Abstract
Pleuropulmonary blastoma (PPB) is a rare primitive malignant lung cancer that occurs in pediatric age. Its main differential diagnosis is congenital cystic pulmonary malformation (CPAM).
A 30-day-old infant with respiratory failure obtained a chest x-ray and a computed tomography scan (CT) which revealed hypertensive pneumothorax with multifocal bilateral cysts. After thoracic drainage, the patient underwent multiple thoracoscopic pulmonary resections. The first histological diagnosis was of type 2 CPAM.
During the radiological follow-up, an increase in the number and dimension of the lesions was detected. Thus, a histological revision was performed, leading to the diagnosis of type I PPB, at nine months. The patient subsequently underwent chemotherapy.
At the five-year follow-up appointment, chest magnetic resonance (MR) and CT scans showed a dimensional increase in size of the lesions, with the risk of recurrent pneumothorax. An upper right lobectomy and wedge resection of the residual cysts were performed.
Control MR scans showed normalization of the lung parenchyma and the patient showed substantial clinical improvement.
Keywords: Children, Pleuropulmonary blastoma, Early surgical resection
1. Introduction
Pleuropulmonary blastoma (PPB) is included in 0.25–0.5% of all primary lung neoplasms [1,2]. Although it is a rare diagnosis, PPB is considered the most frequent primary lung cancer in the pediatric age range [[1], [2], [3]].
A PPB derives from a pulmonary cyst, consisting of respiratory epithelium and sarcomatous mesenchyme [3]. The PPBs are classified into 3 types: type I/Ir, with exclusively multiloculated cystic lesions; type II, composed of cystic and solid areas derived from expansion of mesenchymal cells and type III, a solid tumor displaying a mixed, sarcomatous pattern [2,4,5].
As reported in the International Pleuropulmonary Blastoma Registry (https://www.ppbregistry.org/), the median age at diagnosis of type I PPB is 8 months; 35 months for type 2 and 41 months for type III. The survival rate is 94% for type I PPB; 71% and 53% for type II and III PPB, respectively [3].
The main differential diagnosis of PPBs is congenital cystic pulmonary malformation (CPAM), a group of benign lung anomalies which includes bronchogenic cysts, bronchopulmonary sequestration, congenital lobar emphysema, and congenital cystic adenomatoid malformations [4,6].
A typical clinical presentation is represented by respiratory distress due to air-filled cysts compressing on airways with or without pneumothorax [1].
We hereby present a case of infant PPB which had initially been characterized as CPAM, emphasizing that the differential diagnosis could be challenging. Based on a first misleading histological report of CPAM, an incomplete surgical resection was performed, thus leading to a second surgery.
The typical histological appearance of this tumor is very varied and complex; for this reason, it is important to differentiate it from other benign forms of congenital lung malformations.
Indeed, making a proper diagnosis is crucial as the cornerstone for treatment for PPB is a complete surgical resection, which offers the theoretical advantage of compensatory lung growth and significant functional improvement.
1.1. Clinical case
Our patient was born by caesarean section at 41 weeks, after a regular pregnancy, with normal prenatal ultrasound checks. Family history was silent. His weight was adequate for his gestational age. He was breast-fed and his growth was normal.
