Abstract
Cell-mediated immune processes play a prominent role in the clinical manifestations of syphilis, a sexually transmitted disease of humans caused by spirochetal bacterium Treponema pallidum. The immune cell type that initiates the early immune response to T. pallidum thus far has not been identified. However, dendritic cells (DCs) are the first immune-competent cells to encounter antigens within skin or mucous membranes, the principal sites of early syphilitic infection. In the present study, immature DC line XS52, derived from murine skin, was utilized to examine T. pallidum-DC interactions and subsequent DC activation (maturation). Electron microscopy revealed that T. pallidum was engulfed by DCs via both coiling and conventional phagocytosis and was delivered to membrane-bound vacuoles. The XS52 DC line expressed surface CD14 and mRNA for Toll-like receptors 2 and 4, molecules comprising important signaling components for immune cell activation by bacterial modulins. Both T. pallidum and a synthetic lipopeptide (corresponding to the 47-kDa major membrane lipoprotein) activated the XS52 DC line, as indicated by the secretion of interleukin-12 (IL-12), IL-1β, tumor necrosis factor alpha, and IL-6 and elevated surface expression of CD54. The combined data support the contention that DCs stimulated by T. pallidum and/or its proinflammatory membrane lipoproteins are involved in driving the cellular immune processes that typify syphilis.
Syphilis, a sexually transmitted disease of humans caused by spirochetal bacterium Treponema pallidum, remains a global public health problem, with an estimated 12 million new cases annually (15). A syphilis eradication program for the United States, recently announced, includes the development of a syphilis vaccine (36). However, basic immune mechanisms that might lead to protective immunity in humans (32) are largely unknown and, as such, a more thorough comprehension of syphilis immunology is essential for designing new approaches for the development of a syphilis vaccine.
The first clinical symptom of syphilis typically is the primary genital ulcer (chancre) at the local site of T. pallidum tissue invasion (30); dermal cellular infiltrates are composed chiefly of lymphocytes, macrophages, and plasma cells (30). A key aspect of understanding syphilis immunology has been the identification of the treponemal factor(s) that elicits this intense inflammatory response. Of note, T. pallidum lacks lipopolysaccharide (LPS) (14, 17), a potent proinflammatory agonist. However, treponemes contain abundant membrane lipoproteins (6, 14). There is now a large body of evidence, derived from both in vitro and in vivo studies, to support the notion that T. pallidum's membrane lipoproteins are the principal proinflammatory mediators during syphilitic infection (1, 10, 37, 38, 43, 45, 49, 59). Lipoproteins efficiently activate various immune effector cells, particularly those of the monocyte/macrophage lineage, and their immunomodulatory properties are engendered by the acyl configuration of their N termini. In this regard, in many immune cell activation studies that now have been performed synthetic analogs (lipopeptides) modeled after the N termini of bacterial lipoproteins have been used as surrogates for native lipoproteins (10, 37, 38, 40, 45, 59).
The rabbit experimental model of syphilis has provided for an analysis of the course of cellular events during syphilitic lesion development (29). Following intradermal or intratesticular inoculation of rabbits with T. pallidum, T lymphocytes appear 6 days after infection, reaching maximal numbers on days 10 to 13 (paralleling maximal T. pallidum replication) (29). Macrophages become apparent on day 10 and reach maximal numbers on day 13, coincident with a dramatic clearance of the spirochetes (29). Whereas these former studies provided seminal information about the various immune cell types present in syphilitic lesions, they focused primarily on characterizing immune cells involved at times relatively late (i.e., several to many days) in the cellular processes of syphilis, well beyond the time when key triggering events of the innate immune response occur. The actual cell type(s) that potentiates the early immune response to dermal invasion by T. pallidum thus far has not been identified.
There is now overwhelming evidence that dendritic cells (DCs) (e.g., Langerhans cells of the epidermis) are among the most potent antigen-presenting cells of the immune system and are crucial for the initiation of primary T-cell responses to foreign antigens, particularly bacterial antigens (4, 47). During foreign antigen insult, immature DCs in the peripheral tissues capture antigens (via phagocytosis and pinocytosis) and then, under microenvironmental signals (e.g., cytokines), migrate to the draining lymph nodes while undergoing maturation (4). The maturing DCs, now with a reduced capacity for phagocytosis but with an increased ability to present antigens, enter the lymph nodes where they home to T-cell-rich areas and induce an antigen-specific primary T-cell response (4). Primed T cells then migrate via the efferent pathway back to the site of antigen deposition. In this scenario, DCs act as an important sentinel between the external environment and the host's immune system, as well as serve as the cellular interface for the transition to the adaptive immune response (4, 47).
Given the contemporary understanding of DC biology (4), it is plausible that the DC is the pivotal immune effector cell that initiates the cellular inflammatory response during syphilis. Thus far, DCs in the cellular immune responses to T. pallidum have not been studied. Furthermore, at areas of inflammation in skin and mucous membranes, the principal sites of syphilitic infection, DCs are the first immune-competent cells to encounter antigens (4, 34). The present study thus was designed with two principal objectives. The first was to characterize the interaction of virulent T. pallidum with DCs, with emphasis on examining phagocytic processes, using a well-characterized immature DC line (72) as a model system. The second was to assess the impact of T. pallidum on DC maturation. Additionally, given the proinflammatory properties of T. pallidum membrane lipoproteins (1, 10, 37, 38, 43, 45, 49, 59), we also investigated whether DCs were activated by a synthetic lipopeptide corresponding to T. pallidum's major membrane lipoprotein.
MATERIALS AND METHODS
Reagents.
Salmonella enterica serovar Minnesota R5 LPS (Sigma Chemical Co., St. Louis, Mo.) was suspended in 0.1% triethylamine adjusted to pH 8.0 with 100 mM Tris-HCl. Dilutions of LPS were made in HNEB buffer (20 mM HEPES, 150 mM NaCl, 0.1 mM EDTA, 0.03% bovine serum albumin [BSA] [pH 7.4]). A synthetic hexapeptide (Cys-Gly-Ser-Ser-His-His) (negative control) and a synthetic lipohexapeptide (tripalmitoylglycerylcysteine-Gly-Ser-Ser-His-His) corresponding to the N terminus of the T. pallidum subspecies pallidum 47-kDa lipoprotein (69) were synthesized as described previously (10). For cell activation experiments, quantities of dry hexapeptide or lipopeptide were partially solubilized by extensive vortexing in sterile, endotoxin-free water (45). Synthetic lipopeptide and hexapeptide mixtures contained undetectable levels of endotoxin (≤1 pg of LPS per μg of protein) as measured by the QCL-1000 quantitative chromogenic Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.). Great care was taken throughout to minimize contamination by environmental LPS during the preparation of all buffers and reagents by using baked utensils, disposable plasticware, and pyrogen-free H2O.
Glutaraldehyde (25%), picric acid, osmium tetroxide (4%), tannic acid, and uranyl acetate were purchased from Electron Microscopy Sciences (Ft. Washington, Pa.). Spurr embedding medium (low viscosity) was obtained from Polysciences, Inc. (Warrington, Pa.).
Cultivation and isolation of treponemes.
T. pallidum subspecies pallidum (Nichols strain) was maintained and passaged by intratesticular inoculation of adult male New Zealand White rabbits as previously described (55). Treponemes were extracted from rabbit testes and harvested from testicular tissue debris by differential centrifugation (55). Treponemes were then collected by high-speed centrifugation (55), and the treponemal cell pellet was suspended in RPMI 1640 medium (BioWhittaker) that had been flushed with a reduced-oxygen atmosphere of 3% O2, 5% CO2, and the balance nitrogen. The increase in volume upon suspending the spirochetes in RPMI 1640 was assumed to represent the volume of the spirochetal cell pellet and residual rabbit testicular tissue extract (TEx). Spirochetes in suspension were then enumerated by dark-field microscopy, and the spirochete concentration was adjusted with additional RPMI 1640 medium. TEx, from which spirochetes were removed by high-speed centrifugation (55), was syringe filtered twice through a 0.2-μm-pore-size filter to remove any remaining spirochetes. This TEx preparation, which served as a negative control in cell activation experiments, vastly exceeded (at least 10-fold) the amount of TEx potentially remaining in the T. pallidum preparation (49).
