brief mental deterioration battery
- BNT
Boston naming test
- BSRT
Buschke selective reminding test
- bvFTD
Behavioural‐variant FTD
- BVMT‐R
Brief Visuospatial memory test‐ revised
- Ca2+
Calcium
- CaM
Calmodulin
- CAMCOG
Cambridge cognitive examination
- CBS
Corticobasal syndrome
- CDR
Clinical dementia rating
- CDT
Clock drawing test
- CERAD
Consortium to establish a registry for Alzheimer's disease
- CJD
Creutzfeldt‐Jakob disease (CJD)
- COWAT
Controlled oral word association test
- CNS
Central nervous system
- CPAL
Continuous paired associate learning
- CSF
Cerebrospinal fluid
- CU
Cognitively unimpaired
- CUf
Cognitive unimpaired with familial history of Alzheimer's disease
- CV
Coefficient of variability
- CVLT
California verbal learning test
- DIAN
Dominantly inherited Alzheimer network
- DKEFS
Delis‐Kaplan executive function system
- DLB
Dementia with Lewy bodies
- DSB
Digit span backwards
- DSF
Digit span forwards
- DSM‐III‐R
Diagnostic and statistical manual of mental disorders, 3rd edition revised
- DSS
Digit symbol substitution
- EAD
Early‐onset Alzheimer's disease
- EEG
Electroencephalogram
- ELISA
Enzyme‐linked immunosorbent assay
- EMBASE
Excerpta Medica dataBASE
- FAB
Frontal assessment battery
- FDG
2 [(18)F]fluoro‐2‐deoxy‐D‐glucose
- FTD
Frontotemporal dementia
- GAP‐43
Growth‐associated protein 43
- GENFI
The Genetic Frontotemporal Initiative
- GMCT
Groton maze times chase test
- GML
Groton maze learning test
- GMR
Groton maze learning test delayed recall
- HAD
HIV‐associated dementia
- HAND
HIV‐associated neurocognitive disorder
- HIV
Human immunodeficiency virus
- lvPPA
Logopenic variant primary progressive aphasia
- IWG‐2
The International Working Group 2
- LB
Lewy body
- LTD
Long‐term depression
- LTP
Long‐term potentiation
- MAPT
Microtubule Associated Protein Tau
- MCCB
MATRICS Consensus Cognitive Battery
- MCI
Mild cognitive impairment
- MCI‐AD
Mild cognitive impairment due to Alzheimer's disease
- MCI‐o
Mild cognitive impairment not due to Alzheimer's disease
- MEG
Magnetoencephalography
- MIX
Mixed dementia
- MMSE
Mini‐mental state examination
- MNCD
Mild neurocognitive disorder
- MND
Motor neuron disease
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- MSA
Multiple system atrophy
- NDE
Neuron‐derived exosomes
- NfH
Neurofilament‐heavy
- NfL
Neurofilament‐light
- NfM
Neurofilament‐medium
- nfvPPA
Nonfluent variant primary progressive aphasia
- Ng
Neurogranin
- NIA‐AA
National Institute on Ageing/Alzheimer's Association
- NINCDS‐ADRDA
National Institute of Neurological and Communicative Disorders and the Alzheimer's Disease and Related Disorders Association
- NOS
Not otherwise specified
- NPTX
Neuronal pentraxin
- NPTXR
Neuronal pentraxin receptor
- Nrg1
Neuregulin‐1
- OCL
One‐card learning
- ONB
One‐back memory
- P‐tau
Phosphorylated tau
- PASAT
Paced auditory serial addition test
- PCA
Posterior cortical atrophy
- PD
Parkinson's disease
- PDD
Parkinson's disease dementia
- pDLB
Prodromal dementia with Lewy bodies
- pMCI
Progressive MCI
- pNfH
Phosphorylated neurofilament‐heavy
- PET
Positron emission tomography
- PPA
Primary progressive aphasia
- PSEN
Presenilin
- PSP
Progressive supranuclear palsy
- PVLT
Philadelphia verbal learning test
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- SCI
Subjective cognitive impairment
- SCWT
Stroop colour word test
- sMCI
Stable mild cognitive impairment
- SIMOA
Single molecule array
- SNAP‐25
Synaptosomal‐associated protein 25
- SNARE
Soluble NSF attachment protein receptor
- SV2A
synaptic vesicle glycoprotein 2A
- SVD
Small vessel disease
- svPPA
Semantic variant primary progressive aphasia
- T‐Tau
Total tau
- TMT‐A
Trail making test A
- TMT‐B
Trail making test B
- TWOB
Two‐back memory
- UCSD
University of California San Diego
- VaD
Vascular dementia
- VILIP‐1
Visinin‐like protein‐1
- WAIS
Wechsler adult intelligence scale
- WCST
Wisconsin card sorting test
- WE
Wernicke's Encephalopathy
Supporting information
Supplementary Table S1: Search terms
Supplementary Table S2: National Institute of Health Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies ratings for included studies
ACKNOWLEDGEMENTS
The authors would like to thank Marshall Dozier at The University of Edinburgh and the following authors for providing additional data and clarification for papers included in the current review: Professor Ales Bartos and colleagues, Dr Isabelle Bos and colleagues, Professor Sabina Capellari, Professor Piero Parchi and colleagues, Professor Serge Gauthier, Dr Hua Zhang, and colleagues, Dr Cristina Sanfilippo and colleagues, Dr Alexander Santillo and colleagues, Dr Eugeen Vanmechelen and Dr Henrietta Wellington, colleagues and the following funders: Wolfson Foundation, the Swedish Research Council, the Knut and Alice Wallenberg Foundation, Frimurarstiftelsen, Alzheimerfonden, Hjärnfonden, the Agneta PrytzFolke and Gösta Folke Foundation, the Torsten Söderberg Foundation, NIHR UCL/UCLH Biomedical Research Center and the NIHR Queen Square Dementia Biomedical Research Unit. We would also like to thank Professor Paul Worley and colleagues. Graphical abstract created with Biorender.com. TS and DAG are supported by funding from the University of Edinburgh Translational Neuroscience programme (108890/Z/15/Z). TSJ receives grant funding from the UK Dementia Research Institute, which receives its funding from DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society and Alzheimer's Research UK; the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme under grant agreement no 681181; and two collaborating companies, neither of which had any influence over the current paper. CR is funded by the Innovative Medicines Initiative 115736 (EPAD).
Saunders, T. S. , Gadd, D. A. , Spires‐Jones, T. L. , King, D. , Ritchie, C. , & Muniz‐Terrera, G. (2022). Associations between cerebrospinal fluid markers and cognition in ageing and dementia: A systematic review. European Journal of Neuroscience, 56(9), 5650–5713. 10.1111/ejn.15656
Edited by: Anton Porsteinsson
Funding information Innovative Medicines Initiative; University of Edinburgh Translational Neuroscience Programme, Grant/Award Number: 108890/Z/15/Z; UK Dementia Research Institute
DATA AVAILABILITY STATEMENT
No new data were generated in this systematic literature review.
REFERENCES
- Abu‐Rumeileh, S. , Mometto, N. , Bartoletti‐Stella, A. , Polischi, B. , Oppi, F. , Poda, R. , Stanzani‐Maserati, M. , Cortelli, P. , Liguori, R. , Capellari, S. , & Parchi, P. (2018). Cerebrospinal fluid biomarkers in patients with frontotemporal dementia spectrum: A single‐center study. Journal of Alzheimer's Disease, 66(2), 551–563. 10.3233/JAD-180409 [DOI] [PubMed] [Google Scholar]
- Agarwal, A. , Zhang, M. , Trembak‐Duff, I. , Unterbarnscheidt, T. , Radyushkin, K. , Dibaj, P. , Martins de Souza, D. , Boretius, S. , Brzózka, M. M. , Steffens, H. , Berning, S. , Teng, Z. , Gummert, M. N. , Tantra, M. , Guest, P. C. , Willig, K. I. , Frahm, J. , Hell, S. W. , Bahn, S. , … Schwab, M. H. (2014). Dysregulated expression of neuregulin‐1 by cortical pyramidal neurons disrupts synaptic plasticity. Cell Reports, 8(4), 1130–1145. 10.1016/j.celrep.2014.07.026 [DOI] [PubMed] [Google Scholar]
- Agnello, L. , Gambino, C. M. , Lo Sasso, B. , Bivona, G. , Milano, S. , Ciaccio, A. M. , Piccoli, T. , La Bella, V. , & Ciaccio, M. (2020). Neurogranin as a Novel Biomarker in Alzheimers Disease (pp. 1–9). Laboratory Medicine. 10.1093/labmed/lmaa062 [DOI] [PubMed] [Google Scholar]
- Aisen, P. S. , Petersen, R. C. , Donohue, M. C. , Gamst, A. , Raman, R. , Thomas, R. G. , Walter, S. , Trojanowski, J. Q. , Shaw, L. M. , Beckett, L. A. , Jack, C. R. , Jagust, W. , Toga, A. W. , Saykin, A. J. , Morris, J. C. , Green, R. C. , & Weiner, M. W. (2010). Clinical core of the Alzheimers disease neuroimaging initiative: Progress and plans. Alzheimers and Dementia, 6(3), 239–246. 10.1016/j.jalz.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Shweiki, M. R. , Steinacker, P. , Oeckl, P. , Hengerer, B. , Danek, A. , Fassbender, K. , Diehl‐Schmid, J. , Jahn, H. , Anderl‐Straub, S. , Ludolph, A. C. , Schönfeldt‐Lecuona, C. , & Otto, M. (2019). Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. Journal of Psychiatric Research, 113, 137–140. 10.1016/j.jpsychires.2019.03.019 [DOI] [PubMed] [Google Scholar]
- Albert, M. S. , DeKosky, S. T. , Dickson, D. , Dubois, B. , Feldman, H. H. , Fox, N. C. , Gamst, A. , Holtzman, D. M. , Jagust, W. J. , Petersen, R. C. , Snyder, P. J. , Carrillo, M. C. , Thies, B. , & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimers disease: Recommendations from the National Institute on Aging‐Alzheimers association workgroups on diagnostic guidelines for Alzheimers disease. Alzheimers and Dementia, 7(3), 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea, D. , Vilaplana, E. , Suarez‐Calvet, M. , Illan‐Gala, I. , Blesa, R. , Clarimon, J. , Llado, A. , Sanchez‐Valle, R. , Molinuevo, J. L. , Garcia‐Ribas, G. , Compta, Y. , Marti, M. J. , Pinol‐Ripoll, G. , Amer‐Ferrer, G. , Noguera, A. , Garcia‐Martin, A. , Fortea, J. , & Lleo, A. (2017). CSF sAPP beta, YKL‐40, and neurofilament light in frontotemporal lobar degeneration. Neurology, 89(2), 178–188. [DOI] [PubMed] [Google Scholar]
- Armstrong, M. J. , Litvan, I. , Lang, A. E. , Bak, T. H. , Bhatia, K. P. , Borroni, B. , Boxer, A. L. , Dickson, D. W. , Grossman, M. , Hallett, M. , Josephs, K. A. , Kertesz, A. , Lee, S. E. , Miller, B. L. , Reich, S. G. , Riley, D. E. , Tolosa, E. , Tröster, A. I. , Vidailhet, M. , & Weiner, W. J. (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80(5), 496–503. 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner, A. , Gordon, B. , Fagan, A. , Schindler, S. , Balota, D. , Morris, J. , Hassenstab, J. , & Tales, A. (2020). Neurofilament Light Predicts Decline in Attention but Not Episodic Memory in Preclinical Alzheimer's Disease. Neurofilament Light Predicts Decline in Attention but Not Episodic Memory in Preclinical Alzheimers Disease., 74(4), 1129–1129. 10.3233/JAD-200018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atri, A. (2011). Effective pharmacological Management of Alzheimers Disease. The American Journal of Managed Care, 17(13), S346–S355. https://www.ajmc.com/view/a300_11nov_atri_s346 [PubMed] [Google Scholar]
- Attwell, D. , & Iadecola, C. (2002). The neural basis of functional brain imaging signals. Trends in Neurosciences, 25(12), 621–625. 10.1016/S0166-2236(02)02264-6 [DOI] [PubMed] [Google Scholar]
- Baker, K. , Gordon, S. L. , Grozeva, D. , Van Kogelenberg, M. , Roberts, N. Y. , Pike, M. , Blair, E. , Hurles, M. E. , Chong, W. K. , Baldeweg, T. , Kurian, M. A. , Boyd, S. G. , Cousin, M. A. , & Raymond, F. L. (2015). Identification of a human synaptotagmin‐1 mutation that perturbs synaptic vesicle cycling. Journal of Clinical Investigation, 125(4), 1670–1678. 10.1172/JCI79765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos, A. , Ripova, D. , Svarcova, J. , Cechova, L. , Fialova, L. , & Malbohan, I. (2011). Cerebrospinal fluid and serum autoantibodies against neurocytoskeletal proteins in patients with Alzheimers disease. Journal of Neuroimmunol, 7(4), 100–105. 10.1016/j.jalz.2011.05.341 [DOI] [Google Scholar]
- Bayram, E. , Caldwell, J. Z. K. , & Banks, S. J. (2018). Current understanding of magnetic resonance imaging biomarkers and memory in Alzheimers disease. Alzheimers and Dementia: Translational Research and Clinical Interventions, 4, 395–413. Elsevier Inc. 10.1016/j.trci.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear, M. F. , & Malenka, R. C. (1994). Synaptic plasticity: LTP and LTD. Current Opinion in Neurobiology, 4(3), 389–399. 10.1016/0959-4388(94)90101-5 [DOI] [PubMed] [Google Scholar]
