Abstract
The host cell microfilaments and microtubules (MTs) are known to play a critical role in the life cycles of several pathogenic intracellular microbes by providing for successful invasion and promoting movement of the pathogen once inside the host cell cytoplasm. Orientia tsutsugamushi, an obligate intracellular bacterium, enters host cells by induced phagocytosis, escapes to the cytosol, and then replicates in the cytosol. ECV304 cells infected with O. tsutsugamushi revealed the colocalization of the MT organizing center (MTOC) and cytosolic orientiae by indirect immunofluorescence assay. Using immunofluorescence microscopy in the presence and absence of MT-depolymerizing agents (colchicine and nocodazole), it was shown that the cytosolic oriential movement was mediated by MTs. By transfection study (overexpression of dynamitin [also called p50], which is known to associate with dynein-dependent movement), the movement of O. tsutsugamushi to the MTOC was also mediated by dynein, the minus-end-directed MT-related motor. Although the significance of this movement in the life cycle of O. tsutsugamushi was not proven, we propose that the cytosolic O. tsutsugamushi bacteria use MTs and dyneins to propel themselves from the cell periphery to the MTOC.
Orientia tsutsugamushi, the causative agent of scrub typhus, has been reported to attach to the susceptible host cells in a heparan sulfate glycosaminoglycan-mediated manner (25). After attachment to the host plasma membrane, O. tsutsugamushi induces its own uptake by a process termed induced phagocytosis (40). After entry into the cells, O. tsutsugamushi escapes from the phagocytic vacuole by an unknown mechanism. This probably occurs shortly after phagocytosis, since phagocytic vacuoles containing O. tsutsugamushi have never been seen far from the cell surface (12). Once free in the host cytoplasm, the bacteria replicate in the perinuclear area. Listeriae, shigellae, and rickettsiae also escape from the phagocytic vacuole and replicate in the cytoplasm. These bacteria have independently developed an apparently similar host cell actin-based mechanism that is essential for their intercellular spread (16, 18, 33, 39). In the case of viruses, such as the common type 2 or 5 adenoviruses, they enter a new host cell by receptor-mediated endocytosis and reach the cytosol by acid-dependent disruption of the endosomal membrane (37). It is known that microtubules (MTs) and dyneins are required for directional transport of cytosolic adenovirus to the nucleus (36). Herpes simplex virus 1 (HSV-1) also overcomes the cytosolic barrier by packaging its genome into a capsid structure that is capable of using the minus-end-directed MT-dependent dynein motor (35). Some bacteria, such as Chlamydia trachomatis and Campylobacter jejuni, utilize the host MTs and dyneins during the early events of infection (9, 24). However, it is not clear whether O. tsutsugamushi uses the host cell cytoskeleton for its intracellular movement and intercellular spread.
The MT network of an interphase cell is a dynamic polarized structure. The relatively stable minus ends (from the nucleus to the cell periphery) are localized to the MT organizing center (MTOC), which is typically located at a perinuclear position in cultured cells (30). Motor proteins are needed for a variety of cellular activities, such as positioning of the mitotic spindle apparatus, movement of mitotic chromosomes, vesicular trafficking, and maintenance of cell shape (21, 23, 43). Motors are classified according to the sequences of their motor domains and the directionality of their motion (20, 42). Kinesin superfamily motors typically move towards the MT plus ends, whereas dynein motors mediate minus-end-directed movements. The vast majority of the MT-dependent minus-end-directed transport processes in interphase cells require dynein, which is typically associated with dynactin (43). Dynein is thought to be nonfunctional for minus-end-directed cargo transport unless it is associated with dynactin, a heterooligomeric complex of at least nine different polypeptides (4). This concept has been reinforced experimentally by overexpression of the dynactin component dynamitin (also known as p50), which is thought to dissociate the dynactin complex and severely affects mitosis, as well as vesicular trafficking and organelle distribution in interphase cells (4).
In order to clarify the mode of intracellular movement of O. tsutsugamushi, we performed indirect immunofluorescence staining and cytoskeletal inhibitor assays. Our results with ECV304 cells showed that the movement of O. tsutsugamushi towards the perinucleus depended on intact MTs but did not require dynamic MTs. O. tsutsugamushi bacteria could not recruit and polymerize host actin around their surfaces in the host cell cytoplasm (our unpublished observation). We knew, based on tubulin staining, that this perinuclear region colocalized with the MTOC. When the MT network was disrupted with nocodazole or colchicine, O. tsutsugamushi distributed randomly in the cytoplasm. To understand the association between dynein and O. tsutsugamushi, we overexpressed dynamitin, which significantly reduced the extent of minus-end movements of O. tsutsugamushi. This study, together with a previously published adenovirus study (36), is one of the few examples of the use of the MT network by a nonmembrane-bounded particle or microorganism.