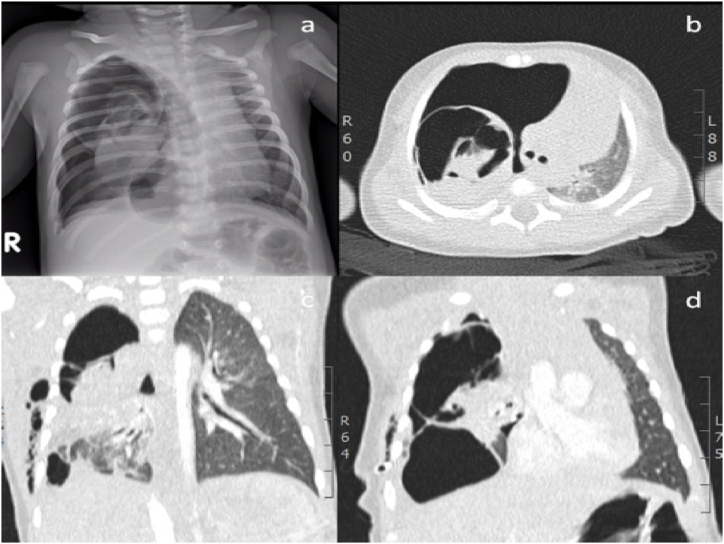
At the age of 30 days, the infant arrived at the emergency room with cough and respiratory distress. He appeared irritable, pale, tachypnoeic and with reduced respiratory sounds; his transcutaneous oxygen saturation was 85%. A chest x-ray was performed and showed the presence of right hypertensive pneumothorax. In addition, the computed tomography (CT) scan confirmed pneumothorax and showed several cystic lesions of the upper and lower right lobes (see Fig. 1).
Fig. 1.
Chest x-ray (a) and CT (b, c, d), showing the presence of right hypertensive pneumothorax with cystic lesions of the upper and lower right lobes.
The infant underwent thoracic drainage and multiple thoracoscopic pulmonary resections of the upper right lobe and of the apical and basal segments of the lower right lobe, saving as much parenchyma as possible. The post-operative course was uncomplicated. The initial histological diagnosis was type 2 congenital cystic pulmonary malformations (CPAM), due to the presence of multiple small sized cysts resembling dilated bronchioles with chronic interstitial inflammation.
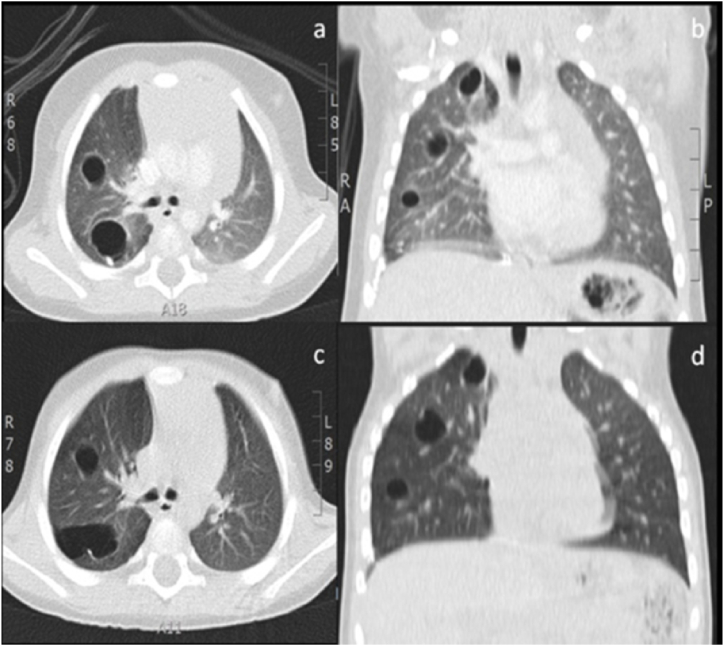
During radiological follow-up, the chest CT scan at nine months detected 13 cystic lesions with air present in all three lobes of the right lung (see Fig. 2). Therefore, a careful histological revision was carried out, identifying small primitive mesenchymal cells in the cyst walls. Indeed, the patient obtained a diagnosis of type I PPB.
Fig. 2.
Chest CT after 3 (a, b) and 9 months from surgery (c, d), showing a progressive increase in number and dimension of the lesions with 13 cysts with air content in the right lung, involving the three lobes.
An abdominal ultrasound was negative, excluding other cancers. DICER-1 mutation was not detected, excluding the predisposition to develop other extrapulmonary neoplasms.
Indeed, after oncological evaluation, the baby underwent chemotherapy according to the European protocols for soft tissue sarcoma (i.e., ifosfamide, vincristine, actinomycin plus doxorubicin) [7,8].
