Abstract
Purpose
The aim of this study is to investigate short-term and long-term effects of coronovirus 19 disease (COVID-19) at inner and outer retinal layers of patients recovered from COVID-19 with Spectral Domain Optical Coherence Tomography (SD-OCT) and compare these to healthy subjects.
Methods
Twenty-seven patients recovered from COVID-19, and age- and gender-matched 27 healthy controls were included in this study. Macular and peripapillary retinal nerve fiber layer (RNFL), ganglion cell-inner plexiform layer (GCIPL), inner nuclear layer (INL), outer plexiform layer (OPL) and outer nuclear layer (ONL) were analyzed with SD-OCT 1 month (V1 visit) and 12 months (V2 visit) after negative result of reverse transcriptase-polymerase chain reaction test.
Results
Macular RNFL thickness in outer ring was thinner at V1 and V2 visits than healthy control (p = 0.049 and p = 0.005). Central and inferonasal quadrants of peripapillary RNFL thicknesses were reduced at V1 and V2 visits compared to controls (p = 0.001 and p = 0.024 for V1 visit; p = 0.001 and p = 0.006 for V2 visit). Thinning in ONL thickness in inner ring was observed at V1 and V2 visits than healthy subjects (p = 0.006 and p = 0.001).
Conclusion
Subclinical localized changes in macular and peripapillary RNFL and outer nuclear layer were demonstrated in early and 12-months follow-up after COVID-19 recovery.
Keywords: SARS-CoV-2, COVID-19, Retina, Optical Coherence Tomography, Neurodegeneration
Introduction
Caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19) has led to a pandemic [1, 2], by infecting millions of people and more than six millions of death as of May 2022 [3].
Although the lungs are the main target organ, it has potential to infect other organs and ocular tissues as well [4–6]. The virus gains entry to host cell by binding to angiotensin converting enzyme-2 (ACE-2), which is widely expressed in human cells [1, 7] and can cause endothelial dysfunction associated with apoptosis, either by direct viral infection of the endothelium or by immune-mediated response [8]. Pathophysiologic mechanisms and clinical manifestations of acute infection are well documented and almost clear. Direct viral toxicity, endothelial cell damage, microvascular injury, disregulation of the immune response, hypercoagulability are possible mechanisms of extrapulmonary manifestations [4].
By the second year of the pandemic, while trying to understand and manage acute infection, new terms for persisting symptoms has come into use. Long– COVID, post-COVID syndrome and chronic COVID syndrome are the new terms ascribed for ongoing persisting symptoms of COVID-19 [9–15].
Increasing numbers of studies have been reporting long-term effects of the disease, mainly subjective symptoms of the patients persisting after the acute period of severe disease [9, 10, 16], nevertheless studies after mild disease are limited [17, 18]. In this prospective cohort study, we intend to investigate evidence for long-COVID syndrome objectively by optical coherence tomography (OCT) imaging, one year after the recovery from mild COVID-19 infection.
In our previous cross-sectional case–control research study [6], we found thinning of the inner and outer retinal layers of recovered COVID-19 patients with Spectral Domain Optical Coherence Tomography (SD-OCT) that is a non-invasive imaging technique, useful for demonstrating subclinical retinal changes in systemic conditions and viral infections as well [19, 20]. The aim of this study is to investigate whether the OCT findings are reversible or persisting and supporting post-COVID syndrome in order to perform a follow-up protocol.
Methods
This study was performed after approval of the Ankara City Hospital Ethics Committee (Ankara, Turkey), with number E1-21-2040. All research procedures were carried out in accordance with the Declaration of Helsinki. Written informed consent was acquired from the participants with local institutional review board requirements. Patients between 18 and 50 years, recovered from COVID-19 who had no history of hospitalization because of PCR positive COVID-19 infection between November and December 2020, were enrolled in the study. Thickness of each retinal layer and peripapillary nerve fiber layers was obtained by SD-OCT after a short while of recovery from COVID-19 and at approximately a 12-months follow-up in retina department of Ankara City Hospital, a tertiary referral hospital, Ankara, Turkey. All patients were health care professionals and none of them had a second PCR positivity of COVID-19 during follow-up period. Patients with any systemic or ocular diseases, history of ophthalmic surgery, any systemic or topical drug administration and more than ± 3 diopter spherical equivalent of refractive errors were excluded from the study. Age- and gender-matched patients, who were received for routine ophthamologic examination, were recruited as healthy control subjects in the study. First visit after a shortwhile of recovery from COVID-19 was defined as V1 visit and approximately 12 months after V1 visit was defined as V2 visit. All patients enrolled in the study underwent a detailed ophthalmological evaluation including best-corrected visual acuity (BCVA) using Snellen charts, anterior segment slit lamp examination, dilated fundus examination, fundus photography and optical coherence tomography images.
