Abstract
Background
Monkeypox is a viral zoonotic disease caused by the monkeypox virus, a double‐stranded DNA‐enveloped virus that can be transmitted from animal to human or human to human. Consequently, it emerged as the most important orthopoxvirus for public health. Based on available online literature, this study reviewed the majority of the data representing the outbreak, diagnosis, treatment, and prevention of monkeypox.
Methods
The literature search was conducted between July 5 and September 15, 2022. In addition to reviewing the databases of World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), Africa CDC, and United Kingdom Health Security Agency monkey pox advice, 43 papers were studied in depth.
Results and Discussion
Human monkeypox was first identified in 1970 in a child in the Democratic Republic of the Congo. Until May 6, 2022, it was endemic in West and Central African countries and infrequently occurred outside of Africa. However, many cases have been identified in several nonendemic countries since May 13, 2022, with no prior human or animal travel from endemic areas; that was the first time to document the cases and long‐term transmission in countries with no epidemiological ties to endemic African countries. Seven travel‐related human monkeypox cases were recorded outside of Africa from September 2018 to November 2021: one in Israel, one in Singapore, and two in the US Youth are most affected. Monkeypox's unanticipated development in places with no known epidemiological linkages raises concerns about the virus's evolution, which permits undetected transmission for a long period.
Conclusion
Monkeypox is no longer a rare, self‐limiting disease limited to endemic countries. Its ever‐changing epidemiology and transmission dynamics have increased the possibility of its evolving into a much deadlier pathogen. Therefore, improved surveillance and detailed case and contact investigation are required to comprehend the ever‐changing epidemiology of monkeypox.
Keywords: endemic, monkeypox, outbreak, transmission, virus
1. INTRODUCTION
The monkeypox virus (MPXV), an orthopoxvirus (OPXV) with symptoms similar to smallpox, is a viral zoonotic disease. 1 , 2 The OPXV genus, which also includes other deadly viruses including variola virus, vaccinia virus, cowpox virus, and others, 3 , 4 contains the enveloped double‐stranded DNA virus MPXV. MPXV is a member of the Poxviridae family, Chordopoxvirinae subfamily, and Poxviridae family. Monkeypox became the most important OPXV for public health after smallpox was eradicated worldwide in 1980. 5 It was first discovered in a Danish laboratory in 1958 as an exanthem on the skin of imported monkeys and is known to produce outbreaks in captive primates. 6 , 7 The natural reservoir for the virus is still unidentified albeit. 5
In 1970, a youngster in the Democratic Republic of the Congo was found to have the first incidence of human monkeypox (HMPX). 1 , 3 With rare outbreaks happening outside of Africa, it progressively became endemic in nations in Central and West Africa. The MPXV received new attention and was deemed a public health emergency of international concern until May 6, 2022, when an unprecedented outbreak of MPXV infection was reported in several nonendemic countries with no known epidemiological links and the number of cases continued to rise with the continued transmission. 1 , 8 The more virulent Congo Basin (CB) clade, or Clade 1, and the West African (WA) clade, or Clade 2, have typically been used to categorize MPXV strains. 9 , 10 A newly emerging clade dubbed MPXV Clade 3 with a predominate B.1 lineage in the 2022 outbreak, however, is shown by a phylogenomic study of the virus genome. 11 The majority of human‐to‐human transmission, which is restricted to men who have sex with men (MSM) via sexual transmission, has likely enhanced infectivity and has been facilitated by MPXV mutations. 1 The MPXV infection causes a number of symptoms, including fever, headache, lymphadenopathy, myalgia, and others, which are followed by a distinctive rash that lasts for 2–3 weeks. 1 , 6 The rashes in the present outbreak, however, vary from earlier ones and do not exhibit any prodromal symptoms. 12 They may not develop sequentially and can be observed in various phases at once, starting with anogenital development that is mainly found in people with MSM. 13 Encephalitis, sepsis, secondary bacterial infection, bronchopneumonia, conjunctivitis, corneal scarring, and miscarriage in pregnancy are all possible complications. 6
Monkeypox has been reported to transmit from either animal to human or human to human. 6 Congenital and nosocomial transmission can also occur. 14 , 15 Persons living in a forested region, taking care of infected animals and in close proximity to infected individuals within the infectious period (21 days) are at increased risk of getting infected. Children, pregnant women, and immunocompromised patients such as human immunodeficiency virus (HIV)‐infected individuals are highly susceptible to MPXV infection. 16 PCR, with or without sequencing and virus isolation, is considered the gold standard technique used to confirm MPXV infection. 6 In remote settings and limited resource areas, most cases are initially diagnosed clinically. Lymphadenopathy is the key feature that distinguishes monkeypox from other OPVX infections. 16 Considering the current surge of monkeypox cases, some next‐generation, including modified vaccinia ankara‐bavarian nordic (MVA‐BN), ACAM2000, and LC16m8 vaccines, were approved with limited use in high‐risk individuals. 1 A selected number of antiviral agents, such as tecovirimat and brincidofovir are approved for treating complicated cases of monkeypox infection under randomized controlled trials or an Expanded Access for an Investigational New Drug protocol. Currently, the majority of mild or uncomplicated monkeypox cases are taken care of with symptomatic treatment and optimizing supportive interventions. 16 The unique characteristics and epidemiology of this current outbreak necessitate increased research efforts to bridge the scientific knowledge gap to halt current and future outbreaks. Therefore, the purpose of this review is to represent current evidence on the epidemiology, clinical characteristics, mode of transmission, diagnosis, management, and prevention of monkeypox (MPX) that may be useful in optimizing surveillance and preparedness in the fight against MPXV infections.
