Abstract
Hypoxia is a prominent feature of malignant tumors and contributes to tumor proliferation, metastasis, and drug resistance in various solid tumors. Therefore, improving tumor oxygenation is crucial for curing tumors. To date, multiple strategies, including oxygen delivering and producing materials, have been designed to increase the oxygen concentration in hypoxic tumors. However, the unsustainable supply of oxygen is still the main obstacle, resulting in a suboptimal outcome in treating oxygen-deprived tumors. Thus, a sufficient oxygen supply is highly desirable in the treatment of hypoxic tumors. Photosynthesis, as the main source of oxygen in nature through the conversion of light energy into chemical energy and oxygen, has been widely studied in scientific research. Moreover, photosynthetic microorganisms have been increasingly applied in cancer therapy by increasing oxygenation, which improves the therapeutic effect of oxygen-consuming tumor therapeutic tools such as radiotherapy and photodynamic therapy. In this review, we summarize recent advances in the design and manufacture of live bacteria as oxygen shuttles for a new generation of hypoxic tumor treatment strategies. Finally, current challenges and future directions are also discussed for successfully addressing hypoxic tumor issues.
Keywords: Oxygen shuttles, Hypoxic tumor, Photosynthesis, Engineered bacteria
Graphical abstract
1. Introduction
Solid tumor interior is far from the blood vessels and is in a state of hypoxia, which is caused by the rapid growth of the tumor [1,2]. Hypoxia not only confers tumor tolerance to conventional therapies, such as radiotherapy (RT) [3,4], chemotherapy [5] and photodynamic therapy (PDT) [[6], [7], [8]], but also facilitates tumors more invasive and metastasis. To overcome tumor hypoxia, various strategies have been developed in recent years. For example, hyperbaric oxygen (HBO) and oxygen (O2)-binding haemoglobin (Hb) has been developed to deliver O2 into the anoxic area for improved tumor oxygenation. In addition, the combination of HBO with RT has also been used for improved RT in clinic [[9], [10], [11]]. However, HBO treatment has serious side effects such as air pressure trauma, lung injury and oxygen poisoning, leading to that HBO has not been widely used in RT [12]. Therefore, novel O2 delivering strategies for the treatment of hypoxic tumors are highly desirable.
With the emergence of nanotechnology, functionalized nanomaterials are gradually being used as adjunctive agents for oxygen-dependent therapies [13,14]. Researchers have designed a variety of nanoscale oxygen shuttles with unique physicochemical properties for increasing intertumoral oxygenation [[15], [16], [17]]. Recent studies have shown that oxygen shuttles with a high affinity for oxygen such as Hb, perfluorocarbon (PFC) and metal-organic framework (MOF) [18,19] could deliver O2 directly to the tumor, and significantly improve the efficacy of oxygen dependent PDT and RT. For example, Cai et al. combined human serum albumin (HSA) with Hb through intermolecular disulfide bonds to obtain a hybrid protein as an oxygen nanocarrier. Photosensitizer chlorin e6 (Ce6) was then encapsulated for enhanced PDT [20]. The nanodrug (C@HPOC) significantly enhanced the effect of PDT while relieving tumor oxygen. In addition, our group has used zirconium (Zr (IV))-based MOF (UIO-66) as an oxygen storage carrier and constructed self-sufficient oxygenated nanoparticles to successfully deliver oxygen and photosensitizer into hypoxic tumor [21]. Under 808 nm laser irradiation, the photothermal agent indocyanine green (ICG) promoted the release of O2 from UIO-66. The released O2 significantly improved the treatment efficiency of PDT against hypoxic tumors. In addition to the improved PDT, increasing the oxygen content in tumors could also increase the sensitivity of RT to hypoxic tumors. For example, Wang et al. built a graded nanodroplet systemusing perfluorooctyl bromide (PFOB) as an oxygen reservoir [22]. Gold nanoparticles wrapped in nanodroplets could concentrate the radiation energy and cause significant DNA damage. Under ultrasound, the burst oxygen release was triggered to reserve the oxygen loss, thus strengthening the RT-induced DNA damage while simultaneously inhibiting DNA repair. In addition to O2 delivering, O2 production in situ by undergoing chemical reactions is also a promising strategy for improved chemotherapy and PDT [23,24]. Hydrogen peroxide (H2O2) has been indicated to over-produce in tumor tissue due to abnormal metabolic processes [[25], [26], [27], [28], [29]]. Some highly active compounds such as manganese dioxide (MnO2) and cerium oxide (CeO2) can react with H2O2 in the tumor microenvironment to produce O2 for hypoxic tumor therapy [[30], [31], [32]]. Liu et al. synthesized a MnO2 nanoparticle based on polyacryllamine hydrochloride (PAH), which was further coupled to the photosensitizer Ce6 to obtain a nanocatalytic drug [7]. This nanocatalytic drug used MnO2 nanoparticles to catalyze the endogenous H2O2 to generate oxygen, thus effectively alleviatingthe tumor oxygen loss and further enhancing the effect of the PDT treatment of Ce6. Photocatalytic nanosystems based on BiO2-x nanosheets (BiO2-x NSs) were also prepared by Gu et al. [33]. Bismuth (Bi), as a high Z element, could effectively improve RT by depositing higher radiation doses in tumor sensibility. Moreover, the BiO2-x NSs could catalyse the breakdown of the highly expressed H2O2 at the tumor site into O2 to alleviate the tumor oxygen loss, thus enhanced the effect of RT. However, due to the low endogenous H2O2 concentration, the oxygen production efficiency is pretty low [[34], [35], [36]]. To overcome this limitation, previously, we have reported a calcium peroxide (CaO2)-based and pH-responsive nanocatalytic for enhanced chemotherapy. In our system, cobalt (Co)-based MOF (ZIF-67) was used to coat doxorubicin (DOX)-loaded CaO2 (CaO2@DOX@ZIF-67) [37]. In tumor acidic environments, CaO2, DOX, and Co2+ would be released rapidly as the outermost ZIF-67 decomposes. Unprotected CaO2 were then reacted with H2O to produce O2 and H2O2, and the increased O2 levels alleviated the intertumoral oxygen deficiency, thus further improving the therapeutic effect of DOX. Although promising, the above oxygen enhancement strategies usually induced a burst release of oxygen within a very short time, and thus the oxygen enhancing capacity was limited. This restricted the improvement of tumor oxygen deficiency through endogenous oxygen production strategies. Another big challenge is that the accumulation of nanodrugs in the hypoxic tumor area is affected by physical barriers such as the dense extracellular matrix, high intratissue fluid pressure and an irregular blood supply [[38], [39], [40]]. The efficient penetration into hypoxic tumor tissue through passive targeting is very limited, which leads to the failure of the desired therapeutic effect [41,42]. Thus, developing hypoxia-targeting oxygen shuttles with efficient tumor penetration and controllable oxygen release ability is highly desirable.
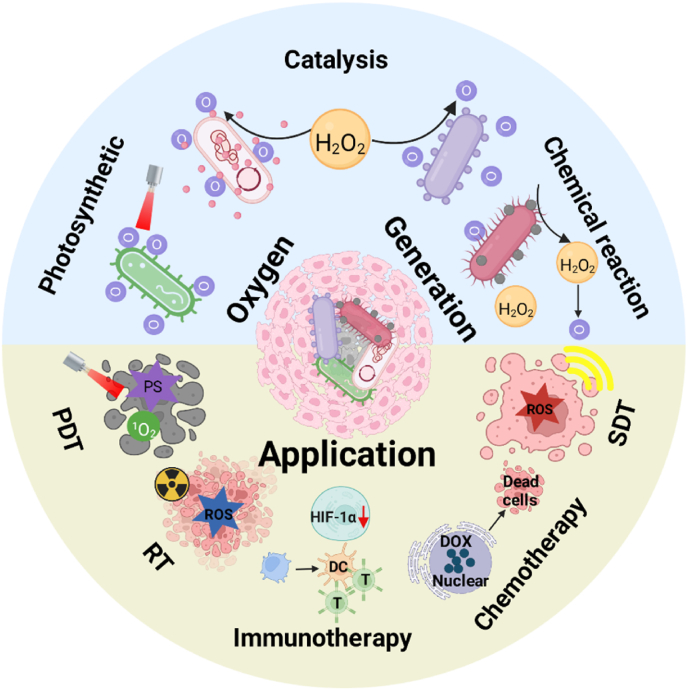
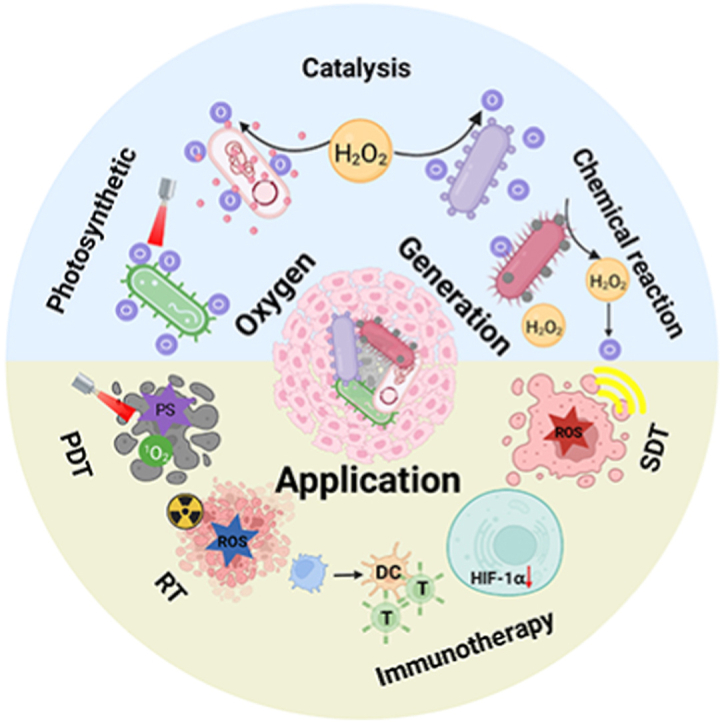
Among the existing targeted therapies, bacterial therapy has been used for more than a century [43,44]. Furthermore, hybrid biological microswimmers have recently been shown to play an active role in targeted delivery and in vitro biomedical applications [45,46]. We have also constructed hypoxia-targeting systems based on facultative anaerobic bacteria and photosynthetic bacteria (PSB) for the treatment of hypoxic tumors [47]. This work was the first report to discover and confirm that PSB has a photothermal transformation capability. Therefore, PSB could be used as both a photothermal agent and hypoxia-targeting carrier to achieve efficient treatment against hypoxic tumors without any post-modification or drug introduction. Moreover, our results showed that PSB with hypoxia and near-infrared chemotaxic capabilities could target tumor core regions. In addition to playing a role as a carrier in cancer therapy, bacteria were also widely used for immunotherapy since William Coley injected heat-killed bacteria (“Coley's Toxins”) into tumor patients to activate the immune system to fight cancer in the late nineteenth century [48,49]. Bacterial-derived molecules such as peptidoglycan, lipopolysaccharide (LPS) and lipoophosphoic acid could provide powerful immunostimulatory signals. They mainly bind to the pattern recognition receptors (PRR) expressed by innate immune cells such as dendritic cells (DCs) and macrophages to activate immune cells for tumor cell elimination [50,51]. For example, Wei et al. attached resiquimod (R848), a toll-like receptor 7/8 (TLR7/8) agonist, on ethylene glycol chitosan-modified Escherichia coli (E. coli) (EcPR848) to enhance the antitumor effect of cancer therapy [52]. EcPR848 could target hypoxic tumors and release R848 after phagocytosis by M2 macrophages. Both R848 and E. coli could polarize M2 macrophages into M1 type for enhanced immunotherapy. In addition, bacteria were also used as tumor vaccine adjuvants. Jing et al. coupled the neoantigen peptide with yeast polysaccharide shell particles by click chemistry to synthesize a novel tumor vaccine delivery system [53]. Bacteria-based tumor vaccines could stimulate the body to produce a potent antigen-specific CD8+ T cell immune response. There are at least two advantages of live bacteria-based oxygen shuttles for enhanced hypoxic tumors therapy: 1) photosynthetic microorganisms can produce oxygen via photosynthesis under light and hypoxic conditions, providing a green and efficient biological oxygen source for hypoxic tumors, and 2) anaerobic or facultative anaerobic bacteria can use their hypoxic properties to target tumors. Thus, the combination of bacterial hypoxia targeting and continuous oxygen production can effectively reverse the hypoxic microenvironment of the tumor and thus achieve the desired therapeutic effect. In this review, we systematically describe the design of live bacteria-based shuttles, including how bacteria increase tumor oxygenation, their underlying mechanisms, their effect on tumor oxygenation and their efficacy for improving various cancer treatments (see Fig. 1 and Table 1).
