Abstract
Objective
We retrospectively analyzed the prevalence and clinical features of splenic infarctions in patients with Philadelphia chromosome-negative myeloproliferative neoplasms (Ph- MPNs).
Patients
Patients diagnosed with essential thrombocythemia (ET), polycythemia vera (PV), prefibrotic/early primary myelofibrosis (pre-PMF), or PMF from January 1996 to October 2020 in Chungnam National University Hospital, Daejeon, Korea, were reviewed.
Results
A total of 347 patients (143 ET, 129 PV, 44 pre-PMF, and 31 PMF patients; 201 men and 146 women) with a median age of 64 (range 15-91) years old were followed up for a median of 4.7 (range 0.1-26.5) years. Fifteen (4.3%) patients exhibited splenic infarctions at the diagnosis. These were most common in PMF patients (12.9%), followed by pre-PMF (9.1%) and PV (5.4%) patients. Multifocal infarcts (60.0%) were most common, followed by solitary (33.3%) and extensive infarcts (6.7%). The cumulative incidence of thrombosis in patients with splenic infarctions tended to be higher than in those lacking infarctions (10-year incidence 46.7% vs. 21.0% in PV; p=0.215; 33.3% vs. 17.9% in pre-PMF; p=0.473) patients, but statistical significance was lacking. Palpable splenomegaly (hazard ratio 14.89; 95% confidence interval 4.00-55.35; p<0.001) was the only independent risk factor for splenic infarction. During follow-up, 5 (1.4%) patients developed splenic infarctions. Conservative treatment adequately controlled the symptoms; no serious complications were noted in any patient.
Conclusion
Splenic infarctions occurred most frequently in patients with PMF; it was rare in patients with ET. The clinical courses were generally mild.
Keywords: splenic infarction, myeloproliferative neoplasm, essential thrombocythemia, polycythemia vera, primary myelofibrosis
Introduction
Philadelphia chromosome-negative myeloproliferative neoplasms (Ph- MPNs) include essential thrombocythemia (ET), polycythemia vera (PV), prefibrotic/early myelofibrosis (pre-PMF), and overt PMF (1). These are all clonal hematological disorders characterized by increased blood cell numbers, frequent thrombotic or hemorrhagic vascular events (2,3), and myelofibrotic or leukemic transformation (4-6).
Palpable splenomegaly is evident in a subpopulation of such patients, but radiological splenomegaly is more common (7,8). Splenomegaly is most common and marked in PMF patients, attributable principally to extramedullary hematopoiesis, and increases the risk for splenic infarction (9). Such infarction is characteristic of many clinical conditions, but the clinical course is generally mild (10-12). Splenic infarctions develop in patients with various hematological disorders; they have been extensively described in patients with sickle cell disease (13) and hematological malignancies, including lymphoma and leukemia (14-17). Such infarctions also develop in patients with Ph- MPN (18); serious complications include splenic rupture, intraperitoneal bleeding, and peritonitis (19-21). However, most data are those of case reports and thus are difficult to generalize and apply in routine clinical practice.
We retrospectively analyzed the prevalence and clinical features of splenic infarctions evident at the diagnosis and during follow-up and the corresponding outcomes, in patients with Ph- MPN.
Materials and Methods
Patient recruitment and data acquisition
Patients diagnosed with ET, PV, pre-PMF, or PMF from January 1996 to October 2020 at Chungnam National University Hospital, Daejeon, Korea, were retrospectively enrolled, and their medical records were reviewed in terms of demographic and laboratory data, including complete blood counts, blood chemistry, driver gene mutations, bone marrow data, cytogenetics, and abdominal computed tomography (CT).
Prognostic stratification of ET and PMF patients employed the International Prognostic Scoring for ET (IPSET) (22) and the International Prognostic Scoring System (IPSS) (23), respectively. For patients diagnosed with ET prior to 2017, diagnoses were revised based on the 2016 World Health Organization criteria (1). Hydroxyurea or anagrelide was used for cytoreduction, based on the standard recommendations, drug availability, and compliance. Except for in low- and very-low-risk patients, low-dose aspirin (100 mg daily) was prescribed to prevent thrombosis.
