Abstract
The performance of gasification for Injera baking was explored in this study, as well as the effects of moisture content, and primary and secondary airflow rates. Primary air is used in the reactor of a biomass gasifier, which creates syngas that is burned by secondary air on the mitad's bottom side. An average temperature of averaged 185 °C at the center and 170 °C away from the center was observed; the size of the cone determines the temperature distribution on the metal surface. The reactor's narrower cone diameter allowed for a greater temperature only in the center and a more variable baked Injera eye appearance. The cone diameter has been reduced to 0.15 m of the mitad diameter to improve the temperature distribution on the mitad surface. The gasifier temperature is 800 °C when the air/fuel ratio is 5.8 kg/kg and the moisture content of the wood is 16%. Gasification is improved by heating the primary air and changing the air-fuel ratio. The findings revealed that pre-heated air is more efficient for gasification and saves money on baking and fuel. Fuel efficiency (0.45) and time savings (0.12) were discovered in the new gasifier. Between gasification temperatures of 650 and 800 °C, an effective Injera baking temperature (170–185 °C) on the mitad surface was attained. Following the tests, the average specific wood fuel consumption (1.414 g/kg), char residue (317 g), and average Injera baking time were calculated. For each test of one baking cycle, this was found at the burning rate capability of both stoves, which is 6 kg/hr. Therefore, the fuel consumption and burning rate of fuel are depending on the amount of airflow rate.
Keywords: Biomass gasifier, Airflow rate, Cone diameter effect, Effect of air-fuel ratio on gasification
Biomass gasifier; Airflow rate; Cone diameter effect; Effect of air-fuel ratio on gasification
1. Introduction
Due to the scarcity of natural resources, the globe is facing challenges with energy and global warming [1], [2]. The so-called energy crisis, which is characterized by the exhaustion of the region's energy resources, has an impact on developing nations [3]. Energy sources that can supply human energy needs are increasingly in demand [4]. The possibility of producing fuel from waste and biomass because they are renewable energy sources is even more alluring. [2]. One of the most significant renewable energy sources is biomass, and gasification offers a viable method for producing clean, low-emission synthetic fuels [5].
The design of gasification stoves ensures that emissions are kept to a minimum, that renewable energy sources are used, that heat is utilized, and that trash is recycled [5]. It is one of the most used methods for converting biomass into energy [6]. In recent years, there has been a lot of interest in the conversion of biomass into energy, fuel, and products due to worries about the damaging effects of greenhouse gas emissions and national security. Biomass is transformed during gasification at high temperatures (650–1200 °C) and with little oxygen. Carbon monoxide (CO) and hydrogen (H2), two species in the producer gas that are crucial for fuel and chemical synthesis in the gasification process, release polluting pollutants [7]. It is necessary to implement a biomass-to-energy conversion gasification system that is environmentally favorable [8].
As several studies have been conducted and revealed, the household is the major energy consumer sector in developing countries [9]. Woody biomass accounts for 87% of the entire annual biomass energy use out of the 90% consumed by households [10]. This biomass solid fuel is used for cooking by people using conventional techniques. Using an open fire to cook releases harmful particles [11] that course several health problems and also deteriorate the rural household economy as well as the local and global environment. As a result of renewed interest in alternative energy sources, climate change and indoor air pollution are attempting to raise public concern about gasification [12]. One of the most significant renewable energy sources is biomass, and gasification offers a viable method for producing clean, low-emission synthetic fuels [13]. These gases' (syngas') exploitable energy content is dependent on gasification temperature. Temperature is influenced by a variety of variables, including moisture content, the air/fuel ratio, and the amount of main and secondary air supplied by gasifiers. With the help of secondary air and sensible heat created during syngas combustion, the novel gasifier design effectively burns syngas for injera baking. The injera was typically baked on a special electric stove or a clay plate called a Mitad that is placed over a three-stone stove. When a fermented dough is thrown onto a heated clay skillet and left there until the temperature of boiling water is achieved, bubbles from the boiling water escape, creating thousands of small craters (eyes) that produce the distinctive texture of injera. The traditional Mitad consists of a griddle plate of “black” clay set on a base of stone and clay [1], [2], [3], [4], [6]. A more effective baking technique is employed to enhance wellbeing and general health [14].
