Abstract
Toxoplasma gondii is an important protozoan pathogen of humans that can cause encephalitis in immunocompromised individuals such as those with AIDS. This encephalitis is due to reactivation of latent infection in T. gondii-seropositive patients. Latent organisms survive within tissue cysts, which are specialized parasitophorous vacuoles containing bradyzoites. The cyst wall of this structure is produced by modification of the parasitophorous vacuole by the parasite and is important in cyst survival. The components of the cyst wall have been poorly characterized. By using immunofluorescence and immunoelectron microscopy, we have identified a monoclonal antibody (MAb 93.18) that reacts with the cyst wall. This antibody recognizes a 116-kDa glycoprotein, which we have termed CST1, containing sugar residues that bind Dolichos biflorans lectin (DBA). CST1 is distinct from T. gondii antigen labeled with succinyl Triticum vulgare lectin (S-WGA) and represents the major DBA-binding component in T. gondii. The carbohydrate components of the tissue cyst, such as CST1, are probably important in both providing stability and facilitating persistence in its host. As is seen in the carbohydrate capsules of fungi, glycoproteins in the T. gondii cyst wall may protect cysts from the immune response of the host. Further characterization of the formation of the cyst wall and its components should lead to insights into the mechanism of tissue cyst persistence and may suggest novel therapeutic approaches to eliminate tissue cysts of this organism.
Toxoplasma gondii is a ubiquitous Apicomplexan parasite of humans and other animals and birds. It is an important opportunistic pathogen of immunocompromised hosts and is a major opportunistic pathogen of the AIDS epidemic (28, 29, 35). Although overwhelming disseminated toxoplasmosis has been reported, the predilection of this parasite for the central nervous system, causing necrotizing encephalitis, constitutes its major threat to patients with human immunodeficiency virus infection (AIDS) (28, 29).
The factors affecting the transition of tachyzoites to the latent bradyzoite stage remain to be defined. Although these stages are well defined morphologically, little is known about how interconversion from one to the other stage occurs or what signal(s) mediates this transformation (43). The identification of early and late bradyzoite differentiation markers is an important avenue of investigation. Several studies using transmission electron microscopy or bradyzoite-specific monoclonal antibodies (MAbs) have demonstrated the development of cyst-like structures in vitro (2, 3, 13, 20, 26, 34, 40, 45). Feeding experiments in cats have demonstrated that tissue culture-derived cysts are biologically identically to cysts obtained from animal tissues (11).
In the brain, T. gondii tissue cysts develop intracellularly within both neurons and astrocytes, persisting for months to perhaps years. Tissue cysts can be 50 to 100 μm in diameter and are separated from the host cell cytoplasm by a thick cyst wall (12). The cyst wall is thought to be important in maintaining the integrity of the cyst in host cells for long periods. The cyst wall consists of a highly invaginated outer membrane underlaid with a dense osmiophilic matrix containing membranous vesicles. It is 200 to 850 nm thick. An association of the host cell intermediate filaments with parasitophorous vacuoles, the membrane-bound compartment in which the tachyzoite stage replicates, has been described in Vero cells, and a similar association of glial fibrillary acidic protein is seen with the bradyzoite (cyst) vacuole (17–19). There is no evidence of structural integration of host cell intermediate filaments in the cyst wall; instead, glial filaments encase the cysts in the host cell during cyst development in host cells in vitro (19). This filament wrapping of tissue cysts may play a role in bradyzoite differentiation and/or cyst stabilization in the host cell cytoplasm.
Study of the T. gondii cyst wall, as well as clarification of the host cell contribution to this structure, is now possible due to the development of the reagents that recognize bradyzoites in vitro (13, 43). These reagents have demonstrated that modification of the parasitophorous vacuole into a developing cyst wall is an early event in differentiation. As early as 1 day postinfection in vitro, bradyzoite-specific cyst wall antigens, such as that recognized by MAb 73.18 (L. M. Weiss, D. LaPlace, H. B. Tanowitz, and M. Wittner, Letter, J. Infect. Dis. 66:213–215, 1992), are already expressed and localized to the cyst wall. In this paper we present data on the localization and carbohydrate modifications of CST1, the 116-kDa cyst wall antigen recognized by MAb 73.18. The carbohydrate modifications of CST1 are probably important in providing stability to the tissue cyst and in facilitating cyst persistence in the host. In addition, as is seen in the carbohydrate capsules of fungi, this glycoprotein may be important in protecting tissue cysts from the immune response of the host.
MATERIALS AND METHODS
Parasite strains, tissue culture, and parasite purification.
T. gondii type II strains ME49 and R5 (an atovaquone-resistant PDS mutant) (41) and a type I strain, RH, were utilized for these studies (21). R5 was used for these studies because it readily differentiates to bradyzoites under stress conditions (e.g., pH change or sodium nitroprusside treatment [43]). Parasites were maintained by serial passage in confluent monolayers of human foreskin fibroblast cells (ATCC CCD-27SK) grown in Dulbecco's modified Eagle's medium (pH 7.1) (Gibco-BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution (Gibco-BRL). To induce the expression of bradyzoite-specific proteins, the culture medium was replaced with pH 8.1 medium 2 h after inoculation, and the medium was changed daily to keep the pH constant (45). Parasites were harvested at 5 to 6 days postinfection and released from the parasitophorous vacuoles by sequential passage of host cells through 23-, 25-, and 26-gauge needles. This material was passaged through a 3.0-μm-pore-size Nuclepore filter and centrifuged at 400 × g for 10 min. Parasites were resuspended in phosphate-buffered saline (PBS) and centrifuged at 400 × g for 10 min, and the pelleted parasites were stored at −70°C for protein analysis and purification.
