Abstract
Obesity rates have rapidly increased worldwide and obesity-related diseases such as hypertension and cardiovascular diseases have become leading factors for global morbidity and mortality. Currently, there are no effective treatments that can prevent or reverse obesity long-term, and hence the prevention of obesity-related adverse effects such as hypertension is critical. Qingda granule (QDG) is a condensed Traditional Chinese Medicine (TCM) formula that has been used clinically for treating hypertension, however, its effectiveness in obesity-induced hypertension and cardiac dysfunction remains explored. Mouse models of obesity via long-term feeding of high-fat high-fructose diet (HFFD) were established to examine the effect and mechanism of QDG in protecting against obesity-induced hypertension and cardiac dysfunction. C57BL/6 mice were fed with either normal diet or HFFD over a period of 16 weeks and administered with either saline or QDG for assessment of obesity-induced blood pressure and cardiac function. QDG administration demonstrated robust anti-hypertensive effects and significantly attenuated HFFD-induced elevations in blood pressures. Moreover, QDG treatment also demonstrated robust cardioprotective effects during obesity-induced hypertension by markedly improving cardiac function and preventing cardiac hypertrophy. QDG protected against obesity-induced hypertension and cardiac dysfunction was due to its ability to prevent adverse chronic activation of Akt signaling pathway during long-term feeding of HFFD. Long-term usage of QDG treatments exhibited no observable side effects and also completely prevented obesity-induced organ damage, demonstrating the feasibility and safety of prolonged use. Our findings thus elucidated the role of QDG in preventing obesity-induced hypertension and cardiac hypertrophy via inhibiting adverse activation of Akt signaling activation. Therefore, our study provides the theoretical basis for the utilization of QDG as both a safe and effective drug in the therapeutic treatment of metabolic diseases such as obesity-induced hypertension.
Keywords: Qingda granule, Obesity, Hypertension, Cardiovascular disease, Akt signaling pathway
Qingda granule; Obesity; Hypertension; Cardiovascular disease; Akt signaling pathway.
1. Introduction
Obesity is commonly defined as an abnormal or excessive accumulation of fat that can have an adverse effect on health. According to estimates by the World Health Organization (WHO), one in five adults worldwide will be obese by 2025 (Mohammed et al., 2018). As the obesity population expands, the incidence of obesity-related chronic diseases including hypertension, cardiovascular disease, and type 2 diabetes have also seen a dramatic increase (O'Neill and O'Driscoll, 2015). Of note, obese people are 3.5 times more likely to develop hypertension than non-obese people, although there are apparent discrepancies in hemodynamic characteristics of lean versus obese hypertensives such as blood volume, vascular peripheral resistance, and sympathetic drive (Seravalle and Grassi, 2017; Modan and Halkin, 1991). Long-term obesity and hypertension can cause cardiovascular hemodynamic changes, cardiomyocyte hypertrophy, and interstitial fibrosis, which lead to cardiac remodeling and dysfunction, and ultimately heart failure (Alpert et al., 2016; Saliba and Maffett, 2019). Although there are no current treatments that can effectively prevent or reverse obesity, the prevention of obesity-induced adverse effects such as hypertension is critical for the improved health and wellbeing of people with obesity.
The underlying cause of obesity-induced hypertension is strongly affected by various factors including insulin resistance, inflammation, and oxidative stress. It has been reported that induction of obesity in mice via a high-fat, high-fructose diet (HFFD) regime results in insulin resistance, hypertension, increased oxidative stress, cardiac contractile dysfunction, and cardiac remodeling (Deng et al., 2007; Li et al., 2009; Panchal et al., 2011a, b; Geetha et al., 2015; Seravalle and Grassi, 2017). In particular, one study reported that rats fed a high-carbohydrate, high-fat diet for 16 weeks exhibited markedly increased systolic blood pressure and endothelial dysfunction, together with cardiac fibrosis and hypertrophy (Panchal et al., 2011a, b). Akt is a major component of the serine-threonine protein kinase family and plays an important role in numerous signaling pathways related to metabolic diseases and survival (Long et al., 2013). Under normal physiological conditions, the heart is constantly regulated and functions normally. But when this balance is disrupted, such as during prolonged hypertension, it leads to pathological cardiac remodeling and heart failure (Chang et al., 2010), and Akt plays a key role in the maintenance of normal cardiac function. During physiological cardiac remodeling caused by short-term Akt activation, heart systolic function is preserved, whereas sustained Akt activation impairs cardiac function, leading to cardiac hypertrophy and decreased contractility (Shiojima et al., 2005). Numerous studies have reported that mice overexpressing Akt results in cardiac hypertrophy and cardiac dysfunction (Condorelli et al., 2002; Cook et al., 2002; Matsui et al., 2002; Unsöld et al., 2015; Kovacic et al., 2003). Therefore, treatments that can inhibit not only sustained Akt activation during obesity, but also prevent obesity-induced hypertension and worsening of cardiac function is crucial.
Qingda granule (QDG) is a TCM condensed formulation composed of four medicinal components that is derived from a famous TCM formula Qingxuan Jiangya Decoction that has been used clinically for the treatment of high blood pressure and hypertension-associated side effects (Xiao et al., 2016; Cheng et al., 2021; Chen et al., 2021). The condensed granulated form of QDG provides improved and easier application and has been shown to reduce cardiac inflammation and myocardial fibrosis as a result of hypertension (Yu et al., 2020). Although previous studies have demonstrated the effectiveness of QDG in the treatment of hypertension, whether QDG also exhibits any effect during obesity-induced hypertension or obesity-induced cardiac dysfunction remains unexplored. A diet-induced model of obesity and hypertension provides a much closer representation of human physiological disease state compared to other divergent models of hypertension such as spontaneous hypertensive rats (SHR), and as such, our study is the first study that examines the anti-hypertensive effect of QDG utilizing an animal model of obesity. High fructose intake causes non-alcoholic fatty liver disease and hepatic steatosis (Ackerman et al., 2005), playing a key role in the pathogenesis of obesity, and hence HFFD that closely mimics a Western diet, provides greater clinical relevance than a high fat only feeding plan. Hence, in this study, we aimed to determine the effects of long-term QDG use in preventing obesity-induced hypertension and cardiac dysfunction, as well as the underlying mechanisms involved.