- Begcevic, I. , Tsolaki, M. , Brinc, D. , Brown, M. , Martinez‐Morillo, E. , Lazarou, I. , Kozori, M. , Tagaraki, F. , Nenopoulou, S. , Gkioka, M. , Lazarou, E. , Lim, B. , Batruch, I. , & Diamandis, E. P. (2018). Neuronal pentraxin receptor‐1 is a new cerebrospinal fluid biomarker of Alzheimers disease progression [version 1; peer review: 4 approved]. F1000Research, 7, 1012. 10.12688/F1000RESEARCH.15095.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin, B. B. , Carlsson, C. M. , Johnson, S. C. , Zetterberg, H. , Blennow, K. , Willette, A. A. , Okonkwo, O. C. , Sodhi, A. , Ries, M. L. , Birdsill, A. C. , Alexander, A. L. , Rowley, H. A. , Puglielli, L. , Asthana, S. , & Sager, M. A. (2012). CSF T‐tau/Aβ42 predicts white matter microstructure in healthy adults at risk for Alzheimers disease. PLoS One, 7(6), e37720. 10.1371/journal.pone.0037720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczki, E. , Branca, R. M. , Francis, P. T. , Pereira, J. B. , Baek, J. H. , Hortobágyi, T. , Winblad, B. , Ballard, C. , Lehtiö, J. , & Aarsland, D. (2018). Synaptic markers of cognitive decline in neurodegenerative diseases: A proteomic approach. Brain, 141(2), 582–595. 10.1093/brain/awx352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni‐Freddari, C. , Fattoretti, P. , Casoli, T. , Meier‐Ruge, W. , & Ulrich, J. (1990). Morphological adaptive response of the synaptic junctional zones in the human dentate gyrus during aging and Alzheimers disease. Brain Research, 517(1–2), 69–75. 10.1016/0006-8993(90)91009-6 [DOI] [PubMed] [Google Scholar]
- Bian, H. , Van Swieten, J. C. , Leight, S. , Massimo, L. , Wood, E. , Forman, M. , Moore, P. , De Koning, I. , Clark, C. M. , Rosso, S. , Trojanowski, J. , Lee, V. M. Y. , & Grossman, M. (2008). CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology, 70(19 PART 2(Issue 19, Part 2), 1827–1835. 10.1212/01.wnl.0000311445.21321.fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke, M. , Andreasson, U. , Rolstad, S. , Nordlund, A. , Lind, K. , Zetterberg, H. , Edman, A. , Blennow, K. , & Wallin, A. (2009). Subcortical Vascular Dementia Biomarker Pattern in Mild Cognitive Impairment. Dementia and geriatric cognitive disorders, 28(4), 348–356. 10.1159/000252773 [DOI] [PubMed] [Google Scholar]
- Blennow, K. , De Leon, M. J. , & Zetterberg, H. (2006). Alzheimers disease. Lancet, 368(9533), 387–403). Elsevier. 10.1016/S0140-6736(06)69113-7 [DOI] [PubMed] [Google Scholar]
- Bogdanovic, N. , Davidsson, P. , Volkmann, I. , Winblad, B. , & Blennow, K. (2000). Growth‐associated protein GAP‐43 in the frontal cortex and in the hippocampus in Alzheimers disease: An immunohistochemical and quantitative study. Journal of Neural Transmission, 107(4), 463–478. 10.1007/s007020070088 [DOI] [PubMed] [Google Scholar]
- Boiten, W. A. , Steenoven, I. V. , Xiao, M. , Worley, P. F. , Lemstra, A. W. , & Teunissen, C. E. (2021). Pathologically decreased CSF levels of synaptic marker NPTX2 in DLB are correlated with levels of alpha‐synuclein and VGF. Cells, 10(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni, B. , Cerini, C. , Archetti, S. , Premi, E. , Cosseddu, M. , Ferrari, M. , Bellelli, G. , Gasparotti, R. , Caimi, L. , Di Luca, M. , & Padovani, A. (2011). Cerebrospinal fluid tau in frontotemporal lobar degeneration: Clinical, neuroimaging, and prognostic correlates. Journal of Alzheimers Disease, 23(3), 505–512. 10.3233/JAD-2010-101407 [DOI] [PubMed] [Google Scholar]
- Bos, I. , Vos, S. , Verhey, F. , Scheltens, P. , Teunissen, C. , Engelborghs, S. , Sleegers, K. , Frisoni, G. , Blin, O. , Richardson, J. C. , Bordet, R. , Tsolaki, M. , Popp, J. , Peyratout, G. , Martinez‐Lage, P. , Tainta, M. , Lleó, A. , Johannsen, P. , Freund‐Levi, Y. , … Visser, P. J. (2019). Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimers disease spectrum. Alzheimers & Dementia, 15(5), 644–654. 10.1016/J.JALZ.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Boutron, I. , Page, M. , Higgins, J. , Altman, D. , Lundh, A. , & Hróbjartsson, A. (2020). Chapter 7: Considering bias and conflicts of interest among the included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. John Wiley & Sons. https://training.cochrane.org/handbook/current/chapter-07 [Google Scholar]
- Braunewell, K. H. , Riederer, P. , Spilker, C. , Gundelfinger, E. D. , Bogerts, B. , & Bernstein, H. G. (2001). Abnormal localization of two neuronal calcium sensor proteins, visinin‐like proteins (VILIPs)‐1 and −3, in neocortical brain areas of Alzheimer disease patients. Dementia and Geriatric Cognitive Disorders, 12(2), 110–116. 10.1159/000051244 [DOI] [PubMed] [Google Scholar]
- Braunewell, K. H. (2012). The visinin‐like proteins VILIP‐1 and VILIP‐3 in Alzheimers disease ‐ old wine in new bottles. Frontiers in Molecular Neuroscience, 5(20), 1–12. 10.3389/fnmol.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmalm, A. , Brinkmalm, G. , Honer, W. G. , Frölich, L. , Hausner, L. , Minthon, L. , Hansson, O. , Wallin, A. , Zetterberg, H. , Blennow, K. , & Öhrfelt, A. (2014). SNAP‐25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimers disease. Molecular Neurodegeneration, 9, 53. 10.1186/1750-1326-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, D. , Plaska, C. R. , Clark, D. P. A. , Zetterberg, H. , Blennow, K. , Verbeek, M. M. , & Pomara, N. (2020). CSF alpha‐synuclein correlates with CSF neurogranin in late‐life depression. The International journal of neuroscience. ((Bruno) School of Psychology, Liverpool John Moores University, Liverpool, United Kingdom (Reichert Plaska, Pomara) Nathan Kline Institute for Psychiatric Research, Orangeburg, NY, United States (Reichert Plaska) Department of Psychology, the Graduate Cen), 131(4), 357–361. 10.1080/00207454.2020.1744596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhave, P. , Blennow, K. , Zetterberg, H. , Stomrud, E. , Londos, E. , Andreasen, N. , Minthon, L. , & Hansson, O. (2009). Longitudinal study of CSF biomarkers in patients with Alzheimers disease. PLoS ONE, 4(7), e6294. 10.1371/journal.pone.0006294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti, S. P. , Ballarini, T. , Sala, A. , Cerami, C. , Presotto, L. , Santangelo, R. , Fallanca, F. , Vanoli, E. G. , Gianolli, L. , Iannaccone, S. , Magnani, G. , Perani, D. , Parnetti, L. , Eusebi, P. , Frisoni, G. , Nobili, F. , Picco, A. , & Scarpini, E. (2018). FDG‐PET and CSF biomarker accuracy in prediction of conversion to different dementias in a large multicentre MCI cohort. NeuroImage: Clinical, 18, 167–177. 10.1016/j.nicl.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto, K. B. , Elahi, F. M. , Bettcher, B. M. , Neuhaus, J. , Bendlin, B. B. , Asthana, S. , Johnson, S. C. , Yaffe, K. , Carlsson, C. , Blennow, K. , Zetterberg, H. , & Kramer, J. H. (2017). Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology, 89(17), 1782–1788. 10.1212/WNL.0000000000004569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M. C. , Park, J. M. , Pelkey, K. A. , Grabenstatter, H. L. , Xu, D. , Linden, D. J. , Sutula, T. P. , McBain, C. J. , & Worley, P. F. (2010). Narp regulates homeostatic scaling of excitatory synapses on parvalbumin‐expressing interneurons. Nature Neuroscience, 13(9), 1090–1097. 10.1038/nn.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, M. , Del Campo, M. , Morrema, T. H. J. , De Waal, M. , Van der Flier, W. M. , Hoozemans, J. J. M. , & Teunissen, C. E. (2018). Contactin‐2, a synaptic and axonal protein, is reduced in cerebrospinal fluid and brain tissue in Alzheimers disease. Alzheimer's Research & Therapy, 10(1), 52. 10.1186/s13195-018-0383-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, M. , Koel‐Simmelink, M. J. , Verberk, I. M. , Killestein, J. , Vrenken, H. , Enzinger, C. , Ropele, S. , Fazekas, F. , Khalil, M. , & Teunissen, C. E. (2018). Contactin‐1 and contactin‐2 in cerebrospinal fluid as potential biomarkers for axonal domain dysfunction in multiple sclerosis. Multiple Sclerosis Journal ‐ Experimental, Translational and Clinical, 4(4), 205521731881953. 10.1177/2055217318819535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. K. , Mecca, A. P. , Naganawa, M. , Finnema, S. J. , Toyonaga, T. , Lin, S. F. , Najafzadeh, S. , Ropchan, J. , Lu, Y. , McDonald, J. W. , Michalak, H. R. , Nabulsi, N. B. , Arnsten, A. F. T. , Huang, Y. , Carson, R. E. , & Van Dyck, C. H. (2018). Assessing synaptic density in Alzheimer Disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurology, 75(10), 1215–1224. 10.1001/jamaneurol.2018.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti, A. , Ricci, M. , Di Biagio, D. , Filippi, L. , Martorana, A. , & Schillaci, O. (2020). The brain metabolic correlates of the main indices of neuropsychological assessment in Alzheimers disease. Journal of Personalized Medicine, 10(2). 10.3390/jpm10020025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, D. , Shum, A. , & Caraveo, G. (2020). GAP‐43 and BASP1 in axon regeneration: Implications for the treatment of neurodegenerative diseases. Frontiers in Cell and Developmental Biology, 8, 567637. 10.3389/fcell.2020.567537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri, A. , & Malenka, R. C. (2008). Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology, 33(1), 18–41. 10.1038/sj.npp.1301559 [DOI] [PubMed] [Google Scholar]
- Colom‐Cadena, M. , Spires‐Jones, T. , Zetterberg, H. , Blennow, K. , Caggiano, A. , DeKosky, S. T. , Fillit, H. , Harrison, J. E. , Schneider, L. S. , Scheltens, P. , De Haan, W. , Grundman, M. , Van Dyck, C. H. , Izzo, N. J. , & Catalano, S. M. (2020). The clinical promise of biomarkers of synapse damage or loss in Alzheimers disease. Alzheimers Research & Therapy, 12, 21. 10.1186/s13195-020-00588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly, S. J. , Mukaetova‐Ladinska, E. B. , Abdul‐All, Z. , da Silva, J. A. , Brayne, C. , Honer, W. G. , & Mann, D. M. A. (2011). Synaptic changes in frontotemporal lobar degeneration: Correlation with MAPT haplotype and APOE genotype. Neuropathology and Applied Neurobiology, 37(4), 366–380. 10.1111/j.1365-2990.2010.01150.x [DOI] [PubMed] [Google Scholar]
- Counts, S. E. , Ikonomovic, M. D. , Mercado, N. , Vega, I. E. , & Mufson, E. J. (2017). Biomarkers for the early detection and progression of Alzheimers Disease. Neurotherapeutics, 14(1), 35–53. 10.1007/s13311-016-0481-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson, P. , & Blennow, K. (1998). Neurochemical dissection of synaptic pathology in Alzheimers disease. International Psychogeriatrics, 10(1), 11–23. 10.1017/S1041610298005110 [DOI] [PubMed] [Google Scholar]
- De Jong, D. , Jansen, R. W. M. M. , Pijnenburg, Y. A. L. , Van Geel, W. J. A. , Borm, G. F. , Kremer, H. P. H. , & Verbeek, M. M. (2007). CSF neurofilament proteins in the differential diagnosis of dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 78(9), 936–938. 10.1136/jnnp.2006.107326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos, A. , Struyfs, H. , Jacobs, D. , Fransen, E. , Klewansky, T. , De Roeck, E. , Robberecht, C. , Van Broeckhoven, C. , Duyckaerts, C. , Engelborghs, S. , & Vanmechelen, E. (2016). The cerebrospinal fluid neurogranin/BACE1 ratio is a potential correlate of cognitive decline in Alzheimers disease. Journal of Alzheimer's disease, 53(4), 1523–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos, A. , Jacobs, D. , Struyfs, H. , Fransen, E. , Andersson, K. , Portelius, E. , Andreasson, U. , De Surgeloose, D. , Hernalsteen, D. , Sleegers, K. , Robberecht, C. , Van Broeckhoven, C. , Zetterberg, H. , Blennow, K. , Engelborghs, S. , & Vanmechelen, E. (2015). C‐terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimers disease. Alzheimers & Dementia, 11(12), 1461–1469. 10.1016/j.jalz.2015.05.012 [DOI] [PubMed] [Google Scholar]
