Abstract
Objective
The prevalence of food allergy (FA) has been increasing in recent years and has become an important public health, food safety, and clinical nutrition problem. However, population-based studies on the prevalence of FA are very limited in China. This study aims to determine the prevalence and pattern of parent-reported FA among school children in Jiangxi Province, China.
Methods
A multicenter cross-sectional study on FA was conducted on primary school children aged 6-11 years old using random cluster sampling with a questionnaire survey. Parent-reported FA was used and defined as individual-reported FA by parent or guardian through a questionnaire in this study.
Result
Among the total of 8,856 (96.36%) complete questionnaires received, 727 (8.2%) children had adverse reactions to food (ARF). The prevalence rates of parent-reported FA and doctor-diagnosed FA were 6.2% and 3.3%, respectively. Animal-derived foods were the main causative source of FA, and the three leading allergenic foods were shrimp, mango, and mollusks. Skin reactions were the most common clinical manifestations of FA, accounting for 63.7%, and 45.32% of the subjects with parent-reported FA experienced severe allergic reactions. There was a significant difference in parent-reported FA between different survey centers, and FA risk increased significantly in children with other allergic diseases (P<0.001) and small family size (P=0.026). The FA prevalence was significantly higher among children aged 8-11 years than those aged 6-7 years (P=0.020).
Conclusions
A high prevalence of parent-reported FA was observed among children in general primary schools in Jiangxi Province, China. Shrimp, mango, and mollusks were the most common causative foods. The main common symptoms of FA were adverse reactions relating to the skin system. The rate of severe allergic reactions was also high in Jiangxi Children with reported FA. Local standards and policies for the prevention and management of FA need to be adjusted on a timely basis according to actual local conditions.
Keywords: food allergy, prevalence, adverse reactions, questionnaire survey, cross-sectional study
Introduction
Food is an indispensable part of life; however, improper diets can cause nutrition, health, and food safety problems. Some studies have shown that up to 41.6% of individuals reported adverse reactions to food (ARF) (1). One of the ARFs is food allergy (FA). In childhood, food allergic reactions are more common compared to nonallergic food hypersensitivity reactions (2).
Food allergy is a complex immune-mediated disorder that affects all ages, especially children. Based on numerous epidemiological studies, FA has become a common chronic disease in children (3) and affects approximately 8% of children (4–8), and even up to 10% in developed countries (9). The prevalence of FA is increasing globally (10–14) and the types of foods causing allergies and serious allergic reactions are growing (2, 15). Therefore, FA is described as the “second wave” of allergic disease after asthma and allergic rhinitis (16).
Food allergy is known to be associated with a negative impact on child growth and psychosocial condition, serious impairment of their quality of life, and is also the main cause of anaphylaxis among patients presenting to hospital emergency departments (10, 17–19). FA not only impacts health, psychological well-being, nutritional status, and the economy (20), but is also an important issue relating to clinical nutrition, food safety, and public health.
The prevalence and trend of FA in the community largely depend on public awareness, diagnostic methods, and dietary habits of the population (21), and vary among different geographic regions. This issue has been well documented in high-income countries (HIC), but information available about it is very limited in many low- and middle-income countries (LMIC) (22). The epidemiology of potentially life-threatening FA in Asia is unclear (23). Currently, few epidemiological studies on FA have been conducted and there is a lack of information from large-scale population-based studies on FA in China. In addition, a small-scale research study reported that the prevalence of confirmed FA in children was approximately 6% in China (24), indicating a consistent upward trend with global prevalence (16). A recent meta-analysis of the Chinese population has also concluded that FA prevalence from 2009–2018 was 8% (95% CI: 6%–11%), higher than that from 1999-2008 (5%; 95% CI: 3 %–7%) (25).
Studies on FA in the general population with a large sample size have not been done in Jiangxi Province. Jiangxi, one of the inland provinces in China, is located in the central southeast of China and covers an area of 166,900 square kilometers. Jiangxi is economically ranked in the middle of the country and accounts for a large share of agriculture, with grain, oilseeds, and vegetables. Jiangxi is a provincial administrative division with 11 prefecture districts and 100 counties, a population of 45.18 million, and 55 ethnic groups, of which the Han account for more than 99%. There are about 7,200 elementary schools with 242,200 teachers and 4,063,100 students.
This survey-based study was designed to estimate the prevalence and pattern of parent-reported FA, identify main foods allergens and understand the most common symptoms of FA. This study also included the prevalence of doctor-diagnosed FA, and the other allergic diseases among children in Jiangxi, China. Findings from this study will form the baseline not only for improving current awareness of FA among children and their parents but also for facilitating the development of an effective strategy to prevent and control FA among school children.
Subjects and methods
Study population
A population-based cross-sectional epidemiological survey on FA was conducted among more than 9000 children using stratified cluster random sampling in Jiangxi Province from January 2020 to May 2021. Three prefectures were randomly selected in Jiangxi and then one district or county was randomly chosen from each selected prefecture as the survey center ( Figure 1 ). The subjects were children aged 6-11 years currently enrolled in primary schools. First, the lists of primary schools were obtained from local education departments, then elementary schools from rural and urban regions were randomly selected. Approximately 3,000 schoolchildren were recruited from primary schools in each survey center using class-based random cluster sampling. A total of 9,000 representative children from 16 primary schools in three survey centers were enrolled in this study. Approximately half of them came from rural regions and the others came from urban regions.
Figure 1.