Driver gene mutation analyses
The Janus kinase 2 mutation (JAK2V617F) was identified via polymerase chain reaction (PCR) and Sanger sequencing (prior to 2010) and using allele-specific real-time quantitative PCR (after 2010) (performed by GC Laboratories, Yongin, Korea). The calreticulin (CALR) mutation of exon 9 was detected via fragment analyses and Sanger sequencing. The myeloproliferative leukemia gene mutation (MPLW515K/L) status was assessed using PCR and Sanger sequencing (GC Laboratories).
Abdominal CT
CT was performed using various scanners and techniques, but most images were obtained using a SOMATOM Sensation 16, SOMATOM Sensation 64, SOMATOM Definition Edge, or a SOMATOM Definition Flash platform (all from Siemens Medical Solutions, Forchheim, Germany). The scanning parameters were section thickness, 3.0-5.0 mm; field of view, 304-360 mm; tube current-time product, 144-486 mAs; and peak voltage, 100-120 kVp. After acquisition of unenhanced scans, contrast-enhanced scans were obtained. A total of 1.2-1.5 mL nonionic contrast material [iopromide (370 mg iodine/mL), Ultravist 370; Bayer Healthcare, Berlin, Germany]/kg body weight was usually injected into the antecubital vein at 3-4 mL/s using a power injector. A bolus-tracking technique was employed to optimize the timing of arterial phase scanning. Late portal phase images were obtained 70-80 seconds after contrast injection. A 20-mL flush of normal saline was administered immediately after contrast injection. Most axial CT images were reconstructed at a section thickness of 3 mm.
Classification of splenomegaly and splenic infarction
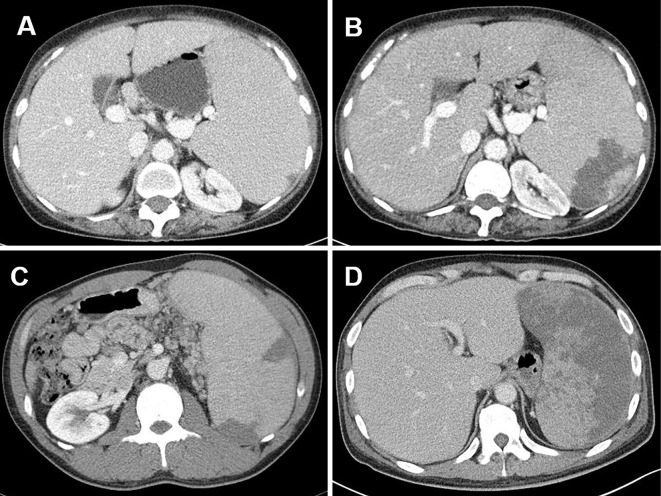
Splenomegaly was defined using previously described criteria (8). In brief, “palpable splenomegaly” indicated that the spleen was palpable below the left costal margin, and “volumetric splenomegaly” indicated that the spleen volume was greater than the mean plus three standard deviations of reference volumes based on both age and body surface area. Splenic infarction was arbitrarily classified into three types by the pattern and extent, as follows: 1) solitary infarct, a single infarct, regardless of size; 2) multifocal infarct, two or more infarcts, regardless of size; and 3) extensive infarct: an infarct involving at least half of the splenic volume (Fig. 1).
Figure 1.
Representative CT images of splenic infarctions. (A) A small solitary infarction. (B) A large solitary infarction. (C) Multifocal infarctions. (D) Extensive infarction.
Definitions of thrombotic and hemorrhagic events
Thrombotic events included cerebrovascular events (ischemic stroke, transient ischemic attack, and venous sinus thrombosis), coronary events (any ischemic heart disease, including acute coronary syndrome), splanchnic events, and peripheral thromboembolism. Hemorrhagic events included any acute bleeding or a need for red cell transfusion on admission. All events that occurred before, at the time of, and after the diagnosis were included in analyses.
Statistical analyses
Descriptive data are presented as means±standard deviations, medians (with ranges), or percentages, and were compared using Student's t-test or the χ2 (Fisher exact) test. The cumulative incidence of thrombotic vascular events by splenic infarction status was estimated, with death serving as a competing risk, using the Fine and Gray model and analyzed using the Gray equality test. Risk factors for splenic infarction were identified via Fine and Gray regression, with death serving as a competing risk.
All statistical analyses were performed using the SPSS software program (ver. 24.0; SPSS, Chicago, USA) or the SAS University edition (SAS Institute, Cary, USA); a p value <0.05 was considered to indicate statistical significance.
