Abstract
Background
New creatinine-based estimated glomerular filtration rate (eGFR) equations, including the 2021 Chronic Kidney Disease Epidemiology Collaboration (2021 CKD-EPI) and European Kidney Function Consortium (EKFC) equations, have been introduced recently. We assessed the performance of the 2021 CKD-EPI and EKFC equations in the Korean population.
Methods
We analyzed 1,654 Korean patients aged ≥18 years who underwent chromium-51-ethylenediamine tetraacetic acid GFR measurements (mGFR). Bias (eGFR–mGFR), root mean square error (RMSE), and proportion of eGFR within 30% of mGFR (P30) of the 2009 CKD-EPI, 2021 CKD-EPI, and EFKC equations were compared. The concordance rate between eGFR and mGFR categories was evaluated. Both eGFR and mGFR categories were classified into six groups ≥90, 89–60, 59–45, 44–30, 29–15, and <15 mL/min/1.73 m2.
Results
The median bias (mL/min/1.73 m2) was 1.8 for the 2009 CKD-EPI equation, 4.8 for the 2021 CKD-EPI equation, and –0.3 for the EKFC equation. The P30 and RMSE were 78.2% and 17.0 for the 2009 CKD-EPI equation, 75.6% and 17.4 for the 2021 CKD-EPI equation, and 80.0% and 16.7 for the EKFC equation, respectively. The overall GFR category concordance rate between eGFR and mGFR was 63.4% for the 2009 CKD-EPI equation, 60.5% for the 2021 CKD-EPI equation, and 61.0% for the EKFC equation.
Conclusions
Among the three eGFR equations, the EKFC equation had the smallest bias and highest P30 in Koreans. The 2009 CKD-EPI equation had a lower bias than the 2021 CKD-EPI equation.
Keywords: Accuracy, Estimated glomerular filtration rate, Creatinine
INTRODUCTION
Over the past few decades, the estimated glomerular filtration rate (eGFR) based on the serum creatinine concentration has been used as a representative index to evaluate overall kidney function in clinical practice, research, and public health assessments [1-5]. Numerous eGFR equations have been introduced, and new eGFR equations are continuously being developed and validated [6]. Advances in hospital and/or laboratory information systems and the introduction of the Modification of Diet in Renal Disease (MDRD) study equation have provided a basis for automated eGFR calculation and reporting in clinical laboratories [6, 7]. In the USA, for example, more than 90% of clinical laboratories currently report the eGFR along with the creatinine concentration [7]. In contrast to the USA, Europe, and Australia, many other countries have low eGFR reporting rates [6, 8, 9].
eGFR equations reflect the characteristics of the population used for equation development. An ethnicity-based constant was applied in the eGFR calculation based on the MDRD equation to account for the finding that the average measured GFR (mGFR) of black participants was 21.2% higher than that of non-black participants with the same serum creatinine concentration, sex, and age [10, 11]. Similarly, in the 2009 Chronic Kidney Disease Epidemiology Collaboration (2009 CKD-EPI) equation introduced after the MDRD equation, the mGFR of black participants was 15.9% higher than that of matched non-black participants, and thus, an ethnicity-based constant was included [12]. However, ethnicity is widely understood as a social rather than a biological factor; therefore, in the context of global efforts to resolve health inequities, ethnicity-based medical decisions have been questioned [13-17]. In the USA, where ethnicity-related issues have received considerable attention, in-depth discussions have arisen regarding the removal of the ethnicity-based constant from the eGFR equation. Consequently, the 2021 CKD-EPI equation no longer includes this constant [1].
The recently developed modified full age spectrum (FAS) equation, or European Kidney Function Consortium (EKFC) equation, can be applied to adults and children over 2 years of age [18]. This equation is an improvement of the previously published FAS equation [19]. The Kidney Disease: Improving Global Outcomes (KDIGO) recommends the 2009 CKD-EPI for adults and the Updated Bedside Schwartz for children; however, the guidelines specifically state that an alternative creatinine-based equation is acceptable if it improves the accuracy of GFR estimates [20]. As the eGFR equations for adults and children differ, it is difficult to assess the continuity of eGFR trends during the transition from adolescence to adulthood [18].
There are few studies on the use of new creatinine-based eGFR equations, such as the 2021 CKD-EPI and EKFC equations, mostly limited to Western populations, with no studies in Asian populations [21, 22]. We evaluated the performance of the above two equations in Koreans and investigated the clinical impact with respect to the estimation of the prevalence of CKD in the Korean general population.
