Abstract
The increasing of chronic diseases and related health management are the main clinical and public health challenges. The long-term nature and the need for continuous monitoring in chronic disease management gave rise to early technological innovations (mobile Health) to improve care management plans, therapeutic adherence, and psychological support to the patient. This review aimed to map the literature on the impact of the use of wearable device on quality of life in patients with chronic diseases. We performed a systematic search of MEDLINE through PubMed, Web of Science, and Scopus of all scientific literature published until January 2022. Based on inclusion and exclusion criteria, a total of 10 papers were included. This review pointed out the relevant focus on the use of wearable device in chronic disease patients highlighting the wearable device impact on several domains including quality of life, Self-Efficacy, Self-Management, and feelings on patients with chronic diseases. The available scientific literature related to the impact of the use of wearable device on quality of life and psychological features in patients with chronic diseases, general underline a need to develop professional healthcare guidelines and tailored intervention on patients with a chronic condition, using mobile Health solutions and trying to fill the lack of knowledge about the topic.
Keywords: Chronic disease, wearable device, quality of life, self-efficacy, self-management, anxiety, depression
Introduction
The increasing of chronic diseases (CDs) is one of the main clinical and public health challenges. According to the World Health Organization (WHO), CDs are the leading cause of mortality globally, representing 63% of all death.1 The prevalence and incidence of CDs led contemporary healthcare systems to face increasingly complex care demand.2 CD management generally requires a long-term care plan. Long-term and continuous monitoring of mental and physical symptoms in CD management gave rise to implement technological solutions in innovative perspective3 in order to improve care management, therapeutic adherence, and quality of life (QoL) and wellbeing of patients.
Personalized medicine based on multidisciplinary and integrated approach improved healthcare effectiveness toward to efficient medical prompts to maximize the adherence and compliance of the patient to the medical long-term treatments. Taking into account that, digital health is part of the personalized medicine process: it can be defined as the use of digital technologies to improve patient outcomes and enable better care delivery.4 Among innovative solutions, mobile Health (m-Health) is becoming a relevant instrument. m-Health has been defined by the Global Observatory for e-Health of the WHO as medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices.1 Among all of them, smart wearable technology not only helps people pursue a healthier lifestyle but also provides a continuous flow of physiological data for disease management by actively recording parameters and tracking the metabolic state.5 m-Health has enormous potential to impact the CD management though the acceptance, availability and technological usability features; taking into account to that, m-Health devices could improve service delivery and impact patient outcomes drawing the continuous health monitoring.3
Among m-Health innovations, wearable devices (WD) featured as autonomous and non-intrusive solutions to promote surveillance, monitoring, or support for biofeedback monitoring in a trustable and comfortable way.6 Wearable means that a device is supported on the human body or in a piece of clothing, suitable for prolonged use as a wearable accessory, allowing individuals to closely monitor changes in their vital signs and provide feedback to help maintain an optimal health-status.7 Wearable systems for continuous health monitoring are the key technology in helping the transition to more proactive and affordable healthcare,7 reducing the high costs of conventional care.
In this context, adherence is critical to achieving improved health outcomes, QoL, and efficacy of health care for integrated CD management.8 While the growing popularity of m-Health is evident, its impact on the QoL and mental health of subjects with CDs has not yet been fully explored. Research generally focused on the development of these devices or on the feasibility of using mobile technology in healthcare, concentrating more on medical and technical parameters of professionals than on psychological dimensions of patients (e.g., emotions and cognitions). The present study aimed to elaborate the systematic review for emerging scientific literature on m-Health and the use of WD for integrated therapeutical solutions to improve CD patients’ QoL. Scope of the study was to propose oriented research perspective for integrated approach for innovative technology into personalized medicine for CD management, as well the exploitation of research topic toward to advanced medicine joining clinical and technological solutions.
Methods
Study design
We conducted a descriptive analysis of the characteristics of the included literature to examine the effects of m-Health and the use of WD in patients with CDs. We performed this review in according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines.9
Search strategy
To identify potentially relevant studies for inclusion, we performed a systematic search of MEDLINE through PubMed, Web of Science, and Scopus in January 2022. Key search terms were: ‘chronic disease’, ‘wearable device’ and ‘quality of life’.
Inclusion and exclusion criteria
To be considered eligible, titles and abstracts of retrieved studies had to refer to patients with CDs and the use of WD for QoL. To be included, studies had to (a be published in the English language; (b) be available in full text; (c) be published in scientific peer-reviewed journals; (d) include psychological measures. In addition, we excluded all reviews on the topic.
Article selection and data extraction
The PICO model defined the research question (patient/population, intervention, comparison, and outcomes); “In CDs applied the WD (P), what were the effect for psychological dimensions (I) compared to the effect on the outcomes in control groups (C) on QoL (O). Two reviewers independently screened all titles, abstracts, and full texts and resolved disagreements by consensus or consultation with a third reviewer. The following data were extracted from each paper: (a) general information: authors, publication year, country, and involved institution (e.g., University, Medical Centre); (b) study characteristics: study design, sample size, monitoring period, CD diagnosis, WD type used; (c) physiological and psychological measures used; (d) data related to the research question of the review: study aim, a summary of outcomes.