Cell lines.
The XS52 cell line is an immature DC line established from the epidermis of a newborn BALB/c mouse; principal features of the XS52 cell line have been described elsewhere (72). It was maintained in XS complete medium composed of RPMI 1640 medium with 10 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, nonessential amino acids, 2-mercaptoethanol (all from Sigma Chemical Co.), 10% heat-inactivated fetal bovine serum (HIFBS) (Gibco-BRL, Gaithersburg, Md.), 0.5 ng of recombinant murine granulocyte-macrophage colony-stimulating factor (R&D Systems, Minneapolis, Minn.), and culture supernatant (10% [vol/vol]) from the NS47 stromal cell line (maintained in RPMI 1640–10% HIFBS) (57). The BALB/c-derived macrophage line RAW 264.7 (RAW) was maintained in Dulbecco modified Eagle medium (BioWhittaker) supplemented with 10% HIFBS and an antibiotic-antimycotic mixture (Sigma Chemical Co.). All cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and air.
Indirect immunofluorescence assays of T. pallidum associated with XS52 cells.
XS52 cells (2.5 × 105/well) were seeded into 24-well flat-bottom tissue culture plates (Falcon, Franklin Lakes, N.J.) containing ethanol-flamed, 12-mm-diameter round coverslips (Fisher Scientific, Pittsburgh, Pa.). The following day, 2.5 × 107 treponemes freshly extracted from rabbit testicular tissue were added to the cells and the coculture was incubated for various time periods. After incubation, cells were washed twice with cold phosphate-buffered saline (PBS) and permeabilized with 95% ethanol for 15 min at 4°C. Cells were then washed twice with PBS containing 1% BSA (PBS-BSA) and blocked with 1× CMRL medium (Gibco-BRL) plus 10% HIFBS (CMRL-HIFBS) for 30 min at room temperature. Monoclonal antibody 11E3, directed against the abundant 47-kDa lipoprotein of T. pallidum (69), was incubated with the cells for 1 h. Coverslips were then washed three times with PBS-BSA and blocked with CMRL–10% HIFBS for 30 min. A fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Jackson Laboratories, West Grove, Pa.) was diluted 1:500 in CMRL–10% HIFBS and incubated with the cells for 30 min in the dark. After incubation, coverslips were washed three times with PBS and once with distilled H2O. Coverslips were allowed to air dry and then were inverted onto slides with Fluoromount mounting medium (Fisher Scientific). Cells were viewed on an Olympus BH2 reflected-light fluorescence microscope, and images were captured digitally via a SPOT camera (Diagnostic Instruments, Inc., Sterling Heights, Mich.) supported by the Adobe PhotoShop, version 5.5, software package.
Electron microscopy.
XS52 cells (5 × 105/tube) were incubated in 1.0 ml of XS complete medium with a designated number of freshly isolated treponemes for various periods of time in 14-ml polypropylene tubes (Falcon). Cells were collected by centrifugation at 300 × g for 5 min, washed twice with cold PBS, and fixed for 1 h in a solution of 2% glutaraldehyde, 3 mM picric acid, and complete NaPi buffer (100 mM sodium phosphate, pH 7.4, containing 3 mM KCl and 3 mM MgCl2). Postfixation and dehydration of samples were performed as described by Kitchens et al. (26). Spurr resin was made according to the manufacturer's instructions. Samples were incubated with a 50% ethanol–50% Spurr mixture overnight at 4°C. This solution was then replaced with full Spurr mixture, and samples were incubated at room temperature for 4 h before being placed in a 60°C oven overnight for embedding. Thin (80-nm) sections were cut and mounted on Formvar-coated copper grids. Sections on grids were stained with uranyl acetate and lead citrate and subsequently viewed with a JEOL 1200EXII electron microscope. For quantitation of types of phagocytic processes, five grids (each containing three thin sections) were examined until a total of 50 phagocytic events were scored; the types of phagocytic processes observed were expressed as percentages of these 50 events.
RNA isolation and Northern blot analysis for tlr2 and tlr4.
Total cellular RNA from XS52 and RAW cells was isolated using TRIzol reagent (Gibco-BRL) according to the manufacturer's directions. RNA was fractionated on a formaldehyde–1% agarose gel, transferred to a nylon membrane, and then UV cross-linked. To obtain hybridization probes, DNA fragments were amplified from purified XS52 DNA by PCR with primers specific for murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′-GTCATCATCTCCGCCCCTTCTG-3′ and 5′ATGCCTGCTTCACCACCTTCTTG-3′) (56), murine Toll-like receptor 2 (TLR2) (5′-TCGACGACTGTACCCTCAATGG-3′ and 5′-CTAAGGTTTGTAGAGAAGGCCAG3′) (18), or murine TLR4 (5′-ACCCAATTGACTTCATTCAAGACC-3′ and 5′-TTGAAATGTTTAGGAACATCTTCTAG-3′) (39). DNA fragments were labeled with [α-32P]dCTP using a random-primer DNA-labeling kit (Boehringer Mannheim, Indianapolis, Ind.). Hybridization with labeled probes was carried out with the UltraHyb (Ambion, Austin, Tex.) solution according to the manufacturer's instructions. X-ray film was exposed to membranes at −70°C to detect radioactive signals.
RT-PCR analysis of XS52 and RAW cells.
Total RNA from 5 × 105 cells was isolated from XS52 and RAW cells using the RNAqueous-4PCR kit (Ambion) followed by DNase treatment. One-step reverse transcription-PCRs (RT-PCRs) were performed with the Titan RT-PCR system (Boehringer Mannheim) according to the manufacturer's recommendations. The 20-μl reaction mixtures included reaction buffer, 2 U of RNase inhibitor, 5 mM dithiothreitol, 0.5 mM deoxynucleoside triphosphates, either 0.4 μM (TLRs) or 0.1 μM (GAPDH) (each) oligonucleotide primer (see above), 50 ng of RNA template, and 1 μl of enzyme mixture. cDNA syntheses were performed by incubating all reaction mixtures at 50°C for 30 min. Amplifications were carried out in a Perkin-Elmer GeneAmp 2400 thermocycler set for the following parameters: 94°C for 2 min; 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; and a final extension step at 72°C for 10 min. For all RT-PCRs, a negative control was performed by omitting reverse transcriptase in the reaction mixtures; positive controls for PCR (not shown) were carried out using 0.5 μg of XS52 DNA as the template; the DNA had been purified with a DNA extraction kit purchased from Stratagene (La Jolla, Calif.). The expected sizes of the PCR products were 327 bp for TLR2, 352 bp for TLR4, and 442 bp for GAPDH. Amplification products were electrophoresed through a 1.5% agarose gel and visualized by ethidium bromide staining upon exposure to UV light.
Flow cytometry.
Unstimulated XS52 cells were stained with either a FITC-conjugated rat anti-mouse CD14 antibody (clone rmC5-3; BD Pharmingen, San Diego, Calif.) or an isotype-matched negative control antibody (clone R3-34; BD Pharmingen). For analysis of CD54 (ICAM-1) expression after cell stimulation, XS52 cells were incubated with various reagents for 24 h and then stained with either a FITC-conjugated hamster anti-mouse CD54 monoclonal antibody (clone 3E2; BD Pharmingen) or an appropriate isotype-matched negative control (clone A19-3; BD Pharmingen). Briefly, 2.5 × 105 cells were blocked with either 3% normal rat serum (CD14 analysis) or 25% normal mouse serum (CD54 analysis) in fluorescence-activated cell sorter (FACS) buffer (7 mM NaN3–5 mM EDTA–1% BSA in PBS) for 15 min on ice. Cells were stained with the appropriate antibody for 30 min on ice in the dark and then washed thoroughly with FACS buffer before suspension in PBS containing 1% paraformaldehyde (Electron Microscopy Sciences). Cells were stored at 4°C in the dark until ready for analysis. Cell-associated fluorescence was analyzed by flow cytometry using a Becton Dickinson FACScan instrument; analyses were gated on live cells by evaluating forward and side scatter. Data were analyzed using the CellQuest software program.