MATERIALS AND METHODS
Study patients and data collection
We reviewed electronic medical records and collected study data. In total, 3,784 patients received GFR measurement using chromium-51-ethylenediamine tetraacetic acid (51Cr-EDTA) between July 2009 and March 2019 at Asan Medical Center, Seoul, Korea. Among them, 2,441 patients had serum creatinine concentration measured on the day of GFR measurement, and 1,702 patients were ≥18 years. For patients with multiple GFR measurements, the first measurement was selected. After excluding cases of mGFR <5 mL/min/1.73 m2 (N=39) and those with missing body mass index (BMI) data (N=9), 1,654 adult patients were enrolled as the final study cohort. The characteristics of the study patients are summarized in Table 1. This study was conducted according to the criteria set by the declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Asan Medical Center (approval number: 2021-1076). This study involved no greater than minimal risk to participants, and the IRB approved an informed consent waiver.
Table 1.
Characteristics of the study patients
| Variable | Overall |
|---|---|
| N | 1,654 |
| Age, yr | 61 (50–69) |
| Age category, N (%) | |
| < 50 yr | 395 (23.9) |
| 50–64 yr | 617 (37.3) |
| ≥ 65 yr | 642 (38.8) |
| Height, cm | 164.5 (156.6–170.0) |
| Weight, kg | 65.0 (57.1–72.6) |
| BMI, kg/m2 | 24.3 (22.1–26.6) |
| BMI category, N (%) | |
| < 20 kg/m2 | 151 (9.1) |
| 20 to < 25 kg/m2 | 817 (49.4) |
| 25 to < 30 kg/m2 | 584 (35.3) |
| ≥ 30 kg/m2 | 102 (6.2) |
| Creatinine, mg/dL | 1.00 (0.80–1.45) |
| mGFR, mL/min/1.73 m2 | 69.1 (43.3–92.1) |
| mGFR category, N (%) | |
| < 15 mL/min/1.73 m2 | 114 (6.9) |
| 15–29 mL/min/1.73 m2 | 142 (8.6) |
| 30–44 mL/min/1.73 m2 | 183 (11.1) |
| 45–59 mL/min/1.73 m2 | 223 (13.5) |
| 60–89 mL/min/1.73 m2 | 544 (32.9) |
| ≥ 90 mL/min/1.73 m2 | 448 (27.1) |
| eGFR, mL/min/1.73 m2 | |
| CKD-EPI | 72.3 (46.8–92.4) |
| 2021 CKD-EPI | 76.6 (49.8–97.1) |
| EKFC | 69.5 (45.3–88.2) |
Continuous variables are expressed as median (25 percentile to 75 percentile) because they are not normally distributed. Conversion factors for units: serum creatinine in mg/dL to μmol/L, 88.4×.
Abbreviations: BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; EKFC, European Kidney Function Consortium; mGFR, measured glomerular filtration rate.
Laboratory measurements and eGFR calculation
All patients were injected intravenously with a single dose of 51Cr-EDTA solution to assess mGFR. Venous blood was drawn 3 and 5 hours after injection. The 51Cr-EDTA plasma clearance rate was determined using the slope-intercept method. The Brochner–Mortensen equation was used to correct body-surface-area-corrected GFR values [23]. 51Cr-EDTA GFR values served as a reference. The serum creatinine concentration was measured by the rate-blanked compensated kinetic Jaffe method (Roche Diagnostics, Indianapolis, IN, USA) using an isotope dilution mass spectrometry-traceable calibrator. eGFR was calculated using the 2009 CKD-EPI [12], 2021 CKD-EPI [1], and 2021 EKFC [18] equations.
Analysis of CKD prevalence in the Korean general population based on the eGFR equations
Data from the Korea National Health and Nutrition Examination Survey (KNHANES), collected from 2019 to 2020, were used to evaluate differences in the prevalence of CKD in the general population according to the eGFR equations. CKD was defined as eGFR <60 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio ≥30 mg/g [20]. The 15,469 KNHANES participants comprised 12,881 adults (≥18 years). In 11,444 participants, serum creatinine, urine albumin, and urine creatinine were measured. We compared the eGFR category proportion and CKD prevalence in these 11,444 adults according to the three eGFR equations.
Statistical analysis
Statistical analysis was performed using MedCalc version 20.106 (MedCalc Software, Ostend, Belgium) and Analyse-it for Microsoft Excel 5.92 (Analyse-it Software, Leeds, UK). P<0.05 was considered to indicate statistical significance.