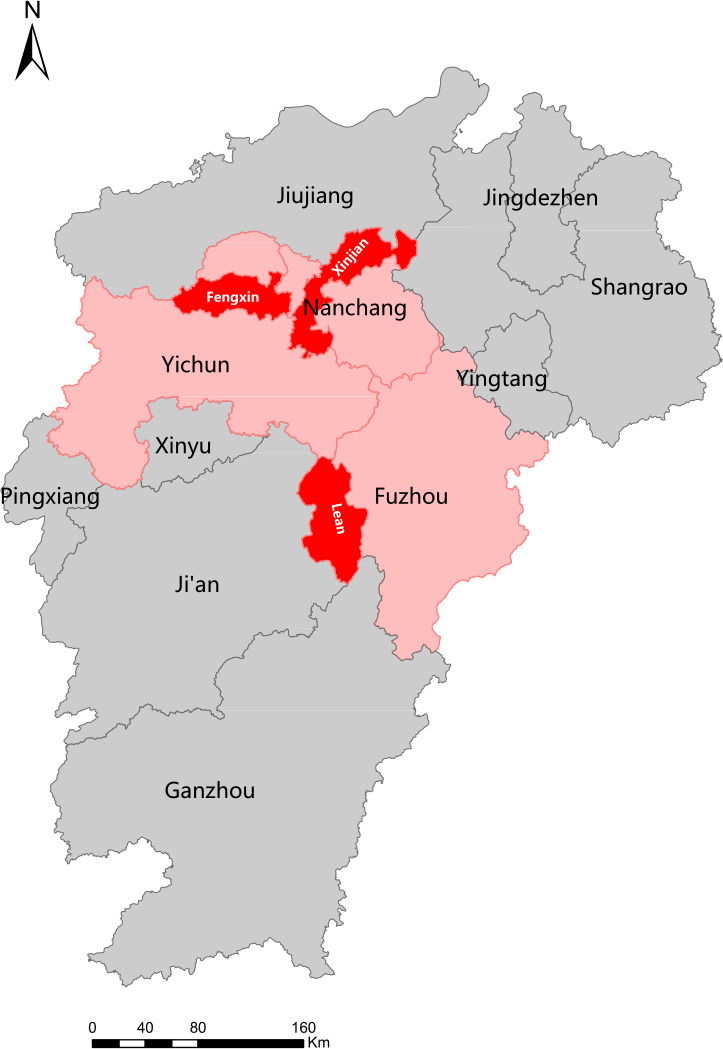
Distribution of survey centers.
Questionnaire
The questionnaire designed for this study was mainly based on the EuroPrevall FA questionnaire (26) with some modifications according to a validation test to accommodate the indigenous need of the local people. Several questions about demography, ARF, FA, doctor-diagnosed FA, and other allergic diseases were the focus of this questionnaire.
Survey method
All the children aged 6-11 years old in grades 1 to 5 from each selected school were invited to participate in this study. The questionnaires were distributed, in the school classes, to their parents or legal guardians for completion. The class teachers and investigators collected and checked the filled questionnaires. Questionnaires with any problems such as incomplete or unclear answers were then returned to respondents for clarification and completion.
Study management
The expert panel of this study formulated and approved standard survey procedures, and all staff were well-trained in the standard procedures and methods and conducted the survey accordingly. The survey data was cross-checked and verified independently by two individuals and was then used to form a database via a web APP (Information Management System of Chinese FA Epidemiological Investigation), which was specifically designed for data capturing in this project.
Definition
Several definitions used in this study were based on the descriptions published previously (27). ARF was defined as the report of an illness or disorder after consuming food: parent-reported FA was defined as FA reported by participants’ parents or guardians, doctor-diagnosed FA referred FA diagnosed by doctors, and multiple FA meant allergies to 3 or over 3 food allergens.
Statistical analysis
The descriptive statistics for categorical variables were presented as frequencies and percentages, while continuous variables were summarized as means ± standard deviation (SD). Pearson’s chi‐squared or Fisher’s exact tests were used for categorical variables. Comparisons of continuous variables were performed with a t-test or one-way ANOVA. The statistical analysis was performed with SPSS version 25.0, and two-tailed P values of <0.05 were considered as statistical significance.
Ethical approval
This study was approved by the Ethical Committees of the China Center for Food Safety Risk Assessment (Number 2019027) and written informed consent was obtained from each child’s legal guardian.
Results
Participants
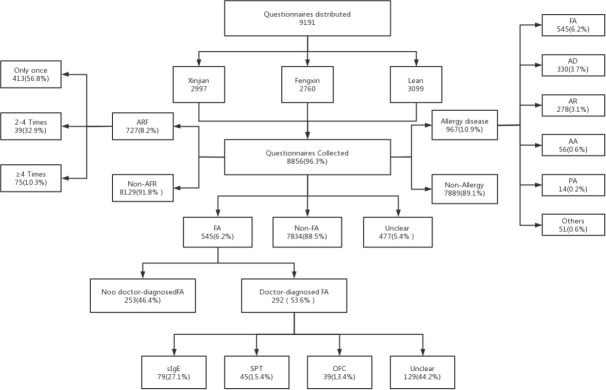
A total of 9,191 questionnaires were randomly distributed to schoolchildren in 16 primary schools from urban and rural regions in Jiangxi, China. Among the 8,856 (96.36%) valid questionnaires collected, 4,658 were from boys, 4,198 were from girls, 4,141 were from urban children, and 4,715 were from rural children. The mean age and height of children were 8.67 ± 1.26 years old and 1.31 ± 0.10 meters, respectively. Table 1 shows the demographic characteristics of the participants.