Data synthesis
General information, study characteristics, physiological and psychological measures used, and data related to the research question of the review were descriptively synthesized. To explore the impact of the use of WD in patients with CDs, results were summarized referring to outcomes on QoL, self-efficacy, self-management, anxiety and depression.
Results
Search results
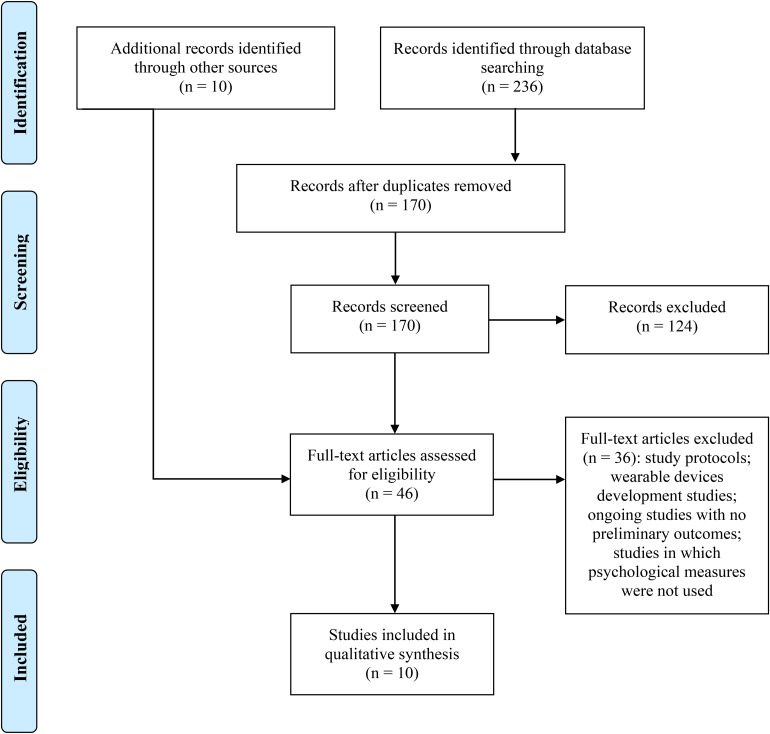
The literature search of PubMed, Web of Science, and Scopus electronic databases provided a total of 236 publications. After removing duplicates, 170 references were identified for screening. Based on the inclusion/exclusion criteria, two reviewers screened for eligibility all titles and abstracts and successfully resolved disagreements by consensus. The full text of the remaining 36 papers potentially worthy of inclusion, were examined comprehensively. To maximize the finding of potentially relevant manuscripts, an additional 10 studies, which met criteria for inclusion were identified by checking citations of eligible articles for supplementary references that were missed in the initial search. Out of a total of 46 articles assessed for eligibility, 36 were excluded and reasons for exclusion were reported. Figure 1 shows all details about the study’s search and selection process. Finally, ten studies10–19 were included.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the studies selection process for the review.
Characteristics of the included studies
Table 1 summarizes the main characteristics of the included studies. We identified from each included study: (a) authors, (b) study design, (c) chronic disease, (d), sample size (e) WD type, and (f) monitoring period.
Table 1.
Main characteristics of the included studies.
| Study design | Authors | Chronic disease | Sample size | Wearable device type | Monitoring period |
|---|---|---|---|---|---|
| Quantitative study design | Varas et al., 2018 | COPD | n = 33 | OMRON Walking Style X Pocket HJ-320e digital pedometer | 12 months |
| J. Lee et al., 2019 | FMS | n = 24 | Painmeter (bracelet for reporting the severity of pain) | 3 months | |
| Moor et al., 2019 | Sarcoidosis | n = 10 | Fitbit Flex 2 | 4 weeks | |
| J.-S. Lee et al., 2020 | MetS | n = 59 | - | 12 weeks | |
| Li et al., 2020 | CKD | n = 49 | Hearth Rate Smart Wristband | 90 days | |
| Lukkahatai et al., 2021 | DM | n = 114 | Garmin Vivofit | 2 days | |
| Z. Xie et al., 2021 | DM HTN CHF |
n = 853 | Fitbit, Apple Watch, Garmin Vivofit | - | |
| Qualitative study design | Andersen et al., 2020 | CHF | n = 27 | Fitbit Alta HR | 4–49 weeks |
| Mixed-method approach (qualitative and quantitative) | Wulfovich et al., 2019 | CDs (general) | n = 200 | Fitbit, Apple Watch, iFit, JawboneUP, Garmin, Samsung Gear, Nike + | 4 weeks |
| Bentley et al., 2020 | COPD | n = 30 | Fitbit | 8 weeks |
COPD: chronic obstructive pulmonary disease; FMS: fibromyalgia syndrome; CKD: chronic kidney disease; MetS: metabolic syndrome; DM: diabetes mellitus; HTN: hypertension; CHF: chronic heart failure; CDs: chronic diseases.
