Abstract
Swinhoe’s tree lizard (Diploderma swinhonis: D. swinhonis) is an arboreal agamid that is native to Taiwan. In Taiwan, the lizard is considered to be a generalist that feeds primarily on ants and a diversity of small insect prey by employing an opportunistic sit-and-wait foraging strategy. In Japan, D. swinhonis is considered as an invasive alien species that was discovered in Hyuga city, Miyazaki Prefecture, in 2016. Despite concerns about the impact of D. swinhonis on native fauna, little information about the diet of this alien species has been published to date. This study, therefore, investigated the feeding ecology of D. swinhonis in Hyuga city to evaluate their potential impact on the ecosystem. Specifically, prey preference was investigated by examining the stomach contents of males, females, and juveniles captured from April to December 2020 and in March 2021. The results showed that the lizards in Hyuga preyed upon a wide variety of invertebrates as in Taiwan, while ants accounted for the largest proportion of the prey items consumed regardless of sex, age or changes in season. These findings indicated that D. swinhonis might cause a decrease in the abundance of the native insect fauna of Hyuga city or competition with native lizards for foods in Hyuga city. Since its impact is not currently apparent, it’s necessary to monitor its effect in the future.
Keywords: feeding ecology, invasive alien species, seasonal change, tree lizard
Examples of invasive alien arboreal lizards are now found all around the world. Species such as brown anoles (Anolis sagrei), green anoles (A. carolinensis) and oriental garden lizards (Calotes versicolor) have caused a variety of ecological problems in the areas that they have invaded [1, 3, 6, 10, 18]. For example, A. carolinensis, which is an arboreal, diurnal, sit-and-wait predator, was introduced to Ogasawara Islands, Japan, where it has caused the local extinction and significant decline of a variety of native insects [26]. Furthermore, populations of native lizards on Ogasawara Islands (e.g., Ogasawara snake-eyed skink, Cryptoblepharus nigropunctatus) have decreased significantly due to predation by, and competition with, A. carolinensis [27]. Anolis carolinensis has a considerably larger head and mouth than C. nigropunctatus, and can eat larger prey [27]. A previous study showed that A. carolinensis has significantly altered the islands’ environment through a decline in primary consumers and the concomitant increase in secondary consumers [27].
The introduction of arboreal, diurnal lizards into the mainland of Japan has also been reported [13, 22]. Invasion of Swinhoe’s tree lizard (Diploderma swinhonis (Günther, 1864): D. swinhonis) was reported on the mainland of Japan in 2006 for the first time [15]. D. swinhonis is a diurnal, arboreal agamid and native to Taiwan and Orchid Island [21, 32]. Currently, in Japan, populations of alien D. swinhonis have been reported in Iwata City, Shizuoka Prefecture, Atsugi City, Kanagawa Prefecture, and Hyuga City, Miyazaki Prefecture [4, 11, 15]. D. swinhonis is regarded as an invasive alien species, and raising, keeping or transporting this species is prohibited by the Japanese Invasive Alien Species Act from 2016. We previously reported the reproductive cycle and size at sexual maturity of the nonnative population of D. swinhonis in Hyuga city [11]; oviposition of averaged clutch size 5.1 ± 1.5 occurs from May to October, and the size (snout-vent length) at sexual maturity is 53.0 mm in males and 50.2 mm in females [11]. The population in Hyuga city is smaller at sexual maturity and has a larger clutch size than the native populations in Taiwan [11].
Despite the concerns for alien arboreal lizards, the diet of alien D. swinhonis distributed in Japan is not well documented. Although the adverse effects of such introductions cannot be entirely understood based on feeding ecology alone, a knowledge of the impacts of introduced alien species can provide crucial information for predicting possible future impacts of these species on native fauna. Studies on the diet of native D. swinhonis in Taiwan have shown that this species is a dietary generalist and a sit-and-wait predator [9, 17, 19]. Although D. swinhonis occasionally feeds on large prey, such as cicadas and other mature lizards [19, 31], it feeds mainly on hymenopteran prey (primarily ants) [19]. In Japan, two studies have examined the diet of nonnative D. swinhonis [4, 15]; however, the sample sizes employed in those studies was small and seasonal changes in diet, sexual differences in feeding preference, and ontogenic shifts in the diet were not examined [4, 15]. To document the whole aspect of the feeding ecology of alien D. swinhonis, we examined the stomach contents of D. swinhonis in Hyuga city. By conducting monthly field surveys and collecting numerous samples, this study provides a more accurate understanding of the feeding ecology of this species. In addition, comparison of the findings with data from Taiwan reveals the changes that were caused by introduction into Japan.
MATERIALS AND METHODS
Field research
Linear transect surveys were conducted in Hyuga city, Miyazaki Prefecture, Japan (32°26’N, 131°39’E) from April 2020 to December 2020 and March 2021. We walked and captured lizards on trees or on the ground. For lizards found in trees, the height of capture was recorded to the nearest 10 cm based on visual estimates.