The oncological follow-up which consisted of clinical checks and chest x-rays showed no signs of relapse. At 5 years old, the patient underwent a pneumological evaluation. The child presented with allergic rhinitis (pollen sensitization) and a few self-limiting infections of the upper respiratory tract. The spirometry was normal.
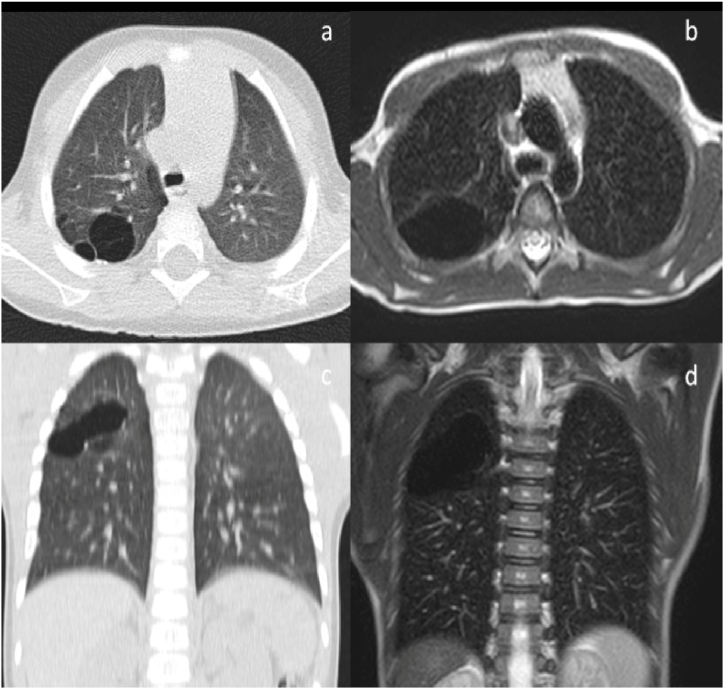
At 8 years old, the patient complained about poor exercise tolerance compared to his peers. The spirometry showed a deterioration of respiratory function. The chest magnetic resonance (MR) scan detected an evident dimensional increase of previous lesions which compressed the diaphragm and led to the risk of recurrent pneumothorax (see Fig. 3). At the age of 8, following a multidisciplinary evaluation with surgeons, oncologists and radiologists, the patient underwent a right superior lobectomy and a wedge resection of cysts localized in the medium and inferior lobes by thoracoscopy. The histology confirmed the absence of oncological relapse.
Fig. 3.
Chest CT (a, c) and MR (b, d) showing a progressive dimensional increase in lesions with risk of recurrent pneumothorax and compression of diaphgram.
Post-operative MR scans showed normalization of the lung parenchyma with the harmonic re-expansion of the medium and inferior right lobes. Now, M. does athletics with no complaints!
2. Discussion
Our paper describes a case of PPB, initially diagnosed as CPAM. After the establishment of International Pleuropulmonary Blastoma Registry, review of several cases has confirmed the possibility that lesions first diagnosed as CPAM were later diagnosed as PPB type I [[1], [2], [3],6,9,10].
As previously described [11,12], in our case, the clinical presentation was respiratory distress due to pneumothorax by the mass effect from air filled cysts.
Therefore, the infant underwent thoracic drainage and multiple thoracoscopic wedge resections based on the suspicion of CPAM. During the follow-up, the progressive increase in number and dimension of the lesions led to histological revision, thus rectifying the diagnosis of type I PPB.
In agreement with literature data [1,3,5], the diagnosis of type I PPB was made at around one year.
PPB is an infrequent lung tumor (0.25–0.5% of primary lung neoplasms) and its pathogenesis is still uncertain [1]. The tumor begins as a cystic lesion with the potential progression to a high-grade primitive sarcoma [3].
The differential diagnosis between PPB and CPAM is challenging [[10], [13]]. Early surgical resection is crucial not only as treatment but also in order to obtain a tumor sample for histological diagnosis [4,15,16].
