Abstract
Ethnopharmacology relevance
Wuzi Yanzong Pill (WYP), a well-known prescription for invigorating the kidney and essence, which is widely used to treat infertility such as oligoasthenospermia. Studies have shown that WYP can be used to treat neurological diseases, but its therapeutic effects and mechanisms for multiple sclerosis (MS) remain unclear.
Aim of the study
Based on the establishment of Cuprizone (CPZ)-induced demyelination model, this study determined the effect of WYP on remyelination by detecting changes in the microenvironment of the central nervous system.
Materials and methods
C57BL/6 mice were divided into three groups. The CPZ group and CPZ + WYP group were fed with 0.2% CPZ feed, and the control group was fed normal feed, for 6 weeks. At the end of the second week, the CPZ + WYP group was gavaged with WYP solution (16 g/kg/d), and the other two groups were gavaged with normal saline twice a day with an interval of 12 h each time, for 4 weeks. Forced swimming and elevated plus maze were used to detect changes in anxiety and depression before and after treatment. Luxol fast blue staining and the expression of MBP were used to evaluate the demyelination of the brain. Western blot was used to detect the expression of microglia and their subtype markers Iba-1, Arg-1, iNOS, the expression of neurotrophic factors BDNF, GDNF, CNTF, and the expression of oligodendrocyte precursor cells NG2. ELISA detected the content of IL-6, IL-1β, IL-10, TGF-β, BDNF, GDNF, CNTF in the brain. The distribution of Iba-1 in the corpus callosum was observed by immunofluorescence.
Results
The results showed that on the basis of improving mood abnormalities and demyelination, WYP reduced the protein content of Iba-1 and iNOS, increased the protein content of Arg-1, and reduce accumulation of microglia in the corpus callosum. In addition, WYP reduced the secretion of IL-6 and IL-1β while promoting the secretion of IL-10 and TGF-β. After WYP intervention treatment, the levels of neurotrophic factors BDNF, GDNF, CNTF increased. Due to the improvement of inflammatory and nutritional environment in the CNS, promoting the proliferation of NG2 oligodendrocyte, increased the expression of MBP, and repairing myelin sheath.
Conclusion
Our results indicated that WYP promoted the proliferation and development of oligodendrocytes by improving the CNS microenvironment, effectively alleviating demyelination.
Keywords: Wuzi Yanzong Pill, Neuroinflammation, Nutritional factors, Oligodendrocyte, Remyelination
Wuzi Yanzong Pill; Neuroinflammation; Nutritional factors; Oligodendrocyte; Remyelination.
1. Introduction
MS is an autoimmune disease, characterized by neuroinflammation, demyelination and neurogliosis (Robinson et al., 2019). The pathogenesis is not completely elucidated, but it may be related to heredity, environment and individual status based on the literature reports (Dobson and Giovannoni, 2019). These factors may trigger the activation of T cells and B cells in the peripheral immune system (Baecher-Allan et al., 2018), and enter the CNS through the BBB, which leads to the activation of glial cells and the formation of neuroinflammation, eventually causing oligodendrocyte loss and axonal degeneration (Macchi et al., 2015). As myelin debris increase in the CNS, they induce the activation of microglia and astrocytes (Sen et al., 2019). At present, the therapeutic drugs of MS mainly act on autoimmune response including FTY720, Teriflunomide and Glatiramer acetate (Martin et al., 2016). There are no drugs targeting myelin protection or myelin regeneration in clinical use.
CPZ is a chelator of copper ions that affects the activity of ion channels, suppresses the complex Ⅳ of mitochondrial respiratory chain and then induces the death of oligodendrocytes, therefore establishing the model of demyelination (Varhaug et al., 2020). Increasing studies have shown that CPZ-induced mouse model is an ideal experimental model for investigating myelin protection and myelin regeneration, because the demyelinating model induced by CPZ lacks T cell-mediated immune attack (Esser et al., 2018). Oligodendrocytes are myelin-forming cells, and OPCs are widely distributed in the brain. At the stage of development or injury in the brain, OPCs will develop, proliferate and migrate to appropriate area, then differentiate into mature oligodendrocytes and wrap myelin sheath around axons (Ceprian and Fulton, 2019), indicating that the proliferation and development of OPCs play an important role in the process of remyelination (Dulamea, 2017). In the process of myelin regeneration, glial cells play an important role in the differentiation and maturation of OPCs. Activated microglia show two subtypes, one is the classical activation state (M1), which has special function of phagocytizing foreign bodies such as myelin debris through secreting IL-6, TNF-α and other pro-inflammatory cytokines. The other is the alternative activation state (M2), which can improve the inflammatory microenvironment of CNS through secreting anti-inflammatory cytokines such as IL-10 and TGF-β (Chu et al., 2018). At the initial stage of inflammation in MS, microglia tended to polarize to M1 phenotype (Rawji and Yong, 2013). Astrocytes are abundant in the CNS, they are innate immune cells as same as microglia. Under physiological circumstance, astrocytes maintain the integrity of the BBB and nutritional support (Ludwin et al., 2016). Neurotrophic factors secreted by astrocytes have neuroprotection function, including BDNF, GDNF and CNTF (Liu and Chopp, 2016). A series of evidence indicate that BDNF promoted OPCs proliferation and differentiation to repair the myelin (Tsiperson et al., 2015). CNTF can upregulate the expression of MOG (Salehi et al., 2013). Besides, the increase of BDNF and CNTF can make up for the failure of myelin repair (Houshmand et al., 2019).
WYP is herbal formula of traditional Chinese medicine known as tonifying the kidney and replenishing essence. It consists of five Chinese herbs, including Lycium barbarum L., Cuscuta chinensis Lam., Rubus chingii Hu., Schisandra chinensis (Turcz.) Baill., and Plantago asiatica L, which is often used for the treatment of infertility and the oligoasthenozoospermia (Zhao et al., 2018). One study indicated that WYP can improve apoptosis induced by microwave radiation through inhibiting the expression of Tip60-p53 (Hu et al., 2019). Another research indicated a modified WYP has potential function of neuroprotection through inhibiting the secretion of inflammatory cytokines including IL-1β, IL-6 and TNF-α (Yu et al., 2017). Besides, the modified WYP can also down-regulate LPS-induced increase of iNOS, NO and COX-2 in astrocytes through inhibiting the NF-κB and JNK/p38MAPK signaling (Zeng et al., 2012). The previous investigations showed that WYP had potential for treating neural tube defect by down-regulating the expression of genes associated with cell apoptosis (Li et al., 2019). Because WYP is shown to has an effect of improving neurological dysfunction in a model of EAE, mainly by inhibiting NF-κB inflammatory signaling and inflammatory cytokines such as IL-6, IL-17 (Zhang et al., 2018), this study aimed to further explore the myelin repairing effect of WYP in a CPZ model of MS and its potential mechanism in regulating neuroinflammation and nutritional environment.
