Abstract
Background
To compare the clinical outcomes of subepithelial connective tissue graft and chorion membrane along with coronally advanced flap in the treatment of gingival recession.
Methods
A total of 12 patients with 24 sites showing isolated bilateral Miller's class I and II gingival recessions were randomly allocated into two treatment sites. One site, connective tissue graft, (n = 12 sites) while on the contra-lateral site, chorion membrane (n = 12 sites) was used with coronally advanced flap. Clinical parameters: probing depth, recession depth, recession width, width of keratinized gingiva, relative attachment level, thickness of keratinized gingiva were recorded at the baseline, 3 months, and 6 months. The amount of root coverage was evaluated after 6 months.
Results
Statistically significant differences were observed between test and control sites in terms of recession depth, recession width, width of keratinized gingiva and thickness of keratinized gingiva at 6 months. The test sites presented 66.17 ± 18.85% and the control site showed 87.17 ± 18.33% of root coverage at 6 months.
Conclusion
Very limited amount of recession coverage with chorion membrane and did not serve as an alternative to connective tissue graft.
Trial registration
CTRI/2017/12/010964.
Keywords: Chorion membrane, Gingival recession, Root coverage, Subepithelial connective tissue graft, Thickness of keratinized gingiva
Graphical abstract
1. Introduction
Apical migration of the gingival margin leads to the exposure of root surface to the oral cavity, resulting in higher susceptibility to root caries, dentinal hypersensitivity, and unfavorable aesthetics.1,2
Among various root-coverage procedures, the subepithelial connective tissue graft technique is considered to be the standard approach. It has an excellent prognosis with good esthetic results.3 However, it is time-consuming, traumatic, and difficult in obtaining grafts of uniform thickness.4
A unique allograft, chorion membrane, was introduced as a root coverage material to overcome all these obstacles. Chorion contains different types of collagen, proteoglycan, laminin, and bioactive factors which help in binding gingival epithelial cells to the root surface. Being a potent stem cell reservoir it promotes cell differentiation, stimulates healing, and helps in revascularization. Thus it has widespread application in periodontology.5, 6, 7
Therefore the aim of the present study was to clinically evaluate the efficacy of chorion membrane (CM) and subepithelial connective tissue graft (SCTG) with coronally advanced flap (CAF) in the treatment of gingival recession. The study clinically compared between CM and SCTG sites in terms of the amount of root coverage as well as evaluated the improvement in gingival thickness.
2. Materials & methods
2.1. Study design and participants
This was an interventional split-mouth study that included 12 systemically healthy subjects with 24 sites. Each patient with 2 bilateral defects was divided into test and control sites. This clinical intervention included: 1) male and female patients in the age range of 18–50 years 2) all the subjects presented with Miller's class I and II bilateral recession on anatomically same tooth having adequate width of keratinized gingival of ≥2 mm 3) subjects with well-aligned teeth and who maintained good plaque control 4) subjects who had fulfilled these criteria's and signed the written informed consent form. The exclusion criteria were followed: 1) history of any systemic disease or medication 2) Miller's class III and IV gingival recession 3) root caries or crowns at CEJ 4) pregnancy/lactation 5) poor oral hygiene (PI < 2) 6) smokers and alcoholics. The study was approved by the University Ethical Committee. Written consent was obtained from all the subjects before the examination. Only qualifying patients who were willing to participate in the study for 6 months were selected from outpatients at the Department of Periodontology of the Institute.
2.2. Sample size calculation
The sample size was determined by a statistical power analysis. 12 patients were selected and 24 sites were estimated based on an earlier conducted clinical study (amnion membrane and connective tissue graft). Significance level and standard deviation = 0.60 (α = 0.05, β = 0.2).8
2.3. Pre-surgical therapy
Before non-surgical therapy (zero-day), Plaque Index (PI) and Gingival Index (GI) were recorded. Proper oral hygiene instruction was given. Phase I therapy was performed. Impressions were taken and casts were poured to prepare the stent. Intraoral periapical radiographs of both sites were taken to confirm the bone level.
2 months post-therapy, a periodontal assessment was carried out and two sites were randomly allocated into test and control sites. The test sites were treated with chorion membrane and coronally advanced flap technique (CM + CAF) and control sites were treated with subepithelial connective tissue graft and coronally advanced flap technique (SCTG + CAF) using a coin-toss method.
2.3.1. Clinical parameters
A single periodontist (R.M) recorded all clinical periodontal measurements at baseline, 3, and 6 months without information of the sites treated. Clinical parameters included: PI, GI, probing depth (PD) measured from the gingival margin to the sulcus depth, recession depth (RD) measured from CEJ to the gingival margin, recession width (RW) measured at the widest point from the mesial gingival margin to the distal gingival margin, the width of keratinized gingiva (WKG) recorded by roll method. Personalized acrylic stents were used for relative attachment level (RAL) recordings to standardize the measurements. A horizontal and vertical groove was made on the stent to place the probe in position. RAL was measured by adding gingival margin level (GML) with PD. The thickness of keratinized gingiva (TKG) was measured at 2 mm apical to the gingival margin with a 25 k endodontic file with a rubber stopper. All the recordings were measured using UNC-15 probe (Hu-Freidy, Chicago, IL).