All studies focused on patients with CDs and concerned the use of different types of WD. Regarding CDs, included studies involving patients with several specific chronic conditions as well as chronic obstructive pulmonary disease (COPD),11,17 fibromyalgia syndrome (FMS),12 sarcoidosis,16 chronic heart failure (CHF),10,19 chronic kidney disease (CKD),14 metabolic syndrome (MetS),13 diabetes mellitus (DM),15,19 and Hypertension.19 Only one study18 considered general chronic conditions. Among WDs, almost all the included studies10–12,14–16,18,19 used wrist-worn activity tracker (n = 8; 80%) (e.g., Fitbit, Apple Watch, Garmin Vivofit), except one17 that used a pedometer attached to the waist. Only one study13 did not mention the WD used.
The 10 selected studies differed in their study design and methods and included research carried out using a quantitative design,12–14,17,20–22 a qualitative design,10 or mixed-methods approach incorporating both qualitative and quantitative methods.11,18 Overall, selected studies involved 1399 patients with CDs, and the monitoring period lasted from 2 days to 12 months.
Source of articles
Table 2 summarizes general information about the publisher of the included studies. We identified for each included paper: (a) source title; (b) subject area; (c) first author's country; (d) first author's institution; and (e) publication year. All papers were published in peer-reviewed journals from 2018 to 2022. Concerning publication year, we noted a growing emerging interest in the recent years showing the general intention to transform healthcare provision in the coming decades, through mobile health software solutions, which can help professionals to early intervene in CD, facilitating real-time monitoring of patients’ vital signs, remotely. Even if the number of identified studies is very limited, we observed the increasing over the time the interest in the use of WDs in patients with CDs and their impact on quality of life. Although these studies involved Korean, Danish, Netherlands, Spanish, Taiwanese, and British researchers, the majority of publications (n = 3; 30%) were undertaken in America.
Table 2.
Publishers of papers included in the present review.
| Number | ||
|---|---|---|
| Publication year | 2018 | 1 |
| 2019 | 3 | |
| 2020 | 4 | |
| 2021 | 2 | |
| Source title | Journal of Medical Internet Research | 3 |
| Asian Pacific Island Nursing Journal | 1 | |
| Frontiers in Psychology | 1 | |
| Health and Technology | 1 | |
| International Journal of Rheumatic Disease | 1 | |
| JMIR mHealth and uHealth | 1 | |
| Journal of Personalized Medicine | 1 | |
| Physiotherapy Research International | 1 | |
| Subject Area (SJR Ranking) * | Medicine – Health Informatics | 4 |
| Medicine | 2 | |
| Engineering – Biomedical Engineering | 1 | |
| Health Professions – Physical Therapy, Sports Therapy and Rehabilitation | 1 | |
| Nursing | 1 | |
| Psychology | 1 | |
| First author's country | United States | 3 |
| Korea | 2 | |
| Denmark | 1 | |
| Netherlands | 1 | |
| Spain | 1 | |
| Taiwan | 1 | |
| United Kingdom | 1 | |
| First author's institution | University | 8 |
| Research Medical Centre | 1 | |
| Department of Internal Medicine | 1 |
Type of articles
Table 3 highlights the details on measures used and outcomes of the included studies. We extracted from each included paper: (a) authors; (b) physiological and medical measures; (c) psychological measures; (d), general measures; and (e) outcomes. All the papers focus on the use of WD in CDs, while outcome tools mainly referred to physiological/medical measures (e.g., disease diagnosis, laboratory data, physical activity tracking, body composition measurement) and psychological measures (e.g., quality of life, self-efficacy, self-management, anxiety, and depression).
Table 3.
Summary of included studies.