The performance of the gasifier is improved by developing efficient sizes of gasifier components and characteristics like the biomass feed door, air flow rate, moisture content of wood fuel, and insulation. Biomasses can be converted into syngas through thermochemical processes like pyrolysis, gasification, and combustion. In the process, components for gas, vapor, and tar are also created [15]. Gasification was the most effective process for the production of syngas [16] and was accomplished in the existence of a gasifying agent (for example air, pure oxygen, steam, or mixtures of these components) at higher temperatures between 500 and 1400 °C. It was appropriate for biomasses with moisture contents under 35%. The direct use of biomass feedstock with moisture levels in the range of 25–60% in the gasifier will result in significant energy losses throughout the entire process. Before they are fed into the gasifier, it was recommended that they be preheated or dried with a moisture content between 10% and 20%. [17]. To obtain a suitable and efficient gas product for industrial and power generation applications, operational parameters such as biomass characteristics, gasification agent flow rate, gasification agent type, the temperature of the feed air, reaction temperatures, and gasifier design in gasification processes should be optimized [18]. The overall effect is the reduction in the calorific value of syngas because the small increase in H2 is not sufficient to compensate for the loss of a significant amount of CO with the increase in moisture content [19]. This affects specific heat capacity and heating values [20].
In Ethiopia, injera is a staple baked meal that needs 180 °C -220 °C [21], [22], [23], [24], [25]. A flat baking pan made of clay soil known as a mitad is used to prepare injera. The Mirt stove was developed by the Ethiopian Energy Studies Research Center in the early 1990s to enhance baking [26]. Due to its up to 40% fuel efficiency and 0.23% overall time savings, mirt stove is being actively advertised and distributed in the nation. According to the findings of the field tests [27], [28], utilizing an enhanced Mirt burner to bake injera can save an average household 570 kilograms of fuel wood annually. The stove is capable of achieving an efficiency of 33% and has an anticipated lifespan of about 4-5 years.
2. Materials and methods
2.1. Design of the gasification system and the experimental setup
The airflow representation, modeling, and schematic diagram of the gasifier stove are displayed in Fig. 1(a–c).
Figure 1.
(a) Airflow representation of gasifier stove (b) the modeling of gasifier stove (c) Detailed diagram of the gasifier.
Wood can be burned in its 400 mm-diameter by 150 mm-depth, which features a grate at the bottom. A typical user's height and injera baking mitad diameter were used to estimate the design's dimensions. The syngas produced during the process was burnt with secondary air on the bottom side of the mitad. The gasifier employs air for gasification that is supplied from the bottom region. Baking injera at home requires an average power output of 3–4 kW, or roughly 10-15 g of biomass each minute. The amount of injera baked using the aforementioned power ranges from 30 to 45 kg. Gasification's power consumption falls between those that are determined by the amount of biomass fuel used and the airflow rate [18], [25]. The gases rise through the hot char bed that has been left in the gas holder beneath the mitad as the air that goes from the bottom through the bed aids in the fiery combustion of the biomass. This combustible gas is burned in the gas holder with secondary air to guarantee full combustion with minimal emissions and the greatest amount of heat transfer to the mitad. A calibrated grate at the bottom regulates the airflow rates. 400 g of wood fragments with a size range of 10 to 15 mm were placed within the reactor. Then primary air was supplied at a predetermined flow rate. To regulate the temperature in gasification, the same experiments were carried out with half of the primary air input holes open and the secondary air holes blocked.
Ash was used as an insulation material to increase heat flow throughout the system and decrease heat losses. Utilizing supplementary air that is provided through a tube, syngas that has been produced in the gap on the bottom side of the mitad surface is burned. This speeds up the burning of the syngas in the support area and raises the temperature of the cooking mitad. In comparison to earlier models, the new gasifier stove differed in terms of its heat source, waste product, utilized airflow, and fuel consumption. A by-product of the open-air fuel burning in the ancient stove was ash. It was impossible to prevent the exhaust gas heat loss; therefore, emissions were just dumped into the atmosphere. On the other hand, the new gasifier's byproduct was char. Char, a byproduct of the new gasifier, held more heat and extended the time that the mitad surface was exposed. When heated secondary air entered the syngas holder from different angles, the flame on the bottom surface of the mitad was disrupted in various directions. To provide the side section of the mitad, we horizontally bent the flame. The air heated up as it moved through the pipe. Warm air was forced into the gas container by ambient air that had cooled, burning syngas there. Fig. 2 shows how the temperature rose while the airflow rate was constant throughout time.
Figure 2.

Relationship diagram between air flow, temperature, and time during gasification.
Through the cone, the flue gas was released from the reactor and burned in a mitad support. Using flow meters, it was estimated that the grate's primary air flow rate and the pipe's secondary air flow rate.
The amount of syngas and char produced in the gasification system was constrained by the moisture content and heating value of wood. Depending on the type of biomass, the size of the reactor, and the amount of biomass fuel loading, different Injera baking temperatures were observed on the surface. To ascertain the difference in temperature before and after the dough was baked on the surface, experimental testing was carried out.