In vivo cyst formation.
BALB/cdm2 mice, which have a deletion of the Ld gene at the HLA-2L locus, were infected intraperitoneally with 20 cysts of T. gondii ME49 (7). Brains were removed from the mice at 4 to 8 weeks postinoculation, and ME49 cysts were isolated from murine brain tissue using isopycnic centrifugation as previously described (10; Weiss et al., Letter). Yields averaged 3,000 cysts per mouse with each cyst containing 500 to 2,000 bradyzoites.
Primary astrocyte culture.
Murine astrocytes from C 57BL/6 × SV129 mice were cultivated from the brains of neonatal (<24-h-old) mice as previously described (16, 19). Murine pups were sacrificed, the brain was removed from the cranium, the forebrain was dissected, and the meninges were removed. The tissue was minced and incubated in 0.25% trypsin for 5 min at 37°C. After 5 min, the trypsin was inactivated with a solution containing DNase and soybean trypsin inhibitors and the tissue was further disrupted by trituration in a 20-ml pipette. The dissociated cells were filtered through a 74-μm-pore-size Nitex mesh, centrifuged at 200 × g, suspended in growth medium at a concentration of 106 cells/ml, and plated onto poly-l-lysine-coated dishes. Astrocytes were maintained in endotoxin-free minimal essential medium (Gibco-BRL) supplemented with 20% fetal bovine serum (Gibco-BRL), 5% glucose, and 100 U of penicillin and streptomycin per ml (Gibco-BRL). The growth medium was changed every 3 days. After 7 days in vitro, a confluent layer of 104 astrocytes/cm2 was reached. By this method, the cells were found to be >95% astrocytes, as judged by positive staining for glial fibrillary acidic protein. Cultures contained <5% microglia, as identified by staining with the lectin BS1-B4 (Sigma, St. Louis, Mo.). Astrocytes were dissociated in trypsin-EDTA, replated onto poly-l-lysine-coated coverslips or 24-well plates at 104 cell/cm, and cultured for 7 to 10 days after replating. These astrocytes were then infected with T. gondii ME49 as described below.
In vitro cyst formation of T. gondii in murine astrocytes.
T. gondii ME49 was used to inoculate murine astrocytes. To induce bradyzoite and cyst formation in vitro, the method previously described by Weiss et al. (45) was used with the following modifications. Briefly, murine astrocytes were infected with 104 ME49 T. gondii, and by 3 days postinoculation, 10 to 25% of the infected cells contained cysts. In some cultures, sodium nitroprusside (50 μM) was added to the culture medium to enhance bradyzoite differentiation and cyst formation.
Bradyzoite-specific antibodies.
Monoclonal hybridomas were prepared as previously described (Weiss et al., Letter) from mice immunized intraperitoneally with 5,000 solubilized (sonicated) ME49 cysts, purified from BALB/c H2dm2 mouse brains, that were emulsified with complete Freund's adjuvant. One of the bradyzoite-specific MAbs identified, MAb 73.18 (immunoglobulin G3 [IgG3]), reacted with the cyst wall of cysts purified from mouse brain (46). Another antibody, MAb 74.1.8 (IgG2b), has been demonstrated to recognize the cytoplasmic antigen BAG1/hsp30 (also known as BAG5) (1, 36). In addition, rabbit antisera to the recombinant bradyzoite-specific antigens MAG1, a matrix antigen (37), and BAG1 (33, 49) were utilized.
Indirect-immunofluorescence staining.
Cultures were washed with Hanks balanced salt solution, fixed in 4% paraformaldehyde for 10 min, and washed three times with PBS. The slides were then permeabilized and blocked for 30 min in a solution containing 10% FBS, 10% lamb serum, and 0.1% Triton X-100. For immunofluorescent staining, cells were incubated in MAb 73.18 (diluted 1:50) for 1 h and then washed three times with PBS. They were then incubated with the secondary antibody rhodamine-conjugated anti-mouse IgG (no. 605-140; Boehringer Mannheim), washed three times with PBS, overlaid with 2.5% DABCO (1,4-diazabicyclo-[2,2,2] octane)–PBS, and viewed with a Nikon epifluorescence microscope. Some cultures were double labeled with MAb 73.18 and 1:100 biotinylated Dolichos biflorus lectin (DBA) (no. L6533; Sigma) and then incubated with streptavidin-Texas red (no. S-6370; Molecular Probes) and fluorescein-conjugated anti-mouse IgG. After being washed three times with PBS, the slides were overlaid with 2.5% DABCO–PBS and then viewed with a Nikon epifluorescence microscope. The lectin and all antibodies were diluted in PBS containing 10% FBS.
Transmission electron microscopy.
Cysts isolated from mouse brain were fixed overnight at 4°C in 2.5% glutaraldehyde buffered in 0.1 M sodium cacodylate (pH 7.2). Following fixation, the cells were rinsed in 0.1 M sodium cacodylate buffer, postfixed in 1% OsO4, dehydrated in a graded ethanol series, placed in propylene oxide, and embedded in Epon. Thin sections were placed on copper grids, stained with 4% uranyl acetate and 0.1% lead citrate, and then examined with a Philips JEOL 1200 transmission electron microscope operated at 80 kV.
Immunoelectron microscopy.