Table 1.
Demographic characteristics and prevalence of reported ARF and FA.
| Variables | Total( n, %) /Mean ± SD | AFR ( n, %) /Mean ± SD | FA ( n, %) /Mean ± SD | Non-FA( n, %) /Mean ± SD | X 2/t value | P value | |
|---|---|---|---|---|---|---|---|
| Total | 8856 (100) | 727 (8.2) | 545 (6.2) | 7834 (88.5) | – | – | |
| Gender | Male | 4658 (52.6) | 392 (8.4) | 297 (6.4) | 4117 (88.4) | 0.771 | 0.380 |
| Female | 4198 (47.4) | 335 (8.0) | 248 (5.9) | 3717 (88.5) | |||
| Age (year) | 8.7 ± 1.26 | 8.8 ± 1.27 | 8.8 ± 1.28 | 8.7 ± 1.25 | 1.787 | 0.074 | |
| Age group | 6-7 | 2861 (32.3) | 207 (7.2) | 159 (5.6) | 2513 (87.8) | 7.869 | 0.020 |
| 8-11 | 5995 (67.7) | 652 (10.9) | 386 (6.4) | 5321 (88.8) | |||
| BMI | 16.6 ± 3.5 | 16.7 ± 3.56 | 16.7 ± 3.33 | 16.6 ± 3.57 | 0.924 | 0.355 | |
| Ethnic | Han | 8825 (99.6) | 724 (8.2) | 542 (6.1) | 7834 (88.8) | 0.344 | 0.558 |
| Others | 31 (0.4) | 3 (9.7) | 3 (9.7) | 24 (77.4) | |||
| Family size | 5.1 ± 1.53 | 5.1 ± 1.43 | 5.0 ± 1.44 | 5.2 ± 1.54 | -2.23 | 0.026 | |
| Residence | Urban | 4141 (46.8) | 339 (8.2) | 279 (6.7) | 3916 (94.6) | 1.879 | 0.170 |
| Rural | 4715 (53.2) | 388 (8.2) | 266 (5.6) | 4215 (89.4) | |||
| Survey center | Fengxin | 2760 (31.2) | 267 (9.7) | 210 (7.6) | 2412 (87.4) | 19.534 | 0.000 |
| Lean | 3099 (35.0) | 252 (8.1) | 189 (6.1) | 2688 (86.7) | |||
| Xinjian | 2997 (33.8) | 208 (6.9) | 146 (4.9) | 2734 (91.2) | |||
| Other allergies (except FA) | 664 (7.5) | 295 (40.6) | 242 (44.4) | 363 (4.6) | 1203.051 | 0.000 | |
| Allergic dermatitis | 330 (3.7) | 98 (13.5) | 148 (27.2) | 155 (2.0) | 926.735 | 0.000 | |
| Allergic rhinitis | 278 (3.1) | 28 (3.9) | 75 (13.8) | 179 (2.3) | 228.317 | 0.000 | |
| Allergic asthma | 56 (0.6) | 33 (4.5) | 22 (4.0) | 28 (0.4) | 116.288 | 0.000 | |
FA, food allergy; ARF, adverse reactions to food.
The bold values mean the data in total or showing statistic difference (P < 0.01).
Prevalence of parent-reported ARF and FA
Our study showed no age difference between the ARF group and the non-ARF group. Among the participants, 727 reported AFR with a mean age of 8.79 ± 1.27, 56.8% experienced ARF only once, and 10.3% experienced it four or more times. Among the non-ARF children (8,129), the mean age was 8.66 ± 1.25. A total of 545 children (6.2%) reported an FA, 88.5% (7,834) reported no FA, and 5.4% (477) did not know if they had an FA. Table 1 summarizes the prevalence of parent-reported ARF and FA. There was no significant difference in FA prevalence between gender, ethnicity, residence, or BMI. However, FA was associated significantly with age group, family size, living region, and other allergic diseases. FA prevalence among 8-11-year-old children was higher than that in 6-7-year-old children (P=0.020), and FA was more common in children with other allergic diseases (P<0.001) or small family size (P=0.026). Figure 2 indicates the overview of participants, distribution of ARF, and allergic diseases. There were 292 doctor-diagnosed FA cases (3.30%) reported, accounting for 53.58% of the parent-reported FAs, of which 15.41% suffered multiple FAs.
Figure 2.
Overview of participation, distribution of ARF, and allergic diseases.
Food allergens and symptoms of FA
This survey revealed over 100 food allergens and animal-derived foods were dominant. As shown in Table 2 , the most common causative foods were shrimp, followed by mango, mollusks, egg, fish, milk, beef, and lamb. Peanuts were not included in the top eight food allergens. The top three leading food allergens accounted for 39.45%, 30.28%, and 22.57%, respectively. Mango, beef, and lamb were determined to be among the top eight food allergens in this study, but they are not included in the recommended list of food allergens in the Chinese GB 7718-2011 National Food Safety Standard. In total, 77 children (0.87%) were reported with multiple FAs, accounting for 14.13% of parent-reported FAs. Table 3 lists the major causative foods of self-reported doctor-diagnosed FA, and it also presents mango, beef, and lamb as the common causative foods.
Table 2.