After 6 months, the percentage of root coverage (RC) was calculated:
| [pre-operative gingival recession depth - post-operative recession depth]/[preoperative recession depth] * 100% |
2.4. Surgical procedure
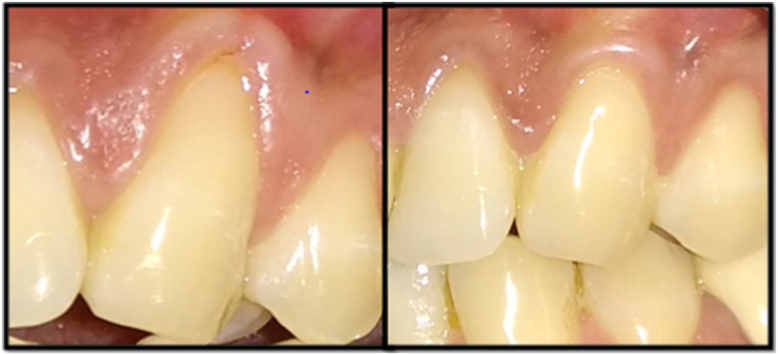
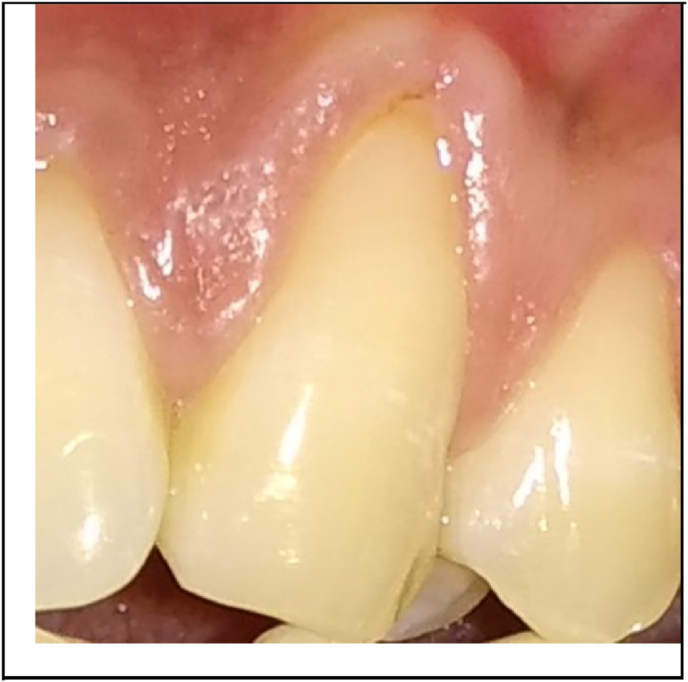
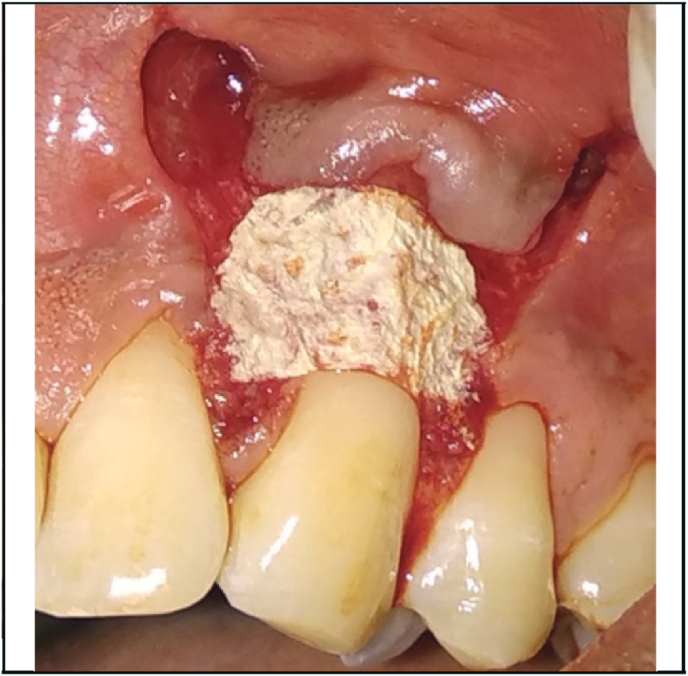
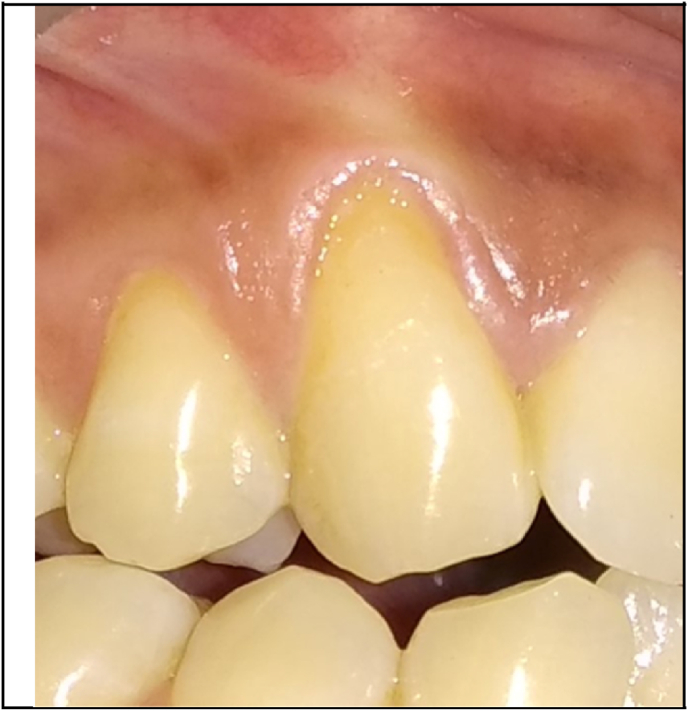
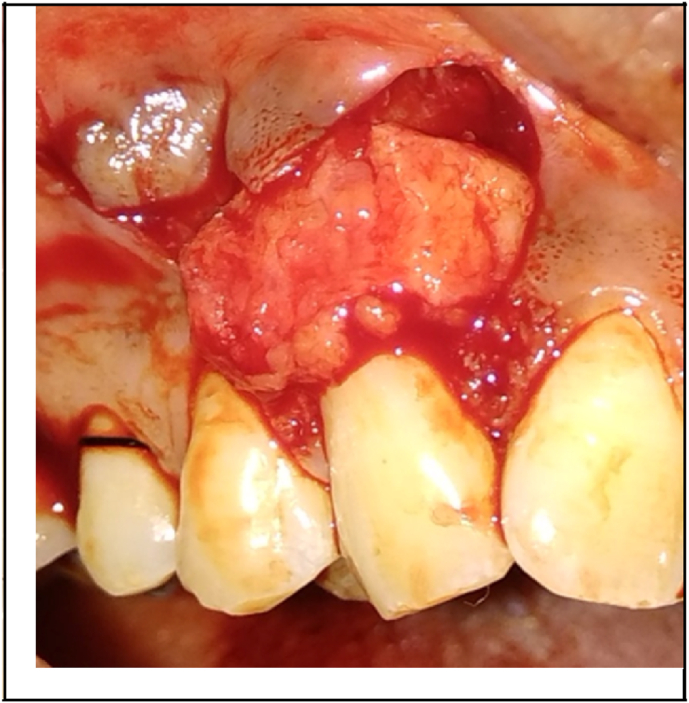
Fig. 1, Fig. 2 showed the pre-operative view of test and control sites. All the surgical procedures were carried out by a single periodontist (S.M). 0.12% chlorhexidine digluconate rinse was used before surgery, and povidone iodine solution for extra-oral antisepsis followed by the application of local anesthesia (2% Lignocaine HCl containing 1: 80,000 adrenaline). The CAF procedure proceeded with the placement of two horizontal incisions mesially and distally at the level of CEJ of the involved tooth. An intra-crevicular incision was given using no. 12 blade. Two vertical releasing incisions were extended beyond the mucogingival junction. Using a 15c blade, split thickness flap was reflected by sharp dissection. Muscle tension was released apically to the bone dehiscence to mobilize the flap. The facial portion of the interdental papillae was de‐epithelialized to form connective tissue bed for the final placement of the flap margin. Thorough root planing was done and convexities were reduced. The test site was treated with freeze-dried CM (commercially available from Tata Memorial Hospital, Mumbai) which was contoured according to the size of the defect while covering at least 2 mm of bone all around the defect. For proper adherence to the recipient