Optical coherence tomography images
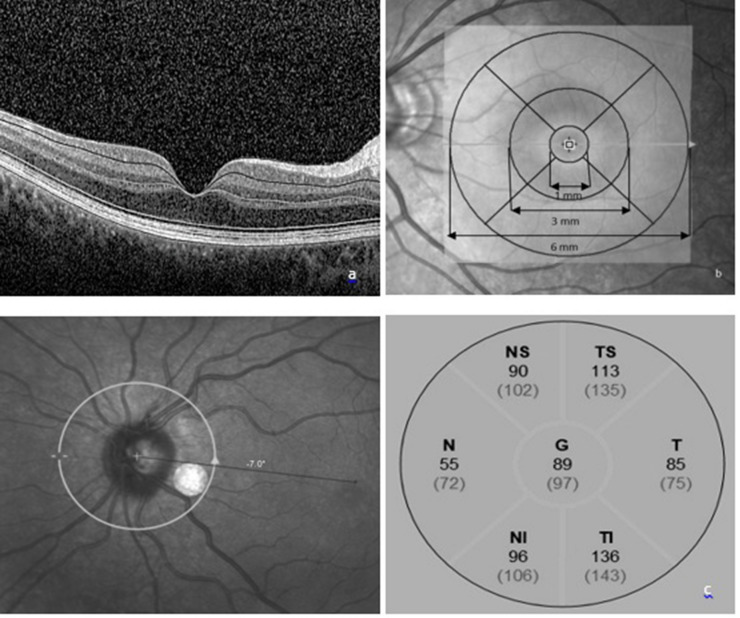
The SD-OCT (HeidelbergEngineering, Heidelberg, Germany) images were performed by an experienced technician. Macula Map X-Y scanning protocol evaluating 6 × 6 mm area centered on the fovea was used for measuring thicknesses of each retinal layer. Each layers, macular ganglion cell–inner plexiform layer complex (GCIPL), macular retinal nerve fiber layer (mRNFL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), were segmented automatically (Fig. 1a) and were measured by the caliper tool embedded in SD-OCT system and studied in three concentric rings centered on the fovea; central (1 mm), inner ring (1–3 mm) and outer ring (3–6 mm) which was determined by the Early Treatment Diabetic Retinopathy Study (ETDRS) [21].
Fig. 1.
a A cross-sectional view of macula SD-OCT scan demonstrating the macular layer segmentation included in the study. b ETDRS grid; the central circle is with a diameter of 1 mm. The inner ring consists of the four quadrants surrounding the central circle with a diameter of 1–3 mm. The outer ring consists of the four quadrants surrounding the inner ring, with a diameter of 3–6 mm. c Analysis of peripapillary RNFL thickness; thickness of all quadrants was included in the study.
Inner and outer rings were segmented into four quadrants (inner/outer superior, inner/outer inferior, inner/outer nasal, and inner/outer temporal) and means of four quadrants in inner and outer rings were calculated and analyzed (Fig. 1b).
Peripapillary retinal nerve fiber layer (pRNFL) thickness was obtained from a circular scan with a diameter of 3.4 mm positioning on middle of the optic disk center; automatically segmented as central, temporal, inferotemporal, superotemporal, nasal, superonasal and inferonasal quadrants (Fig. 1c). OCT images were recorded by using the ‘follow-up’ mode of the eye-tracking-assisted system (Aurorescan, Heidelberg Spectralis) for V2 visit.