Summary of the common food allergens of parent-reported food allergy.
| Food Allergens | n | Proportion % | Prevalence % | Included in GB 7718 |
|---|---|---|---|---|
| Shrimp | 215 | 39.45 | 2.43 | Yes |
| Mango | 165 | 30.28 | 1.86 | No |
| Mollusks | 123 | 22.57 | 1.39 | Yes |
| Egg | 110 | 20.18 | 1.24 | Yes |
| Fish | 65 | 11.93 | 0.73 | Yes |
| Milk | 53 | 9.72 | 0.60 | Yes |
| Beef | 41 | 7.52 | 0.46 | No |
| Lamb | 34 | 6.24 | 0.38 | No |
| Peanut | 30 | 5.50 | 0.34 | Yes |
| Soybean | 19 | 3.49 | 0.21 | Yes |
| Tree Nuts | 15 | 2.75 | 0.17 | Yes |
| Sesame | 15 | 2.75 | 0.17 | No |
| Crab | 12 | 2.20 | 0.14 | Yes |
| Wheat | 9 | 1.65 | 0.10 | Yes |
| Peach | 5 | 0.92 | 0.06 | No |
| Pineapple | 5 | 0.92 | 0.06 | No |
The bold values mean that food allergens are not included in Chinese GB 7718.
Table 3.
Causative foods of the parent-reported doctor-diagnosed food allergy.
| Causative Foods | FA (n) | FA Prevalence (%) | FA Proportion (%) |
|---|---|---|---|
| Total | 292 | 3.30 | 53.58 |
| Shrimp | 95 | 1.07 | 32.53 |
| Mango | 60 | 0.68 | 20.55 |
| Egg | 57 | 0.64 | 19.52 |
| Mollusks | 54 | 0.61 | 18.49 |
| Milk | 33 | 0.37 | 11.30 |
| Fish | 31 | 0.35 | 10.62 |
| Beef | 24 | 0.27 | 8.22 |
| Peanut | 20 | 0.23 | 6.85 |
| Lamb | 14 | 0.16 | 4.79 |
| Soybean | 11 | 0.12% | 3.77 |
| Crab | 9 | 0.10 | 3.08 |
| Nuts | 6 | 0.07 | 2.05 |
| Sesame | 5 | 0.06 | 1.71 |
| Wheat | 4 | 0.05 | 1.37 |
This survey showed that the main clinical manifestations of FA were diverse and heterogeneous, primarily associated with the skin, perioral, respiratory tract, digestive tract, and cardiovascular system. Skin symptoms were particularly prominent, such as rashes and itches, accounting for 85% of the FA reaction. Perioral symptoms accounted for 28.6% of FA cases, while 18% of FA cases presented headaches and dizziness, as shown in Figure 3 . Among the subjects with parent-reported FA, more than 45% (247) experienced severe allergic reactions, among them 12.41% were allergic to multiple foods. Table 4 shows the common symptoms caused by major allergens, and the severe reactions caused by major food allergens are presented in Figure 4 .
Figure 3.
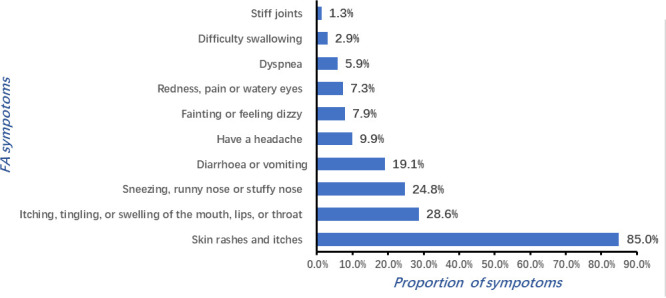
Reaction characteristics and common symptoms of FA.
Table 4.
The prevalence of main clinical symptoms associated with major allergenic foods (%).
| Symptoms | Egg | Milk | Peanut | Fish | Shellfish | Mollusks | Mango | Beef | Mutton |
|---|---|---|---|---|---|---|---|---|---|
| Oropharyngeal symptoms | 13.50 | 11.40 | 26.30 | 17.60 | 20.10 | 19.40 | 35.20 | 12.50 | 23.80 |
| Skin symptoms | 79.80 | 68.20 | 73.70 | 72.50 | 70.70 | 72.80 | 71.00 | 75.00 | 71.40 |
| Gastrointestinal symptoms | 14.60 | 29.50 | 36.80 | 11.80 | 12.00 | 20.40 | 9.00 | 21.90 | 23.80 |
| Nasal symptoms | 14.60 | 22.70 | 10.50 | 15.70 | 16.80 | 19.40 | 12.40 | 34.40 | 47.60 |
| Eye symptoms | 5.60 | 2.30 | 15.80 | 7.80 | 7.10 | 7.80 | 7.60 | 15.60 | 28.60 |
| Difficulty swallowing | 2.20 | 4.50 | 5.30 | 3.90 | 2.20 | 1.00 | 1.40 | 6.30 | 4.80 |
| Dyspnea | 3.40 | 6.80 | 10.50 | 7.80 | 4.90 | 5.80 | 2.10 | 9.40 | 9.50 |
| Stiff joints | 2.30 | 1.10 | 5.30 | 2.00 | 1.60 | 1.00 | 0.70 | 3.10 | 4.80 |
| Fainting and dizziness | 5.60 | 4.50 | 5.30 | 7.80 | 6.00 | 9.70 | 4.80 | 18.80 | 19.00 |
| Headache | 4.50 | 6.80 | 10.50 | 13.70 | 3.30 | 4.90 | 4.10 | 12.50 | 23.80 |
Figure 4.