Fig. 1.
Microorganism-based oxygen shuttles for enhanced hypoxic tumor therapy.
Table 1.
Representative applications and strategies of microorganism-based oxygen shuttles in the treatment of hypoxic tumors.
| Microorganism type | O2 generation mechanism | Mode of cancer therapy | References |
|---|---|---|---|
| Cyanobacteria | Photosynthesis | PDT SDT RT |
[6,64,65,67,68,71,79,80,82] [83] [88,89] |
| Chlorella | Photosynthesis | PTT and Chemotherapy PDT RT CDT |
[96] [99] [101,102] [105] |
| Escherichia coli | Engineered E. coli overexpressed CAT to catalyze endogenous O2 production by H2O2. Magnetic Fe3O4 nanoparticles on the surface of E. coli would catalyse O2 production by H2O2 in situ. E. coli was directly used as the reducing agent to react with the KMnO4 aqueous solution via ultrasonication, and the generated MnOX can react with H2O2 to produce O2. |
PDT and PTT RT CDT PDT and Chemotherapy |
[110] [111] [116] [120] |
| Shewanella oneidensis | The MnO2 nanosheets were modified on the MR-1 surface to catalyze the endogenous H2O2. | Immunotherapy | [114] |
| Spirulina platensis | Photosynthesis | RT and PDT | [117] |
2. Oxygen shuttles produced oxygen for enhanced tumor therapy
2.1. Cyanobacteria-based oxygen production system
2.1.1. Cyanobacteria improved PDT efficiency
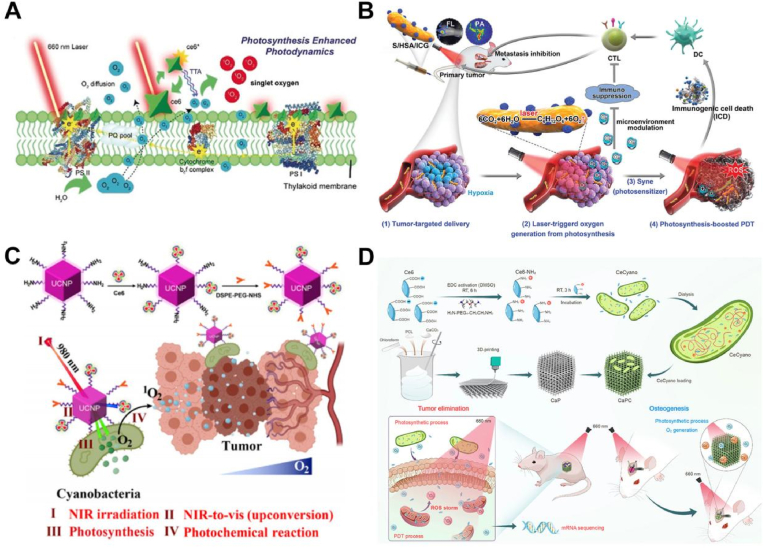
On primitive earth, oxygen began to accumulate in the atmosphere due to bacterial photosynthesis. Algae are among the earliest photosynthetic autotrophs on earth and can efficiently produce O2 by photocatalysis [54]. Microalgae, as photoautotrophs, have recently been used as unique oxygenators for wound healing and hypoxia-related tumor therapy [47,55]. In particular, microalgae have been widely used in the fields of food, biofuels, health products and environmental control [56,57]. Recently, microalgae have been of considerable use in biomedical fields for applications such as bioanalysis, tissue engineering, drug delivery and tumor therapy, and they exhibit high biocompatibility both in vivo and in vitro [[58], [59], [60]]. Studies by Woo et al. used the cyanobacterium Synechococcus elongatus to increase tissue oxygenation, maintain myocardial metabolism, and greatly improved cardiac function [61]. It was also suggested that cyanobacteria therapy was nontoxic and would not cause a meaningful immune response. In addition, Zhou et al. designed Spirulina platensis-based oral drugs in the treatment of two intestinal diseases, colon cancer and colitis [62], which expanded medical microalgae agents’ application. Thus, there was an increasing interest in advances in photosynthetic biomaterials and therapies. However, no previous study has demonstrated their safety in human patients. This gap was a major obstacle to translating photosynthetic therapies into clinical practice. In an early clinical trial, researchers implanted photosynthetic scaffolds containing high concentrations of Chlamydomonas reinhardtii into eight patients with full-thickness skin wounds [63]. Over the subsequent 90 days, the photosynthetic scaffolds did not trigger any harmful local or systemic immune responses and allowed full tissue regeneration. It was the first demonstration that photosynthetic cells could be safely implanted in human patients for tissue regeneration. Therefore, algae could be used as green oxygen donors to improve the oxygenation of the tumor microenvironment (TME). Inspired by this, Shi et al. first injected the photosensitizer Ce6 into cyanobacteria (Syne7942) for enhanced PDT [64]. Fig. 2A showed that under irradiation of 660 nm laser, the biological hybrid carried out continuous photosynthesis through the cyanobacteria, achieved continuous oxygen release and rapid and efficient production of singlet oxygen (1O2), which proved that oxygen production in microbial nanomedicine could enhance PDT for tumor treatment. In addition, Cai et al. used an autotrophy Syne7942 as an oxygen-producing carrier for ICG-coated HSA nanoparticles (HSA/ICG NPs) delivery [65]. Under irradiation with a 660 nm laser, the cyanobacteria produced a large quantity of oxygen through photosynthesis. The oxygen producing in situ effectively reversed the immunosuppressive TME and enhanced the antitumor immune response mediated by ICD (Fig. 2B). In 2015, Synechococcus elongatus UTEX 2973 (S.2973) was isolated, showing the advantages of rapid growth and good light resistance. More importantly, S.2973 exhibited a robust and promising chassis for biomedical applications because its optimal growth temperature was similar to that of humans [66]. Therefore, Zhang et al. encapsulated mesoporous nanoparticles and an S.2973-mediated biologic oxygen pump in a hydrogel (ALG-MI-S.2973) [67]. First, after intratumoral injection of ALG-MI-S.2973, S.2973 efficiently produced O2 under a 640 nm laser, thus relieving the hypoxic TME. After the release of ICG, efficient PDT and tumor growth inhibition were found under an 808 nm laser. In this system, the growth of S.2973 and the triggering PDT were chosen two different lasers, which avoided killing S.2973 with PDT-induced reactive oxygen species (ROS) in the early stage, thus ensuring maximum oxygen production. The 808 nm laser-induced PDT simultaneously cleared S.2973, further ensuring the biosafety of the system. Hao et al. integrated Ce6-containing cyanobacteria onto a 3-dimensional (3D) printed calcium carbonate (CaCO3-PCL) scaffold to build a multifunctional, delicate photosynthetic oxygen autologous platform [68]. First, Ce6 was internalized into photosynthetic cyanobacteria (CeCyano) and then the 3D-printed CaCO3-PCL scaffold was constructed and customized based on personalized data with the assistance of computer-aided design software. The constructed CeCyano cells were then decorated on the cap scaffold to complete the final treatment platform (CAPC). Under 660 nm laser irradiation, the engineered CAPC scaffold generates oxygen through photosynthesis and subsequently activates Ce6, resulting in enough 1O2 production for efficiently combating osteosarcoma (OS) in vivo (Fig. 2D). More importantly, the excess oxygen produced could also promote cell proliferation and differentiation, thus promoted bone regeneration after the synergistic action of OS with the 3D-printed scaffolds. Furthermore, along with the repair of bone injury, the scaffold gradually degraded and released CaCO3 to support new bone generation. Enhanced osteosarcoma PDT efficiency was achieved by enabling photosynthetic oxygenation-induced tumor hypoxia remission and promoting bone regeneration with subsequent local oxygenation.
Fig. 2.
A. Mechanistic diagram of CeCyan generating oxygen and 1O2 by CeCyan under 660 nm laser [64]. B. Schematic illustration of HSA/ICG conjugated Syne as an in situ photocatalyzed oxygen generation system for metastatic tumor immunogenic PDT [65]. C. Schematic illustration of S–UCNP–Ce6 for enhanced PDT of hypoxic tumors [6]. D. The CeCyano-based 3D printed CaCO3-PCL scaffold was used for enhanced PDT of osteosarcoma [66]. Adapted with permission from Ref. [64] Copyright 2019, Wiley-VCH; adapted with permission from Ref. [65] Copyright 2020, Wiley-VCH; adapted with permission from Ref. [6] Copyright 2020, Springer Nature; adapted with permission from Ref. [66] Copyright 2021, Elsevier.
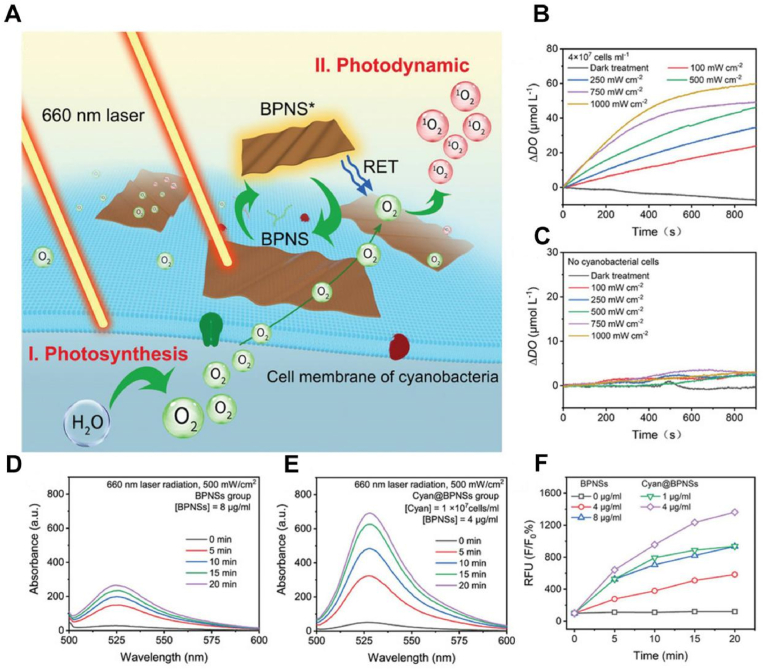
However, traditional photosensitizers (such as porphyrin derivatives) are prone to self-aggregation because of their hydrophobicity under physiological conditions, resulting in low bioavailability and photodynamic yield [69,70]. Black phosphorus nanosheets (BPNSs) have attracted wide attention because of their unique two-dimensional layered structure and physical-chemical properties. Owing to their broad absorption in ultraviolet and visible light, good biocompatibility, and high efficiency of PDT [71,72], two-dimensional BPNSs have broad application prospects in the field of biomedicine, particularly in tumor therapy [73]. Consequently, Shi et al. hybridized cyanobacteria (Cyan) with inorganic nanophotosensitizer BPNSs to obtain a new type of bioreactor (Cyan@BPNSs) (Fig. 3A) [74]. Under irradiation with a 660 nm laser, Cyan continued to produce oxygen in situ through photosynthesis. As shown in Fig. 3B–F, BPNSs induced a large amount of oxidative stress at the tumor site with strong cytotoxicity to tumor cells. However, the application of visible light in vivo is seriously limited due to the short wavelength of visible light and poor penetrating ability. Compared with visible light, near-infrared light (NIR) could penetrate deep tissue and causes less damage to normal tissue [75]. Upconversion nanoparticles (UCNPs) had the ability to convert long wavelength light into short wavelength light, which effectively overcame the limitation of visible light [[76], [77], [78]]. Inspired by this, we have conjugated Ce6 and UCNPs on cyanobacteria to construct a self-oxygenated photodynamic system (Fig. 2C) [6]. As a light-trapping antenna, UCNPs could convert excited light of 980 nm into visible light and 660 nm light, which could be used for oxygen production by cyanobacteria and 1O2 production by photosensitizers, respectively. Our near-infrared-driven self-oxygen nanosystem significantly improved the efficacy of PDT in the treatment of hypoxic tumors. Similarly, Wang and Shi also loaded UCNPs on the surface of cyanobacteria to convert 980 nm wavelength light into red luminescence suitable for inducing oxygen production in cyanobacteria, which greatly enhanced oxygenation in the deep tumor tissue [79,80]. Although UCNPs solved the problem of the low penetration depth of visible light in tissue, the overheating caused by long-term laser irradiation posed an unignored risk for in vivo applications [81]. Therefore, the optimal design of suitable light sources and their adaptability to cyanobacteria oxygen evolution was still a considerable challenge. Shi et al. introduced a new type of blue persistent luminescence material (PLM) into the oxygen production cyanobacteria to construct a novel exogenous radiation-free antitumor platform [82]. First, cyanobacteria were pre-treated with CaAl2O4: Eu, Nd (CAO PLM) in vitro. In the process of UV lamp pre-excitation and LED re-excitation, the broad blue persistent emission was triggered due to the existence of the 5d-4f electron transition and electron trapping of Eu2+. In addition, CAO PLM could store part of the light energy and then act as a light source in vivo to achieve long-term excitation of cyanobacteria and continuous oxygen production while avoiding the side effects of long-term light excitation.