- De Wilde, M. C. , Overk, C. R. , Sijben, J. W. , & Masliah, E. (2016). Meta‐analysis of synaptic pathology in Alzheimers disease reveals selective molecular vesicular machinery vulnerability. Alzheimers and Dementia, 12(6), 633–644. 10.1016/j.jalz.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky, S. T. , & Marek, K. (2003). Looking backward to move forward: Early detection of neurodegenerative disorders. Science, 302(5646), 830–834. 10.1126/science.1090349 [DOI] [PubMed] [Google Scholar]
- DeKosky, S. T. , & Scheff, S. W. (1990). Synapse loss in frontal cortex biopsies in Alzheimers disease: Correlation with cognitive severity. Annals of Neurology, 27(5), 457–464. 10.1002/ana.410270502 [DOI] [PubMed] [Google Scholar]
- Delaby, C. , Alcolea, D. , Carmona‐Iragui, M. , Illan‐Gala, I. , Morenas‐Rodriguez, E. , Barroeta, I. , Altuna, M. , Estelles, T. , Santos‐Santos, M. , Turon‐Sans, J. , Munoz, L. , Ribosa‐Nogue, R. , Sala‐Matavera, I. , Sanchez‐Saudinos, B. , Subirana, A. , Videla, L. , Benejam, B. , Sirisi, S. , Lehmann, S. … Lleó, A. (2020). Differential levels of Neurofilament Light protein in cerebrospinal fluid in patients with a wide range of neurodegenerative disorders. Scientific Reports, 10, 9161. 10.1038/s41598-020-66090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman, K. , Gupta, V. B. , Villemagne, V. L. , Eratne, D. , Graham, P. L. , Fowler, C. , Bourgeat, P. , Li, Q. X. , Collins, S. , Bush, A. I. , Rowe, C. C. , Masters, C. L. , Ames, D. , Hone, E. , Blennow, K. , Zetterberg, H. , & Martins, R. N. (2020). Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimers disease. Alzheimer's & Dementia, 12(1), 1–9. 10.1002/dad2.12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez‐Guerra, F. J. (2010). Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life, 62(8), 597–606. 10.1002/iub.357 [DOI] [PubMed] [Google Scholar]
- Dodds, D. C. , Omeis, I. A. , Cushman, S. J. , Helms, J. A. , & Perin, M. S. (1997). Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin‐ associated calcium‐binding protein 49. Journal of Biological Chemistry, 272(34), 21488–21494. 10.1074/jbc.272.34.21488 [DOI] [PubMed] [Google Scholar]
- Dubois, B. , Feldman, H. H. , Jacova, C. , Hampel, H. , Molinuevo, J. L. , Blennow, K. , Dekosky, S. T. , Gauthier, S. , Selkoe, D. , Bateman, R. , Cappa, S. , Crutch, S. , Engelborghs, S. , Frisoni, G. B. , Fox, N. C. , Galasko, D. , Habert, M. O. , Jicha, G. A. , Nordberg, A. , … Cummings, J. L. (2014). Advancing research diagnostic criteria for Alzheimers disease: The IWG‐2 criteria. The Lancet Neurology, 13(6), 614–629). Lancet Publishing Group. 10.1016/S1474-4422(14)70090-0 [DOI] [PubMed] [Google Scholar]
- Dubois, B. , Slachevsky, A. , Litvan, I. , & Pillon, B. (2000). The FAB: A frontal assessment battery at bedside. Neurology, 55(11), 1621–1626. 10.1212/WNL.55.11.1621 [DOI] [PubMed] [Google Scholar]
- Dulewicz, M. , Kulczyńska‐przybik, A. , & Mroczko, B. (2020). Neurogranin and VILIP‐1 as molecular indicators of neurodegeneration in Alzheimers disease: A systematic review and meta‐analysis. International Journal of Molecular Sciences, 21(21), 1–19). MDPI AG. 10.3390/ijms21218335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecay‐Torres, M. , Estanga, A. , Tainta, M. , Izagirre, A. , Garcia‐Sebastian, M. , Villanua, J. , Clerigue, M. , Iriondo, A. , Urreta, I. , Arrospide, A. , Díaz‐Mardomingo, C. , Kivipelto, M. , & Martinez‐Lage, P. (2018). Increased CAIDE dementia risk, cognition, CSF biomarkers, and vascular burden in healthy adults. Neurology, 91(3), e217–e226. 10.1212/WNL.0000000000005824 [DOI] [PubMed] [Google Scholar]
- Emamzadeh, F. N. (2016). Alpha‐synuclein structure, functions, and interactions. Journal of Research in Medical Sciences, 21(2) Isfahan University of Medical Sciences (IUMS), 21–29. 10.4103/1735-1995.181989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, A. , Turrero, A. , Zuluaga, P. , Gil, P. , Maestú, F. , Campo, P. , & Ortiz, T. (2006). Magnetoencephalographic parietal δ dipole density in mild cognitive impairment: Preliminary results of a method to estimate the risk of developing Alzheimer disease. Archives of Neurology, 63(3), 427–430. 10.1001/archneur.63.3.427 [DOI] [PubMed] [Google Scholar]
- Finnema, S. J. , Nabulsi, N. B. , Eid, T. , Detyniecki, K. , Lin, S. F. , Chen, M. K. , Dhaher, R. , Matuskey, D. , Baum, E. , Holden, D. , Spencer, D. D. , Mercier, J. , Hannestad, J. , Huang, Y. , & Carson, R. E. (2016). Imaging synaptic density in the living human brain. Science Translational Medicine, 8(348), 348ra96. 10.1126/scitranslmed.aaf6667 [DOI] [PubMed] [Google Scholar]
- Finnema, S. J. , Nabulsi, N. B. , Mercier, J. , Lin, S. F. , Chen, M. K. , Matuskey, D. , Gallezot, J. D. , Henry, S. , Hannestad, J. , Huang, Y. , & Carson, R. E. (2018). Kinetic evaluation and test–retest reproducibility of [11C]UCB‐J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. Journal of Cerebral Blood Flow and Metabolism, 38(11), 2041–2052. 10.1177/0271678X17724947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach, G. D. (2007). NRG1 and synaptic function in the CNS. Neuron, 54(4), 495–497. 10.1016/j.neuron.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fuchs, E. , & Cleveland, D. W. (1998). A structural scaffolding of intermediate filaments in health and disease. Science, 279(5350), 514–519. 10.1126/science.279.5350.514 [DOI] [PubMed] [Google Scholar]
- Furley, A. J. , Morton, S. B. , Manalo, D. , Karagogeos, D. , Dodd, J. , & Jessell, T. M. (1990). The axonal glycoprotein TAG‐1 is an immunoglobulin superfamily member with neurite outgrowth‐promoting activity. Cell, 61(1), 157–170. 10.1016/0092-8674(90)90223-2 [DOI] [PubMed] [Google Scholar]
- Gaetani, L. , Blennow, K. , Calabresi, P. , Di Filippo, M. , Parnetti, L. , & Zetterberg, H. (2019). Neurofilament light chain as a biomarker in neurological disorders. Journal of Neurology, Neurosurgery and Psychiatry, 90(8), 870–881. BMJ Publishing Group. 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- Galasko, D. , Xiao, M. , Xu, D. , Smirnov, D. , Salmon, D. P. , Dewit, N. , Vanbrabant, J. , Jacobs, D. , Vanderstichele, H. , Vanmechelen, E. , & Worley, P. (2019). Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimers disease. Alzheimers and Dementia: Translational Research and Clinical Interventions, 5, 871–882. 10.1016/j.trci.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, S. L. , McDaniel, D. , Obideen, M. , Trammell, A. R. , Shaw, L. M. , Goldstein, F. C. , & Hajjar, I. (2019). Racial disparity in cerebrospinal fluid amyloid and tau biomarkers and associated cutoffs for mild cognitive impairment. JAMA Network Open, 2(12), e1917363. 10.1001/jamanetworkopen.2019.17363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam, V. , DAvanzo, C. , Hebisch, M. , Kovacs, D. M. , & Kim, D. Y. (2014). BACE1 activity regulates cell surface contactin‐2 levels. Molecular Neurodegeneration, 9, 4. 10.1186/1750-1326-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENFI‐The Genetic Frontotemporal Initiative . (n.d.). Retrieved October 25, 2020, from https://www.genfi.org/
- Gifford, K. A. , Liu, D. D. , Neal, J. E. , Babicz, M. A. , Thompson, J. L. , Walljasper, L. E. , Wiggins, M. E. , Turchan, M. , Pechman, K. R. , Osborn, K. E. , Acosta, L. M. Y. , Bell, S. P. , Hohman, T. J. , Libon, D. J. , Blennow, K. , Zetterberg, H. , & Jefferson, A. L. (2018). The 12‐word Philadelphia verbal learning test performances in older adults: Brain MRI and cerebrospinal fluid correlates and regression‐based normative data. Dementia and Geriatric Cognitive Disorders Extra, 8(3), 476–491. 10.1159/000494209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens, J. , Bjerke, M. , Van Mossevelde, S. , Van Den Bossche, T. , Goeman, J. , De Vil, B. , Sieben, A. , Martin, J. J. , Cras, P. , De Deyn, P. P. , Van Broeckhoven, C. , Van Der Zee, J. , & Engelborghs, S. (2018). Diagnostic value of cerebrospinal fluid tau, neurofilament, and progranulin in definite frontotemporal lobar degeneration. Alzheimers Research and Therapy, 10(1), 1–10. 10.1186/s13195-018-0364-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno‐Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , Ogar, J. M. , Rohrer, J. D. , Black, S. , Boeve, B. F. , Manes, F. , Dronkers, N. F. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. L. , Knopman, D. S. , Hodges, J. R. , Mesulam, M. M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw, A. A. , Alsema, A. M. , Tijms, B. M. , Borta, A. , Scheltens, P. , Stam, C. J. , & Van der Flier, W. M. (2017). EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiology of Aging, 57, 133–142. 10.1016/j.neurobiolaging.2017.05.017 [DOI] [PubMed] [Google Scholar]
- Hampel, H. , Lista, S. , & Khachaturian, Z. S. (2012). Development of biomarkers to chart all Alzheimers disease stages: The royal road to cutting the therapeutic Gordian knot. Alzheimers & Dementia, 8(4), 312–336. 10.1016/j.jalz.2012.05.2116 [DOI] [PubMed] [Google Scholar]
- Hansson, O. , Hall, S. , Öhrfelt, A. , Zetterberg, H. , Blennow, K. , Minthon, L. , Nägga, K. , Londos, E. , Varghese, S. , Majbour, N. K. , Al‐Hayani, A. , & El‐Agnaf, O. M. (2014). Levels of cerebrospinal fluid α‐synuclein oligomers are increased in Parkinsons disease with dementia and dementia with Lewy bodies compared to Alzheimers disease. Alzheimers Research and Therapy, 6(3), 25. 10.1186/alzrt255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, L. , Bouwman, F. , Burton, E. J. , Barkhof, F. , Scheltens, P. , OBrien, J. T. , Fox, N. C. , Ridgway, G. R. , & Schott, J. M. (2017). Patterns of atrophy in pathologically confirmed dementias: A voxelwise analysis. Journal of Neurology, Neurosurgery and Psychiatry, 88(11), 908–916. 10.1136/jnnp-2016-314978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley, A. , De Leon‐Benedetti, A. , Dong, C. H. , Levin, B. , Loewenstein, D. , Camargo, C. , Rundek, T. , Zetterberg, H. , Blennow, K. , Wright, C. B. , Sun, X. Y. , & Alzheimers Dis, N. (2018). Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology, 90(10), e887, e887. 10.1212/WNL.0000000000005057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig, K. , Kvartsberg, H. , Portelius, E. , Andreasson, U. , Oberstein, T. J. , Lewczuk, P. , Blennow, K. , Kornhuber, J. , Maler, J. M. , Zetterberg, H. , & Spitzer, P. (2015). Neurogranin and YKL‐40: Independent markers of synaptic degeneration and neuroinflammation in Alzheimers disease. Alzheimers Research & Therapy, 7, 74. 10.1186/s13195-015-0161-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, J. , Welsh‐Bohmer, K. A. , Hanson, M. , Crain, B. , Hulette, C. , Earl, N. , & Coleman, R. E. (2000). FDG PET imaging in patients with pathologically verified dementia. Journal of Nuclear Medicine, 41(11), 1920–1928. https://pubmed.ncbi.nlm.nih.gov/11079505/ [PubMed] [Google Scholar]
