Abstract
Sarcomatoid hepatocellular carcinoma (SHCC) is a rare variant of liver cancer that lacks treatment options. The IMbrave trail demonstrated the efficacy of atezolizumab and bevacizumab (A + B) in patients with unresectable hepatocellular carcinoma but excluded patients with sarcomatoid variants. Herein, we describe a case of disease control achieved using the IMbrave regimen in a patient with sarcomatoid hepatocellular carcinoma.
Keywords: atezolizumab, bevacizumab, sarcomatoid, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality and comprises 70% to 90% of primary malignant liver tumors.1-3 Although declining, the incidence4 of HCC remains highest in Eastern countries, mirroring the geographic patterns of hepatitis B virus (HBV) infections.4 For example, HCC incidence is 3 times higher in China than in the United States, and the prevalence of HBV infection is 18% versus <1%, respectively.4 In the United States, the incidence of HCC has quadrupled over the past 40 years secondary to increased rates of obesity, nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH), and hepatitis C virus (HCV) infections.3 Improvements in HCV treatments led to a decrease in mortality between 2009 and 2013.3
Sarcomatoid hepatocellular carcinoma (SHCC) is a rare, aggressive subtype of HCC that confers a poor prognosis.5 This subtype, along with fibrolamellar liver cancer, and mixed cholangiocarcinoma/HCC were excluded from the IMbrave trial.6 The IMbrave trial demonstrated combination therapy with atezolizumab and bevacizumab (A + B) increases in overall survival (OS) when compared with sorafenib, the standard of care since 2007.6,7
Case
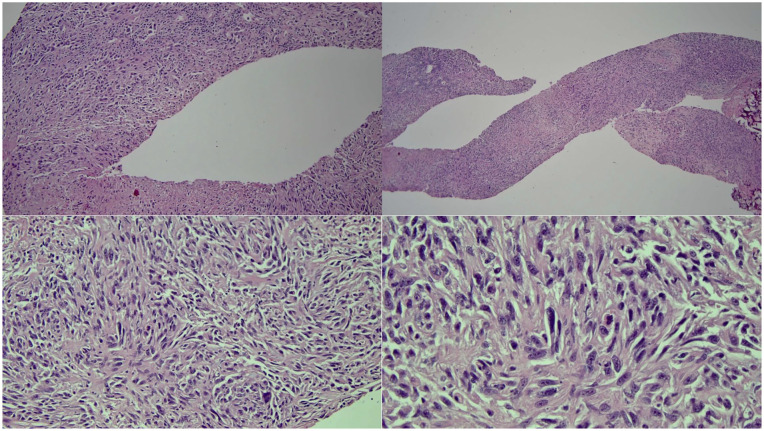
Herein, we present the case of a patient with metastatic SHCC being successfully treated with A + B combination therapy. The patient is a 65-year-old man with a history of intravenous (IV) drug use and HCV cirrhosis diagnosed in 1995. He was treated for HCV in 2015 after developing esophageal varices, which required banding. He received ledipasvir/sofosbuvir and has maintained a sustained virologic response. During routine surveillance, a sub-centimeter Li-RADS 3 lesion in segment 2 of the liver was discovered. Four months later, imaging showed progression of the lesion to 1.8 cm. He denied fever, fatigue, weight loss, abdominal pain, or confusion. His only symptoms were radiculopathy and lower back pain, which were initially attributed to a motor vehicle accident. Five months later, the lesion grew to 4 cm and new porta hepatis lymphadenopathy suspicious for malignancy was found on imaging. He underwent liver and portal lymph node core needle biopsies (Figure 1).
Figure 1.
H&E stains of the liver biopsy show malignant-appearing cells with a largely spindled morphology. The tumor cells show large nuclear/cytoplasmic ratios; large, extremely atypical nuclei with frequent prominent nucleoli; and coarse, clumped chromatin.
Abbreviation: H&E, hematoxylin and eosin.
