Abstract
We encountered a 77-year-old Japanese man who presented with nephrotic-range proteinuria 20 days after receiving ramucirumab treatment for metastatic sigmoid colon cancer. A kidney biopsy showed two characteristic histological findings. The first finding was podocyte injury with cellular crescent-like formation, although focal segmental glomerulosclerosis (FSGS) (collapsing variant) according to the Columbia classification may have been a more appropriate name for this injury, as hypertrophy and hyperplasia of epithelial cells, presumably resulting from podocyte injury, were seen between Bowman's capsule and the glomerular basement membrane (GBM); these changes appeared to be due to the collapse of the GBM rather than to GBM destruction with fibrinoid necrosis. The second finding was endotheliopathy characterized by prominent mesangial interposition via enlargement of the mesangial matrix with mesangiolysis. Proteinuria and renal dysfunction subsided after discontinuation of ramucirumab. Bevacizumab has been reported to induce glomerular microangiopathy with endothelial damage and swelling six months after treatment, but in this case, ramucirumab may have induced focal segmental glomerulosclerosis (FSGS) collapsing variant and glomerular microangiopathy with endotheliopathy via mesangial damage within 1 month. We believe that the damage to the glomerular podocyte and endothelial cells via mesangial damage secondary to ramucirumab in our patient was a different type of glomerular microangiopathy than the endothelial cell damage with enlargement of the subendothelial space caused by bevacizumab.
Keywords: vascular endothelial growth factor receptor 2 antibody, ramucirumab, focal segmental glomerulosclerosis (FSGS) collapsing variant
Introduction
Ramucirumab is an anti-vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal IgG1 antibody and is usually used as a secondary treatment after administration of anti-vascular endothelial growth factor (anti-VEGF) derivatives, e.g. bevacizumab, for the treatment of advanced malignancies. Several reports have described patients who did not develop proteinuria during treatment with bevacizumab but did develop it during treatment with ramucirumab. Glomerular microangiopathy with endothelial injury has been reported to be a characteristic feature of kidney involvement after ramucirumab administration (1-3), but we herein report a case of ramucirumab-induced glomerular microangiopathy that involved not only podocyte damage with cellular crescent-like formation but also endotheliopathy via mesangial damage.
Case Report
A 77-year-old man was admitted to our hospital for the evaluation of proteinuria. At 74 years old, the patient had been diagnosed with sigmoid colon cancer with liver metastasis, and laparoscopy-assisted sigmoid colon resection was performed. One month later, treatment with five cycles of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) plus bevacizumab was started as adjuvant chemotherapy. Right lobectomy for metastatic liver tumors was performed, but one month later, additional liver metastasis was found. Treatment with capecitabine and oxaliplatin (XELOX) was started. At 75 years old, 15 cycles of irinotecan and S-1 (IRIS) plus panitumumab were started, but panitumumab was discontinued at the 11th cycle because of skin damage. At 76 years old, 15 cycles of IRIS plus bevacizumab were started and continued for 10 months. However, because this treatment had little effect on the tumor, IRIS plus ramucirumab therapy was initiated 1 month later, at 77 years old. Twenty days after starting treatment, the patient developed severe pitting edema of bilateral lower legs and nephrotic-range proteinuria. The total dose of ramucirumab was calculated to be 500 mg.
On admission, the patient was 167.0 cm tall and weighed 77.9 kg (weight gain of 10.3 kg); his blood pressure was 173/90 mmHg, and his temperature was 37.0°C. The laboratory findings were as follows (see Table): total protein, 5.9 g/dL; albumin, 2.6 g/dL; creatinine, 1.42 mg/dL (increase from 0.8 mg/dL); and proteinuria, 4.82 g/day.
Table.
Laboratory Data on Admission.