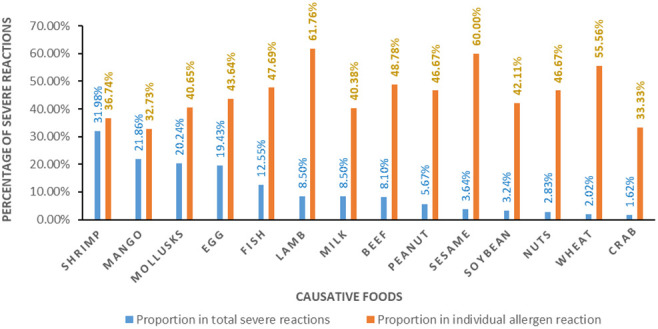
Severe reactions caused by major allergens.
Discussion
Food allergy is affecting the growth and development of children. A recent study from Australia has suggested that the rate of admissions for food anaphylaxis is increasing, and more dominant among elementary and secondary school children than among preschoolers (28). The prevention and treatment of FA are becoming a challenge for doctors, scientists, politicians, and the public (2). Allergen avoidance and drugs in case of an allergic reaction currently remain the standard of care for FA (14). Therefore, a clear understanding of the characteristics and patterns of FA prevalence will help to improve public health services and the prevention and management of FAs.
Prevalence of parent-reported FA
This study showed that 8.2% of parent-reported AFR prevalence among primary school children was higher for Asian children, which previously ranged between 1.2% and 6.5% (23), but was lower than the 10.5% prevalence of hypersensitivity to food among children in Italy, 46.9% among primary school children in Vilnius, Lithuania (2), and 12.6% in Japan (6), but was consistent with the 8% in the United Arab Emirates (2) and 8.1% in Hong Kong (23). The prevalence of parent-reported FA was estimated to be 6% among primary school-age children in Turkey and 7.6% in the USA (29), which are in accordance with our findings. The dietary culture, living environment, and genetic susceptibility are diverse in different countries or regions which might contribute to different distributions and features of FAs in different regions and countries.
Food allergens
Previous studies reported that the most common food allergens responsible for clinical reactions included milk, egg, peanut, tree nuts, mollusks, fish, wheat, and soy, which accounted for 90% of serious allergic reactions (6, 8). The list and order of the common food allergens vary in different countries or regions. For example, fish is a more common food allergen in Scandinavia; while soy is more common in Japan; peanuts in the United States and the United Kingdom; tropical fruits in Southeastern Asia; and seafood in Mediterranean countries. The different observations are most likely due to the differences in cultural traditions, dietary habits, culinary practices, and genetic factors (2).
Our study revealed some common food allergens for both parent-reported FA and doctor-diagnosed FA. Among the 100 FA causative foods analyzed, the top eight offending foods are different from the reports of FA studies in Hong Kong (23) and other regions (2, 29). The prevalence of peanut allergy is as high as 1%-3% among children in the USA, UK, and Australia but is less than 1% in most Asian countries (20, 30). Our study demonstrated that the prevalence of peanut allergy was 0.34%. Consuming peanuts in the form of boiled peanuts and peanut oil are very common in Southern China. The EuroPrevall-INCO Survey revealed that the prevalence of peanut allergy was 10 times higher in British Jewish children than in Israeli Jewish children, suggesting that high peanut consumption in early life could play an important role in explaining the difference (15).
The GB 7718-2011 National Food Safety Standard-General Standard for the Labeling of Prepackaged Foods in China lists eight categories of allergenic substances, referring to the Codex Alimentarius Standard, of recommended foods to encourage enterprises to voluntarily label to inform consumers. Three of the top eight allergenic foods (mango, beef, and lamb) in our study are not in the eight recommended food allergens list of GB 7718-2011. They were also the major food allergens that can cause severe allergic reactions. Since the occurrence and development of FA may change with economic and social development, relevant local standards should be adjusted on a timely basis according to the local situation in order to prevent and manage FA effectively.
Common symptoms of FA
Food allergy is a potentially fatal immune disease (31). Living with FA in childhood is well-recognized as impairing health (32, 33).
This study showed that skin symptoms were particularly prominent, such as rashes and itches, and accounted for 85% of the FA reaction. Perioral symptoms, nasal symptoms, headache, and dizziness were also noted frequently. Ebisawa and their team demonstrated that the most frequent clinical manifestations of FA were skin symptoms (92.0%), followed by respiratory symptoms (33.6%), mucosal symptoms (28.0%), gastrointestinal symptoms (18.6%), and shock (10.4%) (34). However, a similar study concluded that diarrhea and vomiting were the most common reactions of FA among schoolchildren (48.0%); a rash, urticarial rash, or itchy skin were also frequently observed disorders (46.4%) (2). Our study substantiated the previous findings and showed a high rate of severe allergic reactions (>45%) among the children with parent-reported or doctor-diagnosed FA.
Possible risk factors of food allergy
The epidemiological characteristics of FA may be related to the complex interaction of polygenic inheritance and environmental factors. The prevalence of FA ranges from 2% to 10%, based on different variables such as age, geographical location, or ethnicity (2, 29), and declines with the increase in age (22). However, a meta-analysis including 24 studies with 138,740 subjects concluded that the prevalence of FA in children aged 4-17 years was the highest (10%; 95% CI: 7%–14%) in China (25). Our survey study showed that the FA prevalence in schoolchildren was lower compared with that reported in teenagers (12.0%) (21) and college students (15.7%) (35). The FA prevalence among 8-11-year-old children was significantly higher than that among children aged 6-7 years old. Hayashi concluded that the FA prevalence was significantly higher in boys than girls among the schoolchildren (3), but our finding does not support this observation and instead is more consistent with the study among 7–15-year-old children in Japan showing the FA prevalence of 6.3% without difference between boys and girls (3). A previous report noted ethnic differences in the prevalence of FA among African Americans (10); the small sample size of ethnic minorities in this study prevented the assessment of such differences here. Levin et al. reported that exposure to livestock in rural regions could be the strongest protective factor against FA, and the prevalence of allergic diseases was higher in urban regions than in rural regions among South African children (36). This survey showed no difference in FA prevalence between rural children and urban children. A possible explanation may be due to the progress of urbanization in China and the diminishing lifestyle difference between urban and rural regions. Since the living environment and diet habits are quite different among the three survey centers, this may explain the variation in the FA prevalence among them. For example, kiwi is a locally special fruit of Fengxin and known to be an allergen there, however, we found that no child was allergic to it in the other two survey centers. According to the hygiene hypothesis, large family size is a protective factor against FA (17), and our study also showed that the prevalence of FA decreased with an increase in family size.
