Take Home Message
The intracorporeal modified-Y orthotopic neobladder requires a short tract of ileum without ureteral transposition. It has adequate capacity, acceptable postvoid residual of urine, and satisfying compliance. Thus, it seems to provide good continence rates, enhancing quality of life.
Keywords: Bladder cancer, Cystectomy, Urinary diversion, Urodynamics, Robotics, Laparoscopy
Abstract
Background
The intracorporeal orthotopic modified-Y “Bordeaux“ neobladder (iYNB) was first described in 2016. No urodynamic evaluation of this neobladder has yet been performed.
Objective
To present the urodynamic features of the iYNB and incontinence-specific health-related quality of life (HRQoL) outcomes.
Design, setting, and participants
We prospectively assessed 26 patients operated between September 2018 and November 2020.
Surgical procedure
Robotic radical cystectomy for malignant disease of the bladder and iYNB, performed by a single surgeon, were used.
Measurements
Three months after surgery and in November 2021, consenting patients underwent clinical evaluation and multichannel urodynamic study (UDS). The incontinence quality of life (I-QoL) questionnaire was used to evaluate HRQoL. Continence was classified into day- and nighttime, and clinically defined as the use of zero pads. A descriptive statistical analysis was performed.
Results and limitations
The mean age at surgery was 65.4 yr. The mean follow-up period was 27 mo (12–38). The mean time for the neobladder reconstruction was 192 min (110–340). The mean maximum capacity was 431 cm3 (range 200–553). The mean postvoid residual was 101.6 ml (0–310), and the rate of clean intermittent catheterization was 17.6%. With the exception of a significant reduction in the volume of the first sensation of bladder fullness, no other statistically significant changes in the UDS parameters of both the storage and the voiding phase were observed over time. Day- and nighttime continence rates were 58.8% and 23.5%, respectively. The mean postoperative I-QoL score was 103.3 (89–110). Limitations include the small number of patients and short follow-up.
Conclusions
The UDS evaluation of iYNB demonstrates that both the volumetric and the pressure characteristics are acceptable and may enhance quality of life. Prospective studies with larger numbers of patients and longer follow-up are needed to further evaluate the iYNB.
Patient summary
The “Bordeaux“ neobladder provides acceptable urodynamic outcomes. It is associated with high levels of health-related quality of life and good rates of continence in patients.
1. Introduction
Since the early 1900s, surgeons have sought the best method to replace the original bladder when it must be removed because of either benign or malignant disease. Among the various options, the orthotopic neobladder (ONB) was the urinary diversion that most closely resembled the original bladder in terms of both location and function. In fact, the ONB allows for volitional voiding, avoids the need for an appliance, and requires only self-catheterization in a minority of patients [1].
Initially hindered by learning-curve issues and technical difficulty, with the wide diffusion of the robotic surgery and the acquired experience of the surgical teams, the robotic intracorporeal ONB (riONB) has been proved to be well tolerated and reproducible [2]. It allows for complete replication of the “open” surgical technique, when performed by experienced surgeons [2].
Although there are data on the technical efficacy and complications related to the riONB, the assessment of functional outcomes remains a complex task, and the method has not been standardized [3].
In 2016, a modified-Y neobladder was described [4], which resulted in an almost spherical urinary reservoir, with good average maximal capacity, no increased postvoid residual (PVR), and no need for clean intermittent catheterization (CIC). Although functional data were promising, the related evaluations were obtained through voiding charts with no urodynamic evaluation (urodynamic study [UDS]) of the neobladders.
The aim of the current study is to provide the first UDS data of this modified-Y (“Bordeaux”) neobladder.
2. Patients and methods
2.1. Patients
Between September 2018 and November 2020, 26 patients underwent robotic radical cystectomy for malignant disease of the bladder with an riONB (modified-Y) by a single surgeon (F.A.). An extended lymphadenectomy was performed.