No malignancy was identified on liver pathology; however, the lymph node pathology showed a poorly differentiated biphenotypic neoplasm with spindle cell morphology, suggestive of SHCC. The tumor stained positive for cytokeratin (CK) 8/18, vimentin, and demonstrated rare positivity of CK AE1/AE3. Dim focal positivity was seen with DOG1 and focal nonspecific staining was observed with smooth muscle actin (SMA). All other markers suggestive of hepatocyte origin, besides CK 8/18, were negative. The presence of biphenotypic HCC was suspected due to areas staining positive for both conventional and SHCC markers. This diagnosis was corroborated on multi-institutional pathology review.
Further workup revealed that tumor markers, including alpha-fetoprotein (AFP), carbohydrate antigen (CA 19-9), and carcinoembryonic antigen (CEA) were within normal limits. Labs only showed an elevated prothrombin time (PT) of 16.7 and an international normalized ratio (INR) of 1.42. His PET scan demonstrated an F-fluorodeoxyglucose (FDG)-avid segment II liver mass (standard uptake ratio [SUV] max 10), bulky gastrohepatic lymphadenopathy, and multiple lytic bone lesions in the axial and appendicular skeleton consistent with metastatic disease.
The patient was not a candidate for liver resection or transplantation, given the stage of his disease. A magnetic resonance imaging (MRI), ordered to better elucidate the etiology of his radiculopathy and lower back pain, confirmed metastatic disease in the L5 and S1 vertebral bodies, which were likely responsible for his presenting symptoms of pain, weakness, and gait instability. Further MRI evaluation 2 months later revealed the presence of diffuse cervical and thoracic vertebral metastases. Foundation One liquid biopsy revealed a Y2041fs*9 alteration in ATR and a R749C alteration in DNMT3A. Neither mutation was actionable. The peripheral blood sample was inadequate to determine tumor mutational burden. However, no evidence of a blood mutational burden or high microsatellite instability was detected.
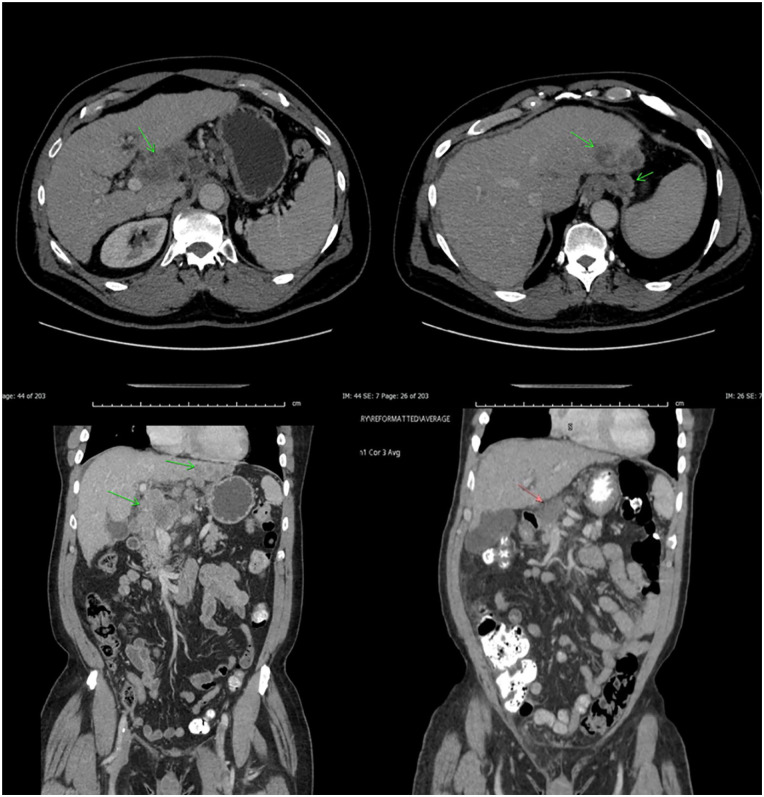
Given the lack of data demonstrating efficacy for any regimen in SHCC, and the epithelial component displaying conventional HCC features on the lymph node biopsy, the patient began atezolizumab (1200 mg IV) and bevacizumab (15 mg/kg IV) therapy every 3 weeks. After 2 months of therapy, imaging showed stability of the visceral and spinal disease, and improvement in the left posterior hepatic mass, which decreased in size from 6.1 × 3.4 cm on prior exam to 5.0 × 2.5 cm. The patient is tolerating therapy and has completed 17/24 treatment cycles while remaining independent in his activities of daily living (Figure 2). He also notes some mild improvement in his radicular symptoms, with tolerable pain levels. Owing to the improvement in his radicular pain from treatment paired with the ongoing COVID-19 pandemic, the patient opted against radiation for his bony metastases.
