Abstract
There are an estimated 500,000 patients treated with full-thickness wounds in the United States every year. Fire-related burn injuries are among the most common and devastating types of wounds that require advanced clinical treatment. Autologous split-thickness skin grafting is the clinical gold standard for the treatment of large burn wounds. However, skin grafting has several limitations, particularly in large burn wounds, where there may be a limited area of non-wounded skin to use for grafting. Non-cellular dermal substitutes have been developed but have their own challenges; they are expensive to produce, may require immunosuppression depending on design and allogenic cell inclusion. There is a need for more advanced treatments for devastating burns and wounds. This manuscript provides a brief overview of some recent advances in wound care, including the use of advanced biomaterials, cell-based therapies for wound healing, biological skin substitutes, biological scaffolds, spray on skin and skin bioprinting. Finally, we provide insight into the future of wound care and technological areas that need to be addressed to support the development and incorporation of these technologies.
Research in wound healing has had a long journey through the ages. Since the age of the caveman, man has been healing his wounds. Early humans discovered that certain herbal remedies would speed up or help the wound healing process and prevent bleeding. Ancient civilizations in Egypt and Greece created their own dressings for the wound healing process from the herbs that were found in their environment. However, moist wound site care protocols began in the mid-20th century. Even today, extensive burns and full-thickness skin wounds are devastating for patients, even when treated quickly in the clinic. There are an estimated 500,000 patients treated with full-thickness wounds in the United States every year, with an overall mortality rate of 4.9% between 1998 and 2007.1,2 The cost of burn injuries is very high. Specifically, studies have shown that the average cost per patient is over $15,250, and ranges as high as $46,069,3,4 approaching $2 billion per year nationally.5 Globally, there are over 11 million burn injuries per year, creating a significant demand for improved therapies.4
Fire-related burn injuries are among the most common and devastating types of trauma and public health crisis globally. Approximately 90% of burn injuries occur in a domestic setting in low- and middle-income countries, or regions that generally lack the necessary infrastructure, but industrial accidents and armed conflict also contribute to the high incidence of burns.6 According to the American Burn Association, 486,000 injuries occur each year, which is one burn every 65 sec.
First degree (superficial) burns involve only the epidermis. The burn site is red, painful, dry, and blister-free. Second-degree (partial-thickness) burns involve the epidermis and part of the dermis. It forms blisters a few hours after the injury and may be swollen and painful. Third degree (full-thickness) burns destroy the epidermis and all layers of the dermis and often also destroy subcutaneous fatty tissue. Some burns can reach deeper structures, such as muscles, tendons, ligaments, and bone, and are classified as fourth-degree or full-thickness burns.7
Deep partial-thickness burn wounds present a difficult diagnostic and prognostic challenge.8 Surgeons often choose a conservative treatment strategy9 of daily local wound care to avoid donor-site scarring that accompanies autologous split-thickness grafting. However, the associated delay in re-epithelialization may prolong the patient’s hospital stay, increase the risk of infection, and lead to poor functional and cosmetic outcomes, as hypertrophic scar formation may complicate delayed wound closure. Early surgical intervention shortens healing time and hospital stay, improves functional outcomes, and limits scar formation.
Autologous split-thickness skin grafting is the clinical gold standard for the treatment of large burn wounds. However, skin grafting has several limitations, particularly in large burn wounds, where there may be a limited area of non-wounded skin to use for grafting. Further limitations, including scarring and contracture at the wound site, pain, donor-site infection, and limited donor sites for injuries that involve more than 20% of total body surface spur the need for further technological development. Thus other treatment methodologies may be needed if the situation requires prompt, aggressive, and large-scale treatment. One alternative treatment method is the use of allografts, however, these patients require extensive immunosuppressive drugs to prevent immune rejection of the graft.10
To address these limitations, non-cellular dermal substitutes have been developed. These technologies are composed of a polymer scaffold-based membrane. Advanced skin substitutes are either dermal substitutes INTEGRA® Dermal Regeneration Template (Integra Life Sciences) and Biobrane® (UDL Laboratories) or complex biological skin tissue equivalents (Dermagraft® [Shire], Apligraf® [Organogenesis], and TransCyte®—Advanced BioHealing). These products come with their own challenges; they are expensive to produce, may require immunosuppression depending on design and allogenic cell inclusion.11,12 Researchers are working to overcome these limitations by utilizing new materials and techniques, including naturally and synthetically derived hydrogels.13 These attempts include the use antimicrobials, growth factors, and cytokines incorporated into hydrogel drug delivery systems to closely mimic the orchestration of the healing process. Furthermore, these technologies have been used to actively promote wound healing by acting as a substrate for endogenous cell migration and proliferation. The use of cell-based therapies, such as the application of stem cells, add additional complexity and therapeutic benefit. Finally, full-thickness skin is being engineered using advanced additive manufacturing techniques as a replacement for autologous skin grafting.
This manuscript provides a brief overview of these recent advances in wound care, using advanced tissue engineering and regenerative medicine tools and approaches, including the use of biomaterials, cell-based therapies for wound healing, biological skin substitutes, biological scaffolds, spray on skin, and skin bioprinting. Finally, we provide insight into the future of wound care and technological areas that need to be addressed to support the development and incorporation of these technologies.
BIOLOGICAL SKIN SUBSTITUTES/SCAFFOLDS
Scaffolds are synthetic building blocks for delivering drugs, cells, and growth factors to the relevant site in the body. They are important tools used in tissue engineering for regeneration of lost or damaged tissues. Scaffolds are generally classified as cellular or acellular based on the presence or absence of living cells. Acellular scaffolds function as a scaffold for cell migration, proliferation, and endogenous matrix production to enable healing and regeneration within wounds. Cellular skin scaffolds consist of living skin substitutes, both normal and engineered, seeded with allogeneic fibroblasts or keratinocytes. They can be produced from a variety of sources, biological or compounded.