Morphology and diet composition
After returning the captured specimens to the laboratory, the lizards were euthanized by intracelomic injection of sodium or calcium pentobarbital. Snout-vent length (SVL) was measured to the nearest 0.1 mm using digital calipers, and body weight (BW) was measured to the nearest 0.01 g using a digital scale. The lizards were dissected and sexed based on the morphology of the genital organ. As shown in Table 1, the stomachs of up to 9 individuals (males, females and juveniles) were removed per month. Based on our previous study, male and female lizards with SVL less than 53 mm and 50.2 mm, respectively, were considered to be juveniles [12]. The dissected stomachs were preserved in 100% ethanol until such time as the stomach contents were examined under a stereomicroscope (Leica MZ6, Leica Microsystems GmbH, Wetzlar, Germany). Almost all of the stomach contents were identified to the level of order. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Miyazaki, Japan (Approval No. 2021-007). The lizards were kept in the facility permitted by the Ministry of the Environment (Permission No. 17000092).
Table 1. The number of lizards used in the experiment.
| 2021 | 2020 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| March | April | May | June | July | August | September | October | November | December | ||
| Male | 0 | 2 | 6 | 6 | 6 | 6 | 6 | 6 | 1 | 2 | 41 |
| Female | 1 | 2 | 6 | 6 | 6 | 6 | 6 | 6 | 5 | 4 | 48 |
| Juvenile | 5 | 3 | 2 | 0 | 2 | 1 | 2 | 2 | 2 | 9 | 28 |
| Total | 6 | 7 | 14 | 12 | 14 | 13 | 14 | 14 | 8 | 15 | 115 |
Males were larger than 53.0 mm in snout-vent length (SVL), and females were larger than 50.2 mm in SVL [12]. Juveniles were smaller than size at sexual maturity and did not sexed.
Statistical analysis
Statistical analyses were performed in R version 4.0.5 (R core team, 2021). Using the mean of each item, the Euclidean distance for each month was calculated. This was used as the degree of dissimilarity, and a hierarchical cluster analysis using the group average method was conducted to identify seasonal differences. Based on this analysis, March to June was defined as season A (spring to early summer), July as season B (summer), August to October as season C (summer to autumn), November (late autumn to winter) as season D, and December (winter) as season E. The Shannon-Wiener index was used to evaluate the diversity in the diet composition. The Jaccard distance was used to assess dissimilarity among seasons, sexes and growth stage. Both indexes were calculated using R package vegan (version 2.5–7). Wilcoxon rank-sum test was used to examine differences among sexes, age classes and seasonal changes in the number of stomach contents. Kruskal-Wallis test was used to examine seasonal changes and sexual or growth differences in the number of prey items of each order or group. The data were shown as the mean ± SD. A value of P<0.05 was considered significant.
RESULTS
Field research and morphology
As shown in Table 1, a total of 117 D. swinhonis specimens were examined. Mean SVL, BW and the height of capture are shown in Table 2. The capture height of males was higher than that of females and juvenile lizards (P<0.05), as shown in Table 2.
Table 2. Snout-vent length (SVL), body weight (BW), and capture height of mature male, female and immature Diploderma swinhonis in Hyuga city.
| SVL (mm) | BW (g) | Capture height (m) | |
|---|---|---|---|
| Male | 78.3 ± 4.5 | 15.0 ± 2.5 | 2.0 ± 1.2* |
| (66.9–85.1) | (8.8–19.8) | (0–5) | |
| Female | 64.5 ± 5.9 | 8.4 ± 2.5 | 1.2 ± 0.8 |
| (53.7–78.8) | (3.3–15.0) | (0–4) | |
| Juveniles | 33.5 ± 5.9 | 1.4 ± 0.7 | 1.1 ± 1.2 |
| (24.7–40.5) | (0.1–3.3) | (0–5) |
SVL: snout-vent length, BW: body weight. Data show mean ± SD. Ranges are shown in parentheses. *P<0.05. Immature D. swinhonis were defined as the snout-vent length of D. swinhonis is more than 53.0 mm (male) or 50.2 mm (female) [12].
Stomach contents
A total of 1875 prey items belonging to 6 classes and at least 17 orders were recorded in the stomachs of D. swinhonis captured in Hyuga city (Table 3). The prey items consisted mainly of Hymenoptera, more than 90% of which were ants (Formicidae), followed by Coleoptera, Hemiptera and various insect larvae, in order of frequency (Table 3). The Shannon-Wiener index was H’=1.35.
Table 3. The diet composition (percentage of each type of dietary item (%N) and occurrence frequency of each prey (F)), the Shannon-Wiener index, and Jaccard distance for total data and by sex and age class of Diploderma swinhonis in Hyuga city.