site, the CM was soaked in normal saline for 1 min (Fig. 3). The contralateral control site was treated with connective tissue graft harvested from the palatal region by trap-door method (Fig. 4). The graft was then immediately transferred to the recipient site and secured with 5–0 vicryl suture. The flaps were coronally advanced and sutured with 5–0 vicryl. 3-0 silk suture placed on donor site. Light finger pressure was applied for 5 min to remove dead spaces. Periodontal pack (Coe-Pak, GC America, Chicago, IL) along with tin foil was placed as a dressing material.
Fig. 1.
Pre-operative view of test site.
Fig. 2.
Pre-operative view of control site.
Fig. 3.
Chorion membrane placed on the test site.
Fig. 4.
CTG secured on the control site.
2.5. Post-surgical care
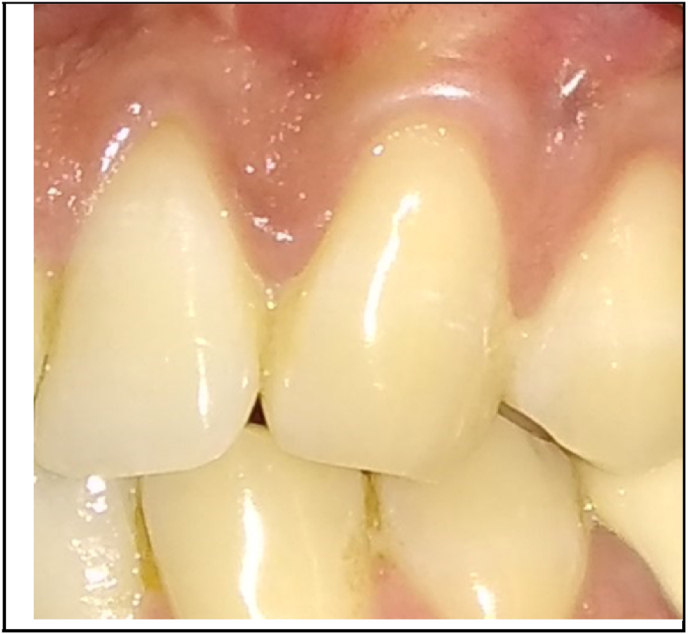
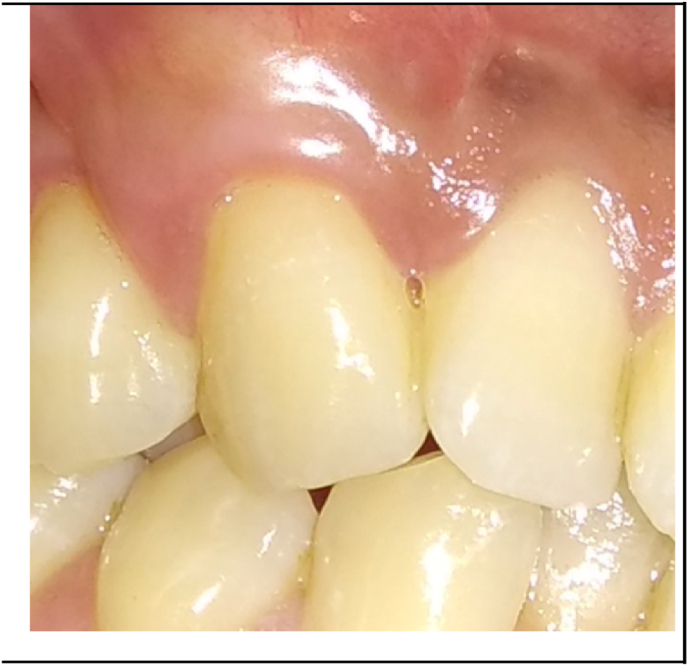
Antibiotics and analgesics were prescribed. Patients were advised to avoid tooth brushing in the treated area for the first 2 weeks and to rinse with 0.12% Chlorhexidine solution three times a day for 4 weeks. The silk sutures were removed after 2 weeks. Patients were instructed to use a soft toothbrush and brushing technique was modified. Recall appointments were scheduled weekly during the first month and then at 3rd and 6th months (Fig. 5, Fig. 6) to assess the clinical parameters.
Fig. 5.
6 months postoperative view of test site.
Fig. 6.
6 months postoperative view of control site.
2.5.1. Statistical analyses