Acellular Scaffolds
Wound healing is highly dependent on the interactions of proliferating cells with the extracellular matrix (ECM) in a process known as dynamic reciprocity.14 In traumatic or chronic wounds, the ECM is often damaged to the point that it no longer adequately supports healing. Acellular dermal matrices (ADM) have been developed to capitalize on the properties of native ECM and promote organized regeneration of host tissue in a wide variety of clinical settings.14
ADM is a product of biological origin composed of a basement membrane and an acellular dermal collagen layer. The ADMs can be allografts derived from human donor skin or xenografts from skin from other mammals. Acellular matrices have been developed as a revolutionary treatment and can be classified as allogeneic, xenogeneic, and synthetic derivatives. These acellular scaffolds help restore the normal wound healing process.15 ADMs have adequate tensile strength and the ability to vascularize and integrate into host tissues with few postoperative complications, making them suitable dermal substitutes. These dermal substitutes provide the benefit of reducing or eliminating the need for autologous tissue grafts and subsequently minimizing donor-site morbidity.16 There are several ADMs available, each with different properties and applications, which are mentioned below. AlloDerm was one of the first ADMs to be introduced in the 1990s and was used as dermal replacement grafts in acute burn patients. ADMs have also been commonly used as a viable alternative for soft tissue augmentation in facial soft tissue reconstruction.17
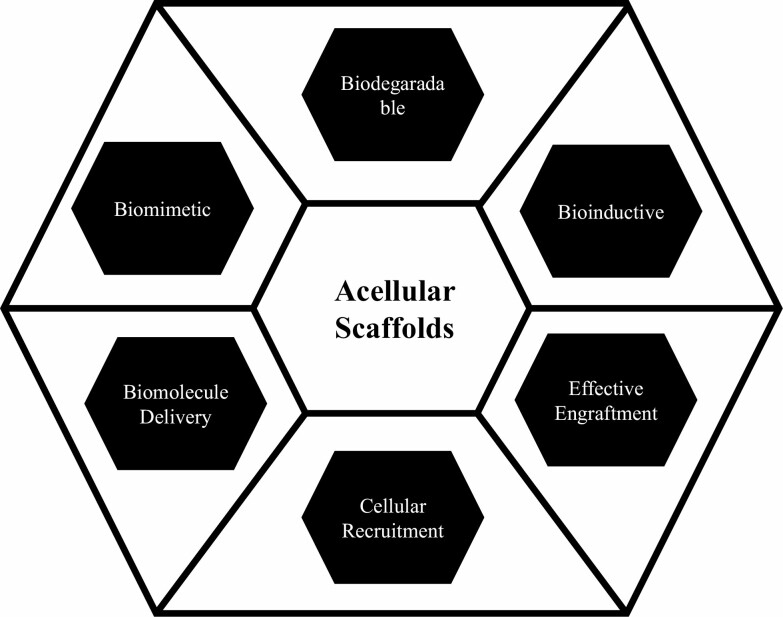
ADMs cannot be used as complete skin substitutes as they lack an epidermal layer, so other acellular bilayer matrices are commercially available, such as Integra (LifeSciences, Plainsboro, NJ, USA) which is made of bovine tendon type I collagen containing a silicone-derived epidermal component and can be used as a temporary bandage or scaffold for tissue regeneration.18 Acellular scaffolds can be subdivided into the following subtypes based on role and functionality (Figure 1).
Figure 1.
Types of acellular scaffolds and their application in tissue engineering.
1) Biodegradable: Biodegradable scaffolds have been proposed and tested for tissue regeneration in vitro or in animal models for such clinical purposes to promote wound healing, stimulate the synthesis of key matrix proteins, reduce scar tissue formation, and for active repair of such an ECM of the skin. Pathologies Biodegradable scaffolds are composed of biodegradable components that degrade after successful function.19,20
2) Biomimetic: Biomimetic scaffolds can be formed using materials that have been designed to elicit specified cellular responses mediated by regulatory factors inside of the engineered environments.21 For example, hydrogels whose matrix composition mirrors that of native articular cartilage. Biomimetic scaffolds, particularly, are guided by the need to restore cell signaling and match the mechanical behavior of the tissue being engineered.22 Biomimetic scaffolds can be used to closely mimic the generation of authentic tissue, which represents the environment of cells in a living organism, while enabling tight control over the cell environment and cellular processes.23 Biomimetic scaffolds can be manufactured for precise control of patterning and mobilization of biological agents such as ligands, hormones, and cytokines.24 The procedure involves culturing and growing stem cells in the infarcted region where they differentiate into various cell types. The expanded cells are inoculated onto a scaffold material once a sufficient number of cells have been acquired. In case of bone repair, scaffolding material complexed with growth factors is implanted into the bone defect. As the scaffold material is gradually degraded and absorbed by the body, the seed cells gradually form new bone tissue with normal physiological structure and function, and finally achieve the purpose of repairing the bone defect.
3) Biomolecule Delivery: Engineering strategies for the design and manufacture of drug delivery systems require the combination and manipulation of materials and drugs to obtain even complex bioactive systems. Examples include 3D drug delivery scaffolds for tissue growth and repair, as well as 3D models for precision cancer medicine provides controlled and gradual release of bioactive molecule.25
4) Mechanical Support: Scaffolds with high mechanical properties are suitable for engineering hard structures such as bone and provide thickness and mechanical support to the infarcted region.
5) Effective Engraftment: Various biomaterials have also been developed as scaffolds to promote stem cell-induced tissue regeneration. The combination of stem cells with biomaterials has been of interest as biomaterials act as scaffolds that can provide these signals, but also facilitate engraftment and long-term viability of stem cells after implantation and also helps to improve retention and localize biodistribution of drugs and therapeutic molecules.
6) Bioinductive: The material helps improve the regenerative or reconstructive capacity of a given tissue or organ and promotes endogenous mechanisms of repair, regeneration, angiogenesis, and vascularization.
Skin Substitutes
Skin substitutes play an important role in plastic surgery, particularly in the treatment of burns and other complex wounds, as they are a valuable resource for restoring skin continuity.26 Complete loss of the skin cover can occur as a result of different conditions including burns, trauma, infections, autoimmune diseases, and complex wounds.27 Loss of the skin barrier increases the risk of infection, water loss, and hypothermia, which increases morbidity, cost, and length of hospital stay, and in some cases, can result in death. The most widely used plastic surgery technique is the autologous skin graft. However, the amount of skin from the donor area is often limited. Therefore, the development of synthetic or biological products as skin substitutes is of interest.28 Skin substitutes are a heterogeneous group of wound dressing materials that aid in closure and replace skin functions, either temporarily or permanently, depending on product characteristics. These substances are alternatives to standard wound dressings in circumstances where standard therapies are undesirable.26
Temporary skin substitutes provide transient physiologic wound closure, including protection from mechanical trauma, physical barrier to bacteria and creation of a moist wound environment.27 To date, a number of biological and synthetic skin substitutes are commercially available, ie, Biobrane®, Integra®, OrCel®, Suprathel®, etc. The synthetic components are mostly organic polymers that are degradable and provide a regenerative environment for tissue regeneration. Biological skin substitutes are cellular products that contain proliferative keratinocytes.28,29
Various skin substitutes are currently available for a variety of clinical applications (Table 1). They can be classified based on cell types used, skin layers mimicked, and synthetic vs biologic.30 Common uses for temporary skin substitutes are; for dressings on donor sites for pain control, to cover superficial wounds to epithelialization, and to provide temporary physiologic closure of deep and full-thickness dermal wounds.
Table 1.