Statistical analysis
The distributions of continuous variables were examined by Shapiro–Wilk’s test and normality plots. OCT measurements were summarized by mean ± standard deviation (mean±SD). Categorical variables were reported as frequency (%) and comparison of categorical variables between groups were determined by using chi-squared test. Independent t test was used for comparing the means of retinal and peripapillary thicknesses between healthy controls and both V1 and V2 visits. Paired t test was used for comparing V1 and V2 visits. All analysis and calculations were performed via IBM SPSS Statistics 22.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). A p < 0.05 was considered as statistically significant.
Results
Fifty-four eyes of 27 patients with 12-months follow-up after recovery from COVID-19 infection; age- and gender-matched 54 eyes of 27 patients as control group were included in the study. The mean time between recovery from COVID-19 infection and V1 visit was 31.18 ± 12.35 days and the mean time between V1 visit and V2 visit was 12.22 ± 0.42 months (min–max 12–13 month). 63% of the patients recovered from COVID-19 infection were female and mean age of the patients was 34.67 ± 7.54 years at V1 visit and 35.67 ± 7.54 years at V2 visit. 66.7% of the healthy subjects were female and mean age was 35.44 ± 4.97 years. There were no statistically significant differences in age and gender between patients and control subjects (p = 0.657, p = 0.899 and p = 0.779) (Table 1).
Table 1.
Comparison of age and gender in groups
| COVID-19 patients (n = 27) |
Healthy subjects (n = 27) |
p value | |
|---|---|---|---|
| Age (years, mean ± SD) | |||
| V1 visit | 34.67 ± 7.54 | 35.44 ± 4.97 | 0.657* |
| V2 visit | 35.67 ± 7.54 | 0.899* | |
| Gender | 16/11 | 18/9 | 0.779ꞎ |
| (Female/Male, %) | (63%/37%) | (66.7%/33.3%) | |
* Independent t test
ꞎχ2 test
Mean retinal thickness in the inner ring and outer ring was significantly reduced at V1 visit compared to healthy subjects (p = 0.013 and p = 0.024). When compared V2 visit with healthy subjects and V1 visit, mRNFL thickness in the outer ring was reduced (p = 0.049 and p = 0.005). Mean mRNFL thickness in outer ring was 37.21 ± 4.60 μm in healthy subjects, 35.55 ± 4.04 μm at V1 visit and 34.70 ± 4.47 μm at V2 visit and was significantly decreased at V1 visit compared to healthy subjects (p = 0.041). Decrease in mean GCIPL thickness in inner ring was statistically significant when compared V1 visit with control group (p = 0.015). No differences were determined in all quadrants INL thickness between visits and healthy controls (p > 0.05, each comparison).
Mean OPL thickness in outer ring was thinner at V2 visit comparing with V1 visit (p = 0.033), while there was no differences in OPL thickness of all other quadrants between visits and controls(p > 0.05, each comparison). ONL thickness in central and inner ring was thinner at V1 visit compared to controls (p = 0.038 and p = 0.006); ONL thickness in inner ring was significantly thinner at V2 visit than healthy subjects (p = 0.001). Central and inferonasal quadrants of pRNFL thickness were reduced at V1 and V2 visits when compared to controls (p = 0.001 and p = 0.024 for V1 visit; p = 0.001 and p = 0.006 for V2 visit) (Table 2).
Table 2.