For every parameter and for every assessment time point, mean values and standard deviations (SD) were calculated. The repeated-measures ANOVA was used to assess the effect of time and treatment on continuous variables. The Bonferroni post hoc test was applied for multiple intra-group comparisons. The inter-group differences were statistically explored using paired sample t-test. The level of significance was set at p < 0.05 and statistical analyses were conducted using commercially available software SPSS (Statistical Package for Social Science version 22).
3. Results
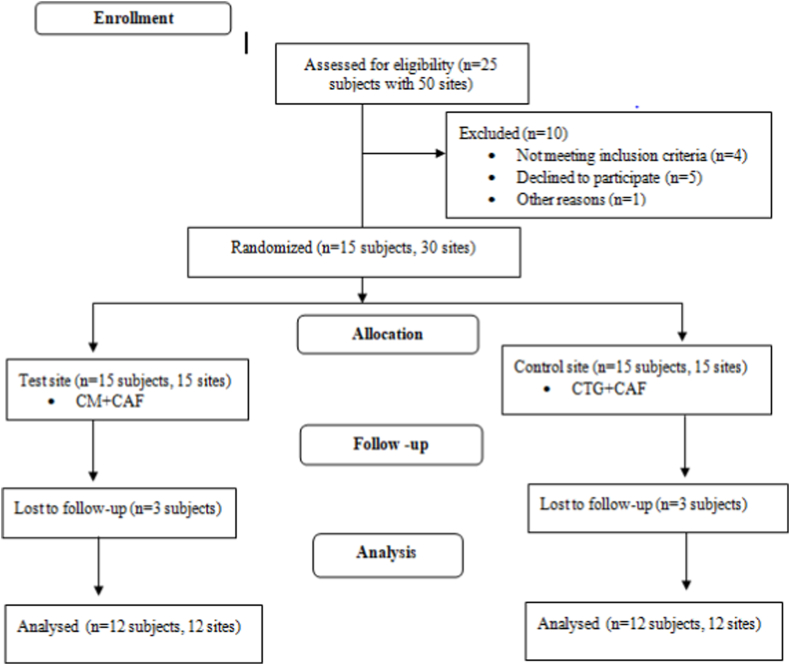
The consort flow chart of the experimental design is presented in Fig. 7. Total 15 subjects (13 males and 2 females), meeting the inclusion criteria with bilateral recession were randomly divided into test and control sites. The test sites (n = 15) were treated CM + CAF and the control sites (n = 15) were treated with SCTG + CAF. 3 subjects lost the 3 months follow-up in the study. As a result, 12 subjects’ mean age of 36.83 ± 8.69 years were analyzed over a period of 6 months.
Fig. 7.
Consort flow chart for patient selection.
The distributions of recession defects according to teeth were: 5 maxillary canines, 1 mandibular canine, 2 maxillary first molars, and 4 mandibular first premolars for the test sites and 5 maxillary canines, 2 maxillary first molars, and 5 mandibular first premolar for the control sites. There were no significant differences in the characteristics of teeth between the sites. Except, in one subject, where canine was considered as the test tooth, bilateral 1st premolar was taken as the control.
Table 1 showed the comparison of mean PI and GI scores at zero-day, baseline, 3, and 6 months. Both showed statistically significant reduction at each time interval as compared to zero-day indicating a good standard of oral hygiene.
Table 1.