MATERIALS AND METHODS
Cells, bacteria, antibodies, and chemicals.
ECV304 cells (human umbilical vein-derived endothelial cell line) were grown in M199, which was supplemented with 10% fetal bovine serum (FBS), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. HeLa cells (human cervical epithelial carcinoma cell line) were grown in Dulbecco's modified Eagle's medium, which was supplemented with 10% FBS, 100 U of penicillin, and 100 μg of streptomycin per ml. O. tsutsugamushi strain Boryong was propagated in monolayers of L929 cells as described previously (28, 34). When more than 90% of the cells were infected, as determined by an indirect immunofluorescent-antibody technique (7), the cells were collected, homogenized using a glass Dounce homogenizer (Wheaton Inc., Millwille, N.J.), and centrifuged at 500 × g for 5 min. The supernatant was stored in liquid nitrogen until use. The titer of infectivity of the inoculum was determined as described previously, with modification (26, 38). Briefly, twofold serially diluted oriential samples were inoculated onto ECV304 cell layers in a 24-well tissue culture plate containing 12-mm-diameter glass coverslips. This plate was centrifuged at 1,450 × g for 10 min at room temperature. After an 8-h incubation in a humidified 5% CO2 atmosphere at 37°C, the culture medium was removed and the cells were washed with phosphate-buffered saline without Ca2+ and Mg2+ (PBS) and then fixed in 100% methanol for 5 min at −20°C and stained as described previously (26). The ratio of infected cells to the counted number of cells was determined microscopically and the number of infected cell-counting units (ICU) of the oriential sample was calculated (8). A total of 2.4 × 106 or 2.4 × 107 ICU of O. tsutsugamushi was used to infect cultured cells in a 24-well tissue culture plate. A mouse anti-α tubulin monoclonal antibody (MAb) (1:200; Sigma Chemical Co., St. Louis, Mo.) was used to label the MTs. A mouse anti-dynamitin MAb (1:100) was kindly provided by R. Vallee (University of Massachusetts, Worcester, Mass.). O. tsutsugamushi was stained with MAb (KI-37) against the Boryong 56-kDa outer membrane protein or human patient polyserum. The fluorescent secondary antibodies (Sigma Chemicals Co.) used were fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) and tetramethylrhodaminyl isothiocyanate-conjugated goat anti-human IgG. Nocodazole and colchicine were purchased from Sigma Chemical Co. Nocodazole was dissolved in dimethyl sulfoxide and colchicine was dissolved in PBS to create a stock solution of 1,000× for each drug.
O. tsutsugamushi infection and drug treatments.
Cells were grown on 12-mm-diameter glass coverslips for 24 h. ECV304 cells were infected with O. tsutsugamushi Boryong (2.4 × 106 or 2.4 × 107 ICU) for an appropriate time. Cells were inoculated with O. tsutsugamushi in a medium containing 10% FBS for 10 min at room temperature (1,450 × g). Oriential infection was initiated by shifting the cells to a humidified 5% CO2 atmosphere at 37°C. In some experiments, the cells were incubated for 1 h before infection in a medium containing 1 μg of colchicine per ml, a drug concentration which was maintained during the infection. For treatment with 10 μg of nocodazole per ml, the cells were incubated for 1 h at 4°C and subsequently incubated for 30 min at 37°C before infection.
Plasmids and DNA experiments.
Green fluorescent protein (GFP)-encoding plasmid DNA (peGFP N1) and dynamitin-encoding DNA (pCMVH50m) (gift of R. Vallee, University of Massachusetts) were transfected into ECV304 or HeLa cells, using Lipofectamine (5 μl/μg of DNA; Gibco-BRL). For transient transfection, cells were grown on coverslips to 50% confluency. These plasmids were mixed with Lipofectamine according to the manufacturer's recommendations. Cells were washed once with serum-free medium and incubated with the DNA-liposome mixture in OPTI-MEM (Gibco-BRL) for 12 h, after which the DNA was removed and replaced with a complete medium. Cells were infected for the assays after 36 h of DNA application. Under these conditions, about 30% of the transfected cells expressed each GFP and dynamitin at 24 h posttransfection. Transfected cells were scored arbitrarily as either bright or dim (corresponding to high and moderate overexpression, respectively) according to the fluorescence intensity.