2.2. Gasification process
To ensure that all of the moisture in the wood had been removed before gasification, the first sample of wood was heated to 105 °C in the presence of oxidizing air and left there for 15 minutes [19].
Atmospheric air lost weight as the temperature was raised to 800 °C at a speed of 35 k/min. The rate of heat transfers through the biomass in the reactor and the airflow rate affected how quickly the biomass degraded or decomposed [20]. The massive biomass molecules break down into lighter molecules at high gasification temperatures, finally forming stable gases (CO, H2, CH4, and lighter hydrocarbons), ash, char, tar, and minor pollutants. Char and tar are the byproducts of insufficient biomass conversion [21]. The heated air in the pipe is converted to steam as it travels through, accelerating the biomass gasification reaction. Equation (1) can be used to represent the overall reaction in the air/steam gasification, which involves several reactions and routes. Equations (2)–(8) are common reactions involved during gasification [4],[22].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
2.3. Wood fuel properties used in gasification
Based on data from the ultimate examination of the literature, the empirical formula of Wood was calculated, as shown in Table 1. By dividing each mass percentage by the atomic weight of the elements, the molar composition may be calculated to create the stoichiometric formula for complete wood fuel gasification, which is used to estimate ER. The composition can then be standardized concerning carbon value [34]. Table 2 shows the normalization of wood constituents based on carbon.
Table 1.
Ultimate analysis and properties of Eucalyptus wood fuel used in gasification.
Table 2.
Normalization of wood constituent concerning carbon [35].
| Element | Wt % | Linearized with C | |
|---|---|---|---|
| C | 48.64 | 48.64/12 = 4.05 | 4.05/4.05 = 1 |
| H | 6.15 | 6.156.15/1 = 6.15 | 6.15/ 4.05 = 1.5 |
| O | 43.49 | 43.49/16 = 2.72 | 2.72/ 4.05 = 0.67 |
| S | 0.00 | 0.00/14 = 0.00 | 0.00/4.05 = 0.00 |
| N | 0.25 | 0.25/32 = 0.0078 | 0.0078/ 4.05 = 0.002 |
The general empirical formula of wood is thus [36]. As can be seen in equation (9), the stoichiometric relation for the complete oxidation of a hydrocarbon fuel is given by:
| (9) |
From the stoichiometric equation for complete combustion of wood fuel
The stoichiometric relation for the complete oxidation of wood fuel becomes
To burn 1 kg of wood fuel, 5.8 kg of air is required for complete combustion. For 6 kg of wood fuel, 34.8 kg/kg of air is required. To gasify wood fuel, 5.8 kg of air will be reduced below one (5.8 ÷ (5.8×6)) = 0.2) by controlling the cover. The air-fuel ratio of biomass for complete combustion is 1. However, the theoretical air (5.8 kg) is limited to 0.2 by controlling the air inlet hole on the grate. The air-fuel ratio can be reduced to (5.8 ÷ (5.8×6) = 0.2). The corresponding emission reduction (ER) of the study is 0.27 which is in the range of commonly used air flow rate (0.3 – 0.4) for gasification [34].
Manually operated sliding grate cover controls primary air flows by limiting the holes on a grate integrated at the tip of the reactor end. Blocking some holes and allowing air to flow through the remaining holes and the effect was tested at 25%, 50%, 75%, and full (100%) grate hole opening. This controlling method is also used to maintain heat loss through the grate after wood biomass ignition is started. As shown in equation (10), the emission reduction (ER) can be calculated by:
| (10) |
The actual air supplied for 1 kg of wood fuel is calculated as
As can be seen in Table 3; when ER is below 1, the fuel is gasified rather than combusted. In practical application, for gasification of biomass ER lies between 0.2- 0.3. Since much lower ER causes excessive char and reduction of heating value. But much higher ER produces too much CO2 and H2O that causes heat loss [37].
Table 3.
Actual air-fuel ratio calculation.
The calculated air/fuel ratio for the experiment should be less than stoichiometric due to the amount of wood fuel used. For 1 kg of wood fuel, 5.8 kg/kg is stoichiometric. 6 kg of wood biomass loaded in the reactor reduced the 5.8 kg/kg air-fuel ratio below stoichiometric. In gasification air-fuel ratio is less than one.
