1. Background
National Natural Science Foundation of China (NSFC), Beijing-Hong Kong Academic Exchange Centre (BHKAEC), and the Chinese University of Hong Kong (CUHK) jointly organized a two-day Academic Symposium on Bone and Joint Degeneration and Regeneration at Cho Yiu Conference Hall of CUHK on November 10 and November 11 (2022), aiming at promoting and/or consolidating Mainland–Hong Kong collaborations among scientists in the musculoskeletal basic and clinical research field. Such collective efforts would facilitate scientific & technological innovation and clinical translation for preventing degeneration and enhancing regeneration of musculoskeletal disorders, a major health challenge in our aging society. The symposium was hosted by CUHK, co-organized by the China Engagement Office and the Department of Orthopaedics and Traumatology (ORT) of CUHK, as well as Journal of Orthopaedic Translation (JOT). It was held in a hybrid mode, including a physical meeting in CUHK for Hong Kong participants and online participation by mainland institutions, with a total of 33 speakers from Hong Kong and Mainland China. It was a milestone event during the challenging pandemic period. Many hot topics were funded by NSFC [1] and the Research Grant Council (RGC) of Hong Kong [2].
2. Opening ceremony
Professor Rocky S. Tuan, Vice-Chancellor and President of CUHK, Mr. Yongtao Zhang, Director of the Hong Kong, Macao and Taiwan Affairs Office of NSFC, Mr. Maozhou Liu, the inspector of the Department of Education and Technology of the Liaison Office of the Central People's Government in the Hong Kong Special Administrative Region, Mr. Hoi Shan Hsu, President of the Beijing-Hong Kong Academic Exchange Centre delivered opening speech. As VIP, Professor Mai-Har Sham, Chairman of the Academic Advisory Committee and Pro-Vice-Chancellor (Research) of CUHK, and Professor Ling Qin, President of the Symposium Organizing Committee, Assistant Dean (Mainland Affairs) of Faculty of Medicine, and Choh-Ming Professor of Orthopaedics and Traumatology of CUHK witnessed the opening ceremony.
Professor Rocky S. Tuan emphasized that the degeneration and regeneration of bone and joint is one of the key research directions of innovative biomedicine. He briefly introduced the exciting achievements made in these areas in recent years. In addition to supporting more cutting-edge research, CUHK has been actively promoting knowledge translation of R&D results, facilitating R&D practice, and enhancing collaboration with industries. CUHK is always open to collaborative scientific research with experts and scholars in the related fields.
Mr. Yongtao Zhang highlighted that the theme of this symposium on bone and joint degeneration and regeneration was of great significance to people's life and health. He trusted that the symposium would provide a platform for sharing and exchange of scientific research between Mainland and Hong Kong, promote more collaboration opportunities, and contribute to biomedical innovation and development in China.
Mr. Hoi Shan Hsu mentioned in his speech that this symposium would bring together experts and scholars from Hong Kong and Mainland to pursue greater breakthroughs based on cutting-edge biotechnologies, to address the unmet clinical needs. Such efforts would accelerate China's development into a leading country in biomedical and health technologies.
3. Keynote speeches
Professor Rocky S. Tuan, Professor Yingze Zhang (academician of the Chinese Academy of Engineering), and Professor Lin Chen (distinguished scholar at the Third Affiliated (Daping) Hospital of the Third Military (Army) Medical University, delivered keynote speeches in the symposium.
Professor Rocky S. Tuan presented his team's innovative work on the mini-Joint, a miniature, microfluidics-enabled platform to replicate human synovial joints. The mini-Joint is the first human cell-derived, multi-tissue system comprising of engineered osteochondral complex (OC), synovial-like fibrous tissue (SFT), and adipose tissue, all of which were bathed in a “simulated synovial fluid” [3]. These tissue components were engineered from human mesenchymal stem cells (MSCs) over 21–28 days, and could maintain their respective phenotypes in a “healthy” mini-Joint for four weeks [3]. To model synovitis, a common manifestation of osteoarthritis (OA), interleukin-1β (IL-1β) was added to the SFT-specific medium for one week. Pathological changes were observed in SFT as well as the other tissues, indicating active tissue crosstalk [3]. Particularly, transcriptomic changes in the chondral component of OC from the inflamed mini-Joint closely resembled those in human OA cartilage [3]. Furthermore, as a proof of concept, the inflamed mini-Joint was employed to test five drugs administered systemically or intra-articularly [3]. Thus, the mini-Joint is a promising organ-on-a-chip (OoC) platform for investigating joint disease mechanisms and testing potential drugs for the treating osteoarthritis (OA) and other joint diseases [3,4].
Professor Yingze Zhang reported that 14.2% of Chinese were elder than 65 years old in 2021. There are about eighty million patients suffering from osteoporosis (OP). Over 2 million of them suffered from OP fractures. The prevalence of OP reached 4.0% per the recent 5 years’ statistics. Meanwhile, there are about 120 million patients suffering from OA. Given the high morbidity rate, the ageing-related musculoskeletal disorders have generated heavy burdens to the healthcare system as well as our society. Professor Zhang and his team have also conducted a systematic analysis on the OA-related publications. They found that there are globally over 900 institutions working on OA investigations. About 1050 papers published during 2010–2022 focused on the transplantation of cartilage, presenting no reliable long-term outcomes. Currently, surgery is still the only therapeutic option for OA [5]. However, the revision rate at 2 years after total joint replacement is 5%–10% [6,7]. Professor Zhang recommended eliminating the mechanical imbalance via osteotomy at the proximal tibia or fibular at an early stage [8,9]. Accordingly, they developed an absorbable β-TCP/PLGA spacer (patent ref. No.: CN206381226U) to cost-effectively provide stability for the tibial osteotomy gap [10].