| Blood | Reference range | |||||
| TP | 5.9 | g/dL | 6.6-8.1 | |||
| Alb | 2.6 | g/dL | 4.1-5.1 | |||
| AST | 28 | U/L | 13-30 | |||
| ALT | 17 | U/L | 10-42 | |||
| LD | 228 | U/L | 124-222 | |||
| ALP | 380 | U/L | 38-113 | |||
| γ-GTP | 111 | U/L | 13-64 | |||
| Amy | 56 | U/L | 44-132 | |||
| Cre | 1.42 | mg/dL | 0.65-1.07 | |||
| eGFR | 38 | mL/min/1.73 m2 | ≥90 | |||
| UA | 8.6 | mg/dL | 3.7-7.0 | |||
| UN | 18 | mg/dL | 8-20 | |||
| TG | 125 | mg/dL | 40-149 | |||
| T-Chol | 214 | mg/dL | 142-248 | |||
| HDL-C | 45 | mg/dL | 40-90 | |||
| LDL-C | 134 | mg/dL | 65-139 | |||
| HbA1c | 5.5 | % | 4.9-6.0 | |||
| Na | 143 | mmol/L | 138-145 | |||
| K | 3.5 | mmol/L | 3.6-4.8 | |||
| Cl | 105 | mmol/L | 101-108 | |||
| Ca | 8.1 | mg/dL | 8.8-10.1 | |||
| P | 3 | mg/dL | 2.7-4.6 | |||
| Fe | 61 | µg/dL | 40-188 | |||
| UIBC | 245 | µg/dL | 173-263 | |||
| T-Bil | 0.6 | mg/dL | 0.4-1.5 | |||
| IgG | 773 | mg/dL | 861-1,747 | |||
| IgA | 241.1 | mg/dL | 93-393 | |||
| IgM | 103.7 | mg/dL | 33-183 | |||
| IgE | 1,155 | mg/dL | ≤170 | |||
| M-protein | Negative | Negative | ||||
| Free light chain | - | |||||
| κ | 51.1 | mg/L | 3.3-19.4 | |||
| λ | 26 | mg/L | 5.7-26.3 | |||
| κ/λ | 1.97 | 0.26-1.65 | ||||
| Cryoglobulin | Negative | Negative | ||||
| CH50 | 61 | U/mL | 31-58 | |||
| C3 | 110 | mg/dL | 73-138 | |||
| Transferrin | 252 | mg/dL | 190-320 | |||
| CRP | 0.4 | mg/dL | ≤0.14 | |||
| Antinuclear antibody | <40 | <40 | ||||
| Anti-dsDNA antibody IgG | 0.9 | IU/mL | <10 | |||
| Anti-SM antibody | Negative | Negative | ||||
| Anti-RNP antibody | Negative | Negative | ||||
| Anti-SS-A antibody | Negative | Negative | ||||
| Anti-SS-B antibody | Negative | Negative | ||||
| Anti-Scl-70 antibody | Negative | Negative | ||||
| PR3-ANCA | <0.5 | IU/mL | <2.0 | |||
| MPO-ANCA | <0.5 | IU/mL | <3.5 | |||
| sIL2-R | 579 | U/mL | 122-496 | |||
| WBC | 4,600 | /µL | 3,300-8,600 | |||
| RBC | 4.58 | ×106/µL | 4.35-5.55 | |||
| Hb | 13.1 | g/dL | 13.7-16.8 | |||
| MCV | 88.4 | fL | 83.6-98.2 | |||
| Plt | 124 | ×103/µL | 158-348 | |||
| BNP | 44.9 | pg/mL | ≤18.4 | |||
| HBs antigen | Negative | Negative | ||||
| HBc antibody | Negative | Negative | ||||
| HCV antibody | Negative | Negative | ||||
| Urine | ||||||
| Specific gravity | 1.019 | 1.002-1.030 | ||||
| pH | 5.5 | 6 | ||||
| Glucose | Negative | Negative | ||||
| RBC | 1-4 | /µL | ≤4 | |||
| WBC | 1-4 | /µL | ≤4 | |||
| NAG | 32.5 | IU/gCre | 0.8-5.0 | |||
| α1-microglobulin | 30.25 | mg/gCre | - | |||
| β2-microglobulin | 0 | mg/gCre | 0.1-1.9 | |||
| Na | 35 | mmol/gCre | 40-400 | |||
| K | 23 | mmol/gCre | 10-120 | |||
| Cl | 48 | mmol/gCre | 50-400 | |||
| Protein | 4.82 | g/day | - |
A kidney biopsy was performed.
