Abstract
A 26-year-old Japanese woman developed a fever, myalgia and gait disturbance one day after receiving the second dose of the mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. A neurological examination revealed symmetrical weakness and myalgia in proximal lower limbs, and a blood examination showed prominent elevation of creatinine kinase. Magnetic resonance imaging (MRI) revealed a high signal intensity in the thigh muscles on short-tau inversion recovery images, and antibody testing revealed positive findings for anti-signal recognition particle (SRP) antibody. Thus, anti-SRP antibody-positive immune-mediated myopathy was diagnosed. We initiated immunotherapy, and she was ultimately able to walk stably.
Keywords: anti-signal recognition particle antibody, immune-mediated necrotizing myopathy, myopathy, SARS-CoV-2, vaccine
Introduction
Since the outbreak of the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, in December 2019, the COVID-19 pandemic has been ravaging the globe (1). Vaccines against SARS-CoV-2 have been an important strategy for managing the pandemic, and inoculations have been administered around the world.
Recently, various autoimmune complications following vaccination, such as myocarditis and Guillain-Barré syndrome, have been reported (2). However, there are very few reports of inflammatory myopathy following vaccination. In particular, cases with positive disease-specific autoantibodies are very rare.
We herein report a case of anti-signal recognition particle (SRP) antibody-positive immune-mediated myopathy after mRNA-1273 SARS-CoV-2 vaccination.
Case Report
A previously healthy 26-year-old Japanese woman had been vaccinated with the first dose of mRNA-1273 vaccine 7 weeks ago and received the second dose 21 days later. One day after receiving the second dose, she developed a fever of 39℃, general malaise, and myalgia. She presented to our hospital due to gait disturbance 26 days after the second vaccination. None of her family members had neurological disorders.
Her body temperature was 37.0℃, blood pressure was 112/67 mmHg, and heart rate was 100 beats per minute. Her respiratory rate was 14 breaths per minute, and SpO2 was 98% in room air. There were no enlarged lymph nodes. A neurological examination revealed symmetrical weakness and myalgia in the proximal lower limbs [Medical Research Council (MRC) grade 2-3/5], with preserved strength in the upper limbs and sensation. Cranial nerves were normal.
A blood examination showed prominent elevation of creatinine kinase (CK) to 16,298 U/L and CK-MB to 632 U/L, but troponin I was normal. The antinuclear antibody test was positive (1:80) with a speckled pattern (Table 1). Her nasopharyngeal specimen tested for SARS-CoV-2 by reverse-transcription polymerase chain reaction was negative at admission. Electrocardiography and echocardiography showed a normal cardiac function, but her vital capacity and forced vital capacity were reduced to 73% and 77%. Magnetic resonance imaging (MRI) revealed a high signal intensity in the bilateral gluteus maximus, quadriceps femoris, and hamstring muscles on short tau inversion recovery imaging (Fig. 1). Needle electromyography showed low amplitude motor unit potentials (MUPs) with a short duration and early recruitment in the semitendinosus muscles, indicating myogenic changes. We suspected anti-SRP antibody-positive myopathy, because of the prominent CK elevation and distribution of muscle damage on the flexion side of the thighs on MRI. Serum anti-SRP antibody was positive (1.3 IU/mL) according to an enzyme-linked immunosorbent assay, but anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibody, anti-aminoacyl tRNA synthetase (ARS) antibody, and anti-mitochondrial M2 antibody were negative. Based on the above results, anti-SRP antibody-positive immune-mediated myopathy was diagnosed.
Table 1.
Laboratory Data on Admission.