2. Materials and methods
2.1. Materials
QDG (Batch: 2012334) was provided by Jiangyin Tianjiang Pharmaceutical Co., Ltd. (Wuxi, China). Phospho-Akt (p-Akt, 66444-1-Ig, USA), Collagen I (14695-1-AP, USA), β-MHC (15862-1-AP, USA) and β-actin (60008-1-Ig, USA) were purchased from Proteintech. α-MHC (ab207926, UK) was obtained from Abcam. Akt (4685S, USA) was obtained from CST. Goat anti-Mouse IgG Secondary Antibody (L3032, USA) and Goat anti-Rabbit IgG Secondary Antibody (L3012, USA) were provided by SAB. Primary antibody dilution buffer (AR1017, USA) and BCA protein assay reagent kit (AR0197, USA) were provided from Boster Biological Technology Co., Ltd. RIPA lysate (P0013B, China) was obtained from Beyotime Biotechnology. Super enhanced chemiluminescence (ECL) was purchased from Super (S6009L, China). HE staining kit was provided by Solarbio (G1120, China).
2.2. Component analysis of QDG powder using high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS)
Fingerprinting analysis was conducted using HPLC-MS/MS to identify the chemical profile of top bioactive compounds in QDG. 0.3 g of QDG powder extract (1 mg/ml) and standards were respectively injected into the HPLC-MS/MS system (LC-30A, Shimadzu, Japan) and separated on a C18 ODS column (1.8μm, 2.1 × 100 mm) with gradient elution. 0.2% 2-Sulfobenzoic Acid Hydrate (A) and acetonitrile (B) was used as mobile phase and the gradient elution procedure was as follows: 0 min, A:B = 97:5; 0.01 min, A:B = 75:30; 37 min, A:B = 95:5; 37.1 min. The flow rate was 0.5 mL/min.
2.3. Preparation of QDG and animal feed
The constituents of QDG consisted of: Gastrodia elata Blume (Orchidaceae), Uncaria rhynchophylla (Miq.) Miq. ex Havil. (Rubiaceae), Scutellaria baicalensis Georgi (Lamiaceae), and Nelumo nucifera Gaertn (Nymphaeaceae), and were mixed at a ratio of 12:10:6:5. QDG (Batch: 2012334) was provided by Jiangyin Tianjiang Pharmaceutical Co., Ltd. (Wuxi, China). The QDG powder was dissolved in 0.9% natural saline solution to a concentration of 0.3 g/ml and stored at −20 °C for subsequent use. For animal experiments, the final concentration of QDG was 0.9 g/kg/day (low-dose group) and 1.8 g/kg/day (high-dose group). Taking the clinical dosage of QDG as a reference, the dosages used for in vivo experiments were obtained by converting the human body surface area to mouse through the "pharmacological experiment method" as recommended by the provided by the United States Food and Drug Administration (USFDA) (Wojcikowski and Gobe, 2014). Obesity-induced hypertension mice model was induced by feeding mouse with high-fat high-fructose diet (HFFD) containing high fat chow (60% calories from fat) supplemented with 20% fructose dissolved in drinking water.
2.4. Animals
The animal experiment protocol in this study was approved by the Animal Care and Use Committee of the Fujian University of Traditional Chinese Medicine (Approval No. FJTCM IACUC 2019040). All animal experiments were conducted in accordance with the institutional guidelines for the ethical care and use of laboratory animals. C57BL/6 male mice (8–10 weeks of age) were provided from SLAC Laboratory Animal Technology Co., Ltd. (Shanghai, China). All animals were kept in SPF environment and maintained at a constant temperature (23 ± 1 °C), under specific pathogen-free conditions, humidity (50–60%) and a 12-hour light/dark cycle, as well as supplemented with food and water on time. The average basal body weight of mice prior to HFFD feeding is approximately 28 g across all groups. Then mice were randomly divided to four groups: Normal Diet, HFFD, HFFD + QDG-L (low-dose, 0.9 g/kg/day) and HFFD + QDG-H (high-dose, 1.8 g/kg/day). HFFD + QDG-L group and HFFD + QDG-H groups were administered via oral gavage at a dose of 0.9 g/kg/day and 1.8 g/kg/day, respectively, once every day for 16 weeks. The control diet group and HFFD group were likewise administered an equal volume of saline solution 6 μl/g/day.
2.5. Blood pressure measurement
Mouse blood pressure was measured using the CODA™ noninvasive blood pressure monitoring system (Kent Scientific, USA) to determine the systolic, diastolic, and mean arterial pressure via tail sleeve plethysmograph method. Mice were kept in a dry and quiet environment prior to and during experiments. Mice were first confined in a warming chamber at a temperature of 35 °C and stabilized for 10 min in order to detect the tail arterial pulsation and reach a stable pulse. Results were obtained from the mean of 15 valid cuff inflation-deflation cycles with distinct sigmoidal systolic/diastolic measurement curves.
2.6. Two-dimensional echocardiography
All mice underwent echocardiography using the Vevo2100 High-Resolution Imaging System (Visual Sonics, Canada). Mice were anesthetized with 2% isoflurane supplemented with oxygen. M-mode images were obtained to determine LV dimensions, including left ventricular internal dimension in diastole (LVID: d) and systole (LVID: s). The evaluation criteria of cardiac function were based on left ventricular ejection fraction (EF%) and fractional shortening (FS%) based on the following formulas: LVEF (%) = (LV Vol: d - LV Vol: s)/LV Vol: d × 100%. LVFS (%) = (LVID: d - LVID: s)/LVID: d × 100%.
2.7. Histology staining using hematoxylin-eosin (HE)
Mice were sacrificed at 16 weeks post-HFFD model. Hearts were perfused using ice cold PBS and 4% paraformaldehyde (PFA), then longitudinally cut and stored in 4% PFA for subsequent morphological experiments. HE staining was performed according to standard protocol. Briefly, hearts were embedded in paraffin and sectioned longitudinally in 6 μm slices. Following staining, slides were visualized using an optical microscope (Leica, Germany) at 400× magnification.