2.4. Biomass energy input and efficiency of the stove
Because heat is utilized to drive off the water in high moisture content gases, less energy is available for reduction processes and converting thermal energy into chemical bond energy in the gas. Low gas heating values derive from high moisture content. Higher heating values (HHV) and lower heating values (LHV) of biomass can be calculated using the Dulong Formula [38].
| (11) |
Where C, H, O, and S are determined from the ultimate analysis
The biomass energy input into gasification (input energy) as mentioned in Equation (12); [39]
| (12) |
Where (kW), (kg/s).
The syngas energy produced can be expressed as
The efficiency of the gasification process is calculated as follows [40]
2.5. Injera baking energy estimation
The amount of heat needed to bake injera is estimated using the sensible heat formula (Equation (13)) [41], [39].
| (13) |
Where (g), (g), (°C), (°C), t = average time for one injera baking (s), for teff at kJ/kg °C and for water at kJ/kg °C.
2.6. Balance of mass and carbon
For this mass balance, the material and carbon flow for all input and output streams were taken into account. The material balance was completed for each process state using Equation (14), which illustrates the rule of conservation of mass [4], [7].
| (14) |
Where, the masses of the input and output streams, respectively, are denoted by and . Additionally, the flow and balance of carbon were calculated. The following mass and carbon balance equation are produced by applying the law of mass conservation to the control volume and is represented by Equations (15) and (16) [4], [7].
| (15) |
| (16) |
Where , , represent the mass input in grams, oxygen, and nitrogen to the system, , , , represent the mass output of gas, total contaminants including tar, char, and liquid condensate, , , , represent the mass output in grams of carbon in gas, char, and liquid condensate. Wood serves as the sole source of carbon input for the input streams, and it is divided across all product streams. Only the carbon from the primary gases was taken into account for the gas product stream.
2.7. Conversion efficiency for carbon
The carbon conversion efficiency of gases was also evaluated in addition to the mass and carbon balances because it is a frequently reported parameter in gasification investigations. Equation (17) defines the carbon conversion efficiency as the mass of the gases over the mass of the biomass (i.e., wood) [7].
| (17) |
| (18) |
Where , , defined in equation (18) is the mass fraction of carbon species (s = CO, CO2, CH4, C2H2, and C2H4) and , is the mass out of the corresponding species in the gas out stream.
Where n and are the number of carbon and molecular weight of gas species (s). , , , , and are 0.43, 0.27, 0.75, 0.92, and 0.86, respectively.
2.8. Energy evaluation
Equation (19) is a representation of the energy evaluation expression. The movement of higher heating values (HHV) from wood into the product stream of the controlled volume is examined in this study's energy analysis [7].
| (19) |
Where is the heating values of wood and and represent the heating values of gas and char outlet streams and represents the energy unrecovered into valuable products expressed in kJ. Equations (20)–(22) are created by using the unique heating properties of wood and char.
| (20) |
| (21) |
| (22) |
Where represents the specific HHV of gas species (s = CO, CO2, CH4, C2H2, and C2H4).
3. Result and discussion
3.1. Effects of gasification operating conditions on the product properties
Gasification temperature is one of the most influential factors affecting temperature on the surface of Mitad. The higher temperature in the gasifier results in a higher temperature on Mitad. Since the contents of H2, CO, CO2, and CH4 in the product gas are affected by the temperature. At temperatures above 650 – 800 °C, the endothermic nature of the H2 production reaction (steam reforming and water-gas reactions) results in an increase in H2 content and a decrease in CH4 content with an increase in temperature [4].
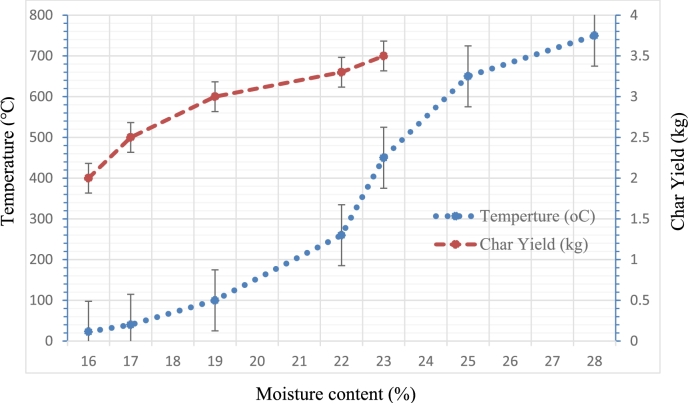
To study the influence of wood fuel moisture content on gasification temperature, the experiment was conducted at different moisture content from 16% to 28%. At a constant airflow rate, increasing the moisture content of fuel slightly increases the gasification temperature in a reactor with time. The gasification process is delayed due to heat loss for evaporation. The higher moisture content of more than 16% requires a more airflow rate to dry wood fuel and increase gasification temperature above 105 °C. Fig. 3 states the effect of moisture content on gasification temperature and char yield. As can be seen in Fig. 3, gasification significantly increases above the moisture dry temperature of 105 °C. Injera baking temperature 185 was achieved after it takes more time above the moisture content of 16% because syngas heating value is low at high moisture content. In this experiment, an average wood fuel moisture content of 14% was used. During gasification, it is vital to regulate the primary and secondary air flow rates to reduce emission factors and promote clean combustion.