2. Materials and methods
2.1. Animals
Male adult mice (8–10 weeks old, weighting 18–20 g) were purchased from the Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All the animals were kept in the pathogen free animal room at suitable temperature (20–24 °C) and humidity with a 12 h dark/12 h light cycle for one week before the formal experiment. All animal procedures were approved by the Council for Laboratory and Ethics Committee of Shanxi University of Traditional Chinese Medicine, Taiyuan, China (2021DW268), and performed according to the International Council for Laboratory Animal Science guidelines.
2.2. CPZ-induced demyelination and administration of WYP
Twenty-four experimental mice were randomly divided into three groups (n = 8/each group): control group (Control), CPZ diet group (CPZ) and CPZ diet plus WYP intervention group (CPZ + WYP). According to the previous study (Vega-Riquer et al., 2019), the demyelinating model was established by feeding a diet containing 0.2% CPZ for six consecutive weeks. WYP was purchased from Tongrentang Pharmacy (Taiyuan, China). The pills were ground to a fine powder and dissolved in normal saline (NS) to prepare drug solution with concentration of 0.32 g/ml. After CPZ was fed for 2 weeks, the drugs should be fully mixed, and then the same volume of normal saline and WYP (0.5 ml, twice a day) were gavaged continuously for 4 weeks (Zhang et al., 2018). Mice fed normal diet were set as control group. Mice were sacrificed at the end of the 6th week.
2.3. Behavioral tests
Behavioral tests were performed in one week before the end of experiment. EPM was used to measure anxiety; a shorter time period spent in the open arms indicates more severe anxiety. Each mouse was put to the center area of an EPM toward to the open arms. The time and distance in the open arms were recorded during a 6 min testing period. FST was used to measure depression of animals. Before the test, a plastic cylinder (height: 30 cm, diameter: 10 cm) was filled with water to 20 cm at 25 ± 1 °C. Cumulative distance traveled and total resting time during 1 min were recorded. All data acquisition and analysis were performed automatically using digital video and Image™ software.
2.4. Tissue preparation
Mice (n = 4/group) were anesthetized with pentobarbital sodium (50 mg/kg) and perfused with normal saline. After fixation with 4% paraformaldehyde, the brain were taken out, fixed in 4% paraformaldehyde for 2 h and dehydrated with 30% sucrose for 24 h at 4 °C before being sliced 10 μm continuous coronal sections. The brain of other mice (n = 4/group) was moved directly after perfusion to obtain protein extract, which was stored at −80 °C.
2.5. LFB staining
Frozen sections of the above brain tissues were placed in a solid blue solution and soaked for 12–18 h at 60 °C. The sections were taken out and washed in a 95% ethanol solution, and then repeatedly immersed in a lithium carbonate solution and 75% ethanol solution for differentiation. They were washed in distilled water to remove the dye, dehydrated in gradient ethanol solutions, and fixed with xylene solution. After natural drying, the sections were sealed with neutral gums and observed under a microscope. After Image-pro Plus 6.0 was used to calculate the average integrated optical density of the corpus callosum, statistical analysis was performed.
2.6. Immunohistochemistry staining
The sections of brain were immersed into the 1% BSA solution to block 1 h at room temperature (RT). After washing with PBS, the slices were incubated with the primary antibodies goat anti-Iba-1 (1:200, Abcam) at 4 °C overnight. After washing off excess antibody, the sections were incubated with the corresponding second antibody for 2 h at RT. The images were observed under fluorescence microscope and analyzed with Image-pro Plus 6.0.
2.7. WB analysis
The concentration of protein was determined by BCA method to prepare protein loading samples. The brain protein extract (30 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to the PVDF membrane. The membrane was blocked with 5% skim milk on a shaker for 1 h. After washing with TBST, the membranes were incubated with the primary antibodies rabbit anti-MBP (1:500, Abcam), goat anti-Iba-1 (1:500, Abcam), rabbit anti-Arg-1 (1:800, Cell Signaling Technology), rabbit anti-iNOS(1:500, Genetex), rabbit anti-BDNF (1:500, Abclonal), rabbit anti-GDNF(1:500, Abcam), rabbit anti-CNTF (1:1000, Abcam), rabbit anti-NG2 (1:1000, Abcam), rabbit anti-β-actin (1:1000, Abclonal) and rabbit anti-Tubulin (1:1000, Abclonal) overnight at 4 °C. After washing with TBST, the membranes were added with the HRP–conjugated goat anti-rabbit (1:3000, Abclonal) and rabbit anti-goat (1:1000, Boster) second antibodies for 2 h at RT. After washing with TBST, immunoblots were developed with an enhanced chemiluminescence systemand measured using Quantity Software (Bio-Rad, Hercules, CA, USA).
2.8. ELISA
The concentrations of IL-6, IL-10, TNF-β (Novus Biologicals), IL-1β (Boster), BDNF, GDNF, and CNTF (Mlbio) in brain homogenate were measured by sandwich ELISA kits, following the manufacturer’s instructions. The BCA method was used for protein quantification.
2.9. Statistical analysis
All statistical analysis were performed by one-way analysis of variance (ANOVA) followed by a Turkey’s multiple comparison test, using GraphPad Prism 6.0 software (Cabit Information Technology Co., Ltd. Shanghai, China). Results were expressed as the mean ± SD. p < 0.05 was considered statistically significant.
