Abstract
Hyper‐inflammation, cytokine storm, and recruitment of immune cells lead to uncontrollable endothelial cell damage in patients with coronavirus disease 2019 (COVID‐19). Sphingosine 1‐phosphate (S1P) signaling is needed for endothelial integrity and its decreased serum level is a predictor of clinical severity in COVID‐19. In this clinical trial, the effect of Fingolimod, an agonist of S1P, was evaluated on patients with COVID‐19. Forty patients with moderate to severe COVID‐19 were enrolled and divided into two groups including (1) the control group (n = 21) receiving the national standard regimen for COVID‐19 patients and (2) the intervention group (n = 19) that prescribed daily Fingolimod (0.5 mg) for 3 days besides receiving the standard national regimen for COVID‐19. The hospitalization period, re‐admission rate, intensive care unit (ICU) administration, need for mechanical ventilation, and mortality rate were assessed as primary outcomes in both groups. The results showed that re‐admission was significantly decreased in COVID‐19 patients who received Fingolimod compared to the controls (p = .04). In addition, the hemoglobin levels of the COVID‐19 patients in the intervention group were increased compared to the controls (p = .018). However, no significant differences were found regarding the intubation or mortality rate between the groups (p > .05). Fingolimod could significantly reduce the re‐admission rate after hospitalization with COVID‐19. Fingolimod may not enhance patients' outcomes with moderate COVID‐19. It is necessary to examine these findings in a larger cohort of patients with severe to critical COVID‐19.
Keywords: COVID‐19, cytokine storm, Fingolimod, sphingosine 1‐phosphate
Fingolimod could reduce the re‐admission rate after hospitalization with COVID‐19.
Abbreviations
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- COVID‐19
coronavirus disease 2019
- FTY720
Fingolimod
- GFR
glomerular filtration rate
- HDL
high‐density lipoproteins
- ICU
intensive care unit
- LPS
lipopolysaccharide
- S1P
Sphingosine 1‐phosphate
1. INTRODUCTION
A growing body of evidence recommends that the main reason for disease severity in coronavirus disease 2019 (COVID‐19) is cytokine storm. 1 Hyper‐inflammation, cytokine storm, and recruitment of immune cells lead to uncontrollable endothelial cell damage, and consequently, acute lung injury (ALI)/Acute respiratory distress syndrome (ARDS). 2 , 3 Multiorgan vascular endothelial dysfunction and its connection with poor prognosis among patients with COVID‐19 are attributed to SARS‐CoV‐2‐induced microvascular endothelial pathology and endotheliitis. 4 Alteration in endothelial permeability and function also occurs in COVID‐19 disease. 5
Sphingosine 1‐phosphate (S1P) is a bioactive lipid and a regulator of various physiological and pathophysiological procedures. The signaling pathway related to S1P and its receptor (S1PR1) is critical for the improvement of endothelial barrier function in the lung. 6 , 7 , 8 RNA viruses including SARS‐1, mouse pulmonary virus, and Influenza H1N1 2009 during their invasion modulate the S1P signaling 9 and S1P1 receptor agonist treatment could suppress global cytokine storm. 10 In line with those studies, it could be speculated a mechanistic connection between serum S1P level as a severity predictor and the progression to a severe inflammatory phase of COVID‐19. Accordingly, it is reported that low serum level of S1P/ high‐density lipoproteins (HDL) has a prognostic value for intensive care unit (ICU) administration and mortality in COVID‐19. 11 Hence, different S1P analogs can inhibit alveolar exudation by stabilizing cell‐matrix adherence, maintaining the integrity of the endothelial cytoskeleton, and tightening the inter‐cellular junction. 12 , 13 , 14
Fingolimod (FTY720), an analog of S1P, is an FDA‐approved therapy for multiple sclerosis (MS), 2010. 15 Fingolimod binds to S1P1R on the endothelium and improves the integrity of the endothelial barrier and restricts the lymphocytes' recruitment toward the inflamed organs and tissues and alveolar space. 16 , 17 Fingolimod by diminishing the cytokine storm and stabilization of pulmonary endothelial integrity can decline the migration of inflammatory immune cells into the lung and prevent pulmonary exudation. 17 This clinical trial aimed to evaluate the effect of Fingolimod on the primary outcomes of COVID‐19 patients.
