Abstract
In Ethiopia, malaria incidence has significantly reduced in the past decade through the combined use of conventional vector control approaches and treatment using antimalarial drugs. However, the sustainability of this achievement is threatened by the shift in biting and resting behaviors and emergence of insecticide resistance by the primary malaria vector. Therefore, continuous monitoring of the behaviour of malaria mosquitoes in different sentinel sites is crucial to design effective prevention and control methods in the local context. Entomological investigations were conducted in three sentinel sites for five consecutive months during the major malaria transmission season. The species composition, population dynamics, biting and resting behaviours of malaria vectors were determined using center for disease control and prevention (CDC) light trap, human landing catch (HLC), pyrethrum spray catch (PSC) and Pitfall shelter collection (PFS). Accordingly, 10 households for CDC, 10 households for PSC, 10 households for PFS and 5 households for HLC from each site were randomly enrolled for mosquito collection. A total of 8,297 anopheline mosquitoes were collected from the three sites, out of which 4,525 (54.5 %) were An. gambiae, s.l. 2,028 (24.4 %) were An. pharoensis, 160 (1.9 %) were An. funestus and the rest 1,584 (19 %) were other anophelines (An. coustani, An. cinerus and An. tenebrosus). No significant variation (P = 0.476) was observed between indoor (25.2/trap-night and outdoor collections (20.1/trap-night). Six hundred seventy six (43.3%) of An. gambiae s.l. (primary vector) were collected between 18:00 and 22:00 h. Biting activity declined between 00:00 and 02:00 h. The national malaria control program should pay close attention to the shifting behavior of vector mosquitoes as the observed outdoor feeding tendency of the vector population could pose challenges to the indoor intervention tools IRS and LLINs.
Keywords: Malaria, An. gambiae, Biting behavior, Resting behavior, Ethiopia
Malaria; An. gambiae; Biting behavior; Resting behavior; Ethiopia.
1. Introduction
The combined use of conventional indoor vector control methods; long lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), has resulted in significant reduction of global malaria mortality and morbidity (Okumu and Moore, 2011). However, these interventions alone are not sufficient to achieve the global malaria strategy of eliminating malaria (Dhiman, 2019) particularly in areas of low malaria transmission (Talisuna et al., 2007). Besides their insufficiency, the sustainability of LLINs and IRS is threatened by emergence of insecticide resistance (Kafy et al., 2017), behavioral shifts of mosquito vectors in relation to their biting venue (Thomsen et al., 2016a), rhythm (Rund et al., 2016) and resting habits (Mahande et al., 2007).
These behavioral shifts are bottlenecks for the transition from malaria prevention and control phase to the elimination phase in many endemic areas (Heggenhougen et al., 2003). It is therefore imperative to understand the biting and resting behavior of malaria mosquitoes in order to design effective vector control strategies considering the local eco-epidemiological context.
In response to the current indoor vector interventions, malaria mosquitoes began shifting their biting location from indoor to the outdoor environment. This phenomenon is witnessed for the major afro-tropical malaria vectors such as Anopheles gambiae s.l. (Carnevale and Manguin, 2021) and An. funestus in different countries of sub-Saharan Africa (SSA) (Sougoufara et al., 2020). Malaria mosquitoes not only shifted their biting location but also biting time from late night to early evening (Mojahedi et al., 2020), making the use of LLINs and IRS less effective. In some malaria endemic regions of SSA, there are some reports stating the substitution of some anthropophilic and endophagic vector species such as An. gambiae s.s. with opportunistic species such as An. arabiensis which is not easily targeted by indoor vector control interventions (Sinka et al., 2010).
2. Methods and materials
2.1. Study sites and duration
Entomological monitoring was conducted in three sites: Medebay Zana (Northern Ethiopia), Mirab Abaya (Southern Ethiopia), and Wondo Genet (South Eastern Ethiopia). The main socio-economic activities such as keeping cattle in indoor settings and socio-agricultural activities such as irrigation, maize cultivation which are factors potential for malaria vector abundance (Asale et al., 2017; Wondwosen et al., 2018). In all three sites, the main socio-economic activities of the local communities are mixed farming involving cultivation of staple food crops like maize, teff and sorghum along with cattle and small-scale stock-raising. This study was conducted from July to December, 2017.
Medebay Zana district is in the Tigray Region of Ethiopia that is found in North West Tigray. It is bordered on the south by the Tekezé River which separates Tahtay Adiyabo from Tselemti, on the southwest by Asgede Tsimbla, on the northwest by Tahtay Koraro, on the north by La’ila’i Adiyabo, and on the east by the Mehakelegnaw (Central) Zone. The administrative center of this Adiyabo woreda is Selek Leka. It is found at 13°99′66′.92″N Latitude and 38.42′25′.86″E Longitude (CSA, 2013). The district is found in a semi-arid tropical mid-highlands belt of Ethiopia with “Weina-dega” agro-climatic zone. The minimum and maximum monthly average temperatures of Medebay zana district are 14.59 °C and 25.61 °C, respectively. The main rainy season with high rainfall intensity, which is locally called kiremit, extends from July to September with a unimodal rainfall pattern. The annual amount of rainfall ranges from 500 to 937 mm (Kinfe et al., 2019).