Figure 2.
Bulky pretreatment tumor (top and bottom left) in liver with periportal adenopathy with obvious regression on posttreatment scans (top and bottom right).
Discussion
Sarcomatoid HCC or spindle cell HCC occurs in 2% of liver cancers.6 Sarcomatoid HCC may arise de novo or occur because of secondary sarcomatous transformation of a typical HCC in patients previously treated with locoregional therapy.6,8 Sarcomatoid transformation may result in the dedifferentiation of HCC accelerated due to selection pressure in the setting of locoregional therapy.6 Sarcomatous HCC in treatment of naive patients, such as our patient, is rare and few case reports are found in the literature.6
The differential diagnosis for spindle cell tumors is extensive and includes metastatic sarcomas,8 primary hepatic sarcoma, hepatic carcinosarcomas (CS), and collision tumors.9 The terms CS and SHCC are sometimes used interchangeably by some, whereas others refer to the former only for heterogeneous tumors with elements, such as leiomyosarcoma, chondrosarcoma, malignant fibrous histiocytoma, fibrosarcoma, or osteosarcoma.10 The World Health Organization (WHO) defines SHCC as consisting of spindle cells or large giant cells11 that are mixed with sarcomatous10 and carcinomatous components, such as HCC or cholangiocarcinoma.8 Sarcomatous elements, such as osteosarcoma or chondrosarcoma, render a diagnosis of CS as opposed to SHCC, which is reserved for a pure malignant cell morphology.12 In clinical practice, distinguishing between CS and SHCC is unnecessary because there is no difference in survival.10 Immunohistochemical markers provide a reliable distinction between the 2 variants.9,10 Distinction relies on the expression of epithelial markers such as keratin6,9 that is retained in the spindle cells of SHCC, whereas CS tumors lack these epithelial markers and are considered truly heterogeneous.10 Sarcomatous components in SHCC tumors stain positive for vimentin.6,11 Positive staining, for cytokeratin 8/18 and vimentin, was noted in our patient supporting SHCC as the diagnosis.
Tumor pathogenesis in SHCC is unclear. Both convergent and divergent mechanisms have been proposed.8,13 The convergent theory is that these are “collision tumors,” which are multiclonal and derived from 2 or more stem cells.8,13 Thompson et al13 lent support to the divergent theory by demonstrating a monoclonal tumor origin confirmed with 2 independent methods of clonality determination. A dedifferentiation theory that conventional neoplastic HCC tumor cells could dedifferentiate into immature, multipotent cells that have the ability to re-differentiate into a sarcomatous tumor has also been proposed.10 Furthermore, sarcomatous transformation may result from repeated necrosis and hepatocyte degeneration from nonsurgical therapies.5 A review of 92 cases over 20 years found that 25 patients were treated with neoadjuvant anticancer therapy, including 15 with transarterial embolization and 8 with transarterial chemoembolization (TACE).8 Our patient, who did not receive any intervention prior to treatment, may have developed SHCC secondary to previous HCV infection, cirrhosis, or antiviral therapy, all of which are considered possibly secondary causes of sarcomatoid transformation.14
Although these tumors are often large, they present insidiously due to lower elevations in liver function studies at presentation.2,5,9,11 In addition, Fibrosis 4 scores, bilirubin, and AFP values are lower for patients with SHCC.2,5,9,11 Although these are positive prognostic indicators for non-sarcomatoid HCC, SHCC patients still demonstrated worse OS and progression-free survival (PFS).5 Other tumor findings include more frequent tumor necrosis, advanced stage, and higher grade at presentation.5,9,11 On imaging, these tumors display large nodules with satellites6 and are less likely to exhibit arterial phase enhancement and portal or delayed phase wash out, the usual dynamic imaging patterns of HCC.5,11 Patients with SHCC may be misdiagnosed as having a hepatic abscess15 or intracholangiocarcinoma (ICC) based on similar imaging findings to SHCC tumors.5,16 For example, a case series identified that 5 of 136 patients