Capture ELISAs for cytokines.
Enzyme-linked immunosorbent assay (ELISA) kits for murine interleukin-6 (IL-6), IL-10, and tumor necrosis factor alpha (TNF-α) were purchased from BD Pharmingen. ELISA kits for murine IL-1β and IL-12p40 were obtained from R&D Systems. All capture ELISAs were performed according to the manufacturer's instructions. After XS52 cells were stimulated with various treatments, cells were removed by centrifugation at 300 × g for 5 min (4°C). In cultures containing viable T. pallidum, an additional centrifugation at 12,000 × g for 10 min was performed to remove bacteria. The supernatants were transferred to new tubes and stored at −70°C until ready for analysis. Supernatants to be assayed by ELISA were appropriately diluted and assayed in duplicate. Readings were taken at 450 nm (corrected at 570 nm) on a Thermomax microplate reader (Molecular Devices, Sunnyvale, Calif.) using the SOFTmax, version 2.3, software package. Statistics were generated using the InStat program (GraphPad Software, San Diego, Calif.).
RESULTS
XS52 cells express CD14, TLR2, and TLR4.
Activation of macrophages by a variety of bacterial modulins is mediated via CD14, a glycosylphosphatidylinositol-linked protein, on the surfaces of select immune cells (25). Recently, it was documented that cellular activation by spirochetes or their membrane lipoproteins (or synthetic lipoprotein analogs) also occurs via signaling through a CD14-dependent pathway (16, 58, 59, 71). CD14 has been shown to be expressed constitutively on XS52 cells (33); upon cytokine-induced maturation of XS52 cells, CD14 is downregulated (73). To verify that our starting cell population was in the CD14-positive immature state, we first assayed for the expression of CD14 on the XS52 cells by flow cytometry. Virtually all XS52 cells expressed CD14 (data not shown), as has been previously reported (73).
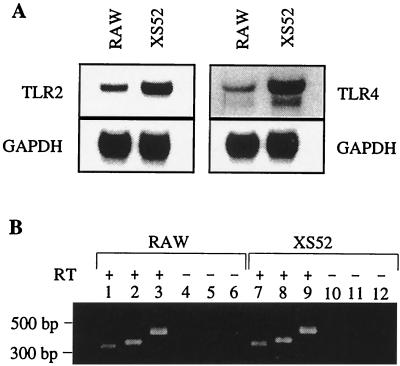
Inasmuch as CD14 is incapable of transducing a signal across the cell membrane, it must employ other accessory molecules, presumably transmembrane proteins, to accomplish this. The TLRs have emerged as the long-sought-after signal-transducing machinery involved in the response of macrophages to various microbial modulins (21). For example, TLR4 has been strongly implicated in the immune response to LPS (20, 39). TLR2 seems to be involved in macrophage responses to bacterial lipoproteins (3, 5, 19). With the macrophage-derived RAW cell line as a positive control, Northern blot analysis was performed with probes specific for either murine TLR4 or TLR2. mRNA for both TLR4 and TLR2 was readily detected from both RAW and XS52 cell lines (Fig. 1A). Membranes reprobed for GAPDH mRNA confirmed equal loading of the samples. On the basis of Northern blotting, the relative amount of mRNA for TLR4 in XS52 cells seemed higher than that for TLR4 in RAW cells, but other experimental factors, such as the specific activity of the probes or stringency of binding, may have accounted for this. The additional faster-migrating band(s) observed in blots of TLR4 mRNA from either XS52 cells or RAW cells has been observed in other studies of TLR4 (24, 39, 41). A slower-migrating band at about 9.5 kb also was detected in the TLR4 blots (not shown), as in other studies (24, 39, 41). As further confirmation of mRNA for these genes, RT-PCR was performed on DNase-treated RNA samples from both cell lines. RT-PCR yielded the appropriately sized amplification products for TLR4 (352 bp) (Fig. 1B, lanes 2 and 8) and TLR2 (327 bp) (lanes 1 and 7). No products were detected when reverse transcriptase was absent from the reactions (Fig. 1B, lanes 4 to 6 and 10 to 12). RT-PCRs for GAPDH were performed as loading controls (Fig. 1B, lanes 3 and 9).
FIG. 1.
XS52 cells express mRNA for tlr2 and tlr4. (A) Total RNA was extracted from RAW cells and XS52 cells and analyzed by Northern blotting with radioactive probes specific for either tlr2 (TLR2) or tlr4 (TLR4). Membranes were rehybridized with a radioactive probe for GAPDH to ensure equal loading of RNA in each gel lane. X-ray film was exposed to membranes for various periods of time to detect radioactive signals (tlr2, 2 h; tlr4, 2 days; GAPDH, 1 h). (B) Detection of tlr2 and tlr4 mRNA in RAW cells and XS52 cells by RT-PCR. DNase-treated RNA (50 ng) was used as the template in each reaction. PCR products in lanes 1, 4, 7, and 10 were amplified with TLR2-specific primers. PCR products in lanes 2, 5, 8, and 11 were amplified using TLR4-specific primers. Products in lanes 3, 6, 9, and 12 were amplified with GAPDH-specific primers. + and −, reaction mixtures containing and lacking reverse transcriptase (RT), respectively. DNA fragment size markers are indicated on the left.
T. pallidum interacts with DCs in a time-dependent manner.
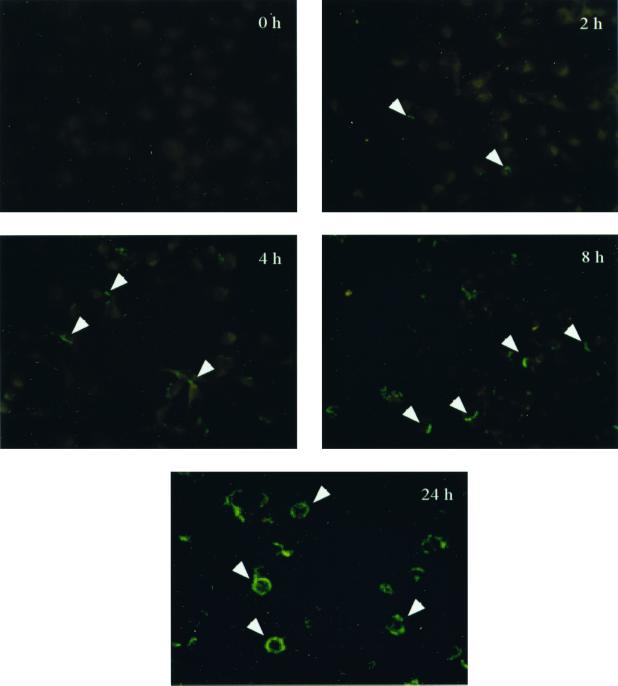
Having determined that XS52 cells possessed cell surface signaling molecules (i.e., CD14 and TLR2) ostensibly required for activation by T. pallidum or its membrane lipoproteins, we next sought to discern to what extent T. pallidum interacted with DCs; such interactions would be requisite for cell activation. To assess this, XS52 cells were exposed to T. pallidum for increasing periods of time. Indirect immunofluorescence utilizing a monoclonal antibody against the abundant 47-kDa membrane lipoprotein of T. pallidum was used to evaluate spirochete-DC interactions. After only 2 h of incubation, individual treponemes attached to DCs could be visualized (Fig. 2). As time progressed, increasing fluorescence was associated with the cells; after 24 h, a ring of intense fluorescence was associated with the perimeters of the cells (Fig. 2).