Mirab Abaya district is a semi-arid area found in southern nations, nationalities and peoples region (SNNPR), Gamo Gofa Zone with its geographic coordinate's 6°17′35.7″N latitude and 37°46′02.9″E longitude. The area has a sub-humid climate with a moderately hot temperature. The district is located at 455 km from Addis Ababa and 56 km Southern Nations, Nationalities from the zonal city, Arba Minch. The district consists of 24 kebeles of which 11% is highland, 27% is midland and 62% lowland. It receives an annual rainfall of 800–1600 mm and the average annual temperature is 27.5 °C. The district is bordered by Lake Abaya which separates it from the Oromia Region on the east and Arba Minch Zuria woreda on the south. Then it is bordered from the west by Chencha woreda, on the northwest by the Borena zone and on the north by the Wolaita Zone. The altitude of the district ranges from 1100 to 2900 m above sea level. The district has an estimated population of 92, 587 (Negash et al., 2019).
Wondo Genet district is located in Oromia region, west Arsi zone, south eastern Ethiopia. The site is located 200 km away from the capital Addis Ababa. Its altitude ranges from 1740 to 1760m above sea level. Its latitude and longitude coordinates are 7°01′66.67″N, 38°58′33.33″E respectively. Its annual minimum and maximum rainfall ranges from 1500to 1750mm, respectively. The mean annual maximum and minimum temperatures range from 11.6 °C and 26.9 °C, respectively.
2.2. Study design
A longitudinal study was deployed to conduct entomological monitoring of Anopheles mosquitoes species composition, density, vector population dynamics, peak biting time and biting places.
2.3. Mosquito sampling and processing
2.3.1. Human landing catches (HLCs)
We used WHO, 1975; revised version of WHO Malaria entomology and vector control guide 2013 and PacMOSSI (2022) SOP for vector sampling procedure and selection of households. In each study sentinel site, five houses were purposely selected for human landing catches. Malaria history of the villages, the availability of vector breeding habitat, and accessibility to road infrastructure were used as criteria in selecting the sentinel houses in each study district. The traditional huts in most rural part of Ethiopia are being replaced with relatively advanced house of iron sheet roof, soil floor and plastered wood wall. This could be partly explained by improved economic status of the community (Asale et al., 2021) or due to comparable quantity bill needed for construction of both types of houses. Thus, in all study areas houses selected for this study were made of iron sheet roof, soil floor and plastered wood wall. In each of the five houses, indoor and outdoor mosquito collections were carried out from 18:00 to 06:00 h. Collections were made at fortnight interval from each house. In each house four trained volunteers of two teams were deployed. All the selected volunteers were adult males. One volunteer did indoor and the other volunteer made outdoors collection. Outdoor mosquito collections were carried out about 8–10 m from each house. In each household one team of collectors worked from 18:00 to 00:00 h followed by the second team from 01:00 to 06:00 h. Every hour, the two volunteers rotated between indoors and outdoors to reduce positional bias and host preference. Mosquitoes landing on exposed legs were aspirated using mouth aspirator. Collections were conducted for 45 min and the volunteers rested for 15 min during which they shifted their positions. The captured mosquitoes were transferred to labeled paper cups. At the end of each collection night, all paper cups with mosquitoes were brought to the field samples identification center where mosquitoes were identified to species using taxonomic keys (Coetzee, 2020).
2.4. CDC light trap catches (LTs)
Ten houses were selected for indoor and outdoor CDC light trap catches from the same village based on their relative location from the breeding site in a similar way to houses selected for HLCs in each of the three study sites following WHO guidelines (WHO, 1975, 2013) Manual on Practical Entomology in Malaria. Mosquitoes were collected indoors and outdoors from 18:00 to 06:00 h from each selected house using standard battery-operated CDC light traps. Traps were hanged from the ceiling at the foot end of the sleeping person at night (Mboera et al., 1998). Each trap was suspended about 1.5 m from the floor. Traps were also hung outdoors 2.5–3 m away from the house. Collection bags were attached to the traps by stretching the open end of the bag over the bottom rim of the trap and a label marked with the date and the site number was placed inside the collection bag. Collection bags were retrieved from traps in each house in the morning between 06:00 and 07:00 h. Mosquitoes were identified by morphological characteristics using taxonomic keys (Coetzee, 2020), and classified according to abdominal status. Absolute mosquitoes’ numbers were converted to capture rate.
2.5. Pyrethrum spray catches (PSCs)
Ten houses were randomly selected in each of the three villages selected for PSCs. Prior to spraying, the heads of households were informed about the purpose and the time of spray and given clear instructions as to what they have to do before and after their houses are sprayed. The collections were performed from 06:00 to 09:00 h every morning. Before mosquito collections, houses were prepared by closing all the eaves, windows and other exit points, removing all animals, all food and small furniture. White cloth sheets were spread on the floor. A commercially available insecticide spray (Baygon aerosol, SC. Johnson & Son Inc, USA) was sprayed in the entire space of the room and then the house was closed for 15 min. Then all the knocked down mosquitoes on the white sheets were collected carefully with forceps and placed in wet paper cups (Ndiath et al., 2011). All Anopheles mosquitoes collected by spray catch method were identified to species using taxonomic keys and the abdominal status of each mosquito was also recorded.
2.6. Pitfall shelter mosquito collection
Outdoor resting mosquitoes were collected using pit-fall traps. Ten houses were randomly selected in each of the three districts and 10 pits were dug under the shade of trees in the compound following the protocol established by WHO (WHO, 1975). Mosquito collections were conducted in the mornings (06:30–09:00 h) by aspirating the resting mosquitoes. The collection was implemented by covering the opening of the pit with untreated bed nets to prevent them from escaping (Massebo et al., 2015).