| Class | Order | Total | Male | Female | Juvenile | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N% | F | N% | F | N% | F | N% | F | |||||
| Insecta | Balattodea | 0.11% | 0.02 | 0.00% | 0.00 | 0.23% | 0.04 | 0.00% | 0.00 | |||
| Coleoptera | 6.24% | 0.44 | 5.84% | 0.49 | 6.64% | 0.41 | 5.96% | 0.43 | ||||
| Dermaptera | 0.11% | 0.02 | 0.15% | 0.02 | 0.11% | 0.02 | 0.00% | 0.00 | ||||
| Diptera | 0.05% | 0.01 | 0.00% | 0.00 | 0.00% | 0.00 | 0.33% | 0.04 | ||||
| Hemiptera | 4.75% | 0.29 | 4.23% | 0.37 | 5.86% | 0.33 | 2.65% | 0.11 | ||||
| Hymenoptera | 75.15% | 0.81 | 77.08% | 0.88 | 73.54% | 0.82 | 75.50% | 0.68 | ||||
| Lepidoptera | 0.05% | 0.01 | 0.15% | 0.02 | 0.00% | 0.00 | 0.00% | 0.00 | ||||
| Mantodea | 0.16% | 0.03 | 0.15% | 0.02 | 0.23% | 0.04 | 0.00% | 0.00 | ||||
| Orthoptera | 0.21% | 0.03 | 0.15% | 0.02 | 0.34% | 0.06 | 0.00% | 0.00 | ||||
| Psocoptera | 0.05% | 0.01 | 0.00% | 0.00 | 0.00% | 0.00 | 0.33% | 0.04 | ||||
| Phasmatodea | 0.11% | 0.02 | 0.00% | 0.00 | 0.23% | 0.04 | 0.00% | 0.00 | ||||
| Arachnida | Araneae | 2.08% | 0.25 | 2.92% | 0.37 | 1.69% | 0.24 | 1.32% | 0.07 | |||
| Dipropoda | 1.49% | 0.17 | 1.02% | 0.15 | 2.36% | 0.29 | 0.00% | 0.00 | ||||
| Chilopoda | 0.59% | 0.07 | 0.44% | 0.07 | 0.56% | 0.06 | 0.99% | 0.07 | ||||
| Gastropoda | Stylommatophora | 0.16% | 0.03 | 0.00% | 0.00 | 0.34% | 0.06 | 0.00% | 0.00 | |||
| Malacostraca | Amphipoda | 0.11% | 0.02 | 0.00% | 0.00 | 0.00% | 0.00 | 0.66% | 0.07 | * | ||
| Malacostraca | Isopoda | 0.75% | 0.08 | 0.29% | 0.05 | 0.90% | 0.06 | 1.32% | 0.14 | |||
| Larva (mainly lepidoptera) | 3.63% | 0.38 | 4.53% | 0.41 | 2.59% | 0.37 | 4.64% | 0.36 | ||||
| Unknown | 4.21% | 0.42 | 3.07% | 0.34 | 4.39% | 0.47 | 6.29% | 0.46 | ||||
| Shannon-Wiener index | 1.35 | 1.25 | 1.42 | 1.22 | ||||||||
| Jaccard distance | vs. female | 0.25 | vs. juvenile | 0.67 | ||||||||
| vs. juvenile | 0.59 | |||||||||||
*P<0.05.
Seasonal changes in stomach contents
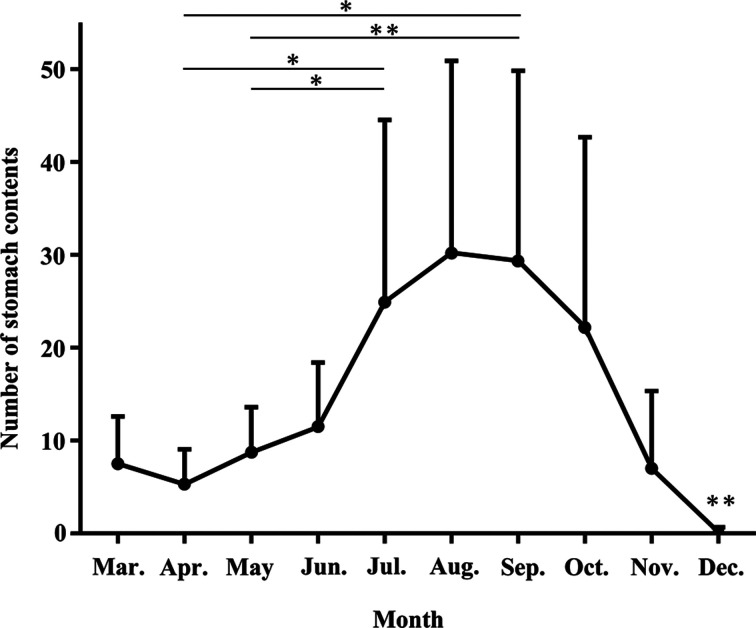
A significant difference was observed in the number of stomach contents among seasons (Fig. 1). Specifically, the number of stomach contents remained low from March to June and increased in July. The values obtained for May and June were significantly different from those for July and September (between May and September P<0.01, others P<0.05). Thereafter, the number of stomach contents decreased from October to November and was the lowest in December when it differed significantly compared to the other months (P<0.01). In December, only one lizard (juvenile) had stomach contents. The stomach contained two items; one was a hymenopteran (an ant) and the other could not be identified (Season E in Table 4).
Fig. 1.
Seasonal changes in the number of stomach contents of Diploderma swinhonis in Hyuga City. Data are shown as the mean + SD. *: P<0.05, **: P<0.01.