Ethics
This study was approved by the Institutional Review Board of Chungnam National University Hospital (approval no. 2020-10-053). The need for informed patient consent was waived given the retrospective nature of the analysis.
Results
Patient characteristics (Table 1)
Table 1.
Patient Characteristics (n=347).
| ET (n=143) | PV (n=129) | Pre-PMF (n=44) | PMF (n=31) | |||||
|---|---|---|---|---|---|---|---|---|
| Age (yr), median (range) | 62 (15-88) | 64 (18-91) | 63.5 (22-88) | 68.5 (40-88) | ||||
| Male, n (%) | 73 (51.0) | 82 (63.6) | 25 (56.8) | 21 (67.7) | ||||
| Palpable splenomegaly, n (%) | 0 (0.0) | 11 (8.5) | 4 (9.1) | 16 (51.6) | ||||
| Laboratory findings | ||||||||
| WBC, ×109/L | 11.0±4.5 | 14.7±6.2 | 14.5±10.2* | 13.7±11.0 | ||||
| Monocyte, ×109/L | 0.6±0.4 | 0.7±0.4 | 0.8±0.4* | 1.0±0.8 | ||||
| Hemoglobin, g/dL | 13.6±2.2 | 18.3±2.5 | 13.0±2.8 | 10.3±2.5 | ||||
| Platelet, ×109/L | 946.5±244.9 | 510.9±288.4 | 1,093.9±461.1* | 424.8±327.5 | ||||
| LDH, ×UNL | 1.1±0.4 | 1.3±0.5 | 1.6±0.7* | 2.0±1.5 | ||||
| Driver gene mutation, n (%) | ||||||||
| JAK2V617F | 80/121 (68.6) | 102/112 (91.1) | 24/37 (64.9) | 15/23 (65.3) | ||||
| CALR | 14/121 (11.6) | - | 5/34 (14.7) | 5/23 (21.7) | ||||
| MPL | 0/12 (0.0) | - | 0/3 (0.0) | 0/3 (0.0) | ||||
| JAK2 exon12 | - | 6/112 (5.4) | - | - | ||||
| Abdomen CT taken at diagnosis, n (%) | 80 (55.9) | 69 (53.5) | 20 (45.5) | 19 (61.3) | ||||
| IPSET, n (%) | ||||||||
| Low | 45 (31.5) | - | - | - | ||||
| Intermediate | 42 (29.4) | - | - | - | ||||
| High | 56 (39.2) | - | - | - | ||||
| IPSS, n (%) | ||||||||
| Low | - | - | 23 (52.3) | 5 (16.1) | ||||
| Intermediate-1 | - | - | 17 (38.6) | 8 (25.8) | ||||
| Intemediate-2 | - | - | 3 (6.8) | 12 (38.7) | ||||
| High | - | - | 1 (2.3) | 6 (19.4) | ||||
| Comorbidity, n (%) | ||||||||
| Hypertension | 51 (35.7) | 77 (59.7) | 22 (50.0) | 11 (35.5) | ||||
| Diabetes mellitus | 19 (13.3) | 32 (24.8) | 9 (20.5) | 7 (22.6) | ||||
| Chronic kidney disease | 21 (14.7) | 30 (23.3) | 9 (20.5) | 4 (12.9) | ||||
| Smoking | 28 (19.6) | 53 (41.1) | 14 (31.8) | 4 (12.9) | ||||
| Treatments, n (%) | ||||||||
| Cytoreductive treatment | 101 (75.4) | 106 (82.2) | 34 (77.3) | 17 (54.8) | ||||
| Aspirin | 128 (89.5) | 121 (93.8) | 38 (86.4) | 12 (38.7) | ||||
| Thrombosis, n (%)** | 37 (25.9) | 39 (30.2) | 13 (29.5) | 1 (3.2) | ||||
| FU (yr), median (range) | 3.6 (0.2-24.1) | 6.2 (0.2-26.5) | 4.8 (0.2-22.7) | 3.2 (0.1-19.7) |
ET: essential thrombocythemia, PV: polycythemia vera, pre-PMF: prefibrotic/early myelofibrosis, LDH: lactate dehydrogenase, UNL: upper normal limit, CT: computed tomography, IPSET: International Prognostic Score for Essential Thrombocythemia, IPSS: International Prognostic Scoring System, FU: follow-up
*p<0.05 compared to ET.