2.7. Mosquito processing
The daily sampled mosquitoes were transported to the field mosquito processing centers and were identified into genus/species based on their morphology using identification keys (Coetzee, 2020), and sorted by abdominal status.
2.8. Data analysis
Data were analyzed using SPSS software package version 25.0 (SPSS Inc., Chicago, IL, USA). The mean mosquito density between indoor and outdoor collection was computed using student t-test. Test of significance was estimated assuming α (two sided) = 0.05 and P-value less than 0.05 was considered significant during the analysis. Mean variation of mosquito densities among different species, sites and months were tested using one way ANOVA. Zero inflated data were log transformed before undertaking the planned analysis in order to fit in to the normal distribution model. Frequency distribution of species composition was determined by excel spreadsheet. Absolute mosquitoes’ numbers were converted to capture rate. The exophilic behavior of mosquitoes collected by PSC was determined using the formula developed by Ameneshewa and Service (1993). Thus, Degree of exophily (DE) was calculated as where, F is the number of fed mosquitoes and HGG is the sum of the gravid and half-gravid mosquitoes collected by PSCs.
3. Results
3.1. Species composition and abundance
A total of 53,450 mosquitoes were collected over a 5 month's period from three study sites (Table 1). Out of the total 53,450 mosquitoes, 8297 were Anopheline and 45,153 were Culicine. Out of the 8297 Anopheline mosquitoes collected using 4 different methods, 4525 were An. gambiae, s.l. 2028 were An. pharoensis, 160 were An. funestus and the rest 1584 were other anophelines (An. coustani, An. cinereus and An. tenebrosus). In terms of collection techniques employed 23,516 mosquitoes were collected using CDC light traps, 19,372 were collected using HLC, 7283 were collected using PFS and the rest 3279 were collected using PSC method.
Table 1.
Mosquito species and abundance using different collection methods.
| Site | Method | An. gambiae s.l. | An. pharoensis | An. funestus | Other anopheline | Culicine |
|---|---|---|---|---|---|---|
| Mirab Abaya | CDC | 158 | 0 | 0 | 0 | 619 |
| HLC | 333 | 81 | 0 | 104 | 2232 | |
| PFS | 101 | 7 | 0 | 2 | 271 | |
| PSC | 48 | 6 | 0 | 0 | 218 | |
| Medebay Zana | CDC | 736 | 0 | 0 | 115 | 326 |
| HLC | 0 | 0 | 0 | 7 | 5 | |
| PFS | 0 | 0 | 0 | 0 | 62 | |
| PSC | 177 | 0 | 0 | 0 | 36 | |
| Wondo Genet | CDC | 465 | 531 | 42 | 717 | 19807 |
| HLC | 1162 | 1168 | 118 | 611 | 13551 | |
| PFS | 44 | 4 | 0 | 9 | 6769 | |
| PSC | 1301 | 231 | 0 | 2 | 1257 | |
| Grand Total | 4525 | 2028 | 160 | 1584 | 45,153 | |
3.2. Vector density
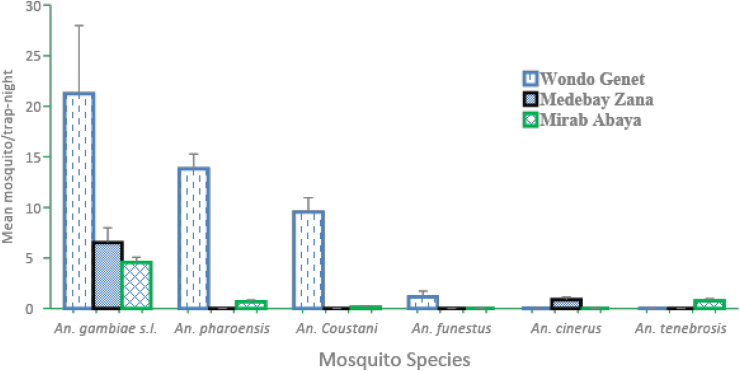
The relative mean density of different Anopheline mosquito density was given in Figure 1. An. gambiae s.l. remains the predominant vector species in three areas, followed by An. pharoensis and An. funestus. Other anophelines with no vectorial history including An. coustani, An. tenebrosis and An. Cinereus were also documented in the study area. Mean separation tests showed that the mean density of An. gambiae s.l. was significantly higher (F = 16.7; P < 0.001) as compared to the mean density of An. pharoensis and An. funestus. Comparison of the primary vector, An. gambiae s.l. across study sites showed that significantly higher mosquito density (F = 5.2; P = 0.006) was collected in the Wondo Genet study site as compared to the rest of Medebay Zana and Mirab Abaya.
Figure 1.
Mean percentage density of Anopheles mosquito's species in each study site.