Cysts isolated from murine brains were fixed in 0.5% glutaraldehyde–2% paraformaldehyde in 0.1 M cacodylate buffer for 1 h at 4°C, rinsed in cacodylate buffer, dehydrated through a graded ethanol series, embedded in LR White, and polymerized for 48 h at 60°C. The tissue blocks were sectioned, placed on nickel grids, and coated with Formvar and carbon. The grids were incubated in blocking buffer (1% bovine serum albumin and 1% Tween 20 in PBS) for 30 min and then incubated in MAb 73.18 (1:20 dilution) for 2 h and washed five times in blocking buffer. They were then incubated for 1 h in anti-mouse IgG conjugated to 20-nm gold particles. Some grids were incubated for 2 h in DBA conjugated to 10-nm gold particles (no. L4643 [Sigma], 1:20 dilution). All antibodies and the lectin were diluted in blocking buffer. Following the incubation with secondary antibody or lectin conjugated to gold, the grids were successively washed in blocking buffer, PBS–1% Tween, and PBS. They were then postfixed in 1% glutaraldehyde for 10 min and washed in PBS and then in water. They were stained with 4% uranyl acetate, counterstained with 0.1% lead citrate, and then examined on a JEOL 1200 transmission electron microscope operated at 80 kV.
Analysis by 2D SDS-PAGE.
Purified T. gondii was resuspended in PBS to a concentration of 2 × 109 to 3 × 109 T. gondii cells/ml, freeze-thawed four times, mixed with an equal volume of first-dimension sample buffer (9.5 M urea, 2% Triton X-100, 5% β-mercaptoethanol, 1.6% Bio-Lyte 5/7 ampholye, 0.4% Bio-Lyte 3/10 ampholyte [Bio-Rad, Hercules, Calif.]), and incubated at room temperature for 15 min. Following incubation, the mixture was centrifuged at 14,000 × g for 10 min and the supernatant was used as crude extract for analysis.
Proteins were separated according to their isoelectric points in the first-dimension tube by using a Bio-Rad mini-Protean II two-dimensional (2D) system employing the method of O'Farrell et al. First-dimension capillary tube gels (4% acrylamide, 9.2 M urea, 20% Triton X-100, 1.6% BioLyte 5/7, 0.4% BioLyte 3/10, 0.01% ammonium persulfate, 0.1% N,N,N′,N′-tetramethylethylerediamine TEMED) were cast 1 day prior to electrophoresis. The tubes were prerun using the following protocol: 200 V for 10 min, 300 V for 15 min, and then 400 V for 15 min. After this prerun, 25 to 50 μl of prepared extract (≈4 × 108 parasites) was loaded into the capillary gel and the tubes were run at 500 V for 10 min followed by 750 V for 3.5 h. The tubes were then placed on the top of a 10% polyacrylamide slab gel and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 100 V for 1.5 to 2 h in the second dimension by method of Laemmli as previously described (48). After electrophoresis, the gels were stained using Coomassie blue or transferred to a nitrocellulose membrane at 100 V for 1.5 h using the method of Towbin as previously described (48).
Immunoblotting and lectin binding.
Nitrocellulose protein blots were washed thoroughly with PBS and blocked for 1 to 2 h in 5% nonfat dried milk in PBS. Immunoblotting was performed by room temperature incubation of these blots for 1 h with 1:200 MAb 73.18 in PBS containing 0.1% Tween 20 (PBS-T). The blots were then washed with PBS, incubated with a goat anti-mouse IgG-IgM-alkaline phosphatase conjugate (Tropix, Bedford, Mass.) at 1:5,000 for 1 h at room temperature, and washed with PBS-T. Antibody binding was visualized using nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) or by chemiluminescence using [disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7] decan}-4-yl)phenyl phosphate (CSPD; Tropix, Bedford, Mass.). Lectin binding was performed in an analogous fashion. After incubation for 1 h with 5% nonfat dried milk, the blots were incubated with either 20 μg of DBA-alkaline phosphatase conjugate per ml or 10 μg of succinyl Triticum vulgare lectin (S-WGA)-horseradish peroxidase conjugate (EY Laboratories, San Mateo, Calif.) per ml for 1 h at room temperature. The blots were then washed in PBS-T, and lectin binding was identified by incubation with NBT-BCIP or CSPD for DBA-alkaline phosphatase and 4-chloro-1-naphthol for S-WGA–horseradish peroxidase.
Protein and carbohydrate analysis.
T. gondii R5 strain extracts grown at pH 8.1 (107 parasites per lane) were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The blots were treated either with 25 mM sodium metaperiodate in 50 mM sodium acetate buffer (pH 4.3) for 1 h in the dark or with 1 mg of proteinase K per ml in 50 mM Tris-HCl buffer (pH 7.5) for 1 h at room temperature. Following this treatment, the blots were washed thoroughly and blocked with 5% nonfat dried milk in PBS prior to antibody incubation.
Purification of CST1 by lectin chromatography.
An extract of T. gondii R5 strain grown at pH 8.1 was prepared by four freeze-thaw cycles in PBS containing 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS). The detergent (CHAPS) was then removed by affinity matrix chromatography using an Extracti-Gel D-CHAPS column (Pierce, Rockford, Ill.). The extract was then loaded on a DBA-Sepharose column (EY Laboratories), and the column was extensively washed with LAC buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.02% NaN3) to remove nonbinding proteins. The bound material was eluted using N-acetyl-d-galactosamine at 60°C, and the eluate was concentrated using an Ultrafree-15 filter (Millipore, Waterford, Mass.) for further analysis.