| Authors | Physiological/medical measures | Psychological measures | General measures | Outcomes |
|---|---|---|---|---|
| Varas et al., 2018 | EST (exercise capacity) Step count (physical activity) Baecke physical activity questionnaire |
SGRQ (quality of life) | Demographic characteristics | This study demonstrates that community-based Pulmonary Rehabilitation training in COPD patients improves exercise capacity, physical activity level, and quality of life over the medium and long term. The benefits obtained in exercise capacity and quality of life indicate that this type of programme both produced a short-term training effect and maintained these benefits over time, suggesting that patients autonomously adopted the regular practice of physical exercise. |
| J. Lee et al., 2019 | Body composition measurement FIQ (disease activity) PtGA VAS (general health-status) |
BDI (depression) EQ-5D-5L (quality of life) |
Demographic characteristics | The PAAS WD was effectively used in FMS patients. No differences were found about depression symptoms and quality of life, but these results could be due to the inclusion of a limited number of patients and the short study period which may not have captured the impact of the WD on QoL and depression. However, the WD accurately reflected pain status between clinic visits, and the use of this system may help to alleviate pain. |
| Moor et al., 2019 | Step count (physical activity) Home spirometry (pulmonary function) KSQ (health status in patients with sarcoidosis) VAS (general health-status) FAS (fatigue in patients with sarcoidosis) |
EQ-5D-5L (quality of life) HADS (anxiety and depression) |
Demographic characteristics Phone interview about opinion on the home monitoring programme and the usability of the device |
Patient satisfaction and adherence to daily spirometry and activity tracking were high. All patients wished to continue the use of the home monitoring programme after the study. However, symptoms measures by HADS, FAS, VAS and QoL (KSQ, EQ-5D-5L) were not significantly different at baseline compared to month one, probably due to the inclusion of a limited number of patients and to the short period study. |
| Wulfovich et al., 2019 | Step count and calories (physical activity) | EMA (self-efficacy) | Demographic characteristics | The results indicate that health apps and WD have the potential to enable better self-management and improve patients' wellbeing but must be further refined to address different human aspects of their use. Specifically, the apps/wearables should be easier to use, more personalized and context-aware for the patient's overall routine and lifestyle choices, and healthcare needs. |
| Andersen et al., 2020 | Step count, sleep and health rate data (physical activity) | Interview on feeling (Anxiety) | Demographic characteristics Interview on knowledge acquired Interview on Evaluation on themselves and their overall health |
Wearable activity trackers actualized patients' experiences across 3 dimensions with a spectrum of contrasting experiences: (a) knowing, which spanned gaining insight and evoking doubts; (b) feeling, which spanned being reassured and becoming anxious; and (c) evaluating, which spanned promoting improvements. The affective dimension of self-tracking when living with a chronic heart disease emerged as loaded with ambivalence: Fitbit data may provide numerical reassurance, which can relieve acute anxiety related to unclear bodily sensations but at the same time heightened attention to these data can also introduce new uncertainties and anxiety. Activity data from WD may be a resource for self-care; however, the data may simultaneously constrain and create uncertainty, fear, and anxiety. |
| Bentley et al., 2020 | Medical Research Council Breathlessness Scale (COPD severity) Step count (physical activity) CHAMPS (physical activity) ISWT (exercise capacity) CAT (symptoms) |
SGRQ (quality of life) PHQ-9 (anxiety and depression) |
Demographic characteristics EQ-5D-3L (cost-effectiveness) SUS (WD usability) |
Overall, the SMART-COPD intervention was well liked and perceived as easy to use into participants' daily lives by those who completed the study. However, there was a high dropout rate which implies high rates of people who were eligible for the intervention but who did not easily adopt the technology. The data suggest that people with COPD who had worse baseline health were more likely to withdraw from the study, which may indicate that this patient group is harder to reach with mHealth interventions. Moreover, participants who withdrew had worse baseline scores on quality of life and depression compared with those who completed. |
| Lee et al., 2020 | Body composition measurement (height, weight, body mass index) Systolic/diastolic pressure Blood sugar level Cholesterol level |
Walker's health-promoting lifestyle profile WHOQOL-BREF (Quality of Life) Self-Efficacy Scale |
Demographic characteristics | The e-Motivate4Change programme based on the use of health apps and WD, selected based on user's need, was associated to increased physical activity, decreased BMI, lower cholesterol, and increased self-efficacy among experimental group, thus effectively promoting a health-related lifestyle. |
| Li et al., 2020 | Body composition measurement Laboratory data (hemogram, serum biochemistry, electrolyte profile, renal function) Step count, distance, consumed calories, and heart rate (physical activity) |
Self-efficacy questionnaire Self-management questionnaire KDQOL-SF (quality of life) |
Demographic characteristics | A self-management intervention that combines WD, a health management platform, and social media could strengthen self-efficacy and self-management, and lead to improvements in quality of life for people with CKD stages 1–4. The effects of this nonpharmacologic intervention were also reflected in a slower decline in eGFR. These results outline a new self-management model to promote healthy lifestyle behaviours in patients with CKD. |
| Lukkahatai et al., 2021 | Body composition measurement Blood pressure Symptom experience Step count (physical activity) Sleep hours |
SDSCA (Self-management) DSES (Self-efficacy) SF-12 (Quality of life) |
Demographic characteristics Questionnaire to measure the experience, acceptability, and satisfaction in using WD |
The results demonstrate the feasibility of the use of the WD among people living with chronic conditions. Participants found that the step count screen provided immediate physical performance feedback that was helpful with their exercise. The behavioural changes, however, could not be examined due to the short duration of the usage. The study did not find significant differences but only slightly higher self-management behaviours and self-efficacy among patients who wore WD than those in the control group but this could be due to the short duration (2 days) of the WD use. Future studies that require longer device usage in larger sample sizes are needed. |
| Xie et al., 2021 | Body composition measurement chronic diagnosis |
Section of the HINTS survey (Self-efficacy, depression) | Demographic characteristics Questionnaire on WD use |
This study examined the association between WD use and self-efficacy for managing health among adults aged 18 and older. Those who used WD in the past month were more likely to have higher self-efficacy (completely confident in managing health) than those who did not use WD. Use of WD can motivate individuals to obtain high self-efficacy to commit to manage their own health. Thus, high self-efficacy is desired for health behaviour change in health-promoting interventions. |
BDI: Beck Depression Inventory; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; CHAMPS: Community Healthy Activities Model Programme for Seniors; DSES: Diabetes Self-efficacy Scale; EST: endurance shuttle test; EQ-5D-3L: EuroQoL 5 Dimensions 3 Level; EQ-5D-5L: EuroQoL-5 Dimension 5 Level; Ex-SRES: Exercise Self-Regulatory Efficacy Scale; FAS: Fatigue Assessment Scale; FIQ: Fibromyalgia Impact Questionnaire; HADS: Hospital Anxiety and Depression Scale; HINTS: Health Information National Trends Survey; ISWT: Incremental Shuttle Walk Test; KDQOL-SF: Kidney Disease Quality of Life Survey; KSQ: King's Sarcoidosis Questionnaire; PAAS: Pain Assessment and Analysis System; PHQ-9, Patient Health Questionnaire-9 (Anxiety and depression); PtGA: Patient global assessment; SDSCA: summary of diabetes self-care activities (self-management behaviours); SGRQ: St George's Respiratory Questionnaire; SF-12: General Health Short Form 12; SUS: System Usability Scale; VAS: Visual Analogue Scale; WHOQOL-BREF: World Health Organization Quality of Life-BREF; WD: wearable device; eGFR: estimated glomerular filtration rate; FMS: fibromyalgia syndrome; CKD: chronic kidney disease; EMA: Ecological Momentary Assessment.