Examples of uses for currently available skin substitutes.
| Product name | Clinical use examples | References |
|---|---|---|
| Epicel | Partial or full-thickness burns | 31 |
| Bioseed-S | to treat chronic Venous leg ulcers | 32 |
| Recell/CellSpray | Burn injury | 33 |
| Stratagraft | Partial and full thickness burns | 34 |
| denovoskin | full thickness burns | 35 |
| Lyphoderm | to treat chronic Venous leg ulcers | 36 |
| ICX-SKN | surgical wounds | 37 |
| Alloderm | Breast Reconstruction | 38 |
| Permacol | Abdominal wall repair | 39 |
| Matriderm | Posttraumatic tissue loss | 40 |
| Biobrane | for burnt auricle | 41 |
| Integra | Scalp reconstruction | 42 |
| EZ Derm | Partial-thickness burns | 43 |
| dermagraft | Venous leg ulcers | 44 |
| Apligraf | Venous leg ulcers | 45 |
| Orcel | to treat burn patient | 46 |
| Hyalomatrix | soft tissue defect | 47 |
| Renoskin | Full thickness wound | 48 |
BIOMATERIALS AND THERAPEUTIC DELIVERY
Hydrogel Materials
Hydrogels have been widely accepted and utilized in clinical settings. One key advantage of these dressings from a regulatory perspective is that these hydrogel-based dressings can be engineered with immunologically inert materials, making them relatively low risk for immune response.49 Some have been shown to regulate gas and fluid exchange critical to the wound healing process.50 Furthermore, these dressing can be manufactured in multiple forms, including gels, sheets, or impregnated into gauze pads. Hydrogel sheets can be used in the treatment of flat wounds as a primary dressing. Alternatively, amorphous hydrogels can be utilized in deep and irregularly shaped wounds. These dressings are typically used alone or in combination with alginate, collagen, fibrin, hyaluronic acid, polyethylene glycol.
Alginate is a naturally occurring polymer that is produce by brown algae. It has been found to have added mechanical strength through ionic and covalent cross-linking by Ca2+ cations.51 Alginate can also be coupled to RGD for improved mechanical properties, which have been strong enough to be used in cartilage engineering designs.52 When placed in vivo, these designs have been shown to promote vascular formation. Additional polymers can be added to alginate to form specialized composite hydrogels, including collagen and PVLE, and in combination improves tissue and cell adhesion.
Collagen protein is abundantly present in human tissue and provides the primary backbone of skin ECM structural integrity.53 Collagen is regularly remodeled to adjust to the biophysical demands place on the tissue.54 Collagen is also a central to hemostasis and wound healing, by providing attachment sites and growth factors that drive clot formation, fibroblast and macrophage proliferation, and keratinocyte attachment and migration across the surface of the wound.55 Given these properties, collagen has been included in many advanced skin tissue products for the treatment of chronic wounds, partial-thickness burns, and full-thickness wounds.56,57 One benefit of collagen treatment is that it is more is more economical than growth factor and cell-based treatments while providing similar, all-be-it less specific, effects.58
Fibrin is a naturally occurring polymer that is formed by the cleavage of fibrinogen by thrombin produced by the coagulation cascade. It forms a self-assembled polymer network and is a critical matrix to the hemostasis phase of wound healing.59 One benefit of the use of fibrin in wound treatment is that cross-linked fibrin adheres to native tissue and then provides binding sites for cell attachment.60 The matrix encourages angiogenesis, which is crucial to accelerating wound healing and providing long-term construct engraftment. Fibrin hydrogel mechanics can be tuned by altering the concentration of fibrin and by mixing it with other hydrogel materials. Clinically, fibrin has been used as a bio-adhesive for the closure of lacerations and surgical wounds.61 It has also been using in skin bioprinting methods as a cell carrier to protect cells from the physical forces of cellular delivery. However, the use of fibrin for these advanced technologies as been limited by the relatively slow cross-linking process and low structural strength.
Hyaluronic acid is a naturally occurring glycosaminoglycan found in the ECM of all tissue, and highly concentrated in mechanically active tissues such as the dermis.62 One benefit of hyaluronic acid in tissue engineering is that it is non-allergic and non-inflammatory; however, it is rapidly degraded in the body.63 One biological advantage of hyaluronic acid is that it has been shown to promote epithelial-mesenchymal transition which can aid in epithelial coverage and time to wound closure.64 Furthermore, the breakdown products can promote angiogenesis.65 Hyaluronic acid is easily modifiable due to its reactive chemical functional groups. It can also strongly retain water and is highly viscous, making it an ideal biomaterial for bioprinting applications.
Therapeutic Delivery
The use of hydrogels is not limited to inert dressing applications. Recent advances have utilized hydrogels as carriers for delivery of therapeutics to wound sites. One method of therapeutic delivery is to deliver growth factors and cytokines that direct the wound healing process.66 The mechanism of wound healing is complex and relies on multiple cytokines and growth factors. These factors drive the interaction of dermal and epidermal cells, extracellular matrix remodeling, and angiogenesis.67 Key growth factors in this process include epidermal growth factor (EGF), fibroblast growth factor (FGF), granulocyte macrophage colony-stimulating factor (GM-CSF), human growth hormone (HGH), insulin-like growth factor (IGF-1) platelet derived growth factor (PDGF), and transforming growth factor (TGF-β1).68,69 Growth factors are necessary for all stages of wound healing; inflammation, proliferation, and cell migration. For the stimulation of angiogenesis and cell proliferation, modulates inflammation and fibroblast proliferation, and extracellular matrix remodeling.70 The addition of factors to otherwise inert hydrogels can promote wound healing by encouraging cells to migrate and proliferate along the hydrogel matrices. PDGFb and HGH have been delivered to wounds in alginate dressings.71 Similarly, delivery endothelial growth factor demonstrated improved vascular growth in wounds treated with alginate beads dosed with the factor.72 Transforming growth factor-β1 (TGF-β1) was used to enhance wound healing.73 Furthermore, the EGF delivered in a cream into partial-thickness incisional wounds was shown to stimulate epidermal regeneration.74 GM-CSF has been shown to be fundamental to wound repair and deficiency of this growth factor results in delayed wound healing and poor scar formation.75
Another method of optimizing wound healing is through delivery of antibiotics and antimicrobials in hydrogel carriers. Antimicrobials have been used extensively to prevent and treat wound site infections.76 By delivering the antibiotics topically, the dressing is less likely to promote bacterial resistance and is unlikely to interfere with the wound healing process.77 Furthermore, the use of local antibiotic delivery can be used in wounds that are complicated by poor blood circulation that would otherwise limit the efficacy of systemic antibiotics.78
The use of paraffin-based ointments, including bisimuth subgallate, has actively aided in the wound healing process.79 Another common antibiotic included in wound dressings is dialkylcarbomoylchloride which found in Cutisorb®. Others include silver impregnated dressings in hydrocolloid, polyurethane, foams, films, and silicone gels.80 Minocycline has been delivered to severe burn wounds in chitosan-polyurethane film dressing.81 In general, antibacterial delivery does not play a direct role in the wound healing process. However, by preventing and treating infections does allow for normal cell division, migration, and differentiation that would otherwise be altered by immune cell involvement in the immunologic defense against bacteria. For example, gentamycin incorporated into silicone gel sheets has been shown to promote epithelialization in superficial burns.82 Furthermore, combination products including growth factors and antibiotics have shown positive effects. One example is a collagen-hyaluronic acid matrix containing FGF and PDGF and tobramycin was shown to enhance wound healing compared to a matrix with antibiotics only.83 Others have shown that adding EFG to silver sulphadiazine treatment reverse impaired wound healing that is seen in wounds treated with silver sulphadiazine alone.84
CELL BASED THERAPIES FOR WOUND HEALING
Skin-Derived Cell Therapies
Cell therapy is the transplantation of autologous or allogeneic cellular material into a patient for the treatment or prevention of diseases. Cell therapy involves the direct administration of the cells into the patient’s body after manipulation or alteration outside the patient’s body for curative purposes. This can either be done by direct cell application, such as cell spray or with complex three dimensional cellular delivery mechanisms such as bioprinting and biopens.