| Complete blood count | Biochemistry test | |||||||
| WBC | 6,100 | /μL | Alb | 4.3 | g/dL | |||
| Hb | 14.3 | g/dL | CK | 16,298 | U/L | |||
| Plt | 271,000 | /μL | CK-MB | 632 | U/L | |||
| Coagulation | Troponin I | 18.8 | pg/mL | |||||
| PT INR | 1.08 | AST | 518 | U/L | ||||
| APTT | 27 | s | ALT | 366 | U/L | |||
| D-dimer | 0.5 | μg/mL | LD | 1,994 | U/L | |||
| ESR | 8.0 | mm/h | ALP | 50 | U/L | |||
| Immunoserological test | γGTP | 9 | U/L | |||||
| CRP | 0.11 | mg/dL | Cr | 0.29 | mg/dL | |||
| IgG | 1,063 | mg/dL | BUN | 6.4 | mg/dL | |||
| IgA | 135 | mg/dL | Na | 141 | mEq/L | |||
| IgM | 146 | mg/dL | Cl | 105 | mEq/L | |||
| ANA | 1:80 | K | 4.3 | mEq/L | ||||
| RF | 0 | IU/mL | Aldolase | 180.6 | U/L | |||
| anti-ARS Ab | 5.0 | index | Ferritin | 72 | ng/mL | |||
| anti-mitochondrial M2 Ab | 1.5 | index | HbA1c | 5.3 | % | |||
| anti-SRP Ab | 1.3 | IU/mL | Glucose | 98 | mg/dL | |||
| anti-HMGCR Ab | <1.0 | IU/mL | ||||||
WBC: white blood cell count, Hb: hemoglobin, Plt: platelet, PT INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time, ESR: erythrocyte sedimentation ratio, CRP: C-reactive protein, Ig: immunoglobulin, ANA: anti nuclear antibodies, RF: rheumatoid factor, ARS: aminoacyl tRNA synthetase, Ab: antibody, SRP: signal recognition particle, HMGCR: 3-hydroxy-3-methylglutary-coenzyme A reductase, Alb: albumin, CK: creatine kinase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LD: lactic dehydrogenase, ALP: alkaline phosphatase, γGTP: γ-glutamyl transpeptidase, Cr: creatinine
Figure 1.
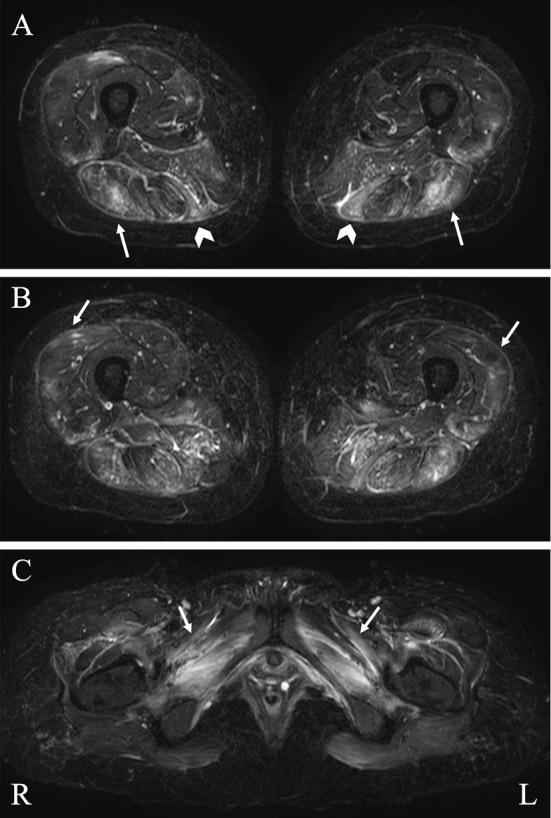
Short tau inversion recovery magnetic resonance images (MRI) of thigh muscles at admission. A high signal intensity is observed in the bilateral biceps femoris (A: arrows), semitendinosus (A: arrowheads), vastus lateralis (B: arrows), and obturator externus and adductor muscles (C: arrows). Changes in signal intensity in muscles are more conspicuous on the flexion side of the thighs, and edematous change was prominent.
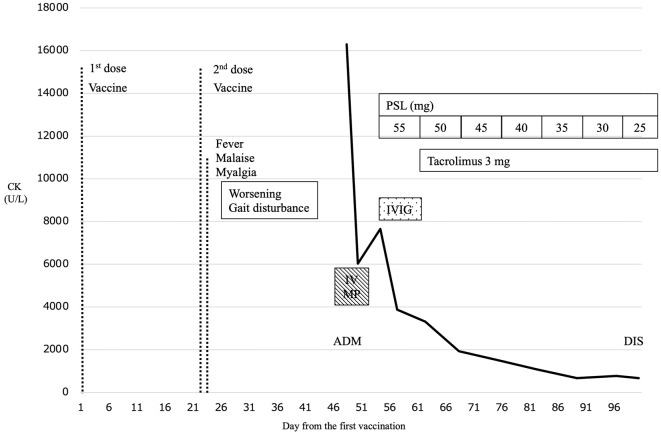
Fig. 2 summarizes the clinical course. She was treated with intravenous methylprednisolone (IVMP) 1 g/day for 3 days and intravenous immunoglobulin (IVIG) 0.4 g/kg for 5 days, followed by oral prednisolone (PSL) 55 mg/day. For tapering PSL, we added tacrolimus 3 mg/day. The serum CK level gradually decreased to 672 U/L at 52 days after starting immunotherapy. Her strength in the lower limbs gradually improved to MRC grade 4-5/5, and she was able to walk independently and stably at 53 days after admission.
Figure 2.