Table 4. The diet composition (percentage of each type of dietary item (%N) and occurrence frequency of each prey (F)), the Shannon-wiener index, and the Jaccard distance for the season of Diploderma swinhonis in Hyuga city.
| Class | Order | Season A | Season B | Season C | Season D | Season E | Significance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring to early summer | Summer | Summer to autumn | Late autumn to winter | Winter | ||||||||
| N% | F | N% | F | N% | F | N% | F | N% | F | |||
| Insecta | Balattodea | 0.00% | 0.00 | 0.57% | 0.14 | 0.00% | 0.00 | 0.00% | 0.00 | 0.00% | 0.00 | ** |
| Coleoptera | 26.06% | 0.80 | 3.72% | 0.57 | 1.08% | 0.29 | 0.00% | 0.00 | 0.00% | 0.00 | ** | |
| Dermaptera | 0.28% | 0.03 | 0.00% | 0.00 | 0.09% | 0.02 | 0.00% | 0.00 | 0.00% | 0.00 | ||
| Diptera | 0.28% | 0.03 | 0.00% | 0.00 | 0.00% | 0.00 | 0.00% | 0.00 | 0.00% | 0.00 | ||
| Hemiptera | 6.52% | 0.33 | 10.32% | 0.57 | 2.60% | 0.29 | 1.79% | 0.13 | 0.00% | 0.00 | ||
| Hymenoptera | 44.48% | 0.83 | 74.50% | 1.00 | 84.93% | 1.00 | 78.57% | 0.75 | 50.00% | 0.07 | ** | |
| Lepidoptera | 0.00% | 0.00 | 0.00% | 0.00 | 0.09% | 0.02 | 0.00% | 0.00 | 0.00% | 0.00 | ||
| Mantodea | 0.00% | 0.00 | 0.00% | 0.00 | 0.27% | 0.07 | 0.00% | 0.00 | 0.00% | 0.00 | ||
| Orthoptera | 0.00% | 0.00 | 0.29% | 0.07 | 0.27% | 0.07 | 0.00% | 0.00 | 0.00% | 0.00 | ||
| Psocoptera | 0.00% | 0.00 | 0.29% | 0.07 | 0.00% | 0.00 | 0.00% | 0.00 | 0.00% | 0.00 | ||
| Phasmatodea | 0.28% | 0.03 | 0.29% | 0.07 | 0.00% | 0.00 | 0.00% | 0.00 | 0.00% | 0.00 | ||
| Arachnida | Araneae | 1.98% | 0.18 | 1.15% | 0.29 | 2.51% | 0.44 | 0.00% | 0.00 | 0.00% | 0.00 | |
| Dipropoda | 1.70% | 0.13 | 0.00% | 0.00 | 1.61% | 0.29 | 7.14% | 0.38 | 0.00% | 0.00 | ||
| Chilopoda | 1.98% | 0.10 | 0.29% | 0.07 | 0.18% | 0.05 | 1.79% | 0.13 | 0.00% | 0.00 | ||
| Gastropoda | Stylommatophora | 0.57% | 0.05 | 0.00% | 0.00 | 0.09% | 0.02 | 0.00% | 0.00 | 0.00% | 0.00 | |
| Malacostraca | Amphipoda | 0.57% | 0.05 | 0.00% | 0.00 | 0.00% | 0.00 | 0.00% | 0.00 | 0.00% | 0.00 | |
| Malacostraca | Isopoda | 1.42% | 0.10 | 0.57% | 0.14 | 0.63% | 0.07 | 0.00% | 0.00 | 0.00% | 0.00 | |
| Larva (mainly lepidoptera) | 8.22% | 0.48 | 4.30% | 0.57 | 2.06% | 0.41 | 1.79% | 0.13 | 0.00% | 0.00 | ||
| Unknown | 5.67% | 0.45 | 3.72% | 0.57 | 3.59% | 0.49 | 8.93% | 0.38 | 50.00% | 0.07 | ||
| Shannon-wiener index | 2.19 | 1.28 | 0.87 | 0.81 | 0.00 | |||||||
| Jaccard distance | vs. B | 0.52 | vs. C | 0.70 | vs. D | 0.95 | vs. E | 0.98 | ||||
| vs. C | 0.80 | vs. D | 0.86 | vs. E | 1.00 | |||||||
| vs. D | 0.85 | vs. E | 1.00 | |||||||||
| vs. E | 1.00 | |||||||||||
March to June was defined as season A (spring to early summer), July as season B (summer), August to October as season C (summer to autumn), November (late autumn to winter) as season D, December (winter) as season E. **P<0.01.