3. Results
3.1. WYP improved behavioral dysfunction in CPZ exposure model
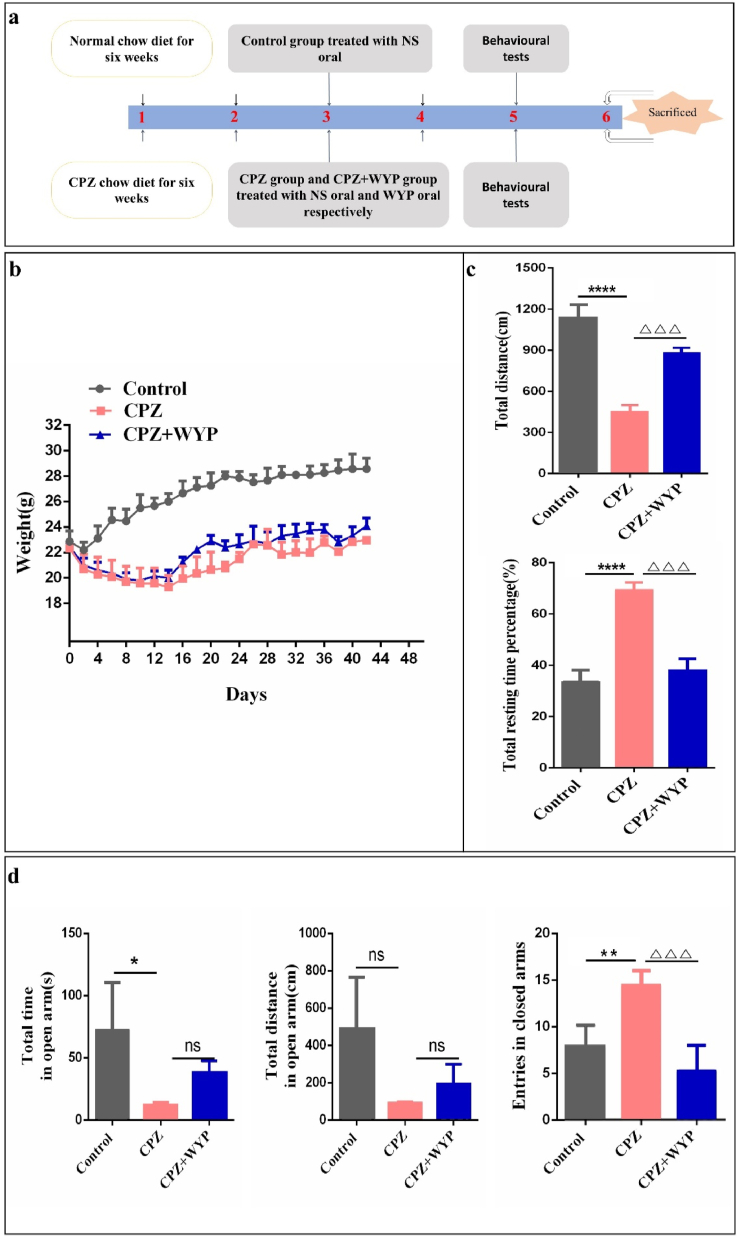
It is known that CPZ feeding for 5–6 weeks can cause oligodendrocytes apoptosis and continuous demyelination in the CNS (Sen et al., 2019). The experimental schedule is shown in Figure 1a. Mice of the CPZ group experienced a significant loss of body weight, especially in the first two weeks after CPZ administration. The loss of body weight of the CPZ + WYP group was slightly less than the CPZ group, but it was not statistically significant (Figure 1b). CPZ administration also induced depression and anxiety in the mice. In the FST, a shorter active time and a shorter travel distance in water indicate more serious depression (Reyes-Mendez et al., 2018). The CPZ + WYP group had a significant decrease in rest time (p < 0.001) and increase in travel distance than the CPZ group (Figure 1c, p < 0.001), suggesting that WYP improved depression in CPZ model. In the EPM, a shorter time and a short travel distance of the mice in the open arms suggest more sever anxiety (He et al., 2019). The time and travel distance in the open arm by the CPZ + WYP group were slightly higher (p > 0.05), but the number of times entering the closed arm was significantly lower than the CPZ group, (p < 0.001), indicating that WYP treatment improved anxiety behavior (Figure 1d).
Figure 1.
WYP improves the abnormal behavior of CPZ-induced demyelination mice. (a) Schematic diagram of the experimental scheme. CPZ group and CPZ + WYP group were fed with 0.2% CPZ chow diet for six weeks, and fed with NS or WYP liquid medicine respectively at the second weekend. (b) The weight change of the three groups. (c) FST was used to test the changes of depression in mice before and after WYP treatment. (d) EMP was used to test the changes in anxiety before and after WYP treatment in mice. The results represent the mean ± SD. Compared with Control group, ns p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. Compared with CPZ + WYP group, ns p > 0.05, △△△p < 0.001.
3.2. WYP attenuated the demyelination in the CNS
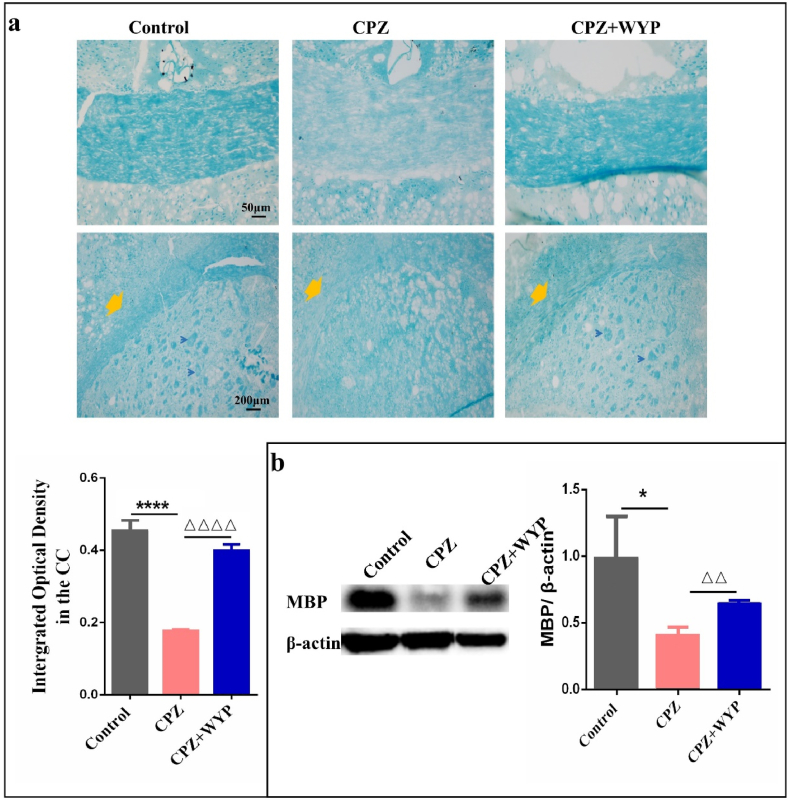
The results of LFB found that the CPZ model had a lighter blue staining in the central part of the CC, EC and striatum regions. After WYP treatment, the blue staining of the middle part of the CC and striatum became darker. The mean density of the corpus callosum in the CPZ + WYP group was higher than that in the CPZ group (Figure 2a, p < 0.0001). MBP is a major protein that composes myelin sheath, and its content reflects the loss and regeneration of myelin sheath in the CNS. The results from WB found that CPZ feeding resulted in the decrease of MBP expression (p < 0.05), which can be increased by WYP intervention (Figure 2b, p < 0.01).
