Abstract
Ethnopharmacological relevance
In Ethiopia, the indigenous medicinal plant Commelina latifolia Hochst. ex C.B. Clarke leaves are used to treat malaria and wounds.
Aim of the study
In this work, the antiplasmodial activity of Commelina latifolia crude leaf extract and solvent fractions against Plasmodium berghei-infected mice was investigated.
Materials and methods
80% methanol was used to extract the leaves of C. latifolia, and the crude extract was fractionated using chloroform, pure methanol, and distilled water. All test compounds were undergone an acute oral toxicity test before being put through Peter’s 4-day suppressive test to see if they have antiplasmodial activity. The hydroalcoholic crude extract and chloroform fraction were additionally assessed for antimalarial activity using curative and prophylactic tests in P. berghei-infected laboratory mice.
Results
All of the tested crude extracts were safe at a dose of 2000 mg/kg. At 400 mg/kg dose both the 80% methanol extract and chloroform fraction exhibited antimalarial activity with parasitemia suppression values of 86.31%, and 76.56% in the four-day suppressive test, 81.97% and 72.05% in Rane's test, and 69.05% and 62.88% in the prophylactic test, respectively.
Conclusion
Collectively, the oral dose of Commelina latifolia is safe, and reveals promising antimalarial activity. The findings backed up the utilization of the plant in traditional medicine to treat malaria.
Keywords: Antimalarial activity, Commelina latifolia, Plasmodium berghei, Traditional medicine
Antimalarial activity; Commelina latifolia; Plasmodium berghei; Traditional medicine.
1. Introduction
Malaria (the most common and dangerous protozoan disease) causes millions of Africans to be hospitalized and die each year. In 2020, approximately 241,000,000 malaria cases were recorded from 85 countries globally, with 228,000,000 cases reported from Africa (WHO, 2021). Currently, there is a global initiative to eradicate malaria. Consequently, a remarkable result in malaria control has been achieved (Hawaria et al., 2019). Malaria continues to be a major public health issue in Ethiopia, where 68% of the population is at risk of infection. Plasmodium falciparum and Plasmodium vivax co-exist as major parasite species in Ethiopia (Taffese et al., 2018). Widespread resistance to the current antimalarials and the costs of the available drugs led many Ethiopians to use medicinal plant preparations against malaria. Traditional and herbal remedies, including the genus Commelina, seem to be the alternative treatment of choice in countries where malaria is endemic (Abebe and Garedew, 2019). Commelina is a genus that comprised around 170 species commonly called dayflowers (Hardy et al., 2009). Plant species in this genus are widely distributed in tropical and temperate regions of the world (Ghosh et al., 2019).
The Perennial herb Commelina latifolia Hochst. ex C.B.Clarke is one of the essential folkloric medicinal plants that has been used for a long history. In Ethiopia, herbal preparations containing Commelina latifolia Hochst. ex C.B.Clarke has been used to treat malaria, wound, and inflammatory diseases (Abebe and Garedew, 2019). First, they crush the dried leaf, boil it with water, and then filter it. It is the filtrate that is ingested for the treatment and prevention of malaria. However, the antimalarial activity of the plant has not been scientifically validated and its phytochemical constituents are not yet identified. Thus, this study aimed to identify the phytochemical components of Commelina latifolia and evaluate its antiplasmodial efficacy in vivo against P. berghei-infected mice.
2. Materials and methods
2.1. Experimental plant
The leaves of C. latifolia were collected from Tepi district, Ethiopia, which is approximately 611 km southwest of Addis Ababa. The National Herbarium, College of Natural and Computational Sciences, Addis Ababa University, confirmed and deposited voucher specimens (GT002).
2.2. Experimental animals and parasites
For the evaluation of the plant extracts, either sex, healthy Swiss albino mice between the ages of 6 and 8 weeks and weighing 24–28 g were employed. The study mice were obtained from the School of Pharmacy, Mizan-Tepi University and acclimatized for a week before the study. They were housed in an air-conditioned cage with a room temperature, a 12-hour light/dark cycle, and unlimited access to regular pellets and water. The Ethiopian Public Health Institute provided chloroquine-sensitive P. berghei (ANKA strain), which was maintained by serially passing blood from infected mice to healthy mice once a week. The handling and caring of the study mice were according to the rules for the care and use of experimental animals (NIH Guidelines for Care and Use of Laboratory Animals, 1996). The procedures were reviewed and approved by the Research and Ethical Review Committee of the School of Pharmacy, College of Medicine and Health Sciences, Mizan-Tepi University.
2.3. Extraction and fractionation
The leaves were thoroughly washed with distilled water, dried for 15 days under shade at room temperature with well-ventilated air condition, and then crushed into a coarse powder using an electrical mill. About 500 g of the powdered plant material was macerated in a mixture of methanol with water (a proportion of 80:20) for 72 h with intermittent stirring and shaking. Filter paper (Whatman number 1) was used to separate the filtrate from the mark, and the residue (mark) was re-macerated twice with fresh solvent. A rotary evaporator (BUCHI Rotavapor™ R-300, Switzerland) was used to evaporate the organic solvent. The extract was further dried using a freeze dryer. The 68.55 g dried extract (13.71% w/w) was transferred into vials and kept at −20 °C until use. The dried hydroalcoholic extract (53.6 g) was then successively extracted with chloroform, absolute methanol, and pure water using a Soxhlet apparatus. A rotary evaporator was used to evaporate the solvents in the chloroform and methanol fractions, while a lyophilizer was used to dry and concentrate the water fraction. The calculated percent yields of the dried chloroform, methanol, and water fractions were 10.4 g (19.40%), 18.4 g (34.33%), and 24.8 g (46.27%). The fractions were stored at −20 °C until use.
2.4. Acute oral toxicity test
Evaluation of the acute oral toxicity of the crude extract and its solvent fractions were performed according to the organization for economic cooperation and development guideline 425 (OECD, 2010). Twenty healthy non pregnant female mice were used to assess the toxicity profile of both the hydromethanolic extract and solvent fractions. Every mouse was fasted for 4 h before and 2 h after administration of the test drugs. First, a 2000 mg/kg dose of the crude extracts was given by oral gavage to a single mouse from each group. The mouse was observed for 24 h for gross behavioral changes such as hair erection, convulsion, poor appetite, lacrimation, tremor, salivation, diarrhea, and mortality after administering the test substance. Since either death or gross behavioral changes was not seen in the first mouse, the same dose was administered to another 4 mice. Each animal was given a single dose and was observed continuously for 4 h with 30 min intervals and then for 14 consecutive days once daily for signs and symptoms of toxicity.
2.5. Phytochemical analysis
The composition of phytoconstituents in the hydromethanolic crude extract and solvent fractions of C. latifolia leaves was investigated to link the plant's antimalarial activity to secondary metabolites (Debella, 2002; Njoku and Obi, 2009).
2.6. In vivo antiplasmodial activity study
2.6.1. Parasite inoculation
Under a microscope, the percentage parasitemia level of donor mice was initially established. To do so, blood was collected from the tails of donor mice that had previously been infected with P. berghei and smeared on frosted microscope slides. The smear fixed with absolute methanol was then stained with Giemsa stain and observed under a microscope. When the donor mice parasitemia level was found to be 30%, the mice were decapitated and their blood was collected onto Petri dishes containing 0.5% trisodium citrate. The collected blood was then diluted with 0.9% normal saline to obtain 5 ×107 P berghei-infected red cells in 1 ml of blood (Fidock et al., 2004). Lastly, each mouse intraperitoneally received 0.2 ml of diluted blood which contains 1 × 107 P. berghei infected erythrocytes.