2. MATERIALS AND METHODS
2.1. Study design and participants
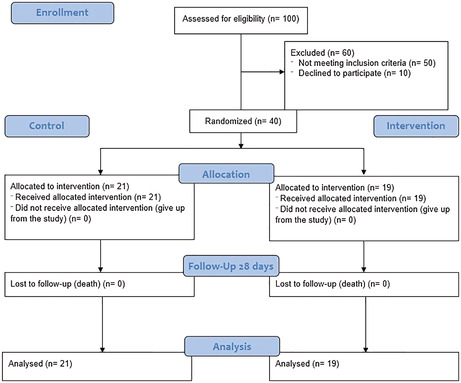
This single‐center, non‐randomized controlled clinical trial was conducted on patients with moderate to severe COVID‐19 admitted to the Infectious Disease Wards of Tabriz University of Medical Sciences (TUOMS), Imam Reza teaching hospital between May to September 2021. Forty patients with COVID‐19 were enrolled and divided into two groups (intervention and control). Inclusion criteria were; patients with moderate to severe SARS‐COV‐2 infection, aged between 18 and 80 years, and positive PCR test for coronavirus. Patients with higher liver enzymes (threefold higher than normal range), active pulmonary tuberculosis, definite fungal and bacterial infections, breast‐feeding and pregnant, severe kidney failure (glomerular filtration rate (GFR) <30 ml/min/1.73 m2), active thrombosis, severe respiratory failure, and immediate need for intubation were excluded from the study. Patients with expected survival duration<24 h who were already enrolled in other clinical trials were excluded as well (Figure 1). This project was approved by the Ethics Committee of TUOMS, Tabriz, Iran (Ethical code: IR.TBZMED.REC.1399.001) and registered at the Iranian Registry of Clinical Trials on 2020‐04‐03 (Registration number: IRCT20200317046797N2).
FIGURE 1.
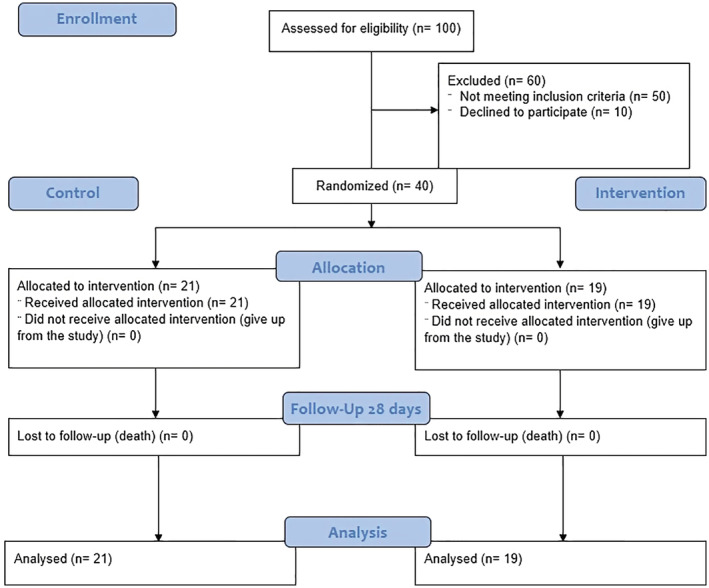
CONSORT diagram 2010
Patients in the control group received the national standard regimen for moderate COVID‐19 infection including Dexamethasone 6 mg daily, Remdesivir 200 mg on the first day, and then 100 mg daily (600 mg in total 5 doses). Moreover, some patients received Famotidine 40 mg twice a day. The intervention group received Fingolimod (0.5 mg) for 3 days in addition to the standard national regimen for COVID‐19. The hospitalization period, re‐admission rate, ICU administration, need for mechanical ventilation, and the mortality rate were assessed as the primary outcomes in both groups.
Laboratory data including lactate dehydrogenase (LDH), neutrophil‐lymphocyte ratio (NLR), liver enzymes [alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT)], bilirubin, serum calcium, sodium, potassium, magnesium, creatine phosphokinase (CPK), C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), CPK‐MB, partial thromboplastin time (PTT), prothrombin time (PT), international normalized ratio (INR), urea, O2 saturation, and serum creatinine were also recorded. The duration of the fever period and the day of hospital discharge were studied as secondary outcomes.
2.2. Statistical analysis
The Shapiro–Wilk test was used for checking the normality of the data distribution. Quantitative variables were presented as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Differences between the groups were compared respectively by independent t‐test or Mann–Whitney U test. SPSS software version 21.0 was used for statistical analysis. p‐values < .05 were considered statistically significant.
3. RESULTS
3.1. Demographic features and clinical symptoms
A total of 19 patients received Fingolimod and 21 patients were in the control group. About 67% of patients in the control group and 53% of patients in the intervention group were males (p = .36). The mean age of all patients was 60.18 ± 18.91 years old and there was not a significant difference between the groups (58.59 vs. 61.88, p = .62). The most common clinical symptom among all patients was cough. However, there was no significant difference between the two arms in terms of clinical symptoms, including; asthma, dry or productive cough, anorexia, anosmia, and fatigue (p > .05). Demographic features and clinical symptoms of patients are shown in Table 1.
TABLE 1.