Overview on the use of m-health for CDs
First, this review aimed to investigate the impact of the use of WD on QoL and psychological features in patients with chronic conditions. Second, we observed the lack of extensive scientific literature on this topic, underlining the need to go beyond acceptability and feasibility studies, to conduct more detailed analyses of the interaction between personal health data from WD and its impact on patients' psychological wellbeing and quality of life.
Outcomes of the included studies showed that the use of WD impact on QoL,11–17 self-management,14,15 self-efficacy,13–15,18,19 anxiety,10,11,16 and depression11,12,16,19 in patients with chronic conditions. An analysis of the impact of m-Health on quality of life and psychological features in patients with CDs is proposed below.
Quality of life indicator for WDs
Almost all of the included studies focused on the impact of QoL related to the use of WD11–17 using St George's Respiratory Questionnaire (SGRQ),23 EuroQoL 5D-5L,24 Kidney Disease Quality of Life survey (KDQOL-SF),25 General Health Short Form 12 (SF-12),26 WHO Quality of Life-BREF (WHOQOL-BREF).1
Overall, the findings we reviewed revealed conflicting results including some pointing out the fundamental role of m-Health interventions, using health apps and WD in improving QoL and healthy lifestyle respect to patients assigned to usual chronic care,11,14,17 and some which found no significant differences among these interventions.13,16
Varas study17 demonstrated that a community-based programme of exercise training through walking and increased physical activity level, using pedometers as feedback, improved exercise capacity, and QoL in COPD patients. Moreover, participants who withdrew (due to difficulties in using technology) from a study on the use of an activity tracker to promote physical activity in the management of COPD had worse QoL baseline scores compared with those who completed the intervention11
However, as observed by Lee et al.12 evaluating the efficacy of a real-time pain monitoring system using a WD, referred to as the Pain Assessment and Analysis System (PAAS), in patients with fibromyalgia, the health-related QoL endpoint was not achieved although the reduction of pain in patients who used the proposed real-time monitoring system was significantly greater than those in the control group. One explanation may be that the pain reduction with PAAS usage was not sufficient to impact more complex outcomes. Lee's study13 implemented the e-Motivate4Change programme with an experimental group to increase knowledge about MetS and promote health-related activities through the use of a WD, and the finding showed no significant differences in QoL among patients who wore WD compared to those in the control group who received the usual chronic care. Also, Moor study20 revealed no significant differences in QoL of patients with sarcoidosis (at baseline compared to month one), through the use of WD and an e-Health application in which patients keep track of their health-related data. One potential explanation of the outcomes of studies that found no significant differences in QoL among patients involved with the use of WD and patients assigned to usual care could be due to the inclusion of a limited number of patients and to the period of the study which may have been too short to impact participants’ QoL.
Self-management and self-efficacy
Several of the included studies evaluated self-management and self-efficacy13–15,18,19 using Self-Efficacy Scale,27 Diabetes Self-efficacy Scale,28 Ecological Momentary Assessment,29 Summary of Diabetes Self-Care Activities,30 or Self-Efficacy and Self-Management questionnaire.
With regard to the self-efficacy index, five studies12–14,21,31 found that specific m-Health interventions, using health apps and WD, significantly increased Self-Efficacy and healthy lifestyle in the experimental groups compared to those who received no treatments and were assigned to usual chronic care. Moreover, a study found that the use of WD with a health management platform and social media support not only strengthened Self-Efficacy, but also Self-Management in the intervention group and slowed the estimated glomerular filtration rate decline in patients with CKD, promoting healthy lifestyle behaviours. Only Lukkahatai's study15 did not find significant differences but only slightly higher self-management behaviours and self-efficacy among patients who wore WD than those in the control group but this could be due to the short duration (2 days) of the WD use.