Cellular source selection is a key challenge for skin-derived cell therapies. Allogeneic cells can be collected at any time and stored and used at the time of need. However, due to the risks of immune rejection, allogeneic cells have some limitations. On the other hand, autologous cells need a certain time to culture the cells to achieve a sufficient number of cells for therapy or on the basis of the wound area. It can provide a lifetime transplant without any type of rejection. However, patients with large lesions require immediate treatment and therefore autologous cell preparation will be time consuming and cannot be considered emergency treatment. Keratinocytes can be isolated from a small punch biopsy and can generate the entire epidermis and can assist in re-epithelialization. Keratinocytes generally take 7–10 days to prepare for spraying.
For the regeneration of the dermis, fibroblasts are the main cells, which help in wound healing and remodeling. Fibroblasts produce collagen, which helps fill in the wound area. The combination of fibroblast cells with melanocytes in the dermis can help pigmentation. Studies have shown that the combination of mesenchymal stem cells with epidermal cells has positive effects on wound healing.85
Spray on Skin
Early excision with autograft coverage may be difficult to achieve in patients with extensive burns due to the limited size of the donor sites. Definitive coverage of all burn wounds may take several weeks while waiting for donor sites to epithelialize for repeat collection or for cultured epithelial autografts (CEA) to grow. Delays may also be due to systemic disease or local wound infection that may develop while awaiting CEA. These delays in autograft coverage have led to the development of an uncultured epithelial autograft that allows the surgeon to greatly expand the amount of coverage they can get from small donor sites with immediate application.86 Furthermore, early difficulty with transferring CEA to wound beds led to the development of a spray technique that allows easy transfer of proliferating keratinocytes to the wound.87 An autologous cell harvesting (ACH) system, first introduced in 2005 (ReCell, Avita Medical, Northridge, CA) allows the surgeon to immediately process a small split-thickness biopsy and deliver harvested keratinocytes, melanocytes, fibroblasts, and Langerhans cells. from the epidermal-dermal junction to the wound bed using a spray technique.
The ACH system has been used in a wide variety of wounds; plastic, reconstructive, burn, and cosmetic procedures, including burns and scalds; donor sites; glabrous lesions; mild to moderate scarring; hypopigmentation (eg, hypopigmented scars, iatrogenic hypopigmentation, and vitiligo); large congenital melanotic nevus; and in cosmetic rejuvenation procedures.88–93 Navarro et al91 demonstrated increased epidermal thickness, confluence, keratin cysts, and blood vessels in histologic analysis of full-thickness wounds treated with the ACH device compared to those treated with medium. culture in the control models. Wounds sprayed with the processed ACH suspension have shown faster and better-quality epithelialization than control wounds.94 Furthermore, these cell suspensions have shown viable and proliferating melanocytes that have the potential to treat hypopigmented lesions.92
The application of CEA allows skin reconstruction even in burn victims with large total body surface area (TBSA) burns.95 A skin biopsy is sent to a specialized laboratory within hours of the burn injury in the ideal case. This procedure facilitates early expansion of skin cells in vitro. Simultaneously, patients undergo intensive care therapy for burn shock, burn wound excision, and wound preparation for CEA administration. In practice, CEAs are available after 7–14 days, depending on the behavior of cell expansion in the laboratory and the body surface area to be covered. CEAs are traditionally delivered on silicone carrier sheets. New methods for producing CEA cell suspensions have recently been introduced.4 These suspensions can be sprayed onto burn wounds using special spray devices and spray nozzles that facilitate even distribution of CEA over wounds.
The cellular spray is designed to treat severe second-degree burns, in which the top two layers of skin are damaged but the subcutaneous tissue remains intact. Third degree burns, which are more serious, are still treated with older skin graft technology. Tissue harvesting, cell segregation, and cell suspension preparation takes approximately 20–30 min in total, during which time the treatment area is prepared. Once processed, the cell suspension is available for immediate use and can cover a treatment area up to 80 times the area of the donor biopsy site. Wood and colleagues96 characterized cell suspension with 75% cell viability in which cells retained their proliferative potential and included melanocytes that could help restore pigmentation.
Cell spray-grafting that could be enabled in an outpatient treatment room setting without general anesthesia would present advantages. The concept of non-cultured cell spray-grafting enabled by the immediate preparation and application of skin cells avoiding in vitro culture is based on the application of single cells sprayed onto the wound that can proliferate in regenerating skin. Fredriksson et al97 used a single cell suspension of cultured human keratinocytes for the in vitro transplantation study based on the study by Rheinwald and Green98. Porous biodegradable microcarriers were used to culture keratinocytes for transplantation into full-thickness skin lesions.99 Other methods of spreading the cell suspension using the syringe or brush were unsuccessful because the cells spread unevenly.100 Different studies were carried out to evenly distribute the cells using the cell spray technique.101 There are also advantages to applying a single cell suspension over the transplant that needs a larger donor area. However, the cell suspension can be expanded at 1:100.102 By improving donor cell isolation, the success rate of therapy can be increased. The use of non-cultured cells in the undifferentiated state results in better regeneration of the epidermis.103 Studies have shown that faster cell isolation does not affect cell viability.96
Full-Thickness Skin Bioprinting
Skin bioprinting is a new technology that has promising potential for tissue engineering applications. Bioprinting is a robotic additive biofabrication approach that allows for automatized and reproducible generation of tissue-like structures.104 This is performed using a computer controlled device to accurately deposit biomaterials which can be cell laden to form anatomically organized tissue structures. The materials can be deposited as droplets, cell aggregates, or encapsulated cells in hydrogels.105 This precise deposition is performed by using 3D CAD/CAM software designs that can mimic tissue organization.106 Among the deposition methods, one that has commonly been employed is printing cell rods by extrusion printing, which allows for layer-by-layer tissue formation and gaps for tubular formations.107 By utilizing hydrogels, the printed structure liquidity allows the rods to fuse into seamless structures.108 The mechanical properties of the hydrogel carrier are further tuned using crosslinking chemistry, which can provide the strength necessary for build additional layers.109
The use of this technology in skin engineering has demonstrated positive early outcomes. Amniotic fluid in stem cells in a hydrogel carrier demonstrated improvements in collagen remodeling and epithelialization.110 This technology has advanced to include six cell types, organized into three biomimetic layers of hypodermis, dermis, and epidermis.111 When implanted into full-thickness wounds in mice, the bioprinted skin accelerated wound closure and generated a normal dermal wound healing environment and collagen structure.112 Preliminary studies utilizing this technology in pigs have also demonstrated improvement in wound healing and robust dermal and epidermal remodeling. However, autologous and allogenic cell sources have similar limitations as autologous and allogenic skin grafts. Autologous cell sources require an autologous tissue harvest site and extensive cell expansion periods. Allogenic cells have the potential for immune rejection, though at a lower rate due to reduced immune cell inclusion. Further advances may make skin bioprinting technology a transformative technology for full-thickness skin replacement nearing clinical translation.