Food allergy is often the first clinical appearance of allergy and the first link of the so-called “march of atopy” (2). It is also associated with a higher risk of severe asthmatic forms in children (37) and more than a third of food-allergic children have asthma (38). Our analysis has also demonstrated that the prevalence of other allergic diseases was higher in children with FAs than in those without FAs. This finding was in agreement with the result reported by Liu et al. (39) Therefore, FA may be a marker of a generalized phenotype for atopic diseases, which may suggest that atopic diseases coexist and may share the same susceptibility genes (10).
This study has several limitations. It is a cross-sectional study, so it could not confirm a causal relationship and the incidence of FA was not available. Another major limitation is that our study was based on parent-reported FA. Recall bias may exist even though many common offending foods were listed in the questionnaire for parents to choose from and retrospective information about the FAs of their children. Some of the reported cases will not be substantiated when clinical confirmation is sought, because other ARF such as food intolerance may be regarded as FAs, so self-reported FA may overestimate FA prevalence by three or four times (40, 41). On the other hand, parent or self-reported FA is affected by many factors, such as economic status, awareness of FA, medical resources, etc., and people may be unaware of FAs or mistake an FA for another ARF, then result in the underestimation of FA prevalence. One study found that approximately 30.0% of allergic reactions occurred in children who were not previously known to have an FA (4). The Food Allergy Working Group of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition recently published the results of a survey that showed much diversity in the diagnostic approach, as well as poor adherence to the existing guidelines (42), but the consensus is that self-reported FA can be an appropriate method for assessing FA prevalence in population-based studies if ancillary testing is not possible (10). It is the initial evidence and an important first step for studies on FA prevalence, so self-reported FA is usually used in studies on FA, especially in a large-scale general population. Nevertheless, this is the first large-scale multicenter study on FA among children with a higher participation rate, high effective response rate, and compliance in Jiangxi Province, China. Strict quality control work was performed during the survey. The result will help to improve awareness of FA in the population and provide insights about the epidemiological characteristics of FA in China, which may form the scientific basis essential for the development of effective approaches for the prevention and control of FA in Jiangxi, and even in wider China. More objective diagnoses of FA including SPT, sIgE, and OFC will be performed in a further study to confirm these findings. The protocol is available in China CDC Weekly (27).
Conclusion
Our study has clearly demonstrated that the prevalence of parent-reported FA is not low among school children in Jiangxi Province, China. More than 45% of the children with parent-reported FA had experienced severe allergic reactions. Three of the eight leading food allergens are not included in the recommended eight food allergens in Chinese GB 7718. These findings suggest that bringing public attention to the importance of carefully reading food allergen labels is necessary to avoid food allergens.Furthermore, health education on FA prevention and treatment should be implemented for school administrators, teachers, medical care personnel, parents, and children in order to increase their awareness and essential knowledge of FA and effectively prevent and control FA in school children. In addition, local standards and policies for preventing and managing FA should be adjusted on a timely basis according to local conditions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Ethical Committees of China Center for Food Safety Risk Assessment and written informed consents were obtained from each child’s legal guardian.
Author contributions
HF, NL, YW, YC conceived and coordinated the study, designed, performed and analyzed the experiments, wrote the paper. YL, JL, JZ and XX, ZC carried out the data collection, data analysis, and revised the paper. All authors contributed to the article and approved the submitted version.
Abbreviations
ARF, Adverse reactions to food; FA, Food allergy; HIC, High-income country; SPT, Skin prick test; sIgE, Specific immunoglobulin E; OFC, Oral food challenge; SD, Standard Deviation.