Immunofluorescence analysis.
The infected monolayer cells were washed three times with PBS and then fixed in 100% methanol for 5 min at −20°C and blocked with nonspecific binding by 10% normal goat serum or 0.1% bovine serum albumin (in PBS). Monolayers were incubated for 30 min at 37°C with primary antibodies diluted in PBS, washed three times with PBS, and subsequently incubated at 37°C with secondary antibodies diluted in PBS. Finally, monolayers were washed three times in PBS. Each stained monolayer was mounted with glycerol-PBS (1/1) and was viewed with a Zeiss fluorescence microscope and a Zeiss confocal laser-scanning microscope. Images of cellular sections were acquired every 1 μm. All images were analyzed and processed with the program Adobe Photoshop 5.0.
RESULTS
O. tsutsugamushi moves to MTOC.
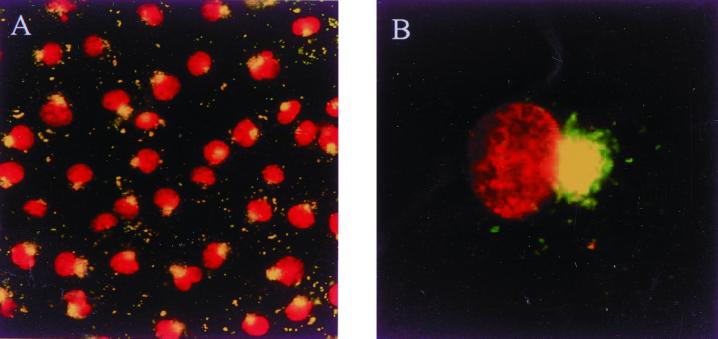
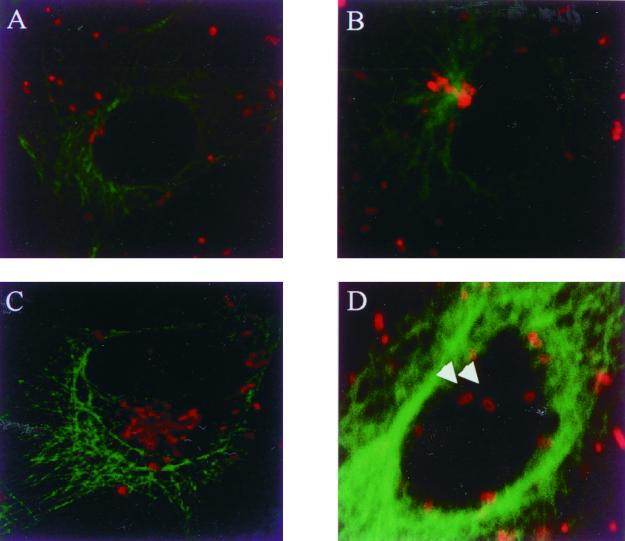
We tried to enhance the infectivity and to induce the synchronized entry to the cultured cells by centrifugation. The infectivity was enhanced about 10 times by centrifugation. Under these conditions, we could more clearly understand the relationships of the orientiae and the cultured cells. At 90 min after infection, O. tsutsugamushi was located in the perinuclear region (Fig. 1). Of a total of 200 examined cells, nearly all (∼100%) had a perinuclear enrichment of the cytosolic orientiae. We were able to furthermore know that this perinuclear region coincides with the MTOC by tubulin staining (Fig. 2B) or by dynamitin staining (data not shown) (11). No perinuclear oriential enrichment was observed at 30 min after infection (Fig. 2A). The orientiae replicated in the perinuclear region (Fig. 2C). To demonstrate the physical interaction between the orientiae and MTs, we conducted confocal microscopy. The orientiae were located quite close to MTs (Fig. 2D), as was clearly shown in the cytoplasmic region below the nucleus, where a relatively small number of MTs were observed. We obtained the same results with various cultured cells like L929 fibroblasts, HeLa epithelial cells, J774 macrophages, and human umbilical vein primary endothelial cells (data not shown).
FIG. 1.