The normal distribution of continuous variables was assessed using the Kolmogorov–Smirnov test, and the mean or median value was reported according to the data distribution. The bias of the three eGFR equations was calculated as eGFR–mGFR. The 95% confidence interval (CI) for the median of non-parametric data was determined [24]. The Wilcoxon signed-rank test was used to compare the bias between eGFR equations.
To evaluate clinical accuracy, the proportion of patients with an eGFR within 30% of the mGFR was calculated and expressed as the P30. The P30 value is an indicator of clinical accuracy that is used to achieve good medical decision-making based on the eGFR [25]. The 95% CI for the P30 was calculated as follows:
where p is the proportion and N is the number of samples.
The P30 values of the three eGFR equations were compared using the McNemar test. The root mean square error (RMSE) was calculated to evaluate the imprecision of the three eGFR equations.
The concordance rate between mGFR and eGFR was assessed for the three equations using the GFR categories of CKD stages recommended by the 2012 KDIGO guidelines (GFR category G1, ≥90 mL/min/1.73 m2; G2, 89–60 mL/min/1.73 m2; G3A, 59–45 mL/min/1.73 m2; G3B, 44–30 mL/min/1.73 m2; G4, 29–15; and G5, <15 mL/min/1.73 m2). Lin’s concordance correlation coefficient (CCC) was used to analyze the concordance rate for each group.
RESULTS
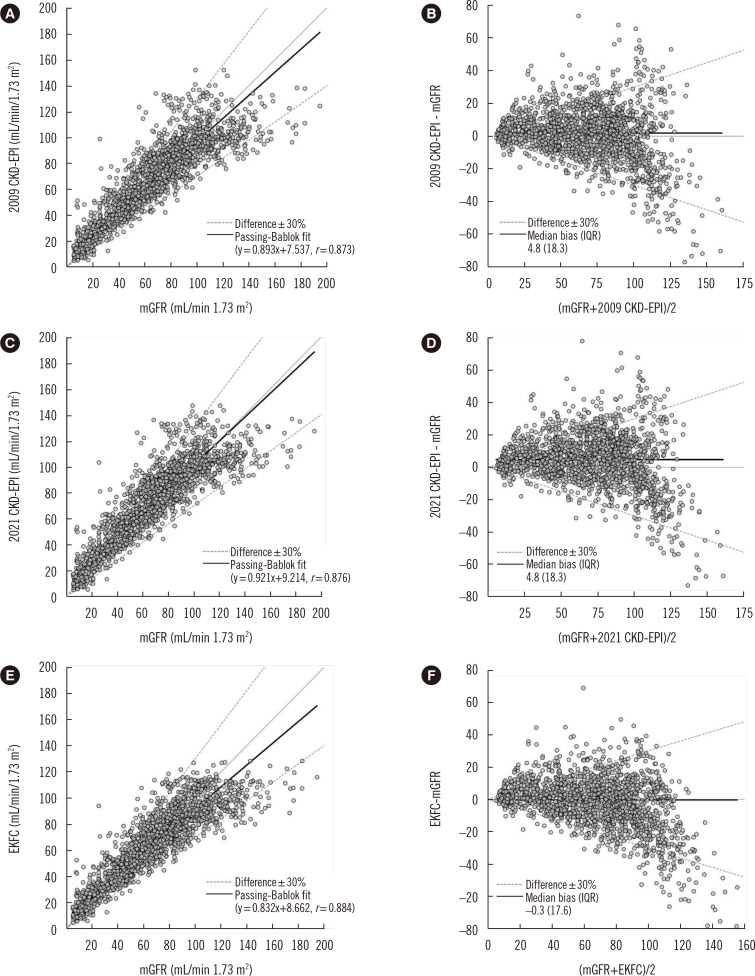
For the group of study patients (N=1,654), the median bias (95% CI) was 1.8 (1.2–2.4) mL/min/1.73 m2 for the 2009 CKD-EPI equation, 4.8 (4.1–5.4) mL/min/1.73 m2 for the 2021 CKD-EPI equation, and –0.3 (–1.2–0.4) mL/min/1.73 m2 for the EKFC equation. The P30 values and RMSE were 78.2% and 17.0 for the 2009 CKD-EPI equation, 75.6% and 17.4 for the 2021 CKD-EPI equation, and 80.0% and 16.7 for the EKFC equation, respectively. The bias of the EKFC equation was significantly lower than that of the other two equations, and its P30 value was the highest. The 2009 CKD-EPI equation had a significantly lower bias and higher P30 than the 2021 CKD-EPI equation (Table 2). The results of subgroup analysis according to sex, age, mGFR, and BMI are presented in Table 2 and Supplemental Data Table S1. Scatter and Bland–Altman plots of the mGFR and three eGFR equations are presented in Fig. 1.