Funding
Supported by CAMS Innovation Fund for Medical Science (CIFMS 2019-I2M-5-024); TCM Science and Technology Project of Jiangxi Province in 2021 (2021B696).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Fox M, Mugford M, Voordouw J, Cornelisse-Vermaat J, Antonides G, de la Hoz Caballer B, et al. Health sector costs of self-reported food allergy in Europe: a patient-based cost of illness study. Eur J Public Health (2013) 23:757–62. doi: 10.1093/eurpub/ckt010 [DOI] [PubMed] [Google Scholar]
- 2. Kavaliūnas A, D R, stukas R, Žagminas Kęstutis, Kummeling I, Mills C. Europrevall survey on prevalence and pattern of self-reported adverse reactions to food and food allergies among primary schoolchildren in Vilnius, lithuania. Prevalence Food Allerg, Medicina (Kaunas) (2012) 48(5):38. doi: 10.3390/medicina48050038. Genė Surkienėubakienė. GŠJŠ, pgJb. [DOI] [PubMed] [Google Scholar]
- 3. Hayashi K, Tsujiguchi H, Hori D, Yamada Y, Shimizu Y, Nguyen TTT, et al. The association between overweight and prevalence of food allergy in Japanese children: a cross-sectional study. Environ Health Prev Med (2021) 26(1):44. doi: 10.1186/s12199-021-00960-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santos MJL, Merrill KA, Gerdts JD, Ben-Shoshan M, Protudjer JLP. Food allergy education and management in schools: A scoping review on current practices and gaps. Nutrients (2022) 14(4):732. doi: 10.3390/nu14040732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ali F. A survey of self-reported food allergy and food-related anaphylaxis among young adult students at Kuwait university, Kuwait. Med Princ Pract (2017) 26(3):229–34. doi: 10.1159/000464361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devdas JM, McKie C, Fox AT, Ratageri VH. Food allergy in children: An overview. Indian J Pediatr (2018) 85(5):369–74. doi: 10.1007/s12098-017-2535-6 [DOI] [PubMed] [Google Scholar]
- 7. Tang R, Wang ZX, Ji CM, Leung PSC, Woo E, Chang C, et al. Regional differences in food allergies. Clin Rev Allergy Immunol (2019) 57(1):d 98–110. doi: 10.1007/s12016-018-8725-9 [DOI] [PubMed] [Google Scholar]
- 8. Heideman K, Poronsky CA-O. Protocols for managing food allergies in elementary and secondary schools. Compr Child Adolesc Nurs (2021) 4:2469–4207. doi: 10.1080/24694193.2021.1883771 [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Wang Z-Z, Geliebter J, Tiwari R, Li X-M. Traditional Chinese medicine for food allergy and eczema. Ann Allergy Asthma Immunol (2021) 126(6):639–54. doi: 10.1016/j.anai.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 10. Wang XY, Zhuang Y, Ma TT, Zhang B, Wang XY. Prevalence of self-reported food allergy in six regions of inner Mongolia, northern China: A population-based survey. Med Sci Monit (2018) 24:1902–11. doi: 10.12659/msm.908365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suaini NHA, Loo EX, Peters RL, Yap GC, Allen KJ, Van Bever H, et al. Children of Asian ethnicity in Australia have higher risk of food allergy and early-onset eczema than those in Singapore. Allergy (2021) 76(10):3171–82. doi: 10.1111/all.14823 [DOI] [PubMed] [Google Scholar]
- 12. Zepeda-Ortega B, Goh A, Xepapadaki P, Sprikkelman A, Nicolaou N, Hernandez REH, et al. Strategies and future opportunities for the prevention, diagnosis, and management of cow milk allergy. Front Immunol (2021) 12:608372. doi: 10.3389/fimmu.2021.608372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol (2014) 133(2):291–307. doi: 10.1016/j.jaci.2013.11.020 [DOI] [PubMed] [Google Scholar]
- 14. Mori F, Giovannini M, Barni S, Jimenez-Saiz R, Munblit D, Biagioni B, et al. Oral immunotherapy for food-allergic children: A pro-con debate. Front Immunol (2021) 12:636612. doi: 10.3389/fimmu.2021.636612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Ogorodova LM, Mahesh PA, Wang MH, Fedorova OS, Leung TF, et al. Comparative study of food allergies in children from China, India, and Russia: The EuroPrevall-INCO surveys. J Allergy Clin Immunol: In Pract (2020) 8(4):1349–58.e1316. doi: 10.1016/j.jaip.2019.11.042 [DOI] [PubMed] [Google Scholar]
- 16. Ma Z, Chen L, Xian R, Fang H, Wang J, Hu Y. Time trends of childhood food allergy in China: Three cross-sectional surveys in 1999, 2009, and 2019. Pediatr Allergy Immunol (2021) 32(5):1073–79. doi: 10.1111/pai.13490 [DOI] [PubMed] [Google Scholar]
- 17. Barni S, Mori F, Giovannini M, de Luca M, Novembre E. In situ simulation in the management of anaphylaxis in a pediatric emergency department. Intern Emerg Med (2019) 14(1):127–32. doi: 10.1007/s11739-018-1891-1 [DOI] [PubMed] [Google Scholar]
- 18. Foong RX, Giovannini M, du Toit G. Food-dependent exercise-induced anaphylaxis. Curr Opin Allergy Clin Immunol (2019) 19(3):224–8. doi: 10.1097/ACI.0000000000000531 [DOI] [PubMed] [Google Scholar]
- 19. Nagakura KI, Sato S, Asaumi T, Yanagida N, Ebisawa M. Novel insights regarding anaphylaxis in children - with a focus on Prevalence,Diagnosis, and treatment. Pediatr Allergy Immunol (2020) 31(8):879–88. doi: 10.1111/pai.13307 [DOI] [PubMed] [Google Scholar]
- 20. Warren C, Lei D, Sicherer S, Schleimer R, Gupta R. Prevalence and characteristics of peanut allergy in US adults. J Allergy Clin Immunol (2021) 147(6):2263–70.e2265. doi: 10.1016/j.jaci.2020.11.046 [DOI] [PubMed] [Google Scholar]