2.8. Quantitative real-time PCR
For quantitative real-time PCR analysis, RNA was extracted using MagZol reagent (Takara, Japan) and reverse transcribed to cDNA using a Prime Script RT reagent Kit according to the manufacturer's instructions (Takara, Japan). Real-time quantitative PCR was performed with SYBR-Green master mix in 96-well optical plates using a Quant Studio 7 Flex Real-Time PCR System (Applied Biosystems, USA). The primer sequences for GDF15 were 5′-CTGGAGACTGTGCAGGCAACTC-3' (forward) and 5′-CATGCAGGCGTGCTTTGATC-3' (reverse). The primer sequences for β-actin were 5′-AGATGACCCAGATCATGTTTGAGA-3' (forward) and 5′-CACAGCCTGGATGGCTACGT-3' (reverse). β-actin was used as the reference gene for determination of relative gene expressions. Relative fold changes were analyzed using the comparative Ct (ΔΔCt) method and normalized to the control group.
2.9. Western blotting assay
Total protein of myocardial tissue was extracted with RIPA lysis buffer containing a cocktail of protease and phosphatase inhibitors. Total protein content of the supernatant was quantified using the BCA protein assay kit. Protein samples were loaded in SDS-PAGE gels and the proteins were transferred onto 0.22 μm PVDF membranes. The membranes were blocked with 5% non-fat milk for 1 h, and then incubated with primary antibodies overnight at 4 °C. Subsequently, membranes were incubated with the respective secondary antibodies (1:5,000) for 1 h at room temperature. Protein expression was visualized via enhanced chemiluminescence. Protein densitometry analysis were analyzed using Image Lab™ Software (version 3.0; Bio-Rad, Hercules, CA, USA).
2.10. Blood biochemical assessment
At the end of the experimental period, mice were sacrificed via intraperitoneal injection of sodium pentobarbital (225 mg/kg) euthanasia solution, and blood was withdrawn by cardiac puncture. Serum was collected by centrifugation, and levels of alanine aminotransferase (ALT), aspartate transaminase (AST), blood urine nitrogen (BUN) and creatinine were assessed by Reagent Kit (Jiancheng Bioengineering Institute, Nanjing, China).
2.11. Statistical analysis
All data were analyzed using SPSS software (SPSS, version 26.0, Chicago, IL, USA). Student's t-test or one-way ANOVA with Bonferroni correction was used to compare differences between two groups, or more than three groups, respectively. Results were expressed as the mean ± standard error. P < 0.05 was considered as statistically significant.
3. Results
3.1. HPLC analysis of QDG extract
The peak retention times of QDG extract were determined by HPLC analysis and quantified using the calibration curves of the corresponding chemical standards. The major components contained in the QDG extract were determined to be gastrodin (main peak at 12.953 min), rhynocholphylline (main peak at 5.623 min) and baicalin (main peak at 9.159 min), as illustrated in (Supplementary Fig. S1).
3.2. QDG attenuates HFFD-induced elevation of blood pressure
We first utilized a high-fat, high fructose (HFFD) feeding regime in order to induce a mouse model of obesity, and subsequently administered either saline or QDG at different doses to determine the effect of QDG in preventing obesity-induced hypertension. There were significant increases in the bodyweight of HFFD-fed mice from week 3 post HFFD-induction compared to those fed a normal diet, and this trend became more obvious at week 6 and onwards during prolonged feeding of HFFD (Figure 1A). The increase in bodyweight corresponded to similar increases in systolic blood pressure (SBP) (Figure 1B), diastolic blood pressure (DBP) (Figure 1C) and mean arterial blood pressure (MAP) (Figure 1D) starting from week 6 in HFFD-fed mice, which also became more pronounced over time. Interestingly, although HFFD-fed mice administered with QDG also became obese with similar bodyweights as those administered with saline (Figure 1A), QDG administration at both dosages significantly attenuated HFFD-induced elevations of blood pressure (Figure 1B-D), which were essentially reversed to baseline levels across all timepoints.
Figure 1.
QDG attenuates HFFD-induced blood pressure elevation. (A) Changes in bodyweight over time in HFFD-fed mice administered with saline, QDG-L or QDG-H. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 for HFFD vs. Normal Diet group, #p < 0.05, ##p < 0.01, ###p < 0.001 for HFFD + QDG-L vs. Normal Diet group, †p < 0.05, ††p < 0.01, †††p < 0.001 for HFFD + QDG-H vs. Normal Diet group, n.s., no significance. Normal Diet, n = 17; HFFD, n = 18; HFFD + QDG-L, n = 12; HFFD + QDG-H, n = 12. Complete list of statistical analyses are detailed in Supplementary Table 1. (B–D) Changes in blood pressures over time in HFFD-fed mice administered with saline, QDG-L or QDG-H, including systolic blood pressure (SBP) (B), diastolic blood pressure (DBP) (C), and mean arterial blood pressure (MAP) (D). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 for Normal Diet group vs. HFFD, #p < 0.05, ##p < 0.01, ###p < 0.001 for HFFD + QDG-L vs. HFFD, †p < 0.05, ††p < 0.01, †††p < 0.001 for HFFD + QDG-H vs. HFFD, n.s., no significance. Data are presented as mean ± sem. Normal Diet, n = 12; HFFD, n = 10; HFFD + QDG-L, n = 13; HFFD + QDG-H, n = 13. Complete list of statistical analyses are detailed in Supplementary Table 1. (E–G) Peak blood pressures levels of mice at 10 weeks post-HFFD induction including SBP (E), DBP (F), and MAP (G) administered with saline, QDG-L or QDG-H. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 12; HFFD, n = 10; HFFD + QDG-L, n = 13; HFFD + QDG-H, n = 13. Complete list of statistical analyses are detailed in Supplementary Table 1.
In particular, after 10 weeks, blood pressures reached a peak in HFFD-fed mice, with SBP of 117.58 ± 4.73 mmHg (Figure 1E), DBP of 88.51 ± 2.72 mmHg (Figure 1F) and MAP of 97.91 ± 3.21 mmHg (Figure 1G), which were approximately 20% higher than mice fed a normal diet. Blood pressures appeared to reach a plateau thereafter until sacrifice at 16 weeks post-HFFD model, however HFFD-fed mice administered with QDG at both low-dose and high-doses effectively prevented the increase in obesity-induced blood pressure upregulation, which remained similar to the level of mice fed a normal diet at all timepoints. Interestingly, a low dose of QDG already provided adequate anti-hypertensive benefits, demonstrating the robust effect of QDG in alleviating obesity-induced hypertension.