2. METHODS
2.1. Searching strategy
From July 5, 2022, through September 15, 2022, we scoured the scholarly literature. Forty‐three studies were read carefully, and databases from the WHO, the CDC, the Africa CDC, and the UK Health Security Advisory Committee on Monkey Pox were also combed through. Figure 1 shows the literature selection process.
Figure 1.
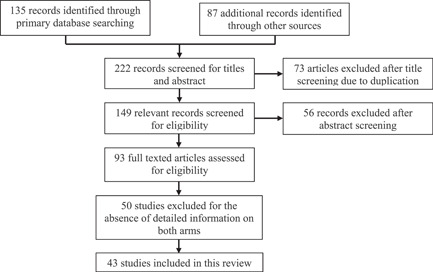
Flow chart of the literature selection process
2.2. Inclusion and exclusion criteria
We include literature containing information about epidemiology, treatment procedures that include vaccines, mode of transmission of monkeypox from one vector to another, diagnosis, and prevention of this deadly disease. We excluded studies containing information on the genetic basis of monkeypox, commentary review, news columns, conference papers, and nonessential information for data extraction.
3. RESULTS AND DISCUSSION
3.1. Ecology
The first case of monkeypox was identified in 1958 in Copenhagen, Denmark after 2 months following their arrival from Singapore. 5 An epidemic at Rotterdam Zoo, in 1964, was one of the earliest indicators of the wide host range of MPXV and its proclivity to infect larger animals in pan‐geographical areas. 17 Following the identification of human MPX in western and central Africa in 1970, cases were mostly reported from forested areas such as tropical rainforest terrain, mangrove forest, freshwater swamp, a mixture of mosaic forests and savannah, or predominantly savannah. 4 , 18 , 19 The Global Commission for Smallpox Eradication recommended in December 1979 that "WHO should organize and assist a special surveillance program on human MPX, its epidemiology, and its ecology." Following this recommendation, studies on the ecology of the MPXV have been strengthened since 1984, resulting in a better understanding of the virus's circulation in wild animals and the mode of transmission to humans. 19 , 20
A series of studies were conducted in various parts of the Democratic Republic of the Congo to identify the MPXV reservoir. One study collected blood samples from 120 sheep and goats, 67 domestic cats, 172 terrestrial rodents, and 21 squirrels during the first survey in Zaire's north (Bumba zone), with only 2 out of 18 squirrels (Funisciurus anerythrus) being seropositive. Seronegative were the remaining domestic animals and terrestrial rodents. 21 Another survey conducted in the late 1980s in the central and western parts of Zaire (DRC) found that MPXV‐specific antibody prevalence rates in primates ranged from 7.3% to 8.0%. It was also reported that higher prevalence rates were observed in Funisciurus squirrels, followed by another squirrel (Heliosciurus rufobrachium), with antibody prevalence rates ranging from 13.0% to 19.4%. 20 Extensive research in the 1980s to identify the MPXV reservoir revealed that infected species have some similar and dissimilar traits based on diet and habitat preferences, with approximately 40% being arboreal, 40% semiterrestrial, and 20% terrestrial. 22
In the 2003 shipment of African rodents that brought MPXV to the United States, cell culture discovered MPXV in 3 out of 6 Funisciurus sp. (rope squirrel), 1 out of 15 Cricetomys sp. (Gambian‐pouched rat), and 8 out of 10 Graphiurus sp. (African dormouse). 17 The black‐tailed prairie dogs (Cynomys ludovicianus), who caught the illness and gave rise to an outbreak of human MPX sickness that resulted in 87 reported (37 laboratory‐confirmed) human cases, were housed in the same enclosure as the afflicted rodents. Along with the diseased animals, hamsters, gerbils, and chinchillas (Cricetus spp., Gerbillus spp., and Chinchilla spp.) were also found to be infected. In a recent investigation, OPXV‐IgG was detected in only 2 (1.9%) of 105 serum samples from animals, including Rodentia (77.1%), Eulipotyphla (21.0%), and Chiroptera (1.9%). The two seropositive rodents belonged to the species Cricetomys emini. 23 Seroactivity suggests that monkeys of the Cercopithecus genus are susceptible to MPXV infection, and squirrels (primarily Funisciurus and Heliosciurus) are a major reservoir of the virus. 17
3.2. Epidemiology
Under the WHO‐enhanced surveillance program, the first case of HMPX infection was identified in 1970 in a 9‐month‐old boy from Zaire's Equateur province (DRC, Central Africa). The boy became ill with a smallpox‐like illness that was later identified as HMPX. 14 , 24 Sporadic outbreaks transmitted from local wildlife to humans continued to be a source of concern in parts of Africa. 5 , 7 , 14 Approximately 923 HMPX cases were reported in eight African countries between 1970 and 1999, including the Democratic Republic of the Congo, Cameroon, Ivory Coast, Liberia, Nigeria, Sierra Leone, the Central African Republic, and Gabon. 2 Cases are classified as suspected, probable, or confirmed. The definition of monkeypox cases is shown in Table 1.
Table 1.
Monkeypox case definitions 25
| Type of cases | Definitions |
|---|---|
| Suspected |
|
| Probable |
A person presenting with an unexplained acute skin rash, mucosal lesions, or lymphadenopathy and one or more of the following
|
| Confirmed | A person with laboratory‐confirmed monkeypox virus infection by detection of unique sequences of viral DNA by real‐time polymerase chain reaction (PCR) and/or sequencing. |
Abbreviations: IgG, immunoglobulin G; MPXV, monkeypox virus; OPXV, orthopoxvirus; PCR, polymerase chain reaction.