3.3. Host seeking behavior
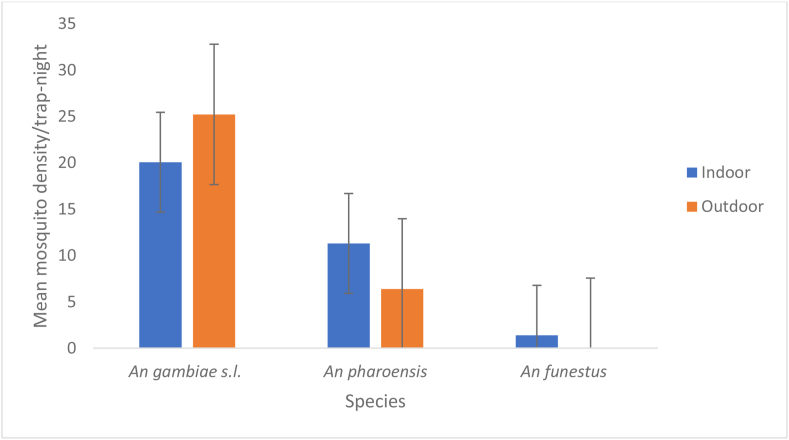
The indoor and outdoor resting mosquito abundance were compared using CDC light trap catches. The mean Anopheline mosquito density collected using CDC were presented in Figure 2 and disaggregated into sites and resting locations. Accordingly, a total of 2764 Anopheline mosquitoes were collected from all three sites. Out of the total 2764 anophelines collected, 1259 mosquitoes were An. gambiae s.l. 531 were An. pharoensis, 42 were An. funestus, 717 were An. coustani, and 115 were An. cinerus. The primary vector An. gambiae s.l. was predominant in all three sites. Hence the indoor, outdoor relative density was compared for An. gambiae s.l. No significant variation (P = 0.476) was observed between indoor and outdoor collections despite slight increase of outdoor catch. The average density of An. gambiae s.l. collected in outdoor and indoor sites were 25.2/trap-night and 20.1/trap-night respectively. Slightly higher mean density (P = 0.05) of An. funestus were collected from indoor collections. No significant variation was observed (P = 0.31) between the indoor and outdoor density of An. Pharoensis.
Figure 2.
Mean mosquito species densities collected indoor and outdoor in study sites by CDC collection.
3.4. Temporal dynamics of anopheline mosquitoes
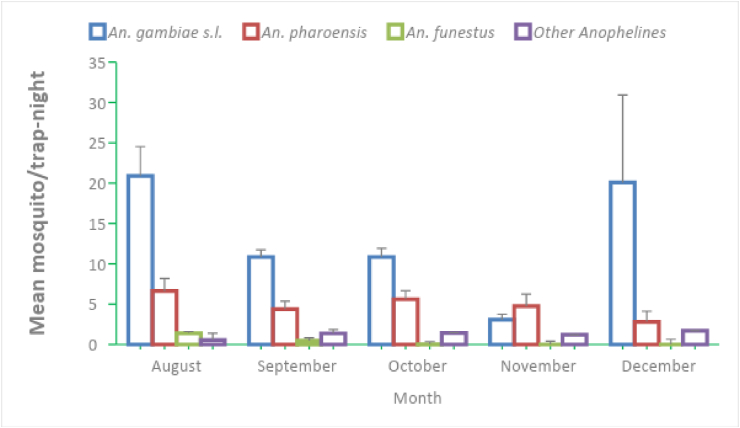
Vector population dynamics in three study sites is presented in Figure 3. Higher mosquito abundance was observed in August and December. The highest 4923 (59.33%) mosquito abundance was documented in the two months combined. Mosquito abundance continuously declined between September and November. The lowest 957 (11.5%) anopheline mosquitoes were collected in November. The abundance of primary vector An. gambiae s.l. was peaked during August and December. Similarly, An. pharoensis peaked during August and its population continuously declined in the following months. Results of one-way ANOVA showed that there was significant difference in mean monthly density of An. gambiae s.l. (F(4,415) = 2.97, p < 0.05). No significant variation was observed among mean monthly densities of An. pharoensis (F(4,415) = 1.19, p = 0.31) and An. funestus (F(4,415) = 1.99, p = 0.09), and An. coustani complex (F(4,415) = 0.96, p = 0.415).
Figure 3.
Mean monthly mosquito population dynamics in study sites for five month.
3.5. Peak biting time
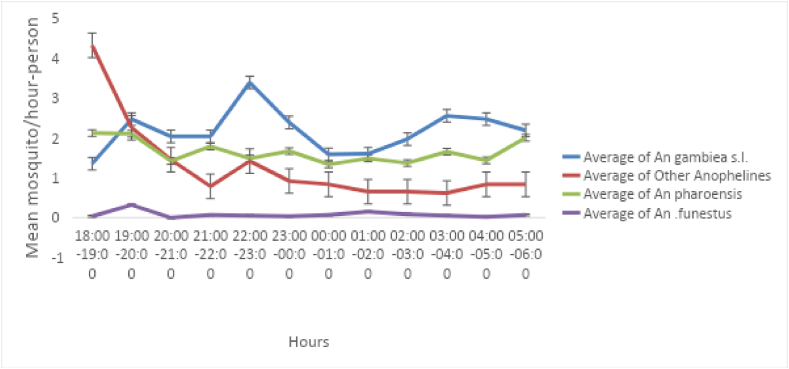
A total of 3,584 anopheline and 15,788 culicine mosquitoes were collected using HLC from the three sites (Figure 4). Among the 3584 anophelines, 1946 mosquitoes were collected from outdoor settings whereas the rest 1,638 mosquitoes were collected from indoor setting. In terms of species composition, 1495 (41.7%) were An. gambiae s.l., 1,249 (34.8%) were An. Pharoensis, 118 (3.3%) were An. funestus, 608 (16.9%) were An. coustani, and 114 (3.2%) were other anophelines (An. tenebrosis and An. cinerus). The peak biting time of major malaria vector mosquitoes was assessed on hourly basis. Hence, 676 (43.3%) of An. gambiae s.l. (primary vector) were collected between 18:00 and 22:00 h. Biting activity declined between 00:00 and 02:00 h. Similarly, 528 (56.6%) of An. pharoensis were collected between 18:00 and 22:00 h. The least catch of An. pharoensis, 128 (13.7%) were collected between 00:00 and 02:00 h. The mean mosquito density per person per hour-night of the rest of Anopheline mosquitoes were given in Figure 4. Early night (18:00–22:00 h) and late night (03:00–05:00 h) are key biting hours as it is documented in all three study areas.