The association of DICER1 mutations is present in approximately 65% of the PPB cases. The DICER1 gene is a member of the ribonuclease III (RNase III) gene family, essential in the processing of microRNAs (miRNAs), which post-transcriptionally regulate gene expression inducing oncogenesis. Germline loss of function mutations in the DICER1 gene leads to the predisposition to develop tumors in other organs (cystic nephroma, rhabdomyosarcoma, thyroid neoplasms, neuroblastoma, embryonal tumors like Wilms tumor and embryonal rhabdomyosarcoma, Sertoli Leydig cell ovarian tumors, juvenile intestinal polyps, intraocular medullo-epithelioma, and nasal chondromesenchymal hamartomas [17]).
These findings could be used to set up a diagnostic algorithm [3,10]. The clinical presentation with pneumothorax, the presence of complex bilateral or multifocal cysts and the mutation of DICER-1 could suggest PPB, while the prenatal diagnosis, the presence of an afferent vessel and pulmonary hyperinflation could suggest CPAM [3,10].
The treatment of type I PPB without extrapulmonary involvement is margin-free exeresis with a radical impact on survival rates [2,4,14]. The use of adjuvant chemotherapy after surgery in patients with type I PPB is controversial [7]. In the case of complete resection, chemotherapy should be used in order to avoid the eventual progression of type I PPB to type II or III; on the contrary, if repeated surgery is not feasible, it should be proposed on an individual basis [4,14,18].
In conclusion, an early radical surgical approach makes the diagnosis certain and offers the theoretical advantage of compensatory lung growth and significant functional improvement.
3. Conclusion
-
•
PPB is a rare primitive primary malignant lung cancer in pediatric age
-
•
Its main differential diagnosis is CPAM
-
•
Early surgical resection makes the diagnosis certain and offers the theoretical advantage of compensatory lung growth and a significant functional improvement, even in the absence of obstructive hyperinflation
Declaration of competing interest
There are no conflicts of interest to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Gbande P., Abukeshek T., Bensari F., et al. Pleuropulmonary blastoma, a rare entity in childhood. BJR Case Rep. 2021 Apr 29;7(4) doi: 10.1259/bjrcr.20200206. PMID: 35047198; PMCID: PMC8749406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messinger Y.H., Stewart D.R., Priest J.R., et al. Pleuropulmonary blastoma: a report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry. Cancer. 2015 Jan 15;121(2):276–285. doi: 10.1002/cncr.29032. Epub 2014 Sep 10. PMID: 25209242; PMCID: PMC4293209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dehner L.P., Schultz K.A., Hill D.A. Pleuropulmonary blastoma: more than a lung neoplasm of childhood. Mo. Med. 2019 May-Jun;116(3):206–210. PMID: 31527943; PMCID: PMC6690274. [PMC free article] [PubMed] [Google Scholar]
- 4.Knight S., Knight T., Khan A., et al. Current management of pleuropulmonary blastoma: a surgical perspective. Children (Basel) 2019 Jul 25;6(8):86. doi: 10.3390/children6080086. PMID: 31349569; PMCID: PMC6721434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung S.S., Donuru A., Kandula V., etal Multimodality imaging of pleuropulmonary blastoma: pearls, pitfalls, and differential diagnosis. Semin. Ultrasound CT MR. 2022 Feb;43(1):61–72. doi: 10.1053/j.sult.2021.05.007. Epub 2021 May 15. PMID: 35164911. [DOI] [PubMed] [Google Scholar]
- 6.Leblanc C., Baron M., Desselas E., et al. Congenital pulmonary airway malformations: state-of-the-art review for pediatrician's use. Eur. J. Pediatr. 2017 Dec;176(12):1559–1571. doi: 10.1007/s00431-017-3032-7. Epub 2017 Oct 19. PMID: 29046943. [DOI] [PubMed] [Google Scholar]