Figure 3.
Effect of moisture content on gasification temperature and char yield.
3.2. Effect of primary airflow rate on gasification temperature
Fig. 4 shows the effect of primary airflow rate on gasification temperature. The effect has been studied at a different air flow rate through the inlets. The present study is only concerned with the air flow rate at a full grate hole opening, the airflow rate at 3/4 grate hole opening, and the airflow rate at 1/2 grate hole opening. The results of airflow rate at all air inlet openings have been clearly shown in Fig. 4. Increasing air supply into the reactor proportionally increased the temperature constant moisture content (14%). At all airflow inlet openings, the syngas flame continued and was stable in the reactor for 26 minutes and achieved a temperature in the range of 650-800 °C. Injera baking mitad surface temperature was 185 °C. The airflow rate can be controlled to continue the stability of syngas in the reactor after the baking temperature (185 °C) has been reached. Since fuel consumption can be saved by maintaining syngas continuity in the reactor.
Figure 4.
Effect of primary airflow rate on gasification temperature.
3.3. Influences of secondary air flow rate on mitad temperature
As can be seen in Table 4, the effect of secondary air on temperature was checked by partial and full air inlet opening situations. The temperature distribution on the mitad surface was measured at different points from the center to the edge in the opposite direction. At every point, the measured value was the same. Temperature changes on the mitad surface between open and closed pipe conditions were due to the air effect on syngas in the support. When syngas in the support was well burnt, the mitad heating was increased. The Conventional wood-fired injera stove temperature is somewhat greater than the gasifier.
Table 4.
Effect of secondary air on mitad surface temperature distribution.
| Pipe situation | Side to center point temperature change on mitad (°C) | ||||||
|---|---|---|---|---|---|---|---|
| open | 170 | 177 | 180 | 185 | 180 | 177 | 170 |
| Closed | 165 | 169 | 176 | 180 | 176 | 169 | 165 |
| Conventional wood- fired Injera stove | 185 | 187 | 200 | 220 | 210 | 190 | 185 |
3.4. Effect of an upper cone-diameter hole on the temperature distribution of the mitad
Fig. 5 shows the Variation of temperature distributions on the baking mitad surface due to the short flame propagation distance on the backside of the mitad. The effect of varying diameters of the cone was examined at 8 cm and 15 cm by comparing the cone diameter concerning the heated surface of mitad diameter. At 8 cm, and 26.3 cm mitad surface around the center from the whole diameter was heated with the size of the cone diameter.
Figure 5.
8 cm and 15 cm cone-diameter effect on mitad temperature distribution.
The flame vertical tech part of mitad gains more heat than horizontal tech on the surface. The laminar flow of flame does not radiate or transfer much more heat to the mitad surface. The baking conditions are all the same, and both burners may burn at a rate of 6 kg per hour. To control emission factors and clean combustion, it is necessary to control primary and secondary air flow rates during gasification. Fig. 6 (a) and (b) show the appearances of injera eyes at different cone diameters. As can be seen in Fig. 6, (a) at 8 cm diameter baked injera has a good eye appearance only around the center. But when the cone diameter is 15 cm, all surface of baked Injera has a good eye appearance and quality as shown in Fig. 6 (b).
Figure 6.
Baked Injera eye appearances.
3.5. Heat transfer between mitad and the baking dough
The bubbles are used to transfer heat from the hot surface of the mitad to the poured dough by absorbing heat from the hot mitad and releasing it into the dough. The bubble buoyancy force penetrates the dough and collapses on the surface of the dough due to the density difference between the dough surface and the environment. The temperature of mitad sub-cooled and its temperature becomes saturated.
Under the gasifier stove of Fig. 7, the experiment on Injera baking was carried out at full grate opening primary airflow rate, open pipe secondary air, and 6 kg of wood fuel loading. Before testing, all necessary data have been recorded in each test. Before testing; ambient temperature, the initial weight of wood fuel input (6 kg), and its moisture content was measured. On testing; the stove starts up and an average of one injera baking time was recorded. After testing has been over, the remaining wood fuel, char produced, and average total Injera baking time was measured. For each test 18 species of Injera were baked. The average injera weight difference in each test is due to baking technique errors in which cooking dough may be more added.