Table 2.
Accuracy and imprecision of three eGFR equations
| Group | eGFR equation | P | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 2009 CKD-EPI [A] | 2021 CKD-EPI [B] | EKFC [C] | [A] vs. [B] | [A] vs. [C] | [B] vs. [C] | |
| All (N = 1,654) | ||||||
| Median bias* (95% CI) | 1.8 (1.2–2.4) | 4.8 (4.1–5.4) | –0.3 (–1.2–0.4) | < 0.001 | < 0.001 | < 0.001 |
| P30† (95% CI), % | 78.2 (76.2–80.2) | 75.6 (73.5–77.6) | 80.0 (78.1–82.0) | < 0.001 | 0.003 | < 0.001 |
| RMSE (95% CI) | 17.0 (16.2–17.8) | 17.4 (16.5–18.1) | 16.7 (15.8–17.5) | |||
| mGFR ≥ 60 mL/min/1.73 m2 (N = 992) | ||||||
| Median bias* (95% CI) | –1.6 (–3.1 to –0.4) | 2.4 (1.1–4.0) | –5.2 (–6.8 to –4.0) | < 0.001 | < 0.001 | < 0.001 |
| P30† (95% CI), % | 88.6 (86.6–90.6) | 88.1 (86.1–90.1) | 89.9 (88.0–91.8) | 0.522 | 0.098 | 0.066 |
| RMSE (95% CI) | 19.3 (18.2–20.4) | 18.9 (17.9–20.0) | 19.4 (18.2–20.5) | |||
| mGFR < 60 mL/min/1.73 m2 (N = 662) | ||||||
| Median bias* (95% CI) | 4.3 (3.4–5.0) | 6.6 (5.4–7.5) | 3.2 (2.6–4.2) | < 0.001 | < 0.001 | < 0.001 |
| P30† (95% CI), % | 62.7 (59–66.4) | 56.8 (53–60.6) | 65.3 (61.6–68.9) | < 0.001 | 0.012 | < 0.001 |
| RMSE (95% CI) | 12.9 (11.6–14.0) | 14.7 (13.4–15.8) | 11.6 (10.5–12.6) | |||
| BMI < 20 (N = 151) | ||||||
| Median bias* (95% CI) | 11.1 (7.8–14.4) | 13.8 (9.8–17.0) | 8.0 (4.8–11.7) | < 0.001 | < 0.001 | < 0.001 |
| P30† (95% CI), % | 47.7 (39.7–55.6) | 45.7 (37.7–53.6) | 55.0 (47.0–62.9) | 0.453 | 0.003 | 0.001 |
| RMSE (95% CI) | 23.0 (19.5–25.9) | 24.6 (21.2–27.6) | 19.6 (16.5–22.3) | |||
| 20 ≤ BMI < 25 (N = 817) | ||||||
| Median bias* (95% CI) | 2.7 (1.9–3.6) | 6.0 (5.0–6.9) | 0.7 (–0.2–1.5) | < 0.001 | < 0.001 | < 0.001 |
| P30† (95% CI), % | 80.2 (77.4–82.9) | 75.4 (72.4–78.4) | 81.4 (78.7–84.1) | < 0.001 | 0.164 | < 0.001 |
| RMSE (95% CI) | 16.0 (14.8–17.1) | 16.7 (15.5–17.7) | 15.5 (14.3–16.7) | |||
| 25 ≤ BMI < 30 (N = 584) | ||||||
| Median bias* (95% CI) | –1.2 (–2.7 to –0.2) | 1.9 (0.7–3.1) | –3.1 (–4.2 to –1.6) | < 0.001 | < 0.001 | < 0.001 |
| P30† (95% CI), % | 82.2 (79.1–85.3) | 81.2 (78–84.3) | 83.6 (80.6–86.6) | 0.377 | 0.215 | 0.087 |
| RMSE (95% CI) | 16.5 (15.0–17.8) | 16.1 (14.8–17.3) | 16.9 (15.3–18.4) | |||
| BMI ≥ 30 (N = 102) | ||||||
| Median bias* (95% CI) | –5.2 (–8.2 to –1.5) | –2.2 (–5.1–2.0) | –6.2 (–9.3 to –3.6) | < 0.001 | < 0.001 | < 0.001 |
| P30† (95% CI), % | 85.3 (78.4–92.2) | 89.2 (83.2–95.2) | 86.3 (79.6–93.0) | 0.219 | 1.000 | 0.508 |
| RMSE (95% CI) | 17.6 (14.7–20.1) | 16.7 (14.0–19.0) | 19.5 (15.9–22.5) | |||
*Bias is calculated as eGFR minus mGFR in units of mL/min/1.73 m2. The Wilcoxon signed-rank test was used–compare the bias between eGFR equations.