Anxiety and depression
Included studies assessed anxiety and depression symptoms10–12,16,19 using Beck Depression Inventory,32 Hospital Anxiety and Depression Scale (HADS),33 Patient Health Questionnaire-9 (PHQ-9),34 or interview on emotional feelings. Diagnosis of CD often introduces negative feelings into patients’ lives.
With regard to depression, the included studies12,16,19 found no significant difference in WD use; differently, Xie et al.21 observed that being depressed showed a significant association with a lower likelihood to have complete confidence in health management when compared to those without depression. Additionally, participants who withdrew from a study on the use of an activity tracker to promote physical activity in the management of COPD had worse depression baseline scores compared with those who completed it,11 and this could be due to lower self-confidence in the management of CD as reported in the study mentioned above.19
Regarding anxiety, as observed by Pedersen et al.35 CHF patients are known to be at an increased risk of being diagnosed with depression and anxiety. In research based on interviews conducted by Andersen et al.10 patients experienced different levels of anxiety and used their Fitbit to reassure themselves about their health and decreased anxiety levels, also helping them engage more in physical activity. However, the affective dimension of self-tracking when living with a chronic heart disease emerged as loaded with ambivalence: Fitbit data may provide numerical reassurance, which can relieve acute anxiety related to unclear bodily sensations but at the same time heightened attention to these data can also introduce new uncertainties and anxiety.10
Discussion
The health management of chronic conditions is a key challenge, and new mobile technologies could offer good solutions.36 Consumer health information technologies such as wearable activity trackers are increasingly being considered to improve chronic care management.37,38 Smartphones and WD are technologies predicted to transform healthcare provision in the future. Technology not only helps people pursue a healthier lifestyle but also provides a continuous flow of health data for diagnosis and treatment by actively recording physiological parameters and monitoring the metabolic state.5 These clinically integrated data-producing devices affect self-care activities as they enable managing symptoms, taking medicine, dealing with the emotional impact, and tackling lifestyle changes.39–41
Despite recent developments and the growing popularity of m-Health, knowledge about the impact on QoL and mental health of patients with CDs is currently underdeveloped. During the screening process of the emerging scientific literature on the use of WD in CD and its impact on patient's quality of life, we observed that research generally focused on the development of new WD or on the feasibility of using mobile technology in healthcare, concentrating more on medical and technical parameters than on psychological outcomes.
In contrast, the included studies showed that m-Health impacts on several domains including QoL, self-efficacy, self-management, and feelings of patients with CDs.
Generally, the findings we reviewed revealed conflicting results about QoL, including some pointing out the central role of m-Health solutions (using health apps and WD) in improving QoL and healthy lifestyle with respect to patients assigned to usual chronic care,11,14,17 and some which found no significant differences among these interventions.13,16 The benefits for chronic patients include an enhancement of their quality of life by reducing the hospitalization time and the patient's cost, and improving their life expectancy by generating fast response alarms.42 However, not finding significant differences could be due to the inclusion of a limited number of patients and the short study period which may not have captured the impact of the WD on QoL.
The included studies, also found that m-Health interventions not only strengthened self-efficacy13–15,18,19 but also self-management.14,22 The use of WD can motivate individuals to achieve high self-efficacy to commit to self-management of their health. Self-efficacy is the self-belief in the ability to manage one's daily life and put in the effort to get the desired behavioural and health outcomes.43 General, it involves attitudes and self-beliefs to cope with a variety of difficult situations in daily life. Self-efficacy significantly contributes to the long-term health status of patients44 and is highly related to the patient's Self-Management efforts and their health outcomes.45 Self-management of health implies management of activities contributing to health including physical activity, diet, medication intake, and management of symptoms.46 It can be defined in terms of learning and practicing the necessary skills to lead an active and emotionally satisfying life in face of a chronic condition that often requires changes that affect different aspects of daily life. Self-Management has been shown to play a crucial role in the reduction of disease exacerbations in chronic patients and improve adherence to rehabilitation. The higher the self-efficacy in health management, the higher the health-related goal will be set and a stronger commitment to achieve the goal will be made.43 So, self-efficacy and self-management play a key role in health behaviour change in health-promoting interventions for chronic patients.
Regarding emotional feelings, although the included studies found no significant differences between WD use and depression,12,16,19 probably due to the short period of the study, the limited number of patients, and the need for a more comprehensive psychological assessment, being depressed can be considered a predictor of a lower self-efficacy for managing health. Moreover, about anxiety, participants felt that having a system to constantly monitor their condition would ease their mind and provide a feeling of security, but at the same time heightened attention to these data could also introduce new uncertainties and anxiety. These findings could be due to the need to introduce psychoeducational interventions in the treatment and management process of CDs and a more detailed psychological assessment for patients.
We, generally, observed the lack of extensive scientific literature on this topic, underlining the need to go beyond acceptability and feasibility studies, to conduct more detailed analyses of the interaction between personal health data from WD and its impact on patients’ psychological wellbeing and quality of life. However, the available scientific literature related to the impact of the use of WD on quality of life and psychological features in patients with CDs, general underline a need to develop professional healthcare guidelines and tailored intervention on patients with a chronic condition, using m-Health solutions and trying to fill the lack of knowledge about the topic.