FIG. 2.
Time-dependent association of T. pallidum with XS52 cells. T. pallidum cells were incubated with XS52 cells at a ratio of 100 spirochetes per DC for the indicated times. Ethanol-fixed samples were probed with a monoclonal antibody specific for the abundant 47-kDa lipoprotein of T. pallidum, followed by staining with a FITC-conjugated goat anti-mouse secondary antibody. Arrowheads, spirochetes or spirochetal antigens associated with XS52 DCs. Results are representative of three independent experiments. Magnification, ×400.
Coiling phagocytosis is the predominant pathway for the uptake of T. pallidum by DCs.
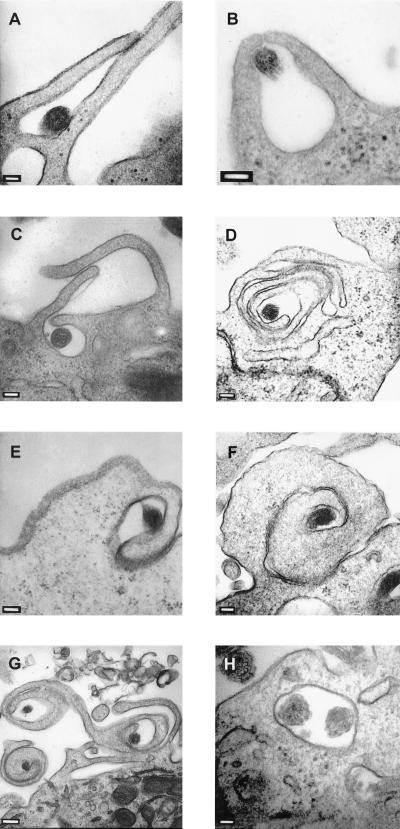
Whereas immunofluorescence assays showed a time-dependent association of treponemes with DCs, they did not reveal whether spirochetes remained outside of the cells or were internalized. To examine possible phagocytic processes, T. pallidum was incubated with XS52 cells for 24 h and interactions of the spirochetes with DCs were assessed by using thin-section transmission electron microscopy (TEM). T. pallidum was readily identified by TEM because of its distinctive dual-membrane structure, endoflagella within the periplasmic space, and the tendency of treponemes to appear more electron dense than eukaryotic cells (27). With a T. pallidum/DC ratio similar to that for immunofluorescence assays (Fig. 2; 100 spirochetes per DC), neither T. pallidum nor phagocytic processes were readily detectable after 24 h of incubation (not shown). To compensate for the lower sensitivity of electron microscopy, spirochete/DC ratios were increased to 500:1 or 1,000:1. Additionally, these increased spirochete/cell ratios were selected on the basis of many other studies showing that only a very small percentage (1 to 3%) of treponemes (within a given T. pallidum population) are capable of interacting with eukaryotic host cells (12, 13, 49, 60, 61, 70). Under these conditions, three distinct types of phagocytic processes could be distinguished. First, conventional phagocytosis, in which two microbe-apposed pseudopods of the cell surrounded the organism and then fused, was observed (Fig. 3A and B); this process accounted for approximately 26% of phagocytic events. The second uptake mechanism involved two or three alternating contradirectional pseudopods (i.e., overlapping pseudopods) (Fig. 3C), which appeared to capture the spirochete less frequently (18%). Upon further inspection, it was observed that the additional overlapping pseudopods appeared to fuse with the cell surface (Fig. 3D). The third type, rotating coiling phagocytosis (Fig. 3E to G), was the predominant mechanism for uptake, accounting for 56% of phagocytic events. Usually, a single pseudopod wrapped around the treponeme (Fig. 3E) and continued coiling to create the characteristic whorl of coiling phagocytosis (Fig. 3F). Occasionally, multiple whorls could be seen within a single TEM field (Fig. 3G); an additional contrarotating pseudopod was sometimes associated with the primary coiling pseudopod (Fig. 3G, right coil). Finally, internalized spirochetes appeared to be within membrane-bound vacuoles (Fig. 3H), although on rare occasions a spirochete which appeared to exist freely in the cytoplasm could be found (not shown).
FIG. 3.
Phagocytosis of T. pallidum by XS52 cells. T. pallidum cells were incubated with XS52 cells at a ratio of 1,000 spirochetes/cell for 24 h. Samples were prepared for thin-section (80 nm) TEM and negatively stained with lead citrate and uranyl acetate. (A and B) Two examples of conventional phagocytosis. (C) Overlapping coiling phagocytosis. (D) Overlapping coiling phagocytosis in the process of internalizing T. pallidum. (E) The initial pseudopod of rotating coiling phagocytosis surrounding a spirochete. (F) A pseudopod whorl of rotating coiling phagocytosis. (G) Three pseudopod whorls of rotating coiling phagocytosis. (H) Two organisms internalized in what appears to be a membrane-bound vacuole. Bars: 100 (A to F) 200 (G), and 50 nm (H).
Additional studies were conducted to acquire a more complete picture of the stages and time course involved in these phagocytic processes. Freshly isolated spirochetes were added to XS52 cell cultures at spirochete/DC ratios of either 500:1 or 1,000:1 for various periods of time (1, 2, 4, 8, and 24 h). At either cell ratio, after 1 h of incubation, spirochetes were neither associated with nor internalized within XS52 cells. After 2 h, a rare spirochete could be visualized within a DC, even though surface associations still were not prevalent (not shown). In cultures incubated for 4 or 8 h, although still not abundant, spirochetes could be found at the surfaces of DCs as well as internalized (not shown). After 24 h, T. pallidum was readily detected at the DC surface (Fig. 3) and large numbers could be detected within DC vacuoles (not shown).
DCs secrete proinflammatory cytokines in response to stimulation by either T. pallidum or a synthetic analog of lipoprotein.
The underlying hypothesis for this study was that immature DCs are activated upon interaction with T. pallidum or its membrane lipoproteins. To assess this, DCs were exposed to either T. pallidum or a synthetic lipopeptide modeled after the N terminus of the 47-kDa lipoprotein. Given that N-terminal acylation is critical for the proinflammatory properties of lipoproteins and lipopeptides, a synthetic hexapeptide (lacking acylation) was included as a negative control in all experiments.