- Hoglund, K. , Kern, S. , Zettergren, A. , Börjesson‐Hansson, A. , Zetterberg, H. , Skoog, I. , & Blennow, K. (2017). Preclinical amyloid pathology biomarker positivity: Effects on tau pathology and neurodegeneration. Translational Psychiatry, 7(1), e995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan, M. R. (2017). A shift from a pivotal to supporting role for the growth‐associated protein (GAP‐43) in the coordination of axonal structural and functional plasticity. Frontiers in Cellular Neuroscience, 11, 266. 10.3389/fncel.2017.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, J. C. , Watts, K. D. , Parker, M. W. , Wu, J. , Kollhoff, A. , Wingo, T. S. , Dorbin, C. D. , Qiu, D. , & Hu, W. T. (2017). Race modifies the relationship between cognition and Alzheimers disease cerebrospinal fluid biomarkers. Alzheimers Research & Therapy, 9(1), 88. 10.1186/s13195-017-0315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R. , Knopman, D. S. , Jagust, W. J. , Shaw, L. M. , Aisen, P. S. , Weiner, M. W. , Petersen, R. C. , & Trojanowski, J. Q. (2010). Hypothetical model of dynamic biomarkers of the Alzheimers pathological cascade. The Lancet Neurology, 9(1), 119–128. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, C. R. Jr. , Bennett, D. A. , Blennow, K. , Carrillo, M. C. , Dunn, B. , Budd Haeberlein, S. , Holtzman, D. M. , Jagust, W. , Jessen, F. , Karlawish, J. , Liu, E. , Luis Molinuevo, J. , Montine, T. , Phelps, C. , Rankin, K. P. , Rowe, C. C. , Scheltens, P. , Siemers, E. , Snyder, H. M. , … Dement Author manuscript, A. (2018). NIA‐AA research framework: Toward a biological definition of Alzheimers disease HHS public access Author manuscript. Alzheimers Dement, 14(4), 535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, R. J. , Rose, J. , Tulloch, J. , Henstridge, C. , Smith, C. , & Spires‐Jones, T. L. (2019). Clusterin accumulates in synapses in Alzheimers disease and is increased in apolipoprotein E4 carriers. Brain Communications, 1(1), fcz003. 10.1093/braincomms/fcz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, R. , & Fasshauer, D. (2012). Molecular machines governing exocytosis of synaptic vesicles. Nature, 490(7419), 201–207. 10.1038/nature11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze, S. , Berron, D. , Smith, R. , Strandberg, O. , Proctor, N. K. , Dage, J. L. , Stomrud, E. , Palmqvist, S. , Mattsson‐Carlgren, N. , & Hansson, O. (2020). Associations of plasma phospho‐Tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurology, 78(2), 149–156. 10.1001/jamaneurol.2020.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze, S. , Stomrud, E. , Palmqvist, S. , Zetterberg, H. , Van Westen, D. , Jeromin, A. , Song, L. , Hanlon, D. , Tan Hehir, C. A. , Baker, D. , Blennow, K. , & Hansson, O. (2016). Plasma β‐amyloid in Alzheimers disease and vascular disease. Scientific Reports, 6, 26801. 10.1038/srep26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, A. L. , Gifford, K. A. , Acosta, L. M. Y. , Bell, S. P. , Donahue, M. J. , Davis, L. T. , Gottlieb, J. , Gupta, D. K. , Hohman, T. J. , Lane, E. M. , Libon, D. J. , Mendes, L. A. , Niswender, K. , Pechman, K. R. , Rane, S. , Ruberg, F. L. , Su, Y. R. , Zetterberg, H. , & Liu, D. (2016). The Vanderbilt memory & aging project: Study design and baseline cohort overview. Journal of Alzheimers Disease, 52(2), 539–559. 10.3233/JAD-150914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic, V. , Johansson, S. E. , Almkvist, O. , Shigeta, M. , Julin, P. , Nordberg, A. , Winblad, B. , & Wahlund, L. O. (2000). Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimers disease. Neurobiology of Aging, 21(4), 533–540. 10.1016/S0197-4580(00)00153-6 [DOI] [PubMed] [Google Scholar]
- Jia, L. , Zhu, M. , Kong, C. , Pang, Y. , Zhang, H. , Qiu, Q. , Wei, C. , Tang, Y. , Wang, Q. , Li, Y. , Li, T. , Li, F. , Wang, Q. , Li, Y. , Wei, Y. , & Jia, J. (2020). Blood neuro‐exosomal synaptic proteins predict Alzheimers disease at the asymptomatic stage. Alzheimers and Dementia, 17(1), 49–60. 10.1002/alz.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas, C. , Segal, J. , Stewart, W. F. , Corrada, M. , & Thal, L. J. (1994). A validation study of the dementia questionnaire. Archives of Neurology, 51(9), 901–906. 10.1001/archneur.1994.00540210073015 [DOI] [PubMed] [Google Scholar]
- Kester, M. I. , Van der Vlies, A. E. , Blankenstein, M. A. , Pijnenburg, Y. A. L. , Van Elk, E. J. , Scheltens, P. , & Van der Flier, W. M. (2009). CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology, 73(17), 1353–1358. 10.1212/WNL.0B013E3181BD8271 [DOI] [PubMed] [Google Scholar]
- Khalil, M. , Pirpamer, L. , Hofer, E. , Voortman, M. M. , Barro, C. , Leppert, D. , Benkert, P. , Ropele, S. , Enzinger, C. , Fazekas, F. , Schmidt, R. , & Kuhle, J. (2020). Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nature Communications, 11(1), 812. 10.1038/s41467-020-14612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W. S. , Kagedal, K. , & Halliday, G. M. (2014). Alpha‐synuclein biology in Lewy body diseases. Alzheimers Research and Therapy, 6(1), 5–8. 10.1186/s13195-014-0073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood, C. M. , MacDonald, M. L. , Schempf, T. A. , Vatsavayi, A. V. , Ikonomovic, M. D. , Koppel, J. L. , Ding, Y. , Sun, M. , Kofler, J. K. , Lopez, O. L. , Yates, N. A. , & Sweet, R. A. (2016). Altered levels of visinin‐like protein 1 correspond to regional neuronal loss in Alzheimer disease and frontotemporal lobar degeneration. Journal of Neuropathology and Experimental Neurology, 75(2), 175–182. 10.1093/jnen/nlv018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsebom, B. E. , Nordengen, K. , Selnes, P. , Waterloo, K. , Torsetnes, S. B. , Gisladottir, B. , Brix, B. , Vanmechelen, E. , Brathen, G. , Hessen, E. , Aarsland, D. , & Fladby, T. (2018). Cerebrospinal fluid neurogranin/beta‐site APP‐cleaving enzyme 1 predicts cognitive decline in preclinical Alzheimers disease. Alzheimer's & Dementia: Translational Research & Clinical Intervention, 4, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie, R. M. , Meyer‐Luehmann, M. , Hashimoto, T. , Adams, K. W. , Mielke, M. L. , Garcia‐Alloza, M. , Micheva, K. D. , Smith, S. J. , Kim, M. L. , Lee, V. M. , Hyman, B. T. , & Spires‐Jones, T. L. (2009). Oligomeric amyloid associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proceedings of the National Academy of Sciences, 106(10), 4012–4017. 10.1073/pnas.0811698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie, R. M. , Hashimoto, T. , Tai, H. C. , Kay, K. R. , Serrano‐Pozo, A. , Joyner, D. , Hou, S. , Kopeikina, K. J. , Frosch, M. P. , Lee, V. M. , Holtzman, D. M. , Hyman, B. T. , & Spires‐Jones, T. L. (2012). Apolipoprotein E4 effects in Alzheimers disease are mediated by synaptotoxic oligomeric amyloid‐β. Brain, 135(7), 2155–2168. 10.1093/brain/aws127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff, A. , Liu, C. , Ginghina, C. , Shi, M. , & Zhang, J. (2013). α‐Synuclein in cerebrospinal fluid of Alzheimers disease and mild cognitive impairment. Journal of Alzheimers Disease, 36(4), 679–688. 10.3233/JAD-130458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvartsberg, H. , Duits, F. H. , Ingelsson, M. , Andreasen, N. , Öhrfelt, A. , Andersson, K. , Brinkmalm, G. , Lannfelt, L. , Minthon, L. , Hansson, O. , Andreasson, U. , Teunissen, C. E. , Scheltens, P. , Van der Flier, W. M. , Zetterberg, H. , Portelius, E. , & Blennow, K. (2015). Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimers disease. Alzheimers & Dementia, 11(10), 1180–1190. 10.1016/j.jalz.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Kvartsberg, H. , Lashley, T. , Murray, C. E. , Brinkmalm, G. , Cullen, N. C. , Höglund, K. , Zetterberg, H. , Blennow, K. , & Portelius, E. (2019). The intact postsynaptic protein neurogranin is reduced in brain tissue from patients with familial and sporadic Alzheimers disease. Acta Neuropathologica, 137(1), 89–102. 10.1007/s00401-018-1910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvartsberg, H. , Portelius, E. , Andreasson, U. , Brinkmalm, G. , Hellwig, K. , Lelental, N. , Kornhuber, J. , Hansson, O. , Minthon, L. , Spitzer, P. , Maler, J. M. , Zetterberg, H. , Blennow, K. , & Lewczuk, P. (2015). Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimers disease patients and healthy controls. Alzheimers Research & Therapy, 7(1), 40. 10.1186/s13195-015-0124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau, S. M. , Harvey, D. , Madison, C. M. , Koeppe, R. A. , Reiman, E. M. , Foster, N. L. , Weiner, M. W. , & Jagust, W. J. (2011). Associations between cognitive, functional, and FDG‐PET measures of decline in AD and MCI. Neurobiology of Aging, 32(7), 1207–1218. 10.1016/j.neurobiolaging.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantero Rodriguez, J. , Karikari, T. K. , Suárez‐Calvet, M. , Troakes, C. , King, A. , Emersic, A. , Aarsland, D. , Hye, A. , Zetterberg, H. , Blennow, K. , & Ashton, N. J. (2020). Plasma p‐tau181 accurately predicts Alzheimers disease pathology at least 8 years prior to post‐mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathologica, 140(3), 267–278. 10.1007/s00401-020-02195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. M. , Blennow, K. , Andreasen, N. , Laterza, O. , Modur, V. , Olander, J. , Gao, F. , Ohlendorf, M. , & Ladenson, J. H. (2008). The brain injury biomarker VLP‐1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clinical Chemistry, 54(10), 1617–1623. 10.1373/clinchem.2008.104497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. J. , Wei, M. , Zhang, C. , Maxeiner, S. , Pak, C. H. , Botelho, S. C. , Trotter, J. , Sterky, F. H. , & Südhof, T. C. (2017). Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. Journal of Neuroscience, 37(5), 1062–1080. 10.1523/JNEUROSCI.2768-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. Q. , Tan, L. , Wang, H. F. , Tan, M. S. , Tan, L. , Xu, W. , Zhao, Q. F. , Wang, J. , Jiang, T. , & Yu, J. T. (2016). Risk factors for predicting progression from mild cognitive impairment to Alzheimers disease: A systematic review and meta‐analysis of cohort studies. Journal of Neurology, Neurosurgery and Psychiatry, 87(5), 476–484. 10.1136/jnnp-2014-310095 [DOI] [PubMed] [Google Scholar]
- Lim, B. , Tsolaki, M. , Soosaipillai, A. , Brown, M. , Zilakaki, M. , Tagaraki, F. , Fotiou, D. , Koutsouraki, E. , Grosi, E. , Prassas, I. , & Diamandis, E. P. (2019). Liquid biopsy of cerebrospinal fluid identifies neuronal pentraxin receptor (NPTXR) as a biomarker of progression of Alzheimers disease. Clinical Chemistry and Laboratory Medicine, 1–7, 57(12), 1875–1881. 10.1515/cclm-2019-0428 [DOI] [PubMed] [Google Scholar]
- Litvan, I. , Agid, Y. , Calne, D. , Campbell, G. , Dubois, B. , Duvoisin, R. C. , Goetz, C. G. , Golbe, L. I. , Grafman, J. , Growdon, J. H. , Hallett, M. , Jankovic, J. , Quinn, N. P. , Tolosa, E. , & Zee, D. S. (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): Report of the NINDS‐SPSP International Workshop. Neurology, 47(1), 1–9. Lippincott Williams and Wilkins. 10.1212/WNL.47.1.1 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Lin, H. , He, X. , Chen, L. , Dai, Y. , Jia, W. , Xue, X. , Tao, J. , & Chen, L. (2020). Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimers disease and mild cognitive impairment. Translational Psychiatry, 10(1), 125. 10.1038/s41398-020-0801-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva, F. (2013). EEG and MEG: Relevance to neuroscience. Neuron, 80(5), 1112–1128. 10.1016/j.neuron.2013.10.017 [DOI] [PubMed] [Google Scholar]
- López, M. E. , Turrero, A. , Cuesta, P. , López‐Sanz, D. , Bruña, R. , Marcos, A. , Gil, P. , Yus, M. , Barabash, A. , Cabranes, J. A. , Maestú, F. , & Fernández, A. (2016). Searching for primary predictors of conversion from mild cognitive impairment to Alzheimers disease: A multivariate follow‐up study. Journal of Alzheimers Disease, 52(1), 133–143. 10.3233/JAD-151034 [DOI] [PubMed] [Google Scholar]