2.2. Multichannel UDS and follow-up
Three months after surgery, consenting patients underwent UDS. Noninvasive uroflowmetry was performed at the start of the procedure. A dual-lumen 7F transurethral catheter was placed into the ONB and PVR was assessed. A 7F rectal catheter was used to transduce the abdominal pressure. All UDS measurements were performed with the patient in a sitting position and according to the standards of the International Continence Society [5]. The ONB was filled at a rate of 50 ml/s with normal saline at room temperature until the maximum cystometric capacity (MCC) of the ONB, defined as abdominal discomfort/“feeling” of bladder fullness. The presence of an increase in intravesical pressure due to residual peristaltic activity was also evaluated as well as leakage of urine during this pressure rise. The change in volume per increase in pressure during the storage phase was used to calculate compliance (ml/cmH2O). At MCC, provocative testing (Valsalva maneuver and coughing) was used to urodynamically assess stress urinary incontinence and the abdominal leak-point pressure [6]. PVR was again determined via catheterization. The parameters noted were maximum enterocystometric capacity of the neobladder, maximal neobladder pressure (maximum pressure during storage, also due to residual peristatic activity), compliance (evaluated at volume 0 and at maximum enterocystometric capacity of the neobladder, in absence of evident peristaltic activity), average flow rate, maximal flow rate (Qmax), and PVR.
All consenting patients were resubmitted to UDS evaluation in November 2021. At the same time point, the continence status was assessed and a frequency-volume chart, kidney ultrasound, incontinence quality of life (I-QoL) questionnaire evaluation [7], and a blood gas analysis were performed. Continence was classified into day- and nighttime, and clinically defined as the use of zero pads. Kidney ultrasound was performed in order to assess for hydronephrosis. The incidence of febrile urinary tract infections, defined as a positive urine culture (≥104 colony-forming units per milliliter of urine) and elevated body temperature (≥38°C), was also evaluated.
2.3. Ethics
The study was conducted in accordance with the Good Clinical Practice rules and the ethical principles contained in the Declaration of Helsinki, as revised in 2013 [8]. All patients provided written informed consent with guarantees of confidentiality. Intra-, peri-, and postoperative pathological and functional data were collected prospectively and inserted in a customized, IRB-approved database (Cod. Prot. CIST_ROB – ID 20289). Data were analyzed anonymously after removal of patient identifiers.
2.4. Statistics
Comparisons in chronological changes in UDS parameters were performed with Mann-Whitney’s U test. All p values of <0.05 were considered statistically significant.
2.5. Modified-Y intracorporeal ONB configuration
As previously described [4], for the modified-Y neobladder, a 40 cm ileal segment is isolated approximately 25 cm from the ileocecal valve (Fig. 1). Ileoileal continuity is re-established through an anisoperistaltic anastomosis of the bowel ends, performed with a single 60-mm stapler line. The most dependent part of the selected ileal segment is brought down to the urethra for a tension-free anastomosis. A posterior reconstruction with a modified Rocco’s stitch is performed. The incised ends of the two limbs are connected, and following their detubularization, the posterior plate of the ONB is created. Subsequently, following a further folding, the anterior reconstruction of the neobladder is completed. All sutures are performed with a V-loc 3-0 stitch.
Fig. 1.
Basic steps for the configuration of the modified-Y intracorporeal ONB. A step-by-step configuration of the modified-Y neobladder. The selected ileal segment is brought down to the pelvis to ensure an adequate mesenteric length to enable tension-free urethroileal anastomosis. Following the completion of this anastomosis on a temporary 18-Fr Foley catheter, the middle antimesenteric part of the ileal segment is opened with scissors. Detubularization is completed and posterior reconstruction is performed. Anterior craniocaudal folding of the neobladder and anterior reconstruction. Ureters are spatulated for approximately 1.5 cm and directly anastomosed to open ends of ONB limbs without an antireflux technique. Before completion of ureter-neobladder anastomosis, two single-J stents are transurethrally or transabdominally inserted on guidewires and advanced to the renal pelvis. The definitive catheter is inserted and the ONB is checked for leaks. ONB = orthotopic neobladder.
For the first 22 cases, a 20-Fr Mercier catheter with two hydrophilic guidewires was placed to assist with single-J stent placement later in the procedure (for transurethrally placed ureteral stents). However, following the incidental removal of the single-J stent in two cases, together with the fact that the early postoperative mobilization of the patients was hindered for the transurethral stents, in the latter four cases, single-J stents were placed percutaneously (transabdominally).
The ureters are spatulated, not crossed-over, and directly anastomosed to the open ends of the neobladder in a refluxing fashion, using two semirunning sutures. Once the suture of the posterior plate of the ureter is completed, the single-J stent is inserted and the ureteroneobladder anastomosis is completed. The neobladder is filled up to 200 cc of saline to test for leaks.