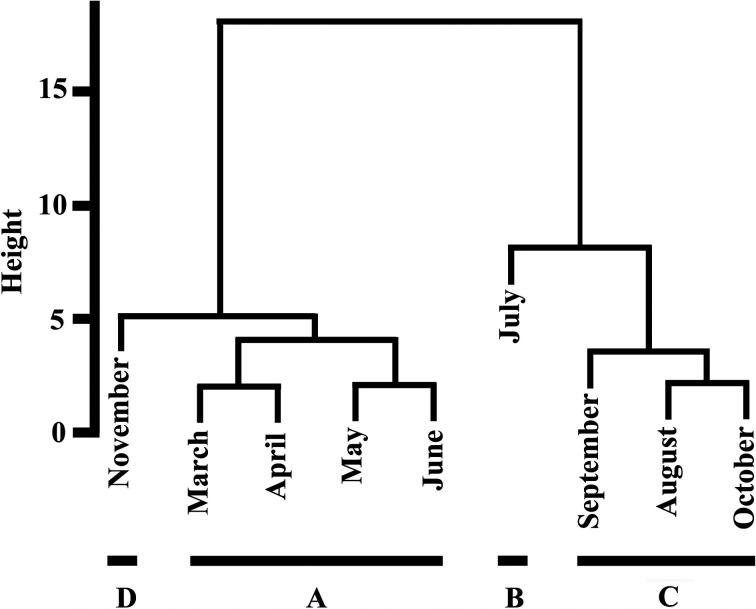
In the hierarchical cluster analysis, we excluded the results for juveniles, as they were not sampled consistently throughout the year (Table 1), as well as the results for December, as there were almost no stomach contents. A hierarchical cluster analysis revealed two clusters and one clade (Fig. 2), with March, April, May, June and November forming one cluster, and August, September and October forming the other cluster. July formed an independent clade.
Fig. 2.
Cluster analysis of stomach contents shown as a dendrogram. Seasonal divisions were indicated by A, B, C and D. The results for juveniles and the results for December were excluded in this analysis because juveniles were not sampled consistently throughout the year, and there were almost no stomach contents in December.
As shown in Table 4, the Shannon-Wiener index (H’) was 2.19 for season A, 1.28 for season B, 0.87 for season C, 0.81 for season D, and 0.00 for season E. The Jaccard distance exceeded 0.70, except for the value between season A and season B, which was 0.52. The results showed that the composition of the diet in each period exhibited seasonal changes.
The composition of the stomach contents in each season was also shown in Table 4. There were significant differences in the number of Hymenoptera, Coleoptera and Blattodea, which the lizards preyed upon throughout the year (Kruskal-Wallis test: P<0.01). Hymenoptera was fewer in season A (spring to early summer) compared to other seasons. In contrast, Coleoptera was more numerous in season A (spring to early summer) than in the other seasons. Blattodea was gained from only two female lizards in season B (July). Although not significant, the seasonal variation in Hemiptera was similar to that of Coleoptera.
Comparison of stomach contents by sex and growth stage
Regardless of sex and growth stage, lizards preyed mainly upon Hymenoptera (N% >73.54, Table 3). The Jaccard distance showed a difference in diet composition between mature and juvenile lizards (male vs. juvenile, 0.59; female vs. juvenile, 0.67). In contrast, index values were similar between sexes (male vs. female, 0.25). As shown in Table 3, the Shannon-Wiener index (H’) was 1.25 for males, 1.42 for females, and 1.22 for juveniles. There was a significant difference between sex and growth stage in the number of Amphipoda, which were gain from only juveniles (P<0.05, Table 3).
Of the 17 taxonomic orders observed in the stomach contents of all lizards, the stomach contents of males contained representatives of 11 orders within 17 orders. The remaining six orders consisted of Blattodea, Diptera, Psocoptera, Phasmatodea, Stylommatophora, and Amphipoda. Imagines of Lepidoptera were gain from only males. Fourteen orders were gain from females’ stomach, and there was no members of Diptera, Lepidoptera, Psocoptera or Amphipoda. The three orders of Blattodea, Phasmatodea and Stylommatophora were gained from only females. From Juveniles’ stomach, arthropods belonging to 9 orders were gained, and the stomachs did not contain members of Blattodea, Dermaptera, Lepidoptera, Mantodea, Orthoptera, Phasmatodea, Diplopoda and Stylommatophora. Diptera, Psocoptera and Amphipoda were gain from only juveniles.
DISCUSSION
The findings of this study showed that, as in Taiwan, D. swinhonis in Hyuga city ate mainly Hymenoptera (primarily ants), although a variety of invertebrates were preyed upon. The findings showed that there were seasonal changes in the diet of D. swinhonis for the first time, with the number of stomach contents increasing significantly during summer and the composition of the diet changing significantly from spring to summer. In other lizards, previous studies have also reported seasonal changes in the number of stomach contents [20, 25, 29]. These changes have often attributed to changes in food availability and/or lizards’ activity throughout the year [5, 12, 23]. In addition, changes in the lizards’ behavior, such as those associated with reproduction, can account for seasonal changes in food intake [8]. In the Japanese temperate zone, ants show phenology in the activity out of their nest. For example, in Tokyo, the number of Formica japonica out of its nest keeps low from May to early July and increases from mid-July to August [7]. Similarly, the community of ants shows the seasonal changes that increase in frequency found in bait traps from April to June and the decrease in frequency after October in Ibusuki City, Kagoshima Prefecture, which is near Hyuga city [33]. It is therefore expected that food availability especially in ants may increase from spring (March–May) to summer (June–August) and that the low abundance of stomach contents observed from March to June may be associated with low food availability at that time. Furthermore, lizards are rarely observed in Hyuga city from November to March, suggesting that they are not active at this time. The low number from November to December may be related to the low activity of lizards.
