Abstract
The pathophysiology of unilateral cortical fluid-attenuated inversion recovery (FLAIR)-hyperintense lesions in anti-myelin oligodendrocyte glycoprotein (MOG)-associated encephalitis with seizures (FLAMES) is unclear. A 26-year-old man was referred because of a seizure. FLAIR showed an increased signal intensity and swelling of the right frontal cortex. His symptoms and imaging abnormalities were improved after intravenous methylprednisolone therapy. MOG antibody was detected both in serum and cerebrospinal fluid (CSF). Therefore, the patient was diagnosed with FLAMES. Myelin basic protein (MBP) was elevated in CSF. The high MBP value in the CSF in the present case suggested that demyelination as well as inflammation can occur in some FLAMES patients.
Keywords: myelin oligodendrocyte glycoprotein antibody, encephalitis, seizure, myelin basic protein, myelitis
Introduction
Unilateral cortical fluid-attenuated inversion recovery (FLAIR)-hyperintense lesions in anti-myelin oligodendrocyte glycoprotein (MOG)-associated encephalitis with seizures (FLAMES) was first reported as a benign form of MOG antibody-associated demyelination (MOGAD) with a good response to immunotherapy. However, the pathophysiology of FLAMES is unclear, and recent reports have shown that this disease entity is not always benign, as relapse sometimes occurred, especially without a follow-up course of corticosteroid tapering.
We herein report a case of FLAMES with subsequent cervical myelitis. Elevated myelin basic protein (MBP) levels in the cerebrospinal fluid (CSF) suggested that demyelination, in addition to inflammation, can occur in FLAMES patients.
Case Report
A 26-year-old man was referred to our hospital because of a suspected brain tumor. He had a history of meningitis at three years old and optic neuritis at five years old.
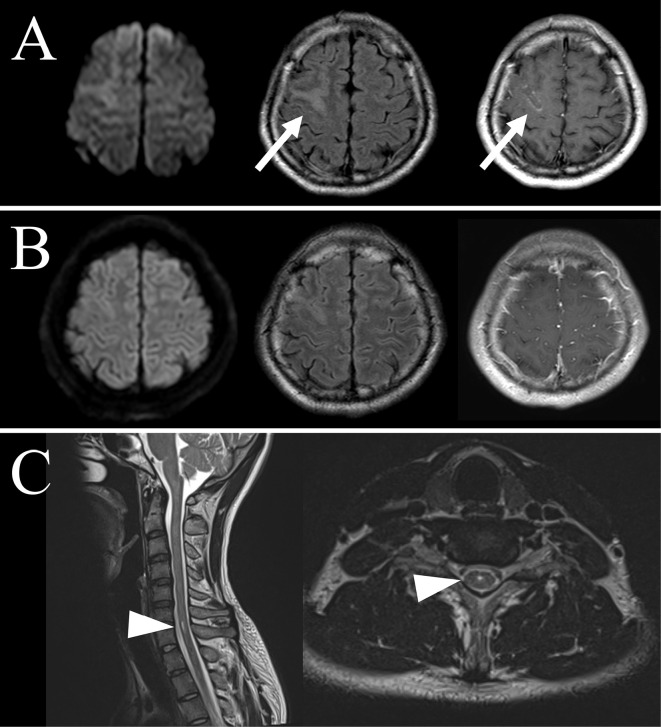
About five weeks before admission, he noticed occasional involuntary movement of his left forearm. Four weeks before admission, he was taken to the emergency department of a local hospital because of a generalized tonic-clonic seizure. He showed no neurological deficit on arrival at the local hospital. Levetiracetam 1,000 mg/day was prescribed, and brain magnetic resonance imaging (MRI) was scheduled. Brain MRI was performed three weeks before admission, showing an increased signal intensity and swelling in the right frontal cortex on FLAIR, with enhancement on T1-weighted imaging with gadolinium injection (Figure A). From 2 weeks before admission, he showed an intermittent fever of around 38°C and headache.
Figure.
Serial brain and cervical magnetic resonance imaging (MRI) findings. (A) Brain MRI at a local hospital; diffusion-weighted imaging (DWI, left panel), fluid-attenuated inversion recovery (FLAIR, center panel), and T1-weighted imaging (T1-WI) with gadolinium injection (right panel). MRI shows increased signal intensity and swelling in the right frontal cortex on FLAIR with enhancement on T1-WI with gadolinium injection (arrows). (B) Brain MRI on Day 16. After high-dose methylprednisolone therapy, the hyperintense signal and swelling on FLAIR are ameliorated, and no gadolinium-enhanced lesion is seen on T1-WI. (C) About 2.5 months later, cervical MRI shows a hyperintense lesion of the cervical cord (arrowheads).