Figure 4.
Mean mosquito densities of different Anopheline species from Medebay Zana, Mirab Abaya and Wondo Genet.
3.6. Abdominal status of Anopheles mosquitoes
Anopheline mosquitoes collected using PSC were analyzed for abdominal status (Table 2). A total of 2587 were collected in 6 months study period. Out of the 2587 total Anopheline mosquitoes collected 1534 (59.3%) were An. gambiae s.l., 227 (8.8) were An. pharoensis, 826 (31.9%) were An. coustani. More mosquitoes caught were found to be fed and the fed to unfed ratio was 1778:799 (≈2:1). The degree of exophily (DE), was consistently higher through out the months with lowest and highest DE documented to be 50% and 100% respectively.
Table 2.
Abdominal status of Anopheline mosquito species in Mirab Abaya, Medebay Zana and Wondo Genet.
| Month | Species | Abdominal status |
F:HGG | ||||
|---|---|---|---|---|---|---|---|
| total | Fed | Unfed | HGG | ||||
| August | An gambiae s.l. | 100 | 76 | 23 | 1 | 76 | 98.6 |
| An pharoensis | 20 | 1 | 17 | 2 | 0.5 | 100 | |
| An coustani | 12 | 0 | 12 | 0 | 0 | 100 | |
| September | An gambiae s.l. | 46 | 36 | 9 | 1 | 36 | 97 |
| An pharoensis | 7 | 2 | 5 | 0 | 2 | 50 | |
| An coustani | 11 | 9 | 2 | 0 | 9 | 89 | |
| October | An gambiae s.l. | 69 | 35 | 30 | 4 | 8.75 | 86 |
| An pharoensis | 13 | 4 | 9 | 0 | 4 | 75 | |
| An coustani | 3 | 0 | 3 | 0 | 0 | 100 | |
| November | An gambiae s.l. | 87 | 50 | 35 | 2 | 25 | 96 |
| An pharoensis | 62 | 62 | 0 | 0 | 62 | 98 | |
| An coustani | 2 | 2 | 0 | 0 | 2 | 50 | |
| December | An gambiae s.l. | 1232 | 1055 | 177 | 0 | 1055 | 100 |
| An pharoensis | 125 | 53 | 72 | 0 | 53 | 98 | |
| An coustani | 798 | 393 | 405 | 0 | 393 | 98 | |
4. Discussion
The current continuous monitoring of malaria vector species composition, behavior and dynamics helps to understand the malaria threat in a certain locality. In this regard, the Ethiopian Public Health Institute and Ministry of Health have established 25 sentinel sites throughout the country in collaboration with development partners to monitor the vector species dynamics, insecticide resistance status, efficacy testing of vector control tools and report any new emerging vector species (FMoH, 2016).
The vector species documentation and determination of their resting, biting or feeding behavior in Medebay Zana, Wondo Genet and Mirab Abaya fills important information gap on the type and abundance of malaria vectors in the aforementioned districts. In this study, six different anopheline species (An. gambiae s.l., An. pharoensis, An. funestus, An. cinereus, An. coustani and An. tenebrosis) were collected and identified from the selected sentinel sites. An. gambiae s.l. was found to be predominant vector species in all three sites and An. pharoensis was identified in Wondo Genet and Mirab Abaya sites. An. funestus was identified only from Mirab Abaya site. The occurrence of An. gambiae s.l. throughout Ethiopia as primary vector has been documented and established for quite long period of time (Zewude and Debusho, 2020; Messenger and Rowland, 2017), and Krafsur, 1977). Both An. pharoensis and An, funestus are known secondary malaria disease vectors in Ethiopia (Krafsur, 1977). Other non-vector anophelines were reported (Massebo et al., 2015).
The host-seeking and biting behavior of vector mosquitoes was assessed using HLC and LTs. In this study, higher density of An. gambiae s.l. was caught from outdoor site as compared to the mean density collected from indoor setting despite the absence of statistical significance. Relatively small number of collections for An. funestus, An. pharoensis, and other non-vector anopheline mosquitoes means we could not confidently discuss mean differences between indoor and outdoor collections for the aforementioned species. With regard to An. gamabiae s.l. presumably An. arabiensis, different studies confirmed conflicting results about their feeding behavior. A study carried out by Taye et al. (2016) documented predominantly indoor host-seeking behavior from Jimma area, where as studies conducted by Degefa et al. (2021) covering Ethiopian context showed exophagic tendency of the vector. Similar study conducted by Taye et al. (2016) showed an equitable split between indoor and outdoor host-seeking behavior of An. arabiensis. Thus, the primary vector An. arabiensis, could be showing flexible behavior of host-seeking in both indoor and outdoor settings with recent reports showing consistent trend of increased outdoor activity. This could be a mechanism adopted by the vector population to evade indoor intervention (LLINs and IRS) (Thomsen et al., 2016b). Anopheles pharoensis, the less important vector however, showed consistently outdoor feeding behavior as it has been documented in other studies (Lelisa et al., 2017; Kibret et al., 2019; Degefa et al., 2021).