However, the number of cases has increased at least 10‐fold in the last 20 years compared with the first 30 years after its discovery. 2 , 5 HMPX was not documented outside of Africa until 2003 when an outbreak of 47 confirmed or probable cases occurred in the United States following contact with infected pet prairie dogs cohoused with infected exotic animals imported from Ghana. 2 , 14 , 26 Among African countries, DRC is the most affected by MPXV, with Nigeria coming in second. 2 MPXV resurfaced in Nigeria on September 22, 2017, after a 40‐year hiatus, with 311 suspected cases and 181 confirmed or probable cases by the end of 2018. Several cases of travel‐associated monkeypox have since been reported in various countries, including Israel (one case in 2018), the United Kingdom (two in 2018, one in 2019), and Singapore (one in 2019), all of which have been linked to exposures in Nigeria. 2 , 14
According to one study, between 2010 and 2019, the median age of MPX cases increased from young children (4 years old) in the 1970s to young adults (21 years old). 2 The median age of infected cases in the Nigerian outbreak in 2018 was around 30. Besides, men were infected at a higher rate than women. The majority of cases in Nigeria were reported from urban and peri‐urban areas in the country's southern regions during the first three decades of the outbreak, whereas the majority of cases in DRC were reported from rural areas, in small villages surrounded by humid evergreen tropical forests. 5 , 6 Clusters of cases in Nigeria have “no epidemiological linkages across states,” and the outbreak is believed to be the result of an underlying epizootic. 6 HMPX was discovered in people who had recently traveled to Nigeria or had contact with someone who had a confirmed MPXV infection outside of Africa following the 2018 Nigeria outbreak. Several countries have reported clusters of monkeypox cases since May 14, 2022, with no direct travel‐related exposure risks, and the source of these infections is unknown. 6 , 27 Given the increasing number of cases with no direct or immediate epidemiological links to endemic areas, as well as community‐wide transmission chains, the WHO Director‐General declared this outbreak a public health emergency of international concern. 8
3.3. Outbreak of monkeypox
3.3.1. Number of cases by country from 1970 to 2019
Table 2 shows the number of monkeypox cases identified in various countries around the world between 1970 and 2019. However, seven travel‐related HMPX cases were reported outside of Africa between September 2018 and November 2021: one in Israel, one in Singapore, and two in the United States. 9 Surprisingly, the infection was imported to the United Kingdom in May 2021 and the United States in July 2021, at a time when there were very few reported cases of monkeypox in Nigeria. Only 32 suspected cases have been reported to authorities in Nigeria since the beginning of 2021. 2 Since September 22, 2017, there have been 558 instances documented as of April 2022. From 2020 through May 2022, the DRC recorded 10,545 suspected cases and 362 related fatalities. 9
Table 2.
Number of cases and case‐fatality rate by decades 9
| Decades | Number of confirmed/probable and/or possible cases | Number of suspected cases | Case fatality rate (%) | Secondary transmission rate (%) | Secondary attack rate (%) |
|---|---|---|---|---|---|
| 1970–1979 | 48 | ‐ | 17 | 9 | 3 |
| 1980–1989 | 357 | 404 | 10 | 28 | 13.9 |
| 1990–1999 | 520 | ‐ | 1.5 (<5) | 78 | ‐ |
| 2000–2009 | 910 | 10,108 |
0.1 (1 death/910) 9 (1/11), Apr–Jun 2003 |
‐ | ‐ |
| 2010–2019 | 280 | 18,788 |
Cameroon: 50 (1/2), Dec 2019 CAR: 23.1 (3/13), Dec 2015–Feb 2016 12 (3/25), 2 Mar 2018–2 June 2019 DRC: 25.6 (10/39), Jul–Dec, 2013 100 (1/1), Dec‐2016 Nigeria: 5 (9 in 181), 2017–19 ROC: 13.6 (3/22), Jan–5 Apr 2017 |
‐ | ‐ |
3.3.2. Multicountry outbreak, 2022
HMPX was endemic in West and Central African countries and infrequently occurred outside of Africa until May 6, 2022. The UK High Consequence Infectious Diseases (HCID) network reported a confirmed monkeypox case with a recent travel history to West Africa on May 6, 2022. 12 Many cases have been reported from several nonendemic countries since May 13, 2022, with no prior human or animal travel from endemic areas (Table 3). 8 This is the first time that cases and long‐term transmission chains have been documented in countries with no epidemiological ties to endemic African countries. The unexpected emergence of monkeypox in several regions with no prior epidemiological links raises concerns about the virus's evolution, which allows it to generate undetected transmission in the communities for a considerable time. 8 , 13 As of September 10, 54,709 laboratory‐confirmed and 397 probable cases, including 18 deaths, had been reported from 102 Member States across all 6 WHO regions since January 1. WHO rates the global risk as Moderate, though countries in the European Region are at high risk and Western Pacific regions are rated as Low‐Moderate. 8 The 10 countries with the highest cumulative number of cases globally as of September 10, 2022, are the United States of America (n = 19,833), Spain (n = 6,749), Brazil (n = 5,525), France (n = 3,646), Germany (n = 3,505), The United Kingdom (n = 3,484), Peru (n = 1,724), Canada (n = 1,289), the Netherlands (n = 1,172), and Colombia (n = 938). These countries account for 87.5% of all reported cases worldwide. 8
Table 3.