RESULTS
MAb 73.18 recognizes a bradyzoite-specific 116-kDa glycoprotein.
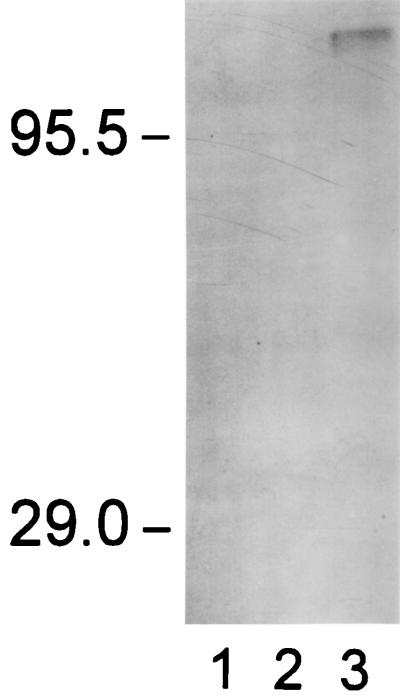
MAb 73.18 reacted with a 116-kDa antigen by immunoblot analysis both in cysts of T. gondii ME49 isolated from mouse brain (data not shown) and in T. gondii R5 cysts developing in vitro (Fig. 1, lane 3). There was no reactivity of MAb 73.18 with either human fibroblasts (lane 1) or T. gondii RH tachyzoites (lane 2). Tachyzoites from either ME49 or R5 at pH 7.1 also had no reactivity with MAb 73.18 (data not shown). A similar-size protein has been demonstrated by radioiodination of intact cysts followed by SDS-PAGE (38, 39). Immunoreactivity of MAb 73.18 to R5 lysate was destroyed by incubation of blots with either 25 mM sodium metaperiodate (pH 4.3 acetate buffer) or proteinase K (1 mg/ml) (data not shown). Thus, MAb 73.18 appears to be directed at a carbohydrate epitope (consistent with its IgG3 subclass) on a glycoprotein found in bradyzoites and cysts of T. gondii. We have termed this 116-kDa reactive antigen CST1.
FIG. 1.
Immunoblot using MAb 73.18. Lanes: 1, Human fibroblast cells; 2, pH 7.1-grown tachyzoites of T. gondii RH; 3, pH 8.1-treated T. gondii R5 purified from human fibroblast cells. Equal amounts of protein were loaded in lanes 1 to 3.
Localization of MAb 73.18 reactivity to the cyst wall.
CST1 is located in the cyst wall (Fig. 2), i.e., the limiting membrane, of the bradyzoite parasitophorous vacuole both in vitro (Fig. 2B) and in vivo (Fig. 2C). In vitro bradyzoites that ruptured out of the parasitophorous vacuole had some cytoplasmic reactivity with MAb 73.18; however, this was of much lower intensity than that seen in the cyst wall. No reactivity was seen in tachyzoites that ruptured out of the tachyzoite parasitophorous vacuole or tachyzoites within this vacuole. During formation of the bradyzoite parasitophorous vacuole, CST1 reactivity (MAb 73.18) was evident on day 1 after exposure to stress conditions (pH 8.1 or SNP) that induce bradyzoite formation. Its expression was as rapid as that of BAG1. Parasite vacuoles positive for CST1 also express the bradyzoite-specific markers BAG1 and MAG1 (49).
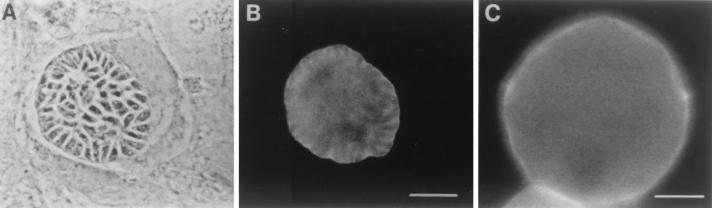
FIG. 2.
Reactivity of MAb 73.18 with T. gondii cysts. (A) Phase-contrast microscopy of cyst from in vitro astrocytes infected with T. gondii ME49. (B) Immunofluorescence microscopy of the cyst in panel A stained with MAb 73.18. Bar, 10 μm. (C) Immunofluorescence microscopy of a cyst, isolated at 8 weeks from the brain of a mouse infected with T. gondii ME49, stained with MAb 73.18. Bar, 15 μm.
Localization of CST1 by electron microscopy.
T. gondii ME49 cysts were isolated from mouse brain and fixed for transmission and immunoelectron microscopy. Transmission electron microscopy of the cyst wall demonstrated a cyst wall, as characterized by an invaginated outer membrane and an underlying dense osmiophilic matrix (Fig. 3A). Immunoelectron microscopy of cysts stained with MAb 73.18 demonstrated labeling of the cyst wall matrix (20-nm gold [Fig. 3B]). A small amount of label was also seen in the cytoplasm of bradyzoites (data not shown), but this did not localize to any specific organelle. Immunoelectron microscopy of cysts stained with DBA also demonstrated labeling of the cyst wall matrix (10-nm gold, [Fig. 3C]).
FIG. 3.
Electron microscopy of the cyst wall of T. gondii cysts isolated from mouse brain. (A) Transmission electron microscopy of the cyst wall. (B) Immunoelectron microscopy of cysts stained with MAb 73.18, showing labeling of the cyst wall matrix (20-nm gold particles). (C) Immunoelectron microscopy of cysts stained with DBA, showing labeling of the cyst wall matrix (10-nm gold particles). Bars, 1 μm.