with SHCC were misclassified as having a hepatic abscess on initial presentation.15 Shared computed tomographic imaging features identified in both hepatic abscess and SHCC patients include peripheral enhancement and central necrosis.14,16 The liquefactive necrosis in SHCC is diffuse with the presence of metastases versus a honeycomb pattern in a hepatic abscess, which may be helpful in making a diagnosis.16 In addition, failure of symptomatic improvement with antibiotic therapy or low levels of CA-19-9 and alkaline phosphatase (ALP) help to rule out hepatic abscess and intrahepatic cholangiocarcinoma (ICC), respectively.16 Furthermore, a study by Wang et al5 of 41 patients with SHCC found that greater than 60% of patients presented with ICC-like imaging patterns. Atypical radiologic and serologic findings associated with SHCC make early detection of this lesion difficult5 and make histologic findings even more important in securing a diagnosis.8
Clinically, patients with SHCC are more likely to display epigastric discomfort, weight loss, and are 10 times as likely to present with fever than their counterparts with typical HCC.5 Fevers were not noted in our patient, but combined with suggestive imaging may delay initial diagnosis in favor of a hepatic abscess.5
As SHCC tumors are generally high grade and poorly differentiated, they carry a worse prognosis.5 They are more frequently associated with an advanced stage at diagnosis, nonspecific symptoms, adjacent organ invasion, and lymph node metastasis than high-grade HCC.5 Due to the late stage at diagnosis, average 5-year survival rates are lower for SHCC than non-sarcomatoid HCC, ranging from 5.7% to 16.1%, as compared with 19.6% to 53.9%, respectively.2,5,11,17-19 In fact, 1 study controlling for demographic factors and treatments administered19 demonstrated that SHCC patients have a worse OS stage for stage as compared with patients with conventional HCC. The worsened OS remained even when compared with conventional HCC tumors of the same American Joint Committee on Cancer (AJCC) stage and grade/differentiation, with the 5-year survival rates being 11.5% and 41.1% for SHCC and HCC, respectively.5 Studies consistently show that SHCC is associated with worse outcomes and is considered an independent unfavorable prognostic indicator.11,18
The literature regarding whether the carcinomatous or sarcomatous component is responsible for the aggressive behavior of these tumors is inconsistent.5 Wang et al5 found that, of 9 patients with 33 positive lymph nodes, 26/33 of these nodes comprised purely carcinomatous elements versus 2/33 purely sarcomatous, and 5/33 mixed carcinomatous and sarcomatous elements. Based on a larger proportion of lymph node, bile duct, and macrovascular invasions containing carcinomatous components, they postulated that the carcinomatous rather than sarcomatoid component is responsible for the more aggressive nature of these tumors. Despite these findings, OS was not correlated with an increased proportion of sarcomatoid tumor elements.5 Conversely, a study by Maeda et al20 of 13 patients found that most metastases and portal lymph node invasions comprised mainly sarcomatous components. The discrepancy may lie in the inclusion of autopsied patients as well as those who underwent TACE preoperatively.5
With early detection, HCC is amenable to curative surgical interventions such as resection and liver transplantation.1 For nonsurgical candidates, treatment options include bland or percutaneous ethanol ablation, radiofrequency ablation, chemoembolization, radioembolization, external beam radiation, hormonal therapy, immunotherapy, thermotherapy, and systemic therapy.2,9,17,21 Although early surgical resection is associated with a reduced risk of death in SHCC patients, surgical intervention is often not feasible due to advanced stage at diagnosis.2 In addition, whereas resection offers the only chance of cure, OS is still poor, with no patients with stage 2, stage 3, or stage 4 disease surviving longer than 2 years.19 In patients who underwent surgical resection of SHCC, they were found to have shorter median recurrence-free survival (5.6 months vs 16.4 months, P < .0001) and OS (10.5 months vs 48.1 months, P < .0001) than those who underwent surgical resection for high-grade HCC tumors.5