3.2. QDG prevents HFFD-induced cardiac dysfunction
Due to the critical involvement of hypertension in causing various cardiovascular diseases, we further examined whether the effects of QDG in lowering blood pressure could also translate to protecting against hypertension-induced cardiac dysfunction in obese mice following prolonged induction of HFFD. We examined the cardiac function of mice after 16 weeks of HFFD induction via echocardiography, which showed significant decreases in left ventricular ejection fraction (EF%) and fractional shortening (FS%) parameters compared with the control group fed a normal diet (Figures 2A and 2B), demonstrating that prolonged HFFD-induced obesity and hypertension does indeed significantly worsen cardiac function. Notably, HFFD-mice administered with QDG at both dosages significantly improved the cardiac function compared to saline-administered mice, to almost the level of mice fed a normal diet. Moreover, M-mode echocardiography showed that QDG-treated mice had significant improvements in LV wall motion compared with saline-administered mice following HFFD for 16 weeks (Figure 2C). These and other cardiac function parameters were summarized, showing a marked overall improvement of the heart in response to QDG treatment following prolonged HFFD induction (Figure 2D). Taken together, these results demonstrate that QDG has a robust ability in preventing long-term HFFD-induced cardiac dysfunction.
Figure 2.
QDG prevents HFFD-induced cardiac dysfunction. (A–D) Echocardiography assessment of cardiac function in mice administered with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks, with ejection fraction (EF%) (A), fractional shortening (FS%) (B) parameters, representative M-mode echocardiographic images of LV end-systolic and end-diastolic dimensions (C), and summary table of cardiac function measurements (D). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 15; HFFD, n = 13; HFFD + QDG-L, n = 13; HFFD + QDG-H, n = 13. Complete list of statistical analyses are detailed in Supplementary Table 1. LVID; d, left ventricular internal dimension; diastolic; LVID; s, left ventricular internal dimension.
3.3. QDG alleviates HFFD-induced cardiac hypertrophy and myocardial tissue damage
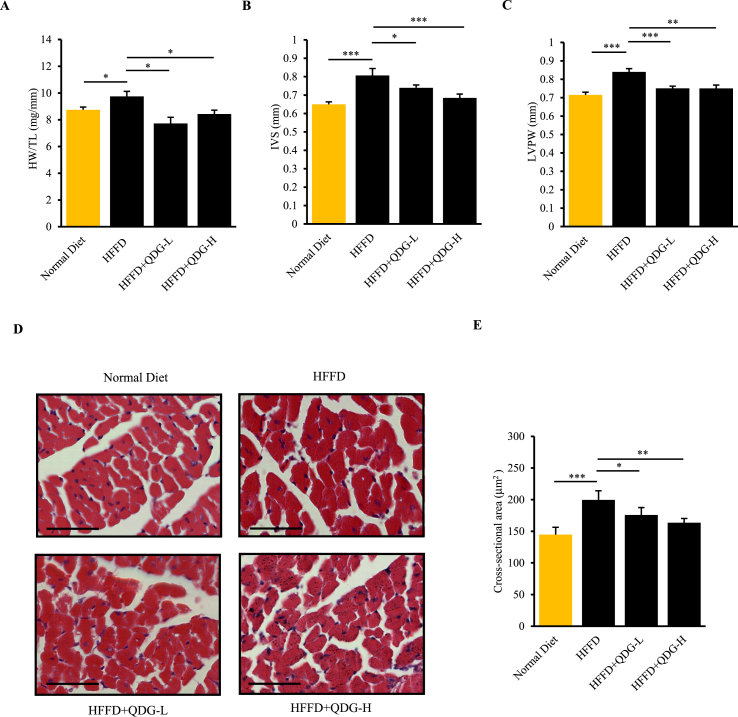
We further examined whether QDG had any effect in preventing hypertension-induced myocardial damage and cardiac hypertrophy in HFFD-fed mice. The ratio of heart weight (HW) to tibial length (TL) can provide a more accurate assessment of cardiac hypertrophy when there are significant weight differences between groups, and hence we evaluated the heart size using the HW:TL ratio (Yin et al., 1982). Indeed, mice fed with HFFD for 16 weeks had significantly increased HW:TL ratio compared to those fed a normal diet, indicating that prolonged obesity-induced hypertension results in the occurrence of cardiac hypertrophy (Figure 3A). Further, echocardiography results showed that left ventricular wall thickness including both interventricular septum thickness (IVS) and posterior wall thickness (LVPW) were significantly increased in HFFD group (Figures 3B and 3C). Of note, HFFD-mice administered with QDG at both dosages had significantly reduced HW:TL ratio, as well LV wall thickness, demonstrating that QDG can effectively prevent obesity-induced cardiac hypertrophy (Figures 3A-3C).
Figure 3.
QDG prevents HFFD-induced cardiac hypertrophy. (A) Heart weight/tibia length ratio (HW:TL) in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 11; HFFD, n = 6; HFFD + QDG-L, n = 4; HFFD + QDG-H, n = 4. Complete list of statistical analyses are detailed in Supplementary Table 1. (B–C) Echocardiography assessment of heart LV wall thickness in mice administered with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks, showing interventricular septum thickness (IVS) (B) and LV posterior wall thickness (LVPW) (C) parameters. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 15; HFFD, n = 13; HFFD + QDG-L, n = 13; HFFD + QDG-H, n = 13. Complete list of statistical analyses are detailed in Supplementary Table 1. (D–E) Representative H&E images (D) and quantification of cardiomyocyte cross-sectional area (E) of hearts in mice administered with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 5; HFFD, n = 6; HFFD + QDG-L, n = 5; HFFD + QDG-H, n = 6. Complete list of statistical analyses are detailed in Supplementary Table 1.