In this study, during season A (from March to June), the proportion and number of Coleoptera and Hemiptera were more numerous while that of Hymenoptera was fewer compared to other seasons. In addition, the Shannon-Wiener index values showed that the highest diversity of stomach contents was observed during season A. Since this high diversity cannot be explained by a decrease in food availability or lizard activity, two hypotheses are proposed to explain this phenomenon. The first is that, as has been reported for other lizard species, the composition of the lizards’ diet changed in response to food availability [12, 24]. In other words, Coleoptera and Hemiptera increased in the microhabitat of D. swinhonis during season A. The other hypothesis is that the food preference and/or feeding strategy employed by the lizards varies depending on their resource requirements. As a result of depleting their fat reserves in winter, the lizards have high energy requirements as they become active again in spring (March or April). Since, in Hyuga city, the formation of vitellogenic ovarian follicles starts in mid-May [11], it is reasonable to assume that lizards require a lot of resources at the onset of spring. Consequently, they may not be able to meet all of their energy needs by foraging on their preferred prey and may be forced to feed on prey that they would not typically feed upon. A similar pattern has been reported in two species of dwarf chameleons (Bradypodion ventrale, Bradypodion taeniabronchum), which have been observed to change their prey selection as food availability decreases during winter [2]. In addition, it is known that D. swinhonis avoid weevils (Pachyrhynchus tobafolius and Kashotonus multipunctatus) that is too hard and unprofitable through laboratory experiment [28]. The seasonal change in diet composition, especially in Coleoptera, may reflect change in food preference depending on resource require and avoidance of unprofitable prey. However, the present results do not provide sufficient support for either of these hypotheses and additional research on seasonal changes in food availability and lizard behavior is necessary.
In this study, D. swinhonis preyed primarily on ants, regardless of sex and age class. Although this suggests there is little difference between sex and age class, the Jaccard distances showed that there was considerably more similarity between the sexes than between adults and juveniles, indicating the likelihood of some ontogenic shifts in diet. It has been reported that ontogenic shifts in diet can promote differences in the use of feeding microhabitat, head size and bite power [24, 30]. In D. swinhonis, females and juveniles use similar microhabitats (low bushes at the bases of trees or near ground) [14], but the results of stomach contents suggested lower similarity between juveniles and females than that between sexes (Table 3). The present result also showed lower diversity of juveniles’ stomach contents than that of adults. However, 43% of juveniles preyed upon Coleoptera, which are hard (Table 3), suggesting that they have jaws that are powerful enough to eat hard prey. Juveniles can chew hard prey but have smaller heads than adults and cannot eat prey large enough to fit in their mouths. Therefore, we suspect that head size is more important for restricting juveniles’ prey and associated with lower diversity of juveniles’ stomach contents than females’. On the other hand, although male D. swinhonis have a larger head than females [16], the Shannon-Wiener index suggested that females fed on a wider variety of prey items than males and juveniles. In the field surveys, we observed that males were found higher above the ground than females (Table 1). In addition, both male and female mature lizards ate relatively large prey, such as cicadas. These findings imply that mature lizards with sufficiently powerful jaws can eat large prey, regardless of their head size, and that the difference in diet composition can be attributed to differences in their microhabitat use, such as perch height.
All of the studies conducted to date on the diet of D. swinhonis, including this study, have shown that Hymenoptera are the favorite prey of this species [9, 17, 19]. However, the frequency of Hymenoptera in stomach contents varied among these studies. In this study, the occurrence frequency (F) of Hymenoptera in the stomach contents was 0.81, whereas frequencies of 0.53, 0.88 and 0.91 were obtained in populations in Taiwan [9, 17, 19]. Huang reported that D. swinhonis on Orchid Island is frequently found along the periphery of forests where crickets are abundant, and that 25% of the lizards surveyed in these areas preyed upon crickets [9]. These results suggest that D. swinhonis is opportunistic and that they eat prey that appears before them [9], and variation in the occurrence frequency (F) of Hymenoptera in the stomach contents reflects this. In terms of relative proportions of prey items in the stomach, the proportion (N%) of Hymenoptera in Hyuga city (75.15%) was lower than that in Taiwan (90.71%) [19]. In addition, the Shannon-Wiener index of lizards’ stomach contents in Hyuga city (H’=1.35) was also higher than that in Taiwan (H’=0.71) [19]. However, the seasonal lowest value (H’=0.87 in summer) was not significantly different from that in Taiwan. Based on these results, we consider that there is no marked difference in the food preference of lizards in Hyuga city and lizards in Taiwan. As shown by the results of the seasonal changes in the composition of the diet, D. swinhonis tends to eat ants when food availability is high. Since the average temperature in the lowland areas of Taiwan is typically higher than that in Hyuga city, food may be more abundant than it is in Hyuga city throughout the years. This is supported by the fact that despite the results in Taiwan containing the samples collected between May to October [19], it showed similar diversity with results in summer in Hyuga city. Therefore, it is assumable that D. swinhonis introduced into Hyuga city does not show a change in food preference. Still, it eats more variety of prey than that in Taiwan, depending on seasonal changes in food availability.