Comparison of mean plaque and gingival scores at different time intervals among study participants.
| Clinical parameter | Zero day (1) Mean ± SD |
Baseline (2) Mean ± SD | 3 months (3) Mean ± SD | 6 months (4) Mean ± SD | Statistical inference† | Post hoc comparison‡ |
|---|---|---|---|---|---|---|
| PI | 1.75 ± 0.4 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | p value: 0.00* | 1 vs 2: 0.001* |
| 1 vs 3: 0.001* | ||||||
| 1 vs 4: 0.001* | ||||||
| 2 vs 3: 1.00 | ||||||
| 2 vs 4: 1.00 | ||||||
| 3 vs 4: 1.00 | ||||||
| GI | 1.28 ± 0.3 | 0.17 ± 0.03 | 0.17 ± 0.04 | 0.17 ± 0.04 | p value: 0.00* | 1 vs 2: 0.00* |
| 1 vs 3: 0.00* | ||||||
| 1 vs 4: 0.00* | ||||||
| 2 vs 3: 1.00 | ||||||
| 2 vs 4: 1.00 | ||||||
| 3 vs 4:1.00 |
†Repeated Measures Analysis of Variance, ‡ Bonferroni post hoc test. 1: zero day, 2: baseline, 3: 3 months, 4: 6 months, p < 0.05 is statistically significant*.
PI: Plaque index; GI: Gingival index.
Intragroup comparison of clinical parameters (mean ± SD) over the 6-month experimental period is presented in Table 2. Both site showed a reduction in mean PD at 3 and 6 months. The mean RD value at the test site decreased from 3.00 ± 0.7 at baseline to 1.08 ± 0.9 and 1.00 ± 0.9 at 3 and 6 months, respectively (p = 0.00). Similarly, in the control site, the mean RD value decreased from 3.33 ± 0.9 at baseline to 0.58 ± 0.8 and 0.42 ± 0.8 at 3 and 6 months, respectively (p = 0.00). Mean RW and WKG at the control sites showed the statistical significant results as compared to test site at 3 and 6 months. Both the sites showed the significant results in RAL gain and an increase in TKG.
Table 2.
Changes in clinical parameters (mean ± SD) over the 6-month experimental period.
| Parameters | Sites | 1 (Mean ± SD) | 2 (Mean ± SD) | 3 (Mean ± SD) | 1vs2 (p value) † | 1vs3 (p value) † | 2vs3 (p value) † | Statistical inference‡ |
|---|---|---|---|---|---|---|---|---|
| PD | Test Control |
1.50 ± 0.7 1.50 ± 0.9 |
0.92 ± 0.5 0.83 ± 0.4 |
0.83 ± 0.6 0.83 ± 0.4 |
0.08 0.07 |
0.04* 0.07 |
1.00 1.00 |
p: 0.004* p: 0.005* |
| RD | Test Control |
3.00 ± 0.7 3.33 ± 0.9 |
1.08 ± 0.9 0.58 ± 0.8 |
1.00 ± 0.9 0.42 ± 0.8 |
0.00* 0.00* |
0.00* 0.00* |
1.00 0.49 |
p: 0.00* p: 0.00* |
| RW | Test Control |
3.50 ± 0.5 3.58 ± 0.7 |
2.75 ± 1.1 1.25 ± 1.4 |
2.67 ± 1.1 1.08 ± 1.4 |
0.17 0.001* |
0.13 0.002* |
1.00 1.00 |
p: 0.01* p: 0.00* |
| WKG | Test Control |
2.33 ± 0.8 2.08 ± 0.3 |
2.42 ± 0.7 2.83 ± 0.7 |
2.50 ± 0.7 2.83 ± 0.7 |
1.00 0.005* |
0.49 0.005 |
1.00 1.00 |
p: 0.23 p: 0.00* |
| RAL | Test Control |
12.92 ± 2.1 13.42 ± 2.4 |
10.42 ± 2.0 10.08 ± 1.7 |
10.25 ± 2.1 9.92 ± 1.6 |
0.00* 0.00* |
0.00* 0.00* |
0.5 0.5 |
p: 0.00* p: 0.00* |
| TKG | Test Control |
1.25 ± 0.6 1.25 ± 0.4 |
1.67 ± 0.5 2.17 ± 0.4 |
1.67 ± 0.5 2.17 ± 0.4 |
0.05* 0.00* |
0.05* 0.00* |
1.00 1.00 |
p: 0.003* p: 0.00* |
†Repeated Measures Analysis of Variance, ‡Bonferroni post hoc test. 1: baseline, 2: 3 months, 3: 6 months, p < 0.05 is statistically significant*.
PD: Probing depth; RD: Recession depth; RW: Recession width; WKG: Width of keratinized gingiva; RAL: Relative attachment level; TKG: Thickness of keratinized gingiva.
Table 3 shows the intergroup comparison. Statistically significant differences between test and control sites in terms of RD, RW, WKG, TKG, and % of RC (p < 0.05).
Table 3.