Figure 2.
WYP reduces the demyelination in CNS. (a) LFB staining method was used to observe the demyelination of the corpus callosum and its tail. (b) WB was used to detect the content of MBP, and reflect the loss of myelin sheath from the protein level. The results represent the mean ± SD. Compared with Control group, ∗p < 0.05, ∗∗∗∗p < 0.0001. Compared with CPZ group, △△p < 0.01, △△△△p < 0.0001.
3.3. WYP promoted phenotypic transformation of microglia and improved inflammatory environment
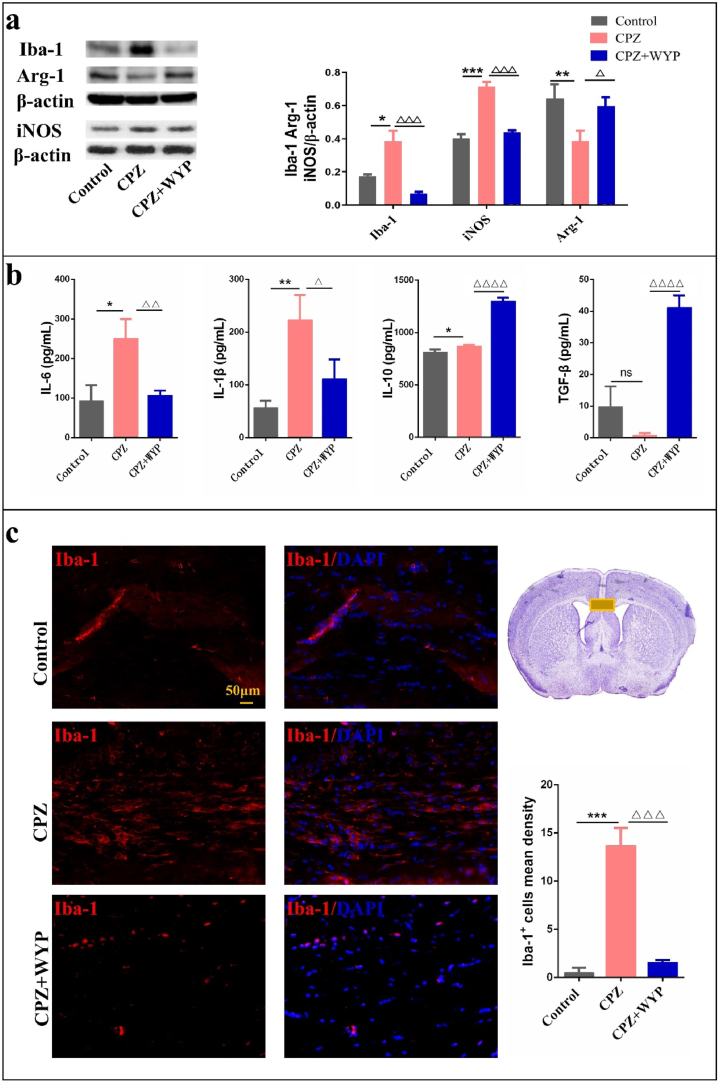
By detecting the M1 and M2 phenotypes of molecules, microglia can be divided into inflammatory M1 cells and anti-inflammatory/repair M2 cells in the brain. iNOS and Arg-1 are markers of M1 and M2 subtype respectively, and their expression reflect the phenotypic transformation of microglia. The results showed that compared with control group, the expression of Iba-1 protein in CPZ group increased significantly (p < 0.05), which was significantly declined by WYP intervention (Figure 3a, p < 0.001). Simultaneously, compared with Control group, the expression of iNOS protein in CPZ group increased (p < 0.001), while the expression of Arg-1 protein decreased (Figure 3a, p < 0.01). Compared with CPZ group, the expression of iNOS protein in CPZ + WYP group decreased (p < 0.001), and the expression of Arg-1 protein increased (Figure 3a, p < 0.05). ELISA results showed that after CPZ modeling, the content of IL-6 and IL-1β increased (p < 0.05, p < 0.01), while the content of IL-10 and TGF-β decreased (Figure 3b, p < 0.05, p > 0.05). After WYP intervention, the content of IL-6 and IL-1β decreased (p < 0.01, p < 0.05), and the content of IL-10 and TGF-β increased (Figure 3b, p < 0.0001). The immunofluorescence results showed that the compared with Control group, Iba-1+ mean density in the CC area increased in CPZ group (p < 0.001), and the Iba-1+ mean density in CPZ + WYP group decreased compared with CPZ group (Figure 3c, p < 0.001).
Figure 3.
WYP promoted the polarization of M2 microglia and promoted the secretion of anti-inflammatory factors. (a) WB detected the protein content of Iba-1, Arg-1 and iNOS in the brain. (b) ELISA was used to detect the content of IL-6, IL-1β, IL-10 and TGF-β in the brain. (c) Observed the expression of Iba-1 in the middle of the corpus callosum by immunofluorescence staining. The results represent the mean ± SD. Compared with Control group, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Compared with CPZ group, △p < 0.05, △△p < 0.01, △△△p < 0.001, △△△△p < 0.0001.