Fig. 3.
A. The process of oxygen generation and photosensitization of Cyan@BPNSs in vitro. B. The relative dissolved oxygen concentration curves for solutions containing cyanobacteria C. and no cyanobacterial cells. D. Fluorescent map of singlet oxygen sensor green (SOSG) at BPNSs and E. Cyan@BPNSs group at different time under laser radiation (660 nm, 500 mW/cm2). F. Relative SOSG fluorescence intensity of different concentrations of BPNSs and Cyan@BPNSs under 660 nm laser irradiation [74]. Adapted with permission from Ref. [74] Copyright 2019, Wiley-VCH.
2.1.2. Oxygen-producing cyanobacteria for enhanced SDT and RT
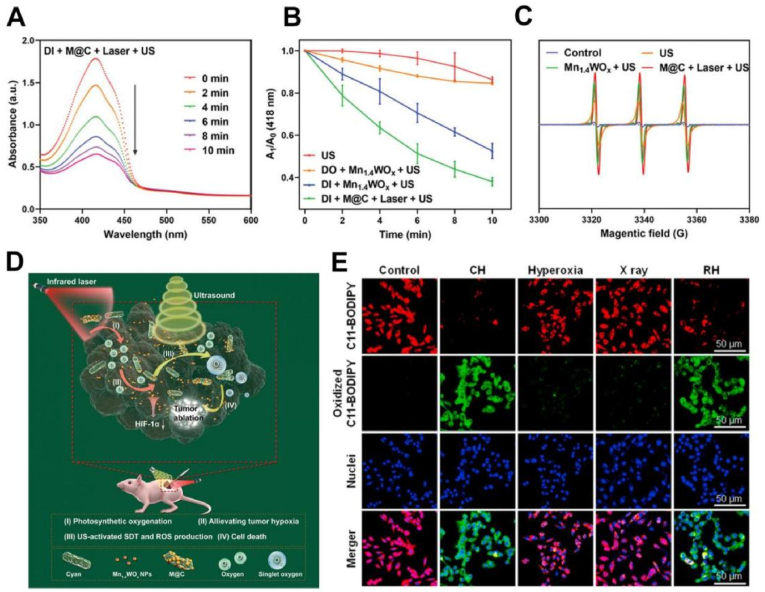
Insufficient oxygen supply has become a major obstacle that restricts the efficiency of acoustic dynamic therapy (SDT). Hu et al. designed an oxygen self-sufficiency system (M@C) with cyan as an oxygen self-donor and ultrasmall anoxic double metal oxide Mn1.4WOX nanoparticles as an acoustic sensitizer to improve the efficacy of SDT on hypoxic tumors (Fig. 4D) [83]. Under light irradiation, cyanobacteria could continuously produce oxygen to relieve tumor hypoxia. Fig. 4A–C showed that the acoustic sensitizer Mn1.4WOX could produce ROS through ultrasound-activated SDT, and the high concentration of O2 significantly improved the efficiency of ROS production to induce apoptosis in cancer cells. Importantly, the abundant photosynthetic oxygenation in tumors suppressed the gene expression of hypoxia-inducible factor-1 (HIF-1α), ultimately achieving the goal of tumor eradication. As the traditional RT is ineffective in treating anaerobic tumors and will damage the normal tissue, many nanomaterials containing high atomic number elements have been used as RT sensitizers to enhance the production of free radicals and thus improve the efficiency of RT [84,85]. However, most of these RT sensitizers were oxygen dependent, and thus the continuous oxygen consumption in tumor tissue significantly limits the production of free radicals, making it difficult to exert the maximum effect [86,87]. Therefore, Zhang et al. introduced photosynthetic deoxygenic cyanobacteria into a radiosensitizing platform based on 2D bismuth nanosheets to effectively regulate the hypoxic TME tolerated by radiotherapy [88]. The continuous photosynthetic oxygen released from cyanobacteria effectively alleviated the hypoxic state of the tumor. Simultaneously, under continuous irradiation with X-rays, the radiosensitizer 2D bismuth produced large amounts of ROS and then broke DNA strands in tumor cells. Notably, newly generated oxygen in TME caused further DNA breaks, which significantly improved the effect of radiotherapy and inhibited tumor growth in vivo. Li et al. prepared photosynthesis microcapsules (PMC) by combining cyanobacteria with UCNPs through electrostatic droplets in alginate microcapsules (MC) [89]. PMC were capable of photosynthesis under radiation from NIR and provided durable and controlled oxygen cooperation in a biological environment. PMC-supplemented O2 inhibited HIF-1α production in cancer cells and significantly inhibited cancer cell replication in a nontoxic manner. Subsequently, they combined PMC with X-rays to increase the sensitivity of malignant melanoma to ionizing radiation. This system could enhance the radiosensitivity of melanoma cells by activating lipid peroxidation and ferroptosis. Driven by NIR (980 nm), PMC could continuously photosynthesize in tumors to build a hyperoxia microenvironment. The combination with X-rays caused lipid peroxidation in melanoma cells and individual tumor graft cells in vivo (Fig. 4E). The accumulation of lipid peroxides would induce ferroptosis-induced cancer cell death that synergized with X-rays. Thus, combination therapy could overcome the intrinsic and acquired drug resistance of melanoma and further suppress tumor metastasis. In conclusion, Cyanobacteria could produce large amounts of oxygen under the light to relieve hypoxic tumors, significantly improving the efficacy of oxygen-dependent therapy.
Fig. 4.
A. The change of 1,3-diphenylisobenzofuran (DPBF) treated with M@C under laser and US irradiation for different times. B. Quantitative analysis of DPBF oxidation after different treatments. C. The electron spin resonance (ESR) spectroscopy detected ROS generation after different treatments. D. Schematic illustration of M@C for enhancing the SDT efficiency in hypoxic tumors [83]. E. Images of the radiation-hyperoxia-induced lipid-peroxidation in cells after different treatments [89]. Adapted with permission from Ref. [83] Copyright 2021, Wiley-VCH; adapted with permission from Ref. [89] Copyright 2019, Elsevier.
2.2. Small algae-based oxygen production system
2.2.1. Chlorellain PDT
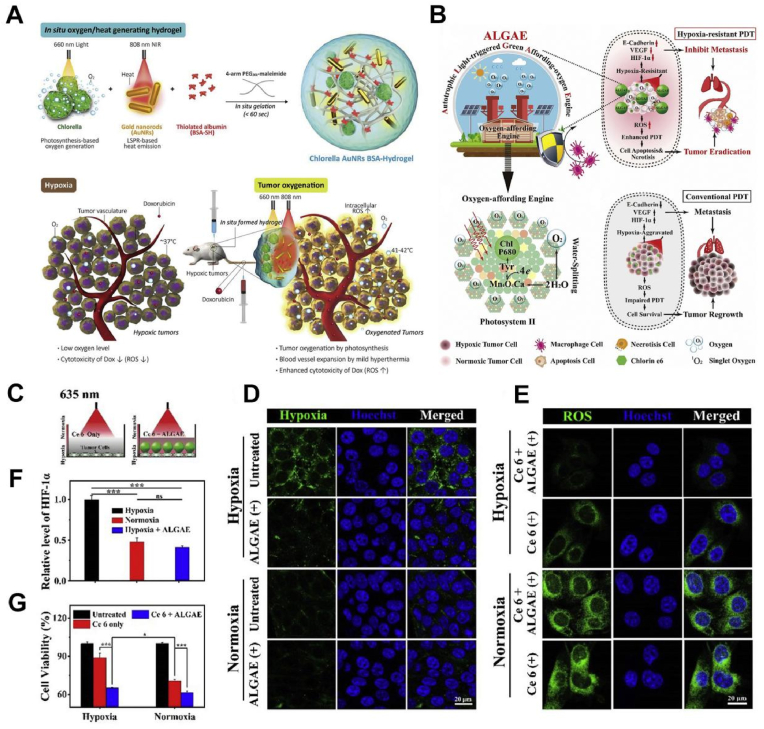
Chlorella pyrenoidosa is a unicellular autotrophic photosynthetic green alga rich in protein, fat, carbohydrates and vitamins, which plays an important role in reducing blood pressure, lowering blood lipids, and enhancing immunity, antioxidation and antitumor activity [90,91]. Chlorella contains a large amount of chlorophyll, which can absorb light over a wide range of wavelengths such that photosynthesis can be carried out at a particular wavelength [92]. Chlorella has been certified as a recognized safe (GRAS) organism by the FDA [93]. Zhao et al. designed a Chlorella-based live scaffold that adapted to irregularly shaped wounds and promoted their healing using an in situ microfluidic-assisted 3D bioprinting strategy [94]. Because photosynthetic protein nucleoli were introduced during 3D printing, this scaffold produced sustainable oxygen under light and promoted cell proliferation, migration and differentiation even under low oxygen conditions. Therefore, printing an active scaffold loaded with microalgae directly onto diabetic wounds could significantly accelerate the closure of chronic wounds by reducing local hypoxia, increasing angiogenesis, and promoting the synthesis of the extracellular matrix (ECM). Wu et al. used live Chlorella and bacteria to fabricate hydrogel patches that produced hydrogen and oxygen and promoted the healing of chronic diabetic wounds by reducing oxidative stress and inflammation [95]. It has been proven that Chlorella possessed good biological safety and great clinical application prospects. Therefore, a new self-oxygen supplying nanosystem based on Chlorella could be constructed to overcome tumor hypoxia. Youn et al. first reported that the characteristics of photosynthetic oxygen production by Chlorella could be used in the treatment of hypoxic breast cancer [96]. First, Chlorella vulgaris produced high levels of oxygen under 660 nm light exposure. The gold nanorods in this system could increase the tumor ambient temperature to 41–42 °C in response to an 808 nm near-infrared laser, which significantly dilated tumor blood vessels and promoted the transport of the chemotherapeutic drugs DOX and oxygen to hypoxic tumors (Fig. 5A). However, the growth factors in Chlorella may be phagocytized by macrophages, resulting in a decline in oxygen production efficiency. Alginate is a type of natural polysaccharide with high adhesion, good biocompatibility and degradability and is therefore widely used in cell transport [97,98]. Jiang et al. coated calcium alginate onto Chlorella and protected Chlorella from being phagocytized, thus realizing a long-term standby oxygen supply (Fig. 5B) [99]. As shown in Fig. 5C, D, F, the system produced oxygen from the decomposition of water and energy conversion after light triggering, which effectively alleviated tumor hypoxia. And then large amounts of ROS could be produced to effectively kill tumor cells in vitro (Fig. 5E, G). It has been demonstrated that Chlorella has excellent potential in hypoxia-tolerant photodynamic therapy.