Intergroup comparison of clinical parameters (mean ± SD) over the 6 months experimental period.
| Parameters | Sites | 1 (Mean ± SD) | 2 (Mean ± SD) | 3 (Mean ± SD) |
|---|---|---|---|---|
| PD | Test Control |
1.50 ± 0.7 1.50 ± 0.9 |
0.92 ± 0.5 0.83 ± 0.4 |
0.83 ± 0.6 0.83 ± 0.4 |
| Statistical inference† | p value: 1.00 | p value: 0.59 | p value: 1.00 | |
| RD | Test Control |
3.00 ± 0.7 3.33 ± 0.9 |
1.08 ± 0.9 0.58 ± 0.8 |
1.00 ± 0.9 0.42 ± 0.8 |
| Statistical inference† | p value: 0.10 | p value: 0.05* | p value: 0.05* | |
| RW | Test Control |
3.50 ± 0.5 3.58 ± 0.7 |
2.75 ± 1.1 1.25 ± 1.4 |
2.67 ± 1.1 1.08 ± 1.4 |
| Statistical inference† | p value: 0.67 | p value: 0.004* | p value:0.003* | |
| WKG | Test Control |
2.33 ± 0.8 2.08 ± 0.3 |
2.42 ± 0.7 2.83 ± 0.7 |
2.50 ± 0.7 2.83 ± 0.7 |
| Statistical inference† | p value: 0.19 | p value: 0.01* | p value: 0.04* | |
| RAL | Test Control |
12.92 ± 2.1 13.42 ± 2.4 |
10.42 ± 2.0 10.08 ± 1.7 |
10.25 ± 2.1 9.92 ± 1.6 |
| Statistical inference† | p value: 0.27 | p value: 0.49 | p value: 0.53 | |
| TKG | Test Control |
1.25 ± 0.6 1.25 ± 0.4 |
1.67 ± 0.5 2.17 ± 0.4 |
1.67 ± 0.5 2.17 ± 0.4 |
| Statistical inference† | p value: 1.00 | p value: 0.007* | p value: 0.007* | |
| % of RC | Test Control |
66.17 ± 18.85 87.17 ± 18.33 |
||
| Statistical inference† | p value: 0.002* |
†Paired sample t-test. 1: baseline, 2: 3 months, 3: 6 months, p < 0.05 is statistically significant*.
PD: Probing depth; RD: Recession depth; RW: Recession width; WKG: Width of keratinized gingiva; RAL: Relative attachment level; TKG: Thickness of keratinized gingiva.
The mean percentage of RC was calculated at 6 months and compared with the baseline. There was 66.17 ± 18.85 and 87.17 ± 18.33 mean percentage of RC at the test and control sites after 6 months follow-up respectively (p = 0.002) (Table 3).
After being treated with chorion, no subjects complained of pain whereas, 7 out of 12 patients complained of severe pain at the control site along with discomfort while taking food. Delayed healing of the palatal wound was noted in 1 subject.
4. Discussion
Out of the numerous surgical techniques for gingival recession, SCTG + CAF is considered the benchmark for root coverage therapy.9,10 A bilayer vascular supply nourishes the graft yielding a better esthetic outcome. However, the downside of this technique has led us to search for other regenerative materials.11 Recent evidence from several in-vivo studies indicated that the placental membrane may be a powerful tool for periodontal regeneration.8,12, 13, 14 With this concept, the study was designed to test a versatile substitute, CM, for root coverage and compared it with SCTG.
The experimental therapy resulted in significant PD reduction gain demonstrating the bioactivity of chorion and its intrinsic healing potential. The mean PD reduction noted was 0.75 mm at 6 months from baseline. The results were similar to the case series reported by Esteves et al., (0.81 ± 0.75).15 This is probably because the matrix of the chorion contains abundant growth factors that promote periodontal regeneration and provide an environment for accelerated healing.16 The study showed a reduction in PD at the control site as well but was not significant after 6 months when compared with baseline. The result is in contrast to the study conducted by Gharoudi et al. in which PD increased by 0.19 mm in the SCTG-treated site. However, when the two sites were compared, our study revealed no significant changes (p > 0.05).
The achieved mean RAL gain at the test sites after 6 months was 2.67 mm. The result was in accordance with studies done by Brain (amnion membrane),17 Chakraborthy et al.13 (chorion membrane), and Esteves et al.15 where 1.2 ± 1.51 mm, 2 mm, and 3.48 ± 1.21 respectively was achieved. The previous studies set down the RAL gain to laminins that may have promoted regeneration and accelerated tissue adhesion, which are key factors in improved healing of gingival lesions. Furthermore, the secretory leukocyte proteinase inhibitor I, lactoferrin, defensin, and elafin which are antimicrobial agents might improve wound healing.
At 3 months the mean RD decreased by 1.92 mm and 2.75 mm in the test and control sites, respectively. On intergroup comparison, significant values were obtained at 3 and 6 months. As limited literature is available on CM, this study was compared with the studies done on amnion, since amnion and chorion are known to share similar properties like immunomodulatory, antimicrobial, anti-inflammatory, and regenerative.18,19 The achieved reduction of RD in the test sites is similar to the studies were done by Ghahroudi et al. and Chakraborthy et al.8,13 The decrease in RD can be explained by the fact that chorion is rich in collagen, proteoglycans, laminin, and fibronectin which promotes cell attachment, growth, and differentiation.20,21
In the present study, there was a statistically significant decrease in RW when compared between the groups at 3 and 6 months (p < 0.05). The result is inconsistent with the study conducted by Chakraborthy et al.13 where the sites treated with CM showed a significant improvement in RW. However, our study showed a significant decrease of RW at the CAF + SCTG treated sites but the result is contradicting the study by Gharoudi et al.8 where the control site treated with CTG showed no significant result.