3.4. WYP influenced the secretion of neurotrophic factors and promoted the growth of OPCs
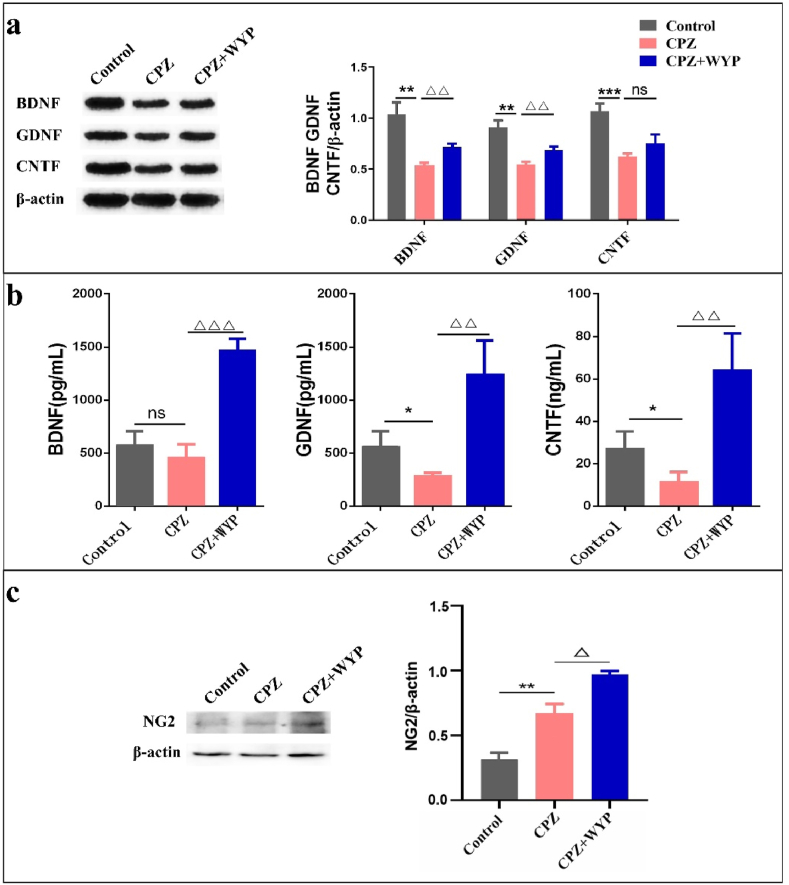
The presence of neurotrophic factors are beneficial to the regeneration and repair of myelin sheath. WB results showed that the protein content of BDNF, GDNF and CNTF decreased in CPZ group (p < 0.01, p < 0.001), while these factors increased in CPZ + WYP group (Figure 4a, p < 0.01). ELISA results also showed that the content of three nutritional factors in CPZ group decreased (p < 0.01), while the content of nutritional factors in CPZ + WYP group increased (Figure 4b, p < 0.01, p < 0.001). By detecting the protein content of the OPCs marker NG2, it was found that the expression level of NG2 was higher in CPZ group than that of Control group (p < 0.01), and the expression level of CPZ + WYP group was increased further than that of CPZ group (Figure 4c, p < 0.05).
Figure 4.
WYP promoted the secretion of neurotrophic factors and the growth of OPCs. (a) WB detected the protein content of BDNF, GDNF and CNTF in the brain. (b) ELISA detected the content of BDNF, GDNF and CNTF in the brain. (c) WB detected the protein expression of NG2. The results represent the mean ± SD. Compared with Control group, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Compared with CPZ group, △p < 0.05, △△p < 0.01, △△△p < 0.001.
4. Discussion
The CPZ-induced demyelination model will cause the activation of microglia and subsequent neuroinflammation will further aggravate myelin damage (Zinnhardt et al., 2019). In this environment, there will be obvious changes in depression and anxiety (Zhang et al., 2019). Modern pharmacological studies have found that WYP has the effect of improving neurological diseases. In our study, CPZ mice showed obvious depressive behaviors, and their depression improved after WYP treatment. The mechanism may be related to remyelination and improvement of neuroinflammation.
In our research, WYP mainly promotes the polarization of M2 microglia, coordinates the secretion of anti-inflammatory cytokines, and improves the inflammatory environment in the CNS. Studies have found that culturing BV2 cells with the serum of MS patients will lead to activation and proliferation, and conversion to M1 type, increasing the secretion of pro-inflammatory factors IL-1β, TNF-α and chemokines CCL2, CCL3, CXCL10, resulting in oligodendrocytes injury and apoptosis (Wu et al., 2018). The CPZ model mimics this pathological change and targets the myelin sheath, causing apoptosis and exfoliation of the myelin sheath, and triggering the activation of M1 microglia in the CNS. Under the electron microscope, it was found that the lost myelin fibers in the CPZ model increased and there were gaps between the myelin layers (Barati et al., 2019a). LFB staining shows demyelinating in the CC and striatum. Iba-1 fluorescence staining shows that microglia mainly aggregates in the CC. It may be due to the presence of myelin fragments that microglia migrate to the myelin injury sites. However, the inflammatory environment caused by excessive activation of microglia is not conducive to the proliferation and maturation of OPCs, and hinders myelin regeneration and repair (Zhao et al., 2020). Modificate the two phenotypes of microglia can improve the loss of myelin sheath in the brain (Barati et al., 2019b). Consistent with the above experimental results, our results demonstrated that, treatment with WYP increased the expression of Arg-1 while decreases the expression of iNOS promotes the transformation of microglia to M2 type. Simultaneously, reducing the secretion of pro-inflammatory factors IL-6 and IL-1β, and promoting the release of anti-inflammatory factors IL-10 and TGF-β, which can improve the inflammatory infiltration of the CC and other injury parts. In order to create favorable conditions for myelin repair.
The presence of neurotrophic factors provides nutrients for cell proliferation and maturation. Overexpression of BDNF can inhibit the activation of microglia, reduce the rise of TNF-α and IL-6, and alleviate the inflammatory infiltration in the hippocampus of mice (Han et al., 2019). The increase of CNTF and BDNF can provide nutritional support for myelin regeneration, combined with anti-apoptotic and anti-inflammatory effects, and jointly improve pathological tissue changes and reduce the clinical score of the EAE model (El-Deeb et al., 2019; Shu et al., 2018). The expression of Stat3 in oligodendrocytes can promote the mature differentiation of oligodendrocyte precursor cells. CNTF can activate Stat3, promote the survival and differentiation of oligodendrocytes, and accelerate the remyelination process (Steelman et al., 2016). During the acute onset of MS, the level of TNF-α in the peripheral blood was significantly increased, and the content of CNTF increased after corticosteroid treatment, which could offset the toxicity of TNF-α to oligodendrocytes (Lindquist et al., 2011). Our results show that, after WYP treatment, the protein content of BDNF, GDNF and CNTF increase in the brain, indicating that WYP promoted the secretion of neurotrophic factors. Our research indicates that, WYP promotes the increase of neurotrophic factors, creating conducive conditions for the proliferation of oligodendrocytes.