2.6.2. Four-day chemosuppressive model
This test was conducted for the antiplasmodial evaluation of test drugs at early infection. For the assessment of the crude extract, thirty inoculated mice were grouped by chance into five groups each having six mice. Group-I, treated with 0.2 ml distilled water, served as negative control, and group-II, treated with chloroquine 25 mg/kg/day, was a positive control group. The remaining mice grouped as group-III, IV, and V were treated with the hydromethanolic extract at a daily dose of 100, 200, and 400 mg/kg, respectively.
Similarly, thirty infected mice were randomly grouped for each fraction into Three treatment and two control groups, each having six mice. For the chloroform fraction, its first group received 0.2 ml of the vehicle (2% Tween 80), while for methanol and aqueous fractions, its first group received 0.2 ml of pure water. Each fraction's second group was treated with chloroquine 25 mg/kg dose per day. Each fraction's treatment groups received their respective fractions at test doses of 100, 200, and 400 mg/kg per day.
All the test drugs were given orally by oral gavage. Treatment was initiated 2 h after infection on day 0 and continued daily until day 3. On day 4, a blood smear was prepared as described in Section 2.6.1 and the parasite number was determined under a microscope (Primo Star, Carl Zeiss, Germany) using an oil immersion objective of x 100 magnification power. The parasitemia was decided by counting 5 fields per slide (Peters, 1965; Hilou et al., 2006). Percent parasitemia and percent suppression was computed by the formula:
2.6.3. Body weight and survival time measurement
The parameters body weight and mean survival days were used to assess the in vivo antiplasmodial activity of all of the extracts. The study mice's body weight was assessed on day 0 before infection and day 4 using a precise digital weighing balance. After inoculation, the death of every mouse was monitored daily from day 1 to day 28. Average body weight and average time to death were then determined for each group using the formula below (Mesfin et al., 2012).
2.6.4. Rectal temperature and packed cell volume measurement
The parameters rectal temperature and packed cell volume (PCV) were also used to evaluate the antimalarial effects of the test extracts in mice. To measure PCV, blood samples drained from the mice's tail were filled in heparinized hematocrit capillary tubes to 75% of their height. The capillary tubes were then closed at their dry end by sealing clay and centrifuged at 11,000 rpm for 5 min in a hematocrit centrifuge. The mice's rectal temperature was measured and recorded using a digital rectal temperature gauge. The mean rectal temperature and PCV were then computed using the following formulas (Fentahun et al., 2017).
2.6.5. Rane's test (established infection test)
Since the hydroalcoholic extract and chloroform fraction of C. latifolia showed promising antiplasmodial activity in early infection tests, their curative effects were further evaluated using a method illustrated by Ryley and Peters (1970). In the evaluation of the test substances (72 h after inoculation), thirty parasitized mice were randomly distributed into five groups, each having six mice. The mice were then treated as described in Section 2.6.2, and the treatment was continued for additional 3 days. To monitor the mean percentage parasitemia levels, a thin blood film was prepared (as described in Section 2.6.1) from day 3 up to day 7 (daily for 5 days). The mice's body weight, rectal temperature, and PCV were also measured on the 4th day before and on the 8th day after treatment. Post-infection, the mice were followed for one month to calculate the mean survival time (Nardos and Makonnen, 2017).
2.6.6. Prophylactic test (repository test)
Since the curative potential of the hydromethanolic crude extract and chloroform fraction of C. latifolia was appreciable, their prophylactic effects were assessed using a procedure depicted by Peters (1965). Like the above tests, 30 mice were arbitrarily divided into 5 groups each having 6 mice for each extract. Each group was treated accordingly from day 0 up to day 3. All the mice were infected on the 5th day (day 4). Blood smears were made and the parasitemia levels were determined on the 8th day (day 7). Both on the 5th day before infection and the 8th day of the experiment, the body weight, temperature, and PCV of the mice were recorded. The survival period of the mice was monitored for a month (28 days) post-infection, and the mean survival time was calculated.
2.7. Data analysis
We fed the data into Microsoft Excel 2019 spreadsheet and then transported to Statistical Package for Social Sciences (SPSS) version 26 for analysis. We used GraphPad Prism 8.0.2 for the construction of graphs. Results were expressed as a mean with a standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to determine statistical significance, and Tukey's post hoc test was used to evaluate mean differences across groups. Statistical significance was defined as a P value of less than 0.05.
3. Results and discussion
3.1. Acute oral toxicity test
The acute oral toxicity study results of the present study revealed that the hydroalcoholic crude extract and solvent fractions of C. latifolia were safe by the oral route at a dose of 2,000 mg/kg/day as no mortality or signs of toxicity were observed within the follow-up period. This indicates the 50% lethal dose (LD50) value of C. latifolia is more than 2,000 mg/kg. Previous studies also reported that the crude extracts of various Commelina species did not show any toxicity and fatality with greater than 2,000 mg/kg/dose (Orni et al., 2018; Rahman et al., 2021).
3.2. Phytochemical screening
According to the preliminary phytochemical screening, all of the secondary metabolites tested, except anthraquinones, were found in the 80% methanolic extract. Cardiovascular glycosides were found in chloroform and pure methanol fractions, whereas alkaloids and tannins were found in all fractions. Furthermore, saponins were found in the chloroform and aqueous fractions, whereas phlobatannins and phenolic compounds were only found in the pure methanol fraction (Table 1).
Table 1.
Phytoconstituent analysis of the hydromethanolic extract and solvent fractions of Commelina latifolia.
| Secondary metabolites | Crude extract |
Solvent fractions |
||
|---|---|---|---|---|
| 80% methanol extract | Chloroform fraction | Methanol fraction | Aqueous fraction | |
| Alkaloids | + | + | + | + |
| Anthraquinones | − | − | − | − |
| Cardiac glycosides | + | + | + | − |
| Flavonoids | + | + | − | − |
| Phenolic compounds | + | − | + | − |
| Phlobatannins | + | − | + | − |
| Saponins | + | + | − | + |
| Tannins | + | + | + | + |
| Terpenoids | + | + | − | − |
+ = present; − = absent.
3.3. Antimalarial activity of 80% methanolic extract
3.3.1. Four-day suppressive test
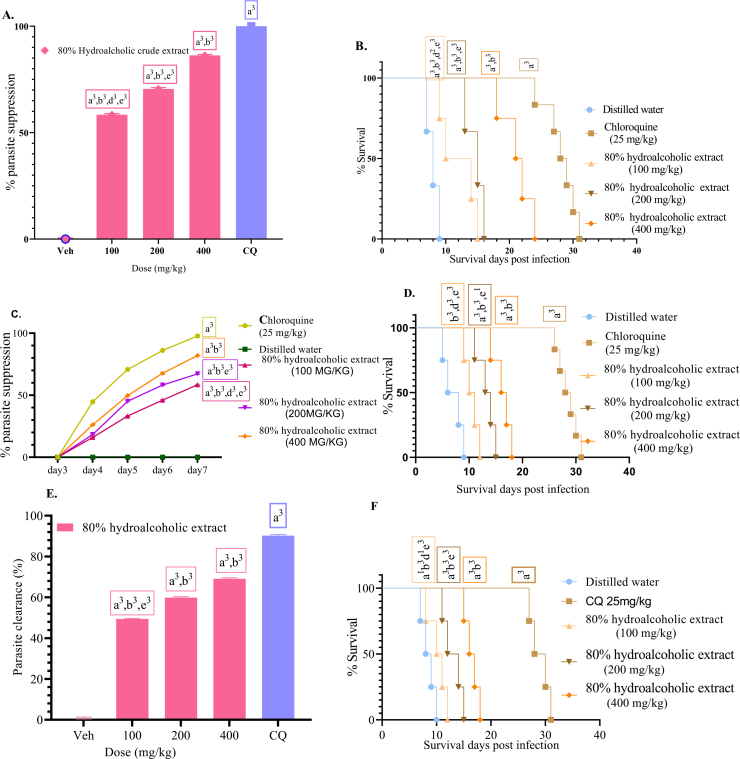
When evaluated against early infection with daily dosages of 100, 200 and 400 mg/kg/day, the hydroalcoholic crude extract of C. latifolia had a dose-dependent chemosuppressive effect, resulting in parasitemia suppression of 58.44%, 70.54%, and 86.31%, respectively (Figure 1a). Various investigations on other medicinal plants have revealed a dose-dependent antimalarial efficacy (Nureye et al., 2021; Tadege et al., 2022). Even at the lowest dose examined, the chemosuppression generated by the hydroalcoholic extract of C. latifolia was statistically significant (P < 0.001). In comparison to the positive control group, all extract doses showed a considerably lower (P < 0001) parasitemia suppression. Furthermore, as compared to the negative control group, the test extracts significantly (P < 0.001) increased mean survival days; however, the values were significantly (P < 0.001) lower than the standard drug (Figure 1b). They also prevented body weight loss, a drop in rectal temperature, and a decrease in PCV caused by an increase in parasitemia (Table 2). However, when compared to the conventional (chloroquine) drug, they showed decreased parasitemia suppression effect (P < 0.001).