Professor Lin Chen introduced the progress in the identification of stem cells or progenitors related to abnormal bone formation, such as osteophytes and heterotopic ossification (HO). Per their experience, stem cells or progenitors, microenvironments, and molecular mechanisms are the trilogy of abnormal bone formation, which share common features with bone development. However, there is controversy on the cell origin of bone development and homeostasis based on commonly used transgenic and transplanting techniques, which can be largely clarified by comprehensive application of multiple techniques such as barcoding-based single-cell RNA-sequencing, endogenous knock-in CreERT2 mice and dual Dre and Cre recombinase-related (Dre/ROX and Cre-loxP) models for more precise genetic modification and lineage tracing. Professor Lin Chen reported that Fgfr3 (a key regulator of skeleton development) positive cells could be a new subset of skeletal stem cells contributing to bone homeostasis and abnormal bone formation. In addition, loss of Fgfr3 result in a pro-inflammatory microenvironment that promote trauma-induced HO [11] and degeneration-induced osteophyte [12]. These data provide insights for the prevention and treatment of ectopic osteogenesis-related diseases by targeting the Fgfr3+ subset of stem cells or using modulators of FGF signaling.
4. Academic sharing session
In this session, the topics were categorized into four main themes: (i) The interactive mechanism between bones and other organs, and between various tissues in bones and joints; (ii) Intrinsic and extrinsic factors affecting the homeostasis of the musculoskeletal system (precise regulation of cell biological functions, response to physicochemical stimuli); (iii) Research status and prospect of tissue engineering and drug delivery systems in bone and articular cartilage repair; (iv) Translational application progress driven by basic research and innovative technologies aiming at clinical problems and new technologies for diagnosis and treatment that need to be tackled in the future. Of note, most studies were multidisciplinary that covered more than one theme.
Joint injury and degeneration Osteoarthritis (OA) is considered as a whole organ disease, affecting not only cartilage itself [13], but also synovium [14], meniscus [14], and ligaments [15]. Professors Di Chen and Guozhi Xiao reported the crucial roles of Wnt/β-catenin and kindlin-2 (focal adhesion protein) in OA. Professor Jian Luo identified that a novel small molecule HL-43 could enhance articular cartilage regeneration via antagonizing prostaglandin E receptor 4 (EP4) [16]. Professor Weiguo Zou found that deficiency of ZMPSTE24 was a key mechanism behind the ageing-related catabolism of cartilage. Professor Jing Qu found that the stabilization of heterochromatin by CLOCK promoted stem cell rejuvenation and cartilage regeneration [17]. CLOCK mRNA was decreased in the joint of 15-month-old mice as compared to 1-month-old mice. Lentivirus mediated over-expression of CLOCK gene attenuated ageing-related articular degeneration in mice. In another study, Professor Jing Qu reported that reactivation of endogenous retroviruses (ERV) might be a potential driver for ageing-related articular degeneration, and intra-articular injection of either lentivirus delivered CRISPR-dCas9/sgERV or Abacavir could attenuate OA, suggesting that resurrection of ERV contributes to OA development and progression [18]. Professors Changhai Ding and Guanghua Lei have conducted extensive clinical studies. For example, Lei found that among patients aged 50 years and older with osteoarthritis, initial prescription of tramadol was associated with a significantly higher rate of mortality over 1 year of follow-up compared with commonly prescribed nonsteroidal anti-inflammatory drugs [19]. Based on clinical evidence, Ding found that greater infrapatellar fat pad (IPFP) volume was associated with greater knee cartilage volume and fewer structural abnormalities, suggesting a protective role of IPFP size in knee OA [20], while IPFP signal intensity alteration and texture score predicted knee structural changes and OA incidence [21,22]. Therefore, we must pay special attention to IPFP in the clinical settings for avoiding resection of normal IPFP in knee surgery. As the debate on the efficacy of hyaluronic acid for OA continues, Professor Hongwei Ouyang and his colleagues have implemented the integration of meta-analysis with bioinformatics analysis [23]. The results suggested that the administered HA activated both systemic and local pro-inflammatory immune responses, possibly limiting its efficacy. In addition, Professors Ouyang and Dongquan Shi also made efforts to classify OA. Indeed, there are distinct types of OA based on the molecular signatures [24,25]. Such data are of importance for guiding future clinical practices, matching well with the expectation of individualized treatment.
Both professors Jiakuo Yu and Qing Jiang focused on the tissue engineering of the meniscus [26,27]. Professor Quanyi Guo has established a non-invasive magnetic resonance imaging (MRI) method to quantitatively and dynamically evaluate the clinical outcomes of tissue-engineered cartilage [28]. The roadmap and experience on how to get the mentioned protocol recommended as a standard by International Organization for Standardization is a good reference for peers [28]. Professors Barbara Pui Chan and Patrick Shu-hang Yung have collaborated for fabricating an all-in-one osteochondral complex in GMP facilities and started to recruit cases in local hospitals (https://www.hku.hk/press/press-releases/detail/13743.html).