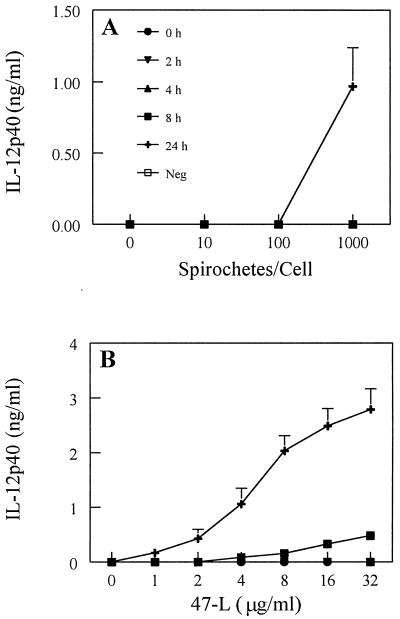
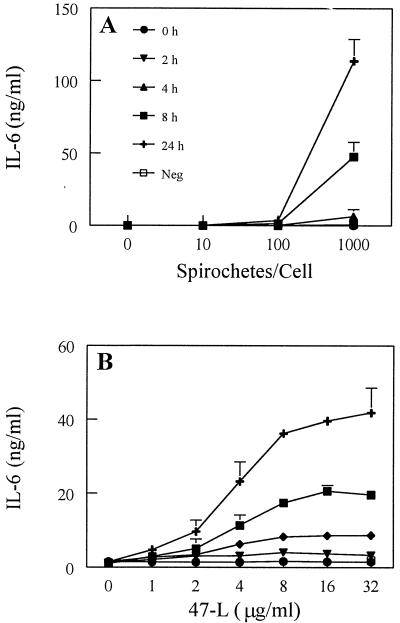
DC activation after exposure to T. pallidum or synthetic lipopeptide was assessed by monitoring the secretion of selected cytokines. It has been established that freshly isolated Langerhans cells tend to upregulate their expression of IL-12 during maturation (23). IL-12 is critical for influencing the transition from the innate to the adaptive type 1 (Th1) T-cell immune response (64). In time- and dose-response experiments, secretion of IL-12p40 was detected when treponemes were incubated with XS52 cells for 24 h at a ratio of 1,000 spirochetes per DC (Fig. 4A). Lower ratios of spirochetes or shorter incubation times failed to elicit IL-12p40 production. An important control for these experiments was rabbit testicular TEx, from which spirochetes were removed. The purpose of this control was to ensure that rabbit cytokines from inflamed testicular tissue, a small portion of which remained in the T. pallidum preparation, were not contributing to the activation of DCs. In general, volumes of 15 to 25 μl of TEx were tested; work from previous cell activation studies with T. pallidum isolated in the same manner (49) have shown comparable treponemal preparations to contain only about 2 to 3 μl of TEx. As such, our experiments were strongly biased towards testing an amount of TEx vastly exceeding (approximately 10-fold) the amount of TEx typically present in a T. pallidum preparation. When XS52 cells were exposed to these high levels of TEx, IL-12p40 was not induced (Fig. 4A). Incubation of the synthetic lipopeptide (47-L) induced the secretion of IL-12p40 in both a time- and dose-dependent fashion (Fig. 4B), with stimulation first observed after 8 h of incubation. The greatest amount of IL-12p40 secretion in response to lipopeptide stimulation occurred at the highest dose (32 μg/ml) and latest (24-h) time point (Fig. 4B).
FIG. 4.
IL-12p40 production by immature DCs stimulated with T. pallidum or the synthetic lipopeptide. XS52 cells were incubated with increasing amounts of either virulent treponemes (A) or a synthetic lipopeptide modeled after the 47-kDa membrane lipoprotein of T. pallidum (47-L) (B). Times examined are denoted in panel A. Cell supernatants were then assayed for cytokine production by capture ELISA. Neg, negative control (either TEx [A] or the 47 hexapeptide [B]) equivalent to the highest dose of stimulus, assayed after 24 h of incubation. Shown are the means ± standard errors of duplicate determinations from two (A) and three (B) independent experiments.
Other proinflammatory cytokines, such as IL-1β and TNF-α, are critical for Langerhans cell migration from the skin to the draining lymph nodes (9). Both of these cytokines were expressed and secreted by XS52 cells upon stimulation with either T. pallidum or the synthetic lipopeptide, but not in response to TEx or the control hexapeptide (Fig. 5 and 6). For IL-1β, when the highest ratio of spirochetes was used, increasing amounts of cytokine were produced as time progressed (Fig. 5A). The IL-1β response to lipopeptide was not marked for up to 8 h of stimulation, but at 24 h the response clearly was dose dependent (Fig. 5B). For TNF-α secretion, intact spirochetes activated cells in a time-dependent manner. The TNF-α response to lipopeptide stimulation was both dose and time dependent (Fig. 6B); a detectable response was observed with as little as 4 μg of lipopeptide/ml and as soon as 2 h after stimulation.
FIG. 5.
IL-1β production by immature DCs stimulated with T. pallidum or the synthetic lipopeptide. XS52 cells were incubated with increasing amounts of either T. pallidum (A) or synthetic lipopeptide (47-L) (B). Times examined are denoted in panel A. Cell supernatants were then assayed for cytokine production by capture ELISA. Neg, negative control (either TEx [A] or the 47 hexapeptide [B]) equivalent to the highest dose of stimulus, assayed after 24 h of incubation. Shown are the means ± standard errors of duplicate determinations for two (A) and three (B) independent experiments.
FIG. 6.
TNF-α production by immature DCs stimulated with T. pallidum or the synthetic lipopeptide. XS52 cells were incubated with increasing amounts of either T. pallidum (A) or synthetic lipopeptide (47-L) (B). Times examined are denoted in panel A. Cell supernatants were then assayed for cytokine production by capture ELISA. Neg, negative control (either TEx [A] or the 47 hexapeptide [B]) equivalent to the highest dose of stimulus, assayed after 24 h of incubation. Shown are the means ± standard errors of duplicate determinations for two (A) and three (B) independent experiments.
IL-6, which includes among its actions costimulatory activity for T lymphocytes (63), is upregulated in both Langerhans cells upon sensitization with oxazolone (8) and monocyte-derived DCs upon stimulation with LPS (67). In the present study, we found that IL-6 also was upregulated in XS52 cells upon exposure to either T. pallidum or synthetic lipopeptide (Fig. 7). Maximal production of IL-6 occurred after 24 h of incubation of XS52 cells with T. pallidum (Fig. 7A). The lipopeptide induced IL-6 production in both a time- and dose-dependent manner (Fig. 7B). LPS, included as a positive control in all experiments, also induced the production of IL-6 as well as TNF-α, IL-1β, and IL-12p40 (data not shown).
FIG. 7.
IL-6 production by immature DCs stimulated with T. pallidum or the synthetic lipopeptide. XS52 cells were incubated with increasing amounts of either T. pallidum (A) or synthetic lipopeptide (47-L) (B). Times examined are denoted in panel A. Cell supernatants were then assayed for cytokine production by capture ELISA. Neg, negative control (either TEx [A] or the 47 hexapeptide [B]) equivalent to the highest dose of stimulus, assayed after 24 h of incubation. Shown are the means ± standard errors of duplicate determinations for two (A) and three (B) independent experiments.
In all experiments, TEx or the synthetic hexapeptide (nonacylated form) did not induce notable secretion of any of the cytokines tested. Finally, we also tested for the secretion of the anti-inflammatory cytokine IL-10 by XS52 cells stimulated with either T. pallidum or synthetic lipopeptide; IL-10 was not detected (limit of detection was 31.3 pg/ml) in response to either agonist (data not shown). The failure to detect IL-10 production by DCs stimulated with the lipopeptide was consistent with results from a recent study employing human DCs (62).
T. pallidum or synthetic lipopeptide increases the expression of CD54 on DCs.
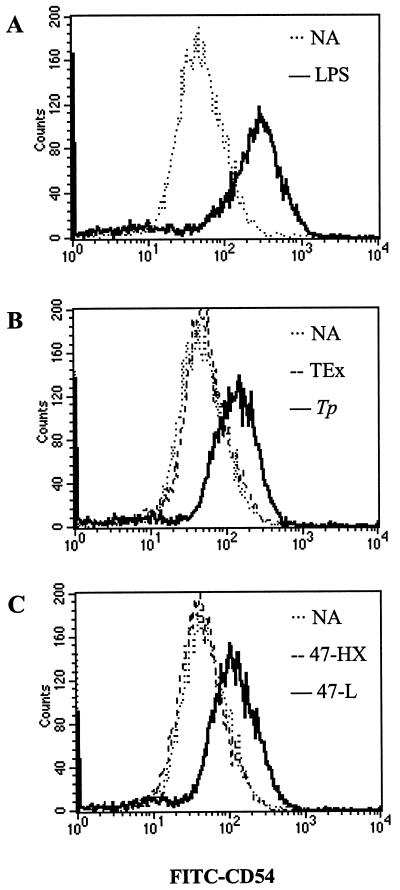
As indicators of DC activation, the above cytokine data implied that T. pallidum and synthetic lipopeptide induced the maturation of XS52 cells. Another indicator of DC maturation is the expression of costimulation molecules important for supporting T cell-DC interactions and subsequent lymphocyte activation that occurs in the regional lymph node. CD54 is a cell surface leukocyte adhesion molecule whose upregulation is associated with DC maturation (66). Stimulation of XS52 cells with LPS (positive control) resulted in a clear increase of CD54 on the cell surface, as analyzed by flow cytometry (Fig. 8A). T. pallidum, but not the TEx control, also induced the upregulation of CD54 (in comparison with an unstimulated population of cells) (Fig. 8B). Accordingly, incubation of DCs with the synthetic lipopeptide also resulted in an augmentation of surface CD54 expression (Fig. 8C), whereas the control hexapeptide had no effect (Fig. 8C).