We obtained a statistically significant increase in mean WKG between test and control sites at 3 and 6 months when compared with baseline. Mean WKG at the test and control sites were 2.50 ± 0.7 and 2.83 ± 0.7 respectively after 6 months. CAF and the keratinocyte growth factor released by the CM might help in keratinization which maintained the mucogingival junction in its position. Many studies have emphasized the positive role of SCTG in increasing keratinized gingival width9,11,22, 23, 24, 25 because of its ability to induce epithelial cell differentiation at the recipient site.26
A thin biotype can be a cause of recession. There was a significant increase in mean TKG by 0.42 mm in CM + CAF treated site and 0.92 mm in SCTG + CAF after 6 months. It was in consensus with the study done by Kothiwale.14 However, the result we obtained is in contrast with the study done by Rehan et al.27 with amnion membrane which was followed up over a period of 18 months with insignificant results. The increase may be due to the presence of large number of pro-angiogenic growth factors which promote endothelial recruitment and better vascularisation.28 Immunohistochemical staining analysis done on CM showed an increased concentration of laminin.29 These helped in binding the epithelium on the root surface.30 The presence of tissue inhibitors of metalloproteinases (TIMPs) suppresses matrix metalloproteinases (MMPs) in turn reduces inflammation and collagenous degradation.28
The mean percentage of RC at the CM + CAF treated site was 66.17 ± 18.85%, while SCTG + CAF treated site showed 87.17 ± 18.33%. Only 1 out of 12 test sites came out with 100% RC while, 7 out of 12 control sites showed complete RC. The achieved RC in the test site was better as compared to the study by Chakraborthy et al.13 Also, the present study differed from the case series which obtained 89.92% ± 15.59% RC.15 The success of CM + CAF is credited to its anti-inflammatory and antimicrobial properties and a large number of growth factors. Also, this allograft contains some cytokines that affect progenitor cells, which may play a vital role in activating cells at the site to participate in regeneration and tissue maturation.28
The present study came across various advantages of CM over SCTG. Elimination of a second surgical site was found to be the most important advantage. CM has a self-adherent nature that reduces surgical time and makes the procedure easier for the clinician and, more comfortable for the patient. Graft rejection is also minimal due to its non-immunogenic property. Since commercially available, an ample amount is available to use in multiple recession cases.
Though there are some limitations of this study. Being technique sensitive procedure, improper handling of flap and harvesting of CTG can be a reason for failure in getting complete root coverage. Only one tooth treated with SCTG in mandibular jaw showed complete coverage. This is probably due to thin mucosa apical to the recession which tends to pull the CAF in downward direction. It is not possible to confirm that periodontal regeneration has indeed occurred in the treated sites, because no histological analysis has been done. Hence RAL and RD have been considered a valid parameter to clinically demonstrate the effectiveness of regenerative procedure.
5. Conclusion
The result of the study showed a very limited amount of recession coverage with chorion membrane and did not serve as an alternative to connective tissue graft. Hence, CTG can still be considered the gold standard. Further long-term clinical trials and histopathologic studies are necessary to know the predictability of the membrane.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
Nil.
Contributor Information
Snigdha Maity, Email: snigdha.ria@gmail.com.
Vidya Priyadharshini, Email: drvidyapd@yahoo.co.in.
References
- 1.Zucchelli G., Mounssif I. Periodontal plastic surgery. Periodontol. 2000 2015;68(1):333–368. doi: 10.1111/prd.12059. [DOI] [PubMed] [Google Scholar]
- 2.Trombelli L. Periodontal regeneration in gingival recession defects. Periodontol. 2000 1999;19(1):138–150. doi: 10.1111/j.1600-0757.1999.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 3.Harris R.J. Grafts : an Evaluation of short- and long- term results. J Periodontol. 2002;73:1054–1059. doi: 10.1902/jop.2002.73.9.1054. [DOI] [PubMed] [Google Scholar]
- 4.Paolantonio M., Dolci M., Esposito P., et al. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: a comparative 1-year clinical study. J Periodontol. 2002;73(11):1299–1307. doi: 10.1902/jop.2002.73.11.1299. [DOI] [PubMed] [Google Scholar]
- 5.Kedige S.D., Gupta A. Gingival biotype enhancement and root coverage using human placental chorion membrane. Clin Adv in Periodontics. 2013;3:237–242. [Google Scholar]
- 6.Gupta A., Kedige S.D., Jain K. Amnion and chorion membranes : potential stem cell reservoir with wide applications in periodontics. Int J Biomaterials. 2015:1–9. doi: 10.1155/2015/274082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra A., Thomas B.S. Amniotic membrane: a novel material for regeneration and repair. Biomimetics Biomater tissue Eng. 2013;18:1–8. [Google Scholar]
- 8.Ghahroudi A.A.R., Khorsand A., Rokn A.R., Sabounchi S.S., Shayesteh Y.S., Soolari A. Comparison of amnion allograft with connective tissue graft for root coverage procedures: a double-blind, randomized, controlled clinical trial. J Int Acad Periodontol. 2013;15:101–112. [PubMed] [Google Scholar]