4.1. Bone physiopathology and regenerative strategies
Professor Xianghang Luo shared two papers recently published in Cell Metabolism [29,30]. The first one was about the identification of grancalcin (GCA) as a negative regulator of bone [29]. In aged rats and mice, the accumulative macrophages and neutrophils in the bone marrow secreted abundant GCA. Genetic deletion of Gca in neutrophils and macrophages delayed skeletal aging. In terms of mechanisms, grancalcin binds to the plexin-b2 receptor and inactivates the phosphorylation of FAK, SRC, and YAP [29]. Another paper reported that bone marrow macrophages-secreted reticulocalbin-2 (RCN2) increased during exercise and further promoted lipolysis, osteogenesis, and lymphopoiesis after binding to its functional receptor composed of neuronilin-2 and integrin β1 to activate cAMP-PKA signaling pathway [30]. Importantly, either grancalcin-neutralizing antibody or recombinant RCN2 can effectively attenuate ageing-related bone loss [29,30]. Professor Chao Wan presented his unpublished findings that global knockout of Cathepsin D (CtsD) dramatically decreased bone mass in mice. At the molecular level, the inactivation of CtsD in MC3T3-E1 cells attenuated osteoblastic differentiation and downregulated LC3B expression, which was accompanied by decreased levels of P62, p-Akt, and p-GSK3β in osteoblasts. Intriguingly, the inactivation of CtsD in RAW264.7 cells increased osteoclast differentiation with decreased LC3B expression but upregulated P62. The results suggest that CtsD mediated autophagy pathway plays important role in regulating of bone mass and homeostasis through the distinct mode of action in osteoblasts and osteoclasts. Professor Xiaochun Bai found that chaperone-mediated autophagy (CMA), a subclass of lysosomes, can degrade a variety of proteins that hinder osteogenic differentiation, such as adipogenic and chondrogenic differentiation determinants TLE3, ZNF423, and SOX9, thus balancing the osteogenic-adipogenic differentiation of stem cells. Furthermore, Professor Bai found that the deletion of RanGAP1 in the fast-proliferating osteogenic precursor cells (Osx-Cre) caused excessive activation of the spindle checkpoint, unbalanced separation of sister chromatids, chromosome instability and formation of aneuploidy, and uncontrolled cell proliferation, resulting in rapid formation of osteosarcoma (7 days after birth). In contrast, when RanGAP1 was knocked out in mature osteoblasts (Ocn-Cre), mice did not appear with bone tumors. RanGAP1 was commonly expressed in human osteosarcoma tissue cells, suggesting that loss of RanGAP1 may be a key driver of osteosarcoma development. Professor Liu Yang focused on the effects of sulfation on bone and joint degeneration. Through a variety of genetically modified mice and animal disease models, she identified key pathogenic mechanisms of sulfation defect, such as endoplasmic reticulum stress response in growth plate chondrocytes, osteoblast mechanosensitivity and lipid metabolism in articular chondrocytes, leading to skeleton development disorder and degeneration of bone and joint. These basic studies provide a valuable theoretical basis for future drug discovery of sulfation disorders.
Professor Jiacan Su integrated the emerging 3D printing skills and cell-based bottom-up fabrication to establish bone organoids, which construct biomimetic and hierarchical structures, including biomineralization and spatiotemporally features [31]. To tackle the challenging drawbacks of exogenous origins and variable composition of Matrigel, his team has also developed various hydrogel alternatives with adjustable material properties (stiffness, viscoelasticity, and charge), including polyethylene glycol (PEG), collagen, skin fibroin, alginate, gelatin, chitosan, and DNA derivative hydrogels, which have shown a promising application in organoid cultures [32]. Future efforts will no doubt bring them closer to model development and disease, as a tool for drug testing, and as a therapeutic approach.
Continuous efforts are made to establish more promising strategies for accelerating bone repair and even regeneration. Professors Ling Qin, Yuxiao Lai, and Jiankun Xu have designed and fabricated innovative magnesium-containing implants and scaffolds [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]. Two multi-centre clinical trials have been coordinated for the highly pure magnesium screw and magnesium powder containing polymer-based 3D scaffolds respectively, under the regulations of the National Medical Products Administration. In addition, based on preclinical models, magnesium also works for challenging conditions such as drug-related delayed fracture healing [40,44] and bone defect after trauma or dissection of bone tumors [45]. The Hong Kong–Mainland team is exploring more clinical indications with funding support from the Areas of Excellence Scheme (Ref. AoE/M402/20) by University Grant Council in Hong Kong, and the Mainland–Hong Kong Joint Funding Scheme by the Ministry of Science and Technology (MOST, Mainland) and Innovation and Technology Commission (ITC, Hong Kong). Professor Xin Zhao delivered an exciting breakthrough in the translation of the biomimicking hyperboloidal structure with the triply periodic minimal surfaces into three-dimensional tissue-engineered bone grafts and revealed that such structure enhanced osteogenesis and angiogenesis to support bone regeneration [46]. Professor Jiankun Xu presented a series of studies to shed light on the crucial role of sensory neuropeptide (calcitonin gene-related peptide, CGRP) on the coupling response of angiogenesis and osteogenesis, essential aspects towards functional bone regeneration [37,39,40,[47], [48], [49], [50]].