Figure 1.
% Parasitemia suppression of the hydroalcoholic crude extract in the early infection (A), Survival days of each mouse treated with the hydroalcoholic crude extract at 100, 200, and 400 mg/kg doses, chloroquine 25 mg/kg (CQ), and distilled water in the early infection (B). % Parasitemia suppression of the hydroalcoholic crude extract in established infection (C). Survival days of each mouse administered with hydroalcoholic crude extract in Rane's test at a dose of 100, 200, 400 mg/kg and, chloroquine 25 mg/kg (CQ), and distilled water (D). % Parasitemia suppression of the hydroalcoholic crude extract in the repository test (E). Survival days of each mouse administered with hydroalcoholic crude extract (100, 200, and 400 mg/kg doses), chloroquine (CQ), and distilled water (F). Values are interpreted as mean ± SEM; n = 6.
Table 2.
Body weight, rectal temperature, and packed cell volume of P. berghei-infected mice treated with hydroalcoholic leaves extract of C. latifolia in the 4-day suppressive test.
| Group | Weight |
Rectal Temperature |
Packed cell volume |
||||||
|---|---|---|---|---|---|---|---|---|---|
| D0 | D4 | % Change | D0 | D4 | % Change | D0 | D4 | % Change | |
| DW | 27.16 ± 0.32 | 22.66 ± 0.21 | −16.55 ± 0.57 | 37.10 ± 0.99 | 31.34 ± 0.10 | −15.52 ± 0.14 | 55.73 ± 0.76 | 46.64 ± 0.66 | −16.31 ± 0.41 |
| CQ 25 | 26.54 ± 0.57 | 26.65 ± 0.60 | .39 ± 0.15 | 36.90 ± 0.11 | 36.78 ± 0.11 | −.31 ± 0.10 | 55.98 ± 0.64 | 55.63 ± 0.62 | −.62 ± 0.17 |
| CLCE100 | 25.95 ± 0.56 | 23.04 ± 0.53 | −11.21 ± 0.75a3b3d2e3 | 37.04 ± 0.06 | 32.32 ± 0.14 | −12.74 ± 0.30a3b3d2e3 | 55.0 ± 600 | 48.36 ± 0.44 | −12.16 ± 0.39a3b3d2e3 |
| CLCE200 | 25.24 ± 0.74 | 23.42 ± 0.66 | −7.1998 ± 0.35a3b3e3 | 36.8 ± 0.07 | 33.62 ± 0.07 | −8.64 ± 0.09a3b3e3 | 55.44 ± 0.49 | 50.7 ± 0.34 | −8.53 ± 0.39a3b3e3 |
| CLCE400 | 26.10 ± 0.67 | 25.16 ± 0.71 | −3.62 ± 0.53a3b3 | 37.00 ± 0.10 | 35.38 ± 0.11 | −4.39 ± 0.10a3b3 | 53.73 ± 0.72 | 51.10 ± 0.78 | −4.91 ± 0.34a3b3 |
Data are expressed as mean ± SEM; n = 5; compared to a, negative control; b, CQ25 mg/kg; c, 100 mg/kg; d, 200 mg/kg; e, 400 mg/kg; 1, P < 0.05; 2, P < 0.01; 3, P < 0.001; DW, distilled water; CQ, chloroquine; CLCE, crude extract of the Commelina latifolia; D0, pre-treatment value on day 0; D4, post-treatment value on day 4.
As the hydroalcoholic crude extract resulted in more than 50% parasite clearance at 200 mg/kg, it can be considered as a potential schizonticidal bullet in treating early malarial infection and has good antimalarial efficacy (Deharo et al., 2001). Prominently, the 20 days of MST achieved by 400 mg/kg dose of the crude extract is immense evidence that the extract reduced the pathogenicity of the malaria parasite. However, the extract did not eliminate the parasite, as it produced MST in less than 28 days (Slack et al., 2012). This may be related to the infection's recurrence. In terms of parasitemia suppression, the hydromethanolic extract outperformed the solvent fractions, which is consistent with Hypoestes forskalei leaves extract (Misganaw et al., 2020) and Ajuga integrifolia (Asnake et al., 2015). This could be due to the hydroalcoholic extract containing more phytochemicals than the fractions (Table 1), as well as synergistic activity. Furthermore, one secondary metabolite may protect others from breakdown, and disrupting this mechanism may reduce solvent fractions' suppressive effects (Traore et al., 2006). Despite their traditional use, this is the first antiplasmodial activity report of C. latifolia. This evidence is further supported by the studies that proved antiplasmodial activity of other species of the same genus such as Commelina benghalensis (Nloga et al., 2014; Orni et al., 2018).
3.3.2. Rane's test
All test doses utilized during treatment resulted in considerable parasitemia suppression (activities against established infection) (58.42%, 67.19%, and 81.97% reduction at a dose of 100, 200, and 400 mg/kg, respectively), demonstrating the hydroalcoholic crude extract of C. latifolia's curative potential. The percentage suppression analysis revealed that the hydroalcoholic crude extract treated groups (all three dosages) had a substantial parasitemia suppressive effect (P < 0.001) when compared to the negative control group, but were less effective (P < 0.001) when compared to the positive control group. Furthermore, a substantially distinct effect (P < 0.00 1) for all treatment groups) was observed in comparison to each other, demonstrating that the crude extract has a dose-dependent effect (Figure 1c). When compared to the vehicle-treated group, the results of mean survival time demonstrated that all three test doses considerably prolonged survival time in a dose-dependent way. The conventional medicine, on the other hand, was more efficacious (P < 0.001) than all of the test substance doses (Figure 1d). The hydroalcoholic crude extract of C. latifolia prevented body weight loss, rectal temperature reduction, and PCV reduction at all doses (Table 3).
Table 3.