SKIN “BIOPENS”
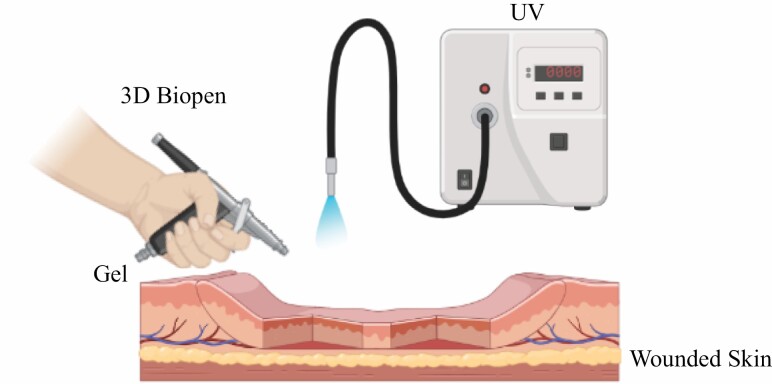
The Biopen is an advanced handheld co-axial extrusion device, which functions as a 3D printer and can create surgical scaffolds, which can then be permanently hardened using ultraviolet light. O’Connell and coworkers113 introduced first prototype of the biopen for in situ fabrication of 3D tissue scaffolds as well as for delivery of primary human stem cells for tissue reconstruction. Biopen can reduce the healing process by delivering live cells along with the growth factors directly on the injured tissue, which will accelerate the regeneration of functional tissue. After dispensing the cells from the biopen on the wound site will multiply and differentiate and will turn into a thriving community of cells in the form of a functional tissue.
Stem cells have the potential to repair and reconstruct the defective tissue, but it is very challenging to apply the stem cells on the defective part with more viability. Stem cells are encapsulated with the gelatin-methacrylamide/hyaluronic acid-methacrylate (GelMA/HAMa) hydrogel ink and pushed through the biopen and deposited on the defective bone or tissue and rapid photo-crosslinking reaction confined the deposition of cells.114 Certain hydrogel parameters need achieved before applying on the tissue such as; sets rapidly and stiff enough to hold the cells at the place as well as provide maximum viable number of the cells.
Duchi and coworkers115 also showed that UVA exposure of 700 mW/cm2 didn’t significantly affect the viability of the cells when compared to the control group without any UVA exposure.
The BioPen works in a similar way to 3D bioprinter and deliver cells within a biopolymer such as alginate, protected by a second outer layer of gel material (Figure 2). The two layers of gel combine in the nozzle of the Biopen as it is extruded onto the bone surface and the clinician draws the defected part of the bone at the site of the injury. After the cells are delivered to the site of the injury, the cells proliferate and differentiate into nerve cells, bone cells, and muscle cells. The Biopen may also seed growth factors for faster recovery.
Figure 2.
Drawing live cells and growth factors directly onto the skin wound using 3D Biopen.
OUTLOOK AND CONCLUSION
Burns are a financial burden health systems and patients alike. The studies discussed in this chapter serve as examples of technologies designed to offer improved patient outcomes following treatment of their injuries. Driving forces in the treatment of these injuries is the shared goal to reduce long-term morbidities associated with these injuries while also improving cosmetic outcomes. One limitation we have come across in our work in the laboratory is the inability to limit contraction during the healing process. In several of the studies described above, contraction contributed greatly to the overall wound closure. While we have shown that the ultimate outcome in these wounds by is the regeneration of a normal dermal collagen network, future studies should focus on optimizing the cellular delivery vehicle in a way that induces more re-epithelization as the primary outcome of wound healing.
Scaling and commercialization cutting edge technologies, including cellularized products, bioprinted tissues, and biopens tissues also present significant hurdles for the translation. This is particularly challenging when treatments are generated in an autologous manner for individual patients. This is further complicated by that fact that most hospitals and clinics do not have clinical-grade cell processing capabilities. To overcome these challenges, updated biomanufacturing logistics or strategically placed biofabrication hubs may be necessary. Perhaps this could be done in a manner similar to United Network for Organ Sharing (UNOS) who have already optimized multi-site coordination, transportation and preservation of human tissues for implantation.115
Finally, these novel technologies will need to continue to demonstrate efficacy and safety through the many ongoing human clinical trials. This will largely be dependent on their ability to closely mimic the biomechanical properties of normal human skin while still allowing for vascular ingrowth and epidermal cell migration and growth. Ultimately, there is great hope for the future of the field, as these technologies mature, and generate more human-like skin than we have seen to date. Close collaboration between researchers and surgeons should continue to develop, allowing for clinical perspective from the early stages of technology development.
Funding: This research was partially supported by NIH/NIAMS 1 F30 AR074866-01A1 (A.M.J).
Supplement sponsorship: This article appears as part of the supplement “Skin Regeneration and Wound Healing in Burn Patients: Are We There Yet?,” sponsored by Mallinckrodt Pharmaceuticals.
Conflict of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Adam M Jorgensen, Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine, Winston Salem, North Carolina, USA.
Naresh Mahajan, Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine, Winston Salem, North Carolina, USA.
Anthony Atala, Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine, Winston Salem, North Carolina, USA.
Sean V Murphy, Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine, Winston Salem, North Carolina, USA.