**Thrombosis before and at the time of diagnosis.
A total of 347 patients (143 ET, 129 PV, 44 pre-PMF, and 31 PMF patients; 201 men and 146 women) with a median age of 64 (range 15-91) years old were enrolled. They were followed for a median of 4.7 (range 0.1-26.5) years. ET patients diagnosed before 2017 were reviewed, and their diagnoses were revised based on the 2016 World Health Organization diagnostic criteria. Of the 129 ET patients, 32 (24.8%) and 11 (8.5%) were re-classified as pre-PMF and PV patients, respectively. Palpable splenomegaly was most common in PMF patients (51.6%), followed by pre-PMF (9.1%) and PV (8.5%) patients. No ET patient exhibited palpable splenomegaly. The white blood cell, monocyte, and platelet counts as well as the lactate dehydrogenase (LDH) level of pre-PMF patients were higher than those of ET patients [14.5±10.2×109/L vs. 11.0±4.5×109/L; 0.8±0.4×109/L vs. 0.6±0.4×109/L; 1,094.9±461.1×109/L vs. 946.5±244.9.4×109/L; and 1.6±0.7×the upper normal limit (UNL) vs. 1.1±0.4×UNL, respectively; all p<0.05]. The JAK2V617F mutation was most common in PV (91.1%) patients, followed by ET (68.6%), PMF (65.3%), and pre-PMF (64.9%) patients. The CALR mutation was most common in PMF patients (21.7%), followed by pre-PMF (14.7%) and ET (11.6%) patients. Abdominal CT data (from the time of the diagnosis) were available for 80 (55.9%) ET, 69 (53.5%) PV, 20 (45.5%) pre-PMF, and 19 (61.3%) PMF patients. ET patients were evenly distributed among the IPSET risk groups. Most pre-PMF patients were in the low (52.3%) or intermediate-1 (38.6%) IPSS risk groups, but many PMF patients were in the intermediate-2 (38.7%) or high (19.4%) risk groups. Cytoreductive therapy was most commonly prescribed for PV patients (82.2%), followed by pre-PMF (77.3%), ET (75.4%), and PMF (54.8%) patients. Most patients with MPN subtypes (other than PMF patients) were placed on low-dose aspirin.
Prevalence of splenic infarction (Table 2)
Table 2.
Prevalence of Splenic Infarction.
| ET (n=143) | PV (n=129) | pre-PMF (n=44) | PMF (n=31) | Total (n=347) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At diagnosis, n (%) | 0 (0.0) | 7 (5.4) | 4 (9.1) | 4 (12.9) | 15 (4.3) | |||||
| After diagnosis, n (%) | 1 (0.7)* | 2 (1.6) | 1 (2.3) | 1 (3.2) | 5 (1.4) | |||||
| Total, n (%) | 1 (0.7) | 9 (7.0) | 5 (11.4) | 5 (16.1) | 20 (5.8) |
ET: essential thrombocythemia, PV: polycythemia vera, pre-PMF: prefibrotic/early primary myelofibrosis
*Developed after transformation into secondary myelofibrosis.
Of the 347 patients, 15 (4.3%) exhibited splenic infarctions at the time of the MPN diagnosis. This was most common in PMF patients (12.9%), followed by pre-PMF (9.1%) and PV (5.4%) patients. No ET patients showed splenic infarction at the diagnosis. During follow-up, 5 (1.4%) of the 347 patients developed splenic infarctions: 1 each of the 143 ET (0.7%), 44 pre-PMF (2.3%), and 31 PMF (3.2%) patients; and 2 (1.6%) of the 129 PV patients. All showed palpable splenomegaly. Overall, 20 (5.8%) patients exhibited splenic infarctions at the diagnosis or during follow-up. No splenic artery or vein thrombosis was observed.
Clinical features of patients presenting with splenic infarctions (Table 3, Fig. 2)
Table 3.