In addition, we performed Hematoxylin & Eosin (H&E) staining to examine the effect of prolonged HFFD feeding-induced hypertension on myocardial tissue damage and cardiac hypertrophy. The staining results showed that HFFD-fed mice had irregular cardiac morphology as evident by the irregular arrangement of myocardial fibers and markedly increased cardiomyocyte cross-sectional area indicative of cardiac hypertrophy compared to mice fed a normal diet (Figures 3D and 3E). In contrast, hearts of HFFD-fed mice administered with QDG at both dosages exhibited regular morphology with neat arrangement of myocardial fibers, as well as normal cardiomyocyte cross-sectional area similar to mice fed a normal diet (Figures 3D and 3E). These results further indicate that QDG has a strong cardioprotective effect in preventing myocardial tissue damage and cardiac hypertrophy during obesity-induced hypertension.
3.4. QDG prevents HFFD-induced cardiac damage by inhibiting adverse Akt signaling activation
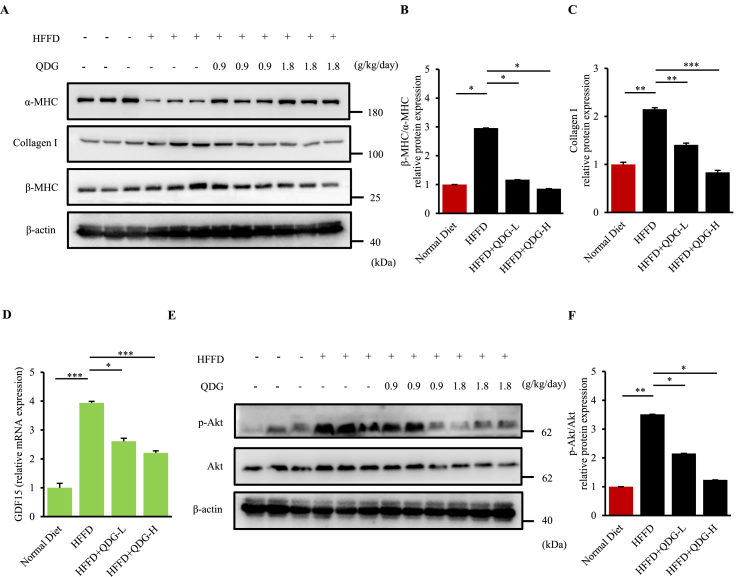
The incidence of pathological cardiac hypertrophy and heart failure results in an alteration in the ratio of the contractile protein isoforms within the heart, with decreased levels of alpha-myosin heavy chain (α-MHC) coinciding with increased levels of beta-myosin heavy chain (β-MHC). Interestingly, Western blot analysis showed that mice fed with HFFD had significantly decreased levels of α-MHC in the heart, corresponding with increased levels of β-MHC as well as β-MHC/α-MHC ratio compared to those fed a normal diet, indicative of impaired cardiac function (Figure 4A). Notably, this was almost completely prevented in HFFD-fed mice administered with QDG at both dosages, suggesting that QDG preserves the cardiac function of mice following long-term HFFD-feeding (Figures 4A and 4B). Furthermore, expressions of fibrotic marker Collagen I (Col-I) as well as stress responsive cytokine growth differentiation factor 15 (GDF15) were also significantly upregulated in the heart following long-term feeding with HFFD (Figures 4C and 4D). Administration of QDG significantly reversed these up-regulations of Col-I and GDF15, suggesting that QDG prevents cardiac fibrosis and cardiac dysfunction following long-term HFFD feeding (Figures 4C and 4D).
Figure 4.
QDG inhibits adverse activation of Akt signaling pathway during long-term HFFD induction. (A–C) Representatives immunoblots (A) and quantification (B–C) showing expressions of β-MHC/α-MHC and Collagen I levels in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. n = 3 for each group. Raw uncropped images are shown in Supplementary Material 1.(D) Real-time PCR analysis showing relative mRNA expression of GDF15 in heart tissue after 16 weeks of HFFD diet induction. β-actin was used as the loading control. Data are presented as mean ± sem. ∗p < 0.05, ∗∗∗p < 0.001. Normal Diet, n = 4; HFFD, n = 6; HFFD + QDG-L, n = 6; HFFD + QDG-H, n = 7. Complete list of statistical analyses are detailed in Supplementary Table 1. (E–F) Representatives immunoblots (E) and quantification (F) showing expressions of p-Akt/Ak levels in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. β-actin was used as the loading control. Data are presented as mean ± sem. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. n = 3 for each group. Raw uncropped images are shown in Supplementary Material 1.
We further investigated the underlying mechanism of QDG in its ability to protect against obesity-induced hypertension. Chronic long-term activation of the Akt signaling pathway such as during metabolic diseases, results in the impairment of cardiac function, cardiac hypertrophy and dysfunction. Thus, we further examined the expressions of total Akt as well as phosphorylated Akt (p-Akt) in the hearts of mice fed on HFFD or normal diet for 16 weeks. Western blot analysis showed that mice fed with HFFD had significantly increased levels of p-Akt/Akt expression compared to those on a normal diet, indicative of the chronic adverse Akt signaling activation as a result of obesity (Figure 4E). Of note, HFFD-mice administered with QDG had significantly decreased levels of p-Akt/Akt expressions and in a dose-dependent manner, becoming most apparent in mice treated with high-dose QDG that was reduced to basically the levels of control mice fed a normal diet (Figure 4E). Densitometry analysis of the p-Akt/Akt ratio showed a similar trend, which further indicated the adverse activation of Akt signaling pathway during prolonged HFFD feeding, which was significantly attenuated by QDG at both dosages (Figure 4F). Taken together, these findings collectively demonstrate that long-term HFFD-induced obesity results in hypertension and cardiac dysfunction, while QDG can strongly prevent obesity-induced hypertension and worsening of cardiac function via inhibiting chronic adverse activation of Akt signaling pathway.