On admission (Day 0), general physical and neurological examinations showed neck stiffness and meningeal irritation signs but were otherwise normal. His blood pressure was 124/85 mmHg, heart rate was 98 beats/min with a regular rhythm, and temperature was 37.0°C. An interictal electroencephalogram was normal. CSF analyses showed pleocytosis (692 /μL, monocytes 346), elevated protein (151 mg/dL), and mild glucose decrease (59 mg/dL, serum glucose 168 mg/dL). The MBP level in the CSF was elevated (179 pg/mL), and oligoclonal band (OCB) was positive. The IgG index was 0.51.
Because infectious meningoencephalitis could not be ruled out, meropenem 6 g/day, vancomycin 2 g/day, and acyclovir 1,500 mg/day were started as empiric therapy. From Day 3, intravenous methylprednisolone therapy (IVMP) (1,000 mg/day for 3 days) was given because immune-mediated encephalitis was suspected based on the negative results for herpes simplex virus DNA and CSF cultures. However, autoantibodies, including anti-aquaporin 4 and anti-N-methyl-D-aspartate receptor, were not detected on enzyme-linked immunosorbent assays. CSF cytology did not show any malignant cells, and enhanced computed tomography of his body did not show any tumor.
After IVMP therapy, his high-grade fever and headache were partially relieved, and follow-up MRI on Day 16 showed no gadolinium-enhanced lesion (Figure B). Therefore, IVMP was considered to be effective, but a course of steroid tapering was not given because no specific autoantibodies were found. A CSF analysis on Day 28 showed improvement in pleocytosis (33 /μL, monocytes 33) and elevated protein (57 mg/dL), but MBP was still elevated (164 pg/mL), and OCB was repeatedly detected. Some of these OCBs had not been noted in the initial examination.
He was discharged on Day 29 without any neurological sequelae. After discharge, MOG antibody was detected in the Day 3 serum (1:512) and in the Day 0 CSF (1:32) using a cell-based assay [the level of anti-MOG antibody was measured using a live cell-based assay method with IgG-specific secondary antibody at Tohoku University Hospital (1)]. The results of serial OCB assessments indicated epitope spreading, but autoantibodies other than anti-MOG antibody, including anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor 1/2, dipeptidyl-peptidase-like protein 6 (DPPX), contactin-associated protein-like 2 (CASPR2), leucine-rich glioma-inactivated 1 (LGI1), and gamma-aminobutyric acid type B (GABAB) receptor, were not detected. Therefore, he was diagnosed with FLAMES.
He felt numbness in his right hand and Lhermitte's sign two weeks after discharge. His symptoms improved gradually without treatment, and 73 days from the initial admission, cervical MRI showed a hyperintense signal in the cervical cord (Figure C). MOGAD in the cervical cord was considered, and he was given oral prednisolone 5 mg/day to prevent recurrence. No relapse has been noted for six months.
Discussion
MOG antibody-positive, unilateral, cerebral cortical encephalitis with epilepsy was first reported by Ogawa et al. (2), and the term FLAMES was recently suggested (3). At first, authors reported FLAMES as having a benign prognosis (2), but subsequently, FLAMES cases with relapse (4-6) or ensuing demyelination (acute disseminated encephalomyelitis, optic neuritis, or myelitis) (6-8) were also reported. In the present case, myelitis of the cervical cord occurred subsequently (1.5 months after FLAMES). The immunotherapy given to the patient consisted only of IVMP and was not followed by a course of steroid tapering because, at that time, MOG antibody positivity had not been identified. Rapid steroid tapering has been reported to be associated with relapse in patients with FLAMES (4,6), as well as MOGAD (9), and the present case may suggest that no course of corticosteroid tapering can lead to subsequent demyelination, even after an excellent response to immunotherapy in patients with FLAMES.