In this study the population density of mosquitoes showed an increase in the month of August and continuously declined between September and November. Then the density rebounded back to show an increase in December. The vector density usually starts to build up in Ethiopia following the exit of the rainy season which starts in June and lasts until August (Woyessa et al., 2012; Ribeiro et al., 1996). The observed density decline starting from September to November could be attributed to the efficacy of the IRS operation conducted prior to the data collection and partly to the diminishing vector breeding habitat following the onset of the dry season. The upsurge of the vector population in the month of December specifically in the Wondo Genet district deviates from the expected trend of having low density given the month has always been considered as dry in Ethiopia. There are many factors which could have prolonged vector abundance and longevity in this particular area. For instance, the area is known for having irrigation schemes and different tributaries which in turn are confirmed to be the center of vector population refugia serving as the bridge between dry and rainy season (Eba et al., 2021; Jaleta et al., 2013).
In this study most of the biting incidence (77%) took place in early night (18:00 to 22:00 h) and down (04:00 to 05:00 h) for primary vector An. gambiae s.l. Similarly, An. pharoensis has shown early and late-night biting behavior. In both cases biting activity dramatically dropped starting from midnight until 02:00 h. Then it started to increase starting at 03:00 h. An. funestus, however, showed even biting activity throughout the night as there was no clear peak in any part of the night. Recent studies from different parts of Ethiopia show a shift in the biting hours of An. arabiensis from late in the evening to early evening before people retire to bed (Kibret et al., 2019; Yohannes and Boelee, 2012). This is in reversal to the biting behavior documented a decade ago by (Taye et al., 2006), when most of the biting incidents were recorded between midnight and before late night. Both outdoor and early night feeding behaviors in recent years by An. arabiensis are consistently being reported from different parts of Ethiopia (Kibret et al., 2019), Western Africa (Reddy et al., 2011; Gatton et al., 2013), and Southern Africa (Norris et al., 2010), Tanzania (Kitau et al., 2012). The repeated application of IRS operations and LLINs utilizations have increased trend of outdoor biting in An. gambiae sensu lato (Kibret et al., 2019). The evening outdoor biting pattern of An. funestus should be also closely watched as this species is known for its indoor biting behavior (Aniedu, 1993). The peak biting hour for An. pharoensis has coincided with that of An. arabiensis i.e. increased during early and late night. One study from the Jimma area showed a similar biting pattern (Lelisa et al., 2017), and a study conducted in central Ethiopia (Ziway area) showed slightly different behavior with biting activity for An. pharoensis progressively decreased as the night falls with peak biting activity registered between 19:00 to 20:00 h (Kenea et al., 2016; Degefa et al., 2021). Despite its biting activity throughout the night An. funestus has not shown any recognizable peak in this study. Other studies however, reported different biting peaks for An. funestus. Kenea et al. (2016), reported two peak biting hours (one before midnight and the second in early morning).
In this study the ratio of fed to unfed vector mosquitoes was found to be 2:1 which indicated the abundance of more fed mosquitoes when compared to unfed mosquitoes. The increased density of fed mosquitoes signals the importance of revision of the quality and quantity of the spray operation conducted prior to the data collection in all the three sites. Blood feeding rate, exophily, endophilly and mortality rate are among key parameters in measuring the efficacy of the ongoing vector control interventions (Asale et al., 2013, 2017). Higher proportion of fed mosquitoes were recorded in the months between June and August in Ethiopia in similar study conducted by Lelisa et al. (2017) and a study conducted by Kulkarn and others in Tanzania (Kulkarni et al., 2006). In this study some key entomological parameters such as information pertaining to EIR and blood meal source are not presented due to limited resource.
5. Conclusions
In this study higher density of An. gambiae s.l. was caught from outdoor settings as compared to the mean density collected from indoor settings despite the absence of statistical significance. An. gambiae s.l., the primary malaria vector in Ethiopia, was found to be biting throughout the night with its peak biting hours registered between 18:00 to 22:00 and 04:00 to 06:00. The higher fed to unfed ratio documented in this study shows the reduced efficacy of IRS applied prior to the data collection. However, robust evaluation of intervention tools should be drawn from longitudinal assessment of a minimum of a year using more sentinel sites. The national malaria control program should keep close eye on the shifting behavior of vector mosquitoes as the observed outdoor feeding tendency of the vector population could pose challenges to the indoor intervention tools IRS and LLINs.
Declarations
Author contribution statement
Alemnesh Hailemariam Bedasso: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Abebe Asale Gutto and Eliningaya John Kweka: Analyzed and interpreted the data; Wrote the paper.
Abate Waldetensai, Araya Eukubay, Getachew Bokore, Solomon Kinde, Fekadu Gemechu, Yared Debebe Desta, Mesfin Aklilu, Geremew Tasew and Fekadu Massebo: Performed the experiments.
Abebe Teshome, Tilahun Kebede and Bedri Abdulatif: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Achamyelesh Sisay and Hiwot Solomon: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Ministry of Health (Global fund).