2.6. Training for voiding
The patients were advised to void every 2 h for the first 3 mo. After this period, voiding records were checked, and the patients were allowed to void according to the patient’s preference.
3. Results
The mean age at surgery was 65.6 yr (49–77) and the mean body mass index was 26.2 (23.1–31). Diabetes mellitus was present in two of 26 cases. The American Society of Anesthesiologists score was 2 in 21/26 patients. Ten patients received neoadjuvant chemotherapy. Nerve sparing was performed in seven cases.
3.1. Perioperative and pathological outcomes
The mean operative time was 395 min (300–560), while the mean time for the ONB was 192 min (110–340). The mean hospital stay was 12 d (7–26). The mean times for first flatus and defecation were 2.7 (1–7) and 4.8 (1–10) d, respectively. The mean times for mono-J and catheter removal were 11 (8–20) and 14 (10–21) d, respectively. No perioperative deaths as well as no cancer recurrence in the urethra or ileal neobladder were found during the follow-up period. Table 1 reports the main perioperative complications and their Clavien-Dindo classification [9].
Table 1.
Complications, grading, and management
| Complication | Number of patients (%) | Grading | Management |
|---|---|---|---|
| Pulmonary embolism | 1 (3.8) | II | Anticoagulants |
| Candiduria | 1 (3.8) | II | Antifungal therapy |
| Incidental removal of mono-J | 1 (3.8) | IIIa | Nephrostomy tube placement under local anesthesia |
| Febrile urinary tract infections | 4 (15.4) | II | Antibiotics |
| Paralytic ileus | 3 (11.5) | II | Nasogastric tube placement and parenteral nutrition |
The mean number of lymph nodes removed was 17 (7–34). Concomitant prostate adenocarcinoma was encountered in nine of 26 cases (35%). Two cases of soft-tissue positive surgical margins were observed (8%). Four patients received adjuvant chemotherapy. Pathology is shown in Table 2.
Table 2.
Pathological outcomes
| pT0 | 1 |
| pTa/T1/CIS | 8 |
| pT2 | 7 |
| pT2 N1 | 1 |
| pT3 | 6 |
| pT4 N0 | 1 |
| pT4a N1 | 1 |
| pT4aN2 | 1 |
CIS = carcinoma in situ.
The mean follow-up was 27 mo (12–38). During follow-up, three patients deceased because of disease progression; no UDS data were available for these patients. Five patients refused any UDS evaluation, while in one patient, placement of the transurethral UDS catheter was impossible because of stenosis of the urethral-neobladder anastomosis. Four patients refused the second UDS evaluation. Thus, 17 patients performed the 3-mo UDS and 13 patients performed both UDS evaluations.
3.2. Three-month UDS (17 patients)
3.2.1. Uroflowmetry
The mean voided volume was 279 ml (64–556). The mean peak flow rate (Qmax) was 16.6 ml/s (5.6–35.3). In all cases, PVR was <50 ml.
3.2.2. (Entero)cystometry storage phase
One patient showed residual peristaltic activity during the cystometry storage phase. The average compliance was 12.9 ml/cmH2O (range 8.9–21.4). The mean MCC was 429 cm3 (150–639). The first sensation of bladder fullness was perceived at an average volume of 368 cm3 (80–500). The mean maximal neobladder pressure was 48.4 cmH2O (21–69.2).
3.2.3. Voiding phase
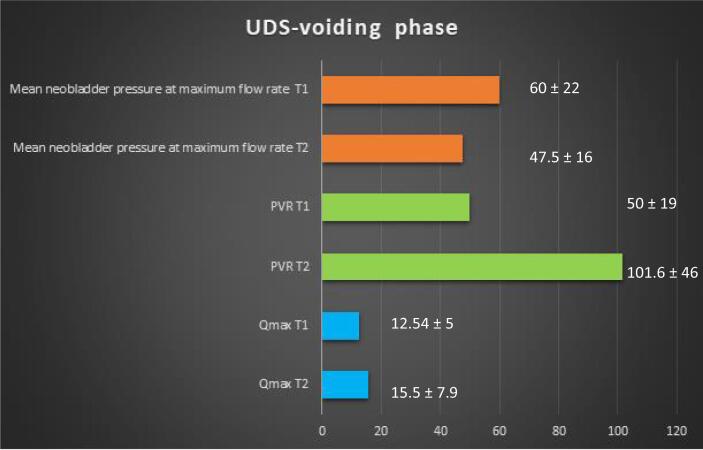
The average flow rate during the voiding phase was 12.5 ml/s (5.2–26.4). The mean PVR after the voiding phase was 50 ml (0–228). The mean neobladder pressure at Qmax was 47.5 cmH2O (6.8–123). CIC was required for two patients for PVR >100 ml.