Body weight, rectal temperature, and packed cell volume of P. berghei-infected mice treated with hydroalcoholic leaves extract of C. latifolia in the Rane's test.
| Group | Bodyweight |
Rectal temperature |
Packed cell volume |
||||||
|---|---|---|---|---|---|---|---|---|---|
| D3 | D7 | %change | D3 | D7 | %change | D3 | D7 | %change | |
| DW | 26.29 ± 0.50 | 22.56 ± 0.50 | −14.20 ± 0.64 | 36.88 ± 0.15 | 32.10 ± 0.24 | −12.96 ± 0.45 | 55.55 ± 0.52 | 48.40 ± 0.68 | −12.88 ± 0.56 |
| CQ | 25.88 ± 0.25 | 26.26 ± 0.24 | 1.47 ± 0.15a3 | 36.83 ± 0.12 | 36.66 ± 0.09 | −0.47 ± 0.12a3 | 54.73 ± 0.67 | 54.37 ± 0.61 | −0.66 ± 0.29a3 |
| CL100 | 26.81 ± 0.17 | 24.47 ± 0.20 | −8.73 ± 0.49a3b3e3 | 36.74 ± 0.12 | 33.12 ± 0.15 | −9.8524 ± 0.31a3b3d3e3 | 54.60 ± 0.64 | 49.20 ± 0.49 | −9.88 ± 0.23a3b3d3e3 |
| CL200 | 25.60 ± 0.50 | 23.94 ± 0.46 | −6.47 ± 0.66a3b3e1 | 36.42 ± 0.14 | 33.80 ± 0.16 | −7.19 ± 0.15a3b3e3 | 55.56 ± 0.91 | 51.70 ± 0.98 | −6.96 ± 0.45a3b3 |
| CL400 | 24.98 ± 0.45 | 24.10 ± 0.55 | −3.55 ± 0.77a3b3 | 36.68 ± 0.22 | 35.24 ± 0.27 | −3.93 ± 0.29a3b3 | 55.32 ± 0.89 | 52.40 ± 0.77 | −5.30 ± 0.48a3b3 |
Data are expressed as mean ± SEM; n = 6; a, compared to negative control; b, to CQ25 mg/kg; c, to 25 mg/kg; d, to 50 mg/kg; e, to 100 mg/kg; 1, P < 0.05; 2, P < 0.01; 3, P < 0.001; DW, distilled water; CQ, chloroquine; CL, crude extract of Commelina latifolia; D3, pre-treatment value on day 3; D7, post-treatment value on day 7.
The antiplasmodial activity of the C. latifolia in the curative test could be due to the antioxidant nature of the plant that neutralizes the oxidative damage provoked by the malaria parasite since the antioxidant property of various species of the genus Commelina have been reported in the literature (Orni et al., 2018; Rahman et al., 2021). The plant's curative potential (Figure 1c) was lower than its suppressive potential (Figure 1a), which is consistent with Maytenus gracilipes methanolic leaves extract (Nureye et al., 2021), Croton macrostachyus (Bantie et al., 2014), and Piper betle (Al-Adhroey et al., 2011). This could be attributed to the constituent(s) short duration of action in inhibiting and destroying exponentially developing parasites in an established infection (Basir et al., 2012).
3.3.3. Prophylactic test
Despite having a lower overall chemo-suppressive impact than chloroquine, all crude extract doses had a dose-dependent significant (P < 0.001) parasitemia suppression effect in the prophylactic test (69.05%, 59.83%, and 49.43% suppression at a dose of 100, 200, and 400 mg/kg, respectively) compared to the negative control (Figure 1e). Although the crude extract significantly reduced parasitemia, it had a less effect than the 4-day suppressive and Rane's tests. This finding is in line with studies performed on other plants (Oluwakanyinsola et al., 2010; Onwusonye and Uwakwe, 2014). Such activity might occur due to the rapid metabolism or elimination of the test drug as it was given earlier to the onset of infection (Lim et al., 2009). It could also be secondary to the in vivo method which does not include the insect vector, the mode of inoculation, and the amount (doses) given that result in quick RBCs infection without the Plasmodium going via the hepatic stages (Adzu et al., 2007). Another possible justification is that the test substances' might exert their effect by metabolic activation of the immune system, and thus the parasite will not be removed entirely (Waako et al., 2005). This could be ascribed to the immunomodulatory effects of the steroids, flavonoids, and saponins (Aherne et al., 2007; Saxena et al., 2013) detected in C. latifolia.
Survival times have changed as a result of the influence, which has been significant. Compared to the negative controls, all treatment groups increased survival time significantly, with the standard drug having the greatest effect (P < 0.001) than the extract-treated groups. The treatment groups exerted their effect in a dose-dependent manner; the observed effect at a dose of 400 mg/kg was more than 200 mg/kg and 100 mg/kg doses (Figure 1f). In comparison to the negative control, the three doses showed protection against body weight loss, a drop in rectal temperature, and PCV. In terms of preventing body weight loss, rectal temperature and PCV reduction, the activity of the positive control (CQ 25) was superior to that of the extract (Table 4).
Table 4.
Body weight, rectal temperature, and packed cell volume of P. berghei-infected mice treated with hydroalcoholic leaves extract of C. latifolia in the prophylactic test.
| GRP | Bodyweight |
Rectal temperature |
Packed cell volume |
||||||
|---|---|---|---|---|---|---|---|---|---|
| D0 | D4 | % Change | D0 | D4 | % Change | D0 | D4 | % Change | |
| DW | 25.74 ± 0.49 | 22.40 ± 0.66 | −13.00 ± 1.53 | 36.80 ± 0.25 | 31.58 ± 0.18 | −14.18 ± 0.28 | 57.11 ± 1.22 | 49.14 ± 1.64 | −14.05 ± 1.07 |
| CQ | 26.02 ± 0.61 | 26.50 ± 0.53 | 1.88 ± 0.43a3 | 36.84 ± 0.12 | 36.68 ± 0.11 | −0.4389 ± 0.13a3 | 58.74 ± 0.21 | 58.23 ± 0.14 | −0.8698 ± 0.15a3 |
| CL100 | 25.75 ± 0.49 | 23.11 ± 0.52 | −10.24 ± 1.02a3b3e3 | 36.98 ± 0.06 | 32.48 ± 0.22 | −12.17 ± 0.52a3b3e3d3 | 56.70 ± 0.81 | 49.98 ± 0.89 | −11.87 ± 0.44a1b3e3 |
| CL200 | 25.80 ± 0.57 | 23.80 ± 0.54 | −7.75 ± 0.67a3b3e3 | 36.69 ± 0.25 | 33.76 ± 0.37 | −7.99 ± 0.98a3b3e3 | 57.56 ± 0.63 | 52.82 ± 0.58 | −8.23 ± 0.32a3b3e2 |
| CL400 | 26.04 ± 0.54 | 24.90 ± 0.45 | −4.35 ± 0.47a3b3 | 36.82 ± 0.24 | 35.04 ± 0.16 | −4.83 ± 0.37a3b3 | 58.79 ± 0.35 | 55.64 ± 0.69 | −5.36 ± 1.12a3b3 |
Data are expressed as mean ± SEM; n = 6; a, compared to negative control; b, to CQ25 mg/kg; c, to 100 mg/kg; d, to 200 mg/kg; e, to 400 mg/kg; 1, P < 0.05; 2, P < 0.01; 3, P < 0.001; DW, distilled water; CQ, chloroquine; CL, crude extract of Commelina latifolia; D0, pre-treatment value on day 0; D4, post-treatment value on day 4.