Kidney biopsy findings
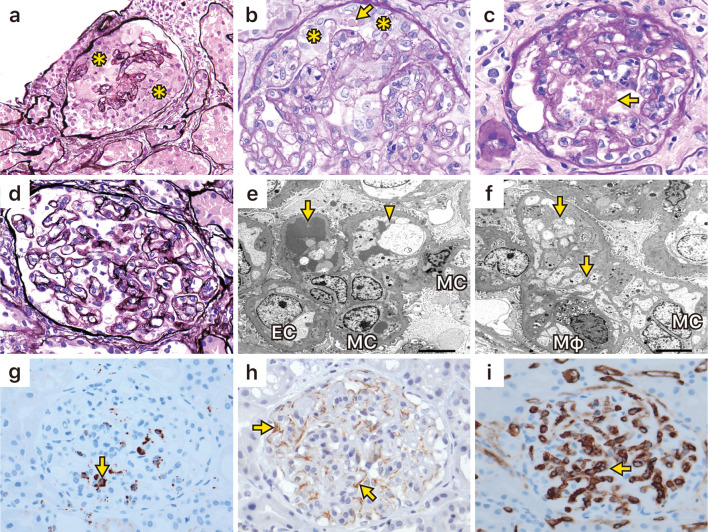
A light microscopic examination showed global sclerosis in 5 out of 32 glomeruli. In five glomeruli, a cellular crescent-like formation comprising two or more layers of activated epithelial cells of Bowman's capsule was noted. Massive hyaline degeneration was also seen in these epithelial cells, but neither fibrinoid necrosis nor disruption of glomerular basement membrane (GBM) was observed. In addition, a double contour of GBM with mesangial interposition was visible in almost all preserved glomeruli (Fig. 1a-d). Fibrosis of the tubulointerstitium was present in 30% of the cortical area, but tubular injury was mild. Moderate fibrous thickening of the interlobular arteries and mild arterial hyalinosis were seen. Immunofluorescence staining was partially positive for IgM, IgA, C3, and C4. Electron microscopy showed that enlargement of the mesangial matrix with deposition of fibrin-like material into the mesangiolysis area had induced interposition of the mesangial matrix into the subendothelial space; however, subendothelial edema with enlargement of the subendothelial space was mild. Electron-dense deposits were not seen (Fig. 1e, f), and only partial foot process effacement was observed.
Figure 1.
Kidney biopsy findings. a and b: Light microscopic examination showed a cellular crescent-like formation occupied by two or more layers of epithelial cells (*) of Bowman’s capsule. b and c: Massive hyaline degeneration (arrows) was also seen in these epithelial cells. d: A double contour of the glomerular basement membrane with mesangial interposition was noted in the preserved glomeruli. e: Electron microscopy showed that enlargement of the mesangial matrix with deposition of fibrin-like material (arrow) into the mesangiolysis area induced interposition of mesangial matrix (arrowhead) into the subendothelial space. Swelling of endothelial cells was noted. f: Infiltrated foamed macrophages and interposition of mesangial matrix (arrows) into the subendothelial space were seen. g: Immunohistological staining for CD68 (arrow), corresponding to macrophages, was identified in the glomerular capillary. h: Staining of vascular endothelial growth factor (arrows), corresponding to podocytes. i: Staining of CD31 showed the preservation of glomerular endothelial cells (arrow). The specimen for CD31 staining did not contain a crescent-like formation. EC: endothelial cells, MC: mesangial cells, Mφ: macrophages
Immunohistological staining was performed according to a previously published method (3). CD68-positive cells, which correspond to macrophages, were seen in the glomerular capillary (Fig. 1g), and VEGF staining was visible in areas that correspond to podocytes on the outer area of the GBM (Fig. 1h). Staining of CD31 showed the preservation of glomerular endothelial cells (Fig. 1i).
Clinical course
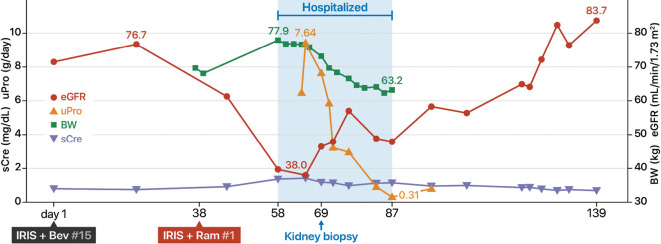
After ramucirumab was discontinued, the proteinuria disappeared within one month, and the renal function improved to the level before the start of treatment (Fig. 2).
Figure 2.
Clinical course.
The diagnosis
This case had two characteristic histological findings. The first finding was endotheliopathy characterized by prominent mesangial interposition via enlargement of the mesangial matrix with mesangiolysis, and the second was podocyte injury with cellular crescent-like formation, with focal segmental glomerulosclerosis (FSGS) (collapsing variant) according to the Columbia classification potentially being a more appropriate name for this injury because the hypertrophy and hyperplasia of epithelial cells between Bowman's capsule and the GBM, probably resulting from podocyte injury, appeared to have been due to the collapse of the GBM rather than GBM destruction with fibrinoid necrosis and because proteinuria and renal dysfunction subsided after the discontinuation of ramucirumab (4,5). Glomerulopathy secondary to ramucirumab was considered a different type of glomerular microangiopathy from the endothelial cell damage with subendothelial edema due to the enlargement of the subendothelial space caused by bevacizumab.