Clinical Features of Patients with Splenic Infarction at Diagnosis (n=15).
| Age (yr), median (range) | 58 (22-88) | |
| Male, n (%) | 10 (66.7) | |
| Symptoms relevant to splenic infarction, n (%) | ||
| Abdominal pain | 8 (53.3) | |
| Vague abdominal discomfort | 2 (13.3) | |
| None | 5 (33.3) | |
| Splenomegaly | ||
| Palpable splenomegaly, n (%) | 8 (53.3) | |
| Volumetric splenomegaly, n (%) | 7 (46.7) | |
| No splenomegaly, n (%) | 0 (0.0) | |
| Extent of splenic infarction, n (%) | ||
| Solitary | 5 (33.3) | |
| Multifocal | 9 (60.0) | |
| Extensive | 1 (6.7) | |
| Laboratory findings | ||
| WBC, ×109/L | 17.1±12.1 | |
| Monocyte, ×109/L | 1.1±0.8 | |
| Hemoglobin, g/dL | 15.2±5.1 | |
| Platelet, ×109/L | 517.6±447.3 | |
| LDH, ×UNL | 2.3±1.7 | |
| Diver gene mutation | ||
| JAK2V617F | 11/14 (78.6) | |
| CALR | 2/14 (14.3) | |
| Comorbidity | ||
| Hypertension | 4 (26.7) | |
| Diabetes mellitus | 3 (20.0) | |
| Chronic kidney disease | 2 (13.3) | |
| Smoking | 6 (40.0) | |
| Thrombotic events, n (%)* | 5 (33.3) | |
| Hemorrhagic event, n (%)* | 1 (6.7) | |
| Follow-up duration (yr), median (range) | 4.3 (0.6-20.5) |
*Events before, at the time of, and after MPN diagnosis.
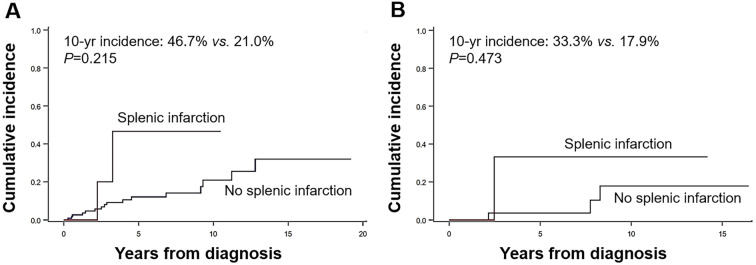
Figure 2.
Cumulative incidence of thrombotic events in patients with polycythemia vera (A) and prefibrotic/early primary myelofibrosis (B) by splenic infarction status at the diagnosis.
The median age of the 15 patients with splenic infarctions at the diagnosis was 58 (range 22-88) years old, and 10 (66.7%) were men. Abdominal pain and vague abdominal discomfort were reported by 8 (53.3%) and 2 (13.3%) patients, respectively. Five (33.3%) patients did not show any symptoms of splenic infarction. Palpable splenomegaly was noted in 8 (53.3%) patients and volumetric splenomegaly in an additional 7 (46.7%); thus, all patients with splenic infarctions showed splenomegaly at the diagnosis. In terms of the extent of infarction, multifocal infarcts (60.0%) were most frequent, followed by solitary infarcts (33.3%) and extensive infarcts (6.7%). During a median follow-up of 4.3 (range 0.6-20.5) years, 5 (33.3%) and 1 (6.7%) of the 15 patients developed thrombotic and hemorrhagic vascular events, respectively. The cumulative incidence of thrombosis in patients with splenic infarctions tended to be higher than that in patients without splenic infarctions; this was true for both PV (10-year cumulative incidence 46.7 vs. 21.0%; p=0.215) and pre-PMF (10-year cumulative incidence 33.3 vs. 17.9%; p=0.473) patients, but statistical significance was lacking.
Clinical outcomes of splenic infarction (Table 4)
Table 4.
Treatment and Outcomes of Patients with Splenic Infarction at Diagnosis (n=15).
| Treatment | ||
| Initial cytoreduction, n, (%) | ||
| Hydroxyurea | 9 (60.0) | |
| Anagrelide | 0 (0.0) | |
| Ruxolitinib | 1 (6.7) | |
| None | 5 (33.3) | |
| Low-dose aspirin, n (%) | 12 (80.0) | |
| Analgesics, n (%) | ||
| NSAID | 2 (13.3) | |
| Narcotic agents | 6 (40.0) | |
| None | 7 (46.7) | |
| Surgical treatment, n (%) | 0 (0.0) | |
| Outcomes | ||
| Complications, n (%) | ||
| Abscess | 0 (0.0) | |
| Fistula | 0 (0.0) | |
| Peritonitis | 0 (0.0) | |
| Others | 0 (0.0) | |
| Radiologic resolution, n (%) | ||
| Residual non-perfused area* | 1/7 (14.3) | |
| No non-perfused area** | 6/7 (85.7) |
NSAID: non-steroidal anti-inflammatory drug
* Computed tomography performed at 23 months after diagnosis.