Comparison of retinal layers between patients recovered from COVID-19 infection in early period and at 12 months and healthy subjects
| V1 visit (n = 27) |
V2 visit (n = 27) |
Healthy controls (n = 27) |
V1 vs. V2 p valueꞎ |
V1 vs. control pvalueꞎꞎ |
V2 vs. control pvalueꞎꞎ |
|
|---|---|---|---|---|---|---|
| Retina (μm, mean ± SD) | ||||||
| Central | 270.26 ± 19.55 | 271.87 ± 24.71 | 276.30 ± 19.55 | 0.364 | 0.102 | 0.295 |
| Inner ring | 337.05 ± 13.83 | 337.04 ± 15.03 | 343.98 ± 14.51 | 0.990 | 0.013* | 0.016 |
| Outer ring | 306.17 ± 10.42 | 305.49 ± 11.85 | 310.89 ± 11.05 | 0.090 | 0.024* | 0.016 |
| mRNFL (μm, mean ± SD) | ||||||
| Central | 12.38 ± 2.54 | 11.98 ± 2.43 | 12.61 ± 1.82 | 0.094 | 0.603 | 0.132 |
| Inner ring | 21.89 ± 1.84 | 21.70 ± 2.30 | 22.45 ± 2.34 | 0.304 | 0.174 | 0.097 |
| Outer ring | 35.55 ± 4.04 | 34.70 ± 4.47 | 37.21 ± 4.60 | 0.041* | 0.049* | 0.005* |
| GCIPL (μm, mean ± SD) | ||||||
| Central | 38.04 ± 10.62 | 37.70 ± 11.57 | 39.52 ± 8.92 | 0.733 | 0.434 | 0.364 |
| Inner ring | 90.91 ± 7.90 | 91.72 ± 7.97 | 94.16 ± 5.43 | 0.166 | 0.015* | 0.067 |
| Outer ring | 69.99 ± 5.55 | 70.45 ± 5.27 | 70.84 ± 4.40 | 0.283 | 0.382 | 0.682 |
| INL (μm, mean ± SD) | ||||||
| Central | 20.43 ± 6.61 | 20.67 ± 6,51 | 20.46 ± 4.84 | 0.687 | 0.754 | 0.837 |
| Inner ring | 40.06 ± 2.99 | 40.02 ± 3.40 | 39.57 ± 2.66 | 0.914 | 0.370 | 0.442 |
| Outer ring | 34.97 ± 1.88 | 35.07 ± 2.16 | 34.88 ± 1.89 | 0.595 | 0.810 | 0.629 |
| OPL (μm, mean ± SD) | ||||||
| Central | 27.46 ± 7.30 | 25.59 ± 6.45 | 25.20 ± 5.16 | 0.102 | 0.067 | 0.730 |
| Inner ring | 32.88 ± 3.08 | 33.07 ± 4.05 | 32.05 ± 3.38 | 0.663 | 0.184 | 0.158 |
| Outer ring | 28.50 ± 2.08 | 27.97 ± 1.77 | 28.00 ± 2.39 | 0.041 | 0.254 | 0.927 |
| ONL (μm, mean ± SD) | ||||||
| Central | 87.39 ± 11.89 | 88.46 ± 11.53 | 92.15 ± 11.69 | 0.472 | 0.038* | 0.102 |
| Inner ring | 69.67 ± 7.51 | 68.78 ± 8.07 | 74.13 ± 8.81 | 0.182 | 0.006* | 0.001* |
| Outer ring | 59.02 ± 7.64 | 58.74 ± 7.00 | 61.21 ± 6.76 | 0.591 | 0.118 | 0.065 |
| pRNFL (μm, mean ± SD) | ||||||
| Central | 99.07 ± 8.06 | 98.87 ± 9.25 | 106.72 ± 14.29 | 0.752 | 0.001* | 0.001* |
| Superotemporal | 133.69 ± 20.33 | 135.15 ± 19.49 | 137.72 ± 17.33 | 0.305 | 0.269 | 0.470 |
| Superonasal | 110.30 ± 19.99 | 110.78 ± 19.99 | 115.59 ± 19.18 | 0.759 | 0.163 | 0.204 |
| Nasal | 70.96 ± 14.30 | 71.17 ± 12.99 | 75.04 ± 15.76 | 0.869 | 0.162 | 0.167 |
| Inferonasal | 112.19 ± 16.31 | 110.37 ± 16.46 | 120.39 ± 20.58 | 0.132 | 0.024* | 0.006* |
| Inferotemporal | 149.65 ± 21.64 | 148.39 ± 20.47 | 154.15 ± 15.87 | 0.361 | 0.221 | 0.105 |
| Temporal | 71.91 ± 11.15 | 72.13 ± 12.70 | 75.78 ± 11.46 | 0.805 | 0.078 | 0.120 |
GCIPL ganglion cell-inner plexiform layer, INL inner nuclear layer, mRNFL macular retinal nerve fiber layer, ONL outer nuclear layer, OPL outer plexiform layer, pRNFL peripapillary retinal nerve fiber layer
ꞎPaired t test
ꞎꞎIndependent t test
* p < 0.05
mRNFL thickness in outer ring and OPL thickness in outer ring varied between V2 visit and V1 visit. To distinguish normal aging process on thickness of retinal layers, the age adjusted linear regression analysis of the mean thickness of these layers was calculated. No differences in mRNFL thickness in outer ring and in OPL thickness in outer ring with age were determined (p = 0.088; R2 = 0.155 and p = 0.973; R2 = 0.457).