FIG. 8.
Upregulation of CD54 on immature DCs stimulated with either T. pallidum or the synthetic lipopeptide. XS52 cells were stimulated for 24 h, stained with a FITC-conjugated anti-CD54 monoclonal antibody, and then analyzed by flow cytometry. (A) LPS (40 ng/ml) was included as a positive control. (B) T. pallidum (Tp) (1,000 spirochetes/cell), but not TEx, induced CD54. (C) The synthetic lipopeptide (47-L) (32 μg/ml), but not the 47 hexapeptide control (47-HX), induced CD54. NA, no application (unstimulated cells). Results are representative of two independent experiments.
DISCUSSION
The results of this study provide compelling evidence that (i) T. pallidum associates with DCs and is phagocytosed and (ii) immature DCs are activated during this process. This is the first study to examine and characterize the interaction of T. pallidum with DCs and provide evidence that treponemal lipoproteins can contribute to, and may largely be responsible for, DC activation. We chose to employ well-characterized immature murine DC line XS52 (72) for our studies given its skin-derived origin, its likeness to Langerhans cells, and the facts that experimental variability could be minimized and that murine cell lines of the monocyte/macrophage lineage are sensitive to stimulation by T. pallidum and/or its membrane lipoproteins (1, 10, 43, 45, 59).
T. pallidum activates human monocytic cells via the involvement of CD14 (58). Membrane-bound CD14, a glycosylphosphatidylinositol-linked protein attached to the outer leaflet of the macrophage membrane, has been shown to recognize diverse microbial modulins (25), including spirochetal lipoproteins (16, 59). CD14 was readily detected on the surfaces of XS52 cells. Additionally, it was of interest to discern whether another set of receptors, TLRs, were expressed by XS52 cells. TLRs are a family of receptors acting downstream of CD14 that have only recently been recognized as the signal-transducing “missing links” on macrophages responding to LPS or other microbial modulins (21). Like CD14, TLRs contain multiple leucine-rich repeats in their extracellular domains; however, unlike CD14, TLRs span the membrane and have a cytoplasmic domain homologous to that of the IL-1 receptor (IL-1R), which is capable of inducing activation of transcription factor NF-κB (35). Substantial evidence implicates TLR4 in the innate response to LPS (20, 39, 41). Of particular relevance to our study, TLR2 has been implicated in the cellular response to bacterial lipoprotein and lipopeptide, including the 47-kDa lipoprotein-based lipopeptide used herein (3, 5, 19, 28, 62). Our analyses revealed that both the tlr4 and tlr2 genes are expressed by XS52 cells; thus, XS52 cells appear to have the signaling module(s) crucial for initiating the innate immune response to lipoproteins, LPS, and likely other bacterial immunomodulatory products.
Electron microscopy revealed that two types of coiling phagocytosis, rotating (i.e., traditional) and overlapping, predominated (74% of phagocytic events) among the pattern of phagocytic processes involved in the uptake of T. pallidum by DCs. In general, coiling phagocytosis, which has been observed for numerous microbes, occurs when a unilateral pseudopod extends from the surface of the eukaryotic cell to continuously wrap around the microbe (50); the resulting pseudopod whorl eventually envelops the organism. Overlapping coiling phagocytosis, also known as overshooting phagocytosis, recently was described and can be utilized by human monocytes, murine DCs, and murine macrophages (53). Overlapping coiling phagocytosis is characterized by stacks of pseudopods; presumably, this is the result of a perturbation of a conventional phagocytic event in that the pseudopods slide over each other rather than fusing (54). It has been suggested that the shape of the microbe may influence the type of coiling mechanism employed, with rotating coiling associated with elongated organisms and overlapping coiling associated with spherical organisms (54). Indeed, it was found that uptake of the elongated, spiral treponemes did occur more often by a rotating mechanism (56% of phagocytic events) than by an overlapping mechanism (18% of phagocytic events). Conventional phagocytosis of T. pallidum also was evident. This dual pattern of coiling and conventional phagocytosis was consistent with reports of phagocytic processes by monocytes/macrophages and DCs for Borrelia burgdorferi (11, 51, 52), suggesting that B. burgdorferi and T. pallidum may have common pathways for uptake by immune cells. Maximal association of T. pallidum with XS52 cells and its subsequent internalization were not observed until 24 h of coincubation, suggesting that adherence and phagocytic mechanisms acted relatively slowly. This slow association and uptake by professional phagocytes have been noted previously in other model systems of T. pallidum phagocytosis (2), suggesting that slow uptake of T. pallidum is not a peculiarity of XS52 cells. Additionally, XS52 cells have been shown previously to have phagocytic properties analogous to those of DCs freshly isolated from skin (46).
The intracellular fate of T. pallidum antigens within professional phagocytes has been largely unknown. Previous work indicates that, upon uptake by rabbit peritoneal macrophages, multiple T. pallidum organisms are found within individual membrane-bound vacuoles (31). In the present study, T. pallidum again was found almost exclusively within membrane-bound vacuoles of DCs. Although individual spirochetes were observed within these vacuoles, it was not uncommon for multiple spirochetes to be within a single vacuole, consistent with the fact that phagocytic surface events were found occasionally to engulf more than one treponeme simultaneously (not shown). Alternatively, it is possible that once spirochetes were internalized, the fusion of multiple vacuoles occurred.
TNF-α, IL-6, IL-1β, and IL-12 were used as markers of DC activation and maturation. In previous studies, T. pallidum and synthetic lipopetides have been shown to induce these same cytokines in both human- and mouse-derived macrophages (45). Importantly, the lipopeptide was found to stimulate DCs in a pattern qualitatively similar to that observed for T. pallidum, again consistent with the contention that the membrane lipoproteins are responsible for the proinflammatory properties of T. pallidum. Although we employed a synthetic lipopeptide modeled after only one highly abundant lipoprotein, the combined proinflammatory activity of T. pallidum actually would be the aggregate of those of its numerous lipoproteins (6, 14). This does not preclude the possibility that other spirochetal components, such as peptidoglycan (48) and glycolipids (45), may contribute to the overall inflammatory response during syphilis.
Upon activation, DCs upregulate a number of costimulatory molecules that interact with cognate receptors on naive T cells within the microenvironment of the lymph node. In particular, the interaction between CD54 on DCs and CD11a/CD18 on T cells not only provides a significant costimulatory signal for the T cell (66) but also allows clustering to occur. As a result, it is estimated that 100 to 3,000 T cells can be activated by a single DC (4). Consistent with our cytokine data indicating maturation of XS52 cells, we observed an upregulation of CD54 on DCs after stimulation with T. pallidum or lipopeptide. Interactions such as those which occur between CD54 and CD11a/CD18 ultimately are pivotal for the transition from the innate to the adaptive immune response.