Colocalization of lectin binding and CST1 by microscopy.
Murine astrocytes were infected with T. gondii ME49 and incubated at pH 8.1 for 3 days to induce bradyzoite formation in vitro. In vitro-derived cysts reacted with MAb 73.18 (Fig. 4A) and with DBA (Fig. 4B). An overlay of the vacuoles stained with both DBA and MAb 73.18 demonstrates a yellow color (Fig. 4C), indicating colocalization of MAb 73.18 and DBA staining.
FIG. 4.
Immunofluorescence of the cyst wall and lectin staining of T. gondii cysts formed in vitro in murine astrocytes. (A) Cysts stained with MAb 73.18 (FITC). (B) DBA staining (Texas red-streptavidin). (C) Overlay of MAb 73.18 and DBA staining; note the yellow color, indicating colocalization of the cyst wall and lectin staining.
Analysis of CST1 by 2D SDS-PAGE and lectin binding.
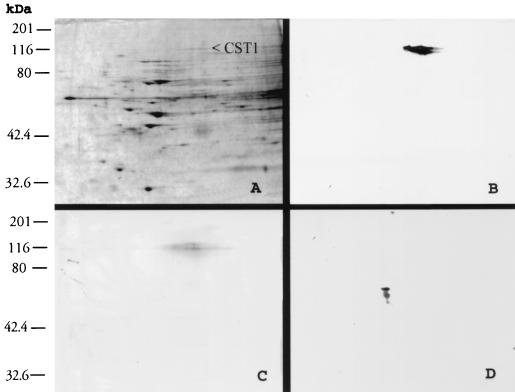
Since the cyst wall is a critical structure in the stability and formation of intact tissue cysts of T. gondii, we sought to purify the CST1 glycoprotein for further analysis. Freeze-thawed extracts of T. gondii R5 grown for 5 days at pH 8.1 were analyzed using 2D SDS-PAGE (Fig. 5). A large number of proteins were evident when a 10% discontinuous polyacrylamide gel was used under reducing conditions (Fig. 5A). After the proteins were transferred to nitrocellulose, an immunoblot was performed using MAb 73.18 (Fig. 5B) and/or a lectin overlay with DBA (20 μg/ml) (Fig. 5C). A single major band was obtained at pI 5.7 and 116 kDa that reacted with both MAb 73.18 (Fig. 5B) and DBA (Fig. 5C). When the lectin S-WGA was used, the 116-kDa protein was not labeled, but instead a different antigen of about 48 kDa was labeled (Fig. 5D). Both DBA and S-WGA have been reported to label the cyst wall of T. gondii (6). This suggests that the carbohydrate modifications recognized by DBA and S-WGA are present on different proteins that make up the cyst wall.
FIG. 5.
2 D electrophoresis of T. gondii R5 grown in human fibroblasts at pH 8.1. (A) Silver-stained 2D gel. The band indicated by < at pI 5.7 and 116 kDa corresponds to CST1, as defined by reactivity to MAb 73.18. (B) Immunoblot of a 2D gel with MAb 73.18, demonstrating that this antibody reacts with a 116-kDa band at pI 5.7. (C) Lectin overlay of a 2D gel using DBA. The identified band is at pI 5.7 and 116 kDa. This band can be superimposed on the immunostained band identified by MAb 73.18. (D) Lectin overlay using S-WGA. This demonstrates that S-WGA localizes to a protein distinct from that identified by DBA and MAb 73.18.
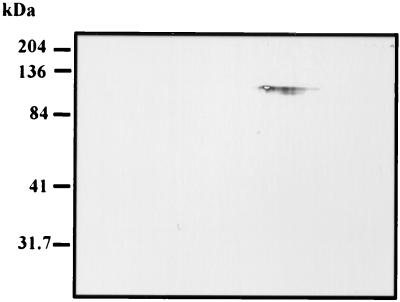
Lectin affinity chromatography was used to further purify CST1. A pH 8.1-grown T. gondii R5 extract was loaded on a DBA-Sepharose column, and the column was extensively washed with LAC buffer to remove nonbinding proteins. No MAb 73.18-reactive material was found in the eluate. The bound material was eluted using N-acetyl-d-galactosamine at 60°C. On 2D electrophoresis, the eluted material was CST1, as defined by MAb 73.18 reactivity (Fig. 6) at 116 kDa, and the corresponding protein band was visible at 116 kDa using SYPRO orange.
FIG. 6.
Immunoblot of DBA chromatography-purified protein. Immunoblot of a 2D gel using the DBA affinity chromatography-purified material (see Materials and Methods) with MAb 73.18, demonstrating that this antibody reacts with a 116-kDa band at pI 5.7, consistent with purification of CST1.
DISCUSSION
The formation of the cyst wall and parasitophorous vacuole matrix is an early event that accompanies bradyzoite differentiation (4, 5, 14, 43). The vacuole, i.e., tissue cyst, in which bradyzoites develop can contain several thousand bradyzoites. The tissue cyst is entirely within a host cell, and the limiting membrane of this host cell can be seen by electron microscopy. The tissue cyst wall is a modification of the limiting membrane of the parasitophorous vacuole and is elastic, argyrophilic, and periodic acid-Schiff reactive (12, 24). The cyst wall and matrix probably protect bradyzoites from harsh environmental conditions and also provide a physical barrier to host immune defenses.