This study elucidated the feeding ecology of D. swinhonis in Hyuga city and showed that the diet of this species exhibits marked seasonal changes. Since D. swinhonis in Hyuga city eat a variety of prey, it is considered to be dietary generalist, as in Taiwan. When food availability is high in its feeding microhabitat, this species typically feeds on ants, like an ant specialist. Prey selection may help to save energy [30]. Based on this study, the most significant concern is the impact on the abundance of ants and the cascading effect of a decrease in the abundance of ants, such as the reorganization of trophic webs. Furthermore, although no lizard mainly eats ants in the Japanese temperate zone, D. swinhonis changes prey in spring, when food availability is low. Therefore, it should be noted that competition may occur for foods with native lizards. However, the impact of D. swinhonis on the ecosystem in Hyuga city is currently unclear. We consider that monitoring the effects of D. swinhonis in Hyuga city is necessary in the future.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Acknowledgments
The study was supported by the Fujifilm Green Fund (MY).
REFERENCES
- 1.Amador L, Ayala-Varela F, Nárvaez AE, Cruz K, Torres-Carvajal O. 2017. First record of the invasive brown anole, Anolis sagrei Duméril & Bibron, 1837 (squamata: iguanidae: dactyloinae), in South America. Check List 13: 1–6. doi: 10.15560/13.2.2083 [DOI] [Google Scholar]
- 2.Carne L, Measey GJ. 2013. Chameleons on the cruise: seasonal differences in prey choice of two dwarf chameleons. Herpetol J 23: 221–227. [Google Scholar]
- 3.Das I, Charles JK, Edwards DS. 2008. Calotes versicolor (Squamata: Agamidae)-a new invasive squamate for Borneo. Curr Herpetol 27: 109–112. doi: 10.3105/1881-1019-27.2.109 [DOI] [Google Scholar]
- 4.Enju M, Iwashita A, Kawasaki M, Tetsuya T, Kato H. 2019. The inhabiting situation of the Swinhoe’s tree lizard, Japalura swinhonis Günther (Squamata, Agamidae) in Atsugi City, Kanagawa. Nat. Hist. Rep. Kanagawa 2019: 85–87 (in Japanese). [Google Scholar]
- 5.Gadsden EH, Palacios-Orona LE. 1997. Seasonal dietary patterns of the Mexican fringe-toed lizard (Uma paraphygas). J Herpetol 31: 1–9. doi: 10.2307/1565321 [DOI] [Google Scholar]
- 6.Goldberg SR, Kraus F. 2018. Reproduction in the green anole, Anolis carolinensis (Squamata: Dactyloidae), from Hawaii. Curr Herpetol 37: 69–74. doi: 10.5358/hsj.37.69 [DOI] [Google Scholar]
- 7.Hayashi K, Hagimoto M, Wada K. 1994. Temopral change in extranidal activity pattern of a formicid ant, Formica japonica (Formicidae: Hymenoptera). Japanese J. Ecol. 44: 171–179 (in Japanese). [Google Scholar]
- 8.Hernández-Salinas U, Ramírez-Bautista A, Cruz-Elizalde R. 2016. Variation in feeding habits of the arboreal lizard Anolis nebulosus (Squamata: Dactyloidae) from island and mainland populations in Mexican pacific. Copeia 104: 831–837. doi: 10.1643/CE-16-390 [DOI] [Google Scholar]
- 9.Huang WS. 2007. Ecology and reproductive patterns of the agamid lizard Japalura swinhonis on an east Asian island, with comments on the small clutch sizes of island lizards. Zool Sci 24: 181–188. doi: 10.2108/zsj.24.181 [DOI] [PubMed] [Google Scholar]
- 10.Hui TH, Lim KK. 2012. Recent introduction of the brown anole Norops Sagrei (Reptilia: Squamata: Dactyloidae) to Singapore. Nat Singap 5: 359–362. [Google Scholar]
- 11.Imatake S, Imaizumi N, Ohashi Y, Matsumura H, Urakawa M, Konaka Y, Kida T, Yanagita T, Fujisaki H, Wakitani S, Yasuda M. 2020. Reproductive cycle and maturation of Swinhoe’s tree lizard (Diploderma swinhonis (Günther, 1864)) in Hyuga City, Miyazaki Prefecture, Japan. J Vet Med Sci 82: 1551–1557. doi: 10.1292/jvms.20-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson DR, Telford SR. 1975. Food habits and predatory role of the Japanese lacertid Takydromus tachydromoides. Copeia 1975: 343. doi: 10.2307/1442889 [DOI] [Google Scholar]
- 13.Jono T, Kawamura T, Koda R. 2013. Invasion of Yakushima island, Japan, by the subtropical lizard Japalura polygonata polygonata (Squamata: Agamidae). Curr Herpetol 32: 142–149. doi: 10.5358/hsj.32.142 [DOI] [Google Scholar]
- 14.Jun-yi L, Kau-hung L. 1982. Population ecology of the lizard Japalura swinhonis formosensis (Sauria: Agamidae) in Taiwan. Copeia 1982: 425. doi: 10.2307/1444624 [DOI] [Google Scholar]