O. tsutsugamushi moves to the perinuclear regions, as shown by conventional immunofluorescence microscopy of ECV304 cells infected with 1 × 106 ICU of O. tsutsugamushi (about 50 orientiae per cell were found). The cells were fixed in methanol and labeled with KI-37, a MAb against the O. tsutsugamushi Boryong 56-kDa protein, and an FITC-conjugated secondary antibody. The nucleus stained by propidium iodide. The yellowish, round spots at the perinucleus are oriential accumulation. (A) Magnification, ×20; (B) magnification, ×100.
FIG. 2.
Subcellular distribution of cytosolic O. tsutsugamushi. Representative immunofluorescence confocal microscopic images of ECV304 cells infected with O. tsutsugamushi for 30 min, 60 min, and 48 h are shown. Cells were fixed in methanol and double-labeled with human polyserum against O. tsutsugamushi (tetramethylrhodaminyl isothiocyanate-conjugated anti-human) and anti-tubulin MAb (FITC-conjugated anti-mouse). (A) ECV304 cells infected at 1 × 105 ICU (about 5 to 10 orientiae per cell were found) for 30 min. The orientiae were scattered throughout the cytoplasm. (B) ECV304 cells infected for 60 min. They began to accumulate at the MTOC. Most intracellular orientiae colocalized with MTOC. The brightest area of MT network in the perinucleus is the MTOC. (C) ECV304 cells infected for 48 h, with numerous orientiae located at the MTOC. (D) Orientiae located quite close to MTs under the nuclear region (arrowheads) at 30 min postinfection. Magnification, ×63.
MTs are associated with movement of O. tsutsugamushi.
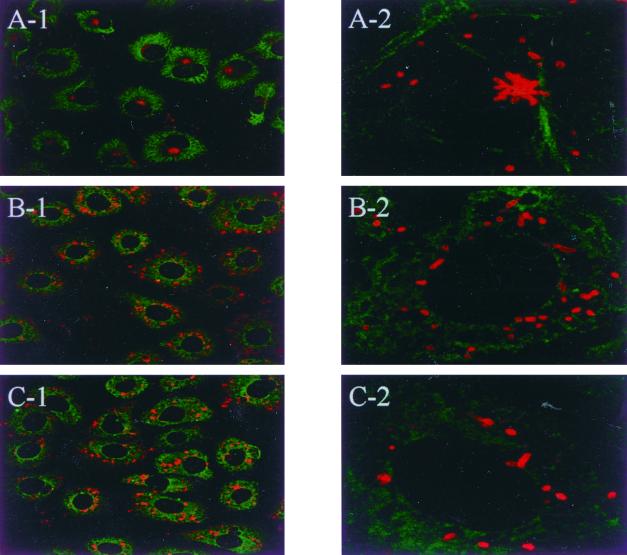
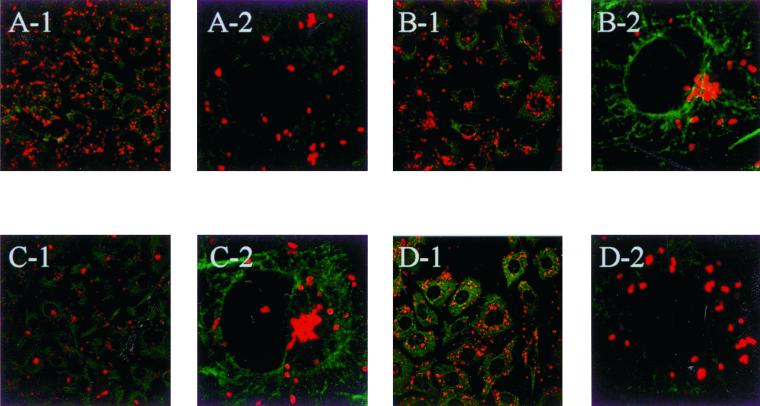
To determine whether MTs are actually needed for the directional transport of orientiae to the MTOC, we first analyzed the requirements of the intact MTs for oriential targeting to the MTOC. ECV304 cells were incubated with 10 μg of nocodazole per ml or 1 μg of colchicine per ml, as described in Materials and Methods. Under these conditions, the MTs were completely depolymerized, as shown by anti-α-tubulin MAb immunostainings. Both nocodazole and colchicine strongly inhibited the localization of orientiae to the perinuclear region, as was observed by confocal microscopy (Fig. 3B). And if these orientiae were further incubated, they replicated at the cell periphery (data not shown). If the MTs were restored by the removal of nocodazole for 4 h, significant oriential targeting to the MTOC, similar to that observed in cells not treated with the drug, was observed (Fig. 4B). If MTs were destroyed by colchicine at 1 or 2 days after infection, perinuclear orientiae dispersed throughout the cytoplasm (Fig. 4D). This may suggest that MTs are needed not only for movement in the cytosol but also for oriential replication. Taxol, a drug that prevents disassembly of MTs, did not affect oriential movement to the MTOC (data not shown), suggesting that the dynamic turnover of MTs was not required for oriential movement in cultured cells. Similar findings with taxol treatment have been reported for HSV-1 (35) and adenovirus type 2 (36). On the other hand, experiments aimed at blocking actin polymerization by using cytochalasin D (to demonstrate the specific MT-mediated movement) were inconclusive. This was due to the insignificant effects of