The clinical course. The patient developed a fever, myalgia, and gait disturbance one day after receiving the second dose of the vaccine. She was admitted to our hospital 47 days after the first vaccination. The patient was diagnosed with immune-mediated myopathy, and intravenous methylprednisolone pulse therapy (IVMP) was given from days 47 to 50 and intravenous immunoglobulin (IVIG) 0.4 g/day from days 55 to 59. Oral prednisolone (PSL) was started at 55 mg/day from day 55 and tapered to 25 mg/day on day 98, with addition of tacrolimus 3 mg/day from day 62. The creatinine kinase level (bold line) declined drastically after the initiation of treatment, and the patient became able to walk independently. She was discharged on day 100 (DIS). ADM: admission, CK: creatinine kinase, DIS: discharge, IVIG: intravenous immunoglobulin, IVMP: intravenous methylprednisolone, PSL: prednisolone
Discussion
We encountered a case of anti-SRP antibody-positive immune-mediated myopathy after mRNA-1273 SARS-CoV-2 vaccination. In this case, inflammatory myopathy was strongly suspected based on myalgia, muscle weakness, MRI findings and electromyography findings, and because anti-SRP antibody was positive at an early stage, a muscle biopsy was not performed. Anti-SRP antibody is a disease-specific autoantibody and can be positive in immune-mediated necrotizing myopathy (IMNM). SRP is an intracellular ribonucleoprotein composed of a 7S RNA molecule and six proteins (9, 14, 19, 54, 68, and 72 kDa) (3). The reaction of autoantibodies to SRP molecules activates complement, and the formation of membrane attack complex (MAC) on the muscle fiber membrane results in necrosis of muscle fibers (4). This antibody level has been reported to correlate with disease activity, such as muscle weakness and the serum CK level (5).
Our case suggests a probable relationship between mRNA-1273 vaccination and anti-SRP antibody-positive immune-mediated myopathy, considering the temporal relationship between the onset of myopathy and the time of vaccination. In the present case, the patient developed myopathy 22 days after the first dose, i.e. the day after the second dose. Three cases of IMNM after SARS-CoV-2 vaccination with CoronaVac and BNT162b2 have been reported (6-8). In those reports, myopathic symptoms appeared 1 day, 10 days, and 14 days after the first vaccination (Table 2). However, IMNM after mRNA-1273 vaccination has not been previously reported. The onset timing of other post-vaccination myopathies has reportedly ranged from 5 hours to 2 months after vaccination (9). In a large survey of neurological complications after SARS-CoV-2 vaccination, Guillain-Barré syndrome, Bell's palsy, and myasthenic disorders showed a significantly increased risk from two to four weeks after vaccination (10). Although myopathy was not described in that study, the onset of myopathy in our case was approximately three weeks after the first dose, so the timing of the onset was similar. However, a case of anti-SRP antibody-positive IMNM two weeks after influenza vaccination has been reported, so IMNM after vaccination may not be specific to the SARS-CoV-2 vaccine (Table 2) (11). Furthermore, a case of IMNM with no antibody test results has been reported (8). In these cases of IMNM after SARS-CoV-2 vaccination, a good prognosis was obtained by adding immunosuppressive drugs and IVIG to steroid therapy from an early stage.
Table 2.
The Previous Reports of Immune-mediated Necrotizing Myopathy after Vaccination.