3.5. Long-term QDG usage has improved lipid profile and no observable side effects
Due to the fact that hypertension is a chronic disease and the development of high blood pressure as a result of obesity can be a lengthy process, any drug treatment regimens must be planned for prolonged usage over a long period of time. Therefore, special attention is required in the observation of any potential side effects during the long-term use. TCM medications are often touted for its benefits of having low side effects, and hence we examined whether long-term usage of QDG would be both safe and effective over a long 4 months (16 weeks) treatment regime. Mice fed with HFFD in combination with either saline or QDG administrations all exhibited regular increases in bodyweight, reaching peaks of approximately 45 g by week 16, which was roughly 50% higher bodyweight than mice fed a normal diet (Figure 1A). Representative images at week 16 showed no distinct differences in hair coat and coloration between the groups, as well as similar body sizes between mice fed with HFFD (Figure 5A). Further, there were no significant differences in mouse body composition, including similar muscle weight from mouse quadriceps muscles between all groups (Figure 5B). Grip strength at week 16 of HFFD-feeding was similar between groups, demonstrating that there were no obesity-induced muscular deficiencies (Figure 5C).
Figure 5.
Long-term use of QDG has no observable side effects. (A) Representative images in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. (B–C) Muscle weight (B) and grip strength (C) in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 15; HFFD, n = 14; HFFD + QDG-L, n = 13; HFFD + QDG-H, n = 13. Complete list of statistical analyses are detailed in Supplementary Table 1. (D–H) Fat weight (D), and serum triglycerides (TG) (E), total cholesterol (TC) (F), low density lipoprotein cholesterol (LDL-C) (G), high-density lipoprotein cholesterol (HDL-C) (H) in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. n.s., no significance, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 11; HFFD, n = 10; HFFD + QDG-L, n = 10; HFFD + QDG-H, n = 13. Complete list of statistical analyses are detailed in Supplementary Table 1. (I–K) Mean daily food intake (I), water intake (J) and fasted blood glucose levels (K) in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 for HFFD vs. Normal Diet group, #p < 0.05, ##p < 0.01, ###p < 0.001 for HFFD + QDG-L vs. Normal Diet group, †p < 0.05, ††p < 0.01, †††p < 0.001 for HFFD + QDG-H vs. Normal Diet group, n.s., no significance. Data are presented as mean ± sem. Normal Diet, n = 11; HFFD, n = 12; HFFD + QDG-L, n = 6; HFFD + QDG-H, n = 6. Complete list of statistical analyses are detailed in Supplementary Table 1.
Moreover, although mice fed with HFFD had significantly greater fat weight from mouse inguinal fat pads compared to mice fed a normal diet, administrations with QDG had no effect on fat mass (Figure 5D). Hence, we further examined the levels of blood cholesterol and triglycerides in these mice, in order to determine whether there were any beneficial effects of QDG on blood cholesterol levels. As expected, HFFD-fed mice had significantly elevated levels of fasted serum total cholesterol (TC), low-density lipoprotein (LDL), and triglycerides (TG), but significantly decreased levels of high-density lipoprotein (HDL) compared to mice fed a normal diet (Figure 5E-H). Surprisingly, HFFD-fed mice administered with QDG had markedly improved trends of all blood cholesterol and triglyceride profiles, including significant reduction in TG but increase in HDL (Figure 5E-H), while the overall trends of LDL and TC are also evidently decreased. These results demonstrate that although QDG administration had no effect on reducing fat mass and overall body weight, it actually improved overall serum lipid profiles and blood cholesterol, suggesting that QDG administered mice were metabolically healthy obese that do not exhibit obesity-induced adverse effects.
Daily food and water intake of mice were also followed up during the 16 weeks feeding period. Mice in each group were housed in individual cages, and hence the mean daily food and water intake in each group were determined based on the total daily food and water intake divided by the number of mice. Although HFFD-fed mice actually had lower mean daily food and water intake than normal diet counterparts, the total energy dietary energy intake of HFFD was adequate for inducing obesity, however, there were almost no differences between HFFD-fed mice administered with either saline or QDG (Figures 5I and 5J). Furthermore, levels of fasted blood glucose were significantly increased in HFFD-fed mice starting from week 6 corresponding to the increases in bodyweight and blood pressures and peaking at week 10 (Figure 5K), but thereafter reverted back to similar levels as HFFD-fed mice administered with QDG, suggesting that the anti-hypertensive and cardioprotective effects of QDG likely had little effect on the control of blood glucose during obesity.
3.6. QDG prevents obesity-induced organ damage
We further performed biochemical analyses of liver and kidney function to examine the adverse effects of long-term HFFD-feeding on organ function. Serum enzymes alanine aminotransferase (ALT) and aspartate transaminase (AST) are often elevated when the liver is damaged. As expected, mice fed long-term with HFFD exhibited significantly elevated levels of serum ALT and AST indicative of liver damage occurrence as a result of obesity (Figures 6A and 6B). Furthermore, serum levels of blood urine nitrogen (BUN) and creatinine can indicate possible kidney damage. Mice fed long-term with HFFD also had significantly elevated levels of both BUN and creatinine, indicating that obesity also resulted in renal insufficiency or kidney damage (Figures 6C and 6D). Notably, mice administered with QDG not only exhibited no adverse effects to HFFD-feeding, but actually significantly reduced these elevations in serum biochemical markers of liver and kidney damage, in a dose-dependent manner (Fig. 6A-D), demonstrating the robust protective ability of QDG treatment in preventing organ damage as a result of HFFD-induced obesity.
Figure 6.
QDG prevents obesity-induced organ damage. (A–D) Serum biochemical liver function markers Alanine aminotransferase (ALT) (A), aspartate transaminase (AST) (B), and kidney function markers blood urine nitrogen (BUN) (C) and creatinine (D) in mice administrated with saline, QDG-L or QDG-H following induction of HFFD for 16 weeks. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± sem. Normal Diet, n = 10; HFFD, n = 19; HFFD + QDG-L, n = 10; HFFD + QDG-H, n = 12. Complete list of statistical analyses are detailed in Supplementary Table 1.
Taken together, these results suggest that long-term usage of QDG can not only effectively prevent HFFD-induced elevations in blood pressure and cardiac dysfunction, but also exhibit no observable side effects while also preventing obesity-induced organ damage, further underlining the feasibility of using QDG as a safe and effective drug for the treatment of obesity-induced hypertension and cardiac dysfunction.