Conclusion
Long-term chronic condition management requires a high level of patient involvement. m-Health (using health apps and WD) might have high potential influence for Qol, self-efficacy, self-management, and psychological aspects such as anxiety and depression. Specifically, they enable more convenient delivery of services for patients’ education, self-monitoring, and strengthening of a healthy lifestyle. However, the implementation of m-Health in the healthcare system is still preliminary phase: in our opinion some key points should be exploited to draw effective integrated and personalized medicine joining pharmacological and technological treatments for better life of CD patients: (1) the lack of robust wearable-to-disease focused data on the efficacy of intervention that might motivate sustained use of WD; (2) incomplete healthcare guidelines; (3) the need of more comprehensive psychological assessment for patients with CDs; (4) the need to create more resources for mental health within-programme.
The present review highlighted the relevance of strategic actions on physiological and psychological interventions to deal with the Quality of Life of CD patients applying m-Health solutions. The efforts of research including clinical and technical professionals should be towards conducting planned research protocol based on the efficacy of integration of technological and medical solutions for advanced clinical treatments; more the need to prioritize the m-Health interventions tailored to patient needs that allow reaching even individuals who do not easily adopt the technology. Moreover, psychology and medicine should work together to address clinical challenge for chronic care management through the implementation of m-Health as an instrument to reduce the healthcare burden while improving patients’ QoL in survivorship.
Footnotes
Author Contributions: DDG handled the conceptualization of the study; JR & FG performed database searching; EC elaborated the database; CF was supervisor for the project and the paper. All authors approved the final version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dina Di Giacomo https://orcid.org/0000-0001-8189-2052
References
- 1.The Whoqol Group. The world health organization quality of life assessment (WHOQOL): development and general psychometric properties. Soc Sci Med 1998; 46: 1569–1585. [DOI] [PubMed] [Google Scholar]
- 2.Graffigna G, Barello S, Riva G, et al. Promozione del patient engagement in ambito clinico-assistenziale per le malattie croniche: raccomandazioni dalla prima conferenza di consenso italiana. Recenti Prog Med 2017; 108: 455–475. [DOI] [PubMed] [Google Scholar]
- 3.Hamine S, Gerth-Guyette E, Faulx D, et al. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res 2015; 17: e3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancela J, Charlafti I, Colloud S, et al. Digital health in the era of personalized healthcare: opportunities and challenges for bringing research and patient care to a new level. Digit Health 2021: 7–31. [Google Scholar]
- 5.Xie Y, Lu L, Gao F, et al. Integration of artificial intelligence, blockchain, and wearable technology for chronic disease management: a new paradigm in smart healthcare. Curr Med Sci. 2021; 41(6): 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa SEP, Rodrigues JJPC, Silva BMC, et al. Integration of Wearable Solutions in AAL Environments with Mobility Support. J Med Syst [Internet] 2015. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84944682178&doi=10.1007%2fs10916-015-0342-z&partnerID=40&md5=fc9b4cf720bd1097cd27dcb27cc57fbe. [DOI] [PubMed] [Google Scholar]
- 7.Otto C, Milenković A, Sanders C, et al. System architecture of a wireless body area sensor network for ubiquitous health monitoring. J Mob Multimed 2006: 307–326. [Google Scholar]
- 8.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med 2012; 157: 785–795. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Med 2009; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen TO, Langstrup H, Lomborg S. Experiences with wearable activity data during self-care by chronic heart patients: qualitative study. J Med Internet Res 2020; 22: e15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentley CL, Powell L, Potter S, et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR MHealth UHealth 2020; 8: e16203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Park SH, Ju JH, et al. Application of a real-time pain monitoring system in Korean fibromyalgia patients: a pilot study. Int J Rheum Dis. 2019; 22: 934–939. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Kang MA, Lee SK. Effects of the e-Motivate4Change program on metabolic syndrome in young adults using health apps and wearable devices: quasi-experimental study. J Med INTERNET Res 2020; 22: e17031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li WY, Chiu FC, Zeng JK, et al. Mobile health app with social Media to support self-management for patients with chronic kidney disease: prospective randomized controlled study. J Med Internet Res 2020; 22: e19452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukkahatai N, Soivong P, Li D, et al. Feasibility of using Mobile technology to improve physical activity among people living with diabetes in Asia. AsianPacific Isl Nurs J 2021; 5: 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moor CC, Gur-Demirel Y, Wijsenbeek MS. Feasibility of a comprehensive home monitoring program for sarcoidosis. J Pers Med. 2019; 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varas AB, Córdoba S, Rodríguez-Andonaegui I, et al. Effectiveness of a community-based exercise training programme to increase physical activity level in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Physiother Res Int J Res Clin Phys Ther. 2018; 23: e1740. [DOI] [PubMed] [Google Scholar]