Data availability statement
The data that has been used is confidential.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ameneshewa B., Service M. Resting habits of Anopheles arabiensis in the awash river valley of Ethiopia. Ann. Trop. Med. Parasitol. 1993;90:515–521. doi: 10.1080/00034983.1996.11813077. [DOI] [PubMed] [Google Scholar]
- Aniedu I. Biting activity and resting habits of malaria vectors in baringo district, Kenya. Anz. Schaedlingskunde Pflanzenschutz Umweltschutz. 1993;66:72–76. [Google Scholar]
- Asale A., Abro Z., Enchalew B., Teshager A., Belay A., Kassie M., Mutero C.M. Community knowledge, perceptions, and practices regarding malaria and its control in Jabi Tehnan district, Amhara Region, Northwest Ethiopia. Malar. J. 2021;20(1):1–13. doi: 10.1186/s12936-021-03996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asale A., Duchateau L., Devleesschauwer B., Huisman G., Yewhalaw D. Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): a systematic review. Infect. Dis. Poverty. 2017;6:160. doi: 10.1186/s40249-017-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale P., Manguin S. Review of issues on residual malaria transmission. J. Infect. Dis. 2021;223 doi: 10.1093/infdis/jiab084. S61–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistictical Agency, Ethiopia. 2013. CSA 2013.pdf. [Google Scholar]
- Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar. J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degefa T., Githeko A.K., Lee M.-C., Yan G., Yewhalaw D. Patterns of human exposure to early evening and outdoor biting mosquitoes and residual malaria transmission in Ethiopia. Acta Trop. 2021;216 doi: 10.1016/j.actatropica.2021.105837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect. Dis. Poverty. 2019;8:14. doi: 10.1186/s40249-019-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eba K., Habtewold T., Yewhalaw D., Christophides G.K., Duchateau L. Anopheles arabiensis hotspots along intermittent rivers drive malaria dynamics in semi-arid areas of Central Ethiopia. Malar. J. 2021;20:154. doi: 10.1186/s12936-021-03697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatton M.L., Chitnis N., Churcher T., Donnelly M.J., Ghani A.C., Godfray H.C.J., Gould F., Hastings I., Marshall J., Ranson H., Rowland M., Shaman J., Lindsay S.W. The Importance of mosquito behavioral adaptations to malaria control in Africa. Evolution. 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggenhougen H.K., Hackethal V., Vivek P. 2003. The Behavioural and Social Aspects of Malaria and its Control 228. [Google Scholar]
- Jaleta K.T., Hill S.R., Seyoum E., Balkew M., Gebre-Michael T., Ignell R., Tekie H. Agro-ecosystems impact malaria prevalence: large-scale irrigation drives vector population in western Ethiopia. Malar. J. 2013;12:350. doi: 10.1186/1475-2875-12-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafy H.T., Ismail B.A., Mnzava A.P., Lines J., Abdin M.S.E., Eltaher J.S., Banaga A.O., West P., Bradley J., Cook J., Thomas B., Subramaniam K., Hemingway J., Knox T.B., Malik E.M., Yukich J.O., Donnelly M.J., Kleinschmidt I. Impact of insecticide resistance in Anopheles arabiensis on malaria incidence and prevalence in Sudan and the costs of mitigation. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E11267–E11275. doi: 10.1073/pnas.1713814114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenea O., Balkew M., Tekie H., Gebre-Michael T., Deressa W., Loha E., Lindtjørn B., Overgaard H.J. Human-biting activities of Anopheles species in south-central Ethiopia. Parasites Vectors. 2016;9:527. doi: 10.1186/s13071-016-1813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibret S., Ryder D., Wilson G.G., Kumar L. Modeling reservoir management for malaria control in Ethiopia. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-54536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinfe T., Tsadik T., Tewolde B., Weldegebreal G., Gebresemaeti K., Solomon M., Goitom A. Evaluation of NPSZnB fertilizer levels on yield and yield component of maize (Zea mays L.) at Laelay Adiyabo and Medebay Zana districts, Western Tigray, Ethiopia. J. Cereals OilSeeds. 2019;10:54–63. [Google Scholar]
- Kitau J., Oxborough R.M., Tungu P.K., Matowo J., Malima R.C., Magesa S.M., Bruce J., Mosha F.W., Rowland M.W. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS One. 2012;7 doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafsur E.S. The bionomics and relative prevalence of Anopheles species with respect to the transmission of plasmodium to man in western Ethiopia1. J. Med. Entomol. 1977;14:180–194. doi: 10.1093/jmedent/14.2.180. [DOI] [PubMed] [Google Scholar]
- Kulkarni M.A., Kweka E., Nyale E., Lyatuu E., Mosha F.W., Chandramohan D., et al. Entomological evaluation of malaria vectors at different altitudes in Hai district, northeastern Tanzania. J. Med. Entomol. 2006;43(3):580–588. doi: 10.1603/0022-2585(2006)43[580:eeomva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lelisa K., Asale A., Taye B., Emana D., Yewhalaw D. Anopheline mosquitoes behaviour and entomological monitoring in southwestern Ethiopia. J. Vector Borne Dis. 2017;54:240. doi: 10.4103/0972-9062.217615. [DOI] [PubMed] [Google Scholar]