Discussion
We encountered a 77-year-old Japanese man who presented with nephrotic-range proteinuria 20 days after receiving ramucirumab treatment for metastatic sigmoid colon cancer. Ramucirumab, a monoclonal antibody against VEGFR2, inhibits VEGFR2 activation by blocking the binding of VEGF-A, VEGF-C, and VEGF-D to VEGFR2. Blocking of VEGF/VEGFR2 signaling has been shown to be efficacious in renal cell and hepatocellular carcinoma and in metastatic colorectal, non-small-cell lung, and metastatic breast cancer (6). Bevacizumab is an anti-VEGF-A monoclonal antibody, and both ramucirumab and bevacizumab inhibit angiogenesis in tumors and suppress tumor growth. However, previous studies have indicated that inhibition of VEGF results in renal injury. In the kidney, VEGFR2 is expressed in podocytes and endothelial cells. In transgenic mouse models, whole-body deletion of Vegfr2 and partial deletion of Vegfa from podocytes induced renal-limited glomerular microangiopathy (7).
Bevacizumab has been found to induce nonthrombotic glomerular microangiopathy, and ramucirumab has been reported also to induce nonthrombotic glomerular microangiopathy (1-3). Yamada et al. reported a 71-year-old woman who developed proteinuria and thrombocytopenia (57,000/μL) 21 days after starting folinic acid, fluorouracil, and irinotecan plus ramucirumab therapy; a kidney biopsy showed glomerular microangiopathy with mesangiolysis and a fibrin-like lesion that was red on Azan staining (1). Fujii et al. reported 5 cases of nephrotic syndrome associated with ramucirumab; the onset of proteinuria was 21 to 112 days after treatment (median value, 31 days). A kidney biopsy of two patients showed diffuse double contours of the GBM and narrowing of the glomerular capillary lumina, and immunohistochemistry showed an increased CD68 expression in the glomerular loop. Electron microscopy revealed double contours of the GBM by electron-dense material in the subendothelial space, probably indicating mesangial interposition due to mesangial proliferation with mesangiolysis. In one case, immunofluorescence microscopy showed segmental fine granular glomerular capillary wall staining for IgA and an electron-dense deposit in the subendothelial space (2). Ozawa et al. reported kidney biopsy results in 9 patients who developed proteinuria 3 to 44 months (median, 10 months) after bevacizumab administration. Glomerular microangiopathy was seen in all cases. On light microscopy, a patchy pattern of hemispherical/spherical amorphous lesions in the subendothelial space was a characteristic finding. Immunohistochemical studies showed positive VEGF staining in the podocyte area, even though VEGF had been inhibited using an anti-VEGF-A monoclonal antibody; the authors were not sure how to explain this finding (3). Toriu et al. reported a case of renal-limited glomerular microangiopathy due to bevacizumab and found that serum VEGF levels were high even after administration of bevacizumab. The authors suggested that the relationship between the high expression of VEGF and renal-limited glomerular microangiopathy may need to be examined more closely in the future. In their patient, the onset of proteinuria was 12 months from the first initiation of the drug, and the proteinuria decreased but did not normalize even after the discontinuation of treatment and actually increased 4 months after the second dose (8). Estrada et al. reported that the mean period between the initiation of bevacizumab and the onset of renal injury was 6.9 months (7). In contrast, our case and previous reports showed that renal injury occurred from 21 days to a median of 31 days after initiation of ramucirumab (1,2). Thus, proteinuria seems to appear earlier with ramucirumab than with bevacizumab.
Nephropathy associated with ramucirumab and bevacizumab has two common features: it is actually glomerular microangiopathy with endothelial cell damage without thrombosis, and it rarely shows extrarenal involvement. However, there are two main differences between the effects of these two agents: proteinuria appears more quickly with ramucirumab than with bevacizumab, and bevacizumab-induced glomerular damage is mainly endothelial injury with subendothelial edema, whereas the kidney biopsy in our case indicated that the glomerular damage caused by ramucirumab mainly affected podocytes and endothelial cells via mesangial injury, while endotheliopathy, characterized by the enlargement of subendothelial space, was mild.
The mechanism underlying the progression from podocyte injury to Bowman's capsule epithelial cell proliferation is unclear. However, the fact that VEGFR2 is expressed in not only endothelial cells but also podocytes may support the hypothesis that anti-VEGFR2 antibodies also affect podocytes.