Demographic features and clinical symptoms of COVID‐19 patients
| Features | Participants | p‐value* | ||
|---|---|---|---|---|
| Control (n = 21) | Intervention (n = 19) | Total (n = 40) | ||
| Sex | ||||
| Male | 14 (67%) | 10 (53%) | 24 (60%) | .36 |
| Female | 7 (33%) | 9 (47%) | 26 (40%) | |
| Age (years) | 61.88 ± 19.35 | 58.59 ± 18.93 | 60.18 ± 18.91 | .62 |
| Asthma | ||||
| Negative | 14 (66.7%) | 18 (94.7%) | 32 (80%) | .10 |
| Mild | 4 (19%) | 1(5.3%) | 5 (12.5%) | |
| Moderate | 2 (9.5%) | 0 (0%) | 2 (5%) | |
| Severe | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Dry cough | ||||
| Negative | 17 (81%) | 18 (94.7%) | 35 (87.5%) | 1.00 |
| Mild | 2 (9.5%) | 1(5.3%) | 3 (7.5%) | |
| Moderate | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Severe | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Productive cough | ||||
| Negative | 19 (90.5%) | 19 (100%) | 38 (95%) | 1.00 |
| Mild | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Anorexia | ||||
| Negative | 17 (81%) | 17 (89.5%) | 34 (85%) | 1.00 |
| Mild | 2 (9.5%) | 2 (10%) | 4 (10%) | |
| Moderate | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Severe | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Anosmia | ||||
| Negative | 20 (95.2%) | 19 (100%) | 39 (97.5%) | 1.00 |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
| Fatigue | ||||
| Negative | 14 (66.7%) | 14 (73.7%) | 28 (70%) | .26 |
| Mild | 3 (14.3%) | 5 (26.3%) | 8 (20%) | |
| Moderate | 3 (14.3%) | 0 (0%) | 3 (7.5%) | |
| Severe | 1 (4.8%) | 0 (0%) | 1 (2.5%) | |
Chi‐square or fisher's exact test was used and p‐value<.05 was considered statistically significant. The severity of symptoms was determined by physicians based on national protocols.
The median admission days of the patients was 7 and 5 days in the control and Fingolimod groups, respectively (p = .42). Only two patients in each group needed ICU admission. Prescription of additional therapies including hemoperfusion (×3), interferon 1‐β, and Methylprednisolone (500 mg) was not significantly different between the groups (p > .05). None of the patients needed intubation and mechanical ventilation. Patients in the Fingolimod group did not need re‐admission but 5 patients (23.8%) in the control group were re‐admitted after their first hospitalization which was statistically significant (p = .04). None of the included patients died because of COVID‐19 infection during the study, and only one patient in the control group died because of his underlying cardiovascular problems. The primary outcomes of patients are shown in Table 2.
TABLE 2.
Primary outcomes of the COVID‐19 patients
| Primary outcomes | Participants | p‐value* | ||
|---|---|---|---|---|
| Control (n = 21) | Intervention (n = 19) | Total (n = 40) | ||
| Admission days1 | 7 (4) | 5 (6) | 5.5 (5) | .42 |
| ICU | ||||
| No | 19 (90.5%) | 17 (89.5) | 36 (90%) | p > .05 |
| Yes | 2 (9.5%) | 2 (10.5%) | 4 (10%) | |
| Additional therapies | ||||
| Hemoperfusion (×3) | 1 (4.8%) | 0 | 1 (2.5%) | .46 |
| Interferon 1‐β | 0 | 1 (5.3%) | 1 (2.5%) | |
| Methylprednisolone (500 mg) | 0 | 1 (5.3%) | 1 (2.5%) | |
| Intubation | ||||
| No | 21 (100%) | 19 (100%) | 40 (100%) | — |
| Yes | 0 | 0 | 0 | |
| Re‐admitted patients | ||||
| No | 16 (76.2%) | 19 (100%) | 35 (87.5%) | .04 |
| Yes | 5 (23.8%) | 0 | 5 (12.5%) | |
1Data are shown as median [interval quartile (IQR)], *: Chi‐square or fisher's exact test was applied and p‐value<.05 was considered statistically significant.
It was revealed that the laboratory measurements were not significantly different between the studied groups (p > .05). Laboratory measurement after the intervention revealed that total WBC, neutrophil count, and CPK, CPK‐MB, and ALP levels were decreased and platelet levels were increased in COVID‐19 patients who received Fingolimod compared to the control group. However, none of the observed differences was statistically significant (p > .05). Hemoglobin levels of patients who received Fingolimod were significantly increased in the intervention group compared to the controls (p = .01). Other biochemical tests are shown in Table 3.
TABLE 3.