Discussion
Alterations in retinal layers of patients recovered from COVID-19 were demonstrated with this study, providing that COVID-19 infection had both temporary and persistant effects in retina in early and long-term periods. At the beginning of the pandemic, thinking to be only a respiratory system virus, making an acute infection, the agent is named as Severe Acute Respiratory Syndrome Coronavirus, letter R represents respiratory, but it is shown that it is more than a respiratory virus [4–6], and letter A represents Acute, nevertheless it seems to be a chronic infection. As COVID-19 pandemic continues and the infected population grows, reports of persistent and prolonged effects after COVID-19 are increasing, those are need to be identified and classified [12–14].
National Institute for Health and Care Excellence (NICE) published a guideline suggesting the following classification: acute COVID-19 for symptoms up to 4 weeks, ongoing symptomatic COVID for symptoms from 4 to 12 weeks, and post-COVID for symptoms continuing for more than 12 weeks. The term “long-COVID” in this guideline is distinguished as both ongoing symptomatic COVID and post-COVID syndrome [12]. Baig proposed that symptoms presenting beyond 3 weeks after the SARS-CoV-2 infection should be considered prolonged or persistent [13]. Another classification offers acute post-COVID for symptoms between weeks (W) 5 and 12; long post-COVID for ongoing symptoms between W12-W24; persistant post-COVID symptoms lasting more than W24 [14]. In this study, we used the term ‘Long-COVID’ in order to describe the sequela of the disease.
In our study, retinal thicknesses in inner ring and outer ring were thinner at V1 visit than healthy controls, but after long-term follow-up, no statistically significant differences were observed between V2 visit and healthy controls and also no differences were obtained between visits. GCIPL thicknesses in inner and outer ring and central ONL thickness were thinner at V1 visit than healthy subjects, but when compared with V2 visit, no differences were found. These results indicated the reduction in retinal thickness in inner and outer ring, GCIPL thickness in inner ring and central ONL thickness of patients recovered from COVID-19 were temporary and did not proceed in long-term follow-up. Bilbao-Malavé et al. demonstrated thinning of parafoveal RNFL and parafoveal ganglion cell layer thicknesses and thickening of foveal thickness in patients with COVID-19 bilateral pneumonia at 6-months follow-up [22]. In our study, thinning of retinal thickness in inner and outer ring was temporary, and no difference was obtained at 12-months follow-up.
mRNFL thicknesses in central, inner ring and outer ring were decreased at V1 and V2 visits when compared with controls and also thinner at V2 visit than V1 visit. Although no significant difference was detected between V1 visit and V2 visit, ONL thickness in inner ring was decreased at V1 visit than controls and V2 visit than controls. Central and inferonasal quadrants of pRNFL thicknesses were reduced in patients recovered from COVID-19 at V1 visit and V2 visit when compared with healthy controls. Decrease in mRNFL thickness in central, inner ring and outer ring, ONL thickness in inner ring and central and inferonasal quadrants of pRNFL thicknesses were persisted and demonstrated in both early and long-term periods. Thinning of these layers had been detected in patients recovered from mild COVID-19 infection with no hospitalization or intensive care history. Bilbao-Malavé et al. demonstrated thinning of parafoveal RNFL thicknesses in hospitalized patients with COVID-19 at 6-months follow-up [22]. Nonetheless in long–term, whether progression in thinning of retinal layers persists needs to be elucidated. This longitudinal study results seem to be favorable in understanding long-term effects of COVID-19 in retinal layers and providing evidence of long-COVID with objective OCT images.