- 7.Bisogno G., Ferrari A., Bergeron C., et al. The IVADo regimen--a pilot study with ifosfamide, vincristine, actinomycin D, and doxorubicin in children with metastatic soft tissue sarcoma: a pilot study of behalf of the European pediatric Soft tissue sarcoma Study Group. Cancer. 2005 Apr 15;103(8):1719–1724. doi: 10.1002/cncr.20928. PMID: 15754335. [DOI] [PubMed] [Google Scholar]
- 8.Bisogno G., Brennan B., Orbach D., et al. Treatment and prognostic factors in pleuropulmonary blastoma: an EXPeRT report. Eur. J. Cancer. 2014 Jan;50(1):178–184. doi: 10.1016/j.ejca.2013.08.015. Epub 2013 Sep 13. PMID: 24041875.) [DOI] [PubMed] [Google Scholar]
- 9.Ghosh M., Islam N., Ghosh A., et al. Pleuropulmonary blastoma developing in a case of misinterpreted congenital pulmonary airway malformation: a case report. Fetal Pediatr. Pathol. 2018 Oct;37(5):377–386. doi: 10.1080/15513815.2018.1520943. Epub 2018 Oct 25. PMID: 30358469. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg A., Hall N.J., Williams G.M., et al. Can congenital pulmonary airway malformation be distinguished from Type I pleuropulmonary blastoma based on clinical and radiological features? J. Pediatr. Surg. 2016 Jan;51(1):33–37. doi: 10.1016/j.jpedsurg.2015.10.019. Epub 2015 Oct 23. PMID: 26561249; PMCID: PMC5031236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adôrno I.F., Santos R.F.T., de Faria B.B., et al. Pleuropulmonary blastoma manifesting as spontaneous pneumothorax: an unusual presentation. Radiol. Bras. 2019 May-Jun;52(3):202–203. doi: 10.1590/0100-3984.2017.0189. PMID: 31210699; PMCID: PMC6561364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ploenes T., Schildhaus H.U., Theegarten D., et al. Pleuropulmonary blastoma misinterpreted as spontaneous pneumothorax in an infant. Ann. Thorac. Surg. 2020 Jul;110(1):e79. doi: 10.1016/j.athoracsur.2020.03.033. Epub 2020 Apr 17. PMID: 32305285. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira C., Himidan S., Pastor A.C., et al. Discriminating preoperative features of pleuropulmonary blastomas (PPB) from congenital cystic adenomatoid malformations (CCAM): a retrospective, age-matched study. Eur. J. Pediatr. Surg. 2011;21:2–7. doi: 10.1055/s-0030-1267923. [DOI] [PubMed] [Google Scholar]
- 14.Grigoletto V., Tagarelli A., Atzeni C., et al. Pleuropulmonary blastoma: a report from the TREPProject. Tumori. 2020 Apr;106(2):126–132. doi: 10.1177/0300891619871344. Epub 2019 Sep 5. PMID: 32270754. [DOI] [PubMed] [Google Scholar]
- 15.Esposito C., Bonnard A., Till H., et al. Thoracoscopic management of pediatric patients with congenital lung malformations: results of a European multicenter survey. J. Laparoendosc. Adv. Surg. Tech. 2021;31(3):355–362. doi: 10.1089/lap.2020.0596. [DOI] [PubMed] [Google Scholar]
- 16.Zamora A.K., Zobel M.J., Ourshalimian S., et al. The effect of gross total resection on patients with pleuropulmonary blastoma. J. Surg. Res. 2020;253:115–120. doi: 10.1016/j.jss.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 17.González I.A., Stewart D.R., Schultz K.A.P., et al. DICER1 tumor predisposition syndrome: an evolving story initiated with the pleuropulmonary blastoma. Mod. Pathol. 2022 Jan;35(1):4–22. doi: 10.1038/s41379-021-00905-8. Epub 2021 Oct 1. PMID: 34599283; PMCID: PMC8695383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisogno G., Sarnacki S., Stachowicz-Stencel T., et al. Pleuropulmonary blastoma in children and adolescents: the EXPeRT/PARTNER diagnostic and therapeutic recommendations. Pediatr. Blood Cancer. 2021 Jun;68(Suppl 4) doi: 10.1002/pbc.29045. Epub 2021 Apr 7. PMID: 33826235. [DOI] [PMC free article] [PubMed] [Google Scholar]