Laboratory findings of the COVID‐19 patients
| Laboratory data | Admission | Patients | p‐value | ||
|---|---|---|---|---|---|
| Control (n = 21) | Intervention (n = 19) | Total (n = 40) | |||
| The percentage of O2 Saturation | Early | 91 (4) | 90 (6) | 90 (88–92) | 1.00 |
| Middle | 94 (5) | 90 (7) | 92 (5) | .10 | |
| Late | 93 (8) | 92 (5) | 93 (6) | .18 | |
| WBC (109/L) | Early | 7.1 (5.8) | 7.1 (5.9) | 7.1 (5.7) | .91 |
| Middle | 7.55 (6.8) | 4.85 (3.5) | 5.75 (4.9) | .24 | |
| Late | 9.4 (5.1) | 5.8 (5.2) | 7.7 (5.6) | .34 | |
| Neutrophil (109/L) | Early | 77.22 ± 10.36 | 70.53 ± 13.91 | 74.27 ± 12.33 | .11 |
| Middle | 72.01 ± 16.55 | 70.77 ± 6.26 | 71.56 ± 13.53 | .80 | |
| Late | 75.21 ± 10.92 | 69.48 ± 15.16 | 72.51 ± 13.0 | .38 | |
| Hemoglobin (mg/dl) | Early | 13.63 ± 1.79 | 14.34 ± 2.15 | 13.97 ± 1.98 | .26 |
| Middle | 12.64 ± 1.75 | 14.42 ± 1.7 | 13.32 ± 1.91 | .01 | |
| Late | 12.56 ± 1.71 | 13.19 ± 1.53 | 12.83 ± 1.63 | .37 | |
| Lymphocyte (109/L) | Early | 18.82 ± 10.24 | 19.78 ± 11.22 | 19.26 ± 10.55 | .79 |
| Middle | 19.24 ± 14.25 | 22.8 ± 7.22 | 20.53 ± 12.09 | .44 | |
| Late | 17.75 ± 9.67 | 20.87 ± 8.04 | 19.13 ± 8.87 | .47 | |
| Platelet (109/L) | Early | 159 (94) | 190 (107) | 168 (102) | .59 |
| Middle | 181 (128) | 130.5 (199) | 156.5 (149) | .58 | |
| Late | 186 (194) | 214.5 (245) | 192 (228) | .69 | |
| LDH (U/L) | Early | 539 (301) | 457 (168) | 486.5 (201) | .76 |
| Middle | 526 (365) | 517 (322) | 521.5 (346) | .80 | |
| Late | 1219 (−) | 410 (−) | 580 (901) | .40 | |
| Urea (mg/dl) | Early | 36 (31) | 35 (24) | 35.5 (24) | .37 |
| Middle | 32 (15) | 38 (33) | 33 (20) | .45 | |
| Late | 37 (22) | 27 (35) | 34 (26) | .20 | |
| Creatinine (mg/dl) | Early | 1.15 ± 0.29 | 1.22 ± 0.42 | 1.18 ± 0.35 | .55 |
| Middle | 1.08 ± 0.27 | 1.24 ± 0.36 | 1.14 ± 0.31 | .35 | |
| Late | 1.12 ± 0.24 | 1.06 ± 0.25 | 1.09 ± 0.24 | .56 | |
| Sodium (mmol/L) | Early | 137.5 (6) | 138 (2) | 138 (5) | .98 |
| Middle | 136.5 (5) | 137.5 (3) | 137 (4) | .30 | |
| Late | 138 (5) | 139 (3) | 138.5 (4) | .44 | |
| Potassium (mmol/L) | Early | 4.1 (0.5) | 4.1 (0.4) | 4.1 (0.5) | .61 |
| Middle | 4.1 (0.7) | 4.35 (0.6) | 4.15 (0.6) | .27 | |
| Late | 4.5 (1.2) | 4.5 (0.6) | 4.5 (1.1) | .58 | |
| Ionized calcium (mg/dl) | Early | 1.06 ± 0.06 | 1.05 ± 0.05 | 1.05 ± 0.058 | .53 |
| Middle | 1.06 ± 0.06 | 1.08 ± 0.07 | 1.07 ± 0.07 | .55 | |
| Late | 1.07 ± 0.07 | 1.11 ± 0.03 | 1.09 ± 0.04 | .38 | |
| Total calcium (mg/dl) | Early | 8.1 (1.3) | 8.55 (1.1) | 8.5 (1.2) | .50 |
| Middle | 8.25 (−) | — | 8.4 (−) | .14 | |
| Magnesium (mg/dl) | Early | 1.86 ± 0.29 | 1.97 ± 0.2 | 1.93 ± 0.23 | .39 |
| Middle | 2.01 ± 0.2 | 1.98 ± 0.23 | 2.0 ± 0.21 | .81 | |
| Late | 2.2 ± 0.42 | 2.17 ± 0.15 | 2.18 ± 0.22 | .94 | |
| Phosphorus (mmol/L) | Early | 2.7 ± 1.12 | 2.93 ± 1.02 | 2.81 ± 1.03 | .71 |