Number of cases and deaths by country as of September 10, 2022 8
| Country name by region | Total confirmed cases | Total probable cases | Total deaths |
|---|---|---|---|
| European Region | |||
| Spain | 6749 | 0 | 2 |
| France | 3646 | 0 | 0 |
| Germany | 3505 | 0 | 0 |
| The United Kingdom | 3484 | 0 | 0 |
| Netherlands | 1172 | 0 | 0 |
| Portugal | 871 | 0 | 0 |
| Italy | 787 | 0 | 0 |
| Belgium | 726 | 0 | 1 |
| Switzerland | 480 | 0 | 0 |
| Austria | 278 | 0 | 0 |
| Israel | 241 | 0 | 0 |
| Denmark | 178 | 0 | 0 |
| Sweden | 165 | 0 | 0 |
| Ireland | 160 | 0 | 0 |
| Poland | 145 | 0 | 0 |
| Norway | 82 | 0 | 0 |
| Hungary | 71 | 0 | 0 |
| Greece | 62 | 0 | 0 |
| Czechia | 58 | 0 | 0 |
| Luxembourg | 53 | 0 | 0 |
| Slovenia | 45 | 0 | 0 |
| Romania | 36 | 0 | 0 |
| Malta | 33 | 0 | 0 |
| Serbia | 31 | 0 | 0 |
| Finland | 30 | 0 | 0 |
| Croatia | 27 | 0 | 0 |
| Slovakia | 14 | 0 | 0 |
| Iceland | 12 | 0 | 0 |
| Estonia | 10 | 0 | 0 |
| Gibraltar | 6 | 0 | 0 |
| Bulgaria | 5 | 0 | 0 |
| Cyprus | 5 | 0 | 0 |
| Lithuania | 5 | 0 | 0 |
| Andorra | 4 | 0 | 0 |
| Latvia | 4 | 0 | 0 |
| Bosnia and Herzegovina | 3 | 0 | 0 |
| Monaco | 3 | 0 | 0 |
| Georgia | 2 | 0 | 0 |
| Greenland | 2 | 0 | 0 |
| Montenegro | 2 | 0 | 0 |
| Republic of Moldova | 2 | 0 | 0 |
| Russian Federation | 1 | 0 | 0 |
| Turkey | 1 | 0 | 0 |
| Regions of America | |||
| United States of America | 19,833 | 0 | 0 |
| Brazil | 5525 | 297 | 2 |
| Peru | 1724 | 0 | 0 |
| Canada | 1289 | 93 | 0 |
| Colombia | 938 | 0 | 0 |
| Mexico | 504 | 0 | 0 |
| Chile | 450 | 1 | 0 |
| Argentina | 170 | 0 | 0 |
| Puerto Rico | 129 | 0 | 0 |
| Bolivia (Plurinational state of) | 89 | 0 | 0 |
| Ecuador | 53 | 1 | 1 |
| Panama | 12 | 0 | 0 |
| Guatemala | 11 | 3 | 0 |
| Dominican Republic | 7 | 1 | 0 |
| Jamaica | 7 | 1 | 0 |
| Uruguay | 7 | 0 | 0 |
| Honduras | 4 | 0 | 0 |
| Costa Rica | 3 | 0 | 0 |
| Venezuela (Bolivarian Republic of) | 3 | 0 | 0 |
| Aruba | 2 | 0 | 0 |
| Bahamas | 2 | 0 | 0 |
| Cuba | 2 | 0 | 1 |
| Guyana | 2 | 0 | 0 |
| Barbados | 1 | 0 | 0 |
| Bermuda | 1 | 0 | 0 |
| Curaçao | 1 | 1 | 0 |
| El Salvador | 1 | 0 | 0 |
| Guadeloupe | 1 | 0 | 0 |
| Martinique | 1 | 0 | 0 |
| Paraguay | 1 | 0 | 0 |
| Saint Martin | 1 | 0 | 0 |
| African Region | |||
| Nigeria | 220 | 0 | 4 |
| Democratic Republic of the Congo | 195 | 0 | 0 |
| Ghana | 76 | 0 | 4 |
| Central African Republic | 8 | 0 | 2 |
| Cameroon | 7 | 0 | 0 |
| South Africa | 5 | 0 | 0 |
| Benin | 3 | 0 | 0 |
| Congo | 3 | 0 | 0 |
| South Sudan | 2 | 0 | 0 |
| Liberia | 2 | 0 | 0 |
| Western Pacific Region | |||
| Australia | 125 | 0 | 0 |
| Singapore | 16 | 0 | 0 |
| New Zealand | 5 | 0 | 0 |
| Japan | 4 | 0 | 0 |
| Philippine | 4 | 0 | 0 |
| China | 4 | 0 | 0 |
| Republic of Korea | 2 | 0 | 0 |
| New Caledonia | 1 | 0 | 0 |
| Eastern Mediterranean Region | |||
| United Arab Emirates | 16 | 0 | 0 |
| Saudi Arabia | 8 | 0 | 0 |
| Lebanon | 8 | 0 | 0 |
| Qatar | 3 | 0 | 0 |
| Morocco | 3 | 0 | 0 |
| Sudan | 2 | 0 | 0 |
| Iran (Islamic Republic of) | 1 | 0 | 0 |
| South‐East Asia Region | |||
| India | 10 | 0 | 1 |
| Thailand | 7 | 0 | 0 |
| Indonesia | 1 | 0 | 0 |
According to detailed confirmed case reports, the outbreak primarily affects young men, with 98.1% (27875/28401) of cases with gender data being males with a median age of 36 years (Interquartile range: 30–43 years). 0.6% (171) of the 28,991 cases with available age data were aged 0–17 years, and 0.2% (46) were aged 0–4 years. The gender description of detailed case reports reveals that 1.9% (526/28401) of all cases with available data are females. 8 Unlike previous outbreaks, the ongoing monkeypox outbreak primarily affects MSM (defined as homosexual or bisexual males) who have reported recent sex with one or more partners, though this is not always the case. There is no evidence of sustained transmission outside these networks. 8 95.1% (12,247/12,878) of cases with sexual orientation were reported to be identified as members of the gay, bisexual and other men who have sex with men community. Sexual encounters were the most frequently reported type of transmission, accounting for 91.3% of all transmission events (8153 out of 8928). The vast majority of patients do not know their HIV status, with 44.6% (5,678/12,721) of those who do know their HIV status reporting being HIV‐positive. 8
Race or ethnicity data are only available for cases reported by the United Kingdom Health Security Agency (UKHSA) and CDC in the European and Americas regions. Race or ethnicity information is available for 59.2% (n = 10,297) of cases, with 33.1% (n = 3413) being Non‐Hispanic White, 31.1% (n = 3218) being Hispanic (of any race), 31.1% (n = 3205) being non‐Hispanic Black, and 3.7% (n = 381) being non‐Hispanic Asian. The CDC reported less than 1% of cases with available race/ethnicity as American Indian or Alaska Native (n = 34), Native Hawaiian or other Pacific Islander (n = 12), or multiple races (n = 34). 28 According to the UKHSA report, the majority of respondents (76.4%) were White, followed by mixed (9.1%), Asian (7.1%), Black (4.3%), and other (0.1%). 29
3.3.3. Evolution of monkeypox