4. Discussion
Hypertension is a chronic disease and the leading risk factor that contributes to cardiovascular morbidity and mortality worldwide (Mortality and Causes of Death, 2015; Collaborators, 2017). Hypertension as a result of obesity may be a slow and gradual process in humans, and the exact mechanism involved in this process remains unelucidated. The relationship between increased body mass index (BMI) and arterial pressure has been demonstrated across various different populations and age groups.
(Liao et al., 2020; Liu et al., 2019; Brown et al., 2000; Weiss et al., 2004). However, it is interestingly to note that that not all obese people suffer from hypertension, which suggests that there may be a complex and multifactorial relationship between obesity and elevated arterial blood pressure. Nevertheless, obesity has been identified as one of the key risk factors for the progression from normotension to prehypertension and more severe hypertension (Vasan et al., 2001; De Marco et al., 2009; Landsberg et al., 2009). Therefore, the early prevention of hypertension is particularly important for all people, in particular those overweight or obese.
Vascular remodeling is fundamentally involved in the development and progression of hypertension. The pathophysiology of vascular remodeling involves alterations in the structure and arrangement of blood vessels that arise due to vasoconstricting peptides such as Angiotensin II (AngII) that promote proliferation of vascular smooth muscle cells (VSMCs). Previous study showed that QDG ameliorated AngII-induced elevations of blood pressure in mice via inhibiting VSMC proliferation by inhibiting the activation of MAPK and Akt signaling pathways (Yu et al., 2020). Due to the critical involvement of the Akt signaling pathway during metabolism, heart development and cardiac homeostasis, enhancement of Akt signal may either be viewed as a blessing or a curse. Although short-term adaptive Akt activation may be beneficial, long-term sustained Akt activation can cause myocardial hypertrophy and heart failure, and is more pronounced in obese people (Chang et al., 2010; Vega et al., 2017). Our findings showed that both Akt and p-Akt levels were significantly upregulated following 16 weeks of continuous feeding with HFFD in mice that also presented significantly decreased cardiac function and other adverse effects, demonstrating that prolonged Akt activation due to obesity-induced hypertension results in cardiac dysfunction. However, it is interesting to note that long-term use of QDG in these obese mice can not only significantly inhibit the adverse activation of Akt and p-Akt, but also prevent the occurrence of cardiac dysfunction. Our findings thus demonstrate that QDG strongly prevents obesity induced hypertension and cardiac dysfunction via inhibiting adverse activation of Akt signaling pathway.
Due to the complex nature of hypertension during obesity, the discovery of drugs that can not only prevent obesity-induced hypertension but also minimize the effects of obesity-induced worsening of cardiac function is urgently needed. TCM formulations have been widely used in China and throughout Asia for the treatment of various diseases, however, few studies have investigated the effects of long-term usage of TCM. Our study administered QDG over a long 16-weeks treatment regime in obese mice showed no observable side effects, indicating that QDG is both a safe and effective drug for the long-term treatment of obesity-induced hypertension and its associated adverse effects.
5. Conclusion
Our findings elucidated the role of QDG in preventing obesity-induced hypertension and cardiac hypertrophy via inhibiting adverse activation of Akt signaling activation and provide a theoretical basis for the long-term utilization of QDG as both a safe and effective drug in the treatment of metabolic diseases such as obesity-induced hypertension.
Declarations
Author contribution statement
Qian Gao: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
En Ma: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Jinxiao Chen; Qiqin Zhao; Jia He; Jun Peng; Weidong Zhu: Contributed reagents, materials, analysis tools or data.
Dan-ni Ren: Conceived and designed the experiments; Analyzed and interpreted the data.
Da Wo: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This study was supported by National Natural Science Foundation of China [82074190, 81903989], Scientific Research Foundation for the High-level Talents, Fujian University of Traditional Chinese Medicine [X2019001-talent, X2021001-talent, X2021002-talent, X2021003-talent], Scientific Research Foundation for the top youth talents of Fujian University of Traditional Chinese Medicine [XQB202201], Natural Science Foundation of Fujian Province for Distinguished Young Scholars [2022J06027].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Dan-ni Ren, Email: danny1217@163.com.
Da Wo, Email: dwo_work@126.com.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Ackerman Z., Oron-Herman M., Grozovski M., Rosenthal T., Pappo O., Link G., Sela B.A. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005 May;45(5):1012–1018. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- Alpert M.A., Omran J., Bostick B.P. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr. Obes. Rep. 2016;5(4):424–434. doi: 10.1007/s13679-016-0235-6. [DOI] [PubMed] [Google Scholar]
- Brown C.D., Higgins M., Donato K.A., Rohde F.C., Garrison R., Obarzanek E., Ernst N.D., Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes. Res. 2000;8(9):605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- Chang Z., Xiao Q., Feng Q., Yang Z. PKB/Akt signaling in heart development and disease. Front. Biosci. 2010;2:1485–1491. doi: 10.2741/e207. [DOI] [PubMed] [Google Scholar]
- Chen X., Long L., Cheng Y., Chu J., Shen Z., Liu L., Li J., Xie Q., Liu H., Wu M., Chen Y., Peng J., Shen A. Qingda granule attenuates cardiac fibrosis via suppression of the TGF-β1/Smad2/3 signaling pathway in vitro and in vivo. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111318. May. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Shen A., Wu X., Shen Z., Chen X., Li J., Liu L., Lin X., Wu M., Chen Y., Chu J., Peng J. Qingda granule attenuates angiotensin II-induced cardiac hypertrophy and apoptosis and modulates the PI3K/AKT pathway. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111022. Jan. [DOI] [PubMed] [Google Scholar]