| Middle | 2.8 ± 0.45 | 2.95 ± 0.41 | 2.91 ± 0.4 | .60 | |
| Late | 3.0 | 2.67 ± 0.43 | 2.74 ± 0.4 | .55 | |
| CPK (U/L) | Early | 142 (657) | 91.5 (145) | 121 (144) | .12 |
| Middle | 185 (900) | 54 (114) | 116 (159) | .41 | |
| Late | 37.5 | 38 (−) | .66 | ||
| CPK‐MB (U/L) | Early | 36 (38) | 27 (26) | 33 (22) | .19 |
| Late | 18 (−) | 1.00 | |||
| ALT (U/L) | Early | 21.5 (19) | 27.5 (29) | 24.5 (22) | .60 |
| Middle | 23.5 (24) | 40 (45) | 27 (41) | .43 | |
| Late | 48 (101) | 53.5 (−) | 53.5 (59) | .82 | |
| AST (U/L) | Early | 33 (25) | 40.5 (35) | 36.5 (27) | .62 |
| Middle | 36 (17) | 46 (63) | 39 (47) | 1.00 | |
| Late | 71 (166) | 81 (−) | 81 (107) | .87 | |
| ALP (U/L) | Early | 216 (83) | 156 (77) | 177 (90) | .05 |
| Middle | 216 (279) | 140.5 (68) | 145.5 (141) | .13 | |
| Late | 154 (−) | 129 (−) | 130 (81) | .78 | |
| ESR (mm/h) | Early | 16 (−) | 21 (−) | 21 (23) | 1.00 |
| Middle | 16 (−) | 21 (44) | .07 | ||
| INR | Early | 1.08 (0.17) | 1.09 (0.16) | 1.08 (0.16) | .88 |
| Middle | 1.13 (0.71) | 1.1 (3.03) | 1.1 (0.18) | .79 | |
| Late | 1.43 (0.4) | 1.3 (0.39) | .34 | ||
| PTT (seconds) | Early | 31 (12) | 33.5 (15) | 32 (11) | .48 |
| Middle | 38 (24) | 40 (35) | 40 (24) | .93 | |
| Late | 37 (38) | 36.5 (35) | 1.00 | ||
| PT (seconds) | Early | 14.4 (32.1) | 14.5 (2.2) | 14.4 (2.2) | .81 |
| Middle | 16 (15.9) | 15.2 (17.7) | 15.8 (12.6) | .90 | |
| Late | 19.2 (5.4) | 0.6 (−) | 17.45 (5.2) | .34 | |
| NLR | Early | 4.81 (7.63) | 4.09 (4.73) | 4.09 (5.58) | .42 |
| Middle | 5.73 (9.37) | 3.41 (1.78) | 3.65 (6.52) | — | |
| Late | 5.52 (7.42) | 4.04 (3.45) | 4.9 (5.57) | .32 | |
| Total bilirubin (μmol/L) | Early | 0.66 ± 0.2 | 0.95 ± 0.59 | — | |
| Middle | 1.35 ± 0.63 | 1.3 ± 0.63 | .58 | ||
| Direct bilirubin (μmol/L) | Early | 0.33 ± 0.057 | 0.42 ± 0.18 | .52 | |
| Middle | 0.55 ± 0.21 | 0.55 ± 0.21 | .27 | ||
Note: Data are shown as median [interval quartile (IQR)] or mean ± standard deviation (SD). p‐value is calculated using the Mann–Whitney U test or independent T‐test in variables with non‐normal or normal distributions, respectively.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BNP, B‐type natriuretic peptide; CPK, creatine phosphokinase; CRP: C‐reactive protein; Early, at the onset of admission; ESR, erythrocyte sedimentation rate; INR, international normalized ratio; Late, at the end of admission; Middle, at the middle of the admission; NLR, neutrophil leukocyte ratio; PLT, platelets; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white‐blood cell.
4. DISCUSSION
Fingolimod could significantly reduce the re‐admission rate of hospitalized COVID‐19. The results suggest that the use of Fingolimod in hospitalized patients with moderate to severe COVID‐19 does not affect the patients' outcomes and the disease prognosis.