RNFL thickness was used to evaluate axonal damage primarily for glaucoma patients whereas for neurodegenerative disorders as indicator of neural damage [23–26]. The current study indicates axonal loss determined by SD-OCT after long-COVID period. COVID-19-induced immune-mediated demyelinating disease, cerebrovascular damage and neurodegeneration were well discussed [27, 28]. Considering retina and brain have similar embryologic origin and anatomical features; RNFL thinning detected in this study might be derived of vascular change in retinal vascular system or neurodegeneration of central nervous system or both of them.
Studies about long-COVID are mainly subjective symptomatic survey studies, emphasizing fatigue, cough, dyspnea, joint pain, chest pain, headaches, myalgia, anosmia, depression and other mental health conditions as common persisting symptoms [9–11, 15–18]. Although most long-COVID studies about survivors after both severe and mild disease demonstrate that long-term effects are independent from disease severity [17, 18], as in our study, fully recovered patients with mild symptoms had retinal thickness alterations. While taking into account aging and co-existence of further common systemic comorbidities; decrease in retinal layers thickness may be a significant burden in understanding retinal pathologies and functional deficits in long–term as emphasized by Teo et al. [29]. Consequences of mild and even asymptomatic SARS-CoV-2 infection indicate a need for systematic follow-up plan and when the large amount of infected population is considered, adapted appropriate health policies should be developed [18, 30].
Ocular signs, symptoms and features of the acute phase of the disease are almost clear, however, the persisting ocular manifestations after COVID-19 infection has not been investigated and published sufficiently yet [31]. The previous studies on quantitative analysis of retinal circulation with optical coherence tomography angiography (OCTA) demonstrated decreased vessel density which might be risk for retinal vascular complications [22, 32–34]. Abrishami et al. reported three months follow-up OCTA results on patients with no hospitalization history [32] and Bilbao-Malavé V et al. declared 6-months follow-up OCTA results on COVID-19 bilateral pneumonia patients that COVID-19 appears to result in subclinical microvascular damage, which manifests in the retinal microcirculation detected by OCTA [22] and that can result in clinically detectable features of micro-vasculopathy as a potential long-term sequelae of the disease [29]. Our previous cross-sectional case–control study demonstrated subclinical localized alterations in inner and outer retinal layers of patients recovered from COVID-19 by SD-OCT [6]. In this cohort, it was determined that alterations were persisting. Providing evidence of long-COVID with objective OCT images, our study is significant and the follow-up time was 12 months and this long follow-up period was a superiority comparing with previous studies.
Ischemic events in different retinal capillary beds after COVID-19 infection had been demonstrated by several studies [35, 8]. In our study, patients with mild symptoms recovered from COVID-19 had decrease in central and inferonasal quadrants of pRNFL thickness; ONL thickness in inner ring and mRNFL thickness in outer ring in early and long-term follow-up visits may indicate subclinic markers of ischemic events in retinal layers.
Also, the existence of SARS-CoV-2 viral RNA in enucleated human retina has been identified and viral RNA of SARS-CoV-2 has been determined in both inner and outer retinal layers and vitreous involvement by COVID-19 has been previously reported [36–38]. These results were consistent with our study that decrease in inner and outer retinal layers thickness had been detected. Several studies denoted microvascular angiopathic complications related to COVID-19 in a wide range metabolisms and organs [39].
In OCTA studies, as a consequence of COVID-19-induced microvascular angiopathy in retina, retinal vascular changes were obtained in early and late periods after recovery. Structural retinal changes are inevitable with microvascular angiopathic process [32, 33]. Neurodegeneration and thinning in retinal layers were obviously demonstrated in Diabetes Mellitus, a microvascular angiopathic disease, without retinal findings [40–42]. When considering all, the changes in retinal layers obtained in this study with structural OCT analysis may be evaluated as the quantitative findings of COVID-19-associated angiopathy in the retina.
Sample size and recruitment criteria of patients with only mild symptoms recovered from COVID-19 were limitations of this study. Inclusion of patients with different severity of disease and comparing these values would be valuable. Our sample also was not random, and health care workers were enrolled in the study. Younger patients with no comorbidities were another limitation of this study.