Oligodendrocytes are the main cells that make up the myelin sheath. MBP is a marker of mature oligodendrocytes, its maturity determines the integrity of the myelin sheath. In MS, whether oligodendrocyte precursor cells can mature into oligodendrocytes is the key for myelin repair and regeneration. Both neuroinflammation and primary oligodendrocyte disease can lead to oligodendrocytes apoptosis and subsequent demyelination (Mallucci et al., 2015). After CPZ feeding for 3 weeks, most of the mature oligodendrocytes apoptosis in the CNS, followed by obvious demyelination (Doan et al., 2013). The amount of NG2 oligodendrocyte in the prefrontal cortex increased, and the growth rate of mature oligodendrocytes and OPCs was unbalanced, inhibiting the transformation of cultured oligodendrocyte precursor cells into mature oligodendrocyte, the expression of MBP reduced (Morita et al., 2014; Xu et al., 2014), and the gene expression of MBP is also down-regulated (Han et al., 2020). After CPZ treating, the existence of inflammatory environment affects the proliferation and maturation speed of OPCs, resulting in the loss of myelin sheath, oligodendrocytes cannot mature into myelin as soon as possible, and myelin repair occurs slower than demyelination. Up-regulating the expression of Ki67 and Olig2 can promote the proliferation, migration and differentiation of oligodendrocyte precursor cells and enhance myelin regeneration (Wang et al., 2020). WYP promotes the development of OPCs by changing the microenvironment of the CNS. WYP promotes the increase of NG2 and MBP protein expression, suggesting that it can promote the remyelination process and speed up myelin repair. It shows that the improvement of the microenvironment provides conditions for the growth and maturation of oligodendrocytes, and promotes myelin repair and regeneration (Figure 5).
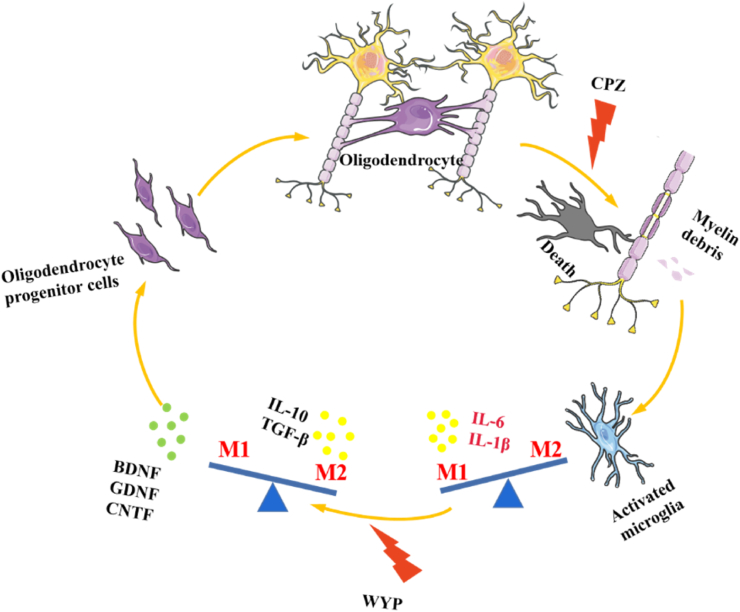
Figure 5.
Schematic diagram of the protective mechanism of WYP on the myelin sheath of CPZ-induced demyelinating mice. Oligodendrocytes wrap around axons to form part of the myelin sheath. CPZ leads to oligodendrocyte apoptosis and myelin sheath exfoliation. The shed myelin fragments activate microglia and polarize to M1 type, secreting inflammation factors. After WYP treatment, microglia are induced to polarize to M2 type and secrete anti-inflammatory factors. At the same time, WYP can induce the secretion of nutritional factors and improve the nutritional environment in the brain. The improvement of the inflammatory environment and nutritional environment promote the increase of oligodendrocyte precursor cells, induces differentiation into mature oligodendrocytes, and repairs the lack of myelin.
5. Conclusion
WYP reverses the expression of activated microglia subtypes, promotes the secretion of anti-inflammatory factors, promotes the secretion of neurotrophic factors, and relieves the neuroinflammatory response in the CNS. As a result, WYP counteract the demyelination sheath caused by CPZ, through creating a good environment for the proliferation and maturation of OPCs, reducing the degree of myelin sheath loss, and then promotes the repair and regeneration of myelin sheath. However, there is still a lack of research on the source of neurotrophic factors in this study, and it is not clear whether more neurotrophic factors secreted by WYP is related to the phenotypic transformation of astrocytes. This is the deficiency in the experiment, and we will continue to improve it in the follow-up study.
Declarations
Author contribution statement
Yan-Rong Li; Meng-Ying Sun: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wei Hang: Performed the experiments; Wrote the paper.
Lu Jia: Performed the experiments.
Qi Xiao: Performed the experiments; Analyzed and interpreted the data.
Hui-Jie Fan: Analyzed and interpreted the data.
Xiao-Ming Jin; Bo Zhang: Contributed reagents, materials, analysis tools or data.
Bao-guo Xiao; Cun-gen Ma; Zhi Chai: Conceived and designed the experiments.
Funding statement
This study was supported by the National Natural Science Foundation of China (No. 81703978, 81102552), the Central Government Guided Local Funding Projects for Science and Technology Development (No. YDZX20201400001483), the Outstanding Youth Talents Program of Shanxi Province (No.[2019] 35), the Natural Science Foundation of Shanxi Province (No. 20210302124293), the Research Project supported by Shanxi Scholarship Council of China (No. 2021-142), the Key science and technology R&D project of Jinzhong (No. Y213004), the Young Scientist Cultivation Program Project, Shanxi University of Chinese Medicine (No. 2021PY-QN-03), the special fund for Science and Technology Innovation Team of Shanxi University of Chinese Medicine (No. 2022TD1013).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Cun-Gen Ma, Email: macungen2001@163.com.
Zhi Chai, Email: chaizhi008@126.com.