An extensive body of evidence supports the notion that the membrane lipoproteins of T. pallidum are major proinflammatory mediators of syphilis (1, 10, 37, 38, 43, 45, 49, 59). Studies of the immunomodulatory activities of lipoproteins are facilitated by using synthetic lipopeptides modeled after the acylated, biologically active N termini of the lipoproteins (10, 37, 38, 45, 59). The use of synthetic lipopeptides as surrogates for native lipoproteins is advantageous for several reasons. First, the immunostimulatory properties of the synthetic lipopeptides are qualitatively similar to those of their native counterparts (10, 37, 38, 45, 59). Second, it is difficult to obtain ample quantities of native lipoprotein from noncultivatable T. pallidum. Third, even though T. pallidum does not synthesize LPS (14, 17), contamination with environmental LPS is a significant problem associated with bacterial lipoprotein purification; lipopeptides are synthesized essentially under endotoxin-free conditions (10). Our results showed that a synthetic lipopeptide indeed was an efficient activator of DCs, consistent with our underlying tenet that treponemal lipoproteins are key inflammatory agonists of syphilis and that DCs likely engage and respond to T. pallidum and its lipoproteins.
Finally, the unique membrane architecture of T. pallidum, which impacts host immunological processes, warrants discussion. T. pallidum has been referred to as a “stealth pathogen” (42) due to its remarkable ability to evade the immune system, resulting in chronic disease. T. pallidum has an inordinate paucity of immune system targets on the surface of its outer membrane (42, 44, 68); its proinflammatory lipoproteins are largely, if not entirely, sequestered below the cell surface (7, 22) and thus are relatively inaccessible to antibodies. It has been proposed that immune cell activation by T. pallidum is a consequence of its uptake and degradation (45, 58). Such actions would release lipoproteins from subsurface compartments and allow interaction of the lipoproteins with receptors on the immune cell. A recent report by Underhill et al. (65) sheds additional light on this potential scenario; upon bacterial stimulation, TLRs were recruited to phagosomes of macrophages and were responsible for subsequent activation of the cells. Therefore, it is plausible that DCs engulf intact T. pallidum into phagosomes, lipoproteins are liberated, and cell activation occurs via the participation of one or more TLRs at the level of the phagosome. Accordingly, we noted in our studies that maximal phagocytosis of treponemes occurred after 24 h of incubation, the time coinciding with maximal secretion of cytokines by DCs upon stimulation with T. pallidum or lipopeptide. This does not preclude, however, the likelihood that normal bacterial cell death, resulting in the release of treponemal lipoproteins, also may contribute to immune cell activation in the skin.
Inasmuch as DC maturation is vital for the transition to the adaptive immune response to infectious agents, our data support the contention that DCs initiate and drive the cellular immune processes of syphilis. The combined results of this study thus provide rationale for investigating further the consequences of T. pallidum activation of DCs in vivo (e.g., human organ cultures). Continuing studies will provide added new insights into the cellular immune processes of syphilis, enhance our understanding of innate immunopathogenesis processes, and elucidate subsequent adaptive mechanisms potentially relevant to syphilis vaccine development.
ACKNOWLEDGMENTS
We thank Kayla Hagman and Martin Goldberg for assistance with treponemes, Dennis Bellotto for electron microscopy thin sectioning, Dale Edelbaum and Richard Kitchens for technical advice, Robert Modlin and Justin Radolf for supportive discussions, and Petra Cravens for critical review of the manuscript.
This work was supported by Public Health Service grant AI-16692 from the Sexually Transmitted Diseases Branch of the National Institute of Allergy and Infectious Diseases, NIH, by an NIH-supported (P30-AR-41940) Pilot and Feasibility Grant from the U.T. Southwestern Skin Disease Research Center, and by grant 010019-0096-1999 (Advanced Technology Program) from the Texas Higher Education Coordinating Board.
REFERENCES
- 1.Akins D R, Purcell B K, Mitra M, Norgard M V, Radolf J D. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993;61:1202–1210. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alder J D, Friess L, Tengowski M, Schell R F. Phagocytosis of opsonized Treponema pallidum subsp. pallidum proceeds slowly. Infect Immun. 1990;58:1167–1173. doi: 10.1128/iai.58.5.1167-1173.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aliprantis A O, Yang R-B, Mark M R, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Brightbill H D, Libraty D H, Krutzik S R, Yang R-B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain N R, Brandt M E, Erwin A L, Radolf J D, Norgard M V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989;57:2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox D L, Akins D R, Porcella S F, Norgard M V, Radolf J D. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 8.Cumberbatch M, Dearman R J, Kimber I. Constitutive and inducible expression of interleukin-6 by Langerhans cells and lymph node dendritic cells. Immunology. 1996;87:513–518. doi: 10.1046/j.1365-2567.1996.504577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cumberbatch M, Dearman R J, Kimber I. Langerhans cells require signals from both tumour necrosis factor-α and interleukin-1β for migration. Immunology. 1997;92:388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeOgny L, Pramanik B C, Arndt L L, Jones J D, Rush J, Slaughter C A, Radolf J D, Norgard M V. Solid-phase synthesis of biologically active lipopeptides as analogs for spirochetal lipoproteins. Pept Res. 1994;7:91–97. [PubMed] [Google Scholar]
- 11.Filgueira L, Nestle F O, Rittig M, Joller J I, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 12.Fitzgerald T J. Attachment of treponemes to cell surfaces. In: Schell R F, Musher D M, editors. Pathogenesis and immunology of treponemal infections. New York, N.Y: Marcel Dekker, Inc.; 1983. pp. 195–228. [Google Scholar]
- 13.Fitzgerald T J, Miller J N, Sykes J A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975;11:1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G G, Dodson R, Gwinn M, Hickey E K, Clayton R, Ketchum K A, Sodergren E, Hardham J M, McLeod M P, Salzberg S, Peterson J, Khalak H, Richardson D, Howell J K, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton M D, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith H O, Venter J C. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 15.Gerbase A C, Rowley J T, Heymann D H, Berkley S F, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74:S12–S16. [PubMed] [Google Scholar]
- 16.Giambartolomei G H, Dennis V A, Lasater B L, Philipp M T. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy P H, Levin J. Lack of endotoxin in Borrelia hispanica and Treponema pallidum. Proc Soc Exp Biol Med. 1983;174:47–52. doi: 10.3181/00379727-174-41702. [DOI] [PubMed] [Google Scholar]
- 18.Heine H, Kirschning C J, Lien E, Monks B G, Rothe M, Golenbock D T. Cells that carry a null allele for Toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 19.Hirschfeld M, Kirschning C J, Schwander R, Wesche H, Weis J H, Wooten R M, Weis J J. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 20.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR-4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 21.Janeway C A, Medzhitov R. Lipoproteins take their Toll on the host. Curr Biol. 1999;9:R879–R882. doi: 10.1016/s0960-9822(00)80073-1. [DOI] [PubMed] [Google Scholar]
- 22.Jones J D, Bourell K W, Norgard M V, Radolf J D. Membrane topology of Borrelia burgdorferi and Treponema pallidum lipoproteins. Infect Immun. 1995;63:2424–2434. doi: 10.1128/iai.63.7.2424-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang K, Kubin M, Cooper K D, Lessin S R, Trinchieri G, Rook A H. IL-12 synthesis by human Langerhans cells. J Immunol. 1996;156:1402–1407. [PubMed] [Google Scholar]
- 24.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 25.Kitchens R L. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. In: Jack R S, editor. CD14 in the inflammatory response. New York, N.Y: Karger; 2000. pp. 61–77. [DOI] [PubMed] [Google Scholar]
- 26.Kitchens R L, Wang P-Y, Munford R S. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J Immunol. 1998;161:5534–5545. [PubMed] [Google Scholar]