Nowadays, bone is recognized as an endocrine organ. Professors Hui Xie and Ren Xu have thoroughly investigated the bone specialized microenvironment [51,52], subsets of stem cells [53], and the remote control of osteo-factors [54,55]. Optineurin (OPTN), a macro-autophagy receptor, is found to play a pivotal role via degrading fatty acid binding protein 3 (FABP3) in bone metabolism [56]. Extracellular vesicles from a child gut can inhibit bone loss, suggesting the existence of a Gut-bone axis [57]. In contrast, extracellular vesicles derived from aged bone matrix favour adipogenesis of mesenchymal stem cells and augment the calcification of vascular smooth muscle cells [58]. More recently, it is reported that the young osteocyte-derived extracellular vesicles can even reduce the progress of Alzheimer's disease [54]. Osteocyte neuropeptide Y promotes bone marrow adipogenesis at the expense of osteogenesis by mesenchymal stem cells (MSCs) [55]. Osteoblasts, instead of osteoclasts, are the major source of skeletal SLIT3, an axon guidance cue involved in osteo-anabolism [59]. At the symposium, Professor Ren Xu also shared his unpublished work that preosteoblasts-produced SLIT2 could regulate the browning of adipose tissue and whole-body energy metabolism. Professor Gang Li has successfully applied the transverse tibial cortex transport technique to facilitate the healing of diabetic foot ulcers in patients [60], presenting with possible mechanisms including (a) systemic factors release to promote stem cells mobilization and wound healing; (b) regulate local inflammation such as macrophages transformation from M1 to M2 phase; (c) improvement in the lymphatic microcirculation functions. All these studies help delineate the complicated cross-talks between bone and other tissues/organs. New concepts on the “bone-vessel axis” and “bone-nerve axis” have been proven and consolidated.
4.2. Physical stimulations
Professor Louis WH Cheung mentioned that ∼70% of osteoporotic hip fracture patients concomitantly suffered from sarcopenia, yet most clinicians usually ignored sarcopenia when treating these patients as there was no promising drug for sarcopenia [61]. Professor Cheung and his team have developed a patented low-magnitude high-frequency vibration treatment to exhibit beneficial effects on accelerating osteoporotic fracture healing, as well as retarding osteoporosis and sarcopenia progression. The restored function of the neuromuscular junction is proposed as a key mechanism behind the efficacy. To prove this, Cheung is coordinating a project supported by a Collaborative Research Fund (Ref. C4032-21 GF).
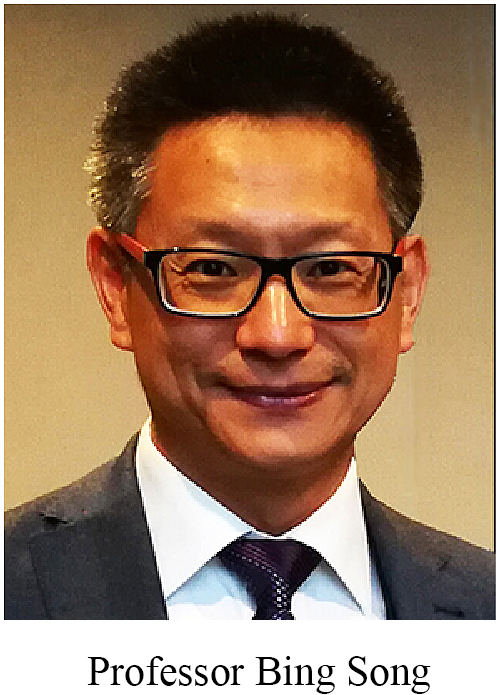
Professor Bing Song used physiological-level electrical stimulation (direct-current electric fields) to modulate the migration, proliferation, and differentiation of stem cells prior and after cell replacement therapy taking place [62]. G protein coupled receptors, PI3K/Akt, and Wnt/β-catenin signaling are responsible to electrical stimulation [63]. Professor Jiankun Xu also demonstrated that electrical stimulation at the lumbar dorsal root ganglion could significantly promote osteoporotic fracture healing in rats by elevating the synthesis and release of CGRP [64].
4.3. Drug delivery systems
Targeting delivery can improve therapeutic efficacy. Professor Jiang Xia added CAP to the exosomal membrane protein Lamp2b of exosomes and such system loading with microRNA-140 could more efficiently bind to chondrocytes to attenuate OA [65]. Similarly, when stem cell-binding peptide E7 was added to Lamp2b, the modified exosome exerted targeting capability to synovial fluid-derived MSCs and thus promoted cartilage regeneration [66]. Professor Chenjie Xu has developed a micro-needle with FDA-approved liquid crystalline polymer for transdermal drug delivery to reduce scar formation in the rabbit ear hypertrophic scar model and patients [67]. To conquer the challenges of cell delivery, his team designed cryogenic micro-needle patches by stepwise cryogenic micro-moulding of cryogenic medium with pre-suspended cells [68]. In the melanoma model of mice, the delivery of ovalbumin-pulsed dendritic cells via the cryomicroneedles could more efficiently boost immunological responses and inhibited tumour growth than intravenous and subcutaneous injections of the cells [68]. These innovative strategies show great potential to be integrated to promote the health of the musculoskeletal system.
5. Round table discussions
Discussions around a number of key issues and hot topics were chaired by Mainland coordinator Professor Di Chen and Hong Kong coordinator Professor Ling Qin.
As a novel enabling technology invented by a multidisciplinary team led by CUHK's President Professor Rocky S. Tuan, the mini-Joint system represents an emerging in vitro model of joint disorders and attracted lots of interest from the speakers and audience. Professor Zhong Alan Li from CUHK's Department of Biomedical Engineering described the technical details and challenges in developing joint-mimicking OoCs. It was suggested that future improvements to the mini-Joint system could include the introduction of neuronal components, application of mechanical signals (e.g., compression and shear), and inclusion of other joint tissues, such as meniscus and ligament. Similar comments were also provided for the bone organoid projects.