Phylogenomic analysis of available MPXV genomes is required to determine their origin, evolutionary trajectory, genetic diversity, and phenotypic characteristics that can be used to guide diagnostics, prophylaxis, and research. 11 In humans, the Congo Basin (CB) clade is more virulent than the WA clade, with respective case fatality rates of 10.6% and 3.6%. 9 , 30 OPVs generally adapt to their hosts through gene loss and gain. 18 Moreover, genome‐sequence length and gene content have a pragmatic correlation with a broad host range but are inversely related to pathogenicity. The WA clade of MPXV (particularly the Nigerian isolates) has larger genomes (197,566–197,792 bp) and content than the CB clade (particularly the Zaire isolates) (196,850–196,959 bp), which may contribute to the WA clade's lower virulence. 18 Furthermore, phylogenetic analysis of MPXV viral sequences associated with the 2022 multicountry outbreak revealed that the virus belongs to a third newly emerging clade: MPXV clade 3 (within the previously designated “WA” clade, which also includes Clade 2) 11 that is made up of the human monkeypox virus‐1A (hMPXV‐1A) clade and the four newly discovered lineages A.1, A.1.1, A.2, and B.1, with lineage B.1 containing all MPXV genomes from the 2022 epidemic. Clade 3 has a lower case‐fatality rate (1%), compared with Clade 2. 11 The main differences between the three clades are associated with coding regions. 31 The 2022 MPXV differs from the associated viruses by an average of 50 single‐nucleotide polymorphisms (SNPs). A divergent branch like this could indicate accelerated evolution, separating the current outbreak virus from the reference sequence. 11
3.4. Mode of transmission
Though the exact mode of MPXV transmission is still uncertain, studies suggest that transmission can occur by three possible means: animal to human, human to human, and from the contaminated environment to human. 16
3.4.1. Animal to human
Animal‐to‐human transmission, also known as zoonotic transmission, occurs through direct contact with infected animals' blood, respiratory droplets, lesion material, and body fluids; inoculation from infected animals' mucocutaneous lesions, particularly when the skin barrier is lost due to scratches, bites, or other trauma; or during handling of infected monkeys, including hunting, skinning, trapping, cooking, and playing with carcasses. Ingestion of inadequately cooked meat from infected animals or nonhuman primates, such as Gambian giant squirrels, terrestrial rodents, rats, rabbits, dormice, porcupines, antelopes and gazelles, and tree squirrels, can also result in transmission. 16 , 24
3.4.2. Human to human
Human‐to‐human transmission can occur through direct contact with an infected individual's skin, mucous membranes, or mucocutaneous lesions, including face‐to‐face, skin‐to‐skin, mouth‐to‐mouth, or mouth‐to‐skin contact, as well as oral or respiratory secretion, droplets requiring prolonged close contact. 1 , 13 , 14 , 15 , 16 , 24 , 32 Long‐term contact puts caregivers and household members at risk of infection. 30 The virus is believed to enter the body via broken skin, mucosal surfaces, or the respiratory tract. 14 , 16 The MPXV has been linked to nosocomial transmission. 14 , 18 , 30 The high prevalence of the current outbreak raises concerns about possible sexual transmission. 1 , 9 , 16 Indeed, high viral loads were found in saliva, rectal swab, sperm, urine, and fecal samples from infected patients. 1 , 16 Transmission can also occur vertically from mother to fetus, known as congenital monkeypox, and can result in a developmental anomaly of the fetus after childbirth. 15 , 32 The concurrent primary zoonotic and secondary human‐to‐human transmission was suggested in studies conducted in Nigeria and Spain. 4 Another study from Spain confirms that monkeypox is a locally transmitted and community‐acquired emerging infectious disease. 33
3.4.3. Environment to human
Direct contact with fomites contaminated by an infected person's lesion fluid, bodily fluids, crust, or scab (e.g., sheets, clothing, towels) may act as a transmission medium. 13 , 14 , 15 , 16 , 18 In general, OPXVs are more resistant to environmental stress and have a high level of environmental stability. Surrogate pox virus can survive in the environment and on various surfaces for up to 56 days, depending on room conditions. However, there is currently a scarcity of data on environmental transmission. There is currently no information on the presence of the MPXV in wastewater. 16
3.4.4. Human to animal
A recent publication in the Lancet describes some convincing evidence of human‐to‐canine transmission of MPXV. The Italian greyhound, who otherwise appeared to be in good health, had frequent and close contact with the two household members from Paris, France, who were infected with MPX, and even shared a bed with them. 34
3.5. Clinical symptoms and comorbidities
Due to MPXV infection, several symptoms such as fever, headache, lymphadenopathy, myalgia, and so on are observed, followed by a characteristic rash that appears simultaneously and progresses sequentially (macules, papules, vesicles, pustules, crusts) for 2–3 weeks. 1 , 6 Table 4 depicts the clinical manifestations of Monkeypox patients.