Numerous studies have been done to examine the benefit of different medications that were allocated based on their mechanism of action to improve the outcomes of patients with COVID‐19. 18 Li and colleagues reported that lopinavir/ritonavir (LPV/r) or arbidol monotherapy exerted little benefit in enhancing the clinical outcomes of mild/moderate COVID‐19 patients. 19 In another randomized clinical trial in COVID‐19 patients with mild‐to‐moderate pneumonia, it has been shown that anakinra (a recombinant human IL‐1 receptor antagonist) could not enhance patients' outcomes and was not effective in reducing the need for non‐invasive or mechanical ventilation or death. 20 Furthermore, it has been reported that aerosol inhalation of interferon kappa (IFN‐κ) and trefoil factor family member 2 (TFF2), a small secreted polypeptide that diminishes inflammatory responses and improves the repair of mucosal injury, in combination with standard treatment effectively could suppress SARS‐CoV‐2 invasion. Moreover, these treatments could improve clinical manifestations in all clinical samples of patients with moderate COVID‐19. 21
The SP1‐S1PR1 signaling pathways are needed for preserving endothelial integrity by mediating the localization of Beta‐catenin and vascular endothelial (VE)‐cadherin at adherent junction sites of endothelial cells. 22 It has been also proposed that the rearrangement of adherents' junction proteins and phosphorylation of intracellular myosin light chain by S1P can improve the endothelial barrier function of the lung. 23 , 24 , 25 , 26 S1P by disturbing the activation and recruitment of lymphocytes can reduce the cytokine storm in viral infection. 27
Since some immunosuppressive therapies have been recommended for COVID‐19 patients, we proposed that Fingolimod (an S1P analog) could positively affect the clinical outcome of COVID‐19. Through its potential effects on tightening the endothelial junction and preventing vascular leakage, 28 , 29 Fingolimod inhibits the trans‐endothelial passage of immune cells. 30 , 31 , 32 Fingolimod could also decrease lipopolysaccharide (LPS)‐induced pulmonary damage 12 and necrotizing pancreatitis in animal models. 33 Likewise, Fingolimod could prevent airway inflammation 34 and inflammatory cell recruitment in vivo. 35 The advantageous effect of Fingolimod has been also reported in MS patients with COVID‐19 and its discontinuation during the infection period could induce a worsening of SARS‐CoV2 infection. 36 Fingolimod can decrease cytokine storm, improve endothelial cell integrity in the lung 37 , 38 and reduce mortality 38 in MS patients infected by SARS‐COV‐2. A case report study did report that there were no statistically significant differences in the frequency of COVID‐19 between MS patients who received Fingolimod or Siponimod and the general population. 39 In the present study, Fingolimod could significantly prevent re‐admission of patients; however, it had no significant effects on the hospitalization period, ICU administration, need for mechanical ventilation, and the mortality rate of patients with moderate to severe COVID‐19. Several studies have evaluated approaches to lessen early re‐admission rates and evaluate the risk factors for re‐admission of patients hospitalized with COVID‐19. 40 Reducing the re‐admission rate will help the clinic to provide better care for patients and decrease burdens on medical services.
The small sample size was the main limitation of this study; hence, the result cannot be generalized to the whole population. Moreover, it is suggested to perform this trial on patients with severe to critical COVID‐19 who require ICU admission. More extensive clinical trials with a large sample size are needed. Moreover, since Fingolimod has a widespread effect on S1PR1 and S1PR3–5, to minimize off‐target effects, more specific S1P analogs such as CYM5542 or RP‐002 are needed to be examined in the future. 41
5. CONCLUSION
It can be concluded that the use of Fingolimod could reduce the re‐admission and increase hemoglobin levels. Fingolimod in hospitalized patients with moderate to severe COVID‐19 does not significantly affect the patients' outcomes and the disease prognosis.
FUNDING INFORMATION
This work was supported by the Kidney Research Center at Tabriz University of Medical Sciences, Tabriz, Iran (Grant #65218).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICS APPROVAL STATEMENT
This project was approved by the Ethics Committee of TUOMS, Tabriz, Iran (Ethical code: IR.TBZMED.REC.1399.001).
PATIENT CONSENT STATEMENT
Informed consent was obtained from all included patients in this study.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
ACKNOWLEDGMENTS
We would like to appreciate the cooperation of the Clinical Research Development unit of Imam Reza General Hospital, Tabriz, Iran in conducting this research.
Teymouri S, Pourbayram Kaleybar S, Hejazian SS, et al. The effect of Fingolimod on patients with moderate to severe COVID‐19. Pharmacol Res Perspect. 2023;11:e01039. doi: 10.1002/prp2.1039
Contributor Information
Mohammadreza Ardalan, Email: ardalan34@yahoo.com, Email: ardalanm@tbzmed.ac.ir.