3.2.4. Continence
Urinary incontinence on provocative testing (coughing) was noted in one patient and on Valsalva maneuver in five of 17 patients. The mean Valsalva leak point pressure was 30.2 cmH2O (12–45).
3.3. November 2021 urinary diary and UDS re-evaluation
3.3.1. Urinary diary
The mean number of voids per day was 5.5 (3–10), while the average number of voids per night was 2 (1–4). Day- and nighttime continence rates were 58.8% (10/17) and 23.5% (4/17), respectively. Seven patients used pads during daytime (5/7 used one pad and 2/7 used two pads). Thirteen patients used pads during nighttime (8/14 used one pad, 2/14 two pads, 2/14 three pads, and 1/14 four pads).
3.3.2. Uroflowmetry
The mean voided volume was 228 ml (43–409). The mean Qmax was 12 ml/s (4–22.6). The average PVR was 43.6 ml (0–510).
3.3.3. Cystometry storage phase
Two of the patients exhibited neobladder overactivity during the cystometry storage phase. The average compliance was 33.1 ml/cmH2O (11–160). The mean MCC was 431 cm3 (median 445, range 200–553). The first sensation of bladder fullness was perceived at an average volume of 337 cm3 (150–500). The mean maximal neobladder pressure was 53.8 cmH2O (3–160).
3.3.4. Voiding phase
The average flow rate during the voiding phase was 15.5 ml/s (3.6–34.1). The mean PVR was 101.6 ml (0–310). The mean neobladder pressure at Qmax was 60 cmH2O (3–150). Urinary incontinence on provocative testing (coughing) was noted in two patients and on Valsalva maneuver in one patient with an abdominal leak point pressure of 45 cmH2O. CIC was required for three patients (3/17, 17.6%) for PVR >100 ml.
3.3.5. I-QoL score
The mean postoperative I-QoL score was 103.3 (89–110).
3.3.6. Kidney ultrasound
Two cases of asymptomatic left hydronephrosis were encountered (grades I and II).
3.3.7. Blood gas analyses
No cases of hyperchloremic acidosis were identified.
3.3.8. Chronological changes in UDS characteristics
Fig. 2, Fig. 3 represent the changes of the parameters of UDS over time.
Fig. 2.
Chronological changes of the main urodynamic parameters of the storage phase, as evaluated in T1 (3 mo after surgery) and T2 (November 2021). The mean values ± standard deviation have been inserted in the chart. UDS = urodynamic study.
Fig. 3.
Chronological changes of the main urodynamic parameters of the voiding phase, as evaluated in T1 (3 mo after surgery) and T2 (November 2021). The mean values ± standard deviation have been inserted in the chart. PVR = postvoid residual of urine; Qmax = maximal flow rate; UDS = urodynamic study.
3.3.9. Composite score
Five of 13 (38.5%) patients demonstrated a combination of optimized outcomes (mean capacity between 300 and 500 ml, compliance >20 ml/cmH2O, PMCC ≤32 cmH2O, PVR <100 ml, no hydronephrosis, use of zero to one pad per day and night, and I-QoL score >100).
4. Discussion
Radical cystectomy with lymph-node dissection and urinary diversion is the gold standard procedure for nonmetastatic muscle-invasive and selected high-risk nonmetastatic bladder cancer [10]. Over the past 15 yr, a number of surgeons have performed cystectomy using laparoscopic or robot-assisted techniques. Initial attempts to perform the diversion completely intracorporeally were fraught with difficulty, with bowel reconfiguration and concerns about time efficiency; the procedure was consequently performed with extracorporeal reconstruction after removal of the bladder and through a small midline incision [11]. While in older series, a totally intracorporeal urinary diversion was performed in <20% of cases [12], this rate has increased significantly to around 50% over the past few years [13], [14].