Table 4.
| Country name | Patients age | Symptoms (physical and hematological) | Comorbidities | Onset of duration/hospital duration | Reference |
|---|---|---|---|---|---|
| London | 38 (IQR: 32–42) |
|
|
3.5 days/8 days (IQR: 3.5–10) | [12] |
| Spain | 35 (IQR: 29–44) |
|
|
32 h/Not mentioned | [33] |
| Germany | 39 (Range: 20–67) |
|
|
4 days/4 days (range 3–6 days) | [35] |
3.6. Diagnosis
3.6.1. Clinical presentation
The early diagnosis of monkeypox is based on clinical signs and epidemiological links. 18 , 36 According to WHO recommendations, any individual meeting with a suspected case, that is, a person of any age who presents in a non‐MPX endemic country with an unexplained acute rash and one or more of the following signs or symptoms for which the common causes of acute eruption do not explain the clinical presentation, should be tested for the presence of MPX. Asthenia, headache, sudden start of fever over 38.5°C, lymphadenopathy, myalgia, and back discomfort should all be evaluated. 13 , 36
3.6.2. Laboratory tests
Cell culture, polymerase chain reaction (PCR), enzyme‐linked immunosorbent assay (ELISA), immunohistochemistry, electron microscope, or Western blot analysis or sequencing are currently available diagnostic tests for MPXV detection, with PCR being used for definitive diagnosis. 15 , 24 Lesion fluid, lesion roof, scab/crust, oropharyngeal tissue or nasopharyngeal tissue swab, tonsillar tissue, punch biopsy kit, whole blood, and acute and convalescent phase sera are all required for laboratory diagnosis. 24 The lesion samples must be stored in a dry, sterile tube in a cool environment. 13 During specimen collection, standard contact and droplet precautions must be implemented, and any potentially contaminated samples with the MPXV must be handled in Biosafety level 2 facilities. 36
3.6.3. Polymerase chain reaction (PCR)
Nucleic acid amplification testing (NAAT), which uses real‐time or conventional PCR to detect unique sequences of viral DNA, is used to confirm monkeypox infection. Positive results from an OPXV PCR assay followed by monkeypox confirmation via PCR and/or sequencing, or positive results from a monkeypox PCR assay in suspected cases, indicate monkeypox infection. 36 To detect MPXV DNA from clinical and veterinary specimens, as well as MPXV‐infected cell cultures, reverse‐transcription polymerase chain reaction (RT‐PCR) targets conserved regions of the extracellular‐envelope protein gene (B6R), DNA polymerase gene, E9L, DNA‐dependent RNA polymerase subunit 18, rpo18, and F3L gene are usually considered as targets. In conjunction with sequencing, RT‐PCR is the technique of choice for routine diagnosis of MPXV. 18 The PCR test is considered the gold standard laboratory test for its specificity and sensitivity, and it is best performed in a Biosafety level‐three facility. 13 , 18
3.6.4. Immunologic assay
Immunological methods for detecting IgG and IgM antibodies, as well as immunohistochemistry for viral antigen detection, can be used to detect OPXV DNA. 18 Unfortunately, because OPXVs are serologically cross‐reactive, none of these tests provide MPX‐specific confirmation. 13 Positive IgM capture enzyme‐linked immunosorbent assay (ELISA) results indicate recent exposure to OPXV in both unvaccinated and vaccinated individuals, whereas positive IgG capture ELISA results indicate previous exposure to OPXV via vaccination or natural infection. 18 IgM and IgG antibodies are detected in serum 5 to 8 days after the onset of the rash, respectively. 18 Immunochemistry analysis can be used to distinguish between OPXV infection and herpes virus infection. 13 , 18
3.6.5. Electron microscope
Electron microscopy with negative staining can be used to examine biopsy specimens from lymph nodes or scab material, vesicular fluid, blood specimens, or viral cultures. 24 MPXV appears intracytoplasmic brick‐shaped under an electron microscope, with lateral bodies and a central core measuring approximately 200–300 nm. Although this method does not provide a definitive diagnosis because OPXV species cannot be distinguished morphologically, it does provide evidence that the virus belongs to the Poxviridae family, which aids in distinguishing it from Herpes and Parapox viruses. 14 , 18 , 24
3.7. Treatment
At the time of writing, no recognized treatment guideline for monkeypox infection is available. 13 , 37 As the disease is generally mild and self‐limiting in nature, bed rest and supportive therapy are often sufficient to reduce the patient's discomfort. 15 , 24 Supportive therapy (Table 5) may include analgesics for pain, antipyretics for fever, or antibiotics for secondary bacterial infections. 15 , 16 In severe cases, however, hospitalization and specialized treatment may be required. 15 , 24 Although no antiviral drugs are commercially available for the treatment of monkeypox, drugs approved for the treatment of smallpox and cytomegalovirus may have activity against the MPXV (Table 6). 37 , 38
Table 5.
| Confirmed patient | Sever/critical condition patient | Monkeypox with other disease condition | Monkeypox with pregnant women |
|---|---|---|---|
|
|
|
|
Abbreviations: PPV, primary preventive vaccination; PEPV, postexposure preventive vaccination.
Table 6.
Common and potent drugs for Monkeypox treatment
| Country name | Drug name | Action mode | Drug development stage | Reference |
|---|---|---|---|---|
| USA | Tecovirimat | Inhibit viral envelope protein P37 and thus viral maturation | Under RCT and EA‐IND protocol for efficacy and safety assessment (Phase‐3) | [16, 37, 38] |
| Canada | ||||
| UK | ||||
| USA | Brincidofovir | Inhibit viral DNA‐polymerase | Under RCT and EA‐IND protocol for efficacy and safety assessment (Phase‐3) | [16, 37, 38] |
| UK | ||||
| USA | Cidofovir | Inhibit viral DNA‐polymerase | Phase‐2 | [16, 15, 24] |
Abbreviations: EA‐IND, Expanded access for an investigational new drug; RCT, randomized controlled trial.