Sepideh Zununi Vahed, Email: sepide.zununi@gmail.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Salton F, Confalonieri P, Campisciano G, et al. Cytokine profiles as potential prognostic and therapeutic markers in SARS‐CoV‐2‐induced ARDS. J Clin Med. 2022;11(11). doi: 10.3390/jcm11112951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pradhan DB. Edaravone: a free radical scavenger with multiple pleotropic actions can be a potential game changer agent in prevention and alleviation of COVID‐19 ‐ induced cytokine storm. J Med Sci Clin Res. 2020;8(7). doi: 10.18535/jmscr/v8i7.41 [DOI] [Google Scholar]
- 3. Baratella E, Ruaro B, Marrocchio C, et al. Interstitial lung disease at high resolution CT after SARS‐CoV‐2‐related acute respiratory distress syndrome according to pulmonary segmental anatomy. J Clin Med. 2021;10(17). doi: 10.3390/jcm10173985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. doi: 10.1016/s0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1‐phosphate in murine endotoxin‐induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169(11):1245‐1251. doi: 10.1164/rccm.200309-1258OC [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1‐phosphate. Microvasc Res. 2009;77(1):39‐45. doi: 10.1016/j.mvr.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sammani S, Moreno‐Vinasco L, Mirzapoiazova T, et al. Differential effects of sphingosine 1‐phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43(4):394‐402. doi: 10.1165/rcmb.2009-0223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oldstone MB, Rosen H. Cytokine storm plays a direct role in the morbidity and mortality from influenza virus infection and is chemically treatable with a single sphingosine‐1‐phosphate agonist molecule. Curr Top Microbiol Immunol. 2014;378:129‐147. doi: 10.1007/978-3-319-05879-5_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci USA. 2014;111(10):3799‐3804. doi: 10.1073/pnas.1400593111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marfia G, Navone S, Guarnaccia L, et al. Decreased serum level of sphingosine‐1‐phosphate: a novel predictor of clinical severity in COVID‐19. EMBO Mol Med. 2021;13(1):e13424. doi: 10.15252/emmm.202013424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1‐phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170(9):987‐993. doi: 10.1164/rccm.200405-684OC [DOI] [PubMed] [Google Scholar]
- 13. Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980‐991. doi: 10.1016/j.cell.2011.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walsh KB, Teijaro JR, Rosen H, Oldstone MB. Quelling the storm: utilization of sphingosine‐1‐phosphate receptor signaling to ameliorate influenza virus‐induced cytokine storm. Immunol Res. 2011;51(1):15‐25. doi: 10.1007/s12026-011-8240-z [DOI] [PubMed] [Google Scholar]
- 15. Pitman M, Woodcock J, Lopez A, Pitson S. Molecular targets of FTY720 (fingolimod). J Curr Mol Med. 2012;12(10):1207‐1219. doi: 10.2174/156652412803833599 [DOI] [PubMed] [Google Scholar]
- 16. Rosen H, Goetzl EJ. Sphingosine 1‐phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560‐570. doi: 10.1038/nri1650 [DOI] [PubMed] [Google Scholar]
- 17. Vahed SZ, Ghiyasvand S, Khatibi SMH, et al. Sphingosine 1 phosphate agonists (SPI); a potential agent to prevent acute lung injury in COVID‐19. Immunopathol Persa. 2021;7(1):e03. doi: 10.34172/ipp.2021.03 [DOI] [Google Scholar]
- 18. Moslemi M, Hejazian SM, Shaddelan M, et al. Evaluating the effect of Edaravone on clinical outcome of patients with severe COVID‐19 admitted to ICU: a randomized clinical trial. Inflammopharmacology. 2022;30(4):1277‐1282. doi: 10.1007/s10787-022-01001-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Xie Z, Lin W, et al. Efficacy and safety of Lopinavir/ritonavir or Arbidol in adult patients with mild/moderate COVID‐19: an exploratory randomized controlled trial. Med (New York, NY). 2020;1(1):105‐113.e4. doi: 10.1016/j.medj.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tharaux P‐L, Pialoux G, Pavot A, et al. Effect of anakinra versus usual care in adults in hospital with COVID‐19 and mild‐to‐moderate pneumonia (CORIMUNO‐ANA‐1): a randomised controlled trial. Lancet Respir Med. 2021;9(3):295‐304. doi: 10.1016/s2213-2600(20)30556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu W, Liu Y, Liu L, et al. An open‐label, randomized trial of the combination of IFN‐kappa plus TFF2 with standard care in the treatment of patients with moderate COVID‐19. EClinicalMedicine. 2020;27:100547. doi: 10.1016/j.eclinm.2020.100547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine‐1‐phosphate. Cell. 1999;99(3):301‐312. doi: 10.1016/s0092-8674(00)81661-x [DOI] [PubMed] [Google Scholar]