- Collaborators GBDCoD Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli G., Drusco A., Stassi G., Bellacosa A., Roncarati R., Iaccarino G., Russo M.A., Gu Y., Dalton N., Chung C., et al. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99(19):12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S.A., Matsui T., Li L., Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J. Biol. Chem. 2002;277(25):22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- De Marco M., de Simone G., Roman M.J., Chinali M., Lee E.T., Russell M., Howard B.V., Devereux R.B. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension: the Strong Heart Study. Hypertension. 2009;54(5):974–980. doi: 10.1161/HYPERTENSIONAHA.109.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J.Y., Huang J.P., Lu L.S., Hung L.M. Impairment of cardiac insulin signaling and myocardial contractile performance in high-cholesterol/fructose-fed rats. Am. J. Physiol. Heart Circ. Physiol. 2007;293(2):H978–987. doi: 10.1152/ajpheart.01002.2006. [DOI] [PubMed] [Google Scholar]
- Geetha R., Radika M.K., Priyadarshini E., Bhavani K., Anuradha C.V. Troxerutin reverses fibrotic changes in the myocardium of high-fat high-fructose diet-fed mice. Mol. Cell. Biochem. 2015;407(1-2):263–279. doi: 10.1007/s11010-015-2474-3. [DOI] [PubMed] [Google Scholar]
- Kovacic S., Soltys C.L., Barr A.J., Shiojima I., Walsh K., Dyck J.R. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J. Biol. Chem. 2003;278(41):39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- Landsberg L., Aronne L.J., Beilin L.J., Burke V., Igel L.I., Lloyd-Jones D., Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment--a position paper of the the Obesity Society and the American Society of Hypertension. Obesity. 2013 Jan;21(1):8–24. doi: 10.1002/oby.20181. [DOI] [PubMed] [Google Scholar]
- Liao Y.Y., Chu C., Wang Y., Zheng W.L., Ma Q., Hu J.W., Yan Y., Wang K.K., Yuan Y., Chen C., Mu J. Sex differences in impact of long-term burden and trends of body mass index and blood pressure from childhood to adulthood on arterial stiffness in adults: a 30-year cohort study. Atherosclerosis. 2020 Nov;313:118–125. doi: 10.1016/j.atherosclerosis.2020.10.003. [DOI] [PubMed] [Google Scholar]
- Li C.J., Zhang Q.M., Li M.Z., Zhang J.Y., Yu P., Yu D.M. Attenuation of myocardial apoptosis by alpha-lipoic acid through suppression of mitochondrial oxidative stress to reduce diabetic cardiomyopathy. Chin. Med. J. 2009;122(21):2580–2586. [PubMed] [Google Scholar]
- Liu Y., Yan Y., Yang X., Li S., Bazzano L., He J., Chen W. Long-term burden of higher body mass index and adult arterial stiffness are linked predominantly through elevated blood pressure. Hypertension. 2019 Jan;73(1):229–234. doi: 10.1161/HYPERTENSIONAHA.118.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H.D., Lin Y.E., Liu M.J., Liang L.Y., Zeng Z.H. Spironolactone prevents dietary-induced metabolic syndrome by inhibiting PI3-K/Akt and p38MAPK signaling pathways. J. Endocrinol. Invest. 2013;36(11):923–930. doi: 10.3275/8946. [DOI] [PubMed] [Google Scholar]
- Matsui T., Li L., Wu J.C., Cook S.A., Nagoshi T., Picard M.H., Liao R., Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J. Biol. Chem. 2002;277(25):22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- Modan M., Halkin H. Hyperinsulinemia or increased sympathetic drive as links for obesity and hypertension. Diabetes Care. 1991 Jun;14(6):470–487. doi: 10.2337/diacare.14.6.470. [DOI] [PubMed] [Google Scholar]
- Mohammed M.S., Sendra S., Lloret J., Bosch I. Systems and WBANs for controlling obesity. J. Healthc. Eng. 2018;2018 doi: 10.1155/2018/1564748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortality G.B.D., Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S., O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015 Jan;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- Panchal S.K., Poudyal H., Arumugam T.V., Brown L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 2011;141(6):1062–1069. doi: 10.3945/jn.111.137877. [DOI] [PubMed] [Google Scholar]
- Panchal S.K., Poudyal H., Iyer A., Nazer R., Alam A., Diwan V., Kauter K., Sernia C., Campbell F., Ward L., et al. High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011;57(1):51–64. doi: 10.1097/FJC.0b013e3181feb90a. [DOI] [PubMed] [Google Scholar]
- Saliba L.J., Maffett S. Hypertensive heart disease and obesity: a Review. Heart Fail. Clin. 2019;15(4):509–517. doi: 10.1016/j.hfc.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Seravalle G., Grassi G. Obesity and hypertension. Pharmacol. Res. 2017;122:1–7. doi: 10.1016/j.phrs.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Shiojima I., Sato K., Izumiya Y., Schiekofer S., Ito M., Liao R., Colucci W.S., Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 2005;115(8):2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsöld B., Bremen E., Didié M., Hasenfuss G., Schäfer K. Differential PI3K signal transduction in obesity-associated cardiac hypertrophy and response to ischemia. Obesity. 2015 Jan;23(1):90–99. doi: 10.1002/oby.20888. [DOI] [PubMed] [Google Scholar]
- Vasan R.S., Larson M.G., Leip E.P., Kannel W.B., Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358(9294):1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- Vega R.B., Konhilas J.P., Kelly D.P., Leinwand L.A. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metabol. 2017;25(5):1012–1026. doi: 10.1016/j.cmet.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R., Dziura J., Burgert T.S., Tamborlane W.V., Taksali S.E., Yeckel C.W., Allen K., Lopes M., Savoye M., Morrison J., et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- Wojcikowski K., Gobe G. Animal studies on medicinal herbs: predictability, dose conversion and potential value. Phytother Res. 2014;28(1):22–27. doi: 10.1002/ptr.4966. [DOI] [PubMed] [Google Scholar]
- Xiao F., He F., Chen H., Lin S., Shen A., Chen Y., Chu J., Peng J. Qingxuan Jiangya decoction reverses vascular remodeling by inducing vascular smooth muscle cell apoptosis in spontaneously hypertensive rats. Molecules. 2016 Jul 22;21(7):956. doi: 10.3390/molecules21070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F.C., Spurgeon H.A., Rakusan K., Weisfeldt M.L., Lakatta E.G. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am. J. Physiol. 1982;243(6):H941–947. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- Yu N., Shen A., Chu J., Huang Y., Zhang L., Lin S., Cai Q., Sankararaman S., Sferra T.J., Chen Y., et al. Qingda granule inhibits angiotensin induced VSMCs proliferation through MAPK and PI3K/AKT pathways. J. Ethnopharmacol. 2020;258 doi: 10.1016/j.jep.2020.112767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.