3.4. Antimalarial activity of solvent fractions
3.4.1. Four-day suppressive test
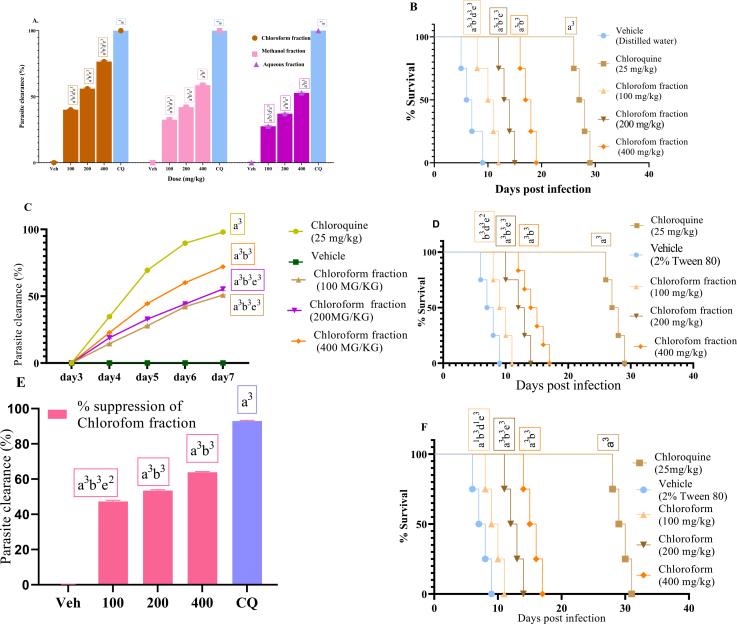
In a four-day suppressive test, the chloroform fraction demonstrated the most antimalarial activity, with a maximum chemo-suppression of 76.56% at a dose of 400 mg/kg, followed by the pure methanol (58.72%) and aqueous (52.86%) (Figure 2a). The higher activity in the chloroform fraction could be attributed to the presence of more phytochemicals in this test substances than in the other fractions (Table 1), or because the study plant's non-polar compounds are more active than its polar ones. This result agrees with the research on the solvent fractions of M. gracilipes and Vernonia amygdalina (Nureye et al., 2021; Bihonegn et al., 2016).
Figure 2.
% Parasitemia suppression of the solvent fraction in the 4-day suppressive test (A); survival days of each mouse during the evaluation of chloroform fraction in the early infection (B); % Parasitemia suppression of chloroform fraction in the established infection on day 3 to day 7 post-infection (C); survival days of each mouse during the evaluation of chloroform fraction in the Rane's test (D); % Parasitemia suppression of chloroform fraction in the prophylactic test (E); and survival days of each mouse during the evaluation of chloroform fraction in the prophylactic test (F); values are interpreted as mean ± SEM; n = 6.
The chloroform fraction outlasted the other solvent fractions in terms of average survival time (Figure 2b). It had fewer chemo-suppressive and mean survival effects than the hydroalcoholic crude extract. Infection-induced reductions in body weight, rectal temperature, and PCV were considerably (P < 0.001) inhibited by this fraction. Chloroquine, the positive control, produced a curative chemo-suppression and a significant stabilization of body weight, rectal temperature, and PCV (Table 5). The fraction's activity on the overall pathology of the disease and its strong parasite clearance is indicated by the better extension of survival days and avoidance of body weight loss and rectal temperature drop. The chloroform fraction is thought to have good antimalarial activity, with a suppression rate of more than 50% at a dose of 200 mg/kg (Deharo et al., 2001).
Table 5.
Body weight, rectal temperature, and packed cell volume of infected animals treated with solvent fractions C. latifolia in four-day suppressive test.
| Group | Weight |
Rectal temperature |
Packed cell volume |
||||||
|---|---|---|---|---|---|---|---|---|---|
| D0 | D4 | %Change | D0 | D4 | %Change | D0 | D4 | %Change | |
| DW | 26.29 ± 0.19 | 21.84 ± 0.32 | −16.90 ± 0.47 | 36.98 ± 0.05 | 32.10 ± 0.19 | −13.19 ± 0.59 | 55.08 ± 0.41 | 46.70 ± 0.66 | −15.24 ± 0.68 |
| CQ | 25.83 ± 0.51 | 25.95 ± 0.52 | 0.47 ± 0.11a3 | 36.68 ± 0.12 | 36.57 ± 0.13 | −.31 ± 0.04a3 | 54.67 ± 0.58 | 54.51 ± 0.57 | −.29 ± 0.13 |
| AF100 | 25.43 ± 0.42 | 23.12 ± 0.32 | −9.07 ± 0.28a3b3e3 | 36.88 ± 0.06 | 33.44 ± 0.13 | −9.33 ± 0.28a3b3d3e3 | 54.94 ± 0.61 | 48.92 ± 0.52 | −10.95 ± 0.55a3b3d3e3 |
| AF200 | 25.00 ± 0.67 | 23.40 ± 0.58 | −6.38 ± 0.19a3b3 | 36.88 ± 0.12 | 34.90 ± 0.16 | −5.37 ± 0.23a3b3e2 | 54.96 ± 0.84 | 50.70 ± 0.83 | −7.76 ± 0.25a3b3e3 |
| AF400 | 25.14 ± 0.88 | 24.26 ± 0.96 | −3.55 ± 0.56a3B2 | 37.02 ± 0.11 | 35.88 ± 0.08 | −3.08 ± 0.32a3b3 | 54.60 ± 0.53 | 52.50 ± 0.63 | −3.85 ± 0.46a3b3 |
| TW80 | 26.07 ± 0.45 | 22.20 ± 0.37 | −14.83 ± 0.48 | 36.98 ± 0.05 | 33.18 ± 0.86 | −10.28 ± 0.96 | 55.29 ± 0.66 | 48.40 ± 0.68 | −12.45 ± 0.69 |
| CQ | 25.91 ± 0.41 | 26.16 ± 0.39 | 0.97 ± 0.22a3 | 36.80 ± 0.11 | 36.60 ± 0.24 | −.53 ± 0.18a3 | 55.27 ± 0.29 | 55.01 ± 0.31 | −.47 ± 0.18a3 |
| MF100 | 25.07 ± 0.43 | 22.26 ± 0.51 | −11.24 ± 0.66a3b3d2e3 | 36.96 ± 0.67 | 33.56 ± 0.17 | −9.19 ± 0.48a2b3 | 56.44 ± 0.56 | 51.40 ± 0.96 | −8.96 ± 0.89a1b3e2 |
| MF200 | 26.14 ± 0.77 | 24.04 ± 0.62 | −7.98 ± 0.65a3b3be3 | 36.80 ± 0.14 | 34.80 ± 0.20 | −5.43 ± 0.41a1b1 | 56.78 ± 0.58 | 53.20 ± 0.66 | −6.31 ± 0.28a1b1e1 |
| MF400 | 25.52 ± 0.88 | 24.64 ± 0.94 | −3.49 ± 0.48a3b3 | 36.92 ± 0.71 | 35.74 ± 0.26 | −3.19 ± 0.65a2 | 54.90 ± 0.62 | 53.20 ± 0.60 | −3.08 ± 0.86a2 |
| TW80 | 26.08 ± 0.65 | 22.64 ± 0.54 | −13.17 ± 0.51 | 36.96 ± 0.13 | 32.54 ± 0.29 | −11.96 ± 0.63 | 55.13 ± 0.50 | 47.40 ± 0.51 | −14.00 ± 0.81 |
| CQ | 25.76 ± 0.52 | 26.15 ± 0.57 | 1.49 ± 0.39a3 | 36.77 ± 0.49 | 36.53 ± 0.73 | −0.65 ± 0.19a3 | 56.01 ± 0.74 | 55.56 ± 0.31 | −0.80 ± 0.33a3 |
| CF100 | 26.11 ± 0.47 | 23.55 ± 0.28 | −9.75 ± 0.95a1b3d1e3 | 36.66 ± 0.22 | 33.14 ± 0.22 | −9.59 ± 0.69a1b3d1e3 | 55.64 ± 0.48 | 49.52 ± 0.50 | −11.00 ± 0.19a1b3d2e3 |
| CF200 | 25.80 ± 0.49 | 23.88 ± 0.54 | −7.46 ± 0.32a3b3fe2 | 36.84 ± 0.12 | 34.20 ± 0.19 | −7.17 ± 0.30a3b3e2 | 55.76 ± 0.59 | 51.49 ± 0.84 | −7.67 ± 0.66a3b3 |
| CF400 | 24.28 ± 0.47 | 23.28 ± 0.46 | −4.12 ± 0.37a3b3 | 36.98 ± 0.08 | 35.54 ± 0.23 | −3.89 ± 0.41a3b3 | 54.54 ± 0.77 | 51.68 ± 0.98 | −5.27 ± 0.63a3b3 |
Data are expressed as mean ± SEM; n = 5; compared to a, negative control; b, CQ25 mg/kg; c, 100 mg/kg; d, 200 mg/kg; e, 400 mg/kg; 1, P < 0.05; 2, P < 0.01; 3, P < 0.001; TW80, 2% Tween80; CQ, chloroquine; AF, aqueous fraction; MF, methanol fraction; CF chloroform fraction; D0, pre-treatment value on day 0; D4, post-treatment value on day 4.