References
- Baecher-Allan C., Kaskow B.J., Weiner H.L. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97(4):742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Barati S., Kashani I.R., Tahmasebi F., Mehrabi S., Joghataei M.T. Effect of mesenchymal stem cells on glial cells population in cuprizone induced demyelination model. Neuropeptides. 2019;75:75–84. doi: 10.1016/j.npep.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Barati S., Ragerdi Kashani I., Moradi F., Tahmasebi F., Mehrabi S., Barati M., Joghataei M.T. Mesenchymal stem cell mediated effects on microglial phenotype in cuprizone-induced demyelination model. J. Cell. Biochem. 2019;120(8):13952–13964. doi: 10.1002/jcb.28670. [DOI] [PubMed] [Google Scholar]
- Ceprian M., Fulton D. Glial cell AMPA receptors in nervous system health, injury and disease. Int. J. Mol. Sci. 2019;20(10) doi: 10.3390/ijms20102450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F., Shi M., Zheng C., Shen D., Zhu J., Zheng X., Cui L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018;318:1–7. doi: 10.1016/j.jneuroim.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Doan V., Kleindienst A.M., McMahon E.J., Long B.R., Matsushima G.K., Taylor L.C. Abbreviated exposure to cuprizone is sufficient to induce demyelination and oligodendrocyte loss. J. Neurosci. Res. 2013;91(3):363–373. doi: 10.1002/jnr.23174. [DOI] [PubMed] [Google Scholar]
- Dobson R., Giovannoni G. Multiple sclerosis - a review. Eur. J. Neurol. 2019;26(1):27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- Dulamea A.O. Role of oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. Adv. Exp. Med. Biol. 2017;958:91–127. doi: 10.1007/978-3-319-47861-6_7. [DOI] [PubMed] [Google Scholar]
- El-Deeb O.S., Ghanem H.B., El-Esawy R.O., Sadek M.T. The modulatory effects of luteolin on cyclic AMP/Ciliary neurotrophic factor signaling pathway in experimentally induced autoimmune encephalomyelitis. IUBMB Life. 2019;71(9):1401–1408. doi: 10.1002/iub.2099. [DOI] [PubMed] [Google Scholar]
- Esser S., Gopfrich L., Bihler K., Kress E., Nyamoya S., Tauber S.C., Clarner T., Stope M.B., Pufe T., Kipp M., Brandenburg L.O. Toll-Like receptor 2-mediated glial cell activation in a mouse model of cuprizone-induced demyelination. Mol. Neurobiol. 2018;55(8):6237–6249. doi: 10.1007/s12035-017-0838-2. [DOI] [PubMed] [Google Scholar]
- Han R., Liu Z., Sun N., Liu S., Li L., Shen Y., Xiu J., Xu Q. BDNF alleviates neuroinflammation in the Hippocampus of type 1 diabetic mice via blocking the aberrant HMGB1/RAGE/NF-κB pathway. Aging Dis. 2019;10(3):611–625. doi: 10.14336/AD.2018.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.R., Kang Y.H., Yoo S.M., Lee M.S., Lee S.H. Diverse gene expressions in the prediction of cuprizone-induced demyelination. Neurotox. Res. 2020;37(3):732–742. doi: 10.1007/s12640-019-00154-3. [DOI] [PubMed] [Google Scholar]
- He Y., An J., Yin J.J., Sui R.X., Miao Q., Ding Z.B., Han Q.X., Wang Q., Ma C.G., Xiao B.G. Ethyl pyruvate enhances spontaneous remyelination by targeting microglia phagocytosis. Int. Immunopharm. 2019;77 doi: 10.1016/j.intimp.2019.105929. [DOI] [PubMed] [Google Scholar]
- Houshmand F., Barati M., Golab F., Ramezani-Sefidar S., Tanbakooie S., Tabatabaei M., Amiri M., Sanadgol N. Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2+ precursor cells in the cuprizone murine model of multiple sclerosis. Daru. 2019;27(2):583–592. doi: 10.1007/s40199-019-00286-z. Tehran University of Medical Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.X., Sun J., Gao Y.J., Fang H., Xu S.Q., Dong J., Wei L.Z., Luo S.B., Shen C.Y., Zhang Q.L., Xie Y.L. Effect of modified Wuzi Yanzong pill on Tip60-mediated apoptosis in Testis of male rats after microwave radiation. Chin. J. Integr. Med. 2019;25(5):342–347. doi: 10.1007/s11655-017-2425-9. [DOI] [PubMed] [Google Scholar]
- Li R.X., Fan H.J., Chai Z., Li Y.R., Sun M.Y., Xiao W.S., Yu J.Z., Li Y.H., Zhang B., Ma C.G., Zhou R. Effects of Wuzi Yanzong pills on the apoptotic pathway of neural tube defects cell model. J. Tradit. Chin. Med. 2019;60(13):1134–1141. [Google Scholar]
- Lindquist S., Hassinger S., Lindquist J.A., Sailer M. The balance of pro-inflammatory and trophic factors in multiple sclerosis patients: effects of acute relapse and immunomodulatory treatment. Mult. Scler.(Houndmills, Basingstoke, England) 2011;17(7):851–866. doi: 10.1177/1352458511399797. [DOI] [PubMed] [Google Scholar]
- Liu Z., Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin S.K., Rao V., Moore C.S., Antel J.P. Astrocytes in multiple sclerosis. Mult. Scler.(Houndmills, Basingstoke, England) 2016;22(9):1114–1124. doi: 10.1177/1352458516643396. [DOI] [PubMed] [Google Scholar]
- Macchi B., Marino-Merlo F., Nocentini U., Pisani V., Cuzzocrea S., Grelli S., Mastino A. Role of inflammation and apoptosis in multiple sclerosis: comparative analysis between the periphery and the central nervous system. J. Neuroimmunol. 2015;287:80–87. doi: 10.1016/j.jneuroim.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Mallucci G., Peruzzotti-Jametti L., Bernstock J.D., Pluchino S. The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis. Prog. Neurobiol. 2015;127–128:1–22. doi: 10.1016/j.pneurobio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Sospedra M., Rosito M., Engelhardt B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur. J. Immunol. 2016;46(9):2078–2090. doi: 10.1002/eji.201646485. [DOI] [PubMed] [Google Scholar]
- Morita S., Tatsumi K., Makinodan M., Okuda H., Kishimoto T., Wanaka A. Geissoschizine methyl ether, an alkaloid from the Uncaria hook, improves remyelination after cuprizone-induced demyelination in medial prefrontal cortex of adult mice. Neurochem. Res. 2014;39(1):59–67. doi: 10.1007/s11064-013-1190-1. [DOI] [PubMed] [Google Scholar]