| literature | Age | Sex | Vaccine | Day from vaccination to onset | CK max (IU/L) | Antibody | Symptoms | Pathological findings | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| (6) | 55 | F | BNT162b2 | 1 day from 1st dose |
7,967 | SRP | Weakness in proximal mucles, myalgia, fatigue | Necrotic fibers with focally aggregated macrophages and MHC-I | PSL, MTX, IVIG | Partially improved |
| (7) | 54 | M | CoronaVac | 14 days from 1st dose | 27,000 | SRP | Weakness in proximal muscles, dysarthria, dysphagia | Necrotic fibers with focally aggregated macrophages and MHC-I | PSL, IVIG | Partially improved |
| (8) | 41 | M | BNT162b2 | 10 days from 1st dose | 12,647 | No data available | Weakness and pain in proximal mucles, dysphagia, dyspnea | Necrotic fibers with infiltration of macrophage and lymphocytes | High dose gluco-corticoids | Improved |
| (11) | 28 | F | Influenza vaccination | 14 days from vaccination | 16,095 | SRP | Weakness in proximal muscles, dysphagia, dyspnea | Necrotic fibers with infiltration of macrophagic | IVMP, IVIG, rituximab | Improved |
| This case | 26 | F | mRNA-1273 | 1 day from 2nd dose (22 days from 1st dose) |
16,298 | SRP | Weakness and pain in proximal lower limbs, fever, fatigue | No data available | PSL, TAC, IVMP, IVIG | Improved |
SRP: signal recognition particle, CK: creatinine kinase, MHC-I: major histocompatibility complex class I, PSL: prednisolone, MTX: methotrexate, IVIG: intravenous immunoglobulin, TAC: tacrolimus, IVMP: intravenous methylprednisolone
One of the causes of the strong immune response to the mRNA vaccines is the stimulation of cytotoxic T cell and proinflammatory cytokine, followed by the induction of antibody production (12). The mRNA-1273 vaccine has been reported to increase antiviral antibody titers approximately 15 days after vaccination (13). In the present case, an immune response was activated after the first vaccination, and abnormal antibodies began to be produced; the second vaccination may have then further activated antibody production, leading to the onset of myopathy. In addition, a case report of cytokine release syndrome (CRS) after BNT162b2 vaccination suggested the possibility of an excessive immune response after vaccination because increased proinflammatory cytokines were observed in parallel with an increased neutralizing antibody titer against SARS-CoV-2 (14). Via a similar mechanism, SARS-CoV-2 vaccination may induce overactivation of innate immune system and humoral immunity, followed by the production of an unexpected antibody. However, whether or not mRNA vaccines induce the production of autoantibodies has not been verified. Furthermore, anti-SRP antibody-positive IMNM is reported to be associated with human leukocyte antigen (HLA)-DRB1*08:03 (15). Although a genetic predisposition may be associated with the development of myopathy upon receiving the vaccine, no epidemiological evidence of this exists at present, and our findings do not exaggerate the risk of vaccination.
The present case suggests a temporal association between mRNA-1273 vaccination and the development of anti-SRP antibody-positive immune-mediated myopathy, but a causal relationship cannot be proven. Since pre-vaccination serum was not available in this case, whether or not anti-SRP antibody newly appeared after vaccination remains unclear. The possibility of coincidental occurrence cannot be totally ruled out. Further surveillance and analyses of immune-mediated myopathy after SARS-CoV-2 vaccination are warranted.
Written informed consent for the publication of the clinical details and clinical images was obtained from the patient and relatives. This observational case report did not require ethics committee approval.
The authors state that they have no Conflict of Interest (COI).
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard [Internet]. [cited 2022 May 25]. Available from: https://covid19.who.int
- 2. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 326: 1390-1399, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anquetil C, Boyer O, Wesner N, Benveniste O, Allenbach Y. Myositis-specific autoantibodies, a cornerstone in immune-mediated necrotizing myopathy. Autoimmun Rev 18: 223-230, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Bergua C, Chiavelli H, Allenbach Y, et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotizing myopathy. Ann Rheum Dis 78: 131-139, 2019. [DOI] [PubMed] [Google Scholar]
- 5. Benveniste O, Drouot L, Jouen F, et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum 63: 1961-1971, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Dodig D, Fritzler MJ, Naraghi A, Tarnopolsky MA, Lu JQ. Immune-mediated necrotizing myopathy after BNT162b2 vaccination in a patient with antibodies against receptor-binding domain of SARS-CoV-2 and signal recognition particle. Muscle Nerve 65: 11-13, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan CY, Toh TH, Toh YF, Wong KT, Shahrizaila N, Goh KJ. A temporal association between COVID-19 vaccination and immune-mediated necrotizing myopathy. Muscle Nerve 65: E24-E26, 2022. [DOI] [PubMed] [Google Scholar]
- 8. Blaise M, Rocher F, Spittler H, et al. Severe necrotizing myopathy after COVID-19 vaccine with BNT162b2 and regimen with ipilimumab plus nivolumab in a patient with advanced melanoma. J Eur Acad Dermatol Venereol 36: e100-e102, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stübgen JP. A review on the association between inflammatory myopathies and vaccination. Autoimmun Rev 13: 31-39, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med 27: 2144-2153, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mamarabadi M, Baisre A, Leitch M, Hsu V, Kanduri JS, Chen S. Case of anti-single recognition particle-mediated necrotizing myopathy after influenza vaccination. J Clin Neuromuscul Dis 19: 211-216, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Oberli MA, Reichmuth AM, Dorkin JR, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett 17: 1326-1335, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383: 2427-2438, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Au L, Fendler A, Shepherd STC, et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med 27: 1362-1366, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohnuki Y, Suzuki S, Shiina T, et al. HLA-DRB1 alleles in immune-mediated necrotizing myopathy. Neurology 87: 1954-1955, 2016. [DOI] [PubMed] [Google Scholar]