3.4.2. Rane's test
The effect of the most active fraction (chloroform fraction) in the four-day suppression test on established parasite infection was investigated further. In the Rane's test, the chloroform fraction showed a dose-dependent decrease (P 0.001) of parasitemia compared to the vehicle-treated group (Figure 2C). The chloroform fraction achieved its maximum inhibition (72.05%) at a dose of 400 mg/kg. The standard treatment, on the other hand, eradicated the parasite to undetectable levels on day 7, and the reduction in parasitemia was much larger (P 0.001) than the negative control and all fraction doses. The extract considerably extended survival days at all doses tested, according to the mean survival time (Figure 2D). Nonetheless, the conventional medicine chloroquine was found to have a much higher curative effectiveness (P 0.001) than the portion. With Rane's model, chloroform fraction suppressed body weight loss, rectal temperature, and PCV drop at all dose levels examined. The positive control, on the other hand, exhibited higher activity (Table 6).
Table 6.
Body weight, rectal temperature, and packed cell volume of infected animals treated with chloroform fraction of C. latifolia in the Rene's test.
| Group | Bodyweight |
Rectal temperature |
Packed cell volume |
||||||
|---|---|---|---|---|---|---|---|---|---|
| D3 | D7 | %Change | D3 | D7 | %Change | D3 | D7 | %CHANGE | |
| TW80 | 24.58 ± 0.46 | 20.82 ± 0.36 | −15.27 ± 0.82 | 36.50 ± 0.25 | 31.76 ± 0.11 | −12.98 ± 0.31 | 56.25 ± 0.81 | 48.78 ± 0.90 | −13.29 ± 0.51 |
| CQ | 25.76 ± 0.57 | 26.11 ± 0.61 | 1.33 ± 0.26a3 | 36.62 ± 0.20 | 36.28 ± 0.26 | −.93 ± 0.26a3 | 55.36 ± 0.89 | 54.60 ± 0.92 | −1.37 ± 0.49a3 |
| CF100 | 26.13 ± 0.47 | 23.68 ± 0.41 | −9.37 ± 0.35a3b3e3 | 36.70 ± 0.24 | 33.08 ± 0.18 | −9.85 ± 0.49a3b3d2e3 | 54.58 ± 0.81 | 49.20 ± 0.73 | −9.84 ± 0.83a2b3d1e3 |
| CF200 | 26.20 ± 0.58 | 24.30 ± 0.59 | −7.27 ± 0.52a3b3e3 | 36.82 ± 0.19 | 33.98 ± 0.27 | −7.72 ± 0.36a3b3e3 | 53.72 ± 1.14 | 49.92 ± 1.13 | −7.08 ± 0.49a3b3e2 |
| CF400 | 25.37 ± 0.77 | 24.60 ± 0.75 | −3.02 ± 0.32a3b3 | 36.70 ± 0.13 | 35.40 ± 0.16 | −3.54 ± 0.29a3b3 | 57.48 ± 0.37 | 55.22 ± 0.41 | −3.93 ± 0.57a3b1 |
Data are expressed as mean ± SEM; n = 6; a, compared to negative control; b, to CQ25 mg/kg; c, to 25 mg/kg; d, to 50 mg/kg; e, to 100 mg/kg; 1, P < 0.05; 2, P < 0.01; 3, P < 0.001; TW80, 2% Tween80; CQ, chloroquine; CF, chloroform fraction; D3, pre-treatment value on day 3; D7, post-treatment value on day 7.
3.4.3. Prophylactic test
In Peter's repository test, the chloroform fraction had a significant effect (P < 0.001) in parasitemia suppression (47.26%53.46%, and 63.88% % suppression at a dose of 100, 200, and 400 mg/kg, respectively) when compared to the placebo agent (Figure 2e). Although less effective than the usual medicine chloroquine, the fraction increased survival time (Figure 2f). The fraction significantly attenuated body weight loss, rectal temperature drop, and PCV reduction. Furthermore, when compared to all extract-treated groups, the chloroquine-treated group had a substantial effect (Table 7). In comparison to the negative control group, substantial effects were detected at 400 mg/kg (P < 0.001) and 200 mg/kg (P < 0.001) test doses in relation to body weight. Compared to the negative control group, all three dosages of chloroform fraction significantly reduced rectal temperature and PCV due to parasite infection (P < 0.001). Similarly, the standard medication had a stronger (P < 0.001) effect on preventing rectal temperature and PCV drop than the fraction-treated groups (Table 7).
Table 7.
Bodyweight, rectal temperature, and packed cell volume of infected animals treated with chloroform fraction of C. latifolia in the prophylactic test.
| Group | Bodyweight |
Rectal temperature |
Packed cell volume |
||||||
|---|---|---|---|---|---|---|---|---|---|
| D0 | D4 | %Change | D0 | D4 | %Change | D0 | D4 | %Change | |
| TW80 | 27.46 ± 0.29 | 23.28 ± 0.36 | −15.24 ± 0.59 | 36.44 ± 0.27 | 31.38 ± 0.17 | −13.87 ± 0.52 | 57.11 ± 0.79 | 49.84 ± 1.16 | −12.77 ± 0.91 |
| CQ | 26.42 ± 0.49 | 26.70 ± 0.49 | 1.06 ± 0.14a3 | 36.78 ± 0.23 | 36.54 ± 0.23a3 | −0.65 ± 0.23a3 | 57.69 ± 0.43 | 56.96 ± 0.45 | −1.26 ± 0.30a3 |
| CF100 | 26.80 ± 0.51 | 24.00 ± 0.45 | −10.43 ± 0.89a3b3d1e3 | 36.33 ± 0.23 | 32.08 ± 0.33 | −11.69 ± 0.82b3d2e3 | 56.49 ± 0.58 | 50.12 ± 0.50 | −11.27 ± 0.4b3d1e3 |
| CF200 | 25.66 ± 0.50 | 24.00 ± 0.32 | −6.41 ± 1.03a3b3 | 36.56 ± 0.12 | 33.66 ± 0.39 | −7.93 ± 1.08a3b3 | 56.10 ± 0.69 | 51.40 ± 0.48 | −8.36 ± 0.34a3b3e1 |
| CF400 | 26.86 ± 0.59 | 25.54 ± 0.66 | −4.94 ± 0.44a3b3 | 36.48 ± 0.45 | 34.50 ± 0.39 | −5.42 ± 0.28a3b3 | 57.03 ± 0.78 | 53.82 ± 0.66 | −5.59 ± 0.94a3b2 |
Data are expressed as mean ± SEM; n = 6; a, compared to negative control; b, to CQ25 mg/kg; c, to 25 mg/kg; d, to 50 mg/kg; e, to 100 mg/kg; 1, p < 0.05; 2, p < 0.01; 3, p < 0.001; TW80, 2% Tween80; CQ, chloroquine; CF, chloroform fraction; D0, pre-treatment value on day 0; D4, post-treatment value on day 4.