Both DBA and S-WGA have been used to demonstrate bradyzoite (cyst) formation in cell culture and are useful alternatives or adjuncts to immunofluorescence with bradyzoite-specific MAbs (6, 22, 43, 46). The cyst wall binds both DBA and S-WGA. This binding can be inhibited by competition with the sugar haptens N-acetylgalactosamine (GalNAc) for DBA and N-acetylglucosamine (GlcNAc) for S-WGA (6). Treatment with chitinase disrupts the cyst wall and eliminates S-WGA binding, consistent with the presence of chitin in this structure (6). Binding of DBA to the cyst wall of cysts of the related coccidian parasite Neospora caninum developing in vitro is also seen (46).
As demonstrated in this study, the 116-kDa antigen identified by MAb 73.18 (CST1) binds to DBA. A similar-size antigen, i.e., 116 kDa, is recognized by the sera of animals with chronic infection (38, 39, 51) and by rat MAb CC2 (15). By 2D electrophoresis, CST1 accounts for all of the DBA-binding activity in the cyst wall. CST1 does not bind S-WGA, and thus there appears to be a second cyst wall component that is responsible for the binding of S-WGA to T. gondii cysts. With the exception of glycosylphosphatidylinositol anchors, T. gondii proteins do not usually have carbohydrate modifications, and in fact little to no lectin binding is seen to the surface of either bradyzoites or tachyzoites (13). The carbohydrate modifications of CST1 are probably important in providing stability to the cyst and its persistence in the host. In addition, as is seen in the carbohydrate capsules of fungi (9), cyst wall glycoproteins may be important in protecting the tissue cysts from the immune response of the host either by being poorly immunogenic or by masking other epitopes.
In extracellular protozoa, such as Giardia lamblia and Entamoeba histolytica, trophozoites undergo fundamental biologic changes to survive outside the intestine of their host by differentiating into environmentally resistant cyst stages that contain carbohydrates in their cyst walls (23, 32). In G. lamblia, this is triggered by lipid starvation and other stresses, and formation of the cyst wall is associated with induction of hsp78/BiP, a heat shock protein (30, 31). In T. gondii, bradyzoite differentiation is also triggered by environmental stress (44, 45, 47) and is associated with heat shock protein expression. It is interesting that in the T. gondii EST database (http://www.cbil.upenn.edu /ParaDBs/Toxoplasma/index.html), 12 of the 13 clones with identity to hsp78/BiP expression are found in the ME49 bradyzoite in vivo EST library and only one is from the RH tachyzoite library. hsp 78/BiP may act as a chaperone for glycoproteins involved in the formation of the cyst wall during bradyzoite differentiation. In G. lamblia, the cyst wall is carbohydrate rich and contains GalNAc. Cyst formation is associated with the expression of UDP-N-acetylglucosamine pyrophosphorylase (8). Similarly, in yeast the unfolded-protein response is associated with induction of glycosylation enzymes (42). It is possible that tissue cyst formation in T. gondii is also associated with the induction of enzymes for the carbohydrate modification of proteins. Gene homologs associated with glycosylation, e.g., UDP-N-acetylglucosamine-1-phosphate transferase (GenBank no. N59933), are present in the T. gondii EST database. These pathways may offer new therapeutic targets for treatment of the bradyzoite stage of T. gondii.
Assembly of the cyst wall in T. gondii, as in G. lamblia, yeast, or bacteria, is likely to involve the expression and assembly of a series of genes and proteins in a sequential manner (27). This process is most probably dependent on both the timing of expression and the spatial localization of proteins during the assembly process. To date, only two genes have been identified that correspond to proteins identified in either the cyst wall or parasitophorous vacuole matrix of T. gondii. MAG1 (GenBank no. U09029) is a 65-kDa protein expressed in the matrix of the parasitophorous vacuole between bradyzoites and is also seen at the edge of the cyst wall by immunoelectron microscopy (37). The dense-granule protein GRA5 (GenBank no. L06091) (25) is found in both tachyzoites and bradyzoites within dense granules and the parasitophorous vacuole matrix. In tachyzoites, faint staining of the parasitophorous membrane is seen using antibody to GRA5; however, in bradyzoites, there is strong staining of the cyst wall, i.e., the limiting parasitophorous membrane (24). Like BAG1, both MAG1 and GRA5 appear early in bradyzoite vacuole formation, with antibody staining being evident by 1 day following stress induction (24). Other dense-granule antigens (GRA1 and GRA2) have been found in the matrix of both tachyzoite and bradyzoite vacuoles. While no other cyst wall-reactive MAbs have been reported, several MAbs have been identified that recognize bradyzoite-specific molecules localized to the parasitophorous vacuole matrix of developing cysts. These include a 29-kDa matrix antigen identified by MAb E7B2 (50) and a 19-kDa matrix antigen recognized by MAb1.23.29 (L. M. Weiss, unpublished data). The corresponding genes have not been identified.
The present paper describes the initial characterization of CST1, a glycosylated protein component of the cyst wall. Further studies on this protein, including identification of the corresponding gene, as well as investigation of the nature of the carbohydrate modifications of the cyst wall, are in progress. The cyst wall is the defining structure of the T. gondii tissue cyst and is most probably responsible for the persistence of bradyzoites in tissue. This modification of the limiting membrane of the parasitophorous vacuole may limit the communication of this intracellular parasite with its host cells. Additionally, the cyst wall probably limits antigen presentation to the host, contributing to the persistence of this intracellular parasite. Studies on the structure and function of the cyst wall may suggest novel therapeutic strategies for the elimination or prevention of latency during T. gondii infection.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI39454 (L.M.W.) and NIH Training grant T32AI07501 (Y.W.Z.).