Figure 7.
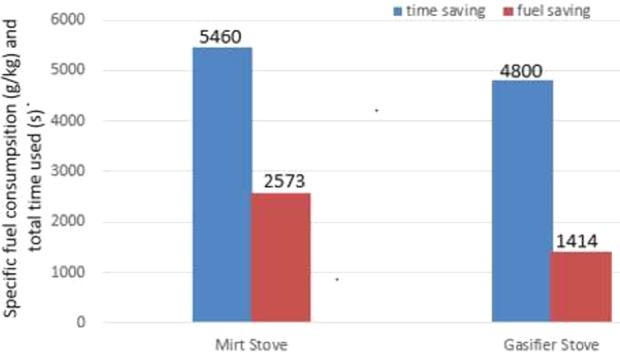
Comparison of fuel and time consumption of gasifier and mirt stove.
3.6. Fuel and time-saving comparison of mirt and gasifier stove
The control cooking test (CCT) is established to reflect the performance of the new gasifier stove at the standard baking methods. It is used to assess the performance of the new stove relative to the common or traditional stoves at the same local meal preparation. It compares the specific wood consumption of different stoves with new stoves during the preparation of the same quantities of food, ingredients, and fuel to identify deviations in the specific fuel consumption [19], [33]. Table 5 shows the controlled cooking test results by comparing the mirt stove with the gasifier stove. Comparisons have been done between the two stoves concerning time and wood fuel consumption.
Table 5.
Results of control cooking Test (CCT) comparing two stoves.
| 1. CCT Results: Stove 1 | Units | Test 1 | Test 2 | Test 3 | Mean | St.Dev |
|---|---|---|---|---|---|---|
| Total weight of food cooked | gram | 1.20 | 1.280 | 1.280 | 1.293 | 23 |
| Weight of char remaining | gram | 400 | 570 | 500 | 490 | 85 |
| Equivalent dry wood consumed | gram | 3.237 | 3.024 | 3.719 | 3.326 | 356 |
| Specific fuel consumption | g/kg | 2.452 | 2.362 | 2.905 | 2.573 | 291 |
| Total cooking time | Minute | 94 | 89 | 91 | 91 | 3 |
| 2. CCT Results: Stove 2 | Units | Test 1 | Test 2 | Test 3 | Mean | St.Dev |
| Total weight of food cooked | gram | 1302 | 1270 | 1370 | 1314 | 51 |
| Weight of char remaining | gram | 300 | 350 | 300 | 317 | 29 |
| Equivalent dry wood consumed | gram | 1911 | 1752 | 1911 | 1858 | 92 |
| Specific fuel consumption | g/kg | 1468 | 1379 | 1395 | 1414 | 47 |
| Total cooking time | Minute | 79 | 82 | 79 | 80 | 2 |
| 3. Comparison of stove 1 and Stove 2 | Units | % difference | T-test | Sig.@95% | ||
| Specific fuel consumption | g/kg | 45 | 6.81 | Yes | ||
| Total cooking time | minute | 12 | 6.43 | Yes |
Stove type/model: stove 1: Mirt stove
Stove type/model: stove 2: Gasifier stove
Wood species: Eucalyptus Globulus (southern Blue Gum, Fe)
As can be seen in the controlled cooking test (CCT) result in Table 5, the performance efficiency of the gasifier stove in terms of wood fuel and time-saving as compared to the mirt stove is 45% and 12% respectively. This saving was found at the burning rate capacity of both stoves which are performed at 6 kg/hr for each test one baking cycle. In a gasifier stove, this amount of fuel loading (6 kg) provides syngas flame which is continuous and stable for 26 minutes. During this time, 18 pieces of injera were baked.
3.7. Fuel and time-saving
As shown in Control cooking test (CCT) result in Fig. 7, the average specific fuel consumption of Mirt is 2573 g/kg while the Gasifier is 1414 g/kg. Because in Gasifier, fuel consumption was saved by controlling the air flow rate supplied through the grate by the controller, and Syngas flame continuity has been maintained at an improved gasification process below the stoichiometric air-fuel ratio. In a mirt stove: after wood fuel has been started to fire, much amount of airflow continuity bends the vertical direction of fire flame out of the pot surface. It causes baking temperature loss at the front of the mitad. That is the flame leaves through outlets and edges as a loss. Therefore, the mirt stove consumes more wood fuel.