- López‐Sanz, D. , Serrano, N. , & Maestú, F. (2018). The role of magnetoencephalography in the early stages of Alzheimers disease. Frontiers in Neuroscience, 12, 572. 10.3389/fnins.2018.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah, E. , Crews, L. , & Hansen, L. (2006). Synaptic remodeling during aging and in Alzheimers disease. Journal of Alzheimers Disease, 9, 91–99. 10.3233/jad-2006-9s311 [DOI] [PubMed] [Google Scholar]
- Masliah, E. , Mallory, M. , Hansen, L. , Richard, D. T. , Alford, M. , & Terry, R. (1994). Synaptic and neuritic alterations during the progression of Alzheimers disease. Neuroscience Letters, 174(1), 67–72. 10.1016/0304-3940(94)90121-X [DOI] [PubMed] [Google Scholar]
- Masuda, T. (2017). Contactin‐2/TAG‐1, active on the front line for three decades. Cell adhesion and Migration, 11(5–6), 524–531). Taylor and Francis Inc. 10.1080/19336918.2016.1269998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathuranath, P. , Nestor, P. J. , Berrios, G. E. , Rakowicz, W. , & Hodges, J. R. (2000). A brief cognitive test battery to differentiate Alzheimers disease and frontotemporal dementia. Neurology, 55(11), 1613–1620. 10.1212/01.wnl.0000434309.85312.19 [DOI] [PubMed] [Google Scholar]
- Mattsson, N. , Andreasson, U. , Zetterberg, H. , Blennow, K. , Weiner, M. W. , Aisen, P. , Toga, A. W. , Petersen, R. , Jack, C. R. , Jagust, W. , Trojanowki, J. Q. , Shaw, L. M. , Beckett, L. , Green, R. C. , Saykin, A. J. , Morris, J. C. , Khachaturian, Z. , Sorensen, G. , Carrillo, M. , … Fargher, K. (2017). Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurology, 74(5), 557–566. 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson, N. , Insel, P. S. , Palmqvist, S. , Portelius, E. , Zetterberg, H. , Weiner, M. , Blennow, K. , & Hansson, O. (2016). Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimers disease. EMBO Molecular Medicine, 8(10), 1184–1196. 10.15252/emmm.201606540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson, N. , Schöll, M. , Strandberg, O. , Smith, R. , Palmqvist, S. , Insel, P. S. , Hägerström, D. , Ohlsson, T. , Zetterberg, H. , Jögi, J. , Blennow, K. , & Hansson, O. (2017). 18 F‐AV‐1451 and CSF T‐tau and P‐tau as biomarkers in Alzheimers disease. EMBO Molecular Medicine, 9(9), 1212–1223. 10.15252/emmm.201707809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson‐Carlgren, N. , Janelidze, S. , Palmqvist, S. , Cullen, N. , Svenningsson, A. L. , Strandberg, O. , Mengel, D. , Walsh, D. M. , Stomrud, E. , Dage, J. L. , & Hansson, O. (2020). Longitudinal plasma p‐tau217 is increased in early stages of Alzheimers disease. Brain, 143(11), 3234–3241. 10.1093/brain/awaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson‐Carlgren, N. , Palmqvist, S. , Blennow, K. , & Hansson, O. (2020). Increasing the reproducibility of fluid biomarker studies in neurodegenerative studies. Nature Communications, 11(1), 1–11. 10.1038/s41467-020-19957-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, J. , Gill, A. J. , Douglas, S. D. , Kolson, D. L. , & Cns, H. I. V. A.‐R. T. E. (2015). Central and peripheral markers of neurodegeneration and monocyte activation in HIV‐associated neurocognitive disorders. Journal of Neurovirology, 21(4), 439–448. 10.1007/s13365-015-0333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann, G. , Drachman, D. , Folstein, M. , Katzman, R. , Price, D. , & Stadlan, E. M. (1984). Clinical diagnosis of alzheimers disease: Report of the NINCDS‐ADRDA work group⋆ under the auspices of department of health and human services task force on alzheimers disease. Neurology, 34(7), 939–944. 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- McKiernan, E. F. , & OBrien, J. T. (2017). 7T MRI for neurodegenerative dementias in vivo: A systematic review of the literature. In Journal of neurology, neurosurgery and psychiatry (Vol. 88, 7, pp. 564–574). BMJ Publishing Group. 10.1136/jnnp-2016-315022 [DOI] [PubMed] [Google Scholar]
- Mecca, A. P. , Chen, M. K. , ODell, R. S. , Naganawa, M. , Toyonaga, T. , Godek, T. A. , Harris, J. E. , Bartlett, H. H. , Zhao, W. , Nabulsi, N. B. , Wyk, B. C. V. , Varma, P. , Arnsten, A. F. T. , Huang, Y. , Carson, R. E. , & Van Dyck, C. H. (2020). In vivo measurement of widespread synaptic loss in Alzheimers disease with SV2A PET. Alzheimers and Dementia, 16(7), 974–982. 10.1002/alz.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter, L. , Dopper, E. G. , Jiskoot, L. C. , Sanchez‐Valle, R. , Graff, C. , Benussi, L. , Ghidoni, R. , Pijnenburg, Y. A. , Borroni, B. , Galimberti, D. , Laforce, R. J. , Masellis, M. , Vandenberghe, R. , Ber, I. L. , Otto, M. , Van Minkelen, R. , Papma, J. M. , Rombouts, S. A. , Balasa, M. , … Van Swieten, J. C. (2016). Neurofilament light chain: A biomarker for genetic frontotemporal dementia. 3(8), 36–636. 10.1002/acn3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter, L. , Gendron, T. F. , Sias, A. C. , Jiskoot, L. C. , Russo, S. P. , Donker Kaat, L. , Papma, J. M. , Panman, J. L. , Van der Ende, E. L. , Dopper, E. G. , Franzen, S. , Graff, C. , Boxer, A. L. , Rosen, H. J. , Sanchez‐Valle, R. , Galimberti, D. , Pijnenburg, Y. , Benussi, L. , Ghidoni, R. & Lee, S. E. (2018). Poly(GP), neurofilament and grey matter deficits in C9orf72 expansion carriers. Annals of Clinical and Translational Neurology, 5(5), 583–597. 10.1002/acn3.559 [DOI] [PMC free article] [PubMed]
- Meeter, L. , Steketee, R. M. , Salkovic, D. , Vos, M. , Grossman, M. , McMillan, C. T. , Irwin, D. J. , Boxer, A. L. , Rojas, J. C. , Olney, N. T. , Karydas, A. , Miller, B. L. , Pijnenburg, Y. A. L. , Barkhof, F. , Sanchez‐Valle, R. , Llado, A. , Borrego‐Ecija, S. , Diehl‐Schmid, J. , Grimmer, T. , … Van Swieten, J. C. (2019). Clinical value of cerebrospinal fluid neurofilament light chain in semantic dementia. Journal of Neurology, Neurosurgery and Psychiatry, 90(9), 997–1004. 10.1136/jnnp-2018-319784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter, L. , Vijverberg, E. , Del Campo, M. , Rozemuller, A. J. M. , Kaat, L. D. , de Jong, F. J. , van der Flier, W. M. , Teunissen, C. E. , & van Swieten, J. C. (2018). Clinical value of neurofilament and phospho‐tau/tau ratio in the frontotemporal dementia spectrum. Neurology, 90(14), e1231–e1239. 10.1212/WNL.0000000000005261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, M. , Syrjanen, J. A. , Blennow, K. , Zetterberg, H. , Skoog, I. , Vemuri, P. , Machulda, M. M. , Graff‐Radford, J. , Knopman, D. S. , Jack, C. R. , Petersen, R. C. , & Kern, S. (2019). Comparison of variables associated with cerebrospinal fluid neurofilament, total‐tau, and neurogranin. Alzheimer's & Dementia, 15(11), 1437–1447. 10.1016/j.jalz.2019.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, M. , Syrjanen, J. A. , Blennow, K. , Zetterberg, H. , Vemuri, P. , Skoog, I. , Machulda, M. M. , Kremers, W. K. , Knopman, D. S. , Jack, C. Jr. , Petersen, R. C. , & Kern, S. (2019). Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology, 93(3), e252–e260. 10.1212/WNL.0000000000007767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, M. M. (2020). Consideration of sex differences in the measurement and interpretation of Alzheimer Disease‐related biofluid‐based biomarkers. The Journal of Applied Laboratory Medicine, 5(1), 158–169. 10.1373/jalm.2019.030023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, M. M. , Hagen, C. E. , Wennberg, A. M. V. , Airey, D. C. , Savica, R. , Knopman, D. S. , Machulda, M. M. , Roberts, R. O. , Jack, C. R. , Petersen, R. C. , & Dage, J. L. (2017). Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic study on aging. JAMA Neurology, 74(9), 1073–1080. 10.1001/jamaneurol.2017.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, M. M. , Hagen, C. E. , Xu, J. , Chai, X. , Vemuri, P. , Lowe, V. J. , Airey, D. C. , Knopman, D. S. , Roberts, R. O. , Machulda, M. M. , Jack, C. R. , Petersen, R. C. , & Dage, J. L. (2018). Plasma phospho‐tau181 increases with Alzheimers disease clinical severity and is associated with tau‐ and amyloid‐positron emission tomography. Alzheimers and Dementia, 14(8), 989–997. 10.1016/j.jalz.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minger, S. L. , Honer, W. G. , Esiri, M. M. , Mcdonald, B. , Keene, J. , Nicoll, J. A. R. , Carter, J. , Hope, T. , & Francis, P. T. (2001). Synaptic pathology in prefrontal cortex is present only with severe dementia in Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 60(10), 929–936. 10.1093/jnen/60.10.929 [DOI] [PubMed] [Google Scholar]
- Mitchell, A. J. (2009). A meta‐analysis of the accuracy of the mini‐mental state examination in the detection of dementia and mild cognitive impairment. Journal of Psychiatric Research, 43(4), 411–431. 10.1016/j.jpsychires.2008.04.014 [DOI] [PubMed] [Google Scholar]
- Mouton‐Liger, F. , Dumurgier, J. , Cognat, E. , Hourregue, C. , Zetterberg, H. , Vanderstichele, H. , Vanmechelen, E. , Bouaziz‐Amar, E. , Blennow, K. , Hugon, J. , & Paquet, C. (2020). CSF levels of the BACE1 substrate NRG1 correlate with cognition in Alzheimers disease. Alzheimer's Research & Therapy, 12(1), 88. 10.1186/s13195-020-00655-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaetova‐Ladinska, E. B. , Xuereb, J. H. , Garcia‐Sierra, F. , Hurt, J. , Gertz, H. J. , Hills, R. , Brayne, C. , Huppert, F. A. , Paykel, E. S. , McGee, M. A. , Jakes, R. , Honer, W. G. , Harrington, C. R. , & Wischik, C. M. (2009). Lewy body variant of Alzheimers disease: Selective neocortical loss of t‐SNARE proteins and loss of MAP 2 and α‐Synuclein in medial temporal lobe. The Scientific World Journal, 9, 1463–1475. 10.1100/tsw.2009.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai, K. K. , Misner, D. , & Ranscht, B. (2002). Contactin supports synaptic plasticity associated with hippocampal long‐term depression but not potentiation. Current Biology, 12(3), 181–190. 10.1016/S0960-9822(02)00680-2 [DOI] [PubMed] [Google Scholar]
- Najera, K. , Fagan, B. M. , & Thompson, P. M. (2019). SNAP‐25 in major psychiatric disorders: A review. Neuroscience, 420, 79–85). Elsevier Ltd. 10.1016/j.neuroscience.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Nakamura, A. , Kaneko, N. , Villemagne, V. L. , Kato, T. , Doecke, J. , Doré, V. , Fowler, C. , Li, Q. X. , Martins, R. , Rowe, C. , Tomita, T. , Matsuzaki, K. , Ishii, K. , Ishii, K. , Arahata, Y. , Iwamoto, S. , Ito, K. , Tanaka, K. , Masters, C. L. , & Yanagisawa, K. (2018). High performance plasma amyloid‐β biomarkers for Alzheimers disease. Nature, 554(7691), 249–254. 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health . (2014). Quality assessment tool for observational cohort and cross‐sectional studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Neary, D. , Snowden, J. S. , Gustafson, L. , Passant, U. , Stuss, D. , Black, S. , Freedman, M. , Kertesz, A. , Robert, P. H. , Albert, M. , Boone, K. , Miller, B. L. , Cummings, J. , & Benson, D. F. (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554. 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Neve, R. L. , Finch, E. A. , Bird, E. D. , & Benowitz, L. I. (1988). Growth‐associated protein GAP‐43 is expressed selectively in associative regions of the adult human brain. Proceedings of the National Academy of Sciences of the United States of America, 85(10), 3638–3642. 10.1073/pnas.85.10.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor, A. , & Zahid, S. (2017). A review of the role of synaptosomal‐associated protein 25 (SNAP‐25) in neurological disorders. International Journal of Neuroscience, 127(9), 805–811. 10.1080/00207454.2016.1248240 [DOI] [PubMed] [Google Scholar]