Precision diagnosis and treatment is the common objective of most projects as presented in the symposium. Cross-talks between multiple molecules, cell types, and tissues are the foundations. This symposium has provided a unique opportunity for sharing the up-to-date findings from the individual principal investigator. It is apparent that our interests are partially overlapped. Therefore, we should consider forming task force groups to investigate each theme as mentioned above.
Professors Hongwei Ouyang and Dongquan Shi emphasized the heterogeneity of OA. All the professors agreed that single team could not resolve all the questions. Professor Guanghua Lei suggested establishing a Mainland–Hong Kong alliance of OA investigations. The alliance would be responsible for the central management of the tissue bank, then assign subgroups of investigators to determine synovium fluid, synovium, cartilage, subchondral bone, etc, respectively. Through this approach, it would be also easier to reach a consensus on drug efficacy.
Professor Changhai Ding agreed that revised classifications of OA could better serve patients. However, there would still be a long way to go to establish practical guidelines. The bottleneck is a lack of accepted biomarkers. Approaches from in vitro mini-Joint system and different in vivo animal models to collecting samples and cohort studies to verify different phenotypes should be adopted. Professors Qing Jiang, Dongquan Shi, and Gang Li suggested that the markers in synovium fluid should be thoroughly analyzed first to make the classification approach easy to perform by the clinicians. Professor Di Chen commented that since it might be difficult to sort out the endogenous marker, alternative, exogenous labeling approaches would likely be more feasible. Professor Guozhi Xiao emphasized that whether it is the right time to promote the revised OA classifications in clinics, anyway, it is worthwhile to implement more in-depth mechanistic investigations. Professor Lin Chen addressed that the classifications of OA based on fundamental (molecular) research should collaborate with the clinically practical (imaging and pathological) guidelines gradually, and the dynamic changes of OA types, that is, it may shift to another type after treatment or progression. The selection of animal models and establishment of new OA models will be crucial. Therefore, Professor Lin Chen suggested starting experiments in the “mini-Joint” system reported by Professors Rocky S. Tuan and Zhong Alan Li.
Professors Hui Xie and Ren Xu also suggested forming a BONE alliance to better understand the complexity of cellular interactions, exosomes, and signaling transduction.
Professor Quanyi Guo commented that support from regulatory bodies is needed to accelerate the clinical translation of magnesium-based medical implants, and relatively mature products should be promoted to clinical practice as soon as possible to serve the patients. In the future, efforts should be made to empower structural bionics and individual customization.
Last but not the least, Professors Ling Qin and Gang Li introduced the development of the Journal of Orthopaedic Translation and invited submissions of high-quality manuscripts, such as perspectives, reviews, and original work, from the speakers’ groups, for a special issue to be published in July 2023. They also announced that CUHK would host the 2023 Tissue Engineering and Regenerative Medicine International Society Asia Pacific Conference (TERMIS-AP, conference website: https://ap2023.termis.org/) and welcomed submissions of relevant work for sharing with national and international colleagues in the field of tissue engineering and regenerative medicine. At the end of the NSFC-CUHK joint symposium, Professor Ling Qin showed a video in which TERMIS-AP 2023 Conference Chair Professor Rocky Tuan invited all to Hong Kong for the academic gathering during October 16–19, 2023, at Hong Kong Science Park.
6. Conclusions and future perspectives
In view of the current statues of basic research, clinical investigation, and industrial involvement for implementing the concept of precision medicine in tissue engineering and regenerative medicine, we still need to strengthen the following aspects in musculoskeletal research:
-
1)
Explore the risk factors that affect the occurrence and development of bone and joint degeneration in different groups of the population and formulate more precise prevention and intervention measures from the micro-level (gene, cell) to the macro-perspective (individual, group) to attenuate the progression of these diseases.
-
2)
Based on the basic status of the affected individual(s), the severity of degeneration, functional and imaging-based changes, physical and chemical indicators, and other factors, an individualized step-by-step treatment plan can be formulated for the patients.
-
3)
To resolve the deficiencies and drawbacks of surgical (especially revision surgery) techniques, implants, and instruments, we should leverage the latest technological development in the fields of materials, chemistry, physics, and artificial intelligence. Standards, guidelines, and consensus should be established on top of a series of proof-of-concept and translational studies to push forward innovative treatments, technologies, and products. With the guidance and support from policy makers and regulatory bodies, industry-academia collaboration and bench-to-bedside translation will be accelerated.
-
4)
Only by employing advanced technologies in related fields and through multidisciplinary integration, will we be able to clarify the spatiotemporal changes accompanying the initiation and development of degenerative bone and joint diseases at the molecular, cellular, and tissue levels from more diverse perspectives and in broader fields. Such efforts will enable us to better describe the molecular mechanisms underlying bone and joint diseases, explore related signaling pathways and key factors, identify more effective targets for theragnostic, and develop innovative biomaterials and drugs for treating bone and joint degeneration and disorders.

































Acknowledgements
We appreciate the great support and coordination from the organizers and co-organizers to ensure the accomplishment of this NSFC-CUHK Academic Symposium on Bone and Joint Degeneration and Regeneration.
Organizers: National Natural Science Foundation of China (NSFC), the Beijing-Hong Kong Academic Exchange Centre, and the Chinese University of Hong Kong (CUHK).