** Computed tomography performed at a median of 18 months (range: 9-80 months) after diagnosis.
Of the 15 patients with splenic infarctions at the MPN diagnosis, 10 (66.7%) received cytoreductive therapy but 5 (33.3%) did not. Low-dose aspirin was prescribed for 12 (80.0%) patients. For pain control, non-steroidal anti-inflammatory drugs and narcotic agents were given to 2 (13.3%) and 6 (40.0%) patients, respectively. No analgesic was prescribed for 7 (46.7%) patients. No complication (an abscess, fistula, splenic rupture, intraperitoneal bleeding, or peritonitis) was observed during follow-up. Of the 7 patients who underwent follow-up abdominal CT after the MPN diagnosis, 1 (14.3%) showed a residual non-perfused spleen area 23 months after the diagnosis; the others lacked non-perfused areas at a median of 18 months (range 9-80 months) after the MPN diagnosis. No complications were noted in the five patients who developed splenic infarctions during follow-up.
Risk factors for splenic infarction at the diagnosis (Table 5)
Table 5.
Fine and Gray Regression Analysis Seeking Risk Factors at Diagnosis for Developing Splenic Infarction (n=347).
| Univariate analysis | Multivariate analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||||||
| Age >60yr | 0.58 | 0.21-1.63 | 0.300 | - | - | - | ||||||
| Male | 1.48 | 0.49-4.42 | 0.486 | - | - | - | ||||||
| Palpable splenomegaly | 14.35 | 5.52-46.09 | <0.001 | 14.89 | 4.00-55.35 | <0.001 | ||||||
| WBC >12.0 ×109/L | 0.52 | 0.25-2.03 | 0.521 | - | - | - | ||||||
| WBC >25.0 ×109/L | 3.04 | 0.63-14.62 | 0.166 | - | - | - | ||||||
| Monocyte >1.0 ×109/L | 2.32 | 0.71-7.60 | 0.165 | |||||||||
| Platelet >1,000 ×109/L | 0.25 | 0.93-1.90 | 0.177 | - | - | - | ||||||
| LDH >1.5 ×UNL | 3.46 | 1.21-9.88 | 0.020 | 2.73 | 0.88-8.45 | 0.082 | ||||||
| Positive JAK2V617F | 1.25 | 0.04-4.61 | 0.736 | - | - | - | ||||||
| Positive CALR mutation | 0.53 | 0.06-5.07 | 0.580 | |||||||||
| PV | 1.51 | 0.53-4.26 | 0.440 | - | - | - | ||||||
| pre-PMF | 2.66 | 0.81-8.74 | 0.108 | - | - | - | ||||||
| PMF | 4.11 | 1.23-12.78 | 0.022 | 0.61 | 0.13-2.92 | 0.538 | ||||||
| Hypertension | 0.41 | 0.13-1.30 | 0.129 | - | - | - | ||||||
| Diabetes mellitus | 1.05 | 0.29-3.82 | 0.945 | - | - | - | ||||||
| Chronic kidney disease | 0.67 | 0.15-3.05 | 0.604 | - | - | - | ||||||
| Smoking | 1.71 | 0.59-4.95 | 0.320 | - | - | - | ||||||
| Thrombosis before or at diagnosis | 0.4 | 0.0-91.81 | 0.235 | - | - | - | ||||||
HR: hazard ratio, CI: confidence interval, LDH: lactate dehydrogenase, UNL: upper normal limit, PV: polycythemia vera, pre-PMF: prefibrotic/early primary myelofibrosis
A Fine and Gray regression analysis was performed to identify risk factors for splenic infarction at the diagnosis. In univariate analyses, palpable splenomegaly [hazard ratio (HR) 14.35; 95% confidence interval (CI) 5.52-46.09; p<0.001], a high LDH level (>1.5-fold the UNL) (HR 3.46; 95% CI 1.21-9.88; p=0.020), and PMF (HR 4.11; 95% CI 1.23-12.78; p=0.22) were identified as risk factors. In multivariate analyses, palpable splenomegaly (HR 14.89; 95% CI 4.00-55.35; p<0.001) was the only risk factor.