- Mahande A., Mosha F., Mahande J., Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar. J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massebo F., Balkew M., Gebre-Michael T., Lindtjørn B. Zoophagic behaviour of anopheline mosquitoes in southwest Ethiopia: opportunity for malaria vector control. Parasites Vectors. 2015;8:645. doi: 10.1186/s13071-015-1264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger L.A., Rowland M. Insecticide-treated durable wall lining (ITWL): future prospects for control of malaria and other vector-borne diseases. Malar. J. 2017;16:213. doi: 10.1186/s12936-017-1867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojahedi A.R., Safari R., Yarian M., Pakari A., Raeisi A., Edalat H., Beniardelan M., Poudat A., Zaim M., Basseri H.R. Biting and resting behaviour of malaria vectors in Bandar-Abbas County, Islamic Republic of Iran. East. Mediterr. Health J. 2020;26:1218–1226. doi: 10.26719/emhj.19.104. [DOI] [PubMed] [Google Scholar]
- Ndiath M.O., Mazenot C., Gaye A., Konate L., Bouganali C., Faye O., Sokhna C., Trape J.-F. Methods to collect Anopheles mosquitoes and evaluate malaria transmission: a comparative study in two villages in Senegal. Malar. J. 2011;10:270. doi: 10.1186/1475-2875-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash B., Alemu B., Yohannes B., Hunegnaw A., Yalew A. Experimental study on insecticide treated net effect on cattle in Mirab Abaya, southern Ethiopia. Appl. J. Hygiene. 2019;8(2):48–57. [Google Scholar]
- Norris L.C., Fornadel C.M., Norris D.E. Centers for disease control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am. J. Trop. Med. Hyg. 2010;83:838–842. doi: 10.4269/ajtmh.2010.10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu F.O., Moore S.J. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar. J. 2011;10:208. doi: 10.1186/1475-2875-10-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PacMOSSI . James Cook University; Australia: 2022. Standard Operating Procedure for Vector Surveillance, Processing and Storage; p. 13. [Google Scholar]
- Reddy M.R., Overgaard H.J., Abaga S., Reddy V.P., Caccone A., Kiszewski A.E., Slotman M.A. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar. J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J.M.C., Seulu F., Abose T., Kidane G., Teklehaimanot A. Vol. 74. 1996. Temporal and Spatial Distribution of Anopheline Mosquitos in an Ethiopian Village: Implications for Malaria Control Strategies; p. 7. [PMC free article] [PubMed] [Google Scholar]
- Rund S., O’Donnell A., Gentile J., Reece S. Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects. 2016;7:14. doi: 10.3390/insects7020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M.E., Rubio-Palis Y., Manguin S., Patil A.P., Temperley W.H., Gething P.W., Van Boeckel T., Kabaria C.W., Harbach R.E., Hay S.I. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic précis. Parasites Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sougoufara S., Ottih E.C., Tripet F. The need for new vector control approaches targeting outdoor biting anopheline malaria vector communities. Parasites Vectors. 2020;13:295. doi: 10.1186/s13071-020-04170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talisuna A.O., Okello P.E., Erhart A., Coosemans M., D’Alessandro U. Intensity of malaria transmission and the spread of plasmodium falciparum–resistant malaria: a review of epidemiologic field evidence. Am. J. Trop. Med. Hyg. 2007;77:170–180. [PubMed] [Google Scholar]
- Taye A., Hadis M., Adugna N., Tilahun D., Wirtz R.A. Biting behavior and Plasmodium infection rates of Anopheles arabiensis from Sille, Ethiopia. Acta Trop. 2006;97:50–54. doi: 10.1016/j.actatropica.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Taye B., Lelisa K., Emana D., Asale A., Yewhalaw D. Seasonal dynamics, longevity, and biting activity of anopheline mosquitoes in southwestern Ethiopia. J. Insect Sci. 2016;16(1) doi: 10.1093/jisesa/iev150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen E.K., Koimbu G., Pulford J., Jamea-Maiasa S., Ura Y., Keven J.B., Siba P.M., Mueller I., Hetzel M.W., Reimer L.J. Mosquito behaviour change after distribution of bednets results in decreased protection against malaria exposure. J. Infect. Dis. 2016;jiw615 doi: 10.1093/infdis/jiw615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen E.K., Koimbu G., Pulford J., Jamea-Maiasa S., Ura Y., Keven J.B., Siba P.M., Mueller I., Hetzel M.W., Reimer L.J. Mosquito behaviour change after distribution of bednets results in decreased protection against malaria exposure. J. Infect. Dis. 2016 doi: 10.1093/infdis/jiw615. jiw615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondwosen B., Birgersson G., Tekie H., Torto B., Ignell R., Hill S.R. Sweet attraction: sugarcane pollen-associated volatiles attract gravid Anopheles arabiensis. Malar. J. 2018;17:90. doi: 10.1186/s12936-018-2245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyessa A., Deressa W., Ali A., Lindtjørn B. Prevalence of malaria infection in Butajira area, south-central Ethiopia. Malar. J. 2012;11:84. doi: 10.1186/1475-2875-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO Press, World Health Organization; 20 Avenue Appia, 1211 Geneva 27, Switzerland: 2013. WHO Malaria Entomology and Vector Control. Guide for Participants. [Google Scholar]
- WHO . WHO Press, World Health Organization; 20 Avenue Appia, 1211 Geneva 27, Switzerland: 1975. Manual on Practical Entomology in Malaria. Part II: Methods and Techniques. [Google Scholar]
- Yohannes M., Boelee E. Early biting rhythm in the afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med. Vet. Entomol. 2012;26:103–105. doi: 10.1111/j.1365-2915.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- Zewude B.T., Debusho L.K. Review. 2020. Weighted multilevel models for malaria indicator survey (preprint) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.