Co-organizers: China Engagement Office of CUHK, Department of Orthopaedics and Traumatology (ORT), and Journal of Orthopaedic Translation (JOT).
We appreciate the great efforts and technical supports from Ms. Wing Wong, Ms. Nicole Yang, Ms. Tiara Lau, Ms. Waie Pao, Ms. Mable Law, and Ms. Cathy Lo of CUHK China Engagement Office, and Professors Jiankun XU and Wenxue Tong for recording the conference minutes.
Contributor Information
Di Chen, Email: di.chen@siat.ac.cn.
Ling Qin, Email: lingqin@cuhk.edu.hk.
References
- 1.Lin J., Chen L., Dou D. Progress of orthopaedic research in China over the last decade. J Orthop Translat. 2020;24:131–137. doi: 10.1016/j.jot.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://www.ugc.edu.hk/eng/rgc/funding_opport/
- 3.Li Z., Lin Z., Liu S., Yagi H., Zhang X., Yocum L., et al. Human mesenchymal stem cell-derived miniature joint system for disease modeling and drug testing. Adv Sci. 2022;9(21) doi: 10.1002/advs.202105909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z.A., Sant S., Cho S.K., Goodman S.B., Bunnell B.A., Tuan R.S., et al. Synovial joint-on-a-chip for modeling arthritis: progress, pitfalls, and potential. Trends Biotechnol. 2022 doi: 10.1016/j.tibtech.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C., Schmid C.H., Iversen M.D., Harvey W.F., Fielding R.A., Driban J.B., et al. Comparative effectiveness of tai chi versus physical therapy for knee osteoarthritis: a randomized trial. Ann Intern Med. 2016;165(2):77–86. doi: 10.7326/M15-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L., Zheng J.J.Y., Li G., Yuan J., Ebert J.R., Li H., et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. J Orthop Translat. 2020;24:66–75. doi: 10.1016/j.jot.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo K., Au M., Ni J., Wen C. Association between hypertension and osteoarthritis: a systematic review and meta-analysis of observational studies. J Orthop Translat. 2022;32:12–20. doi: 10.1016/j.jot.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin D., Chen W., Wang J., Lv H., Ma W., Dong T., et al. Mechanism and influencing factors of proximal fibular osteotomy for treatment of medial compartment knee osteoarthritis: a prospective study. J Int Med Res. 2018;46(8):3114–3123. doi: 10.1177/0300060518772715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F., Ma W., Chen J., Cong W., Zhang Y., Yu T., et al. Prognostic factors for medial open-wedge high tibial osteotomy with spacer implantation in patients with medial compartmental knee osteoarthritis. J Orthop Surg Res. 2022;17(1):50. doi: 10.1186/s13018-022-02934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R., Li S., Yin Y., Guo J., Chen W., Hou Z., et al. Open-Wedge HTO with absorbable beta-TCP/PLGA spacer implantation and proximal fibular osteotomy for medial compartmental knee osteoarthritis: new technique presentation. J Invest Surg. 2021;34(6):653–661. doi: 10.1080/08941939.2019.1670296. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D., Huang J., Sun X., Chen H., Huang S., Yang J., et al. Targeting local lymphatics to ameliorate heterotopic ossification via FGFR3-BMPR1a pathway. Nat Commun. 2021;12(1):4391. doi: 10.1038/s41467-021-24643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuang L., Wu J., Su N., Qi H., Chen H., Zhou S., et al. FGFR3 deficiency enhances CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7 and aggravates joint destruction in mice. Ann Rheum Dis. 2020;79(1):112–122. doi: 10.1136/annrheumdis-2019-215696. [DOI] [PubMed] [Google Scholar]
- 13.Teunissen M., Meij B.P., Snel L., Coeleveld K., Popov-Celeketic J., Ludwig I.S., et al. The catabolic-to-anabolic shift seen in the canine osteoarthritic cartilage treated with knee joint distraction occurs after the distraction period. J Orthop Translat. 2023;38:44–55. doi: 10.1016/j.jot.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan W., Maimaitimin M., Zhao F., Fan Y., Yang S., Li Y., et al. The transplantation of particulated juvenile allograft cartilage and synovium for the repair of meniscal defect in a lapine model. J Orthop Translat. 2022;33:72–89. doi: 10.1016/j.jot.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu S.C., Cheng W.H., Cheuk Y.C., Mok T.Y., Rolf C., Yung S.H., et al. Development of vitamin C irrigation saline to promote graft healing in anterior cruciate ligament reconstruction. Journal of Orthopaedic Translation. 2013;1(1):67–77. [Google Scholar]
- 16.Jin Y., Liu Q., Chen P., Zhao S., Jiang W., Wang F., et al. A novel prostaglandin E receptor 4 (EP4) small molecule antagonist induces articular cartilage regeneration. Cell Discov. 2022;8(1):24. doi: 10.1038/s41421-022-00382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang C., Liu Z., Song M., Li W., Wu Z., Wang Z., et al. Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration. Cell Res. 2021;31(2):187–205. doi: 10.1038/s41422-020-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X., Liu Z., Sun L., Ren J., Wu Z., Jiang X., et al. Resurrection of human endogenous retroviruses during aging reinforces senescence. bioRxiv. 2021:2021. doi: 10.1016/j.cell.2022.12.017. 02.22.432260. [DOI] [PubMed] [Google Scholar]
- 19.Zeng C., Dubreuil M., LaRochelle M.R., Lu N., Wei J., Choi H.K., et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969–982. doi: 10.1001/jama.2019.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai J., Xu J., Wang K., Zheng S., He F., Huan S., et al. Association between infrapatellar fat pad volume and knee structural changes in patients with knee osteoarthritis. J Rheumatol. 2015;42(10):1878–1884. doi: 10.3899/jrheum.150175. [DOI] [PubMed] [Google Scholar]