nocodazole, colchicine, and taxol on the invasion process; however, cytochalasin D almost completely inhibited the invasion of orientiae into the cells (data not shown). And we accidentally found that the orientiae were colocalized with double-sided centrosomes in metaphase cells (data not shown). Perhaps the orientiae moved to the centrosome as they would to a eukaryotic chromosome. As both nocodazole and colchicine affected the network of MT, we concluded that MTs are directly or indirectly involved in the transport of orientiae from the cell periphery to the MTOC in ECV304 cells and also in HeLa cells (data not shown).
FIG. 3.
Inhibitory effects of oriential movement in the presence of MT-disrupting agents; immunofluorescence confocal microscopy of ECV304 cells infected with 2 × 105 ICU (about 10 to 20 orientiae per cell were found) of O. tsutsugamushi for 90 min. O. tsutsugamushi and MT were fixed and stained as described for Fig. 2. Under control conditions (A-1 and A-2) most cytosolic O. tsutsugamushi accumulated around the MTOC. If the cells were infected in the presence of nocodazole (10 μg/ml) (B-1 and B-2) or colchicine (1 μg/ml) (C-1 and C-2), there was no accumulation of O. tsutsugamushi at the MTOC. Orientiae were scattered throughout the entire cytoplasm. Magnification: for panels A-, B-, and C-1, ×40; for panels A-, B-, and C-2, ×100.
FIG. 4.
Nocodazole reversibility and MT disruption after movement to the MTOC. O. tsutsugamushi and MT were fixed and stained as described for Fig. 2. In the presence of nocodazole (10 μg/ml) the number of orientiae localized to the MTOC was drastically reduced (A-1 and A-2), whereas after a 4-h chase in nocodazole-free medium, the MT network was reconstituted and the orientiae accumulated around the MTOC (B-1 and B-2). Orientiae replicated in the perinuclear region after 24 h of infection (C-1 and C-2), but if the cells were treated with colchicine (1 μg/ml), after 24 h of infection, the orientiae were scattered throughout the cytoplasm. (D-1 and D-2). Magnification: for panels A-1, B-1, C-1, and D-1, ×40; for panels A-2, B-2, C-2, and D-2, ×100.
Dynein-dynactin significantly contribute to minus-end-directed movement of O. tsutsugamushi.
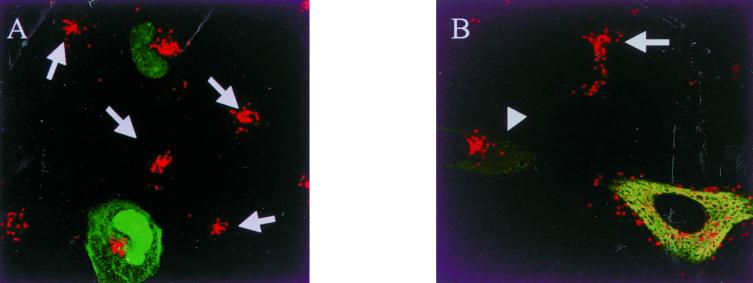
The major minus-end-directed motor complex of interphase cells is cytoplasmic dynein (21, 43). The motor activity can be experimentally dislodged from the cargo by overexpression of dynamitin, a component of the dynein-associated dynactin complex (1, 5, 11). We therefore tested if transient expression of human dynamitin in HeLa cells or ECV304 cells (data not shown) affected the movement of cytosolic O. tsutsugamushi. Control HeLa cells transfected with GFP-encoding plasmid DNA showed efficient movement to the MTOC of orientiae at 90 min after infection (Fig. 5A). However, of a total of 529 examined cells, the orientiae moved to the MTOC in nearly all (∼100%) of the nontransfected HeLa cells (383 cells), while they moved to the MTOC in a very low number (about 14%) of cells transfected with the dynamitin plasmids at high level of overexpression (53 cells). With moderate dynamitin overexpression (93 cells), high levels (about 80%) of movement to the MTOC occurred, probably due to incomplete inactivation of the dynactin complex (5).