Fig. 5.
A. Scheme showing in situ rapid gelation of Chlorella AuNRs BSA-Gel that generated oxygen for overcoming hypoxia [96]. B. Schematic diagram of the hypoxia-resistant PDT induced by the ALGAE system. C. Schematic illustration of the cellular experiment with 4T1 cells treated with Ce6, Ce6-ALGAE under 635 nm laser light. D. The images that were stained by the hypoxic probe in cells irradiated with or without ALGAE by confocal microscopy (LSCM). E. The cellular expression of HIF-1α with or without ALGAE after irradiation. F. LSCM images of the intracellular ROS production. G. Cell viability of conventional PDT with or without ALGAE under hypoxic and normoxic conditions [99]. Adapted with permission from Ref. [96] Copyright 2019, Elsevier; Adapted with permission from Ref. [99] Copyright 2019, Elsevier.
2.2.2. Chlorella in RT and SDT
The oxygen production system based on Chlorella plays an important role in PDT-based antitumor strategy, and researchers have further explored their potential applications in tumor RT. Zhou et al. first designed an erythrocyte membrane-modified microalgae (RBCM-Algae) to enhance the therapeutic efficacy of RT-mediated hypoxia-tolerant tumors [100]. Fig. 6B showed that RBCM-Algae could produce O2 when stimulated by red light, which increased the oxygenation of the local tumor tissue. Furthermore, under laser irradiation, RBCM-Algae would breakdown, resulting in the release of chlorophyll from the microalgae. Subsequently, under 650 nm irradiation, free chlorophyll could be used as a photosensitizer to produce ROS, thus enhancing PDT efficiency. They found that microalgae coated with calcium phosphate had good biocompatibility compared with silicon dioxide (SiO2), which could continuously supply oxygen and significantly improve the RT effect [101,102]. Both photosynthesis and PDT required photoexcitation while the poor penetration of light into biological tissues limited their efficiency. Generally, the construction of heterojunction structures (type II heterojunctions) is the most direct and effective means to improve the efficiency of light energy conversion [103,104]. Mei et al. constructed functionalized Chl cells (Chl-BP-Fe) of BPNSs through the Lego building block method and improved the oxygen production of Chl cells, which then enhanced the photodynamic effect of BPNSs [105]. The BPNSs adsorbed by polyaspartate (PASP) was first used as Lego building blocks and then loaded onto the Chl surface by the complexation reaction of Fe3+ with PASP. Under 658 nm laser irradiation, Chl cells were able to reduce tumor hypoxia and improve the efficiency of PDT through their own photosynthesis system. Importantly, the type of heterojunction with a suitable band structure was cleverly constructed between BPNSs and chlorophyll from Chl cells, greatly improving the efficiency of light penetration into the tissue. Moreover, Fe3+ between Lego blocks could both consume glutathione and reduce the resistance of tumor cells to ROS. This also catalyzed the reaction of Fe2+ with H2O2 to produce hydroxyl radicals, thus improving the efficiency of chemodynamic therapy (CDT)-mediated death of tumor cells. In summary, Chlorella could not only produce oxygen via photosynthesis, but also have reliable biosafety. Therefore, the Chlorella-based oxygen shuttle has the most potential in clinical applications.
Fig. 6.
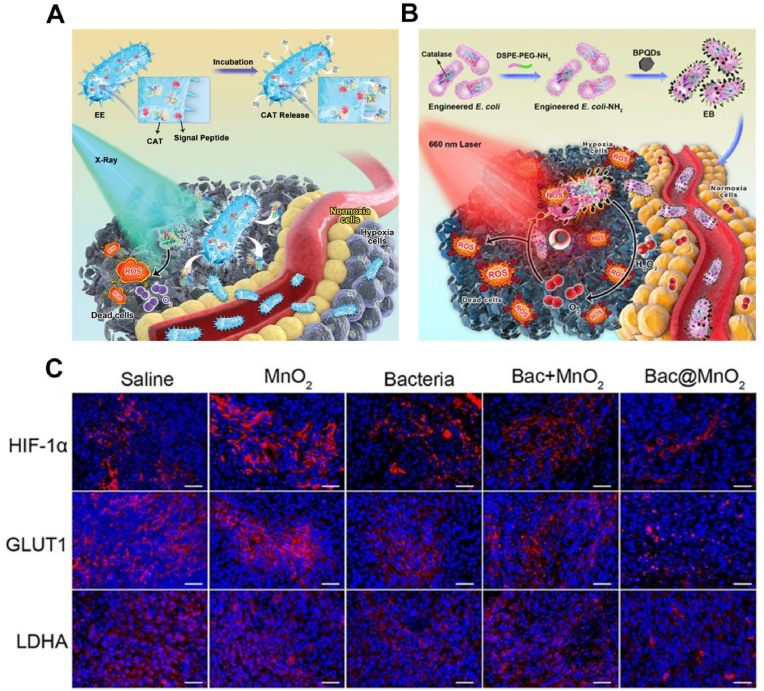
A. Engineered E. coli of secreted CAT for the treatment of hypoxic tumors [112]. B. Bacteria carrying CAT could relieve tumor hypoxia and thus enhance PDT [112]. C. Immunofluorescence staining of GLUT1, HIF-1α, and LDHA of mice tumor tissues after different treatments [114]. Adapted with permission from Ref. [114] Copyright 2021, Wiley-VCH; Adapted with permission from Ref. [112] Copyright 2020, American Chemical Society; Adapted with permission from Ref. [114] Copyright 2020, Wiley-VCH.
2.3. Bacteria engineering for oxygen supplying
Researchers have combined the ability of facultative anaerobic bacteria or obligatory anaerobic bacteria to colonize tumors with the oxygen production performance of photosynthetic bacteria to greatly increase the intratumor oxygen content [106]. To expand bacterial therapy, researchers have focused on genetically engineered common bacteria for O2 producing [107,108]. For example, bacteria were genetically engineered to produce enzymes that could catalyze H2O2 to generate O2. The facultative anaerobic bacterium E. coli DH5α is a genetically modified competent and it's easy to absorb exogenous genes, which makes it suitable for gene modification and targeted delivery [109]. Zhao et al. genetically modified E. coli by plasmid transfection to obtain overexpressed human catalase (CAT) in E. coli, which catalyzed O2 production from endogenous H2O2 in situ to enhance the PDT effect [110]. In addition, PDT mediated by bacterial surface-loaded nanodopamine (pDA) also selectively ablated tumors under near-infrared irradiation. The integration of transgenic bacteria and pDA/Ce6 showed a potent antitumor effect. At the same time, Zhao et al. also used these engineered bacteria to achieve continuous oxygen supply in solid tumors and obtained positive RT effects (Fig. 6A) [111]. In addition to the genetic modification of bacteria to express CAT, bacteria could also be engineered nanoenzymes on their surface to catalyze O2 production (Fig. 6B) [112]. Under natural environmental conditions, facultative anaerobic Shewanella oneidensis MR-1 (S. oneidensis MR-1) could transfer electrons from some organic compounds (such as formic acid, amino acids, and lactic acid) to the terminal electron receptors of redox-active minerals (Fe3+ and Mn4+) and then respire in the absence of molecular oxygen [113]. Given this unique property of MR-1, Zhang et al. modified MnO2 nanosheets on MR-1 to construct a biohybrid bacterium (Bac@MnO2) [114]. MnO2 nanoflowers with larger surface areas could serve as electron acceptors to provide larger reaction regions for lactate. Simultaneously, MnO2 could catalyze the conversion of H2O2 into O2, which both improved tumor hypoxia and prevented lactate production by downregulating the expression of glucose transporter type 1 (GLUT1), HIF-1α, and interfering lactate dehydrogenase A (LDHA), thus effectively inhibited tumor growth (Fig. 6C).
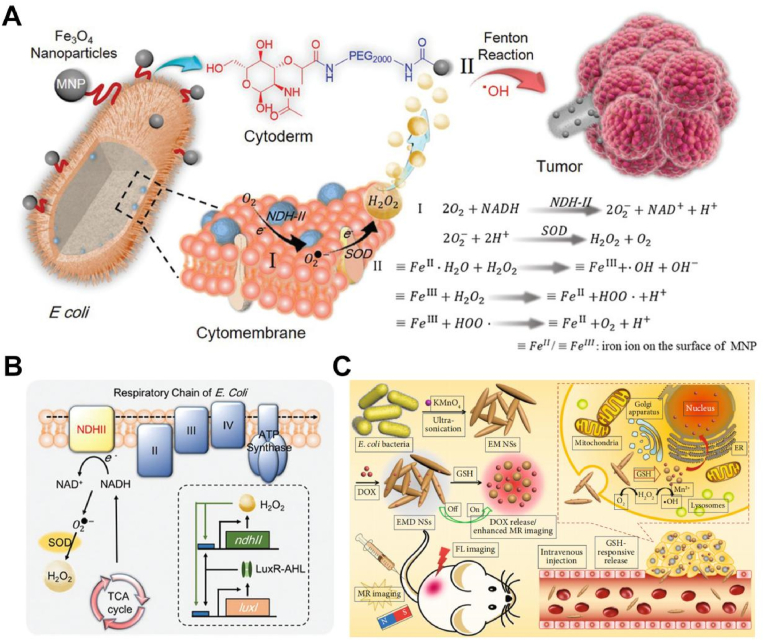
However, due to the limited content of endogenous H2O2 limiting the application of CAT and nanowaves in situ catalysis, the introduction of exogenous H2O2 is crucial to improve the treatment of hypoxic tumors [115]. Inspired by magnetic bacteria, Zhang et al. modified magnetic ferroferric oxide (Fe3O4) nanoparticles (MNPs) on the E. coli surface and developed an engineered bacteria-based integrated bioreactor (Ec-pE@MNPs) (Fig. 7A) [116]. The engineered bacterium overexpressed the respiratory chain enzyme NDH-2, which received electrons from nicotinamide adenine dinucleotide (NADH) and then transferred the electrons to O2 to produce H2O2 (Fig. 7B). Subsequently, the MNPs on the surface of E. coli catalyzed H2O2 to produce O2 in situ via a Fenton-like reaction. Moreover, E. coli-pE produced continuous H2O2 while producing large amounts of hydroxyl radicals (•OH) to induce tumor cell apoptosis. This bacterial manipulation allowed a bacterium to deliver different drugs for different situations to treat hypoxic tumors. Zhou et al. modified magnetic Fe3O4 NPs on the surface of magnetically engineered Spirulina platensis via a dip-coating process and developed a photosynthetic biohybrid nanopolymer system (PBNs) [117]. This system had a high tumor aggregation rate due to the presence of magnetic Fe3O4 NPs. Subsequently, PBNs that colonize the anoxic region of the tumor could regulate the tumor TME by forming oxygen in situ through photosynthesis. In addition, the chlorophyll released from PBNs treated with RT can act as photosensitizers to produce cytotoxic reactive oxygen species under laser irradiation, thus achieving powerful PDT. Inspired by the strong oxidant potassium permanganate (KMnO4), MnO4− is reduced to MnO2 [118,119]. For the first time, Wu et al., used E. coli as a template and reducing agent to build a tumor microenvironment-responsive nanospindle (EM NSs) at room temperature and loaded the chemotherapeutic drug DOX (EMD NSs) by ultrasonication with (KMnO4 at room temperature (Fig. 7C) [120]. The MnOx structure in the EMD NSs could react with GSH in the TME to cause EMD NSs breakdown with the simultaneous release of DOX and Mn2+. Subsequently, the released DOX could eliminate cancer cells, and Mn2+, with Fenton-like properties, could convert endogenous H2O2 into highly toxic hydroxyl radicals to induce apoptosis. The MnO2 in EMD NSs could also react with H2O2 in the TME to produce a large amount of O2, alleviating tumor hypoxia while overcoming hypoxia-induced chemoresistance. After genetic modification, E. coli, Shewanella oneidensis, and Spirulina platens could produce oxygen to improve the hypoxic TEM. Interestingly, these oxygen shuttles could also rely on oxygen targeting accurately reach tumors, greatly inhibiting tumor growth.