We thank Kami Kim for helpful discussions on the preparation of the manuscript and the Analytical Imaging Facility of the Albert Einstein College of Medicine for assistance on transmission electron and confocal microscopy.
REFERENCES
- 1.Bohne W, Gross U, Ferguson D J, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 2.Bohne W, Heesemann J, Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohne W, Holpert M, Gross U. Stage differentiation of the protozoan parasite Toxoplasma gondii. Immunobiology. 1999;201:248–254. doi: 10.1016/S0171-2985(99)80065-5. [DOI] [PubMed] [Google Scholar]
- 5.Bohne W, Parmley S F, Yang S, Gross U. Bradyzoite-specific genes. Curr Top Microbiol Immunol. 1996;219:81–91. doi: 10.1007/978-3-642-51014-4_9. [DOI] [PubMed] [Google Scholar]
- 6.Boothroyd J C, Black M, Bonnefoy S, Hehl A, Knoll L J, Manger I D, Ortega-Barria E, Tomavo S. Genetic and biochemical analysis of development in Toxoplasma gondii. Philos Trans R Soc London Ser B. 1997;352:1347–1354. doi: 10.1098/rstb.1997.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown C R, Hunter C A, Estes R G, Beckmann E, Forman J, David C, Remington J S, McLeod R. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 8.Bulik D A, van Ophem P, Manning J M, Shen Z, Newburg D S, Jarroll E L. UDP-N-acetylglucosamine pyrophosphorylase, a key enzyme in encysting Giardia, is allosterically regulated. J Biol Chem. 2000;275:14722–14728. doi: 10.1074/jbc.275.19.14722. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 10.Cornelissen A W, Overdulve J P, Hoenderboom J M. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981;83:103–108. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- 11.Dubey J P. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J Eukaryot Microbiol. 1997;44:592–602. doi: 10.1111/j.1550-7408.1997.tb05965.x. . (Erratum, 45:367, 1998.) [DOI] [PubMed] [Google Scholar]
- 12.Dubey J P, Lindsay D S, Speer C A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross U. Toxoplasma gondii. Berlin, Germany: Springer-Verlag KG; 1996. [Google Scholar]
- 14.Gross U, Bohne W, Luder C G, Lugert R, Seeber F, Dittrich C, Pohl F, Ferguson D J. Regulation of developmental differentiation in the protozoan parasite Toxoplasma gondii. J Eukaryot Microbiol. 1996;43:114S–116S. doi: 10.1111/j.1550-7408.1996.tb05033.x. [DOI] [PubMed] [Google Scholar]
- 15.Gross U, Bormuth H, Gaissmaier C, Dittrich C, Krenn V, Bohne W, Ferguson D J. Monoclonal rat antibodies directed against Toxoplasma gondii suitable for studying tachyzoite-bradyzoite interconversion in vivo. Clin Diagn Lab Immunol. 1995;2:542–548. doi: 10.1128/cdli.2.5.542-548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halonen S K, Chiu F, Weiss L M. Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun. 1998;66:4989–4993. doi: 10.1128/iai.66.10.4989-4993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halonen S K, Lyman W D, Chiu F C. Growth and development of Toxoplasma gondii in human neurons and astrocytes. J Neuropathol Exp Neurol. 1996;55:1150–1156. doi: 10.1097/00005072-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Halonen S K, Weidner E. Overcoating of Toxoplasma parasitophorous vacuoles with host cell vimentin type intermediate filaments. J Eukaryot Microbiol. 1994;41:65–71. doi: 10.1111/j.1550-7408.1994.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 19.Halonen S K, Weiss L M, Chiu F C. Association of host cell intermediate filaments with Toxoplasma gondii cysts in murine astrocytes in vitro. Int J Parasitol. 1998;28:815–823. doi: 10.1016/s0020-7519(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 20.Hoff R L, Dubey J P, Behbehani A M, Frenkel J K. Toxoplasma gondii cysts in cell culture: new biologic evidence. J Parasitol. 1977;63:1121–1124. [PubMed] [Google Scholar]
- 21.Howe D K, Sibley L D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 22.Knoll L J, Boothroyd J C. Molecular biology's lessons about Toxoplasma development: stage-specific homologs. Parasitol Today. 1998;14:490–493. doi: 10.1016/s0169-4758(98)01347-7. [DOI] [PubMed] [Google Scholar]
- 23.Kreier J P, Baker J R. Parasitic protozoa. 2nd ed. 1–10. San Diego, Calif: Academic Press, Inc.; 1991. [Google Scholar]
- 24.Lane A, Soete M, Dubremetz J F, Smith J E. Toxoplasma gondii: appearance of specific markers during the development of tissue cysts in vitro. Parasitol Res. 1996;82:340–346. doi: 10.1007/s004360050123. [DOI] [PubMed] [Google Scholar]
- 25.Lecordier L, Mercier C, Torpier G, Tourvieille B, Darcy F, Liu J L, Maes P, Tartar A, Capron A, Cesbron-Delauw M F. Molecular structure of a Toxoplasma gondii dense granule antigen (GRA 5) associated with the parasitophorous vacuole membrane. Mol Biochem Parasitol. 1993;59:143–153. doi: 10.1016/0166-6851(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay D S, Toivio-Kinnucan M A, Blagburn B L. Ultrastructural determination of cystogenesis by various Toxoplasma gondii isolates in cell culture. J Parasitol. 1993;79:289–292. [PubMed] [Google Scholar]