Although critiqued [15], plenty of reports in the published literature suggest that the riONB is a low-pressure spherical reservoir with adequate capacity, associated with a satisfactory continence rate [16], [17], [18]. Papers describing the functional outcomes of the riONB include small series of up to 70 patients [3]. The outcomes on continence vary on the basis of the definition. Tyritzis et al [19], in a series of 70 patients submitted to the Karolinska modified Studer neobladder, reported rates of day- and nighttime continence rates of 90% and 75%, respectively, at 12 mo, with a continence definition of zero to one pad per day. With a strict definition of continence as zero pads per day, the University of South Carolina group reported an overall rate of continent patients of 17%, with 79% of patients using two or fewer pads per day [20]. Concerning the U-shaped neobladder, continence defined as one or fewer pad per day was reported for 65% and 18% of patients [21]. In the study of Asimakopoulos et al [4] reporting outcomes of a highly selected population of 40 young men with very localized bladder cancer who underwent nerve-sparing surgery and modified-Y neobladder, 100% and 72.5% of, respectively, day- and nighttime continence rates were achieved after 12 mo, with a continence definition of zero pads per day.
In the current study, day- and nighttime continence rates were 58.8% (10/17) and 23.5% (4/17), respectively. During daytime, the majority of the incontinent patients (71.4%) used one pad per day. With a broader definition of continence of zero to one pad per day, the relative rates would be 15/17 (88%) and 12/17 (70.6%) for day- and nighttime continence, respectively.
The rate of CIC after neobladder varies broadly in literature from 4% to 43% at 5 yr [22], but seems to be dependent on which cutoff of PVR the authors consider to be clinically significant. CIC is recommended when PVR routinely exceeded 100 ml [23]. The rate of CIC in our study was 17.6%. Voiding after orthotopic urinary diversion requires the concomitant combination of abdominal straining with pelvic muscle floor relaxation. Thus, the voiding difficulty may be due to the inability to relax the pelvic floor muscles, inadequate abdominal straining, and neobladder neck positioning since urethral angulation may also be responsible for urinary retention [24].
Although criticized as of limited interest in ileal neobladder [25], UDS probably represent the most objective method in assessing their functional outcomes. Currently, there is no consensus about the urodynamic assessment of the intestinal neobladder. The same parameters applied to an intact bladder are used without considering that the intestine was not originally conceived to store or void urine. Urodynamic results depend on the type, length, and configuration of the intestinal segment used. They are also dependent on the time elapsed after surgery since the overall capacity seems to increase during the first 6–9 mo [22].
Furthermore, there are some difficulties in conducting the examination and interpretation of the results of enterocystometry. First of all, this relates to the difficulty in assessing the sensitivity of the neobladder as a subjective part of the examination; patients have impaired feeling for the need to void but instead feel pressure in the place of the neobladder. Enterocystometric capacity is determined by a strong desire to void or when it is absent with an onset of leakage beside the cystometric catheter.
In the study of Satkunasivam et al [20], the median capacity was 514 ml (330–1001) for a 60-cm Studer ONB. These values relate to normal compliance (median 33 ml/cmH2O) and no neobladder overactivity, but seem to be correlated with a higher PVR after UDS (268 ml). In contrast, the U-shaped ONB described by Koie et al [26] showed a mean maximum capacity of 285 ml for a 40-cm U-shaped neobladder. The maximum ONB pressure was 26.5 ml/cmH2O, which translates into a much poorer compliance with respect to that in the study of Satkunasivam et al [20]; however, the median PVR after UDS was only 29 ml.
The mean maximum capacity in our study was 431 cm3 (range 200–553). The first sensation of bladder fullness was perceived at an average volume of 337 cm3 (150–500). Two of the patients exhibited neobladder overactivity during the cystometry storage phase. The average compliance was 33.1 ml/cmH2O (range 11–160, median value 26). The mean PVR after UDS was 101.6 ml (0–310).
Concerning upper urinary tract repercussions, in our study, two of 17 (11.8%) patients showed hydronephrosis of the left kidney, of grades I and II, not requiring further treatment.