Fig. 7.
A. The scheme of bacteria-based Fenton-like bioreactor was capable of oxygen production and used for enhanced CDT [115]. B. Mechanism of NDH-2 expression based on synthetic biology, and the function of NDH-2 played in the respiratory chain of bacteria [115]. C. Schematic illustration of the fabrication of EMD NSs that enhanced CDT combination therapy [120]. Adapted with permission from Ref. [115] Copyright 2019, Wiley-VCH; adapted with permission from Ref. [120] Copyright 2020, Science Partner Journals.
3. Conclusions and perspectives
The development of live microorganism-based oxygen shuttles have facilitated the rapid development of novel advanced bioactive materials for high-performance hypoxic tumor therapy. In this review, we summarize the recent advances in innovative live microorganism-based oxygen shuttles for the treatment of hypoxic tumors. Photosynthetic microorganism-based oxygen shuttles possessed inherent advantages in cancer treatment over traditional oxygen shuttles. The controlled and continuous oxygen produced by photosynthetic microorganisms in response to light could increase the oxygen concentration at the tumor site and reverse the immunosuppressive TME, thus greatly improving the therapeutic effect of oxygen-dependent treatment modalities. Thus, oxygen shuttles constructed by photosynthetic microorganisms showed excellent oxygen encapsulation rate and loading capacity compared to conventional oxygen shuttles. However, Cyanobacteria and Chlorella were mostly administered through i.v., the low tumor targeting ability of photosynthetic microorganisms would lead to insufficient oxygen production. Genetically engineered bacteria were also developed for oxygen production. However, it should be noted that although these engineered bacteria greatly improved their tumor accumulation, some inherent disadvantages of engineered bacteria such as the instability of gene expression and the introduction of resistance genes. Although many attenuated live bacteria have been developed, such as Salmonella and E. coli, their residual toxicity has still been a concerned even after the removal of toxin genes. It should be further noted that Cyanobacteria and Chlorella are superior to engineered bacteria in biocompatibility. Although Microalgae have been used in the treatment of various diseases such as cardiovascular diseases, brain diseases, human wound healing, and gut-related diseases, it still needs more studies to assess their safety and therapeutic efficacy before clinical applications. In addition, we discussed in detail how oxygen production increased the therapeutic efficacy in hypoxic tumors. Notably, the novel oxygen shuttle has made significant progress in the treatment of hypoxic tumors, but there are still numerous challenges involved in the clinical translation of bacteria.
Bacterial virulence remains a prominent obstacle to the application of bacterial therapies in clinic, and even attenuated species may have some adverse effects in a dose-dependent manner. Furthermore, it is likely that most administered bacteria will be cleared by the mesh endothelial system before colonization in tumor site, thereby affecting therapeutic efficacy. With the rapid development of synthetic biology, an increasing number of targeted and controlled biological loads have been developed to transport drugs and imaging agents. Bacteria can be genetically engineered to reduce their inherent virulence without affecting tumor targeting, thereby increasing maximum tolerance. In addition, transgenic bacterial strains that respond to various stimuli (such as chemicals, temperature, light, osmolarity, and pH) can be generated which ensures a controlled release of cargo after sensing specific environmental signals. Stimulus response strategies further increase the in-situ accumulation of bacteria and minimize diffusion to normal tissues. In addition, bacterial surface modification of therapeutic agents, nanoparticles, liposomes, and micelles can enhance their targeted aggregation, and the biofilm surface modification of the nanoparticles improves their biocompatibility and targeting. Similarly, bacterial toxicity and immunogenicity can be mitigated by wrapping cells with highly biocompatible nanomaterials or biomics, which prevent them from being rapidly cleared by the immune system. What is more noteworthy is that the related bacteria in the intestinal microbiome are expected to be linked to the tumor treatment. Thus, attenuated or nontoxic probiotic strains can be developed to replace existing pathogens. As naturally avirulent strains, L. lactis and L. casei are widely used in the food industry and commonly regarded as beneficial strains for maintaining microbe balance in the intestine. They are facultatively anaerobic and can replace pathogenic Salmonella to serve as magic bullets targeting tumor hypoxia.
The instability of gene-engineered bacteria also contributes to their poor therapeutic efficacy. Bacteria possess a large genetic toolbox to enable rapid and simple modifications while it is also a primary drawback that hinders their application in clinical practice. Bacteria are prone to lose genes in repeat passage or in response to a complicated growth environment. In addition, after foreign gene editing, it is likely to affect their inherent biofunction, such as oxygen concentration-sensing. Therefore, to ensure the biosafety and stability of engineered live bacterial drugs, the following basic problems must be considered in the design: (1) avoid introducing resistance genes; (2) do not use plasmid-based gene editing systems as they are easy to lose and cause horizontal gene transfer, permanently integrate the gene circuit into the genome.
The oxygen production efficiency of microorganisms is highly important in the treatment of hypoxic tumors in vivo. Thus, how to accurately monitor the efficiency of oxygen production by microorganisms in vivo has been regarded as another big challenge. Due to the complicated biological environment of the human body, it is necessary to explore innovative methods to track bacterial biodistribution and their oxygen production capacity in vivo. Synthetic biology and imaging techniques can be used to monitor the survival and oxygen production of bacteria in vivo [121,122]. The oxygenation effect of tumors in vivo can be investigated by combined photoacoustic (PA) and B-mode ultrasound (US) imaging using oxygen saturation mode (sO2). Reliably, many probes have been developed for gas detection, such as a wide variety of probes of CO, NO and H2S. We believe these advanced O2 tracking techniques would accelerate their clinical applications of oxygen shuttles.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32271420, 21977024, and 31971304), the Beijing-Tianjin-Hebei Basic Research Cooperation Project (19JCZDJC64100), Cross-Disciplinary Project of Hebei University (DXK201916). One Hundred Talent Project of Hebei Province (E2018100002). Science Fund for Creative Research Groups of Nature Science Foundation of Hebei Province (B2021201038), Natural Science Foundation of Hebei Province (B2020201091), Guangdong Basic and Applied Basic Research Foundation (2021B1515120065).
Contributor Information
Xinjian Yang, Email: jianxinyang123@163.com.
Zhenhua Li, Email: zhenhuali@hbu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Bhandari V., Hoey C., Liu L.Y., Lalonde E., Ray J., Livingstone J., Lesurf R., Shiah Y.J., Vujcic T., Huang X.Y., Espiritu S.M.G., Heisler L.E., Yousif F., Huang V., Yamaguchi T.N., Yao C.Q., Sabelnykova V.Y., Fraser M., Chua M.L.K., Kwast T.V., Liu S.K., Boutros P.C., Bristow R.G. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019;51:308–318. doi: 10.1038/s41588-018-0318-2. [DOI] [PubMed] [Google Scholar]
- 2.Michiels C., Tellier C., Feron O. Cycling hypoxia: a key feature of the tumor microenvironment. Bba. Mol. Cell. Res. 2016;1866:76–86. doi: 10.1016/j.bbcan.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y., Tu W.Z., Shen M.W., Chen X.M., Ning Y.S., Li J.J., Chen T.F., Wang H., Yin F.F., Liu Y., Shi X.Y. Targeted tumor hypoxia dual-mode CT/MR imaging and enhanced radiation therapy using dendrimer-based nanosensitizers. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 4.Gao M., Liang C., Song X.J., Chen Q., Jin Q.T., Wang C., Liu Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv. Mater. 2017;29 doi: 10.1002/adma.201701429. [DOI] [PubMed] [Google Scholar]
- 5.Luo X.Y., Cao J., Yu J.M., Dai D.Q., Jiang W., Feng Y.H., Hu Y. Regulating acidosis and relieving hypoxia by platelet membrane-coated nanoparticle for enhancing tumor chemotherapy. Front. Bioeng. Biotechnol. 2022;12 doi: 10.3389/fbioe.2022.885105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y.H., Liu H.F., Dai X.Y., Li H., Zhou X.H., Chen S.Z., Zhang J.C., Liang X.J., Li Z.H. Cyanobacteria-based near-infrared light-excited self-supplying oxygen system for enhanced photodynamic therapy of hypoxic tumors. Nano Res. 2021;14:667–673. [Google Scholar]
- 7.Zhu W.W., Dong Z.L., Fu T.T., Liu J.J., Chen Q., Li Y.G., Zhu R., Xu L.G., Liu Z. Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy. Adv. Funct. Mater. 2016;26:5490–5498. [Google Scholar]
- 8.Yang Y.Y., Liu X., Ma W., Xu Q., Chen G., Wang Y.F., Xiao H.H., Li N., Liang X.J., Yu M., Yu Z.Q. Light-activatable liposomes for repetitive on-demand drug release and immunopotentiation in hypoxic tumor therapy. Biomaterials. 2021;265 doi: 10.1016/j.biomaterials.2020.120456. [DOI] [PubMed] [Google Scholar]
- 9.Henk J.M., Kunklerdagger P.B., Smith C.W. Radiotherapy and hyperbaric oxygen in head and neck cancer final report of first controlled clinical trial. Lancet. 1977;310:101–103. doi: 10.1016/s0140-6736(77)90116-7. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa K., Kohshi K., Ishiuchi S., Matsushita M., Yoshimi N., Murayama S. Old but new methods in radiation oncology: hyperbaric oxygen therapy. Int. J. Clin. Oncol. 2013;18:364–370. doi: 10.1007/s10147-013-0537-6. [DOI] [PubMed] [Google Scholar]
- 11.Rockwell S., Irvin C.G., Kelley M., Hughes C.S., Yabuki H., Porter E., Fischer J.J. Effects of hyperbaric oxygen and a perfluorooctylbromide emulsion on the radiation responses of tumors and normal tissues in rodents. Inr. J. Radiation. Onrolog. Bml. Phys. 1992;22:81–93. doi: 10.1016/0360-3016(92)90986-r. [DOI] [PubMed] [Google Scholar]