- 18.Wulfovich S, Fiordelli M, Rivas H, et al. “I Must Try Harder”: Design Implications for Mobile Apps and Wearables Contributing to Self-Efficacy of Patients With Chronic Conditions. Front Psychol [Internet] 2019. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85074489289&doi=10.3389%2ffpsyg.2019.02388&partnerID=40&md5=5b41f82949d25271423368a9f327f60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Z, Yadav S, Jo A. The association between electronic wearable devices and self-efficacy for managing health: a cross sectional study using 2019 HINTS data. Health Technol [Internet] 2021. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85099935585&doi=10.1007%2fs12553-021-00525-x&partnerID=40&md5=d27b9be7d42f5bf0adb87465349123f1. [Google Scholar]
- 20.Moor CC, Gür-Demirel Y, Wijsenbeek MS. Feasibility of a comprehensive home monitoring program for sarcoidosis. J Pers Med 2019; 9: E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y, Lu L, Gao F, et al. Integration of artificial intelligence, blockchain, and wearable technology for chronic disease management: a new paradigm in smart healthcare. Curr Med Sci 2021; 41: 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukkahatai N, Soivong P, Li D, et al. Feasibility of using Mobile technology to improve physical activity among people living with diabetes in Asia. AsianPacific Isl Nurs J 2021; 5: 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med 1991; 85: 25–31. discussion 33. [DOI] [PubMed] [Google Scholar]
- 24.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hays RD, Kallich JD, Mapes DL, et al. Development of the kidney disease quality of life (KDQOLTM) instrument. Qual Life Res 1994; 3: 329–338. [DOI] [PubMed] [Google Scholar]
- 26.Chariyalertsak S, Wansom T, Kawichai S, et al. Reliability and validity of Thai versions of the MOS-HIV and SF-12 quality of life questionnaires in people living with HIV/AIDS. Health Qual Life Outcomes 2011; 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherer M, Maddux JE, Mercandante B, et al. The self-efficacy scale: construction and validation. Psychol Rep 1982; 51: 663–671. [Google Scholar]
- 28.Ritter PL, Lorig K, Laurent DD. Characteristics of the Spanish-and English-language self-efficacy to manage diabetes scales. Diabetes Educ 2016; 42: 167–177. [DOI] [PubMed] [Google Scholar]
- 29.Hektner JM, Schmidt JA, Csikszentmihalyi M. Experience sampling method: Measuring the quality of everyday life. Sage, 2007. [Google Scholar]
- 30.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000; 23: 943–950. [DOI] [PubMed] [Google Scholar]
- 31.Wulfovich S, Fiordelli M, Rivas H, et al. “I must try harder”: design implications for mobile apps and wearables contributing to self-efficacy of patients with chronic conditions. Front Psychol 2019; 10: 2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8: 77–100. [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen SS, Von Känel R, Tully PJ, et al. Psychosocial perspectives in cardiovascular disease. Eur J Prev Cardiol 2017; 24: 108–115. [DOI] [PubMed] [Google Scholar]
- 36.Castelnuovo G, Zoppis I, Santoro E, et al. Managing chronic pathologies with a stepped mHealth-based approach in clinical psychology and medicine. Front Psychol 2015; 6: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piwek L, Ellis DA, Andrews S, et al. The rise of consumer health wearables: promises and barriers. PLoS Med. febbraio 2016; 13: e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin G, Jarrahi MH, Fei Y, et al. Wearable activity trackers, accuracy, adoption, acceptance and health impact: a systematic literature review. J Biomed Inform. maggio 2019; 93: 103153. [DOI] [PubMed] [Google Scholar]
- 39.Barlow J, Wright C, Sheasby J, et al. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 2002; 48: 177–187. [DOI] [PubMed] [Google Scholar]
- 40.Nunes F, Verdezoto N, Fitzpatrick G, et al. Self-care technologies in HCI: trends, tensions, and opportunities. ACM Trans Comput-Hum Interact TOCHI 2015; 22: 1–45. [Google Scholar]
- 41.Schermer M. Telecare and self-management: opportunity to change the paradigm? J Med Ethics 2009; 35: 688–691. [DOI] [PubMed] [Google Scholar]
- 42.Lloret J, Parra L, Taha M, et al. An architecture and protocol for smart continuous eHealth monitoring using 5G. Comput Netw [Internet] 2017. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85019548377&doi=10.1016%2fj.comnet.2017.05.018&partnerID=40&md5=8e2b25cb5e085fcf790c885ddcecc07d. [Google Scholar]
- 43.Bandura A, Freeman WH, Lightsey R. Self-efficacy: The exercise of control. Springer, 1999. [Google Scholar]
- 44.Cameron JE, Voth J, Jaglal SB, et al. “In this together”: social identification predicts health outcomes (via self-efficacy) in a chronic disease self-management program. Soc Sci Med 2018; 208: 172–179. [DOI] [PubMed] [Google Scholar]
- 45.Strecher VJ, McEvoy DeVellis B, Becker MH, et al. The role of self-efficacy in achieving health behavior change. Health Educ Q 1986; 13: 73–92. [DOI] [PubMed] [Google Scholar]
- 46.Morgan HM, Entwistle VA, Cribb A, et al. We need to talk about purpose: a critical interpretive synthesis of health and social care professionals’ approaches to self-management support for people with long-term conditions. Health Expect Int J Public Particip Health Care Health Policy. aprile 2017; 20: 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]