Quality of life was assessed by the I-QoL questionnaire that covers the most important concerns related to the symptoms associated with urinary incontinence, from social life to intimate relationships and psychophysical health [7]. This disease-specific, 22-item tool included questions that allow evaluations of both distress and impact of urinary incontinence in three domains: (1) avoidance and limiting behavior (eight items), (2) social embarrassment (five items), and (3) psychosocial impact (nine items). Each of the 22 items has a five-point Likert-type response scale (1 = extremely, 2 = quite a bit, 3 = moderately, 4 = a little, and 5 = not at all). The higher scores indicate better quality of life. In our study, the mean postoperative I-QoL score was 103.3 (89–110), indicating overall high satisfaction of the patients harboring the presented neobladder.
Concerning the chronological changes of the intracorporeal orthotopic modified-Y “Bordeaux“ neobladder, at a mean follow-up of 22 mo, no statistically significant changes in the UDS parameters of the voiding phase were observed. Concerning the parameters of the storage phase, there was only a significant reduction in the volume of the first sensation of bladder fullness.
Overall, nearly 40% of the evaluated patients satisfied the composite criteria of optimal volumetric, UDS, and continence outcomes.
5. Conclusions
The analysis of the parameters of both invasive and noninvasive UDS demonstrate that the intracorporeal modified-Y ONB has adequate capacity, acceptable PVR, and satisfying compliance. Thus, it seems to decrease the possibility of abnormalities of the upper urinary tract and avoid the metabolic complications, enhancing quality of life. Prospective studies with larger numbers of patients and longer follow-up periods are needed to determine the characteristics of this ONB over time.
Author contributions: Anastasios D. Asimakopoulos had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Asimakopoulos.
Acquisition of data: Morini, Gubbiotti, Annino.
Analysis and interpretation of data: Asimakopoulos, Annino.
Drafting of the manuscript: Asimakopoulos.
Critical revision of the manuscript for important intellectual content: Asimakopoulos, Agrò, Piechaud, Gaston, Annino.
Statistical analysis: Asimakopoulos, Gubbiotti, Annino.
Obtaining funding: None.
Administrative, technical, or material support: Giommoni, Gubbiotti.
Supervision: None.
Other: Video: Annino, Asimakopoulos.
Financial disclosures: Anastasios D. Asimakopoulos certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: We thank Dr. Lorenzo Dutto, an urologist in the Department of Urology of the Queen Elisabeth’s University Hospital (Glasgow, UK), for his kind help in the linguistic revision of the manuscript.
Associate Editor: Véronique Phé
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.11.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wein A.J., Kavoussi L.R., Campbell M.F., et al. ed. 12. Elsevier Saunders; Philadelphia, PA: 2020. Campbell-Walsh-Wein urology. [Google Scholar]
- 2.Goh A.C., Gill I.S., Lee D.J., et al. Robotic intracorporeal orthotopic ileal neobladder: replicating open surgical principles. Eur Urol. 2012;62:891–901. doi: 10.1016/j.eururo.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 3.Benamran D., Phé V., Drouin S.J., et al. Functional outcomes obtained with intracorporeal neobladder after robotic radical cystectomy for cancer: a narrative review. J Robot Surg. 2020;14:813–820. doi: 10.1007/s11701-020-01070-x. [DOI] [PubMed] [Google Scholar]
- 4.Asimakopoulos A.D., Campagna A., Gakis G., et al. Nerve sparing, robot-assisted radical cystectomy with intracorporeal bladder substitution in the male. J Urol. 2016;196:1549–1557. doi: 10.1016/j.juro.2016.04.114. [DOI] [PubMed] [Google Scholar]
- 5.Rosier P.F.W.M., Schaefer W., Lose G. International Continence Society good urodynamic practices and terms 2016: urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol Urodyn. 2017;36:1243–1260. doi: 10.1002/nau.23124. [DOI] [PubMed] [Google Scholar]
- 6.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20; Int Urogynecol J 2010;21:5–26. [DOI] [PubMed]