†P30 is the proportion of eGFR within 30% of mGFR. The P30 of three eGFR equations was compared using McNemar test.
Abbreviations: BMI, body mass index (kg/m2); Chronic Kidney Disease Epidemiology Collaboration; CI, confidence interval; eGFR, estimated glomerular filtration rate; EKFC, European Kidney Function Consortium; mGFR, measured glomerular filtration rate; RMSE, root mean square error.
Fig. 1.
Scatter and Bland–Altman plots of the concordance rate between mGFR and eGFR. (A, B) for 2009 CKD-EPI, (C, D) for 2021 CKD-EPI, and (E, F) for EKFC.
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; EKFC, European Kidney Function Consortium; mGFR, measured glomerular filtration rate; IQR, interquartile range.
The overall GFR category concordance rate between eGFR and mGFR and Lin’s CCC were 63.4% and 0.895 for the 2009 CKD-EPI equation, 60.5% and 0.884 for the 2021 CKD-EPI equation, and 61.0% and 0.891 for the EKFC equation, respectively. There was no significant difference in Lin’s CCC for group concordance (Table 3).
Table 3.
Concordance rate between eGFR and mGFR categories
| eGFR, mL/min/1.73 m2 | N participants | Overall group concocrdance rate (%) | Lin’s CCC for group agreement (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| mGFR, mL/min/1.73 m2 | |||||||||
|
| |||||||||
| <15 | 15–29 | 30–44 | 45–59 | 60–89 | ≥90 | ||||
| 2009 CKD-EPI | < 15 | 70 | 13 | 1 | 0 | 0 | 0 | 63.4 | 0.895 (0.885–0.904) |
| 15–29 | 37 | 73 | 11 | 0 | 0 | 0 | |||
| 30–44 | 3 | 44 | 90 | 43 | 5 | 0 | |||
| 45–59 | 4 | 9 | 70 | 93 | 48 | 0 | |||
| 60–89 | 0 | 2 | 11 | 81 | 371 | 96 | |||
| ≥ 90 | 0 | 1 | 0 | 6 | 120 | 352 | |||
| 2021 CKD-EPI | < 15 | 62 | 11 | 1 | 0 | 0 | 0 | 60.5 | 0.884 (0.873–0.894) |
| 15–29 | 42 | 61 | 7 | 0 | 0 | 0 | |||
| 30–44 | 6 | 57 | 81 | 25 | 2 | 0 | |||
| 45–59 | 4 | 10 | 72 | 87 | 30 | 0 | |||
| 60–89 | 0 | 2 | 22 | 103 | 324 | 62 | |||
| ≥ 90 | 0 | 1 | 0 | 8 | 188 | 386 | |||
| EKFC | < 15 | 68 | 12 | 1 | 0 | 0 | 0 | 61.0 | 0.891 (0.881–0.901) |
| 15–29 | 41 | 78 | 12 | 1 | 0 | 0 | |||
| 30–44 | 3 | 41 | 99 | 50 | 5 | 0 | |||
| 45–59 | 2 | 9 | 61 | 103 | 73 | 0 | |||
| 60–89 | 0 | 1 | 10 | 66 | 382 | 169 | |||
| ≥ 90 | 0 | 1 | 0 | 3 | 84 | 279 | |||
Abbreviations: CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; EKFC, European Kidney Function Consortium; Lin’s CCC, Lin’s concordance correlation coefficient; mGFR, measured glomerular filtration rate.
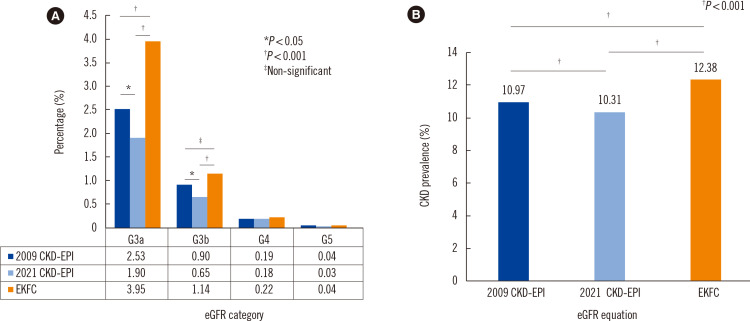
The proportion of eGFR category G3 (G3a and G3b) in the Korean general population was 3.4% according to the 2009 CKD-EPI equation, 2.6% according to the 2021 CKD-EPI equation, and 5.1% according to the EKFC equation (Fig. 2A). The prevalence of CKD in the general population was 11.0%, 10.3%, and 12.4%, respectively (Fig. 2B). There was a significant difference in the prevalence of CKD stage 3 depending on the eGFR equation. A detailed comparison of the data for each eGFR category and the differences in CKD prevalence are summarized in Supplemental Data Fig. S1 and Supplemental Data Table S2.