References
- 1. Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Rep 2008;1–39. [PubMed] [Google Scholar]
- 2. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Rep 2008;1–38. [PubMed] [Google Scholar]
- 3. Miller SF, Bessey P, Lentz CW, Jeng JC, Schurr M, Browning S. National burn repository 2007 report: a synopsis of the 2007 call for data. J Burn Care Res 2008;29:862–70; discussion 871. Discussion 71. [DOI] [PubMed] [Google Scholar]
- 4. Forjuoh SN. Burns in low- and middle-income countries: a review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns 2006;32:529–37. [DOI] [PubMed] [Google Scholar]
- 5. Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2011;37:1087–100. [DOI] [PubMed] [Google Scholar]
- 6. Stewart IJ, Sosnov JA, Snow BDet al. Hypertension after injury among burned combat veterans: a retrospective cohort study. Burns 2017;43:290–6. [DOI] [PubMed] [Google Scholar]
- 7. Kagan RJ, Peck MD, Ahrenholz DH, et al. Surgical management of the burn wound and use of skin substitutes: an expert panel white paper. J Burn Care Res 2013;34:e60–79. [DOI] [PubMed] [Google Scholar]
- 8. Magnusson M, Papini RP, Rea SM, Reed CC, Wood FM. Cultured autologous keratinocytes in suspension accelerate epithelial maturation in an in vivo wound model as measured by surface electrical capacitance. Plast Reconstr Surg 2007;119:495–9. [DOI] [PubMed] [Google Scholar]
- 9. Jackson D, Topley E, Cason JS, Lowbury EJ. Primary excision and grafting of large burns. Ann Surg 1960;152:167–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holavanahalli RK, Helm PA, Kowalske KJ. Long-term outcomes in patients surviving large burns: the skin. J Burn Care Res 2010;31:631–9. [DOI] [PubMed] [Google Scholar]
- 11. Vyas KS, Vasconez HC. Wound healing: biologics, skin substitutes, biomembranes, and scaffolds. Healthcare. 2014;2:356–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis JS. Skin transplantation. Johns Hopkins Hosp Rep 1910;15:307–96. [Google Scholar]
- 13. Skardal A, Murphy SV, Crowell K, Mack D, Atala A, Soker S. A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery. J Biomed Mater Res B Appl Biomater 2017;105:1986–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirsner RS, Bohn G, Driver VRet al. Human acellular dermal wound matrix: evidence and experience. Int Wound J 2015;12:646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho H, Blatchley MR, Duh EJ, Gerecht S. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev 2019;146:267–88. [DOI] [PubMed] [Google Scholar]
- 16. Patel S, Ziai K, Lighthall JG, Walen SG. Biologics and acellular dermal matrices in head and neck reconstruction: a comprehensive review. Am J Otolaryngol 2022;43:103233. doi: 10.1016/j.amjoto.2021.103233. [DOI] [PubMed] [Google Scholar]
- 17. Kridel RW. Acellular human dermis for facial soft tissue augmentation. Facial Plast Surg Clin N Am 2001;9:413–37. [PubMed] [Google Scholar]
- 18. Hughes OB, Rakosi A, Macquhae F, Herskovitz I, Fox JD, Kirsner RS. A review of cellular and acellular matrix products: indications, techniques, and outcomes. Plast Reconstr Surg 2016;138:138S–47S. [DOI] [PubMed] [Google Scholar]
- 19. Hahn MS, Teply BA, Stevens MM, Zeitels SM, Langer R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials 2006;27:1104–9. [DOI] [PubMed] [Google Scholar]
- 20. Kriesel KJ, Thiebault SL, Chan RWet al. Treatment of vocal fold scarring: rheological and histological measures of homologous collagen matrix. Ann Otol Rhinol Laryngol 2002;111:884–9. [DOI] [PubMed] [Google Scholar]
- 21. Chen C, Bang S, Cho Yet al. Research trends in biomimetic medical materials for tissue engineering: 3D bioprinting, surface modification, nano/micro-technology and clinical aspects in tissue engineering of cartilage and bone. Biomater Res 2016;20:10. doi: 10.1186/s40824-016-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016;98:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skylar-Scott MA, Liu M-C, Wu Y, Dixit A, Yanik MF. Guided homing of cells in multi-photon microfabricated bioscaffolds. Adv Healthc Mater 2016;5:1233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moeinzadeh S, Jabbari E. Morphogenic peptides in regeneration of load bearing tissues. Adv Exp Med Biol 2015;881:95–110. [DOI] [PubMed] [Google Scholar]
- 25. Shafiee A. Design and fabrication of three-dimensional printed scaffolds for cancer precision medicine. Tissue Eng Part A 2020;26:305–17. [DOI] [PubMed] [Google Scholar]
- 26. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care 2007;20:493–508; quiz 509–510. [DOI] [PubMed] [Google Scholar]
- 27. Ferreira MC, Tuma P, Carvalho VF, Kamamoto F. Complex wounds. Clinics (Sao Paulo, Brazil) 2006;61:571–8. [DOI] [PubMed] [Google Scholar]
- 28. Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns 2007;33:405–13. [DOI] [PubMed] [Google Scholar]
- 29. Whitaker IS, Prowse S, Potokar TS. A critical evaluation of the use of Biobrane as a biologic skin substitute: a versatile tool for the plastic and reconstructive surgeon. Ann Plast Surg 2008;60:333–7. [DOI] [PubMed] [Google Scholar]
- 30. Atiyeh BS, Hayek SN, Gunn SW. New technologies for burn wound closure and healing—review of the literature. Burns 2005;31:944–56. [DOI] [PubMed] [Google Scholar]
- 31. Wright KA, Nadire KB, Busto P, Tubo R, McPherson JM, Wentworth BM. Alternative delivery of keratinocytes using a polyurethane membrane and the implications for its use in the treatment of full-thickness burn injury. Burns 1998;24:7–17. [DOI] [PubMed] [Google Scholar]
- 32. Vanscheidt W, Ukat A, Horak Vet al. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant: a multinational randomized controlled clinical trial. Wound Repair Regen 2007;15:308–15. [DOI] [PubMed] [Google Scholar]
- 33. Peirce SC, Carolan-Rees G. ReCell® spray-on skin system for treating skin loss, scarring and depigmentation after burn injury: a NICE Medical Technology Guidance. Appl Health Econ Health Policy 2019;17:131–41. [DOI] [PubMed] [Google Scholar]
- 34. Gibson ALF, Holmes JH, Shupp JWet al. A phase 3, open-label, controlled, randomized, multicenter trial evaluating the efficacy and safety of StrataGraft® construct in patients with deep partial-thickness thermal burns. Burns 2021;47:1024–37. [DOI] [PubMed] [Google Scholar]
- 35. Varkey M, Ding J, Tredget EE. Advances in skin substitutes-potential of tissue engineered skin for facilitating anti-fibrotic healing. J Funct Biomater 2015;6:547–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harding KG, Krieg T, Eming SAet al. Efficacy and safety of the freeze-dried cultured human keratinocyte lysate, LyphoDerm 0.9%, in the treatment of hard-to-heal venous leg ulcers. Wound Repair Regen 2005;13:138–47. [DOI] [PubMed] [Google Scholar]
- 37. Boyd M, Flasza M, Johnson PA, Roberts JSC, Kemp P. Integration and persistence of an investigational human living skin equivalent (ICX-SKN) in human surgical wounds. Regen Med 2007;2:363–70. [DOI] [PubMed] [Google Scholar]