Previous reports suggested that anti-VEGF antibody causes glomerular microangiopathy characterized by double contours of GBM, mesangial interposition, mesangiolysis, and endothelial swelling with subendothelial edema originating from endothelial damage (with abundant expression of synaptopodin, nephrin, and RelA in podocytes). In contrast, the inhibition of VEGFR2 by tyrosine kinase inhibitors, such as sunitinib, results in collapsing glomerulopathy resembling minimal change nephrotic syndrome or FSGS and is characterized by podocyte foot process effacement and GBM wrinkling originating from podocyte injury (with reduced expression of synaptopodin, nephrin, and RelA in podocytes) (9-11). In our case, glomerular injury secondary to ramucirumab showed crescent-like formation and endotheliopathy via mesangial injury, although foot process effacement due to podocyte injury and glomerular endothelial damage, with enlargement of the subendothelial space, was mild; thus, the endotheliopathy in our case may have been a different type of endotheliopathy (11).
Nakano et al. recently reported a case of nephropathy caused by ramucirumab. Their case showed non-thrombotic glomerular microangiopathy, including collapsed glomeruli, with crescent-like lesions due to epithelial cell proliferation, as in our case. In addition, the glomerular lesions were reported to be endothelial cell hyperplasia and non-specific dense substances around the mesangium (12). Thus, our patient is the second case showing simultaneous endotheliopathy and FSGS (collapsing variant).
In conclusion, in our case, podocytes showed positive staining for VEGF, suggesting that VEGF may be overexpressed after the binding of VEGF-A to VEGFR2 is blocked by the administration of ramucirumab. However, how this finding is related to podocyte injury remains unclear. Podocyte injury may be strongly associated with GBM collapse, which leads to hypertrophy and hyperplasia of epithelial cells between Bowman's capsule and GBM, and may also be associated with endotheliopathy via mesangial injury. The finding that nephropathy normalized after drug discontinuation in our patient suggests that the renal damage caused by ramucirumab is reversible.
This investigation was conducted in accordance with the Declaration of Helsinki. The patient gave her informed consent for this case report to be published.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Kazuho Honda (Department of Anatomy, Showa University School of Medicine), Yukiko Kanetsuna (Department of Pathology, International University of Health and Welfare), Kensuke Joh (Renal Pathology, Jikei University School of Medicine), and Yutaka Yamaguchi (Yamaguchi's Pathology Laboratory) for valuable discussions on the diagnostic and therapeutic strategy.
References
- 1.Yamada R, Okawa T, Matsuo K, Suzuki M, Mori N, Mori K. Renal-limited thrombotic microangiopathy after switching from bevacizumab to ramucirumab: a case report. BMC Nephrol 20: 14, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii T, Kawasoe K, Tonooka A, Ohta A, Nitta K. Nephrotic syndrome associated with ramucirumab therapy: a single-center case series and literature review. Medicine (Baltimore) 98: e16236, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozawa M, Ohtani H, Komatsuda A, Wakui H, Takahashi N. VEGF-VEGFR2 inhibitor-associated hyaline occlusive glomerular microangiopathy: a Japanese single-center experience. Clin Exp Nephrol 25: 1193-1202, 2021. [DOI] [PubMed] [Google Scholar]
- 4.Nair R. Focal segmental glomerulosclerosis: cellular variant and beyond. Kidney Int 70: 1676-1678, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Stokes MB, Valeri AM, Markowitz GS, D'Agati VD. Cellular focal segmental glomerulosclerosis: clinical and pathologic features. Kidney Int 70: 1783-1792, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8: 579-591, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol 30: 187-200, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toriu N, Sekine A, Mizuno H, et al. Renal-limited thrombotic microangiopathy due to bevacizumab therapy for metastatic colorectal cancer: a case report. Case Rep Oncol 12: 391-400, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izzedine H, Escudier B, Lhomme C, et al. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine (Baltimore) 93: 333-339, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzedine H, Mangier M, Ory V, et al. Expression patterns of RelA and c-mip are associated with different glomerular diseases following anti-VEGF therapy. Kidney Int 85: 457-470, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz GS, Bomback AS, Perazella MA. Drug-induced glomerular disease: direct cellular injury. Clin J Am Soc Nephrol 10: 1291-1299, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano Y, Kumagai J, Nagahama K, Fujisawa H. A case of ramucirumab-induced renal failure with nephrotic-range proteinuria and its pathological findings. BMJ Case Rep 14: e239603, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]