Generally, the cardinal sign and symptom of mice infected with malaria is a fall in blood glucose level, as well as a decrease in body weight, rectal temperature, and PCV (Langhorne et al., 2002). The plant materials with antiplasmodial action are expected to attenuate the drop in body mass, body temperature, and hematocrit caused by the Plasmodium species due to an increase in parasite density. In the current study, the protection of body weight loss by all doses of crude extract and chloroform fraction of C. latifolia in all models, and absolute methanol and aqueous fractions in the 4-day suppressive test were statistically plausible as compared to the respective placebo treatment. This effect might be more attributed to the study plant's nutritional value than other factors (Maroyi, 2020). A study done on different plant species of the same genus (Commelina diffusa) displayed the presence of carbohydrates, vitamin C, vitamin B3, vitamin B2, Na, Ca, and Mg in this genus (Rahman et al., 2021). Thus, vitamins and minerals that might be present in C. latifolia leaves could contribute to body weight increment in extract-treated mice by improving appetite (food intake capacity). The result might have also been attributed to the improvement in PCV, body temperature, and parasite clearance in extract-treated animals as revealed in the result tables.
In the present study, the prevention of decline in body temperature of infected mice by all doses of hydroalcoholic extract and chloroform fraction of C. latifolia in all models, and pure methanol and water fractions in the 4-day suppressive model were statistically significant as compared to the respective placebo-treated groups. This bioactivity perhaps designates the plant's capacity to improve some pathology caused by malaria that brings a fall in rectal temperature (Mengiste et al., 2012). Once again, the analgesic and anti-inflammatory activities (involved in the regulation of body temperature) demonstrated by other species of Commelina (C. benghalensis and C. diffusa) could also be exhibited by the leaf extract of C. latifolia (Hossain et al., 2014; Rahman et al., 2021).
Similarly, all test substances significantly prohibited PCV reduction as compared to the respective negative control agents in all tests. This outcome might result from the noteworthy parasite containment brought by the active component(s) present in the extract because the mount in blood parameters is generally related to the decline in parasitemia load (Mequanint, 2014). On the other hand, the detected antioxidant metabolites (flavonoids, saponins, phenols, and tannins) and other undetected metabolites of the plant could also be in charge of the prevention of anemia because these phytodrugs might have the ability to stabilize the erythrocyte membrane by stopping membrane phospholipid oxidation through counteracting the hemolytic effect of saponins (Mpiana et al., 2010; Yang et al., 2005).
Commelina species are known for their anti-inflammatory activities (Hossain et al., 2014; Rahman et al., 2021), which might significantly contribute to the antiplasmodial activity of the study plant. Even if the active compounds have yet to be isolated, the antimalarial action of C. latifolia could be ascribed to one or more of its phytoconstituents. As suggested in various investigations, the antiparasitic effect of the study plant against Plasmodium berghei might have been exerted by its secondary metabolites (terpenoids, tannins, steroids, saponins, flavonoids, and alkaloids) (Arise et al., 2012; Soh et al., 2012). In the process of eliciting antiplasmodial property, saponins and flavonoids may disrupt the biological membranes of the parasite and interrupt the integrity of cellular components. Terpenoids and flavonoids may inhibit the process of protein synthesis made by the Plasmodium while alkaloids and terpenoids may induce a cytotoxic effect through intercalating with the parasite DNA and stop cell division. The phytometabolites like steroids may suppress the growth and multiplication of test parasites by hindering the entry of essential nutrients into the cell (Fenta and Kahaliw, 2019; Misganaw et al., 2020; Alafid et al., 2019).
4. Conclusion and recommendation
The results of the present study confirmed that C. latifolia is with relative lack of toxicity and good antimalarial activity. The solvent fractions also resulted in antimalarial activity with various degrees of chemosuppression with the chloroform fraction being relatively more effective. Our results confirm the traditional claim of the plant in treating malaria by traditional users in Ethiopia. In the future, sub-acute and chronic toxicity tests should be conducted to check its long-term safety profile. It is also essential to identify and isolate active constituents that explain the possessed antimalarial activity in the leaves of C. latifolia.
4.1. Limitation of the study
Due to financial limitations, isolation and characterization of major components of the most active fractionate responsible for the antiplasmodial activity of the plant was not carried out, which could be used as a lead compound/s in the discovery and development of effective drugs for the treatment of malaria.
Declarations
Author contribution statement
Getnet Bizuayehu Tadege: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data, Wrote the paper.
Semere Welday Kahssay: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Nebeyi Fisseha: Contributed reagents, materials, analysis tools or data.
Dehnnet Abebe: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dejen Nureye: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Dejen Nureye was supported by Mizan-Tepi University (MTU-2021-37).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Mizan-Tepi University for its funding.
References
- Abebe D., Garedew B. Ethnobotanical survey of plants traditionally used for malaria prevention and treatment in indigenous villages of Tepi Town South West Ethiopia. J. Pharmacogn. Phytochem. 2019;11:9–16. [Google Scholar]
- Adzu B., Haruna A.K., Salawu O.A., Katsayal U.D., Njan A. In vivo antiplasmodial activity of ZS-2A: a fraction from chloroform extract of Zizyphus spina-christi root bark against Plasmodium berghei berghei in mice. Int. J. Biol. Chem. Sci. 2007;1(3):281–286. [Google Scholar]
- Aherne S.A., Daly T., O’Connor T., O’Brien N.M. Immunomodulatory effects of β-sitosterol on human Jurkat T cells. Planta Med. 2007;73(9):797–1034. [Google Scholar]
- Al-Adhroey A., Nor Z., Al-Mekhlafi H., et al. Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules. 2011;16:107–118. doi: 10.3390/molecules16010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafid F., Edrah S.M., Meelad F.M., Belhaj S., Altwair K., Maizah N.R. Evaluation of phytochemical constituents and antibacterial activity of Thymelaea hirsuta Endl, and that utilised as a conventional treatment of infertility and diabetic in Libya. World J. Pharmaceut. Res. 2019;8(11):72–88. [Google Scholar]
- Arise R., Malomo S., Lawal M. Comparative antimalarial and toxicological effects of artemisinin with methanolic extract ofcarica papaya leaves and bark of alstonia broonaiin animal models. Adv. Nat. Appl. Sci. 2012;6(2):116–123. [Google Scholar]
- Asnake S., Teklehaymanot T., Hymete Evaluation of the antiplasmodial properties of selected plants in Southern Ethiopia. BMC Compl. Alternative Med. 2015;15(1):448. doi: 10.1186/s12906-015-0976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantie L., Assefa S., Engdawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Compl. Alternative Med. 2014;14:79. doi: 10.1186/1472-6882-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basir R., Rahiman S.F., Hasballah K., Chong W.C., Talib H., Yam M. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran. J. Parasitol. 2012;7:62. [PMC free article] [PubMed] [Google Scholar]
- Bihonegn T., Giday M., Yimer G., Animut A., Sisay M. Antimalarial activity of hydromethanolic extract and its solvent fractions of Vernonia amygdalina leaves in mice infected with Plasmodium berghei. SAGE Open Med. 2016;7:1–10. doi: 10.1177/2050312119849766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debella A. Manual for Phytochemical Screening of Medicinal Plants, 38–57. Department of Drug Research, Ethiopia Health and Nutrition Research Institute; Addis Ababa, Ethiopia: 2002. Preliminary screening techniques of secondary metabolites. [Google Scholar]