- 15.Kato H, Oba T, Oba S, Etoh H, Tahira Y. 2013. The reproduction and feeding habitats of the Swinhoe’s tree lizard, Japalura swinhonis Günther (Squamata, Agamidae) from Iwata City, Shizuoka Prefecture, Japan. Nat. Hist. Tokai Dist. 2013: 35–38 (in Japanese). [Google Scholar]
- 16.Kuo C, Lin Y, Lin Y. 2009. Sexual size and shape dimorphism in an agamid lizard, Japalura swinhonis (Squamata: Lacertilia: Agamidae). Zool Stud 48: 351–361. [Google Scholar]
- 17.Kuo CY, Lin YS, Lin YK. 2007. Resource use and morphology of two sympatric Japalura lizards (Iguania: Agamidae). J Herpetol 41: 713–723. doi: 10.1670/06-197.1 [DOI] [Google Scholar]
- 18.Matyot P. 2004. The establishment of the crested tree lizard, Calotes versicolor (DAUDIN, 1802) (Squamata: Agamidae), in Seychelles. Phelsuma 12: 35–47. [Google Scholar]
- 19.Norval G, Huang SC, Mao JJ, Goldberg SR, Slater K. 2012. Additional notes on the diet of Japalura swinhonis (Agamidae) from southwestern Taiwan, with comments about its dietary overlap with the sympatric Anolis sagrei (Polychrotidae). Basic Appl Herpetol 26: 87–97. [Google Scholar]
- 20.Ortiz MF, Ugarte ANM, Isaías HS. 2001. Diet and reproductive biology of the viviparous lizard Sceloporus torquatus torquatus (Squamata: Phrynosomatidae). J Herpetol 35: 104–112. doi: 10.2307/1566029 [DOI] [Google Scholar]
- 21.Ota H. 1991. Taxonomic redefinition of Japalura swinhonis Günther (agamidae: squamata) with a description of a new subspecies of J.polygonata from Taiwan. Herpetologica 47: 280–294. [Google Scholar]
- 22.Ota H, Hoshino I, Sueyoshi T. 2006. Colonization by the subtropical lizard, Japalura polygonata polygonata (Squamata: Agamidae), in southeastern Kyushu, Japan. Curr Herpetol 25: 29–34. doi: 10.3105/1345-5834(2006)25[29:CBTSLJ]2.0.CO;2 [DOI] [Google Scholar]
- 23.Pal A, Swain MM, Rath S. 2007. Seasonal variation in the diet of the fan-throated lizard, Sitana ponticeriana (Sauria: Agamidae). Herpetol Conserv Biol 2: 145–148. [Google Scholar]
- 24.Sagonas K, Pafilis P, Lymberakis P, Valakos ED. 2015. Trends and patterns in the feeding ecology of the widespread balkan green lizard Lacerta trilineata (Squamata: Lacertidae) in insular and continental Greece. North-West J Zool 11: 117–126. [Google Scholar]
- 25.Schwarzkopf L. 1996. Decreased food intake in reproducing lizards: a fecundity-dependent cost of reproduction? Austral Ecol 21: 355–362. doi: 10.1111/j.1442-9993.1996.tb00622.x [DOI] [Google Scholar]
- 26.Sugiura S. 2016. Impacts of introduced species on the biota of an oceanic archipelago: the relative importance of competitive and trophic interactions. Ecol Res 31: 155–164. doi: 10.1007/s11284-016-1336-0 [DOI] [Google Scholar]
- 27.Toda M, Takahashi H, Nakagawa N, Sukigara N. 2010. Ecology and control of the green anole (Anolis carolinensis), an invasive alien species on the Ogasawara Islands. pp. 145–152. In: Restoring the Oceanic Island Ecosystem (Kawakami K, Okochi I eds), Springer, Tokyo. [Google Scholar]
- 28.Tseng HY, Lin CP, Hsu JY, Pike DA, Huang WS. 2014. The functional significance of aposematic signals: geographic variation in the responses of widespread lizard predators to colourful invertebrate prey. PLoS One 9: e91777. doi: 10.1371/journal.pone.0091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitt LJ, Lacher TE. 1981. Behavior, habitat, diet and reproduction of the iguanid lizard Polychrus acutirostris in the caatinga of northeastern Brazil. Herpetologica 37: 53–63. [Google Scholar]
- 30.Vitt LJ, Janalee PC. 2014. Foraging Ecology and Diets. pp. 291–318. In: Herpetology 4th ed. (Vitt LJ, Caldwell JP eds.), Academic Press, Cambridge. [Google Scholar]
- 31.Wang CM, Hsu JY, Huang WS. 2014. Japalura swinhonis (Swinhole’s japalura) and Takydromus sauteri (Sauter’s grass lizard) predation. Herpetol Rev 45: 501–502. [Google Scholar]
- 32.Wang K, Che J, Lin S, Deepak V, Aniruddha DR, Jiang K, Jin J, Chen H, Siler CD. 2019. Multilocus phylogeny and revised classification for mountain dragons of the genus Japalura s.l. (Reptilia: Agamidae: Draconinae) from Asia. Zool J Linn Soc 185: 246–267. doi: 10.1093/zoolinnean/zly034 [DOI] [Google Scholar]
- 33.Yamane S. 2019. Seasonal change in the foraging activity of ants in a residential area of mainland Kagoshima, Southwest Japan (Insecta, Hymenoptera, Formicidae). Nat. Kagoshima 45: 361–366 (in Japanese). [Google Scholar]