- 21.Han W., Aitken D., Zhu Z., Halliday A., Wang X., Antony B., et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016;75(10):1783–1788. doi: 10.1136/annrheumdis-2015-208360. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Fu S., Gong Z., Zhu Z., Zeng D., Cao P., et al. MRI-Based texture analysis of infrapatellar fat pad to predict knee osteoarthritis incidence. Radiology. 2022;304(3):611–621. doi: 10.1148/radiol.212009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao K., Wen Y., Bunpetch V., Lin J., Hu Y., Zhang X., et al. Hype or hope of hyaluronic acid for osteoarthritis: integrated clinical evidence synthesis with multi-organ transcriptomics. J Orthop Translat. 2022;32:91–100. doi: 10.1016/j.jot.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan C., Pan Z., Zhao K., Li J., Sheng Z., Yao X., et al. Classification of four distinct osteoarthritis subtypes with a knee joint tissue transcriptome atlas. Bone Res. 2020;8(1):38. doi: 10.1038/s41413-020-00109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv Z., Yang Y.X., Li J., Fei Y., Guo H., Sun Z., et al. Molecular classification of knee osteoarthritis. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.725568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z.Z., Chen Y.R., Wang S.J., Zhao F., Wang X.G., Yang F., et al. Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus. Sci Transl Med. 2019;11(487) doi: 10.1126/scitranslmed.aao0750. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Yang L., Zhang K., Zhu L., Wang X., Jiang Q. Three-dimensional finite-element analysis of aggravating medial meniscus tears on knee osteoarthritis. J Orthop Translat. 2020;20:47–55. doi: 10.1016/j.jot.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L., Guo X., Chen J., Zhen Z., Cao B., Wan W., et al. Key considerations on the development of biodegradable biomaterials for clinical translation of medical devices: with cartilage repair products as an example. Bioact Mater. 2022;9:332–342. doi: 10.1016/j.bioactmat.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C.J., Xiao Y., Sun Y.C., He W.Z., Liu L., Huang M., et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell Metabol. 2021;33(10):1957–19573 e6. doi: 10.1016/j.cmet.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Peng H., Hu B., Xie L.Q., Su T., Li C.J., Liu Y., et al. A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis. Cell Metabol. 2022;34(8):1168–11682 e6. doi: 10.1016/j.cmet.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Chen S., Chen X., Geng Z., Su J. The horizon of bone organoid: a perspective on construction and application. Bioact Mater. 2022;18:15–25. doi: 10.1016/j.bioactmat.2022.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S.L., Wu X.M., Wang X.H., Su J.C. Hydrogels for bone organoid construction: from a materiobiological perspective. J Mater Sci Technol. 2023;136:21–31. [Google Scholar]
- 33.Luo Y., Zhang C., Wang J., Liu F., Chau K.W., Qin L., et al. Clinical translation and challenges of biodegradable magnesium-based interference screws in ACL reconstruction. Bioact Mater. 2021;6(10):3231–3243. doi: 10.1016/j.bioactmat.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J., Hu P., Zhang X., Chen J., Wang J., Zhang J., et al. Magnesium implantation or supplementation ameliorates bone disorder in CFTR-mutant mice through an ATF4-dependent Wnt/beta-catenin signaling. Bioact Mater. 2022;8:95–108. doi: 10.1016/j.bioactmat.2021.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian L., Sheng Y., Huang L., Chow D.H., Chau W.H., Tang N., et al. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials. 2018;180:173–183. doi: 10.1016/j.biomaterials.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Wu Y., Li H., Liu Y., Bai X., Chau W., et al. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials. 2018;157:86–97. doi: 10.1016/j.biomaterials.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Ye L., Xu J., Mi J., He X., Pan Q., Zheng L., et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120984. [DOI] [PubMed] [Google Scholar]
- 38.Zhu W.Y., Guo J., Yang W.F., Tao Z.Y., Lan X., Wang L., et al. Biodegradable magnesium implant enhances angiogenesis and alleviates medication-related osteonecrosis of the jaw in rats. J Orthop Translat. 2022;33:153–161. doi: 10.1016/j.jot.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng N.Y., Xu J.K., Ruan Y.C., Chang L., Wang X.L., Yao H., et al. Magnesium facilitates the healing of atypical femoral fractures: a single-cell transcriptomic study. Mater Today. 2022;52:43–62. [Google Scholar]