Fig. 2.
Distribution of eGFR categories and CKD stages according to eGFR equation in the Korean general population. Data from the KNHANES 2019–2020. eGFR (mL/min/1.73 m2) categories: G3a, 59–45; G3b, 44–30; G4, 29–15; G5, <15. CKD was defined as an eGFR <60 mL/min/1.73 m2 or urinary albumin-to-creatinine ratio >30 mg/g. For details, see Supplemental Data Fig. S1 and Supplemental Table S2. *P<0.05, †P<0.001, ‡not significant.
Abbreviations: eGFR, estimated glomerular filtration rate; CDK, chronic kidney disease; KNHANES, Korea National Health and Nutrition Examination Survey
DISCUSSION
Among the three eGFR equations evaluated, the EKFC equation had the smallest bias, and the bias of the 2021 CKD-EPI equation was significantly greater than that of the 2009 CKD-EPI equation in Koreans. The performance of the eGFR equations can be affected by various factors, such as study participant characteristics, the mGFR measurement method, and the uncertainty in the serum creatinine concentration measurement [6].
Pottle, et al. [18] reported that the overall bias of the EKFC equation was lower than that of the 2009 CKD-EPI equation for Europeans, whereas Levey, et al. [22] found a lower bias of the 2009 CKD-EPI equation than of the EKFC equation for Americans. In the present study, the median bias of the EKFC equation was –0.3 mL/min/1.73 m2, which was the lowest among the three eGFR equations. The 95% CI was –1.2 to 0.4, including 0; there was no significant difference from the zero bias.
In a previous study comparing the accuracies of the 2009 CKD-EPI and 2021 CKD-EPI equations in a non-black population, Inker, et al. [1] reported a median bias (eGFR–mGFR in mL/min/1.73 m2) and P30 of 0.5 and 89.5%, respectively, for the 2009 CKD-EPI equation and of 3.9 and 86.5%, respectively, for the 2021 CKD-EPI equation. Meeusen, et al. [21] reported a median bias and P30 of –3.1 mL/min/1.73 m2 and 78.6%, respectively, for the 2009 CKD-EPI equation and of –0.2 mL/min/1.73 m2 and 78.7%, respectively, for the 2021 CKD-EPI equation. Thus, in these studies targeting Western populations, the 2021 CKD-EPI equation overestimated the eGFR by approximately 3 mL/min/1.73 m2 compared to the 2009 CKD-EPI equation, whereas the P30 was similar or slightly lower in non-black participants. This pattern was consistent with the results of the present study. The tendency for a difference in bias between the two equations persisted in subgroup analysis according to sex, age, mGFR, serum creatinine concentration, and BMI category.
According to a systematic review, there is insufficient medical evidence supporting the use of a black ethnicity modifier in eGFR calculations [26]. The Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease of the National Kidney Foundation-American Society of Nephrology in the United States recommended that the 2021 CKD-EPI equation be immediately applied in eGFR calculation in American adults [27]. We agree that eliminating ethnicity as a variable in the eGFR equation leads to a more equitable diagnosis and treatment of kidney disease. As Koreans are non-black, all clinical laboratories in Korea that use the 2009 CKD-EPI equation have applied the so-called unifying 2009 CKD-EPI (only for whites or others) to all Koreans. As noted above, in Koreans, the bias of the 2021 CKD-EPI equation was higher than that of the 2009 CKD-EPI equation. Therefore, additional research and discussion are needed to support the adoption of the 2021 CKD-EPI equation in clinical practice in Korea.
In the subgroup analysis based on BMI, all three eGFR equations had a relatively high bias for the BMI <20 kg/m2 group. This group had a higher serum creatinine concentration and a lower mGFR than the other groups, resulting in a larger bias of the eGFR equation. In the subgroup analysis based on mGFR category, the bias in the mGFR ≥90 mL/min/1.73 m2 group was relatively high. In this group, the serum creatinine concentration was lower than in the other groups. However, the creatinine assay based on the non-enzymatic method overestimates the serum creatinine concentration and consequently underestimates the eGFR, especially in low-concentration samples [28]. This characteristic of the creatinine assay may explain the relatively high bias of the eGFR equation in the mGFR ≥90 mL/min/1.73 m2 group.