- Rawji K.S., Yong V.W. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin. Dev. Immunol. 2013;2013 doi: 10.1155/2013/948976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Mendez M.E., Castro-Sánchez L.A., Dagnino-Acosta A., Aguilar-Martínez I., Pérez-Burgos A., Vázquez-Jiménez C., Moreno-Galindo E.G., Álvarez-Cervera F.J., Góngora-Alfaro J.L., Navarro-Polanco R.A., Alamilla J. Capsaicin produces antidepressant-like effects in the forced swimming test and enhances the response of a sub-effective dose of amitriptyline in rats. Physiol. Behav. 2018;195:158–166. doi: 10.1016/j.physbeh.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Robinson R.R., Dietz A.K., Maroof A.M., Asmis R., Forsthuber T.G. The role of glial-neuronal metabolic cooperation in modulating progression of multiple sclerosis and neuropathic pain. Immunotherapy. 2019;11(2):129–147. doi: 10.2217/imt-2018-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi Z., Hadiyan S.P., Navidi R. Ciliary neurotrophic factor role in myelin oligodendrocyte glycoprotein expression in Cuprizone-induced multiple sclerosis mice. Cell. Mol. Neurobiol. 2013;33(4):531–535. doi: 10.1007/s10571-013-9918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M.K., Mahns D.A., Coorssen J.R., Shortland P.J. Behavioural phenotypes in the cuprizone model of central nervous system demyelination. Neurosci. Biobehav. Rev. 2019;107:23–46. doi: 10.1016/j.neubiorev.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Shu J., He X., Li H., Liu X., Qiu X., Zhou T., Wang P., Huang X. The beneficial effect of human amnion mesenchymal cells in inhibition of inflammation and induction of neuronal repair in EAE mice. J. Immunol. Res. 2018;(2018) doi: 10.1155/2018/5083797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman A.J., Zhou Y., Koito H., Kim S., Payne H.R., Lu Q.R., Li J. Activation of oligodendroglial Stat3 is required for efficient remyelination. Neurobiol. Dis. 2016;91:336–346. doi: 10.1016/j.nbd.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiperson V., Huang Y., Bagayogo I., Song Y., VonDran M.W., DiCicco-Bloom E., Dreyfus C.F. Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination. ASN Neuro. 2015;7(1) doi: 10.1177/1759091414566878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varhaug K.N., Krakenes T., Alme M.N., Vedeler C.A., Bindoff L.A. Mitochondrial complex IV is lost in neurons in the cuprizone mouse model. Mitochondrion. 2020;50:58–62. doi: 10.1016/j.mito.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Vega-Riquer J.M., Mendez-Victoriano G., Morales-Luckie R.A., Gonzalez-Perez O. Five decades of cuprizone, an updated model to replicate demyelinating diseases. Curr. Neuropharmacol. 2019;17(2):129–141. doi: 10.2174/1570159X15666170717120343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yang H., Zang C., Dong Y., Shang J., Chen J., Wang Y., Liu H., Zhang Z., Xu H., Bao X., Zhang D. CXCR2 antagonism promotes oligodendrocyte precursor cell differentiation and enhances remyelination in a mouse model of multiple sclerosis. Neurobiol. Dis. 2020;134 doi: 10.1016/j.nbd.2019.104630. [DOI] [PubMed] [Google Scholar]
- Wu M., Xu L., Wang Y., Zhou N., Zhen F., Zhang Y., Qu X., Fan H., Liu S., Chen Y., Yao R. S100A8/A9 induces microglia activation and promotes the apoptosis of oligodendrocyte precursor cells by activating the NF-κB signaling pathway. Brain Res. Bull. 2018;143:234–245. doi: 10.1016/j.brainresbull.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Xu H., Yang H.J., Li X.M. Differential effects of antipsychotics on the development of rat oligodendrocyte precursor cells exposed to cuprizone. Eur. Arch. Psychiatr. Clin. Neurosci. 2014;264(2):121–129. doi: 10.1007/s00406-013-0414-3. [DOI] [PubMed] [Google Scholar]
- Yu Q., Song F.J., Chen J.F., Dong X., Jiang Y., Zeng K.W., Tu P.F., Wang X.M. Antineuroinflammatory effects of modified Wu-Zi-Yan-Zong prescription in beta-amyloid-stimulated BV2 microglia via the NF-kappaB and ERK/p38 MAPK signaling pathways. Evid. base Compl. Alternative Med.: eCAM. 2017;2017 doi: 10.1155/2017/8470381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng K.W., Zhang T., Fu H., Liu G.X., Wang X.M. Modified Wu-Zi-Yan-Zong prescription, a traditional Chinese polyherbal formula, suppresses lipopolysaccharide-induced neuroinflammatory processes in rat astrocytes via NF-kappaB and JNK/p38 MAPK signaling pathways. Phytomedicine. 2012;19(2):122–129. doi: 10.1016/j.phymed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang R.N., Chai Z., Fan H.J., Li Y.Y., Li R.X., Li Y.R., Wang Q., Yu J.Z., Li Y.H., Liu C.Y., Xiao B.G., Ma C.G. Preventive and therapeutic effect and its mechanism of Wuzi Yanzong Pills on EAE mouse. China J.Tradit. Chin. Med. Pharm. 2018;33(4):1316–1319. [Google Scholar]
- Zhang Y., Bi X., Adebiyi O., Wang J., Mooshekhian A., Cohen J., Wei Z., Wang F., Li X.M. Venlafaxine improves the cognitive impairment and depression-like behaviors in a cuprizone mouse model by alleviating demyelination and neuroinflammation in the brain. Front. Pharmacol. 2019;10:332. doi: 10.3389/fphar.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M.P., Shi X., Kong G.W.S., Wang C.C., Wu J.C.Y., Lin Z.X., Li T.C., Chan D.Y.L. The therapeutic effects of a traditional Chinese medicine formula Wuzi Yanzong pill for the treatment of oligoasthenozoospermia: a meta-analysis of randomized controlled Trials. Evidence-Based complementary and alternative medicine. eCAM. 2018;2018 doi: 10.1155/2018/2968025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Bao X.Q., Zhang Z., Liu H., Zhang D. Phloroglucinol derivative compound 21 attenuates cuprizone-induced multiple sclerosis mice through promoting remyelination and inhibiting neuroinflammation. Sci. China Life Sci. 2020;63(6):905–914. doi: 10.1007/s11427-019-9821-2. [DOI] [PubMed] [Google Scholar]
- Zinnhardt B., Belloy M., Fricke I.B., Orije J., Guglielmetti C., Hermann S., Wagner S., Schäfers M., Van der Linden A., Jacobs A.H. Molecular imaging of immune cell dynamics during de- and remyelination in the cuprizone model of multiple sclerosis by [(18)F]DPA-714 PET and MRI. Theranostics. 2019;9(6):1523–1537. doi: 10.7150/thno.32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.