- 12.van den Brenk H.A.S., Jamieson D. Brain damage and paralysis in animals exposed to high pressure oxygen-pharmacological and biochemical observations. Biochem. Pharmacol. 1964;13:165–182. doi: 10.1016/0006-2952(64)90134-0. [DOI] [PubMed] [Google Scholar]
- 13.Yu L., Wang Z.J., Mo Z.M., Zou B.H., Yang Y.Y., Sun R., Ma W., Yu M., Zhang S.J., Yu Z.Q. Synergetic delivery of triptolide and Ce6 with light-activatable liposomes for efficient hepatocellular carcinoma therapy. Acta Pharm. Sin. B. 2021;11(7):2004–2015. doi: 10.1016/j.apsb.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D.D., Wu H.H., Lim W.Q., Phua S.Z.F., Xu P.P., Chen Q.W., Guo Zhen, Zhao Y.L. A mesoporous nanoenzyme derived from metal–organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv. Mater. 2019;31 doi: 10.1002/adma.201901893. [DOI] [PubMed] [Google Scholar]
- 15.Feng L.Z., Betzer O., Tao D.L., Sadan T., Popovtzer R., Liu Z. Oxygen nanoshuttles for tumor oxygenation and enhanced cancer treatment. CCS Chem. 2019;1:239–250. [Google Scholar]
- 16.Gao M., Liang C., Song X.J., Chen Q., Jin Q.T., Wang C., Liu Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv. Mater. 2017;29 doi: 10.1002/adma.201701429. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C., Chen W.H., Liu L.H., Qiu W.X., Yu W.Y., Zhang X.Z. An O2 self-supplementing and reactive-oxygen-species-circulating amplified nanoplatform via H2O/H2O2 splitting for tumor imaging and photodynamic therapy. Adv. Funct. Mater. 2017;27 [Google Scholar]
- 18.Cai X.C., Xie Z.X., Ding B.B., Shao S., Liang S., Pang M.L., Lin J. Monodispersed copper(I)-based nano metal-organic framework as a biodegradable drug carrier with enhanced photodynamic therapy efficacy. Adv. Sci. 2019;6 doi: 10.1002/advs.201900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y.H., Cheng H., Jiang C.X., Qiu X.F., Wang K.K., Huan W., Yuan A.H., Wu J.H., Hu Y.Q. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumor growth inhibition in photodynamic therapy. Nat. Commun. 2015;6:8785. doi: 10.1038/ncomms9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z.K., Liu L.L., Liang R.J., Luo Z.Y., He H.M., Wu Z.H., Tian H., Zheng M.B., Ma Y.F., Cai L.T. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano. 2018;12:8633–8645. doi: 10.1021/acsnano.8b04371. [DOI] [PubMed] [Google Scholar]
- 21.Gao S.T., Zheng P.L., Li Z.H., Feng X.C., Yan W.X., Chen S.Z., Guo W.S., Liu D.D., Yang X.J., Wang S.X., Liang X.J., Zhang J.C. Biomimetic O2-evolving metal-organic framework nanoplatform for highly efficient photodynamic therapy against hypoxic tumor. Biomaterials. 2018;178:83e94. doi: 10.1016/j.biomaterials.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W., Li Q., Xiao L., Dou J.X., Liu Y., Yu W.H., Ma Y.C., Li X.Q., You Y.Z., Tong Z.T., Liu H., Liang H., Lu L.G., Xu X.D., Yao Y.D., Zhang G.Q., Wang Y.C., Wang J. Hierarchical multiplexing nanodroplets for imaging guided cancer radiotherapy via DNA damage enhancement and concomitant DNA repair prevention. ACS Nano. 2018;12:5684–5698. doi: 10.1021/acsnano.8b01508. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W.T., Li S.H., Liu X.N., Yang C.Y., Hu N., Dou L.N., Zhao B.X., Zhang Q.Y., Suo Y.R., Wang J.L. Oxygen-generating MnO2 nanodots-anchored versatile nanoplatform for combined chemo-photodynamic therapy in hypoxic cancer. Adv. Funct. Mater. 2018;28 [Google Scholar]
- 24.Hao H.S., Yu M., Yi Y.F., Sun S.J., Huang X.Y., Huang C.Y., Liu Y.Q., Huang WeX., Wang J.Q., Zhao J., Wu M.Y. Mesoporous calcium peroxide-ignited NO generation for amplifying photothermal immunotherapy of breast cancer. Chem. Eng. J. 2022;437 [Google Scholar]
- 25.Liu Q.Y., Shi L.Y., Liao Y., Cao X.S., Liu X.Q., Yu Y.X., Wang Z.F., Lu X.H., Wang J.W. Ultrathin-FeOOH-coated MnO2 sonosensitizers with boosted reactive oxygen species yield and remodeled tumor microenvironment for efficient cancer therapy. Adv. Sci. 2022;9 doi: 10.1002/advs.202200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enríquez G.V., Arroyo A., Grijalva M., Zafra R.I.A., Camacho J. Molecular and cellular effects of hydrogen peroxide on human lung cancer cells: potential therapeutic implications. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/1908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M.X., Zhang F., Wang C.Q., Yin N., Wang Y.T., Qin G.X., Xu Q.L., Gong J.H., Liu H.Z., Duan X.R. Target-binding accelerated response for sensitive detection of basal H2O2 in tumor cells and tissues via a dual-functional fluorescence probe. Anal. Chem. 2022;94:5962–5969. doi: 10.1021/acs.analchem.2c00400. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D.D., Wei W., Liu Y., Pu Y.P., Liu S.Q. Dual imaging of poly (ADP-ribose) polymerase-1 and endogenous H2O2 for the diagnosis of cancer cells using silver-coated gold nanorods. Anal. Chem. 2021;93:16248–16256. doi: 10.1021/acs.analchem.1c04155. [DOI] [PubMed] [Google Scholar]
- 29.Chen G., Yang Y.Y., Xu Q., Ling M.G., Lin H.M., Ma W., Sun R., Xu Y.C., Liu X.Q., Li N., Yu Z.Q., Yu M. Self-amplification of tumor oxidative stress with degradable metallic complexes for synergistic cascade tumor therapy. Nano Lett. 2020;20(11):8141–8150. doi: 10.1021/acs.nanolett.0c03127. [DOI] [PubMed] [Google Scholar]
- 30.Shen J., Yu H.Z., Shu Y.M., Ma M., Chen H.R. A robust ROS generation strategy for enhanced chemodynamic/photodynamic therapy via H2O2/O2 self-Supply and Ca2+ overloading. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 31.Yang K., Qi S.L., Yu X.Y., Bai B., Zhang X.Y., Mao Z.W., Huang F.H., Yu G.C. A hybrid supramolecular polymeric nanomedicine for cascade-amplified synergetic cancer therapy. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202203786. [DOI] [PubMed] [Google Scholar]
- 32.Wang G., Gao J.Y., Fu Y.K., Ren Z.H., Huang J., Li X., Han G.R. Implantable composite fibres with self-supplied H2O2 for localized chemodynamic therapy. Chem. Eng. J. 2020;388 [Google Scholar]
- 33.Liu H.M., Cheng R., Dong X.H., Zhu S., Zhou R.Y., Yan L., Zhang C.Y., Wang Q., Gu Z.J., Zhao Y.L. BiO2−x nanosheets as radiosensitizers with catalase-like activity for hypoxia alleviation and enhancement of the radiotherapy of tumors. Inorg. Chem. 2020;59:3482–3493. doi: 10.1021/acs.inorgchem.9b03280. [DOI] [PubMed] [Google Scholar]
- 34.Xu B.L., Cui Y., Wang W.W., Li S.S., Lyu C.L., Wang S., Bao W.E., Wang H.Y., Qin M., Liu Z., Wei W., Liu H.Y. Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv. Mater. 2020;32 doi: 10.1002/adma.202003563. [DOI] [PubMed] [Google Scholar]
- 35.Zhu D.M., Chen H., Huang C.Y., Li G.X., Wang X., Jiang W., Fan K.L. H2O2 self-producing single-atom nanozyme hydrogels as light-controlled oxidative stress amplifier for enhanced synergistic therapy by transforming “cold” tumors. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 36.Sang Y.J., Cao F.F., Li W., Zhang L., You Y.W., Deng Q.Q., Dong K., Ren J.S., Qu X.G. Bioinspired construction of a nanozyme-based H2O2 homeostasis disruptor for intensive chemodynamic therapy. J. Am. Chem. Soc. 2020;142:5177–5183. doi: 10.1021/jacs.9b12873. [DOI] [PubMed] [Google Scholar]
- 37.Gao S.T., Jin Y., Ge K., Li Z.H., Liu H.F., Dai X.Y., Zhang Y.H., Chen S.Z., Liang X.J., Zhang J.C. Self-supply of O2 and H2O2 by a nanocatalytic medicine to enhance combined chemo/chemodynamic therapy. Adv. Sci. 2019;6 doi: 10.1002/advs.201902137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi H., Choyke P.L. Super enhanced permeability and retention (SUPR) effects in tumors folloeing near infrared photoimmunotherapy. Nanoscale. 2016;8:12504–12509. doi: 10.1039/c5nr05552k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang J., Islam R., Islam W., Yin H.Z., Subr V., Etrych T., Ulbrich K., Maeda H. Augmentation of EPR effect and efficacy of anticancer nanomedicine by carbon monoxide generating agents. Pharmaceutics. 2019;11:343. doi: 10.3390/pharmaceutics11070343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang D., Sun L.N., Huang L., Chen Y.Z. Nanodrug delivery systems modulate tumor vessels to increase the enhanced permeability and retention effect. J. Personalized Med. 2021;11:124. doi: 10.3390/jpm11020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q., Feng L.Z., Liu J.J., Zhu W.W., Dong Z.L., Wu Y.F., Liu Z. Intelligent albumin-MnO2 nanoparticles as pH-/H2O2 -responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016;28:7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- 42.Zhong Y., Su T., Shi Q.X., Feng Y.R., Tao Z., Huang Q.X., Li L., Hu L.Q., Li S.F., Tan H., Liu S., Yang H. Co-administration of iRGD enhances tumor-targeted delivery and anti-tumor effects of paclitaxel-loaded PLGA nanoparticles for colorectal cancer treatment. Int. J. Nanomed. 2019;14:8543–8560. doi: 10.2147/IJN.S219820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang L.H., Bettegowda C., Huso D.L., Kinzler K.W., Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl. Acad. Sci. USA. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zu C., Wang J.S. Tumor-colonizing bacteria: a potential tumor targeting therapy. Crit. Rev. Microbiol. 2014;40:225–235. doi: 10.3109/1040841X.2013.776511. [DOI] [PubMed] [Google Scholar]
- 45.Geng Z.M., Cao Z.P., Liu R., Liu K., Liu J.Y., Tan W.H. Aptamer-assisted tumor localization of bacteria for enhanced biotherapy. Nat. Commun. 2021;12:6584. doi: 10.1038/s41467-021-26956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W.F., He C.T., Qiao N., Guo Z.F., Hu S.L., Song Y.S., Wang H.R., Zhang Z.R., Ke B.W., Sun X. Dual drugs decorated bacteria irradiate deep hypoxic tumor and arouse strong immune responses. Biomaterials. 2022;286 doi: 10.1016/j.biomaterials.2022.121582. [DOI] [PubMed] [Google Scholar]
- 47.Zheng P.L., Fan M., Liu H.F., Zhang Y.H., Dai X.Y., Li H., Zhou X.H., Hu S.Q., Yang X.J., Jin Y., Yu N., Guo S.T., Zhang J.C., Liang X.J., Cheng K., Li Z.H. Self-propelled and near-infrared-phototaxic photosynthetic bacteria as photothermal agents for hypoxia-targeted cancer therapy. ACS Nano. 2021;15:1100–1110. doi: 10.1021/acsnano.0c08068. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z.J., Zhu Y.J., Dong Z.L., Hao Y., Wang C.J., Li Q.G., Wu Y.M., Feng L.Z., Liu Z. Engineering bioluminescent bacteria to boost photodynamic therapy and systemic anti-tumor immunity for synergistic cancer treatment. Biomaterials. 2022;281 doi: 10.1016/j.biomaterials.2021.121332. [DOI] [PubMed] [Google Scholar]