- Oeckl, P. , Halbgebauer, S. , Anderl‐Straub, S. , von Arnim, C. A. F. , Diehl‐Schmid, J. , Froelich, L. , Grimmer, T. , Hausner, L. , Denk, J. , Jahn, H. , Steinacker, P. , Weishaupt, J. H. , Ludolph, A. C. , & Otto, M. (2020). Targeted mass spectrometry suggests beta‐synuclein as synaptic blood marker in Alzheimers disease. 19(3), 1318. 10.1021/acs.jproteome.9b00824 [DOI] [PubMed] [Google Scholar]
- Öhrfelt, A. , Brinkmalm, A. , Dumurgier, J. , Zetterberg, H. , Bouaziz‐Amar, E. , Hugon, J. , Paquet, C. , & Blennow, K. (2019). A novel ELISA for the measurement of cerebrospinal fluid SNAP‐25 in patients with Alzheimer's disease. Neuroscience, 420, 136–144. 10.1016/j.neuroscience.2018.11.038 [DOI] [PubMed] [Google Scholar]
- Öhrfelt, A. , Brinkmalm, A. , Dumurgier, J. , Brinkmalm, G. , Hansson, O. , Zetterberg, H. , Bouaziz‐Amar, E. , Hugon, J. , Paquet, C. , & Blennow, K. (2016). The pre‐synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimers disease. 8(1), 41. 10.1186/s13195-016-0208-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson, B. , Lautner, R. , Andreasson, U. , Öhrfelt, A. , Portelius, E. , Bjerke, M. , Hölttä, M. , Rosén, C. , Olsson, C. , Strobel, G. , Wu, E. , Dakin, K. , Petzold, M. , Blennow, K. , & Zetterberg, H. (2016). CSF and blood biomarkers for the diagnosis of Alzheimers disease: A systematic review and meta‐analysis. The Lancet Neurology, 15(7), 673–684. 10.1016/S1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- Ortega, R. L. , Dakterzada, F. , Arias, A. , Blasco, E. , Naudí, A. , Garcia, F. P. , & Piñol‐Ripoll, G. (2019). Usefulness of CSF biomarkers in predicting the progression of amnesic and nonamnesic mild cognitive impairment to Alzheimers Disease. Current Aging Science, 12(1), 35–42. 10.2174/1874609812666190112095430 [DOI] [PubMed] [Google Scholar]
- Osborn, K. E. , Khan, O. A. , Kresge, H. A. , Bown, C. W. , Liu, D. , Moore, E. E. , Gifford, K. A. , Acosta, L. M. Y. , Bell, S. P. , Hohman, T. J. , Blennow, K. , Zetterberg, H. , & Jefferson, A. L. (2019). Cerebrospinal fluid and plasma neurofilament light relate to abnormal cognition. Alzheimers Dement (Amst), 11, 700–709. 10.1016/j.dadm.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osera, C. , Pascale, A. , Amadio, M. , Venturini, L. , Govoni, S. , & Ricevuti, G. (2012). Pentraxins and Alzheimers disease: At the interface between biomarkers and pharmacological targets. Ageing Research Reviews, 11(2), 189–198. 10.1016/j.arr.2011.12.004 [DOI] [PubMed] [Google Scholar]
- Ottoy, J. , Niemantsverdriet, E. , Verhaeghe, J. , De Roeck, E. , Struyfs, H. , Somers, C. , wyffels, L. , Ceyssens, S. , Van Mossevelde, S. , Van den Bossche, T. , Van Broeckhoven, C. , Ribbens, A. , Bjerke, M. , Stroobants, S. , Engelborghs, S. , & Staelens, S. (2019). Association of short‐term cognitive decline and MCI‐to‐AD dementia conversion with CSF, MRI, amyloid‐ and 18 F‐FDG‐PET imaging. NeuroImage: Clinical, 22, 101771. 10.1016/j.nicl.2019.101771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovod, V. , Ramsey, K. N. , Mawuenyega, K. G. , Bollinger, J. G. , Hicks, T. , Schneider, T. , Sullivan, M. , Paumier, K. , Holtzman, D. M. , Morris, J. C. , Benzinger, T. , Fagan, A. M. , Patterson, B. W. , & Bateman, R. J. (2017). Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers and Dementia, 13(8), 841–849. 10.1016/j.jalz.2017.06.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankonin, M. S. , Sohi, J. , Kamholza, J. , & Loeba, J. A. (2009). Differential distribution of neuregulin in human brain and spinal fluid. Brain Research, 1258, 1–11. 10.1016/j.brainres.2008.12.047 [DOI] [PubMed] [Google Scholar]
- Pereira, J. B. , Westman, E. , Hansson, O. , & Alzheimers Disease Neuroimaging Initiative . (2017). Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimers disease. Neurobiology of Aging, 58, 14–29. 10.1016/j.neurobiolaging.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Petersen, A. , & Gerges, N. Z. (2015). Neurogranin regulates CaM dynamics at dendritic spines. Scientific Reports, 5(1), 11135. 10.1038/srep11135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen, R. C. , Aisen, P. S. , Beckett, L. A. , Donohue, M. C. , Gamst, A. C. , Harvey, D. J. , Jack, C. R. , Jagust, W. J. , Shaw, L. M. , Toga, A. W. , Trojanowski, J. Q. , & Weiner, M. W. (2010). Alzheimers disease neuroimaging initiative (ADNI): Clinical characterization. Neurology, 74(3), 201–209. 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, A. , Keir, G. , Warren, J. , Fox, N. , & Rossor, M. N. (2007). A systematic review and meta‐analysis of CSF neurofilament protein levels as biomarkers in dementia. Neuro‐Degenerative Diseases, 4(2–3), 185–194. 10.1159/000101843 [DOI] [PubMed] [Google Scholar]
- Petzold, A. (2005). Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. Journal of the Neurological Sciences, 233(1–2), 183–198. 10.1016/j.jns.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Phelps, M. E. , Huang, S. C. , Hoffman, E. J. , Selin, C. , Sokoloff, L. , & Kuhl, D. E. (1979). Tomographic measurement of local cerebral glucose metabolic rate in humans with (F‐18)2‐fluoro‐2‐deoxy‐D‐glucose: Validation of method. Annals of Neurology, 6(5), 371–388. 10.1002/ana.410060502 [DOI] [PubMed] [Google Scholar]
- Pickett, E. K. , Herrmann, A. G. , McQueen, J. , Abt, K. , Dando, O. , Tulloch, J. , Jain, P. , Dunnett, S. , Sohrabi, S. , Fjeldstad, M. P. , Calkin, W. , Murison, L. , Jackson, R. J. , Tzioras, M. , Stevenson, A. , dOrange, M. , Hooley, M. , Davies, C. , Colom‐Cadena, M. , … Spires‐Jones, T. L. (2019). Amyloid Beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimers Disease. Cell Reports, 29(11), 3592, e5–3604. 10.1016/j.celrep.2019.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portelius, E. , Olsson, B. , Höglund, K. , Cullen, N. C. , Kvartsberg, H. , Andreasson, U. , Zetterberg, H. , Sandelius, Å. , Shaw, L. M. , Lee, V. M. Y. , Irwin, D. J. , Grossman, M. , Weintraub, D. , Chen‐Plotkin, A. , Wolk, D. A. , McCluskey, L. , Elman, L. , McBride, J. , Toledo, J. B. , … Blennow, K. (2018). Cerebrospinal fluid neurogranin concentration in neurodegeneration: Relation to clinical phenotypes and neuropathology. Acta Neuropathologica, 136(3), 363–376. 10.1007/s00401-018-1851-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portelius, E. , Zetterberg, H. , Skillbäck, T. , Törnqvist, U. , Andreasson, U. , Trojanowski, J. Q. , Weiner, M. W. , Shaw, L. M. , Mattsson, N. , Blennow, K. , & Alzheimers Disease Neuroimaging Initiative . (2015). Cerebrospinal fluid neurogranin: Relation to cognition and neurodegeneration in Alzheimers disease. Brain: A Journal of Neurology, 138(11), 3373–3385. 10.1093/brain/awv267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, R. , Patterson, B. W. , Elbert, D. L. , Ovod, V. , Kasten, T. , Sigurdson, W. , Mawuenyega, K. , Blazey, T. , Goate, A. , Chott, R. , Yarasheski, K. E. , Holtzman, D. M. , Morris, J. C. , Benzinger, T. L. S. , & Bateman, R. J. (2013). Increased in vivo amyloid‐b42 production, exchange, and loss in presenilin mutation carriers. Science Translational Medicine, 5(189), 189ra77. 10.1126/scitranslmed.3005615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine, A. , Clark, L. , Berman, S. , Koscik, R. , Mueller, K. , Norton, D. , Nicholas, C. , Blennow, K. , Zetterberg, H. , Jedynak, B. , Bilgel, M. , Carlsson, C. , Christian, B. , Asthana, S. , & Johnson, S. (2016). Associations between performance on an abbreviated CogState battery, other measures of cognitive function, and biomarkers in people at Risk for Alzheimers disease. Journal of Alzheimer's disease, 54(4), 1395–1408. 10.3233/JAD-160528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall, J. , Mörtberg, E. , Provuncher, G. K. , Fournier, D. R. , Duffy, D. C. , Rubertsson, S. , Blennow, K. , Zetterberg, H. , & Wilson, D. H. (2013). Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: Results of a pilot study. Resuscitation, 84(3), 351–356. 10.1016/j.resuscitation.2012.07.027 [DOI] [PubMed] [Google Scholar]
- Randolph, C. (1998). Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). The Psychological Corporation. http://www.harcourt-uk.com/product [DOI] [PubMed] [Google Scholar]
- Rascovsky, K. , Hodges, J. R. , Knopman, D. , Mendez, M. F. , Kramer, J. H. , Neuhaus, J. , Van Swieten, J. C. , Seelaar, H. , Dopper, E. G. P. , Onyike, C. U. , Hillis, A. E. , Josephs, K. A. , Boeve, B. F. , Kertesz, A. , Seeley, W. W. , Rankin, K. P. , Johnson, J. K. , Gorno‐Tempini, M. L. , Rosen, H. , … Miller, B. L. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, P. H. , Mani, G. , Park, B. S. , Jacques, J. , Murdoch, G. , Whetsell, W. , Kaye, J. , & Manczak, M. (2005). Differential loss of synaptic proteins in Alzheimers disease: Implications for synaptic dysfunction. Journal of Alzheimers Disease, 7(2), 103–117. 10.3233/JAD-2005-7203 [DOI] [PubMed] [Google Scholar]
- Rekart, J. L. , Quinn, B. , Mesulam, M. M. , & Routtenberg, A. (2004). Subfield‐specific increase in brain growth protein in postmortem hippocampus of Alzheimers patients. Neuroscience, 126(3), 579–584. 10.1016/j.neuroscience.2004.03.060 [DOI] [PubMed] [Google Scholar]
- Remnestål, J. , Just, D. , Mitsios, N. , Fredolini, C. , Mulder, J. , Schwenk, J. M. , Uhlén, M. , Kultima, K. , Ingelsson, M. , Kilander, L. , Lannfelt, L. , Svenningsson, P. , Nellgård, B. , Zetterberg, H. , Blennow, K. , Nilsson, P. , & Häggmark‐Månberg, A. (2016). CSF profiling of the human brain enriched proteome reveals associations of neuromodulin and neurogranin to Alzheimers disease. Proteomics ‐ Clinical Applications, 10(12), 1242–1253. 10.1002/prca.201500150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riascos, D. , Nicholas, A. , Samaeekia, R. , Yukhananov, R. , Mesulam, M. M. , Bigio, E. H. , Weintraub, S. , Guo, L. , & Geula, C. (2014). Alterations of Ca2+−responsive proteins within cholinergic neurons in aging and Alzheimers disease. Neurobiology of Aging, 35(6), 1325–1333. 10.1016/j.neurobiolaging.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, C. W. , Muniz‐Terrera, G. , Kivipelto, M. , Solomon, A. , Tom, B. , & Molinuevo, J. L. (2020). The European prevention of Alzheimers dementia (EPAD) longitudinal cohort study: Baseline data release V500.0. The Journal of Prevention of Alzheimers Disease, 7(1), 8–13. 10.14283/jpad.2019.46 [DOI] [PubMed] [Google Scholar]
- Roberts, R. O. , Geda, Y. E. , Knopman, D. S. , Cha, R. H. , Pankratz, V. S. , Boeve, B. F. , Ivnik, R. J. , Tangalos, E. G. , Petersen, R. C. , & Rocca, W. A. (2008). The Mayo Clinic study of aging: Design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology, 30(1), 58–69. 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher, A. B. , Chapon, F. , Blaizot, X. , Baron, J. C. , & Chavoix, C. (2003). Resting‐state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: A study in baboons. NeuroImage, 20(3), 1894–1898. 10.1016/j.neuroimage.2003.07.002 [DOI] [PubMed] [Google Scholar]
- Rojas, J. , Bang, J. , Lobach, I. , Tsai, R. , Rabinovici, G. , Miller, B. , & Boxer, A. (2018). CSF neurofilament‐light chain and phosphorylated‐tau predict disease progression in PSP. 90(4), e273–e281. 10.1212/WNL.0000000000004859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolstad, S. , Berg, A. I. , Eckerstrom, C. , Johansson, B. , & Wallin, A. (2015). Differential impact of neurofilament light subunit on cognition and functional outcome in memory clinic patients with and without vascular burden. Journal of Alzheimer's Disease, 45(3), 873–881. 10.3233/JAD-142694 [DOI] [PubMed] [Google Scholar]