- 21. Pereira B, Venter C, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J Allergy Clin Immunol (2005) 116(4):884–92. doi: 10.1016/j.jaci.2005.05.047 [DOI] [PubMed] [Google Scholar]
- 22. Ontiveros N, Valdez-Meza EE, Vergara-Jiménez MJ, Canizalez-Román A, Borzutzky A, Cabrera-Chávez F. Self-reported prevalence of food allergy in Mexican schoolchildren: A population-based study. Allergol Immunopathol (Madr) (2016) 44(6):563–70. doi: 10.1016/j.aller.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 23. Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Self-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol (2009) 20(4):339–46. doi: 10.1111/j.1399-3038.2008.00801.x [DOI] [PubMed] [Google Scholar]
- 24. Hu Y, Chen J, Li H. Comparison of food allergy prevalence among Chinese infants in chongqing, 2009 versus 1999. Pediatr Int (2010) 52(5):820–4. doi: 10.1111/j.1442-200X.2010.03166.x [DOI] [PubMed] [Google Scholar]
- 25. Luo J, Zhang Q, Gu Y, Wang J, Liu G, He T, et al. Meta-analysis: Prevalence of food allergy and food allergens — China, 2000–2021. China CDC Weekly (2022) 4(34):766–70. doi: 10.46234/ccdcw2022.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kummeling I, Mills ENC, Clausen M, Dubakiene R, Pérez CF, Fernández-Rivas M, et al. The EuroPrevall surveys on the prevalence of food allergies in children and adults: background and study methodology. Allergy (2009) 64(10):1493–7. doi: 10.1111/j.1398-9995.2009.02046.x [DOI] [PubMed] [Google Scholar]
- 27. Feng H, Chen Y, Chen H, Liu C, Zhou W, Wang L, et al. A methodology of epidemiologic study in the general population focusing on food allergy — China, 2020. China CDC Weekly (2022) 4(34):749–55. doi: 10.46234/ccdcw2022.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sasaki M, Koplin JJ, Dharmage SC, Field MJ, Sawyer SM, McWilliam V, et al. Prevalence of clinic-defined food allergy in early adolescence: The SchoolNuts study. J Allergy Clin Immunol (2018) 141(1):391–8.e394. doi: 10.1016/j.jaci.2017.05.041 [DOI] [PubMed] [Google Scholar]
- 29. Celebioglu E, Akarsu A, Sahiner UM. IgE-mediated food allergy throughout life. Turk J Med Sci (2021) 51(1):49–60. doi: 10.3906/sag-2006-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US Prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol (2010) 125(6):1322–6. doi: 10.1016/j.jaci.2010.03.029 [DOI] [PubMed] [Google Scholar]
- 31. Esther B, Florsheim ZAS, Khoury-Hanold W, Medzhitov R. Food allergy as a biological food quality control system. Cell (2021) 3(18):1440–54. doi: 10.1016/j.cell.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 32. Vreeken-Ross SC, Smith HE, Jones CJ. A review of freely available online support for food allergy-related emotional well-being. Clin Exp Allergy (2021) 51(7):942–46. doi: 10.1111/cea.13868 [DOI] [PubMed] [Google Scholar]
- 33. Anvari S, Miller J, Yeh CY, Davis CM. IgE-mediated food allergy. Clin Rev Allergy Immunol (2019) 57(2):244–60. doi: 10.1007/s12016-018-8710-3 [DOI] [PubMed] [Google Scholar]
- 34. Ebisawa M, Ito K, Fujisawa T, Committee for Japanese Pediatric Guideline for Food Allergy, T. J. S. o. P. A., Clinical, I. Japanese Society of, A . Japanese Guidelines for food allergy 2020. Allergol Int (2020) 69(3):370–86. doi: 10.1016/j.alit.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 35. Feng H, Liu Y, Wu Y. Self-reported food allergy and its familial aggregation in college students. Chin J Food Hygiene (2021) 33(3):291–5. doi: 10.13590/j.cjfh.2021.03.009 [DOI] [Google Scholar]
- 36. Levin ME, Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, et al. Environmental factors associated with allergy in urban and rural children from the south African food allergy (SAFFA) cohort. J Allergy Clin Immunol (2020) 145(1):415–26. doi: 10.1016/j.jaci.2019.07.048 [DOI] [PubMed] [Google Scholar]
- 37. Olabarri M, Vazquez P, Gonzalez-Posada A, Sanz N, Gonzalez-Peris S, Diez N, et al. Risk factors for severe anaphylaxis in children. J Pediatr (2020) 225:193–7.e5. doi: 10.1016/j.jpeds.2020.06.021 [DOI] [PubMed] [Google Scholar]
- 38. Simpson AB, Glutting J, Yousef E. Food allergy and asthma morbidity in children. Pediatr Pulmonol (2007) 42(6):489–95. doi: 10.1002/ppul.20605 [DOI] [PubMed] [Google Scholar]
- 39. Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the national health and nutrition examination survey 2005-2006. J Allergy Clin Immunol (2010) 126(4):798–806.e713. doi: 10.1016/j.jaci.2010.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Althumiri NA, Basyouni MH, AlMousa N, AlJuwaysim MF, BinDhim NF, Alqahtani SA. Prevalence of self-reported food allergies and their association with other health conditions among adults in Saudi Arabia. Int J Environ Res Public Health (2021) 18(1):347. doi: 10.3390/ijerph18010347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loh W, Tang M. The epidemiology of food allergy in the global context. Int J Environ Res Public Health (2018) 15:2043. doi: 10.3390/ijerph15092043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toca MC, Morais MB, Vazquez-Frias R, Becker-Cuevas DJ, Boggio-Marzet CG, Delgado-Carbajal L, et al. Consensus on the diagnosis and treatment of cow's milk protein allergy of the Latin American society for pediatric gastroenterology, hepatology and nutrition. Rev gastroenterologia Mexico (English) (2022) 87(2):235–50. doi: 10.1016/j.rgmxen.2022.01.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.