- 23. Dudek SM, Jacobson JR, Chiang ET, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1‐phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279(23):24692‐24700. doi: 10.1074/jbc.M313969200 [DOI] [PubMed] [Google Scholar]
- 24. Sun X, Shikata Y, Wang L, et al. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1‐phosphate‐induced endothelial barrier enhancement. Microvasc Res. 2009;77(3):304‐313. doi: 10.1016/j.mvr.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abbasi T, Garcia JG. Sphingolipids in lung endothelial biology and regulation of vascular integrity. Handb Exp Pharmacol. 2013;216:201‐226. doi: 10.1007/978-3-7091-1511-4_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiong Y, Hla T. S1P control of endothelial integrity. Curr Top Microbiol Immunol. 2014;378:85‐105. doi: 10.1007/978-3-319-05879-5_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosen H, Oldstone MBA. The riddle of the sphinx: why sphingosine‐1‐phosphate may help define molecular mechanisms underlying risk stratification for serious COVID‐19 infections. EMBO Mol Med. 2021;13(1):e13533. doi: 10.15252/emmm.202013533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez T, Estrada‐Hernandez T, Paik JH, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor‐induced vascular permeability. J Biol Chem. 2003;278(47):47281‐47290. doi: 10.1074/jbc.M306896200 [DOI] [PubMed] [Google Scholar]
- 29. English D, Kovala AT, Welch Z, et al. Induction of endothelial cell chemotaxis by sphingosine 1‐phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8(6):627‐634. doi: 10.1089/152581699319795 [DOI] [PubMed] [Google Scholar]
- 30. Imeri F, Fallegger D, Zivkovic A, et al. Novel oxazolo‐oxazole derivatives of FTY720 reduce endothelial cell permeability, immune cell chemotaxis and symptoms of experimental autoimmune encephalomyelitis in mice. Neuropharmacology. 2014;85:314‐327. doi: 10.1016/j.neuropharm.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 31. Camp SM, Chiang ET, Sun C, et al. Pulmonary endothelial cell barrier enhancement by novel FTY720 analogs: Methoxy‐FTY720, Fluoro‐FTY720, and beta‐glucuronide‐FTY720. Chem Phys Lipids. 2016;194:85‐93. doi: 10.1016/j.chemphyslip.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 32. Hemdan NY, Weigel C, Reimann CM, Graler MH. Modulating sphingosine 1‐phosphate signaling with DOP or FTY720 alleviates vascular and immune defects in mouse sepsis. Eur J Immunol. 2016;46(12):2767‐2777. doi: 10.1002/eji.201646417 [DOI] [PubMed] [Google Scholar]
- 33. Liu HB, Cui NQ, Wang Q, Li DH, Xue XP. Sphingosine‐1‐phosphate and its analogue FTY720 diminish acute pulmonary injury in rats with acute necrotizing pancreatitis. Pancreas. 2008;36(3):e10‐e15. doi: 10.1097/MPA.0b013e31815f3905 [DOI] [PubMed] [Google Scholar]
- 34. Sawicka E, Zuany‐Amorim C, Manlius C, et al. Inhibition of Th1‐ and Th2‐mediated airway inflammation by the sphingosine 1‐phosphate receptor agonist FTY720. J Immunol. 2003;171(11):6206‐6214. doi: 10.4049/jimmunol.171.11.6206 [DOI] [PubMed] [Google Scholar]
- 35. Fujino M, Funeshima N, Kitazawa Y, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305(1):70‐77. doi: 10.1124/jpet.102.045658 [DOI] [PubMed] [Google Scholar]
- 36. Gomez‐Mayordomo V, Montero‐Escribano P, Matías‐Guiu JA, González‐García N, Porta‐Etessam J, Matías‐Guiu J. Clinical exacerbation of SARS‐CoV2 infection after fingolimod withdrawal. J Med Virol. 2021;93(1):546‐549. doi: 10.1002/jmv.26279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barzegar M, Mirmosayyeb O, Nehzat N, et al. COVID‐19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm 2020;7(4):e753. doi: 10.1212/nxi.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Foerch C, Friedauer L, Bauer B, Wolf T, Adam EH. Severe COVID‐19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord. 2020;42:102180. doi: 10.1016/j.msard.2020.102180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sullivan R, Kilaru A, Hemmer B, et al. COVID‐19 infection in Fingolimod‐ or Siponimod‐treated patients: case series. Neurol Neuroimmunol Neuroinflamm. 2021;9(1). doi: 10.1212/nxi.0000000000001092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green H, Yahav D, Eliakim‐Raz N, et al. Risk‐factors for re‐admission and outcome of patients hospitalized with confirmed COVID‐19. Sci Rep. 2021;11(1):17416. doi: 10.1038/s41598-021-96716-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naz F, Arish M. Battling COVID‐19 pandemic: Sphingosine‐1‐phosphate analogs as an adjunctive therapy? Front Immunol. 2020;11:1102. doi: 10.3389/fimmu.2020.01102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