- 41.Li Y., Pan Q., Xu J., He X., Li H.A., Oldridge D.A., et al. Overview of methods for enhancing bone regeneration in distraction osteogenesis: potential roles of biometals. J Orthop Translat. 2021;27:110–118. doi: 10.1016/j.jot.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai Y., Li Y., Cao H., Long J., Wang X., Li L., et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019;197:207–219. doi: 10.1016/j.biomaterials.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Li C., Zhang W., Deng J., Nie Y., Du X., et al. 3D-printed NIR-responsive shape memory polyurethane/magnesium scaffolds with tight-contact for robust bone regeneration. Bioact Mater. 2022;16:218–231. doi: 10.1016/j.bioactmat.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y., Huang Z., Wang Y., Xu W., Chen H., Xu J., et al. The efficacy and safety of denosumab in postmenopausal women with osteoporosis previously treated with bisphosphonates: a review. J Orthop Translat. 2020;22:7–13. doi: 10.1016/j.jot.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long J., Zhang W., Chen Y., Teng B., Liu B., Li H., et al. Multifunctional magnesium incorporated scaffolds by 3D-Printing for comprehensive postsurgical management of osteosarcoma. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120950. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y., Xu T., Bei H.P., Zhang L., Tang C.Y., Zhang M., et al. Gaussian curvature-driven direction of cell fate toward osteogenesis with triply periodic minimal surface scaffolds. Proc Natl Acad Sci U S A. 2022;119(41) doi: 10.1073/pnas.2206684119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J., Xu J., Wang X., Sheng L., Zheng L., Song B., et al. Magnesium-pretreated periosteum for promoting bone-tendon healing after anterior cruciate ligament reconstruction. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120576. [DOI] [PubMed] [Google Scholar]
- 48.Xu J., Wang J., Chen X., Li Y., Mi J., Qin L. The effects of calcitonin gene-related peptide on bone homeostasis and regeneration. Curr Osteoporos Rep. 2020;18(6):621–632. doi: 10.1007/s11914-020-00624-0. [DOI] [PubMed] [Google Scholar]
- 49.Mi J., Xu J., Yao H., Li X., Tong W., Li Y., et al. Calcitonin gene-related peptide enhances distraction osteogenesis by increasing angiogenesis. Tissue Eng. 2021;27(1–2):87–102. doi: 10.1089/ten.TEA.2020.0009. [DOI] [PubMed] [Google Scholar]
- 50.Mi J., Xu J.K., Yao Z., Yao H., Li Y., He X., et al. Implantable electrical stimulation at dorsal root ganglions accelerates osteoporotic fracture healing via calcitonin gene-related peptide. Adv Sci. 2021 doi: 10.1002/advs.202103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie H., Cui Z., Wang L., Xia Z., Hu Y., Xian L., et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20(11):1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu R., Yallowitz A., Qin A., Wu Z., Shin D.Y., Kim J.M., et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24(6):823–833. doi: 10.1038/s41591-018-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debnath S., Yallowitz A.R., McCormick J., Lalani S., Zhang T., Xu R., et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562(7725):133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang Y.L., Wang Z.X., Liu X.X., Wan M.D., Liu Y.W., Jiao B., et al. The protective effects of osteocyte-derived extracellular vesicles against Alzheimer's disease diminished with aging. Adv Sci. 2022;9(17) doi: 10.1002/advs.202105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y., Chen C.Y., Liu Y.W., Rao S.S., Tan Y.J., Qian Y.X., et al. Neuronal induction of bone-fat imbalance through osteocyte neuropeptide Y. Adv Sci. 2021;8(24) doi: 10.1002/advs.202100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Z.Z., Hong C.G., Hu W.B., Chen M.L., Duan R., Li H.M., et al. Autophagy receptor OPTN (optineurin) regulates mesenchymal stem cell fate and bone-fat balance during aging by clearing FABP3. Autophagy. 2021;17(10):2766–2782. doi: 10.1080/15548627.2020.1839286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J.H., Chen C.Y., Liu Z.Z., Luo Z.W., Rao S.S., Jin L., et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. 2021;8(9) doi: 10.1002/advs.202004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z.X., Luo Z.W., Li F.X., Cao J., Rao S.S., Liu Y.W., et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun. 2022;13(1):1453. doi: 10.1038/s41467-022-29191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li N., Inoue K., Sun J., Niu Y., Lalani S., Yallowitz A., et al. Osteoclasts are not a source of SLIT3. Bone Res. 2020;8:11. doi: 10.1038/s41413-020-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G., Ling S., Li H., Zhang Y., Hu J. How to perform minimally invasive tibial cortex transverse transport surgery. J Orthop Translat. 2020;25:28–32. [Google Scholar]
- 61.Wang J., Cui C., Chim Y.N., Yao H., Shi L., Xu J., et al. Vibration and beta-hydroxy-beta-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J Cachexia Sarcopenia Muscle. 2020;11(2):564–577. doi: 10.1002/jcsm.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song B., Zhao M., Forrester J.V., McCaig C.D. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci U S A. 2002;99(21):13577–13582. doi: 10.1073/pnas.202235299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao M., Song B., Pu J., Wada T., Reid B., Tai G., et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442(7101):457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 64.Mi J., Xu J.K., Yao Z., Yao H., Li Y., He X., et al. Implantable electrical stimulation at dorsal root ganglions accelerates osteoporotic fracture healing via calcitonin gene-related peptide. Adv Sci. 2022;9(1) doi: 10.1002/advs.202103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 66.Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- 67.Yeo D.C., Balmayor E.R., Schantz J.T., Xu C. Microneedle physical contact as a therapeutic for abnormal scars. Eur J Med Res. 2017;22(1):28. doi: 10.1186/s40001-017-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang H., Chew S.W.T., Zheng M., Lio D.C.S., Wiraja C., Mei Y., et al. Cryomicroneedles for transdermal cell delivery. Nat Biomed Eng. 2021;5(9):1008–1018. doi: 10.1038/s41551-021-00720-1. [DOI] [PubMed] [Google Scholar]