3.8. Prevention
The Centers for Disease Control and Prevention (CDC) recommend the following measures to reduce one's risk of contracting monkeypox: avoiding close, skin‐to‐skin contact with someone who has monkeypox‐like rashes; avoiding contact with objects and materials that a case with monkeypox has used; washing one's hands frequently; and using an alcohol‐based hand sanitizer before eating and touching one's face. 39
To prevent the community transmission of MPXV infection, isolation of patients with suspected or confirmed MPX with mild, uncomplicated disease are recommended for the duration of the infectious period, as long as a home assessment determines infection prevention control (IPC) criteria are met in the home environment. If proper isolation and IPC measures cannot be guaranteed at home, then isolation may need to be arranged in a healthcare or other designated facility with patient's informed consent and approval from the caregiver and household members. 16
Monkeypox prevention is solely dependent on infection control measures such as surveillance, active contact tracing, isolation practices and quarantining of infected animals, early case‐finding, improved nursing, adequate facilities and staffing, and self‐monitoring by contacts. Along with these measures, immunization of high‐risk contacts with postexposure preventive vaccination (PEPV) and primary preventive vaccination (PPV) could be effective options. 14 , 40 , 41 Despite the current risk and benefit assessments, and regardless of vaccine supply, mass vaccination is neither required nor recommended to prevent monkeypox. 40
3.8.1. Vaccines
Prior immunization with the smallpox vaccine may effectively prevent monkeypox infection and improve clinical manifestation when administered early in the incubation period. 15 , 42 Prior vaccination with the vaccinia virus is known to provide 85% protection against the MPXV and reduce the severity of the infection, but patients with compromised immune systems should exercise caution. 15 , 24 , 42 MVA‐BN ACAM2000 and LC16m8 vaccines are currently available for monkeypox infection. 1 , 15 , 24
MVA‐BN
MVA‐BN is a third‐generation live viral vaccine derived from the modified vaccinia Ankara‐Bavarian Nordic (MVA‐BN strain), an attenuated, nonreplicating OPXV produced by Bavarian Nordic. 1 , 39 , 41 In 2019, it was licensed by the United States Food and Drug Administration as Smallpox, Monkeypox Vaccine for monkeypox infection and as IMVANEX for smallpox in Europe, though the United Kingdom has been using it off‐label in response to monkeypox cases. 41 , 42 It has a better safety profile because of its decreased ability to replicate in host cells and the lack of a lesion at the site of immunization. 13 , 37 It is now indicated for high‐risk populations, such as HIV patients, patients with hematological conditions, atopic dermatitis, and immunocompromised individuals aged 18 years, for the prevention of smallpox and monkeypox, and is administered subcutaneously in two doses, 28 days apart, without producing a take. 9 , 37 , 41 , 42 If necessary, MVA‐BN should be used as a PPV or PEPV against MPX infection. 41
ACAM2000
ACAM2000 is a replication‐competent live vaccinia virus vaccine produced in cell culture from a clone of Dryvax, a first‐generation vaccine indicated for smallpox immunization. 1 , 37 , 42 The Food and Drug Administration (FDA) approved it in August 2007. 41 , 42 It may behave similarly, and subsequent monkeypox infection may be milder even after several years of vaccination, with a lower incidence of complications. 24 ACAM2000 vaccination has been linked to a rash, fever, headache, body aches, and myopericarditis. 9 , 41 The vaccine is not recommended for pregnant people, who have eczema or atopic dermatitis, or immune deficiencies (e.g., HIV infection). 9 However, the CDC recommends ACAM2000 vaccination for non‐variola OPXV infections, including monkeypox. 42
LC16m8
LC16m8 is an attenuated vaccinia virus that contains attenuated strains with a better safety profile than first‐ and second‐generation smallpox vaccines. It also stimulates antibody production adequately in atopic and immunocompromised patients. 15 , 24 It has fewer side effects than ACAM2000 and is approved for use in Japan. 24
PPV
PPV is only available as a vaccine and is generally recommended for individuals at high risk of exposure, such as gay or bisexual men, MSM, or individuals who have multiple sexual partners; and health workers, laboratory personnel working with OPXVs, and outbreak response team members designated by appropriate public health and antiterror health authorities. 1 , 37 , 40 Persons at higher risk of severe diseases, such as immunocompromised people, pregnant women, or children who are potential contacts or members of the same household as people with monkeypox, should be offered vaccination with appropriate vaccine on a case‐by‐case basis, in addition to those at high risk of exposure. 10
PEPV
PEPV is recommended for high and medium‐risk contact cases, and an appropriate second and third generation vaccine is currently available. 1 , 40 PEPV should be administered within 4 days of first exposure and up to 14 days in the absence of symptoms to prevent disease onset and mitigate disease severity. 40
4. CONCLUSION
The monkeypox outbreak in 2022 highlights the fact that monkeypox is no longer a rare, self‐limiting disease limited to endemic countries. Its ever‐changing epidemiology and transmission dynamics have increased the possibility of it evolving into a much deadlier pathogen than it is now. Improved surveillance, as well as detailed case and contact investigation, are required to comprehend the ever‐changing epidemiology of monkeypox. Extensive research is required to understand the disease's current and potential future trends, as well as other OPXV infections. Laboratory diagnostics, particularly serological assays, may be useful in understanding community exposure and subclinical infection. Characterizing ecology, zoonosis, epidemiology, and the clinical and public health aspects of monkeypox can give some insights into reducing the disease burden.