- 7.Symonds T. A review of condition-specific instruments to assess the impact of urinary incontinence on health-related quality of life. Eur Urol. 2003;43:219–225. doi: 10.1016/s0302-2838(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 8.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witjes J.A., Bruins H.M., Cathomas R., et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 11.Xylinas E., Green D.A., Otto B., et al. Robotic-assisted radical cystectomy with extracorporeal urinary diversion for urothelial carcinoma of the bladder: analysis of complications and oncologic outcomes in 175 patients with a median follow-up of 3 years. Urology. 2013;82:1323–1329. doi: 10.1016/j.urology.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed K., Khan S.A., Hayn M.H., et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2014;65:340–347. doi: 10.1016/j.eururo.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Hussein A.A., May P.R., Jing Z., et al. Outcomes of intracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol. 2018;199:1302–1311. doi: 10.1016/j.juro.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 14.Lenfant L., Verhoest G., Campi R., et al. Perioperative outcomes and complications of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy for bladder cancer: a real-life, multi-institutional French study. World J Urol. 2018;36:1711–1718. doi: 10.1007/s00345-018-2313-8. [DOI] [PubMed] [Google Scholar]
- 15.Hautmann R.E., Herr H.W., Pruthi R.S., Aron M. Robotic radical cystectomy—is the diversion the Achilles' heel? J Urol. 2014;192:1601–1603. doi: 10.1016/j.juro.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Studer U.E., Burkhard F.C., Schumacher M., et al. Twenty years’ experience with an ileal orthotopic low pressure bladder substitute—lessons to be learned. J Urol. 2006;176:161–166. doi: 10.1016/S0022-5347(06)00573-8. [DOI] [PubMed] [Google Scholar]
- 17.Madersbacher S., Möhrle K., Burkhard F., Studer U.E. Long-term voiding pattern of patients with ileal orthotopic bladder substitutes. J Urol. 2002;167:2052–2057. [PubMed] [Google Scholar]
- 18.Hautmann R.E., Volkmer B.G., Schumacher M.C., Gschwend J.E., Studer U.E. Long-term results of standard procedures in urology: the ileal neobladder. World J Urol. 2006;24:305–314. doi: 10.1007/s00345-006-0105-z. [DOI] [PubMed] [Google Scholar]
- 19.Tyritzis S.I., Hosseini A., Collins J., et al. Oncologic, functional, and complications outcomes of robot-assisted radical cystectomy with totally intracorporeal neobladder diversion. Eur Urol. 2013;64:734–741. doi: 10.1016/j.eururo.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 20.Satkunasivam R., Santomauro M., Chopra S., et al. Robotic intracorporeal orthotopic neobladder: urodynamic outcomes, urinary function, and health-related quality of life. Eur Urol. 2016;69:247–253. doi: 10.1016/j.eururo.2015.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canda A.E., Atmaca A.F., Altinova S., Akbulut Z., Balbay M.D. Robot-assisted nerve-sparing radical cystectomy with bilateral extended pelvic lymph node dissection (PLND) and intracorporeal urinary diversion for bladder cancer: initial experience in 27 cases. BJU Int. 2012;110:434–444. doi: 10.1111/j.1464-410X.2011.10794.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh V., Mandal S., Patil S., Sinha R.J., Gupta D.K., Sankhwar S.N. Urodynamic and continence assessment of orthotropic neobladder reconstruction following radical cystectomy in bladder cancer; a prospective, blinded North Indian tertiary care experience. South Asian J Cancer. 2014;3:223–226. doi: 10.4103/2278-330X.142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M., Takeda A., Saito S., et al. Long-term functional outcomes of ileal and sigmoid orthotopic neobladder procedures. Urology. 2007;69:74–77. doi: 10.1016/j.urology.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Al Salhi Y., Fuschi A., Martoccia A., et al. Impact of early self-clean intermittent catheterization in orthotopic ileal neobladder: prospective randomized study to evaluate functional outcomes, continence status and urinary tract infections. Minerva Urol Nephrol. 2022 doi: 10.23736/S2724-6051.22.04944-8. In press. [DOI] [PubMed] [Google Scholar]
- 25.Burkhard F.C. The devil is in the details: still true. Eur Urol. 2016;69:254–255. doi: 10.1016/j.eururo.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 26.Koie T., Ohyama C., Yoneyama T., et al. Robotic cross-folded U-configuration intracorporeal ileal neobladder for muscle-invasive bladder cancer: initial experience and functional outcomes. Int J Med Robot Comput Assist Surg. 2018;14:e1955. doi: 10.1002/rcs.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.