As can be seen in Fig. 7, the mirt stove takes more average cooking time than a gasifier is 5460 s. This is due to the startup time consumption of the mirt, flame continuity gap, and wood fuel feeding technique problems that occurred in the mirt stove.
4. Conclusion
As the experimental result shows that Injera baking temperature was achieved between gasifier temperatures 650-800 °C, at full grate hole opening primary air flow rate, 15 cm cone diameter size, and an average wood fuel moisture content of 14%. The fuel fed into the gasifier stove should have a moisture content of less than 16% to produce high heating value syngas in the gasifier by reducing its stoichiometric air-fuel ratio. Syngas heating value was stable and continued for 26 minutes between this gasifier temperature range with 6 kg of fuel loading and 18 injera has to be baked. Mirt stove was used as a base for comparison of efficiency test since the new gasifier is designed for injera baking application rather than biogas production. The gasifier stove can contribute to fuel and time saving compared to traditional and mirt stoves to reduce gas emissions to the atmosphere and reduce global warming. This is important for the conservation of the remaining forests and biodiversity in addition to shortening the cooking time for extra work for the development of the country. The stoichiometric of burning air syngas ratio at different secondary air pipe diameters with specified gasification temperature, air flow rate, and moisture content of wood fuel would be better studied as father work. And the effect of the insulator on gasification temperature change is suggested to be done as further work.
Declarations
Author contribution statement
Tayachew Nega: Conceived and designed the experiments; contributed reagents, materials, analysis tools of data. Nigus Gabbiye habtu: Performed the experiments; contributed reagents, materials, analysis tools of data. Assefa Tesfaye: analyzed and interpreted the data; wrote the paper. Getahun Tassew Melesse: analyzed and interpreted the data. Ermias Aswessie: contributed reagents, materials, analysis tools of data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Forsythe H.D.M., Madyira D.M. Procedia Manufacturing, vol. 35. 2019. Experimental performance assessment of a solar powered baking oven; pp. 535–540. [Google Scholar]
- 2.Inayat A., et al. Parametric study for production of dimethyl ether (DME) as a fuel from palm wastes. Energy Proc. 2017;105:1242–1249. [Google Scholar]
- 3.Hassen A.A., Amibe D.A., Nydal O.J. Performance investigation of solar powered injera baking oven for indoor cooking. ISES Solar World Congress Proceedings; Kassel, Germany; 2011. [Google Scholar]
- 4.Kumar A., Jones D.D., Hanna M.A. Thermochemical biomass gasification: a review of the current status of the technology. Energies. 2009;2(3):556–581. [Google Scholar]
- 5.Tesfaye A., Habtu N.G. Fabrication and performance evaluation of solar tunnel dryer for ginger drying. Int. J. Photoenergy. 2022;2022 [Google Scholar]
- 6.Varunkumar S., Rajan N., Mukunda H. Experimental and computational studies on a gasifier based stove. Energy Convers. Manag. 2012;53(1):135–141. [Google Scholar]
- 7.Abdoulmoumine N., Kulkarni A., Adhikari S. Effects of temperature and equivalence ratio on mass balance and energy analysis in Loblolly pine oxygen gasification. Energy Sci. Eng. 2016;4(4):256–268. [Google Scholar]
- 8.Borello D., et al. Modeling and experimental study of a small scale olive pomace gasifier for cogeneration: energy and profitability analysis. Energies. 2017;10(12):1930. [Google Scholar]
- 9.Ejigu N.A. 2016. Energy modelling in residential houses: A case study of single family houses in Bahir Dar city, Ethiopia. [Google Scholar]
- 10.Tadesse M. The developmental patterns of injera baking stoves: review on the efficiency, and energy consumption in Ethiopia. Int. J. Mech. Eng. 2020;7(1):7–16. [Google Scholar]
- 11.Vigolo V., Sallaku R., Testa F. Drivers and barriers to clean cooking: a systematic literature review from a consumer behavior perspective. Sustainability. 2018;10(11):4322. [Google Scholar]
- 12.Tesfaye A., Workie F., Kumar V.S. Production and characterization of coffee husk fuel briquettes as an alternative energy source. Adv. Mater. Sci. Eng. 2022;2022 [Google Scholar]
- 13.Boujjat H., Rodat S., Abanades S. Solar-hybrid thermochemical gasification of wood particles and solid recovered fuel in a continuously-fed prototype reactor. Energies. 2020;13(19):5217. [Google Scholar]
- 14.A. Tesfaye, M.A. Khader, Strategies for promoting the use of renewable energy in Ethiopia, in: IEEE AFRICON, Addis Ababa, Ethiopia, 14-17 September, 2015, IEEE.