Discussion
We found that a small proportion of Ph- MPN patients exhibited splenic infarctions at the diagnosis. The prevalence varied among the subtypes, being most frequent in patients with PMF, followed by patients with pre-PMF and PV; it was rare in ET patients. Only a few patients (1.4%) developed such infarctions during follow-up. To our knowledge, this is the first report on the prevalence of splenic infarction in Ph- MPN patients. However, our work has inevitable limitations because of its retrospective design. Importantly, the prevalence at the diagnosis may be underestimated, as only 54.2% of patients underwent abdominal CT. Given that 5 (33.3%) of the 15 patients with splenic infarctions were asymptomatic, some patients who did not undergo abdominal CT may have had infarctions. Thus, a prospective study using abdominal CT is warranted to determine the exact prevalence of splenic infarction at the time of the MPN diagnosis.
Whether or not abdominal CT is appropriate for every patient, regardless of symptoms or signs, at the time of the MPN diagnosis remains controversial (24); abdominal CT is not included in the standard MPN diagnostic algorithm (25,26). However, asymptomatic splanchnic thrombosis is sometimes encountered in clinical practice (24). Furthermore, we observed that not only palpable but also volumetric splenomegaly (as revealed by CT) had certain clinical implications (8). Overall, the clinical utility of abdominal CT at the MPN diagnosis remains unclear. All 15 patients with splenic infarctions showed palpable splenomegaly or volumetric splenomegaly on abdominal CT. This indicates that splenic infarction was caused by splenic enlargement itself (27) and was not a thrombotic vascular event. It was not surprising that splenic infarction was most common in PMF patients, in whom splenomegaly is more frequent and marked than in other patients (9,27-29).
Conservative treatment adequately controlled the symptoms, with no serious complications noted in any patient. Thus, splenic infarction is not very serious and does not require particular attention. However, some patients complained of severe abdominal pain, and several case reports have described serious complications (splenic rupture, peritoneal bleeding, and peritonitis) in MPN patients (19-21). In addition, abscess formation and functional asplenia attributable to splenic infarction have been reported in patients with other clinical conditions (30-33).
We found that the cumulative incidence of thrombotic vascular events in PV and pre-PMF patients with splenic infarctions at the diagnosis tended to be higher than in those without splenic infarctions; however, the differences were not statistically significant. This may reflect the small numbers of patients with infarctions; a further evaluation is thus warranted. A Fine and Gray regression analysis revealed that palpable splenomegaly was the only independent risk factor for infarction, again indicating that infarction is closely associated with splenomegaly. Given that approximately half of MPN patients underwent abdominal CT at the time of the diagnosis, the results should be interpreted with caution. A well-designed prospective study is required.
In summary, splenic infarction occurred in subpopulations of Ph- MPN patients with volumetric or palpable splenomegaly (4.3% at the diagnosis and 1.4% during follow-up). Infarctions were most frequent in patients with PMF, followed by pre-PMF and PV; they were rare in ET patients. The clinical courses were generally mild, the symptoms were adequately controlled with conservative treatment, and complications were rare.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127: 2391-2405, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Rungjirajittranon T, Owattanapanich W, Ungprasert P, Siritanaratkul N, Ruchutrakool T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer 19: 184, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stein BL, Martin K. From Budd-Chiari syndrome to acquired von Willebrand syndrome: thrombosis and bleeding complications in the myeloproliferative neoplasms. Blood 134: 1902-1911, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Mudireddy M, Shah S, Lasho T, et al. Prefibrotic versus overtly fibrotic primary myelofibrosis: clinical, cytogenetic, molecular and prognostic comparisons. Br J Haematol 182: 594-597, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Yogarajah M, Tefferi A. Leukemic transformation in myeloproliferative neoplasms: a literature review on risk, characteristics, and outcome. Mayo Clin Proc 92: 1118-1128, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Hong J, Lee JH, Byun JM, et al. Risk of disease transformation and second primary solid tumors in patients with myeloproliferative neoplasms. Blood Adv 3: 3700-3708, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tremblay D, Schwartz M, Bakst R, et al. Modern management of splenomegaly in patients with myelofibrosis. Ann Hematol 99: 1441-1451, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee MW, Yeon SH, Ryu H, et al. Volumetric splenomegaly in patients with essential thrombocythemia and prefibrotic/early primary myelofibrosis. Int J Hematol 114: 35-43, 2021. [DOI] [PubMed] [Google Scholar]