- Rolstad, S. , Jakobsson, J. , Sellgren, C. , Ekman, C. J. , Blennow, K. , Zetterberg, H. , Palsson, E. , & Landen, M. (2015). Cognitive performance and cerebrospinal fluid biomarkers of neurodegeneration: A study of patients with bipolar disorder and healthy controls. PLoS One, 10(5), e0127100. 10.1371/journal.pone.0127100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren, L. E. , Karlsson, J. E. , Sjögren, M. , Blennow, K. , & Wallin, A. (1999). Neurofilament protein levels in CSF are increased in dementia. Neurology, 52(5), 1090–1093. 10.1212/wnl.52.5.1090 [DOI] [PubMed] [Google Scholar]
- Sager, M. A. , Hermann, B. , & La Rue, A. (2005). Middle‐aged children of persons with Alzheimers disease: APOE genotypes and cognitive function in the Wisconsin registry for Alzheimers prevention. Journal of Geriatric Psychiatry and Neurology, 18(4), 245–249. 10.1177/0891988705281882 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (2019). Trajectories of normal cognitive aging. Psychology and Aging, 34(1), 17–24. 10.1037/PAG0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancesario, G. , Di Lazzaro, G. , Alwardat, M. , Biticchi, B. , Basile, V. , Salimei, C. , Luigi Colona, V. , Sinibaldi Salimei, P. , Bernardini, S. , Mercuri, N. , Pisani, A. , & Schirinzi, T. (2020). Amyloid‐beta42/Neurogranin Ratio as a Potential Index for Cognitive Impairment in Parkinsons Disease. ((Sancesario, Mercuri, Pisani) IRCCS Fondazione Santa Lucia, Rome, Italy (Sancesario, Biticchi, Basile) Department of Experimental Medicine and Surgery, University of Roma Tor Vergata, Rome, Italy (Di Lazzaro, Alwardat, Salimei, Luigi Colona, Bernardini, M). 76(3), 1171–1178. 10.3233/JAD-200344 [DOI] [Google Scholar]
- Sandelius, Å. , Portelius, E. , Källén, Å. , Zetterberg, H. , Rot, U. , Olsson, B. , Toledo, J. B. , Shaw, L. M. , Lee, V. M. Y. , Irwin, D. J. , Grossman, M. , Weintraub, D. , Chen‐Plotkin, A. , Wolk, D. A. , McCluskey, L. , Elman, L. , Kostanjevecki, V. , Vandijck, M. , McBride, J. , … Blennow, K. (2019). Elevated CSF GAP‐43 is Alzheimers disease specific and associated with tau and amyloid pathology. Alzheimers & Dementia, 15(1), 55–64. 10.1016/j.jalz.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo, C. , Forlenza, O. , Zetterberg, H. , & Blennow, K. (2016). Increased neurogranin concentrations in cerebrospinal fluid of Alzheimers disease and in mild cognitive impairment due to AD. Journal of Neural Transmission, 123(12), 1443–1447. 10.1007/s00702-016-1597-3 [DOI] [PubMed] [Google Scholar]
- Santillo, A. , Lundgren, S. , Xu, C. , Orhan, F. , Fatouros‐Bergman, H. , Blennow, K. , Zetterberg, H. , Portelius, E. , Cervenka, S. , J, E. G. , Erhardt, S. , & Engberg, G. (2019). Neurogranin as a potential synaptic marker in the cerebrospinal fluid of patients with a first episode psychosis. Schizophr Res, 208, 490–492. 10.1016/j.schres.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Savage, M. J. , Kalinina, J. , Wolfe, A. , Tugusheva, K. , Korn, R. , Cash‐Mason, T. , Maxwell, J. W. , Hatcher, N. G. , Haugabook, S. J. , Wu, G. , Howell, B. J. , Renger, J. J. , Shughrue, P. J. , & McCampbell, A. (2014). A sensitive Aβ oligomer assay discriminates Alzheimers and aged control cerebrospinal fluid. Journal of Neuroscience, 34(8), 2884–2897. 10.1523/JNEUROSCI.1675-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling, C. S. , Hall, T. , Berisha, F. , Klepac, K. , Karydas, A. , Coppola, G. , Kramer, J. H. , Rabinovici, G. , Ahlijanian, M. , Miller, B. L. , Seeley, W. , Grinberg, L. T. , Rosen, H. , Meredith, J. , & Boxer, A. L. (2014). Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Annals of Neurology, 75(1), 116–126. 10.1002/ana.24052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, S. , Li, Y. , Todd, K. W. , Herries, E. M. , Henson, R. L. , Gray, J. D. , Wang, G. , Graham, D. L. , Shaw, L. M. , Trojanowski, J. Q. , Hassenstab, J. J. , Benzinger, T. L. S. , Cruchaga, C. , Jucker, M. , Levin, J. , Chhatwal, J. P. , Noble, J. M. , Ringman, J. M. , Graff‐Radford, N. R. , … Dominantly Inherited Alzheimer Network . (2019). Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimers disease. Alzheimer's & Dementia, 15(5), 655–665. 10.1016/j.jalz.2018.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano, N. , López‐Sanz, D. , Bruña, R. , Garcés, P. , Rodríguez‐Rojo, I. C. , Marcos, A. , Crespo, D. P. , & Maestú, F. (2020). Spatiotemporal oscillatory patterns during working memory maintenance in mild cognitive impairment and subjective cognitive decline. International Journal of Neural Systems, 30(1), 1950019. 10.1142/S0129065719500199 [DOI] [PubMed] [Google Scholar]
- Shi, L. , & Bergson, C. M. (2020). Neuregulin 1: An intriguing therapeutic target for neurodevelopmental disorders. Translational Psychiatry, 10(190), 190. 10.1038/s41398-020-00868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, L. I. , Tayler, H. M. , & Love, S. (2015). Synaptic protein levels altered in vascular dementia. Neuropathology and Applied Neurobiology, 41(4), 533–543. 10.1111/nan.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, J. B. , Fedgchin, M. , Daly, E. J. , De Boer, P. , Cooper, K. , Lim, P. , Pinter, C. , Murrough, J. W. , Sanacora, G. , Shelton, R. C. , Kurian, B. , Winokur, A. , Fava, M. , Manji, H. , Drevets, W. C. , & Van Nueten, L. (2016). A double‐blind, randomized, placebo‐controlled, dose‐frequency study of intravenous ketamine in patients with treatment‐resistant depression. American Journal of Psychiatry, 173(8), 816–826. 10.1176/appi.ajp.2016.16010037 [DOI] [PubMed] [Google Scholar]
- Sjogren, M. , Blomberg, M. , Jonsson, M. , Wahlund, L. O. , Lind, K. , Rosengren, L. , Blennow, K. , & Wallin, A. (2001). Neurofilament protein in cerebrospinal fluid: A marker of white matter changes. Journal of Neuroscience Research, 66(3), 510–516. 10.1002/jnr.1242 [DOI] [PubMed] [Google Scholar]
- Sjogren, M. , Rosengren, L. , Minthon, L. , Davidsson, P. , Blennow, K. , & Wallin, A. (2000). Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology, 54(10), 1960–1964. 10.1212/WNL.54.10.1960 [DOI] [PubMed] [Google Scholar]
- Skillback, T. , Farahmand, B. , Bartlett, J. W. , Rosen, C. , Mattsson, N. , Nagga, K. , Kilander, L. , Religa, D. , Wimo, A. , Winblad, B. , Rosengren, L. , Schott, J. M. , Blennow, K. , Eriksdotter, M. , & Zetterberg, H. (2014). CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology, 83(21), 1945–1953. 10.1212/WNL.0000000000001015 [DOI] [PubMed] [Google Scholar]
- Slaets, S. , Vanmechelen, E. , Le Bastard, N. , Decraemer, H. , Vandijck, M. , Martin, J.‐J. , De Deyn, P. P. , & Engelborghs, S. (2014). Increased CSF α‐synuclein levels in Alzheimers disease: Correlation with tau levels. Alzheimers & Dementia, 10(5), S290–S298. 10.1016/j.jalz.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Sokoloff, L. (1977). Relation between physiological function and energy metabolism in the central nervous system. Journal of Neurochemistry, 29(1), 13–26. 10.1111/j.1471-4159.1977.tb03919.x [DOI] [PubMed] [Google Scholar]
- Soldan, A. , Moghekar, A. , Walker, K. A. , Pettigrew, C. , Hou, X. R. , Lu, H. Z. , Miller, M. I. , Alfini, A. , Albert, M. , Xu, D. S. , Xiao, M. F. , Worley, P. , & Biocard Res, T. (2019). Resting‐state functional connectivity is associated with cerebrospinal fluid levels of the synaptic protein NPTX2 in non‐demented older adults. Frontiers in Aging Neuroscience, 11, 132. 10.3389/fnagi.2019.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, A. , Kivipelto, M. , Molinuevo, J. L. , Tom, B. , & Ritchie, C. W. (2018). European prevention of Alzheimers dementia longitudinal cohort study (EPAD LCS): Study protocol. BMJ Open, 8(12), e021017. 10.1136/bmjopen-2017-021017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector, R. , Robert Snodgrass, S. , & Johanson, C. E. (2015). A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. In Experimental Neurology, 273, 57–68. 10.1016/j.expneurol.2015.07.027 [DOI] [PubMed] [Google Scholar]
- Sudo, F. K. , De Souza, A. S. , Drummond, C. , Assuncao, N. , Teldeschi, A. , Oliveira, N. , Rodrigues, F. , Santiago‐Bravo, G. , Calil, V. , Lima, G. , Erthal, P. , Bernardes, G. , Monteiro, M. , Tovar‐Moll, F. , & Mattos, P. (2019). Inter‐method and anatomical correlates of episodic memory tests in the Alzheimers Disease spectrum. PLoS ONE, 14(10), e0223731. 10.1371/journal.pone.0223731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman, M. A. , Zetterberg, H. , Blennow, K. , Tripodis, Y. , McKee, A. C. , Stein, T. D. , Martin, B. , Palmisano, J. N. , Steinberg, E. G. , Simkin, I. , Budson, A. E. , Killiany, R. , OConnor, M. K. , Au, R. , Qiao Qiu, W. W. , Goldstein, L. E. , Kowall, N. W. , Mez, J. , Stern, R. A. , & Alosco, M. L. (2020). A longitudinal examination of plasma Neurofilament light and Total tau for the clinical detection and monitoring of Alzheimers Disease. Neurobiology of Aging, 94, 60–70. 10.1016/j.neurobiolaging.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Dong, C. , Levin, B. , Crocco, E. , Loewenstein, D. , Zetterberg, H. , Blennow, K. , Wright, C. B. , & Alzheimers Disease Neuroimaging Initiative . (2016). APOE ε4 carriers may undergo synaptic damage conferring risk of Alzheimers disease. Alzheimers & Dementia, 12(11), 1159–1166. 10.1016/j.jalz.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, A. , Willette, A. A. , & Alzheimers Disease Neuroimaging Initiative . (2016). Neuronal Pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimers disease spectrum. Brain Behav Immun, 58, 201–208. 10.1016/j.bbi.2016.07.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasoff‐Conway, J. M. , Carare, R. O. , Osorio, R. S. , Glodzik, L. , Butler, T. , Fieremans, E. , Axel, L. , Rusinek, H. , Nicholson, C. , Zlokovic, B. V. , Frangione, B. , Blennow, K. , Ménard, J. , Zetterberg, H. , Wisniewski, T. , & De Leon, M. J. (2015). Clearance systems in the brain ‐ Implications for Alzheimer disease. In Nature reviews neurology Vol. 11, (8), pp. 457–470. Nature Publishing Group. 10.1038/nrneurol.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitsdottir, U. D. , Jonsdottir, M. K. , Lund, S. H. , Darreh‐Shori, T. , Snaedal, J. , & Petersen, P. H. (2020). Association of glial and neuronal degeneration markers with Alzheimers disease cerebrospinal fluid profile and cognitive functions. Alzheimers Research and Therapy, 12(1), 1–14. 10.1186/s13195-020-00657-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry, R. D. , Masliah, E. , Salmon, D. P. , Butters, N. , DeTeresa, R. , Hill, R. , Hansen, L. A. , & Katzman, R. (1991). Physical basis of cognitive alterations in alzheimers disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology, 30(4), 572–580. 10.1002/ana.410300410 [DOI] [PubMed] [Google Scholar]
- Tible, M. , Sandelius, Å. , Höglund, K. , Brinkmalm, A. , Cognat, E. , Dumurgier, J. , Zetterberg, H. , Hugon, J. , Paquet, C. , & Blennow, K. (2020). Dissection of synaptic pathways through the CSF biomarkers for predicting Alzheimer disease. Neurology, 95(8), e953–e961. 10.1212/WNL.0000000000010131 [DOI] [PubMed] [Google Scholar]