- 49.Yin T., Diao Z.Y., Blum N.T., Qiu L., Ma A.Q., Huang P. Engineering bacteria and bionic bacterial derivatives with nanoparticles for cancer therapy. Small. 2022;18 doi: 10.1002/smll.202104643. [DOI] [PubMed] [Google Scholar]
- 50.Fritz J.H., Girardin S.E., Fitting C., Werts C., Lecreulx D.M., Caroff M., Cavaillon J.M., Philpott D.J., Conquy M.A. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 2005;35:2459–2470. doi: 10.1002/eji.200526286. [DOI] [PubMed] [Google Scholar]
- 51.Huang X.H., Pan J.M., Xu F.N., Shao B.F., Wang Y., Guo X., Zhou S.B. Bacteria-based cancer immunotherapy. Adv. Sci. 2021;8 doi: 10.1002/advs.202003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei B.C., Pan J.M., Yuan R.T., Shao B.F., Wang Y., Guo X., Zhou S.B. Polarization of tumor-associated macrophages by nanoparticle-loaded Escherichia coli combined with immunogenic cell death for cancer immunotherapy. Nano Lett. 2021;21:4231–4240. doi: 10.1021/acs.nanolett.1c00209. [DOI] [PubMed] [Google Scholar]
- 53.Jing Z., Wang S.Q., Xu K.Y., Tang Q., Li W.J., Zheng W., Shi H.B., Su K.L., Liu Y.T., Hong Z.Y. A potent micron neoantigen tumor vaccine GP-neoantigen induces robust antitumor activity in multiple tumor models. Adv. Sci. 2022;9 doi: 10.1002/advs.202201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soo R.M., Hemp J., Parks D.H., Fischer W.W., Hugenholtz P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science. 2017;355:1436–1440. doi: 10.1126/science.aal3794. [DOI] [PubMed] [Google Scholar]
- 55.Zhao E.M., Liu H.F., Jia Y.R., Xiao T.S., Li J.X., Zhou G.Q., Wang J., Zhou X.H., Liang X.J., Zhang J.C., Li Z.H. Engineering a photosynthetic bacteria-incorporated hydrogel for infected wound healing. Acta Biomater. 2022;140:302–313. doi: 10.1016/j.actbio.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Chen C., Tang T., Shi Q.W., Zhou Z.D., Fan J.H. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022;126:99–112. [Google Scholar]
- 57.Liber J.A., Bryson A.E., Bonito G., Du Z.Y. Harvesting microalgae for food and energy products. Small Methods. 2020 [Google Scholar]
- 58.Wang Y., Xue Y.N., Zhang T., Fang Q.Q., Jin M.Y., Wang X.F., Wang Z.C., Hu Y.Y., Zhao W.Y., Lou D., Tan W.Q. Photosynthetic biomaterials: applications of photosynthesis in algae as oxygenerator in biomedical therapies. Bio-Des. Manuf. 2021;4:596–611. [Google Scholar]
- 59.Shahid A., Khurshid M., Aslam B., Muzammi S., Mehwish H.M., Rajoka M.S.R., Hayat H.F., Sarfraz M.H., Razzaq M.H., Nisar M.A., Waseem M. Cyanobacteria derived compounds: emerging drugs for cancer management. J. Basic Microbiol. 2021:1–18. doi: 10.1002/jobm.202100459. [DOI] [PubMed] [Google Scholar]
- 60.Silva M.R.O.B., Silva G.M., Silva A.L.F., Lima L.R.A., Bezerra R.P., Marques D.A.V. Bioactive compounds of arthrospira spp. (Spirulina) with potential anticancer activities: a systematic review. ACS Chem. Biol. 2021;16:2057–2067. doi: 10.1021/acschembio.1c00568. [DOI] [PubMed] [Google Scholar]
- 61.Cohen J.E., Goldstone A.B., Paulsen M.J., Shudo Y., Steele A.N., Edwards B.B., Patel J.B., MacArthur J.W., Hopkins M.S., Burnett C.E., Jaatinen K.J., Thakore A.D., Farry J.M., Truong V.N., Bourdillon A.T., Stapleton L.M., Eskandari A., Fairman A.S., Hiesinger W., Esipova T.V., Patrick W.L., Ji K., Shizuru J.A., Woo Y.J. An innovative biologic system for photon-powered myocardium in the ischemic heart. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1603078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong D.N., Zhang D.X., Chen W., He J., Ren C.J., Zhang X.C., Kong N., Tao W., Zhou M. Orally deliverable strategy based on microalgal biomass forintestinal disease treatment. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abi9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obaíd M.L., Camacho J.P., Brenet M., Orovio R.C., Carvajal F., Martorell X., Werner C., Simón V., Varas J., Calderón W., Guzmán C.D., Bono M.R., Martín S.S., Zajjur A.E., Egaña J.T. A first in human trial implanting microalgae shows safety of photosynthetic therapy for the effective treatment of full thickness skin wounds. Front. Med. 2021;8 doi: 10.3389/fmed.2021.772324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huo M.F., Wang L.Y., Zhang L.L., Wei C.Y., Chen Y., Shi J.L. Photosynthetic tumor oxygenation by photosensitizer-containing Cyanobacteria for enhanced photodynamic therapy. Angew. Chem. Int. Ed. 2020;59:1906–1913. doi: 10.1002/anie.201912824. [DOI] [PubMed] [Google Scholar]
- 65.Liu L.L., He H.M., Luo Z.Y., Zhou H.M., Liang R.J., Pan H., Ma Y.F., Cai L.T. In situ photocatalyzed oxygen generation with photosynthetic bacteria to enable robust immunogenic photodynamic therapy in triple-negative breast cancer. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 66.Yu J.J., Liberton M., Cliften P.F., Head R.D., Jacobs J.M., Smith R.D., Koppenaal D.W., Brand J.J., Pakrasi H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015;5:8132. doi: 10.1038/srep08132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun T., Zhang Y.Y., Zhang C.N., Wang H.J., Pan H.Z., Liu J., Li Z.X., Chen L., Chang J., Zhang W.W. Cyanobacteria-based bio-oxygen pump promoting hypoxia-resistant photodynamic therapy. Front. Bioeng. Biotechnol. 2020;8:237. doi: 10.3389/fbioe.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He C., Dong C.H., Hu H., Yu L.D., Chen Y., Hao Y.Q. Photosynthetic oxygen-self-generated 3D-printing microbial scaffold enhances osteosarcoma elimination and prompts bone regeneration. Nano Today. 2021;41 [Google Scholar]
- 69.Wang C.C., Zhao P.R., Jiang D.W., Yang G.L., Xue Y.D., Tang Z.G., Zhang M., Wang H., Jiang X.W., Wu Y.L., Liu Y.Y., Zhang W.A., Bu W.B. In-situ catalytic reaction for solving the aggregation of hydrophobic photosensitizers in tumor. ACS Appl. Mater. Interfaces. 2020;12:5624–5632. doi: 10.1021/acsami.9b21589. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y., Wang L.Z., Shi J.L. Two-dimensional non-carbonaceous materials-enabled efficient photothermal cancer therapy. Nano Today. 2016;11:292–308. [Google Scholar]
- 71.Xue C.C., Sutrisno L., Li M.H., Zhu W., Fei Y., Liu C.H., Wang X., Cai K.Y., Hu Y., Luo Z. Implantable multifunctional black phosphorus nanoformulation-deposited biodegradable scaffold for combinational photothermal/chemotherapy and wound healing. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120623. [DOI] [PubMed] [Google Scholar]
- 72.Li B.S., Cui Lai, Zeng G.G., Huang D.L., Qin L., Zhang M.M., Cheng M., Liu X.G., Yi H., Zhou C.Y., Huang F.L., Liu S.Y., Fu Y.K. Black phosphorus, a rising star 2D nanomaterial in the post-graphene era: synthesis, properties, modifications, and photocatalysis applications. Small. 2019;15 doi: 10.1002/smll.201804565. [DOI] [PubMed] [Google Scholar]
- 73.Chen T., Zeng W.W., Tie C.J., Yu M., Hao H.S., Deng Y., Li Q.Q., Zheng H.R., Wu M.Y., Mei L. Engineered gold/black phosphorus nanoplatforms with remodeling tumor microenvironment for sonoactivated catalytic tumor theranostics. Bioact. Mater. 2022;10:515–525. doi: 10.1016/j.bioactmat.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qi F.G., Ji P.H., Chen Z.X., Wang L.P., Yao H.L., Huo M.F., Shi J.L. Photosynthetic Cyanobacteria-hybridized black phosphorus nanosheets for enhanced tumor photodynamic therapy. Small. 2021;17 doi: 10.1002/smll.202102113. [DOI] [PubMed] [Google Scholar]
- 75.Hamblin M.R. Upconversion in photodynamic therapy: plumbing the depths. Dalton Trans. 2018;47:8571–8580. doi: 10.1039/c8dt00087e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu J.H., Shi J.L., Gao Y.Q., Yang W., Liu P., Liu Q.H., He F., Wang C.X., Li T., Xie R., Zhu J.X., Yang P.P. 808 nm near-infrared light-excited UCNPs@mSiO2-Ce6-GPC3 nanocomposites for photodynamic therapy in liver cancer. Int. J. Nanomed. 2019;14:10009–10021. doi: 10.2147/IJN.S221496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J., Xu L.G., Wang C.Y., Yang R., Zhuang Q., Han X., Dong Z.L., Zhu W.W., Peng R., Liu Z. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11:4463–4474. doi: 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 78.Li Y., Tang J.L., Pan D.X., Sun L.D., Chen C.Y., Liu Y., Wang Y.F., Shi S., Yan C.H. A versatile imaging and therapeutic platform based on dual-band luminescent lanthanide nanoparticles toward tumor metastasis inhibition. ACS Nano. 2016;10:2766–2773. doi: 10.1021/acsnano.5b07873. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X.Y., Zhang Y.Y., Zhang C.N., Yang C., Tian R., Sun T., Zhang W.W., Chang J., Wang H.J. An injectable hydrogel co-loading with cyanobacteria and upconversion nanoparticles for enhanced photodynamic tumor therapy. Colloids Surf., B. 2021;201 doi: 10.1016/j.colsurfb.2021.111640. [DOI] [PubMed] [Google Scholar]
- 80.Huo M.F., Liu P.L., Zhang L.L., Wei C.Y., Wang L.Y., Chen Y., Shi J.L. Upconversion nanoparticles hybridized Cyanobacterial cells for near-infrared mediated photosynthesis and enhanced photodynamic therapy. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 81.Lismont M., Dreesen L., Wuttke S. Metal-organic framework nanoparticles in photodynamic therapy: current status and perspectives. Adv. Funct. Mater. 2017;27 [Google Scholar]
- 82.Chang M.Q., Feng W., Ding L., Zhang H.G., Dong C.H., Chen Y., Shi J.L. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioact. Mater. 2022;10:131–144. doi: 10.1016/j.bioactmat.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu S.T., Feng W., Dong C.H., Song X.R., Gao X., Guo J.H., Chen Y., Hu Z.Q. Photosynthetic oxygenation-augmented sonodynamic nanotherapy of hypoxic tumors. Adv. Healthc. Mater. 2022;11 doi: 10.1002/adhm.202102135. [DOI] [PubMed] [Google Scholar]
- 84.Gilreath C., Boerma M., Qin Z.Q., Hudson M.K., Wang S.Z. The hypoxic microenvironment of breast cancer cells promotes resistance in radiation therapy. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.629422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan L., Gao P., Zhou W.H., Mei C.M., Huang Y.Y., Yu X.F., K Chu P., Chen T.F. Sequentially triggered delivery system of black phosphorus quantum dots with surface charge switching ability for precise tumor radiosensitization. ACS Nano. 2018;12:12401–12415. doi: 10.1021/acsnano.8b06483. [DOI] [PubMed] [Google Scholar]