- 27.Konishi H, Yoshii Z, Cox D L. Electron microscopy of Treponema pallidum (Nichols) cultivated in tissue cultures of Sf1Ep cells. Infect Immun. 1986;53:32–37. doi: 10.1128/iai.53.1.32-37.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 29.Lukehart S A, Baker-Zander S A, Lloyd R M C, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980;124:461–467. [PubMed] [Google Scholar]
- 30.Lukehart S A, Holmes K K. Syphilis. In: Isselbacher K J, Braunwald E, Wilson J, Martin J B, Fauci A S, Kasper D L, editors. Harrison's principles of internal medicine. New York, N.Y: McGraw Hill, Inc.; 1994. pp. 726–737. [Google Scholar]
- 31.Lukehart S A, Miller J N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978;121:2014–2024. [PubMed] [Google Scholar]
- 32.Magnuson H J, Thomas E W, Olansky S, Kaplan B I, de Mello L, Cutler J C. Inoculation syphilis in human volunteers. Medicine. 1956;35:33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Mahnke K, Becher E, Ricciardi-Castagnoli P, Luger T A, Schwarz T, Grabbe S. CD14 is expressed by subsets of murine dendritic cells and upregulated by lipopolysaccharide. In: Ricciardi-Castagnoli P, editor. Dendritic cells in fundamental and clinical immunology. New York, N.Y: Plenum Press; 1997. pp. 145–159. [DOI] [PubMed] [Google Scholar]
- 34.McWilliams A S, Nelson D, Thomas J A, Holt P G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medzhitov R, Preston-Hurlburt P, Janeway C A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 36.National Center for HIV, STD, and TB Prevention. The national plan to eliminate syphilis from the United States. Atlanta, Ga: Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 37.Norgard M V, Arndt L L, Akins D R, Curetty L L, Harrich D A, Radolf J D. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-κB. Infect Immun. 1996;64:3845–3852. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norgard M V, Riley B S, Richardson J A, Radolf J D. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect Immun. 1995;63:1507–1515. doi: 10.1128/iai.63.4.1507-1515.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poltorak A, He X, Smirnova I, Liu M-Y, van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 40.Prass W, Ringsdorf H, Bessler W, Wiesmuller K-H, Jung G. Lipopeptides of the N-terminus of Escherichia coli lipoprotein: synthesis, mitogenicity and properties in monolayer experiments. Biochim Biophys Acta. 1987;900:116–128. doi: 10.1016/0005-2736(87)90283-5. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radolf J D. Role of outer membrane architecture in immune evasion by Treponema pallidum and Borrelia burgdorferi. Trends Microbiol. 1994;2:307–311. doi: 10.1016/0966-842x(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 43.Radolf J D, Norgard M V, Brandt M E, Isaacs R D, Thompson P A, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis: analysis using a CAT reporter construct. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 44.Radolf J D, Norgard M V, Schulz W W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci USA. 1989;86:2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 46.Reis e Sousa C, Stahl P D, Austyn J M. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rescigno M, Rittig M, Citterio S, Matyszak M K, Foti M, Granucci F, Martino M, Fascio U, Rovere P, Ricciardi-Castagnoli P. Interaction of dendritic cells with bacteria. In: Lotze M T, Thomson A W, editors. Dendritic cells. San Diego, Calif: Academic Press; 1999. pp. 403–419. [Google Scholar]
- 48.Rietschel E T, Schletter J, Weidemann B, El-Samalouti V, Mattern T, Zahringer U, Seydel U, Brade H, Flad H-D, Kusumoto S, Gupta D, Dziarski R, Ulmer A J. Lipopolysaccharide and peptidoglycan: CD14-dependent bacterial inducers of inflammation. Microb Drug Resist. 1998;4:37–44. doi: 10.1089/mdr.1998.4.37. [DOI] [PubMed] [Google Scholar]
- 49.Riley B S, Oppenheimer-Marks N, Hansen E J, Radolf J D, Norgard M V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992;165:484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- 50.Rittig M G, Burmester G-R, Krause A. Coiling phagocytosis: when the zipper jams, the cup is deformed. Trends Microbiol. 1998;6:384–388. doi: 10.1016/s0966-842x(98)01343-2. [DOI] [PubMed] [Google Scholar]
- 51.Rittig M G, Jagoda J C, Wilske B, Murgia R, Cinco M, Repp R, Burmester G R, Krause A. Coiling phagocytosis discriminates between different spirochetes and is enhanced by phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor. Infect Immun. 1998;66:627–635. doi: 10.1128/iai.66.2.627-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rittig M G, Krause A, Haupl T, Schaible U E, Modolell M, Kramer M D, Lutjen-Drecoll E, Simon M M, Burmester G R. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect Immun. 1992;60:4205–4212. doi: 10.1128/iai.60.10.4205-4212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rittig M G, Schroppel K, Seack K-H, Sander U, N'Diaye E-N, Maridonneau-Parini I, Solbach W, Bogdan C. Coiling phagocytosis of trypanosomatids and fungal cells. Infect Immun. 1998;66:4331–4339. doi: 10.1128/iai.66.9.4331-4339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rittig M G, Wilske B, Krause A. Phagocytosis of microorganisms by means of overshooting pseudopods: where do we stand? Microbes Infect. 1999;1:727–735. doi: 10.1016/s1286-4579(99)80074-4. [DOI] [PubMed] [Google Scholar]
- 55.Robertson S M, Kettman J R, Miller J N, Norgard M V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols) Infect Immun. 1982;36:1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabath D E, Broome H E, Prystowsky M B. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 57.Schuhmachers G, Xu S, Bergstresser P R, Takashima A. Identity and functional properties of novel skin-derived fibroblast lines (NS series) that support the growth of epidermal-derived dendritic cell lines. J Investig Dermatol. 1995;105:225–230. doi: 10.1111/1523-1747.ep12317512. [DOI] [PubMed] [Google Scholar]
- 58.Sellati T J, Bouis D A, Caimano M J, Feulner J A, Ayers C, Lien E, Radolf J D. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J Immunol. 1999;163:2049–2056. [PubMed] [Google Scholar]
- 59.Sellati T J, Bouis D A, Kitchens R L, Darveau R P, Pugin J, Ulevitch R J, Gangloff S C, Goyert S M, Norgard M V, Radolf J D. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that utilized by lipopolysaccharide. J Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- 60.Thomas D D, Baseman J B, Alderete J F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985;161:514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas D D, Navab M, Haake D A, Fogelman A M, Miller J N, Lovett M A. Treponema pallidum invades intracellular junctions of endothelial cell monolayers. Proc Natl Acad Sci USA. 1988;85:3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thoma-Uszynski S, Kiertscher S M, Ochoa M T, Bouis D A, Norgard M V, Miyake K, Godowski P J, Roth M D, Modlin R L. Activation of Toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165:3804–3810. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 63.Tosato G, Pike S E. Interferon-beta 2/interleukin 6 is a co-stimulant for human T lymphocytes. J Immunol. 1988;141:1556–1562. [PubMed] [Google Scholar]
- 64.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 65.Underhill D M, Ozinsky A, Hajjar A M, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 66.van Seventer G A, Shimizu Y, Horgan K J, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–4586. [PubMed] [Google Scholar]
- 67.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 68.Walker E M, Zampighi G A, Blanco D R, Miller J N, Lovett M A. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989;171:5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weigel L M, Brandt M E, Norgard M V. Analysis of the N-terminal region of the 47-kilodalton integral membrane lipoprotein of Treponema pallidum. Infect Immun. 1992;60:1568–1576. doi: 10.1128/iai.60.4.1568-1576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong G H W, Steiner B, Faine S, Graves S. Factors affecting the attachment of Treponema pallidum to mammalian cells in vitro. Br J Vener Dis. 1983;59:21–29. doi: 10.1136/sti.59.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wooten R M, Morrison T B, Weis J H, Wright S D, Thieringer R, Weis J J. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- 72.Xu S, Ariizumi K, Caceres-Dittmar G, Edelbaum D, Hashimoto K, Bergstresser P R, Takashima A. Successive generation of antigen-presenting, dendritic cell lines from murine epidermis. J Immunol. 1995;154:2697–2705. [PubMed] [Google Scholar]
- 73.Yamada N, Katz S I. Generation of mature dendritic cells from a CD14+ cell line (XS52) by IL-4, TNF-α, IL-1β, and agonistic anti-CD40 monoclonal antibody. J Immunol. 1999;163:5331–5337. [PubMed] [Google Scholar]