FIG. 5.
Dynamitin overexpression inhibits cytosolic movement of O. tsutsugamushi. HeLa cells transiently transfected with a plasmid DNA expressing GFP (A) or with a dynamitin-expressing plasmid (B). The orientiae were internalized for 90 min at 37°C and cells were processed for indirect immunofluorescence microscopy using anti-dynamitin MAb and FITC-goat anti-mouse IgG. (A) O. tsutsugamushi accumulated in the cytosol both in nontransfected cells (arrows) and GFP-transfected cells. (B) O. tsutsugamushi did not accumulate in the cytosol in dynamitin-overexpressing cells. O. tsutsugamushi accumulate in the cytosol both in moderately overexpressed cells (arrowhead) and nontransfected cells (arrow). Magnification, ×63.
DISCUSSION
Bacterial entry into the mammalian cells may involve microfilament-dependent mechanisms or MT-dependent mechanisms or both. Once internalized, some bacteria, such as listeriae, shigellae, and rickettsiae, use host cell actin to move within the cells and to spread to adjacent cells (16). All these bacteria escape from the phagosomal membrane. However some bacteria, such as C. trachomatis (9) and C. jejuni (24), involve host cell MTs and dyneins. These bacteria cannot escape the phagosomal vacuole. In the case of viruses, vaccinia virus (10) uses actin for its movement but adenovirus and HSV-1 use the MT network.
In our study, we showed that O. tsutsugamushi moved to the MTOC within 90 min after infection, indicating an MT-dependent mode of oriential redistribution. We further confirmed the location of MTOC using anti-dynamitin MAb immunostaining (11). Treatment with nocodazole or colchicine, which both depolymerize MT prior to the infection, reduced oriential accumulation at the MTOC. When the MT network was disrupted by colchicine treatment at 1 or 2 days after infection, perinuclear O. tsutsugamushi dispersed to the cell periphery. These results provide evidence that O. tsutsugamushi interacts in a specific manner with the host cell MT and that MTs may be involved in its intracellular movement and replication. Our results furthermore identify the major minus-end-directed motor complex, dynein-dynactin, as a key mediator of the cytosolic movement of O. tsutsugamushi. We did not demonstrate that the oriential movement follows phagosomal escape. Because O. tsutsugamushi has been known to escape from the phagosome rapidly after penetration into the cytoplasm, we think naked orientiae interact with MT network (12). These results are somewhat surprising because other phagosomal escaping bacteria, rickettsiae in particular, use host cell actin for their movement. However, it has been reported that MTs are associated with the intracellular movement and spread of the periodontopathogen Actinobacillus actinomycetemcomitans, which enters epithelial cells by an actin-dependent mechanism, escapes from the host cell vacuole, and spreads intracellularly and to adjacent epithelial cells via intracellular protrusions (31).
C. trachomatis serovar L2 utilizes the host cell MT network during the early events of infection (9). The fact that C. trachomatis appears to require a perinuclear location for effective growth is in agreement with the results obtained by Hackstadt et al. (17), who proved that the chlamydial inclusion disrupts the normal Golgi trafficking by intercepting endogenously synthesized sphingomyelin, which is normally destined for the plasma membrane. So, we suspect that the movement of orientiae to the MTOC is associated with their effective growth.
MTs are formed from molecules of tubulin, each of which is a heterodimer consisting of two closely related and tightly linked globular polypeptides called α-tubulin and β-tubulin. MTs have long been known to be involved in some of the membrane trafficking steps of both endocytic and secretory pathways within eukaryotic cells (27). In vitro motility assays were used to identify and isolate two classes of MT-dependent motor proteins, the kinesins and the cytoplasmic dyneins. Like other MT-based motors, the major minus-end-directed motor dynein converts the energy of ATP hydrolysis into vectorial movement (20–22). Dynein is a protein complex having a molecular mass greater than a million daltons and is composed of several heavy, light, and intermediate chains (32). The two head domains are ATPase motors that bind to MTs, while the tails generally bind to specific cell components and thereby specify the type of cargo. Dynein attaches to cargo, such as cellular vesicles and mitotic chromosomes, via the dynactin complex. Dynactin is a large protein complex with a short actin-like filament and an arm capable of interacting with cytoplasmic dynein and with MTs. Overexpression of the dynactin component dynamitin has been shown to disrupt the dynactin complex on the kinetochore of mitotic chromosomes (11) and to affect vesicular trafficking in interphase cells (1, 5).