The present case showed a high CSF MBP value. CSF MBP in FLAMES cases has not been reported to be elevated (2,6), even though patients with anti-MOG antibody and presenting transverse myelitis, optic neuritis, and acute disseminated encephalomyelitis often show elevated CSF MBP levels (10). The underlying mechanisms involved in FLAMES are not well known; even whether an anti-MOG antibody directly causes encephalitis and seizure or just coexists with another autoimmune disorder is a matter of debate (2,11). Furthermore, antigen-specificity of T cells can affect pathophysiological mechanisms (demyelination-dominant or inflammation-dominant) in patients with anti-MOG antibody. (12) One report of a FLAMES case with a brain biopsy showed inflammatory changes without demyelination or MOG loss (7), whereas another showed inflammatory changes with mild loss of MOG immunoreactivity (13). In the second case report, the authors suggested that their case was in an early stage of demyelination. During demyelination, MOG is degraded quickly, whereas MBP seems to take more time to be degraded (14). The present case showed repeated high CSF MBP values, probably because of the relatively long duration from the symptom onset to immunotherapy.
Another possible explanation is that the elevated CSF MBP value reflected subsequent myelitis, as most transverse myelitis patients with anti-MOG antibody show elevated MBP levels in the CSF (10). However, whether and from when MBP elevation in CSF precedes myelitis is unclear at present.
One more possible explanation is that the elevated MBP level was due to seizure attacks involving non-specific myelin damage (15), although MBP levels were still elevated one month after the seizure attack in the present case, and MBP has not been reported to be elevated even after seizure in other FLAMES cases (2,6). The present case suggests that demyelination, as well as inflammation, can occur in some FLAMES patients. Measuring the CSF MBP level in FLAMES patients is likely to be valuable in terms of evaluating the pathophysiology and perhaps predicting subsequent MOGAD.
In conclusion, we encountered a FLAMES case with elevated CSF MBP, followed by myelitis of the cervical cord. A short course of corticosteroid tapering after IVMP might be needed not only to prevent relapse but also to prevent subsequent MOGAD. The high CSF MBP value in the present case suggests that demyelination, as well as inflammation, can occur in some FLAMES patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 82: 474-481, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm 4: e322, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Budhram A, Mirian A, Le C, Hosseini-Moghaddam SM, Sharma M, Nicolle MW. Unilateral cortical FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES): characterization of a distinct clinico-radiographic syndrome. J Neurol 266: 2481-2487, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Numa S, Kasai T, Kondo T, et al. An adult case of anti-myelin oligodendrocyte glycoprotein (MOG) antibody-associated multiphasic acute disseminated encephalomyelitis at 33-year intervals. Intern Med 55: 699-702, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto D, Uchiyama T, Ohashi T, Iizuka T. Case of steroid-responsive unilateral encephalitis with anti-myelin oligodendrocyte glycoprotein antibodies. Neurol Clin Neurosci 5: 101-102, 2017. [Google Scholar]
- 6. Sugimoto T, Ishibashi H, Hayashi M, et al. A case of anti-MOG antibody-positive unilaterally dominant meningoencephalitis followed by longitudinally extensive transverse myelitis. Mult Scler Relat Disord 25: 128-130, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Fujimori J, Takai Y, Nakashima I, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry 88: 534-536, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Wang L, ZhangBao J, Zhou L, et al. Encephalitis is an important clinical component of myelin oligodendrocyte glycoprotein antibody associated demyelination: a single-center cohort study in Shanghai, China. Eur J Neurol 26: 168-174, 2019. [DOI] [PubMed] [Google Scholar]
- 9. Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 89: 127-137, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaneko K, Sato DK, Nakashima I, et al. Myelin injury without astrocytopathy in neuroinflammatory disorders with MOG antibodies. J Neurol Neurosurg Psychiatry 87: 1257-1259, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Russ JB, Timbie CM, Li Y, Gonzalez-Giraldo E. Clinical reasoning: an 11-year-old girl with focal seizures, fevers, and unilateral, enhancing cortical lesions. Neurology 95: e3153-e3159, 2020. [DOI] [PubMed] [Google Scholar]
- 12. Spadaro M, Winklmeier S, Beltran E, et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol 84: 315-328, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Ikeda T, Yamada K, Ogawa R, et al. The pathological features of MOG antibody-positive cerebral cortical encephalitis as a new spectrum associated with MOG antibodies: a case report. J Neurol Sci 392: 113-115, 2018. [DOI] [PubMed] [Google Scholar]
- 14. van der Valk P, De Groot CJ. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol 26: 2-10, 2000. [DOI] [PubMed] [Google Scholar]
- 15. Levin SD, Hoyle NR, Brown JK, Thomas DG. Cerebrospinal fluid myelin basic protein immunoreactivity as an indicator of brain damage in children. Dev Med Child Neurol 27: 807-813, 1985. [DOI] [PubMed] [Google Scholar]