As best we know, this is the first longitudinal study to follow thickness of each retinal layer in patients recovered from COVID-19. This study exhibits peristant long-term effects of COVID-19 on subclinic alterations of peripapillary and macular RNFL and ONL thicknesses. COVID-19 pandemic continues to be a challenging process on health care systems globally. As the infected population grows and reports of persistent and prolonged multisystemic effects after COVID-19 increase, planned health programs are needed for alleviating unpreceeding consequences of COVID-19. These programs can only form with objective studies. Multicentered studies with larger population and including patients with different severity of disease are further required to understand the pathogenesis of retinal alterations caused by COVID-19.
Author contributions
Both authors contributed to the study conception and design. Material preparation, data collection were performed by Esra Dag Seker, analysis was performed by Inci Elif Erbahceci Timur. The first draft of the manuscript was written by Esra Dag Seker and Inci Elif Erbahceci Timur and both authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Competing ınterests
The authors have no relevant financial or non-financial interest to disclose.
Ethics approval
This study was performed in line with the principles of Declaration of Helsinki. Approval was granted by the Ethics Committee of Ankara City Hospital (2021/ E1-21-2040).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Esra Dağ Şeker, Email: dr.esra.seker@gmail.com.
İnci Elif Erbahçeci Timur, Email: inci_elif84@hotmail.com.
References
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bchetnia M, Girard C, Duchaine C, et al. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review of the current global status. J Infect Public Health. 2020;13(11):1601–1610. doi: 10.1016/j.jiph.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO COVID-19 dashboard. Available from: https://who.sprinklr.com/. Accessed March 5, 2022.
- 4.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol. 2020;98(8):e951–e959. doi: 10.1111/aos.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dag Seker E, Erbahceci Timur IE. COVID-19: more than a respiratory virus, an optical coherence tomography study. Int Ophthalmol. 2021;41(11):3815–3824. doi: 10.1007/s10792-021-01952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carfì A, Bernabei R, Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandal S, Barnett J, Brill SE, et al. ARC Study Group (2021) 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 76(4):396–398. [DOI] [PMC free article] [PubMed]
- 11.Jennings G, Monaghan A, Xue F, et al. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 2021;10(24):5913. doi: 10.3390/jcm10245913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence (NICE) (2020), Royal College of General Practitioners, Healthcare Improvement Scotland SIGN. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. National Institute for Health and Care Excellence; London, UK. Accessed on 30 December 2020. Available online: www.nice.org.uk/guidance/ng188
- 13.Baig AM. Chronic COVID syndrome: need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. 2021;93(5):2555–2556. doi: 10.1002/jmv.26624. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Defining post-COVID symptoms (post-acute COVID, Long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18(5):2621. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero-Duarte Á, Rivera-Izquierdo M, Guerrero-Fernández de Alba I, et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19(1):129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend L, Dowds J, O'Brien K, et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Kessel SAM, Olde Hartman TC, Lucassen PLBJ, et al. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. 2022;39(1):159–167. doi: 10.1093/fampra/cmab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Martin E, Garcia-Campayo J, Puebla-Guedea M, et al. Fibromyalgia is correlated with retinal nerve fiber layer thinning. PLoS One. 2016;11(9):e0161574. doi: 10.1371/journal.pone.0161574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Invernizzi A, Agarwal AK, Ravera V, et al. Comparing optical coherence tomography findings in different aetiologies of infectious necrotising retinitis. Br J Ophthalmol. 2018;102(4):433–437. doi: 10.1136/bjophthalmol-2017-310210. [DOI] [PubMed] [Google Scholar]
- 21.Grading diabetic retinopathy from stereoscopic color (1991) Fundus photographs—an extension of the modified Airlie House classification. ETDRS reportnumber 10. Early treatment diabetic retinopathy study research group. Ophthalmology 98:786–806 [PubMed]