Prior to 2020, there were 2 first-line therapies for unresectable HCC, sorafenib and lenvatinib, both of which only modestly improve OS and are associated with significant side effects.1 Programmed death 1 (PD-1) inhibitors are used as second-line therapy due to a lack of OS benefit when compared with sorafenib despite showing promising results in phases 1 and 2 studies and response rates of 15% to 20% in phase 3 studies.1 The IMbrave150 trial,7 a global, phase 3 clinical trial showed that A + B resulted in better objective response rate and OS than sorafenib alone (29.8% and 19.2 months vs 11.3% and 13.4 months, respectively) with an acceptable side effect profile. In addition, 25 patients (7.7%) achieved a complete response. Although this trial was practice-changing for the treatment of patients with unresectable HCC, patients with rare variants, such as sarcomatoid, fibrolamellar, and mixed cholangiocarcinoma and HCC were excluded from participation.7
Currently, no guidelines exist to support a standardized approach to therapy for patients with SHCC.9,18 Although the variant was not excluded from 2 phase 3 trials, SHARP and REFLECT, which investigate the efficacy of sorafenib versus placebo and lenvatinib versus sorafenib, respectively, there were not enough patients to produce a meaningful subgroup analysis.6 This also applies for second-line HCC therapy in the RESORCE, CELESTIAL, and REACH-2 trials investigating regorafenib, cabozantinib, and ramucirumab, respectively.6 However, in a study of 72 patients with SHCC, chemotherapy was the only therapy associated with increased OS on multivariate analysis.22 Notably, a study by Liao et al11 found that SHCC patients treated with chemotherapy and sorafenib had worse OS than those with conventional HCC.
Case reports prior to the publication of the IMbrave trial outline chemotherapy regimens with varying success. A study by Ma etal14 utilized gemcitabine and ifosfamide in combination with intrapleural chemotherapy and radiation as palliative treatment for a patient with metastatic SHCC with an accompanying right-sided malignant pleural effusion. Gemcitabine and ifosfamide were delivered IV every 3 weeks for 6 cycles following 2 weeks of intrapleural cisplatin (90 mg) and 1 week of interleukin (2 000 000 U) in combination with radiation therapy and thermal therapy for a resultant pleural effusion. This regimen eased the patient’s symptoms and resulted in an OS of 8 months.14 A retrospective review by Ma et al14 of 28 patients demonstrated that the median OS for patients receiving surgical treatment was 15.6 months compared with 7.6 months for patients receiving palliative treatment, indicating that early surgical intervention provides the best prognosis.14 Another patient received 4 cycles of albumin-bound paclitaxel (105 mg/m2 IV) and gemcitabine (850 mg/m2 IV); however, tumor progression was noted on enhanced MRI.16 Subsequently, the patient received immunotherapy and targeted therapy with a PD-1 inhibitor (130 mg/m2 IV for 30 min on day 1) and anlotinib (10 mg orally on days 1-14), however efficacy was limited, and the patient expired 3 months after initiation of therapy.16
The success of treating the sarcomatoid variant of clear cell renal cell carcinoma (ccRCC) with A + B provides a rationale for use in this patient. The IMmotion15123 trial, a multicenter, open-label phase 3 trial, investigated A + B versus sunitinib in ccRCC and histologic variants. The sarcomatoid subgroup demonstrated an OR of 49% (n = 33 of 68). Improved PFS was maintained in the sarcomatoid group regardless of PD-L1 expression, suggesting that histology is an independent predictor of response to this regimen.23 These results were supported in a phase 2 trial, which demonstrated a response rate of >50% in patients with sarcomatoid differentiation.24 The anecdotal treatment response in our patient suggests that sarcomatoid HCC may demonstrate similar successes to the ccRCC sarcomatoid population. Robust investigation of novel treatments and combinations are of critical importance in this subtype.