- 38. Tierney BP, De La Garza M, Jennings GR, Weinfeld AB. Clinical outcomes of acellular dermal matrix (SimpliDerm and AlloDerm Ready-to-Use) in immediate breast reconstruction. Cureus 2022;14:e22371. doi: 10.7759/cureus.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dirani M, Chahine E, D’Alessandro A, Chouillard M-A, Gumbs AA, Chouillard E. The use of Permacol® biological mesh for complex abdominal wall repair. Minerva Surg 2022;77:41–9. [DOI] [PubMed] [Google Scholar]
- 40. Fulchignoni C, Rocchi L, Cauteruccio M, Merendi G. Matriderm dermal substitute in the treatment of post traumatic hand’s fingertip tissue loss. J Cosmet Dermatol 2022;21:750–7. [DOI] [PubMed] [Google Scholar]
- 41. Haines M, Allan J, Wijewardana A, Cha J, Vandervord J. Treating the burnt auricle using Biobrane. ANZ J Surg 2021;91:453–4. [DOI] [PubMed] [Google Scholar]
- 42. Mogedas-Vegara A, Agut-Busquet E, Yébenes Marsal M, Luelmo Aguilar J, Escuder de la Torre Ò. Integra as firstline treatment for scalp reconstruction in elderly patients. J Oral Maxillofac Surg 2021;79:2593–2602. [DOI] [PubMed] [Google Scholar]
- 43. Troy J, Karlnoski R, Downes Ket al. The use of EZ Derm® in partial-thickness burns: an institutional review of 157 patients. Eplasty 2013;13:e14. [PMC free article] [PubMed] [Google Scholar]
- 44. Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J 2013;10:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eudy M, Eudy CL, Roy S. Apligraf as an alternative to skin grafting in the pediatric population. Cureus 2021;13:e16226. doi: 10.7759/cureus.16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Still J, Glat P, Silverstein P, Griswold J, Mozingo D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns 2003;29:837–41. [DOI] [PubMed] [Google Scholar]
- 47. Coban YK. Combination of negative pressure wound therapy and hyalomatrix application for soft tissue defect of the great toe. Int J Low Extrem Wounds 2012;11:155–6. [DOI] [PubMed] [Google Scholar]
- 48. Philandrianos C, Andrac-Meyer L, Mordon Set al. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012;38:820–9. [DOI] [PubMed] [Google Scholar]
- 49. Rahmanian-Schwarz A, Beiderwieden A, Willkomm LMet al. A clinical evaluation of Biobrane((R)) and Suprathel((R)) in acute burns and reconstructive surgery. Burns. 2011;37:1343–8. [DOI] [PubMed] [Google Scholar]
- 50. Lloyd EC, Rodgers BC, Michener Met al. Outpatient burns: prevention and care. Am Fam Physician 2012;85:25–32. [PubMed] [Google Scholar]
- 51. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012;37:106–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Daly AC, Cunniffe GM, Sathy BN, Jeon O, Alsberg E, Kelly DJ. 3D Bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater 2016;5:2353–62. [DOI] [PubMed] [Google Scholar]
- 53. Chattopadhyay S, Raines RT. Collagen-based biomaterials for wound healing. Biopolymers 2014;101:821–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Asz_odi A, Legate KR, Nakchbandi I, F€assler R.. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- 55. Mian M, Beghe F, Mian E. Collagen as a pharmacological approach in wound healing. Int J Tissue React 1992;14:1–9. [PubMed] [Google Scholar]
- 56. Thomas S, Loveless PA. A comparative study of twelve hydrocolloid dressings. World Wide Wounds. 1997;1:1–12. [Google Scholar]
- 57. Thomas S. Hydrocolloids. J Wound Care 1992;1:27–30. [DOI] [PubMed] [Google Scholar]
- 58. Thomas A, Harding KG, Moore K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-a. Biomaterials 2000;21:1797–802. [DOI] [PubMed] [Google Scholar]
- 59. Noori A, Ashrafi SJ, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomed 2017;12:4937–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Litvinov RI, Weisel JW. Fibrin mechanical properties and their structural origins. Matrix Biol 2017;60–61:110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller R, Wormald JCR, Wade RG, Collins DP. Systematic review of fibrin glue in burn wound reconstruction. Br J Surg 2019;106:165–73. [DOI] [PubMed] [Google Scholar]
- 62. Walimbe T, Panitch A, Sivasankar PM. A review of hyaluronic acid and hyaluronic acid-based hydrogels for vocal fold tissue engineering. J Voice 2017;31:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hemshekhar M, Thushara RM, Chandranayaka S, Sherman LS, Kemparaju K, Girish KS. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol 2016;86:917–28. [DOI] [PubMed] [Google Scholar]
- 64. Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004;4:528–39. [DOI] [PubMed] [Google Scholar]
- 65. West DC, Kumar S. Hyaluronan and angiogenesis. Ciba Found Symp 1989;143:187–201; discussion 201. discussion 20172815. [DOI] [PubMed] [Google Scholar]
- 66. Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 2017;8:217–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yamada KM, Clark RAF. Provisional matrix. In: Clark RAF, editor. The molecular and cellular biology of wound repair. 2nd ed. London: Plenum Press; 1996. p. 51–942. [Google Scholar]
- 68. Steenfos HH. Growth factors and wound healing. Scand J Plast Reconstr Surg 1994;28:95–105. [DOI] [PubMed] [Google Scholar]
- 69. Greenhalgh DG. The role of growth factors in wound healing. J Trauma Inj Infect Crit Care 1996;41:159–67. [DOI] [PubMed] [Google Scholar]
- 70. Komarcevic A. The modern approach to wound treatment. Med Pregl 2000;53:363–8. [PubMed] [Google Scholar]
- 71. Koempel JA, Gibson SE, O’Grady K, Toriumi DM. The effect of platelet-derived growth factor on tracheal wound healing. Int J Pediatr Otorhinolaryngol 1998;l46:1–8. [DOI] [PubMed] [Google Scholar]
- 72. Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Control Release 2004;96:463–72. [DOI] [PubMed] [Google Scholar]
- 73. Puolakkainen P, Twardzik DR, Ranchalis JE, Pankey SC, Reed MJ, Gombotz WR. The enhancement in wound healing by transforming growth factor-b1 (TGF-b1) depends on the topical delivery system. J Surg Res 1995;58:321–9. [DOI] [PubMed] [Google Scholar]
- 74. Brown G, Curtsinger L, White Met al. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg 1988;208:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grzybowski J, Oldak E, Antos-Bielska M, Janiak MK, Pojda Z. New cytokine dressings. I. Kinetics of the in vitro rhG-CSF, rhGM-CSF, and rhEGF release from the dressings. Int J Pharm 1999;184:173–8. [DOI] [PubMed] [Google Scholar]
- 76. O’Meara S, Callum N, Majid M, Sheldon T. Systematic reviews of wound care management (3) antimicrobial agents for chronic wounds (4) diabetic foot ulceration. Health Technol Assess 2000;4:1–237. [PubMed] [Google Scholar]
- 77. Doillon CJ, Silver FH. Collagen-based wound dressing: effect of hyaluronic acid and fibronectin on wound healing. Biomaterials 1986;7:3–8. [DOI] [PubMed] [Google Scholar]
- 78. Boateng JS, Matthews KH, Eccleston HNESGM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci 2008;8:2892–923. [DOI] [PubMed] [Google Scholar]
- 79. Lee-Min Mai LM, Lin CY, Chen CY, Tsai YC. Synergistic effect of bismuth subgallate and borneol, the major components of Sulbogin1 on the healing of skin wound. Biomaterials 2003;24:3005–12. [DOI] [PubMed] [Google Scholar]