- 22.Bilbao-Malavé V, González-Zamora J, Saenz de Viteri M, et al. Persistent retinal microvascular ımpairment in COVID-19 bilateral pneumonia at 6-months follow-up assessed by optical coherence tomography angiography. Biomedicines. 2021;9(5):502. doi: 10.3390/biomedicines9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lederer DE, Schuman JS, Hertzmark E, et al. Analysis of macular volume in normal and glaucomatous eyes using optical coherence tomography. Am J Ophthalmol. 2003;135(6):838–43. doi: 10.1016/S0002-9394(02)02277-8. [DOI] [PubMed] [Google Scholar]
- 24.Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139(1):44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 25.Cheung CY, Ikram MK, Chen C, et al. Imaging retina to study dementia and stroke. Prog Retin Eye Res. 2017;57:89–107. doi: 10.1016/j.preteyeres.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Mutlu U, Bonnemaijer PWM, Ikram MA, et al. Retinal neurodegeneration and brain MRI markers: the Rotterdam Study. Neurobiol Aging. 2017;60:183–191. doi: 10.1016/j.neurobiolaging.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Nagu P, Parashar A, Behl T, et al. CNS implications of COVID-19: a comprehensive review. Rev Neurosci. 2020;32(2):219–234. doi: 10.1515/revneuro-2020-0070. [DOI] [PubMed] [Google Scholar]
- 28.Ferini-Strambi L, Salsone M. COVID-19 and neurological disorders: are neurodegenerative or neuroimmunological diseases more vulnerable? J Neurol. 2021;268(2):409–419. doi: 10.1007/s00415-020-10070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teo KY, Invernizzi A, Staurenghi G, et al. COVID-19-related retinal micro-vasculopathy—a review of current evidence. Am J Ophthalmol. 2021;235:98–110. doi: 10.1016/j.ajo.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins V, Sohaei D, Diamandis EP, et al. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58(5):297–310. doi: 10.1080/10408363.2020.1860895. [DOI] [PubMed] [Google Scholar]
- 31.Costa ÍF, Bonifácio LP, Bellissimo-Rodrigues F, et al. Ocular findings among patients surviving COVID-19. Sci Rep. 2021;11(1):11085. doi: 10.1038/s41598-021-90482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrishami M, Hassanpour K, Hosseini S, et al. Macular vessel density reduction in patients recovered from COVID-19: a longitudinal optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2021;12:1–9. doi: 10.1007/s00417-021-05429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turker IC, Dogan CU, Guven D, et al. Optical coherence tomography angiography findings in patients with COVID-19. Can J Ophthalmol. 2021;56(2):83–87. doi: 10.1016/j.jcjo.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zapata MÁ, Banderas García S, Sánchez-Moltalvá A, et al. Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br J Ophthalmol. 2022;106(4):559–563. doi: 10.1136/bjophthalmol-2020-317953. [DOI] [PubMed] [Google Scholar]
- 35.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye (Lond) 2020;34:2352–3. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casagrande M, Fitzek A, Puschel K, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020;28:721–5. doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- 37.ZagoFilho LA, Lima LH, Melo GB, et al. Vitritis and outer retinal abnormalities in a patient with COVID19. Ocul Immunol Inflamm. 2020;28:1–3. doi: 10.1080/09273948.2020.1821898. [DOI] [PubMed] [Google Scholar]
- 38.Araujo-Silva CA, Marcos AAA, Marinho PM, et al. Presumed SARS-CoV-2 viral particles in the human retina of patients with COVID-19. JAMA Ophthalmol. 2021;139(9):1015–1021. doi: 10.1001/jamaophthalmol.2021.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nalugo M, Schulte LJ, Masood MF, et al. Microvascular angiopathic consequences of COVID 19. Front Cardiovasc. Med. 2021;8:26. doi: 10.3389/fcvm.2021.636843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aschauer J, Pollreisz A, Karst S, et al. Longitudinal analysis of microvascular perfusion and neurodegenerative changes in early type 2 diabetic retinal disease. Br J Ophthalmol. 2020;106:528–533. doi: 10.1136/bjophthalmol-2020-317322. [DOI] [PubMed] [Google Scholar]
- 41.Ambiya V, Kumar A, Bhavaraj VR, et al. Study of inner retinal neurodegeneration in diabetes mellitus using spectral domain optical coherence tomography. Eur J Ophthalmol. 2021;30:11206721211048793. doi: 10.1177/11206721211048793. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Martin E, Cipres M, Melchor I, et al. Neurodegeneration in patients with Type 2 diabetes mellitus without diabetic retinopathy. J Ophthalmol. 2019;2019:1825819. doi: 10.1155/2019/1825819. [DOI] [PMC free article] [PubMed] [Google Scholar]