- 27.Losick R, Shapiro L, editors. Cold Spring Harbor monograph series. 16. Microbial development. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 28.Luft B J, Brooks R G, Conley F K, McCabe R E, Remington J S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984;252:913–917. [PubMed] [Google Scholar]
- 29.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 30.Lujan H D, Mowatt M R, Byrd L G, Nash T E. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proc Natl Acad Sci USA. 1996;93:7628–7633. doi: 10.1073/pnas.93.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lujan H D, Mowatt M R, Conrad J T, Nash T E. Increased expression of the molecular chaperone BiP/GRP78 during the differentiation of a primitive eukaryote. Biol Cell. 1996;86:11–18. doi: 10.1111/j.1768-322x.1996.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 32.Lujan H D, Mowatt M R, Nash T E. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol Mol Biol Rev. 1997;61:294–304. doi: 10.1128/mmbr.61.3.294-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAllister M M, Parmley S F, Weiss L M, Welch V J, McGuire A M. An immunohistochemical method for detecting bradyzoite antigen (BAG5) in Toxoplasma gondii-infected tissues cross-reacts with a Neospora caninum bradyzoite antigen. J Parasitol. 1996;82:354–355. [PubMed] [Google Scholar]
- 34.McHugh T D, Gbewonyo A, Johnson J D, Holliman R E, Butcher P D. Development of an in vitro model of Toxoplasma gondii cyst formation. FEMS Microbiol Lett. 1993;114:325–332. doi: 10.1111/j.1574-6968.1993.tb06593.x. [DOI] [PubMed] [Google Scholar]
- 35.McLeod R, Mack D, Brown C. Toxoplasma gondii—new advances in cellular and molecular biology. Exp Parasitol. 1991;72:109–121. doi: 10.1016/0014-4894(91)90129-k. [DOI] [PubMed] [Google Scholar]
- 36.Parmley S F, Weiss L M, Yang S. Cloning of a bradyzoite-specific gene of Toxoplasma gondii encoding a cytoplasmic antigen. Mol Biochem Parasitol. 1995;73:253–257. doi: 10.1016/0166-6851(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 37.Parmley S F, Yang S, Harth G, Sibley L D, Sucharczuk A, Remington J S. Molecular characterization of a 65-kilodalton Toxoplasma gondii antigen expressed abundantly in the matrix of tissue cysts. Mol Biochem Parasitol. 1994;66:283–296. doi: 10.1016/0166-6851(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 38.Smith J E, editor. NATO ASI series H. 78. Toxoplasmosis. Heidelberg, Germany: Springer-Verlag, KG; 1993. [Google Scholar]
- 39.Smith J E, McNeil G, Zhang Y W, Dutton S, Biswas-Hughes G, Appleford P. Serological recognition of Toxoplasma gondii cyst antigens. Curr Top Microbiol Immunol. 1996;219:67–73. doi: 10.1007/978-3-642-51014-4_7. [DOI] [PubMed] [Google Scholar]
- 40.Soete M, Fortier B, Camus D, Dubremetz J F. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993;76:259–264. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- 41.Tomavo S, Boothroyd J C. Interconnection between organellar functions, development and drug resistance in the protozoan parasite, Toxoplasma gondii. Int J Parasitol. 1995;25:1293–1299. doi: 10.1016/0020-7519(95)00066-b. [DOI] [PubMed] [Google Scholar]
- 42.Travers K J, Patil C K, Wodicka L, Lockhart D J, Weissman J S, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 43.Weiss L M, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–D405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss L M, Laplace D, Takvorian P, Tanowitz H B, Wittner M. The association of the stress response and Toxoplasma gondii bradyzoite development. J Eukaryot Microbiol. 1996;43:120S. doi: 10.1111/j.1550-7408.1996.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 45.Weiss L M, Laplace D, Takvorian P M, Tanowitz H B, Cali A, Wittner M. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 46.Weiss L M, Ma Y F, Halonen S, McAllister M M, Zhang Y W. The in vitro development of Neospora caninum bradyzoites. Int J Parasitol. 1999;29:1713–1723. doi: 10.1016/s0020-7519(99)00130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss L M, Ma Y F, Takvorian P M, Tanowitz H B, Wittner M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect Immun. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss L M, Udem S A, Tanowitz H, Wittner M. Western blot analysis of the antibody response of patients with AIDS and Toxoplasma encephalitis: antigenic diversity among Toxoplasma strains. J Infect Dis. 1988;157:7–13. doi: 10.1093/infdis/157.1.7. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y W, Kim K, Ma Y F, Wittner M, Tanowitz H B, Weiss L M. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Mol Microbiol. 1999;31:691–701. doi: 10.1046/j.1365-2958.1999.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y W, Smith J E. Toxoplasma gondii: identification and characterization of a cyst molecule. Exp Parasitol. 1995;80:228–233. doi: 10.1006/expr.1995.1028. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y W, Smith J E. Toxoplasma gondii: reactivity of murine sera against tachyzoite and cyst antigens via FAST-ELISA. Int J Parasitol. 1995;25:637–640. doi: 10.1016/0020-7519(94)00139-f. [DOI] [PubMed] [Google Scholar]