Conclusion
This case is an example of SHCC being successfully managed with A + B combination therapy. The results obtained in this patient suggest that further investigation of this combination is warranted. The authors encourage the contribution of further case reports using this combination in SHCC patients.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
References
- 1. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. [DOI] [PubMed] [Google Scholar]
- 2. Giannis D, Morsy S, Geropoulos G, Esagian SM, Sioutas GS, Moris D. The epidemiology, staging and outcomes of sarcomatoid hepatocellular carcinoma: a SEER population analysis. In Vivo. 2021;35(1):393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fenton SE, Burns MC, Kalyan A. Epidemiology, mutational landscape and staging of hepatocellular carcinoma. Chin Clin Oncol. 2021;10(1):2. [DOI] [PubMed] [Google Scholar]
- 4. Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol. 2020;10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang JP, Yao ZG, Sun YW, et al. Clinicopathological characteristics and surgical outcomes of sarcomatoid hepatocellular carcinoma. World J Gastroenterol. 2020;26(29):4327-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wege H, Schulze K, von Felden J, Calderaro J, Reig M; Rare Liver Tumors Working Group of the European Reference Network on Hepatological Diseases (ERN RARE-LIVER). Rare variants of primary liver cancer: fibrolamellar, combined, and sarcomatoid hepatocellular carcinomas. Eur J Med Genet. 2021;64(11):104313. [DOI] [PubMed] [Google Scholar]
- 7. Finn RS, Qin S, Ikeda M, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_suppl):267-267. [Google Scholar]
- 8. Giunchi F, Vasuri F, Baldin P, Rosini F, Corti B, D’Errico-Grigioni A. Primary liver sarcomatous carcinoma: report of two cases and review of the literature. Pathol Res Pract. 2013;209(4):249-254. [DOI] [PubMed] [Google Scholar]
- 9. Numbere N, Zhang D, Agostini-Vulaj D. A rare histologic subtype of hepatocellular carcinoma, sarcomatoid hepatocellular carcinoma: report of a case. Hepat Oncol. 2020;8(2):HEP33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang QB, Cui BK, Weng JM, Wu QL, Qiu JL, Lin XJ. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg. 2012;16(9):1715-1726. [DOI] [PubMed] [Google Scholar]
- 11. Liao SH, Su TH, Jeng YM, et al. Clinical manifestations and outcomes of patients with sarcomatoid hepatocellular carcinoma. Hepatology. 2019;69(1):209-221. [DOI] [PubMed] [Google Scholar]
- 12. Kwon JH, Kang YN, Kang KJ. Carcinosarcoma of the liver: a case report. Korean J Radiol. 2007;8(4):343-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Am J Surg Pathol. 1996;20(3):277-285. [DOI] [PubMed] [Google Scholar]
- 14. Ma Q, Jiang L, Bonda S, Luo D, Zhang W. A rare case of hepatic sarcomatoid carcinoma: exceeding expectations in a stage IV primary hepatic sarcomatoid carcinoma patient. Int J Clin Exp Pathol. 2019;12(1):378-383. [PMC free article] [PubMed] [Google Scholar]
- 15. Ji W, Xing Y, Ma J, et al. Primary liver sarcomatoid carcinoma: a case series and literature review. J Hepatocell Carcinoma. 2021;8:1117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Z, Lv K, Zhao Y, et al. Sarcomatoid hepatocellular carcinoma mimicking hepatic abscess: a case report. Medicine (Baltimore). 2020;99(39):e22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morisue R, Kojima M, Suzuki T, et al. Sarcomatoid hepatocellular carcinoma is distinct from ordinary hepatocellular carcinoma: clinicopathologic, transcriptomic and immunologic analyses. Int J Cancer. 2021;149(3):546-560. [DOI] [PubMed] [Google Scholar]
- 19. Wu L, Tsilimigras DI, Farooq A, et al. Management and outcomes among patients with sarcomatoid hepatocellular carcinoma: a population-based analysis. Cancer. 2019;125(21):3767-3775. [DOI] [PubMed] [Google Scholar]
- 20. Maeda T, Adachi E, Kajiyama K, et al. Spindle cell hepatocellular carcinoma: a clinicopathologic and immunohistochemical analysis of 15 cases. Cancer. 1996;77(1):51-57. [DOI] [PubMed] [Google Scholar]
- 21. Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(suppl 3):S104-S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zakka K, Jiang R, Alese OB, et al. Clinical outcomes of rare hepatocellular carcinoma variants compared to pure hepatocellular carcinoma. J Hepatocell Carcinoma. 2019;6:119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404-2415. [DOI] [PubMed] [Google Scholar]
- 24. McGregor BA, McKay RR, Braun DA, et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol. 2020;38(1):63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]