Although we have detailed models of motor interactions with MTs for kinesin (44) and dynein (14) and an increasing structural understanding of kinesin's directionality (6, 19), a more detailed knowledge of the mechanism of dynein motor directionality and of its interactions with simple cargo is desired (36). Cytoplasmic dyneins have already been reported to be involved in vesicular transport, with their role including trafficking of the intermediate compartment (19), Golgi vesicles (13, 29, 41), mature phagosomes (3), and endosomes (2, 15), but we know very little about MT-dependent trafficking of nonmembranous organelles. Adenovirus (36) and HSV-1 (35) provide good examples of MT-dependent trafficking of nonmembranous particles. O. tsutsugamushi may serve to provide another example. Further studies should seek to identify oriential surface proteins and the dynactin complex involved.
The growth cycle of O. tsutsugamushi can be summarized as follows. It begins by binding to the host cell surface via the cell surface heparan sulfate. After the orientiae are captured into the phagosomes, they escape from the phagosome by an unknown mechanism. After entering into the cytoplasm, O. tsutsugamushi moves as a naked cytosolic bacterium along the MTs to the MTOC, replicating in the perinuclear region. After sufficient replications have taken place in the perinuclear region, O. tsutsugamushi is released from the host cell in a manner similar to the budding process of enveloped viruses.
ACKNOWLEDGMENTS
We are grateful to R. Vallee for pCMVH50m and MAb to dynamitin. We also thank all the members of the laboratory.
This work was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Seoul, Republic of Korea (HMP-00-B-20200-0009).
REFERENCES
- 1.Ahmad F J, Echeverri C J, Vallee R B, Baas P W. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blocker A, Severin F F, Burkhardt J K, Bingham J B, Yu H, Olivo J C, Schroer T A, Hyman A A, Griffiths G. Molecular requirements for bi-directional movement of phagosomes along microtubules. J Cell Biol. 1997;137:113–129. doi: 10.1083/jcb.137.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkhardt J K. The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex. Biochim Biophys Acta. 1998;1404:113–126. doi: 10.1016/s0167-4889(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt J K, Echeverri C J, Nilsson T, Vallee R B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Case R B, Pierce D W, Hom-Booher N, Hart C L, Vale R D. The directional preference of kinesin motors is specified by an element outside of the motor catalytic domain. Cell. 1997;90:959–966. doi: 10.1016/s0092-8674(00)80360-8. [DOI] [PubMed] [Google Scholar]
- 7.Chang W H, Kang J S, Lee W K, Choi M S, Lee J H. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990;28:685–688. doi: 10.1128/jcm.28.4.685-688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho N H, Seong S Y, Huh M S, Han T H, Koh Y S, Choi M S, Kim I S. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun. 2000;68:594–602. doi: 10.1128/iai.68.2.594-602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clausen J D, Christiansen G, Holst H U, Birkelund S. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol Microbiol. 1997;25:441–449. doi: 10.1046/j.1365-2958.1997.4591832.x. [DOI] [PubMed] [Google Scholar]
- 10.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 11.Echeverri C J, Paschal B M, Vaughan K T, Vallee R B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewing E P, Jr, Takeuchi A, Shirai A, Osterman J V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978;19:1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fath K R, Trimbur G M, Burgess D R. Molecular motors and a spectrin matrix associate with Golgi membranes in vitro. J Cell Biol. 1997;139:1169–1181. doi: 10.1083/jcb.139.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee M A, Heuser J E, Vallee R B. An extended microtubule-binding structure within the dynein motor domain. Nature. 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- 15.Goodson H V, Valetti C, Kreis T E. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- 16.Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti P J, Cossart P. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112:1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- 17.Hackstadt T, Rockey D D, Heinzen R A, Scidmore M A. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzen R A, Hayes S F, Peacock M G, Hackstadt T. Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells. Infect Immun. 1993;61:1926–1935. doi: 10.1128/iai.61.5.1926-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henningsen U, Schliwa M. Reversal in the direction of movement of a molecular motor. Nature. 1997;389:93–96. doi: 10.1038/38022. [DOI] [PubMed] [Google Scholar]