- 9. Song MK, Park BB, Uhm JE. Understanding splenomegaly in myelofibrosis: association with molecular pathogenesis. Int J Mol Sci 19: 898, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaroch MT, Broughan TA, Hermann RE. The natural history of splenic infarction. Surgery 100: 743-750, 1986. [PubMed] [Google Scholar]
- 11. Nores M, Phillips EH, Morgenstern L, Hiatt JR. The clinical spectrum of splenic infarction. Am Surg 64: 182-188, 1998. [PubMed] [Google Scholar]
- 12. Antopolsky M, Hiller N, Salameh S, Goldshtein B, Stalnikowicz R. Splenic infarction: 10 years of experience. Am J Emerg Med 27: 262-265, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Conn HO. Sickle-cell trait and splenic infarction associated with high-altitude flying. N Engl J Med 251: 417-420, 1954. [DOI] [PubMed] [Google Scholar]
- 14. Boddana PV, Tomson CR, Austen B, et al. A case of lymphoma presenting as splenic infarction. BMJ Case Rep 2009: bcr.09.2008.0849, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldschmidt N, Gural A, Ben Yehuda D. Extensive splenic infarction, deep vein thrombosis and pulmonary emboli complicating induction therapy with all-trans-retinoic acid (ATRA) for acute promyelocytic leukemia. Leuk Lymphoma 44: 1433-1437, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Dyer MJ, Majid A, Walewska R, et al. Splenic infarction associated with rapidly progressive chronic lymphocytic leukemia with complex karyotype and ATM mutation. Leuk Res 35: e55-e57, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Drummond D, Lenoir M, Petit AY. Splenic infarction in a child revealing chronic myeloid leukemia. Eur J Pediatr 171: 1141-1142, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Charan VD. Splenic infarction in polycythemia vera: can the spleen be saved? J Assoc Physicians India 63: 94, 2015. [PubMed] [Google Scholar]
- 19. Downer WR, Peterson MS. Massive splenic infarction and liquefactive necrosis complicating polycythemia vera. AJR Am J Roentgenol 161: 79-80, 1993. [DOI] [PubMed] [Google Scholar]
- 20. Friedrich EB, Kindermann M, Link A, Bohm M. Splenic rupture complicating periinterventional glycoprotein IIb/IIIa antagonist therapy for myocardial infarction in polycythemia vera. Z Kardiol 94: 200-204, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Tzeng CW, Wang YC, Hsieh CH. Peritonitis resulting from ruptured splenic abscess after splenic infarction in a patient with polycythemia vera. Am Surg 78: E474-E475, 2012. [PubMed] [Google Scholar]
- 22. Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood 120: 1197-1201, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 113: 2895-2901, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Passamonti F. How I treat polycythemia vera. Blood 120: 275-284, 2012. [DOI] [PubMed] [Google Scholar]
- 25. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol 95: 1599-1613, 2020. [DOI] [PubMed] [Google Scholar]
- 26. Tefferi A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am J Hematol 96: 145-162, 2021. [DOI] [PubMed] [Google Scholar]
- 27. Mesa RA. How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood 113: 5394-5400, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Cervantes F. How I treat splenomegaly in myelofibrosis. Blood Cancer J 1: e37, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Xu J, Gale RP, et al. Prognostic impact of splenomegaly on survival of Chinese with primary myelofibrosis. Leuk Res 38: 1207-1211, 2014. [DOI] [PubMed] [Google Scholar]
- 30. Altemeier WA, Macmillan BG. Massive splenic infarction with acute necrosis and abscess following ligation of the hepatic and splenic arteries for portal hypertension; case report. Am Surg 20: 739-743, 1954. [PubMed] [Google Scholar]
- 31. Warrier I, Ravindranath Y. Splenic infarction and total functional asplenia in disseminated varicella. J Pediatr 109: 305-307, 1986. [DOI] [PubMed] [Google Scholar]
- 32. Wang CC, Lee CH, Chan CY, Chen HW. Splenic infarction and abscess complicating infective endocarditis. Am J Emerg Med 27: 1021. e3-e5, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Krepis P, Maritsi DN, Tsolia MN, Vakaki M, Kossiva L. Massive splenic infarction and autosplencetomy: first presentation of homozygous sickle cell disease in a toddler. J Pediatr Hematol Oncol 42: 371-372, 2020. [DOI] [PubMed] [Google Scholar]