- Uhlén, M. , Fagerberg, L. , Hallström, B. M. , Lindskog, C. , Oksvold, P. , Mardinoglu, A. , Sivertsson, Å. , Kampf, C. , Sjöstedt, E. , Asplund, A. , Olsson, I. M. , Edlund, K. , Lundberg, E. , Navani, S. , Szigyarto, C. A. K. , Odeberg, J. , Djureinovic, D. , Takanen, J. O. , Hober, S. , … Pontén, F. (2015). Tissue‐based map of the human proteome. Science, 347(6220), 1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- van der Ende, E. L. , Xiao, M. , Xu, D. , Poos, J. , Panman, J. , Jiskoot, L. , Meeter, L. , Dopper, E. G. , Papma, J. , Heller, C. , Convery, R. , Moore, K. , Bocchetta, M. , Neason, M. , Peakman, G. , Cash, D. , Teunissen, C. , Graff, C. , Synofzik, M. , … Van Swieten, J. (2020). Neuronal pentraxin 2: A synapse‐derived CSF biomarker in genetic frontotemporal dementia. J Neurol Neurosurg Psychiatry, 91(6), 612–621. 10.1136/jnnp-2019-322493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende, Emma L. , Meeter, L. , Stingl, C. , Van Rooij, J. G. J. , Stoop, M. P. , Nijholt, D. A. T. , Sanchez‐Valle, R. , Graff, C. , Öijerstedt, L. , Grossman, M. , McMillan, C. , Pijnenburg, Y. A. L. , Laforce, R. , Binetti, G. , Benussi, L. , Ghidoni, R. , Luider, T. M. , Seelaar, H. , & Van Swieten, J. C. (2019). Novel CSF biomarkers in genetic frontotemporal dementia identified by proteomics. Annals of Clinical and Translational Neurology, 6(4), 698–707. 10.1002/acn3.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Der Flier, W. , & Scheltens, P. (2018). Amsterdam dementia cohort: Performing research to optimize care. Journal of Alzheimers Disease, 62(3), 1091–1111. IOS Press. 10.3233/JAD-170850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenoven, I. , Koel‐Simmelink, M. J. , Vergouw, L. J. , Tijms, B. , Piersma, S. , Pham, T. , Bridel, C. , Ferri, G.‐L. , Cocco, C. , Noli, B. , Worley, P. , Xiao, M.‐F. , Xu, D. , Oeckl, P. , Otto, M. , van Der Flier, W. , De Jong, F. , Jimenez, C. , Lemstra, A. , & Teunissen, C. (2020). Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: A proteomic approach. Mol Neurodegener, 15(1), 36. 10.1186/s13024-020-00388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergallo, A. , Bun, R. S. , Toschi, N. , Baldacci, F. , Zetterberg, H. , Blennow, K. , Cavedo, E. , Lamari, F. , Habert, M. O. , Dubois, B. , Floris, R. , Garaci, F. , Lista, S. , Hampel, H. , Audrain, C. , Auffret, A. , Bakardjian, H. , Baldacci, F. , Batrancourt, B. , … Younesi, E. (2018). Association of cerebrospinal fluid α‐synuclein with total and phospho‐tau181 protein concentrations and brain amyloid load in cognitively normal subjective memory complainers stratified by Alzheimers disease biomarkers. Alzheimers and Dementia, 14(12), 1623–1631. 10.1016/j.jalz.2018.06.3053 [DOI] [PubMed] [Google Scholar]
- Vogl, C. , Tanifuji, S. , Danis, B. , Daniels, V. , Foerch, P. , Wolff, C. , Whalley, B. J. , Mochida, S. , & Stephens, G. J. (2015). Synaptic vesicle glycoprotein 2A modulates vesicular release and calcium channel function at peripheral sympathetic synapses. European Journal of Neuroscience, 41(4), 398–409. 10.1111/ejn.12799 [DOI] [PubMed] [Google Scholar]
- Wallin, A. , Nordlund, A. , Jonsson, M. , Lind, K. , Edman, Å. , Göthlin, M. , Stålhammar, J. , Eckerström, M. , Kern, S. , Börjesson‐Hanson, A. , Carlsson, M. , Olsson, E. , Zetterberg, H. , Blennow, K. , Svensson, J. , Öhrfelt, A. , Bjerke, M. , Rolstad, S. , & Eckerström, C. (2016). The Gothenburg MCI study: Design and distribution of Alzheimers disease and subcortical vascular disease diagnoses from baseline to 6‐year follow‐up. Journal of cerebral blood flow and metabolism, 36(1), 114–131). Nature Publishing Group. 10.1038/jcbfm.2015.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. (2019). Association of cerebrospinal fluid Neurogranin with Alzheimers disease. Aging Clinical and Experimental Research, 31(2), 185–191. 10.1007/s40520-018-0948-3 [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Zhou, W. , & Zhang, J. (2018). Levels of cortisol in CSF are associated with SNAP‐25 and tau pathology but not amyloid‐β. Frontiers in Aging Neuroscience, 10, 383. 10.3389/fnagi.2018.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Zhang, J. , & Pan, T. (2018). APOE ε4 is associated with higher levels of CSF SNAP‐25 in prodromal Alzheimers disease. Neuroscience Letters, 685, 109–113. 10.1016/j.neulet.2018.08.029 [DOI] [PubMed] [Google Scholar]
- Wattmo, C. , Blennow, K. , & Hansson, O. (2020). Cerebro‐spinal fluid biomarker levels: Phosphorylated tau (T) and total tau (N) as markers for rate of progression in Alzheimers disease. BMC Neurology, 20(1), 10. 10.1186/s12883-019-1591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, H. , Paterson, R. W. , Portelius, E. , Törnqvist, U. , Magdalinou, N. , Fox, N. C. , Blennow, K. , Schott, J. M. , & Zetterberg, H. (2016). Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology, 86(9), 829–835. 10.1212/WNL.0000000000002423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington, H. , Paterson, R. W. , Suárez‐González, A. , Poole, T. , Frost, C. , Sjöbom, U. , Slattery, C. F. , Magdalinou, N. K. , Lehmann, M. , Portelius, E. , Fox, N. C. , Blennow, K. , Zetterberg, H. , & Schott, J. M. (2018). CSF neurogranin or tau distinguish typical and atypical Alzheimer disease. Annals of Clinical and Translational Neurology, 5(2), 162–171. 10.1002/acn3.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J. K. , Yang, X. , & Baum, J. (2018). Interactions between the Intrinsically Disordered Proteins β‐Synuclein and α‐Synuclein. Proteomics, 18(21–22), e1800109. Wiley‐VCH Verlag. 10.1002/pmic.201800109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad, B. , Palmer, K. , Kivipelto, M. , Jelic, V. , Fratiglioni, L. , Wahlund, L. O. , Nordberg, A. , Bäckman, L. , Albert, M. , Almkvist, O. , Arai, H. , Basun, H. , Blennow, K. , De Leon, M. , Decarli, C. , Erkinjuntti, T. , Giacobini, E. , Graff, C. , Hardy, J. , … Petersen, R. C. (2004). Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256(3), 240–246. 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- Wolk, D. A. , & Dickerson, B. C. (2011). Fractionating verbal episodic memory in Alzheimers disease. NeuroImage, 54(2), 1530–1539. 10.1016/j.neuroimage.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman, M. A. , Sittaramane, V. K. , Essner, J. J. , Yost, H. J. , Chandrasekhar, A. , & Halloran, M. C. (2008). Transient axonal glycoprotein‐1 (TAG‐1) and laminin‐α1 regulate dynamic growth cone behaviors and initial axon direction in vivo. Neural Development, 3, 6. 10.1186/1749-8104-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . (2020). Dementia fact sheet. https://www.who.int/news-room/fact-sheets/detail/dementia
- Xiao, M.‐F. , Xu, D. , Craig, M. T. , Pelkey, K. A. , Chien, C.‐C. , Shi, Y. , Zhang, J. , Resnick, S. , Pletnikova, O. , Salmon, D. , Brewer, J. , Edland, S. , Wegiel, J. , Tycko, B. , Savonenko, A. , Reeves, R. H. , Troncoso, J. C. , McBain, C. J. , Galasko, D. , & Worley, P. F. (2017). NPTX2 and cognitive dysfunction in Alzheimers Disease. eLife, 6, e23798. 10.7554/eLife.23798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y. , Liu, T. , Ai, J. , Chen, D. , Zhuo, Y. , Zhao, G. , He, S. , Wu, J. , Han, Y. , & Yan, T. (2019). Changes in centrality frequency of the default mode network in individuals with subjective cognitive decline. Frontiers in Aging Neuroscience, 11, 118. 10.3389/fnagi.2019.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. C. , Chiu, M. J. , Chen, T. F. , Chang, H. L. , Liu, B. H. , & Yang, S. Y. (2018). Assay of plasma phosphorylated tau protein (threonine 181) and total tau protein in early‐stage Alzheimers disease. Journal of Alzheimers Disease, 61(4), 1323–1332. 10.3233/JAD-170810 [DOI] [PubMed] [Google Scholar]
- Yiannopoulou, K. G. , & Papageorgiou, S. G. (2013). Current and future treatments for Alzheimers disease. Therapeutic Advances in Neurological Disorders, 6(1), 19–33. 10.1177/1756285612461679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, G. N. , Lee, H. W. , Cho, J. Y. , & Suk, K. (2009). Neuronal pentraxin receptor in cerebrospinal fluid as a potential biomarker for neurodegenerative diseases. Brain Research, 1265, 158–170. 10.1016/j.brainres.2009.01.058 [DOI] [PubMed] [Google Scholar]
- Yoo, B. C. , Cairns, N. , Fountoulakis, M. , & Lubec, G. (2001). Synaptosomal proteins, beta‐soluble N‐ethylmaleimide‐sensitive factor attachment protein (Beta‐SNAP), gamma‐SNAP and synaptotagmin I in brain of patients with down syndrome and Alzheimers disease. Dementia and Geriatric Cognitive Disorders, 12(3), 219–225. 10.1159/000051261 [DOI] [PubMed] [Google Scholar]
- Zetterberg, H. (2016). Neurofilament light: A dynamic cross‐Disease fluid biomarker for neurodegeneration. Neuron, 91(1), 1–3. 10.1016/j.neuron.2016.06.030 [DOI] [PubMed] [Google Scholar]
- Zetterberg, H. (2019). Blood‐based biomarkers for Alzheimers disease—An update. Journal of Neuroscience Methods, 319, 2–6. Elsevier B.V. 10.1016/j.jneumeth.2018.10.025 [DOI] [PubMed] [Google Scholar]
- Zetterberg, H. , & Burnham, S. C. (2019). Blood‐based molecular biomarkers for Alzheimers disease. Molecular Brain, 12(1), 26. 10.1186/s13041-019-0448-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg, H. , Mörtberg, E. , Song, L. , Chang, L. , Provuncher, G. K. , Patel, P. P. , Ferrell, E. , Fournier, D. R. , Kan, C. W. , Campbell, T. G. , Meyer, R. , Rivnak, A. J. , Pink, B. A. , Minnehan, K. A. , Piech, T. , Rissin, D. M. , Duffy, D. C. , Rubertsson, S. , Wilson, D. H. , & Blennow, K. (2011). Hypoxia due to cardiac arrest induces a time‐dependent increase in serum amyloid β levels in humans. PLoS ONE, 6(12), e28263. 10.1371/journal.pone.0028263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg, H. , Skillbäck, T. , Mattsson, N. , Trojanowski, J. Q. , Portelius, E. , Shaw, L. M. , Weiner, M. W. , Blennow, K. , & Alzheimers Disease Neuroimaging Initiative . (2016). Association of Cerebrospinal Fluid Neurofilament Light Concentration with Alzheimer Disease Progression. JAMA Neurology, 73(1), 60–67. 10.1001/jamaneurol.2015.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg, H. , Wilson, D. , Andreasson, U. , Minthon, L. , Blennow, K. , Randall, J. , & Hansson, O. (2013). Plasma tau levels in Alzheimers disease. Alzheimers Research and Therapy, 5(2), 9. BioMed Central. 10.1186/alzrt163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Ng, K. P. , Therriault, J. , Kang, M. S. , Pascoal, T. A. , Rosa‐Neto, P. , Gauthier, S. , & Alzheimers Disease Neuroimaging Initiative . (2018). Cerebrospinal fluid phosphorylated tau, visinin‐like protein‐1, and chitinase‐3‐like protein 1 in mild cognitive impairment and Alzheimers disease. Translational Neurodegeneration, 7(1), 23. 10.1186/s40035-018-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Therriault, J. , Kang, M. S. , Ng, K. P. , Pascoal, T. A. , Rosa‐Neto, P. , Gauthier, S. , & Alzheimers Disease Neuroimaging Initiative . (2018). Cerebrospinal fluid synaptosomal‐associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimers disease. Alzheimers Res Ther, 10(1), 80. 10.1186/s13195-018-0407-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B. , Teramukai, S. , Yoshimura, K. , & Fukushima, M. (2009). Validity of cerebrospinal fluid biomarkers as endpoints in early‐phase clinical trials for Alzheimers disease. Journal of Alzheimers Disease: JAD, 18(1), 89–102. 10.3233/JAD-2009-1124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Search terms
Supplementary Table S2: National Institute of Health Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies ratings for included studies
Data Availability Statement
No new data were generated in this systematic literature review.





