- 20.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 21.Hirokawa N, Noda Y, Okada Y. Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr Opin Cell Biol. 1998;10:60–73. doi: 10.1016/s0955-0674(98)80087-2. [DOI] [PubMed] [Google Scholar]
- 22.Howard J. Molecular motors: structural adaptations to cellular functions. Nature. 1997;389:561–567. doi: 10.1038/39247. [DOI] [PubMed] [Google Scholar]
- 23.Hoyt M A, Hyman A A, Bahler M. Motor proteins of the eukaryotic cytoskeleton. Proc Natl Acad Sci USA. 1997;94:12747–12748. doi: 10.1073/pnas.94.24.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu L, Kopecko D J. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect Immun. 1999;67:4171–4182. doi: 10.1128/iai.67.8.4171-4182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ihn K S, Han S H, Kim H R, Huh M S, Seong S Y, Kang J S, Han T H, Kim I S, Choi M S. Cellular invasion of Orientia tsutsugamushi requires initial interaction with cell surface heparan sulfate. Microb Pathog. 2000;28:227–233. doi: 10.1006/mpat.1999.0344. [DOI] [PubMed] [Google Scholar]
- 26.Kee S H, Choi I H, Choi M S, Kim I S, Chang W H. Detection of Rickettsia tsutsugamushi in experimentally infected mice by PCR. J Clin Microbiol. 1994;32:1435–1439. doi: 10.1128/jcm.32.6.1435-1439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly R B. Microtubules, membrane traffic, and cell organization. Cell. 1990;61:5–7. doi: 10.1016/0092-8674(90)90206-t. [DOI] [PubMed] [Google Scholar]
- 28.Kim I S, Seong S Y, Woo S G, Choi M S, Kang J S, Chang W H. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J Clin Microbiol. 1993;31:2057–2060. doi: 10.1128/jcm.31.8.2057-2060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippincott-Schwartz J. Cytoskeletal proteins and Golgi dynamics. Curr Opin Cell Biol. 1998;10:52–59. doi: 10.1016/s0955-0674(98)80086-0. [DOI] [PubMed] [Google Scholar]
- 30.Mandelkow E, Mandelkow E M. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 31.Meyer D H, Rose J E, Lippmann J E, Fives-Taylor P M. Microtubules are associated with intracellular movement and spread of the periodontopathogen Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:6518–6525. doi: 10.1128/iai.67.12.6518-6525.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milisav I. Dynein and dynein-related genes. Cell Motil Cytoskelet. 1998;39:261–272. doi: 10.1002/(SICI)1097-0169(1998)39:4<261::AID-CM2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Prevost M C, Lesourd M, Arpin M, Vernel F, Mounier J, Hellio R, Sansonetti P J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992;60:4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seong S Y, Huh M S, Jang W J, Park S G, Kim J G, Woo S G, Choi M S, Kim I S, Chang W H. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect Immun. 1997;65:1541–1545. doi: 10.1128/iai.65.4.1541-1545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suomalainen M, Nakano M Y, Keller S, Boucke K, Stidwill R P, Greber U F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensson U. Role of vesicles during adenovirus 2 internalization into HeLa cells. J Virol. 1985;55:442–449. doi: 10.1128/jvi.55.2.442-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura A, Urakami H. Easy method for infectivity titration of Rickettsia tsutsugamushi by infected cell counting. Nippon Saikingaku Zasshi. 1981;36:783–785. [PubMed] [Google Scholar]
- 39.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urakami H, Tsuruhara T, Tamura A. Electron microscopic studies on intracellular multiplication of Rickettsia tsutsugamushi in L cells. Microbiol Immunol. 1984;28:1191–1201. doi: 10.1111/j.1348-0421.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 41.Vaisberg E A, Grissom P M, McIntosh J R. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vale R D, Fletterick R J. The design plan of kinesin motors. Annu Rev Cell Dev Biol. 1997;13:745–777. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- 43.Vallee R B, Sheetz M P. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- 44.Woehlke G, Ruby A K, Hart C L, Ly B, Hom-Booher N, Vale R D. Microtubule interaction site of the kinesin motor. Cell. 1997;90:207–216. doi: 10.1016/s0092-8674(00)80329-3. [DOI] [PubMed] [Google Scholar]