- 9.Oates T.W., Robinson M., Gunsolley J.C. Surgical therapies for the treatment of gingival recession. a systematic review. Ann Periodontol. 2003;8:303–320. doi: 10.1902/annals.2003.8.1.303. [DOI] [PubMed] [Google Scholar]
- 10.Chambrone L., Sukekava F., Araújo M.G., Pustiglioni F.E., Chambrone L.A., Lima L.A. Root-coverage procedures for the treatment of localized recession-type defects: a cochrane systematic review. J Periodontol. 2010;81:452–478. doi: 10.1902/jop.2010.090540. [DOI] [PubMed] [Google Scholar]
- 11.Harris R.J. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol. 2004;75:734–743. doi: 10.1902/jop.2004.75.5.734. [DOI] [PubMed] [Google Scholar]
- 12.Shetty S.S., Chatterjee A., Bose S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J Indian Soc Periodontol. 2014;18:102–106. doi: 10.4103/0972-124X.128261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborthy S., Sambashivaiah S., Kulal R., Bilchodmath S.P. Amnion and chorion allografts in combination with coronally advanced flap in the treatment of gingival recession: a clinical study. J Clin Diagn Res. 2015;9:98–101. doi: 10.7860/JCDR/2015/12971.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kothiwale S., Rathore A., Panjwani V. Enhancing gingival biotype through chorion membrane with innovative step in periodontal pocket therapy. Cell Tissue Bank. 2016;17:33–38. doi: 10.1007/s10561-015-9524-7. [DOI] [PubMed] [Google Scholar]
- 15.Esteves J., Bhat K.M., Thomas B., Jothi M.V., Jadhav T. Efficacy of human chorion membrane allograft for recession coverage- A case series. J Periodontol. 2015;86:1–22. doi: 10.1902/jop.2014.140025c. [DOI] [PubMed] [Google Scholar]
- 16.Holtzclaw D.J., Toscano N.J. Amnion–Chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: a retrospective observational report. Clin Adv Periodontics. 2013;3:131–137. [Google Scholar]
- 17.Brain G. A novel dehydrated amnion allograft for use in the treatment of gingival recession: an observational case series. J Implant Adv Clin Dent. 2009;1:42–56. [Google Scholar]
- 18.Mishra S., Singh S. Human amniotic membrane : can it be a ray of hope in periodontal regeneration. Paripex - Indian J Res. 2014;3:118–121. [Google Scholar]
- 19.Sharma M., Kotwal B., Mahajan N., Kharyal S. Amniotic membrane in periodontics : an insight. Int J Sci Stud. 2017;4:211–214. [Google Scholar]
- 20.Rinastiti M., Harijadi, Santoso A.L.S., Sosroseno W. Histological evaluation of rabbit gingival wound healing transplanted with human amniotic membrane. Int J Oral Maxillofac Surg. 2006;35:247–251. doi: 10.1016/j.ijom.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Engel J., Furthmayr H. Electron microscopy and other physical methods for the characterization of extracellular matrix components: laminin, fibronectin, collagen IV, collagen VI, and proteoglycans. Methods Enzymol. 1987;145:3–78. doi: 10.1016/0076-6879(87)45003-9. [DOI] [PubMed] [Google Scholar]
- 22.Joly J.C., Carvalho A.M., da Silva R.C., Ciotti D.L., Cury P.R. Root coverage in isolated gingival recessions using autograft versus allograft: a pilot study. J Periodontol. 2007;78:1017–1022. doi: 10.1902/jop.2007.060428. [DOI] [PubMed] [Google Scholar]
- 23.Han J.S., John V., Blanchard S.B., Kowolik M.J., Eckert G.J. Changes in gingival dimensions following connective tissue grafts for root coverage : comparison of two procedures. J Periodontol. 2008;79:1346–1354. doi: 10.1902/jop.2008.070472. [DOI] [PubMed] [Google Scholar]
- 24.Cordioli G., Mortarino C., Chierico A., Grusovin M.G., Majzoub Z. Comparison of 2 techniques of subepithelial connective tissue graft in the treatment of gingival recessions. J Periodontol. 2001;72:1470–1476. doi: 10.1902/jop.2001.72.11.1470. [DOI] [PubMed] [Google Scholar]
- 25.Bouchard P., Etienne D., Ouhayoun J.P., Nilveus R. Subepithelial connective tissue grafts in the treatment of gingival recessions. a comparative study of 2 procedures. J Periodontol. 1994;65:929–936. doi: 10.1902/jop.1994.65.10.929. [DOI] [PubMed] [Google Scholar]
- 26.Karring T., Lang N.P., Loe H. The role of gingival connective tissue in determining epithelial differentiation. J Periodontal Res. 1975;10:1–11. doi: 10.1111/j.1600-0765.1975.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 27.Rehan M., Khatri M., Bansal M., Puri K., Kumar A. Comparative evaluation of coronally advanced flap using amniotic membrane and platelet-rich fibrin membrane in gingival recession : an 18-month clinical study. Contemp Clin Dent. 2018;9:188–194. doi: 10.4103/ccd.ccd_799_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koob J.T., Lim J.J., Massee M., Zabek N., Robert R., Gurtner G. Angiogenic properties of dehydrated human amnion/chorion allografts : therapeutic potential for soft tissue repair and regeneration. Vasc Cell. 2014;6:1–10. doi: 10.1186/2045-824X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant-Greenwood G.D. Current matrix of the human fetal membranes : structure the extracellular and function. Placenta. 1998;19:1–11. doi: 10.1016/s0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 30.Mullen L.M., Richards D.W., Quaranta V. Evidence that laminin-5 is a component of the tooth surface internal basal lamina, supporting epithelial cell adhesion. J Periodontal Res. 1999;34:16–24. doi: 10.1111/j.1600-0765.1999.tb02217.x. [DOI] [PubMed] [Google Scholar]