- 86.Dong X.H., Cheng R., Zhu S., Liu H.M., Zhou R.Y., Zhang C.Y., Chen K., Mei L.Q., Wang C.Y., Su C.J., Liu X.F., Gu Z.J., Zhao Y.L. A heterojunction structured WO2.9-WSe2 nanoradiosensitizer increases local tumor ablation and checkpoint blockade immunotherapy upon low radiation dose. ACS Nano. 2020;14:400–416. doi: 10.1021/acsnano.9b08962. [DOI] [PubMed] [Google Scholar]
- 87.Gong F., Chen J.W., Han X., Zhao J.Y., Wang M.Y., Feng L.Z., Li Y.G., Liu Z., Cheng L. Core-shell TaOx@MnO2 nanoparticles as a nano-radiosensitizer for effective cancer radiotherapy. J. Mater. Chem. B. 2018;6:2250–2257. doi: 10.1039/c8tb00070k. [DOI] [PubMed] [Google Scholar]
- 88.Chai R., Yu L.D., Dong C.H., Yin Y.P.C., Wang S., Chen Y., Zhang Q. Oxygen-evolving photosynthetic cyanobacteria for 2D bismuthene radiosensitizer-enhanced cancer radiotherapy. Bioact. Mater. 2022;17:276–288. doi: 10.1016/j.bioactmat.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang J., Wang W.L., Zheng H.Z., Chen X., Liu X., Xie Q.Q., Cai X.M., Zhang Z.L., Li R.B. Nano-enabled photosynthesis in tumors to activate lipid peroxidation for overcoming cancer resistances. Biomaterials. 2022;285 doi: 10.1016/j.biomaterials.2022.121561. [DOI] [PubMed] [Google Scholar]
- 90.Bae M.J., Shin H.S., Chai O.H., Hanc J.G., Shon D.H. Inhibitory effect of unicellular green algae (Chlorella vulgaris) water extract on allergic immune response. J. Sci. Food Agric. 2013;93:3133–3136. doi: 10.1002/jsfa.6114. [DOI] [PubMed] [Google Scholar]
- 91.Bito T., Okumura E., Fujishima M., Watanabe F. Potential of Chlorella as a dietary supplement to promote human health. Nutrients. 2020;12:2524. doi: 10.3390/nu12092524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kubatka P., Kapinova A., Kruzliak P., Kello M., Vybohov D., Kajo K., Novak M., Chripkova M., Adamkov M., Pec M., Mojzis J., Bojkova B., Kassayova M., Stollarov N., Dobrota D. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition. 2015;31:560–569. doi: 10.1016/j.nut.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 93.Gouda M., Tadda M.A., Zhao Y.L., Farmanullah F., Chu B.Q., Li X.L., He Y. Microalgae bioactive carbohydrates as a novel sustainable and eco-friendly source of prebiotics: emerging health functionality and recent technologies for extraction and detection. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.806692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X.C., Yang C.Y., Yu Y.R., Zhao Y.J. In situ 3D bioprinting living photosynthetic scaffolds for autotrophic wound healing. Research. 2022 doi: 10.34133/2022/9794745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen H.H., Guo Y.F., Zhang Z.W., Mao W.X., Shen C.Y., Xiong W., Yao Y.F., Zhao X.Z., Hu Y.Q., Zou Z.G., Wu J.H. Symbiotic algae-bacteria dressing for producing hydrogen to accelerate diabetic wound healing. Nano Lett. 2022;22:229–237. doi: 10.1021/acs.nanolett.1c03693. [DOI] [PubMed] [Google Scholar]
- 96.Lee C., Lim K., Kim S.S., Thien L.X., Leeb E.S., Ohc K.T., Choid H.G., Youn Y.S. Chlorella-gold nanorods hydrogels generating photosynthesis-derived oxygen and mild heat for the treatment of hypoxic breast cancer. J. Contr. Release. 2019;294:77–90. doi: 10.1016/j.jconrel.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Lakkakula J.R., Gujarathi P., Pansare P., Tripathi S. A comprehensive review on alginate-based delivery systems for the delivery of chemotherapeutic agent: Doxorubicin. Carbohydr. Polym. 2021;259 doi: 10.1016/j.carbpol.2021.117696. [DOI] [PubMed] [Google Scholar]
- 98.Yan X., Li F., Hu K.D., Xue J.Z., Pan X.F., He T., Dong L., Wang X.Y., Wu Y.D., Song Y.H., Xu W.P., Lu Y. Nacre-mimic reinforced Ag@reduced graphene oxide-sodium alginate composite film for wound healing. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou T.J., Xing L., Fan Y.T., Cui P.F., Jiang H.L. Light triggered oxygen-affording engines for repeated hypoxia-resistant photodynamic therapy. J. Contr. Release. 2019;307:44–54. doi: 10.1016/j.jconrel.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 100.Qiao Y., Yang F., Xie T.T., Du Z., Zhong D.N., Qi Y.C., Li Y.Y., Li W.L., Lu Z.M., Rao J.H., Sun Y., Zhou M. Engineered algae: a novel oxygen-generating system for effective treatment of hypoxic cancer. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aba5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li W.L., Zhong D.N., Hua S.Y., Du Z., Zhou M. Biomineralized biohybrid-algae for tumor hypoxia modulation and cascade radio-photodynamic therapy. ACS Appl. Mater. Interfaces. 2020;12(40):44541–44553. doi: 10.1021/acsami.0c14400. [DOI] [PubMed] [Google Scholar]
- 102.Zhong D.N., Li W.L., Hua S.Y., Qi Y.C., Xie T.T., Qiao Y., Zhou M. Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in-situ modulating hypoxia and cascade radio-phototherapy. Theranostics. 2021;11:3580–3594. doi: 10.7150/thno.55441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ji X.Y., Wang J., Kang Y., Mei L., Su Z.G., Wang S.M., Ma G.H., Shi J.J., Zhang S.P. Enhanced solar energy harvest and electron transfer through intra-and inter-molecular dual-channel in chlorosome-mimicking supramolecular self-assemblies. ACS Catal. 2018;8:10732–10745. [Google Scholar]
- 104.Ji X.Y., Kang Y., Fan T.J., Xiong Q.Q., Zhang S.P., Tao W., Zhang H. An antimonene/Cp∗Rh(phen)Cl/black phosphorus hybrid nanosheet-based Z-scheme artificial photosynthesis for enhanced photo/bio-catalytic CO2 reduction. J. Mater. Chem. 2020;8:323–333. [Google Scholar]
- 105.Ou M.T., Lin C.C., Wan Y., Lu Y.T., Wang W.Y., Li Z.M., Zeng W.W., Zeng X.W., Ji X.Y., Mei L. Heterojunction engineered bioactive chlorella for cascade promoted cancer therapy. J. Contr. Release. 2022;345:755–769. doi: 10.1016/j.jconrel.2022.03.059. [DOI] [PubMed] [Google Scholar]
- 106.Chen W.F., Wang Y., Qin M., Zhang X.D., Zhang Z.R., Sun X., Gu Z. Bacteria-driven hypoxia targeting for combined biotherapy and photothermal therapy. ACS Nano. 2018;12:5995–6005. doi: 10.1021/acsnano.8b02235. [DOI] [PubMed] [Google Scholar]
- 107.Fan J.X., Niu M.T., Qin Y.T., Sun Y.X., Zhang X.Z. Progress of engineered bacteria for tumor therapy. Adv. Drug Deliv. Rev. 2022;185 doi: 10.1016/j.addr.2022.114296. [DOI] [PubMed] [Google Scholar]
- 108.Naganandhini S., John Kennedy Z., Uyttendaele M., Balachandar D. Persistence of pathogenic and non-pathogenic escherichia coli strains in various tropical agricultural soils of India. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lugo F.I., Estrada J.V., Alvis C.A., López J.O., Horcasitas M.C.M. Developing strategies to increase plasmid DNA production in Escherichia coli DH5α using batch culture. J. Biotechnol. 2016;233:66–73. doi: 10.1016/j.jbiotec.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 110.Deng X.Y., Yang W.B., Shao Z.W., Zhao Y.L. Genetically modified bacteria for targeted phototherapy of tumor. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120809. [DOI] [PubMed] [Google Scholar]
- 111.Huang C.Y., Wang F.B., Liu L., Jiang W., Liu W., Ma W., Zhao H. Hypoxic tumor radiosensitization using engineered probiotics. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202002207. [DOI] [PubMed] [Google Scholar]
- 112.Ding S.J., Liu Z.M., Huang C.Y., Zeng N., Jiang W., Li Q. Novel engineered bacterium/black phosphorus quantum dot hybrid system for hypoxic tumor targeting and efficient photodynamic therapy. ACS Appl. Mater. Interfaces. 2021;13:10564–10573. doi: 10.1021/acsami.0c20254. [DOI] [PubMed] [Google Scholar]
- 113.Wang S.Q., Shi L., Yu S.J., Pang H.W., Qiu M.Q., Song G., Fu D., Hu B.W., Wang X.X. Effect of Shewanella oneidensis MR-1 on U(VI) sequestration by montmorillonite. J. Environ. Radioact. 2022;242 doi: 10.1016/j.jenvrad.2021.106798. [DOI] [PubMed] [Google Scholar]
- 114.Chen Q.W., Wang J.W., Wang X.N., Fan J.X., Liu X.H., Li B., Han Z.Y., Cheng S.X., Zhang X.Z. Intervening tumor progression by coupling bacteria respiration with tumor metabolism. Angew. Chem., Int. Ed. 2020;59:21562–21570. doi: 10.1002/anie.202002649. [DOI] [PubMed] [Google Scholar]
- 115.W.T. Li, X.L. Zhou, S.K. Liu, J.L. Zhou, H. Ding, S.L. Gai, R.M. Li, L. Zhong, H.J. Jiang, P.P. Yang, Biodegradable nanocatalyst with self-supplying Fenton-like ions and H2O2 for catalytic cascade-amplified tumor therapy, ACS Appl. Mater. Interfaces 13 (2021), 50760-50773. [DOI] [PubMed]
- 116.Fan J.X., Peng M.Y., Wang H., Zheng H.R., Liu Z.L., Li C.X., Wang X.N., Liu X.H., Cheng S.X., Zhang X.Z. Engineered bacterial bioreactor for tumor therapy via Fenton-like reaction with localized H2O2 generation. Adv. Mater. 2019;31 doi: 10.1002/adma.201808278. [DOI] [PubMed] [Google Scholar]
- 117.Zhong D.N., Li W.L., Qi Y.C., He J., Zhou M. Photosynthetic biohybrid nanoswimmers system to alleviate tumor hypoxia for FL/PA/MR imaging-guided enhanced radio-photodynamic synergetic therapy. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 118.Pan J.B., Wang Y.Q., Pan H.Y., Zhang C., Zhang X.G., Fu Y.Y., Zhang X.J., Yu C.S., Sun S.K., Yan X.P. Mimicking drug-substrate interaction: a smart bioinspired technology for the fabrication of theranostic nanoprobes. Adv. Funct. Mater. 2017;27 [Google Scholar]
- 119.Felfoul O., Mohammadi M., Taherkhani S., Lanauze D., Xu Y.Z., Loghin D., Essa S., Jancik S., Houle D., Lafleur M., Gaboury L., Tabrizian M., Kaou N., Atkin M., Vuong T., Batist G., Beauchemin N., Radzioch D., Martel S. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumor hypoxic regions. Nat. Nanotechnol. 2016;11:941–947. doi: 10.1038/nnano.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bao Y.W., Hua X.W., Zeng J., Wu F.G. Research; 2020. Bacterial template synthesis of multifunctional nanospindles for glutathione detection and enhanced cancer-specific chemo-chemodynamic therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma W., Chen Q.L., Xu W.G., Yu M., Yang Y.Y., Zou B.H., Zhang Y.S., Ding J.X., Yu Z.Q. Self-targeting visualizable hyaluronate nanogel for synchronized intracellular release of doxorubicin and cisplatin in combating multidrug-resistant breast cancer. Nano Res. 2021;14(3):846–857. [Google Scholar]
- 122.Qi S.L., Zhang H.Y., Zhang X.Y., Yu X.Y., Wang Y.J., Meng Q.F., Yang K., Bai B., Tian R., Zhu S.J., Rao L., Huang F.H., Yu G.C. Supramolecular engineering of cell membrane vesicles for cancer immunotherapy. Sci. Bull. 2022;67:1898–1909. doi: 10.1016/j.scib.2022.08.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.