- 15.Akhator P., Obanor A., Sadjere E. Design and development of a small-scale biomass downdraft gasifier. Niger. J. Technol. 2019;38(4):922–930. [Google Scholar]
- 16.Klavins M., Bisters V., Burlakovs J. Small scale gasification application and perspectives in circular economy. Environ. Climate Technol. 2018;22(1):42–54. [Google Scholar]
- 17.Bantu A.A., et al. Design of an improved cooking stove using high density heated rocks and heat retaining techniques. J. Renew. Energy. 2018;2018 [Google Scholar]
- 18.Ahmad A.A., et al. Assessing the gasification performance of biomass: a review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016;53:1333–1347. [Google Scholar]
- 19.Gautam G. 2010. Parametric study of a commercial-scale biomass downdraft gasifier: experiments and equilibrium modeling. [Google Scholar]
- 20.Yosef A. Addis Ababa University; 2008. Assessing environmental benefits of mirt stove with particular reference to indoor air pollution (Carbon Monoxide & Suspended Particulate Matter) and energy conservation. [Google Scholar]
- 21.Burlon F. Energy efficiency of combined ovens. Energy Proc. 2015;82:986–993. [Google Scholar]
- 22.Tesfay A.H., Kahsay M.B., Nydal O.J. Solar powered heat storage for injera baking in Ethiopia. Energy Proc. 2014;57:1603–1612. [Google Scholar]
- 23.Barnes D.F., Golumbeanu R., Diaw I. 2016. Beyond Electricity Access. [Google Scholar]
- 24.Tekle A. Experimental assessment on thermal performance and efficiency of pyra-box solar cooker. J. Energy Technol. Policy. 2014;4(7):58–65. [Google Scholar]
- 25.Panwar N., Kaushik S., Kothari S. State of the art of solar cooking: an overview. Renew. Sustain. Energy Rev. 2012;16(6):3776–3785. [Google Scholar]
- 26.Nega T., Tesfaye A., Paramasivam P. Design and CFD modeling of gasifier stove combined with heat exchanger for water heating application. AIP Adv. 2022;12(4):045121. [Google Scholar]
- 27.Molino A., Chianese S., Musmarra D. Biomass gasification technology: the state of the art overview. J. Energy Chem. 2016;25(1):10–25. [Google Scholar]
- 28.Pieratti E. University of Trento; 2011. Biomass gasification in small scale plants: experimental and modelling analysis. [Google Scholar]
- 29.Asadullah M., et al. Biomass gasification to hydrogen and syngas at low temperature: novel catalytic system using fluidized-bed reactor. J. Catal. 2002;208(2):255–259. [Google Scholar]
- 30.Asadullah M. Barriers of commercial power generation using biomass gasification gas: a review. Renew. Sustain. Energy Rev. 2014;29:201–215. [Google Scholar]
- 31.Wang J.-J., et al. Energy and exergy analyses of an integrated CCHP system with biomass air gasification. Appl. Energy. 2015;142:317–327. [Google Scholar]
- 32.Ishaq H., Dincer I. Design and performance evaluation of a new biomass and solar based combined system with thermochemical hydrogen production. Energy Convers. Manag. 2019;196:395–409. [Google Scholar]
- 33.Agrawal S. 2014. Carbonization of eucalyptus wood and characterization of the properties of chars for application in metallurgy. [Google Scholar]
- 34.Moka V.K. 2012. Estimation of calorific value of biomass from its elementary components by regression analysis. [Google Scholar]
- 35.A.E. Elmubarak, et al., Occupational Risks among the Municipal Solid Waste Collectors in Khartoum Locality.
- 36.Yusoff S. Renewable energy from palm oil–innovation on effective utilization of waste. J. Clean. Prod. 2006;14(1):87–93. [Google Scholar]
- 37.Musse D., Bogale W., Assefa B. International Conference on Advances of Science and Technology. Springer; 2019. Modeling of gasification of refuse derived fuel: optimizations and experimental investigations. [Google Scholar]
- 38.El-Mahallawy F., Habik S.-D. Elsevier; 2002. Fundamentals and technology of combustion. [Google Scholar]
- 39.Basu P. Academic Press; 2010. Biomass gasification and pyrolysis: practical design and theory. [Google Scholar]
- 40.Skreiberg Ø., Georges L. Wood stove material configurations for increased thermal comfort. Energy Proc. 2017;142:488–494. [Google Scholar]
- 41.James Rivas A.M.C. Kansas State University; 2012. The Effect of Biomass, Operating Conditions, and Gasifier Design on the Performance of an Updraft Biomass Gasifier. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.