There were significant differences in the prevalence of CKD in the general Korean population according to the eGFR equation. For example, the proportion of the CKD stage 3 eGFR category was 3.4% for the 2009 CKD-EPI equation, 2.6% for the 2021 CKD-EPI equation, and 5.1% for the EKFC equation. In particular, there was a roughly two-fold difference in the proportion between the 2021 CKD-EPI and the EKFC equations, most likely due to the aforementioned bias trend in the eGFR equations. In the GFR category concordance rate analysis between eGFR and mGFR, the concordance rate of CKD G3 was the highest for the EKFC equation (45.3%), followed by the 2009 CKD-EPI equation (41.0%), and the 2021 CKD-EPI equation (37.7%). However, there was no significant difference in the GFR category concordance rate between the three equations. In the mGFR ≥90 mL/min/1.73 m2 group (GFR category G1), there was no significant difference in the concordance rate between the 2009 CKD-EPI and 2021 CKD-EPI equations; however, the concordance rate of the EKFC equation was significantly lower than that of the other two equations (EKFC vs. 2009 CKD-EPI, 62.3% vs. 78.6%, P<0.05; EKFC vs. 2021 CKD-EPI, 62.3% vs. 86.2%, P<0.001).
The prevalence of CKD may vary depending on the eGFR equation used to calculate it [29, 30]. Ideally, GFR should be measured using exogenous markers, as this will provide a more accurate assessment of CKD prevalence [30]. However, this is practically impossible. Therefore, the eGFR equation with the smallest bias should be applied instead. To compare CKD prevalence across countries or regions, the same eGFR equation must be used. However, it should be taken into account that the accuracy of the same eGFR equation may vary from country to country or from region to region.
This study had several limitations. First, data from one tertiary medical hospital were used, without external validation. Second, cystatin C-related equations could not be included because of a lack of laboratory data. The National Kidney Foundation Laboratory Engagement Working Group recommends using the cystatin C marker in conjunction with creatinine to increase the accuracy of the eGFR [7]. Third, the use of the Jaffe method for creatinine is not recommended and may introduce additional bias compared to the enzymatic method. Fourth, mGFR is not a perfect method for determining the actual GFR. Finally, CKD was defined based on single eGFR and albuminuria measurements.
In conclusion, among the three eGFR equations, the EKFC equation had the smallest bias and the highest P30 in Koreans. The 2009 CKD-EPI equation had a lower bias than the 2021 CKD-EPI equation. The introduction of the 2021 CKD-EPI equation serves as a reminder that all patients have the right to equal medical decision-making, regardless of ethnicity. The use of the eGFR equation, with its minimal bias, is good laboratory practice to evaluate kidney function.
Supplemental Materials
ACKNOWLEDGMENTS
None.
Footnotes
AUTHOR CONTRIBUTIONS
Research area and study design: Jeong TD and Min WK; data acquisition: Hong J; data analysis and interpretation: Hong J and Jeong TD; statistical analysis: Jeong TD; supervision or mentorship: Lee W and Chun S. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
No potential conflicts of interest relevant to this article were reported.
RESEARCH FUNDING
This work was supported by the Ewha Womans University Research Grant of 2020.