- 80. The Annual UK Drug Tariff. 1988. The Stationary Office. London, UK: The UK Drug Tariff. [Google Scholar]
- 81. Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm 2007;330:138–45. [DOI] [PubMed] [Google Scholar]
- 82. Sawada Y, Ara M, Yotsuyanagi T, Sone K. Treatment of dermal depth burn wounds with an antimicrobial agent-releasing silicone gel sheet. Burns 1990;16:347–52. [DOI] [PubMed] [Google Scholar]
- 83. Park S-N, Kim JK, Suh H. Evaluation of antibiotic loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials 2004;25:3689–98. [DOI] [PubMed] [Google Scholar]
- 84. Lee ARC, Leem H, Jaegwan L, Park KC. Reversal of silver sulfadiadine-impaired wound healing by epidermal growth factor. Biomaterials 2005;26:4670–6. [DOI] [PubMed] [Google Scholar]
- 85. Chang M, Liu J, Guo Bet al. Auto micro atomization delivery of human epidermal organoids improves therapeutic effects for skin wound healing. Front Bioeng Biotechnol 2020;8:110. doi: 10.3389/fbioe.2020.00110. https://www.frontiersin.org/article/10.3389/fbioe.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. O’connor N, Mulliken J, Banks-Schlegel S, Kehinde O, Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981;317:75–78. doi: 10.1016/S0140-6736(81)90006-4. [DOI] [PubMed] [Google Scholar]
- 87. Duncan CO, Shelton RM, Navsaria H, Balderson DS, Papini RPG, Barralet JE. In vitro transfer of keratinocytes: Comparison of transfer from fibrin membrane and delivery by aerosol spray. J Biomed Mater Res B Appl Biomater 2005;73:221–8. [DOI] [PubMed] [Google Scholar]
- 88. Cervelli V, Spallone D, Lucarini L, Palla L, Brinci L, De Angelis B. Treatment of stable vitiligo hands by ReCell system: a preliminary report. Eur Rev Med Pharmacol Sci 2010;14:691–4. [PubMed] [Google Scholar]
- 89. Goodman GJ. An automated autologous cell transplantation method for the treatment of hypopigmented scarring. Dermatol Surg 2008;34:578–81. [DOI] [PubMed] [Google Scholar]
- 90. Mulekar SV, Ghwish B, Al Issa A, Al Eisa A. Treatment of vitiligo lesions by ReCell vs. conventional melanocyte-keratinocyte transplantation: a pilot study. Br J Dermatol 2008;158:45–9. [DOI] [PubMed] [Google Scholar]
- 91. Navarro FA, Stoner ML, Park CSet al. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J Burn Care Rehabil 2000;21:513–8. [DOI] [PubMed] [Google Scholar]
- 92. Navarro FA, Stoner ML, Lee HB, Park CS, Wood FM, Orgill DP. Melanocyte repopulation in full-thickness wounds using a cell spray apparatus. J Burn Care Rehabil 2001;22:41–6. [DOI] [PubMed] [Google Scholar]
- 93. O’Neill TB, Rawlins J, Rea S, Wood F. Treatment of a large congenital melanocytic nevus with dermabrasion and autologous cell suspension (ReCELL®): a case report. J Plast Reconstr Aesthet Surg 2011;64:1672–6. [DOI] [PubMed] [Google Scholar]
- 94. Magnusson M, Papini RP, Rea SM, Reed CC, Wood FM. Cultured autologous keratinocytes in suspension accelerate epithelial maturation in an in vivo wound model as measured by surface electrical capacitance. Plast Reconstr Surg 2007;119:495–9. [DOI] [PubMed] [Google Scholar]
- 95. Gallico GG, O’Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 1984;311:448–51. [DOI] [PubMed] [Google Scholar]
- 96. Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns 2012;38:44–51. [DOI] [PubMed] [Google Scholar]
- 97. Fredriksson C, Kratz G, Huss F. Transplantation of cultured human keratinocytes in single cell suspension: a comparative in vitro study of different application techniques. Burns 2008;34:212–9. [DOI] [PubMed] [Google Scholar]
- 98. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975;6:331–43. [DOI] [PubMed] [Google Scholar]
- 99. Seland H, Gustafson C-J, Johnson H, Junker JPE, Kratz G. Transplantation of acellular dermis and keratinocytes cultured on porous biodegradable microcarriers into full-thickness skin injuries on athymic rats. Burns 2011;37:99–108. [DOI] [PubMed] [Google Scholar]
- 100. Fredriksson C, Kratz G, Huss F. Transplantation of cultured human keratinocytes in single cell suspension: a comparative in vitro study of different application techniques. Burns 2008;34:212–9. [DOI] [PubMed] [Google Scholar]
- 101. Gwak S-J, Kim S-S, Sung K, Han J, Choi CY, Kim B-S. Synergistic effect of keratinocyte transplantation and epidermal growth factor delivery on epidermal regeneration. Cell Transplant 2005;14:809–17. [DOI] [PubMed] [Google Scholar]
- 102. Esteban-Vives R, Corcos A, Choi MSet al. Cell-spray auto-grafting technology for deep partial-thickness burns: problems and solutions during clinical implementation. Burns 2018;44:549–59. [DOI] [PubMed] [Google Scholar]
- 103. Gustafson C-J, Birgisson A, Junker Jet al. Employing human keratinocytes cultured on macroporous gelatin spheres to treat full thickness-wounds: an in vivo study on athymic rats. Burns 2007;33:726–35. [DOI] [PubMed] [Google Scholar]
- 104. Visconti RP, Kasyanov V, Gentile C, Zhang J, Markwald RR, Mironov V. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin Biol Ther 2010;10:409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng 2007;13:1905–25. [DOI] [PubMed] [Google Scholar]
- 106. Mironov V, Kasyanov V, Drake C, Markwald RR. Organ printing: promises and challenges. Regen Med 2008;3:93–103. [DOI] [PubMed] [Google Scholar]
- 107. Jakab K, Damon B, Neagu A, Kachurin A, Forgacs G. Three-dimensional tissue constructs built by bioprinting. Biorheology 2006;43:509–13. [PubMed] [Google Scholar]
- 108. Jakab K, Damon B, Marga Fet al. Relating cell and tissue mechanics: implications and applications. Dev Dyn 2008;237:2438–49. [DOI] [PubMed] [Google Scholar]
- 109. Skardal A, Zhang J, McCoard L, Oottamasathien S, Prestwich GD. Dynamically crosslinked gold nanoparticle—hyaluronan hydrogels. Adv Mater 2010;22:4736–40. [DOI] [PubMed] [Google Scholar]
- 110. Skardal A, Mack D, Kapetanovic Eet al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 2012;1:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jorgensen AM, Varkey M, Gorkun A, et al. Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Eng Part A. 2020;26:512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jorgesen AM, Varkey M, Gorkun A, et al. Bioprinted skin integrates and forms epidermal rete ridges in full-thickness wounds. J Investig Dermatol 2020;140:S105. doi: 10.1016/j.jid.2020.03.821. [DOI] [Google Scholar]
- 113. O’Connell CD, Di Bella C, Thompson Fet al. Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 2016;8:015019. doi: 10.1088/1758-5090/8/1/015019. [DOI] [PubMed] [Google Scholar]
- 114. Duchi S, Onofrillo C, O’Connell CDet al. Handheld co-axial bioprinting: application to in situ surgical cartilage repair. Sci Rep 2017;7:5837. doi: 10.1038/s41598-017-05699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jorgensen AM, Yoo JJ, Atala A. Solid organ bioprinting: strategies to achieve organ function. Chem Rev 2020;120:11093–127. doi: 10.1021/acs.chemrev.0c00145. https://pubs.acs.org/doi/10.1021/acs.chemrev.0c00145. [DOI] [PMC free article] [PubMed] [Google Scholar]