- Deharo E., Bourdy G., Quenevo C., Munoz V., Ruiz G., Sauvain M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tacana Indians. J. Ethnopharmacol. 2001;77:91–98. doi: 10.1016/s0378-8741(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Fenta M., Kahaliw W. Evaluation of antimalarial activity of hydromethanolic crude extract and solvent fractions of the leaves of Nuxia congesta R. Br. Ex fresen (buddlejaceae) in Plasmodium berghei infected mice. J. Exp. Pharmacol. 2019;(11):121–134. doi: 10.2147/JEP.S230636. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentahun S., Makonnen E., Awas T., Giday M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Compl. Alternative Med. 2017;17:13. doi: 10.1186/s12906-016-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock D.A., Rosenthal P.J., Croft S.L., Brun R., Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug. 2004;3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Dutta A., Biswas M., Biswas S., Hazra L., Nag S.K., Sil S.Y., Chatterjee S. Phytomorphological, chemical and pharmacological discussions about Commelina benghalensis Linn. (Commelinaceae): a review. J. Pharm. Innov. 2019;8:12–18. [Google Scholar]
- Hardy C.R., Sloat L.L., Faden R.B. Floral organogenesis and the developmental basis for pollinator deception in the Asiatic dayflower, Commelina communis (Commelinaceae) Am. J. Bot. 2009;96:1236–1244. doi: 10.3732/ajb.0800344. [DOI] [PubMed] [Google Scholar]
- Hawaria D., Getachew H., Zhong G., Demissew A., Habitamu K., Raya B., Lee M.C., Yewhalaw D., Yan G. Ten years malaria trend at Arjo-Didessa sugar development site and its vicinity, Southwest Ethiopia: a retrospective study. Malar. J. 2019;18:1–11. doi: 10.1186/s12936-019-2777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilou A., Nacoulma O.G., Guiguemde T.R. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J. Ethnopharmacol. 2006;103:236–240. doi: 10.1016/j.jep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hossain F., Saha S., Slam M., Nasrin S., Adhikari S. Analgesic and anti-infammatory activity of Commelina benghalensis linn. Turk. J. Pharm. Sci. 2014;11(1):25–32. [Google Scholar]
- Langhorne J., Quin S., Sanni L. Malaria Immunology. second ed. Karger publisher; 2002. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology; pp. 204–228. [DOI] [PubMed] [Google Scholar]
- Lim H.-S., Im J.-S., Cho J.-Y. Pharmacokinetics of hydroxychlor-oquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob. Agents Chemother. 2009;53(4):1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroyi A.A. Gardenia ternifolia Schum. & Thonn. (Rubiaceae): review of medicinal uses, phytochemistry and biological activities. Pharm. Nutr. Sci. 2020;11(4):5876–5885. [Google Scholar]
- Mengiste B., Meyasu M., Kelbessa U. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against P. berghei in mice model. MEJS. 2012;4(1):47–63. [Google Scholar]
- Mequanint G. Antimalarial activity of methanolic extract of Phytolacca dodecandra leaves against Plasmodium berghei infected Swiss albino mice. Int. J. Pharm. Clin. 2014;3(3):39–45. [Google Scholar]
- Mesfin A., Giday M., Animut A., Teklehaymanot T. Ethnobotanical study of antimalarial plants in Shinile District, Somali Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J. Ethnopharmacol. 2012;139:221–227. doi: 10.1016/j.jep.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Misganaw D., Amare G., Mengistu G. Chemo suppressive and curative potential of Hypoestes forskalei against plasmodium berghei: evidence for in vivo antimalarial activity. J. Exp. Pharmacol. 2020;12:313–323. doi: 10.2147/JEP.S262026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpiana P.T., Ngbolua K.N., Bokota M.T., et al. In vitro effects of anthocyanins extract from Justicia secunda VAHL on the solubility of hemoglobin S and membrane stability of sickle erythrocytes. Blood Transfusion. 2010;8:248–254. doi: 10.2450/2009.0120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardos A., Makonnen E. In vivo antiplasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota. Malar. J. 2017;l:1–8. doi: 10.1186/s12936-017-1677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Guidelines for Care and Use of Laboratory Animals . Department of Health and Human Services; USA, Md, Bethesda: 1996. National Institutes of Health, Office of Science and Health Reports, Guide for Care and Use of Laboratory Animals 83-23. [Google Scholar]
- Njoku V.O., Obi C. Phytochemical constituents of some selected medicinal plants. AJPAC. 2009;3(11):228–233. [Google Scholar]
- Nlôga N., Yebga N., Bum N. Antiplasmodial effects of Commelina benghalensis/Steganotaenia araliacea plants extract on the human population in Ngaoundre (Cameroon) J. Med. Sci. 2014;14(2):68–74. [Google Scholar]
- Nureye D., Kedir M.S., Muluye R.A., Hammeso W.W., Tekalign E. In vivo antiplasmodial activity of hydromethanolic leaf extract and solvent fractions of Maytenus gracilipes (Celastraceae) against Plasmodium berghei in mice. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e08457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD . The Organization of Economic Co-operation and Development, France; Paris: 2010. Guidelines for Testing of Chemicals. Guideline 425: Acute Oral Toxicity. [Google Scholar]
- Oluwakanyinsola S., Adeniyi T., Babayi H. Antimalarial activity of ethanolic stem bark extract of Faidherbia Albida (Del) a. Chev (Mimosoidae) in mice. Arch. Appl. Sci. Res. 2010;2(5):261–268. [Google Scholar]
- Onwusonye J., Uwakwe A. The antiplasmodial activity of methanol root bark extract of Alstonia boonei against Plasmodium berghei infectionin mice. Indian J. Sci. Res. 2014;3(7):199–201. [Google Scholar]
- Orni P.R., Shetu H.J., Khan T., Rashed S.S.B., Dash P.R. A comprehensive review on Commelina benghalensis L. (Commelinaceae) Int. J. Pharmacogn. 2018;5(10):637–645. [Google Scholar]
- Peters W. Drug resistance in Plasmodium berghei. I. Chloroquine resistance. Exp. Parasitol. 1965;17:80–89. doi: 10.1016/0014-4894(65)90012-3. [DOI] [PubMed] [Google Scholar]
- Rahman M., Mannan A., Nijhu R.S., Khatun A. Traditional uses, phytochemistry and pharmacology of Commelina diffusa Burm: an updated systematic review. J. Pharmacogn. Phytochem. 2021;10(4):53–59. [Google Scholar]
- Ryley J.F., Peters W. The antimalarial activity of some quinolone esters. Ann. Trop. Med. Parasitol. 1970;64:209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- Saxena M., Saxena J., Nema R. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013;1(6):168–182. [Google Scholar]
- Slack R.D., Mott B.T., Woodard L.E., Tripathi A., Sullivan D., Nenortas E. Malaria-infected mice are completely cured by one 6 mg/kg oral dose of a new monomeric trioxane sulfide combined with mefloquine. J. Med. Chem. 2012;55:291–296. doi: 10.1021/jm201214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh P., Witkowski B., Gales A. Implication of glutathione in the in vitro antiplasmodial mechanism of action of Ellagic Acid. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadege G., Alebachew Y., Hymete A., Tadesse S. Identification of lobetyolin as a major antimalarial constituent of the roots of Lobelia giberroa Hemsl. Int. J. Parasitol-Drug. 2022;18:43–51. doi: 10.1016/j.ijpddr.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffese H.S., Hemming-Schroeder E., Koepfli C., Tesfaye G., Lee M.C., Kazura J., et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect. Dis. Poverty. 2018;7(6):1–9. doi: 10.1186/s40249-018-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore M., Guiguemde A., Yago I. Investigation of antiplasmodial compounds from two plants, Cochlospermum tinctorium A. Rich and Gardenia sokotensis hutch. Afr. J. Trad. CAM. 2006;3(4):34–41. [Google Scholar]
- Waako P.J., Gumede B., Smith P., Folb P.I. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. Et Thonn. J. Ethnopharmacol. 2005;99(1):137–143. doi: 10.1016/j.jep.2005.02.017. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), World Health Organization; Geneva: 2021. World Malaria Report 2021. [Google Scholar]
- Yang Z.-G., Sun H.-X., Fang W.-H. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (AMS) on the immune responses to ovalbumin in mice. Vaccine. 2005;23(44):5196–5203. doi: 10.1016/j.vaccine.2005.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.