REFERENCES
- 1.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–49. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Strengths and limitations of estimated and measured GFR. Nat Rev Nephrol. 2019;15:784. doi: 10.1038/s41581-019-0213-9. [DOI] [PubMed] [Google Scholar]
- 4.Nichols GA, Ustyugova A, Déruaz-Luyet A, O'Keeffe-Rosetti M, Brodovicz KG. Health care costs by type of expenditure across eGFR stages among patients with and without diabetes, cardiovascular disease, and heart failure. J Am Soc Nephrol. 2020;31:1594–601. doi: 10.1681/ASN.2019121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD Chronic Kidney Disease Collaboration, author. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–33. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller WG, Jones GRD. Estimated glomerular filtration rate; laboratory implementation and current global status. Adv Chronic Kidney Dis. 2018;25:7–13. doi: 10.1053/j.ackd.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Miller WG, Kaufman HW, Levey AS, Straseski JA, Wilhelms KW, Yu HE, et al. National Kidney Foundation Laboratory Engagement Working Group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: practical guidance for clinical laboratories. Clin Chem. 2022;68:511–20. doi: 10.1093/clinchem/hvab278. [DOI] [PubMed] [Google Scholar]
- 8.Kim SK, Jeong TD, Park S, Lee YW, Min WK. Current status of estimated glomerular filtration rate reporting in Korea. J Lab Med Qual Assur. 2019;41:201–6. doi: 10.15263/jlmqa.2019.41.4.201. [DOI] [Google Scholar]
- 9.Costa NR, Carvalho ARM, Pinto CMA, Andriolo A, Guerra IC. Laboratory diagnosis of chronic kidney disease in adults: an overview of hospitals inserted in the Portuguese National Health System. J Bras Patol Med Lab. 2017;53:388–96. doi: 10.5935/1676-2444.20170062. [DOI] [Google Scholar]
- 10.Madero M, Sarnak MJ. Creatinine-based formulae for estimating glomerular filtration rate: is it time to change to chronic kidney disease epidemiology collaboration equation? Curr Opin Nephrol Hypertens. 2011;20:622–30. doi: 10.1097/MNH.0b013e32834ba210. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado C, Baweja M, Burrows NR, Crews DC, Eneanya ND, Gadegbeku CA, et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN task force. J Am Soc Nephrol. 2021;32:1305–17. doi: 10.1681/ASN.2021010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet. 2020;396:1125–8. doi: 10.1016/S0140-6736(20)32076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight-reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874–82. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 16.Oni-Orisan A, Mavura Y, Banda Y, Thornton TA, Sebro R. Embracing genetic diversity to improve black health. N Engl J Med. 2021;384:1163–7. doi: 10.1056/NEJMms2031080. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JPA, Powe NR, Yancy C. Recalibrating the use of race in medical research. JAMA. 2021;325:623–4. doi: 10.1001/jama.2021.0003. [DOI] [PubMed] [Google Scholar]
- 18.Pottel H, Björk Bjork J, Courbebaisse M, Couzi L, Ebert N, Eriksen BO, et al. Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: a cross-sectional analysis of pooled data. Ann Intern Med. 2021;174:183–91. doi: 10.7326/M20-4366. [DOI] [PubMed] [Google Scholar]
- 19.Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016;31:798–806. doi: 10.1093/ndt/gfv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:19–62. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 21.Meeusen JW, Kasozi RN, Larson TS, Lieske JC. Clinical impact of the refit CKD-EPI 2021 creatinine-based eGFR equation. Clin Chem. 2022;68:534–9. doi: 10.1093/clinchem/hvab282. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Tighiouart H, Inker LA. Improving glomerular filtration rate estimation-across the age and diversity spectrum. Ann Intern Med. 2021;174:265–7. doi: 10.7326/M20-6983. [DOI] [PubMed] [Google Scholar]
- 23.Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS British Nuclear Medicine Society, author. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun. 2004;25:759–69. doi: 10.1097/01.mnm.0000136715.71820.4a. [DOI] [PubMed] [Google Scholar]
- 24.Campbell MJ, Gardner MJ. Calculating confidence intervals for some non-parametric analyses. Br Med J (Clin Res Ed) 1988;296:1454–6. doi: 10.1136/bmj.296.6634.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Kidney Foundation, author. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 26.Marzinke MA, Greene DN, Bossuyt PM, Chambliss AB, Cirrincione LR, McCudden CR, et al. Limited evidence for use of a black race modifier in eGFR calculations: a systematic review. Clin Chem. 2022;68:521–33. doi: 10.1093/clinchem/hvab279. [DOI] [PubMed] [Google Scholar]
- 27.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–88.e1. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Jeong TD, Cho EJ, Lee K, Lee W, Yun YM, Chun S, et al. Recent trends in creatinine assays in Korea: long-term accuracy-based proficiency testing survey data by the Korean Association of External Quality Assessment Service (2011-2019) Ann Lab Med. 2021;41:372–9. doi: 10.3343/alm.2021.41.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora P, Rajagopalan S, Patel N, Nainani N, Venuto RC, Lohr JW. The MDRD equation underestimates the prevalence of CKD among blacks and overestimates the prevalence of CKD among whites compared to the CKD-EPI equation: a retrospective cohort study. BMC Nephrol. 2012;13:4. doi: 10.1186/1471-2369-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delanaye P, Cavalier E, Mariat C, Maillard N, Krzesinski JM. MDRD or CKD-EPI study equations for estimating prevalence of stage 3 CKD in epidemiological studies: which difference? Is this difference relevant? BMC Nephrol. 2010;11:8. doi: 10.1186/1471-2369-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.