AUTHOR CONTRIBUTIONS
Jannatul Ferdous: Data curation; validation; writing – original draft. Md. Abdul Barek: Data curation; validation; writing – original draft. Md. Shafiul Hossen: Data curation; visualization; writing – original draft. Khokon Kanti Bhowmik: Validation; visualization; writing – review & editing. Mohammad Safiqul Islam: Conceptualization; supervision; writing – review & editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Mohammad Safiqul Islam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Ferdous J, Barek MA, Hossen MS, Bhowmik KK, Islam MS. A review on monkeypox virus outbreak: new challenge for world. Health Sci Rep. 2022;6:e1007. 10.1002/hsr2.1007
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Patauner F, Gallo R, Durante‐Mangoni E. Monkeypox infection: an update for the practicing physician: monkeypox infection. Eur J Intern Med. 2022;104:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16:e0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meo SA, Ali Jawaid S. Human monkeypox: fifty‐two years based analysis and updates. Pak J Med Sci. 2022;38(6):1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yinka‐Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ihekweazu C, Yinka‐Ogunleye A, Lule S, Ibrahim A. Importance of epidemiological research of monkeypox: is incidence increasing? Expert Rev Anti Infect Ther. 2020;18(5):389‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Neglected Trop Dis. 2019;13(10):e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petersen BW, Damon IK. Orthopoxviruses: vaccinia (smallpox vaccine), variola (smallpox), monkeypox, and cowpox. In: Bennett JE, Raphael D, Martin JB, eds. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 8th ed. Elsevier; 2014. [Google Scholar]
- 8. World Health Organization . Monkeypox Outbreak: Global Trends. World Health Organization; 2022. [Google Scholar]
- 9. Xiang Y, White A. Monkeypox virus emerges from the shadow of its more infamous cousin: family biology matters. Emerg Microbes Infect. 2022;11(1):1768‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Multi‐Country Outbreak of Monkeypox: External Situation Report. World Health Organization; 2022. [Google Scholar]
- 11. Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi‐country outbreak of monkeypox virus. Nature Med. 2022;28(8):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel A, Bilinska J, Tam JC, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Gennaro F, Veronese N, Marotta C, et al. Human monkeypox: a comprehensive narrative review and analysis of the public health implications. Microorganisms. 2022;10(8):1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33(4):1027‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheema AY, Ogedegbe OJ, Munir M, Alugba G, Ojo TK. Monkeypox: a review of clinical features, diagnosis, and treatment. Cureus. 2022;14(7):26756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization . Clinical Management and Infection Prevention and Control for Monkeypox: Interim Rapid Response Guidance. World Health Organization; 2022. [Google Scholar]
- 17. Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8(2):129‐157. 10.2217/fvl.12.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the democratic Republic of Congo. Proc Natl Acad Sci. 2010;107(37):16262‐16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66(6):747‐752. [PMC free article] [PubMed] [Google Scholar]
- 21. KHODAKEVICH L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. The Lancet. 1986;327:98‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parker S, Nuara A, Buller RML, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2(1):17‐34. [DOI] [PubMed] [Google Scholar]
- 23. Doshi RH, Guagliardo SAJ, Doty JB, et al. Epidemiologic and ecologic investigations of monkeypox, likouala department, republic of the Congo, 2017. Emerging Infect Dis. 2019;25(2):281‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fowotade A, Fasuyi TO, Bakare RA. Re‐emergence of monkeypox in Nigeria: a cause for concern and public enlightenment. African Journal of Clinical and Experimental Microbiology. 2018;19(4):307‐313. [Google Scholar]
- 25. World Health Organization . Monkeypox Outbreak Toolbox. World Health Organization; 2022. [Google Scholar]
- 26. Simpson K, Heymann D, Brown CS, et al. Human monkeypox–after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077‐5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention (CDC) . Monkeypox virus infection in the United States and other non‐endemic countries—2022. Published online May 20, 2022. https://emergency.cdc.gov/han/2022/han00466.asp
- 28. Data UC . Multi‐National Monkeypox Outbreak Technical Report 2 . US Department of Health and Human Services, CDC; 2022. https://emergency.cdc.gov/han/2022/han00466.asp
- 29. Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22(9):1321‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen E, Abubakar I, Ihekweazu C, et al. Monkeypox—Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post‐eradication era. Int J Infect Dis. 2019;78:78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luna N, Ramírez AL, Muñoz M, et al. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: emergence of a novel viral lineage? Travel Med Infect Dis. 2022;49:102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kannan S, Shaik Syed Ali P, Sheeza A. Monkeypox: epidemiology, mode of transmission, clinical features, genetic clades and molecular properties. Eur Rev Med Pharmacol Sci. 2022;26:5983‐5990. [DOI] [PubMed] [Google Scholar]
- 33. Orviz E, Negredo A, Ayerdi O, et al. Monkeypox outbreak in Madrid (Spain): clinical and virological aspects. J Infect. 2022;85:412‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seang S, Burrel S, Todesco E, et al. Evidence of human‐to‐dog transmission of monkeypox virus. The Lancet. 2022;400(10353):658‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffmann C, Jessen H, Wyen C, et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: a large outbreak cohort in Germany. HIV Med . 10.1007/s15010-022-01923-7 [DOI] [PubMed]
- 36. World Health Organization . Laboratory Testing for the Monkeypox Virus Interim Guidance. World Health Organization; 2022. [Google Scholar]
- 37. O'Shea J, Filardo TD, Morris SB, Weiser J, Petersen B, Brooks JT. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection—United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1023‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reynolds M, McCollum A, Nguete B, Shongo Lushima R, Petersen B. Improving the care and treatment of monkeypox patients in low‐resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9(12):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther. 2022;7(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Health Organization . Vaccines and Immunization for Monkeypox: Interim Guidance. World Health Organization; 2022. [Google Scholar]
- 41. Petersen E, Zumla A, Hui D, et al. Vaccination for monkeypox prevention in persons with high‐risk sexual behaviours to control on‐going outbreak of monkeypox virus clade 3. Int J Infect Dis. 2022;122:569‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


